Abstract
Background
Nocturnal hypoglycemia is a significant problem. From 50% to 75% of hypoglycemia seizures occur at night. Despite the development of real-time glucose sensors (real-time continuous glucose monitor [CGM]) with hypoglycemic alarms, many patients sleep through these alarms. The goal of this pilot study was to assess the feasibility using a real-time CGM to discontinue insulin pump therapy when hypoglycemia was predicted.
Methods
Twenty-two subjects with type 1 diabetes had two daytime admissions to a clinical research center. On the first admission their basal insulin was increased until their blood glucose level was <60 mg/dL. On the second admission hypoglycemic prediction algorithms were tested to determine if hypoglycemia was prevented by a 90-min pump shutoff and to determine if the pump shutoff resulted in rebound hyperglycemia.
Results
Using a statistical prediction algorithm with an 80 mg/dL threshold and a 30-min projection horizon, hypoglycemia was prevented 60% of the time. Using a linear prediction algorithm with an 80 mg/dL threshold and a 45-min prediction horizon, hypoglycemia was prevented 80% of the time. There was no rebound hyperglycemia following pump suspension.
Conclusions
Further development of algorithms is needed to prevent all episodes of hypoglycemia from occurring.
Introduction
The term “closed loop pancreas” (CLP) as used in this study refers to a system in which a subcutaneous continuous glucose monitor (CGM) controls delivery of insulin via an insulin pump. The system requires an accurate CGM, an accurate insulin delivery system, and algorithms to adjust insulin delivery. Although CGM values are now approaching adequate accuracy1 and insulin pumps deliver insulin with precision, there have been relatively few clinical studies of algorithms to adjust insulin delivery.2,3
It is likely that initial CLP systems will be “partial” systems focusing on a particular aspect of diabetes management. Hypoglycemia is one of the major rate-limiting factors in diabetes management.4 In the Diabetes Control and Complications Trial, 55% of severe hypoglycemia (SH) occurred during sleep,5 and in children, approximately 75% of SH episodes occur during sleep.6 Although alarms for pending hypoglycemia are a part of many CGM systems, an initial study showed that 71% of alarms were not responded to during sleep.7 SH episodes are a well-recognized cause of death in people with diabetes.8,9 It is thus not unreasonable to expect that an early partial CLP might be aimed at preventing SH episodes during sleep. The purpose of this pilot study was to evaluate algorithms to discontinue insulin delivery when pending hypoglycemia was predicted.
Research Design and Methods
Subjects for this pilot study were recruited at the Barbara Davis Center (Aurora, CO) and the Stanford Medical Center (Stanford, CA). The protocol was approved by the local institutional review boards, and all subjects and parents and/or guardians signed an informed consent form and an assent form if necessary.
Twenty-two subjects with type 1 diabetes (T1D) were enrolled in the initial phase of this pilot study. All subjects had been diagnosed with T1D for at least 1 year and had used an insulin pump for at least 3 months. Potential subjects who had an SH event in the previous 18 months were excluded from the study.
Subjects were trained on the use of the FreeStyle Navigator® (Abbott Diabetes Care, Alameda, CA) and given two systems to be worn during their admissions to the Clinical Research Center (CRC). Subjects were admitted in the morning to the CRC on two occasions and had at least one functioning and calibrated Navigator at the time of their admission. During the initial visit, the basal insulin infusion rates were increased by approximately 25% increments to induce a steady decline in blood glucose (BG) level to <60 mg/dL. BG was measured from an intravenous line every 15–30 min depending on the BG level and the rate of fall. Patients were treated with oral glucose when their venous BG level was <60 mg/dL. Based on their insulin sensitivity from the first admission, basal rates were again increased on the second admission to produce a similar glucose rate of fall and a projected BG of <60 mg/dL. One-minute glucose readings from the Navigator receiver display were manually entered into an Excel (Microsoft, Redmond, WA) spreadsheet. The spreadsheet contained two hypoglycemic prediction algorithms. One was a modification of the current Navigator alarm, which uses a short-term linear extrapolation and uncertainty threshold to predict hypoglycemia (linear prediction [LP] alarm). The modification from the alarm on current receivers allowed for greater uncertainty, and therefore it was more sensitive in predicting hypoglycemia. The second algorithm was developed at Stanford and is based on multiple empirical, statistical models that are used to estimate future glucose values (statistical prediction [SP] alarm). From these models a probability of hypoglycemia is generated to produce an alarm. For each subject only one of the hypoglycemia prediction algorithms was used during the second admission. When the hypoglycemia prediction algorithm predicted a future BG of <80 mg/dL the insulin pump was suspended for 90 min. After 90 min, the pump basal insulin infusion was resumed at the usual infusion rate, and subjects were observed for 2 h in a fasting state to assess for subsequent hyperglycemia. Serum ketones were measured every ½ h during the pump suspension and for the 2 h after basal insulin infusion was resumed. During the pump suspension, BG levels were measured from a venous line every 10 min.
To determine how long basal insulin caused a negative rate of change in glucose levels after basal insulin was suspended, we measured the glucose rate of change after pump suspension in those subjects who did not require treatment for hypoglycemia. The time from when the pump was suspended until the rate of change reached 0 was considered the duration of the basal insulin hypoglycemic effect.
Results
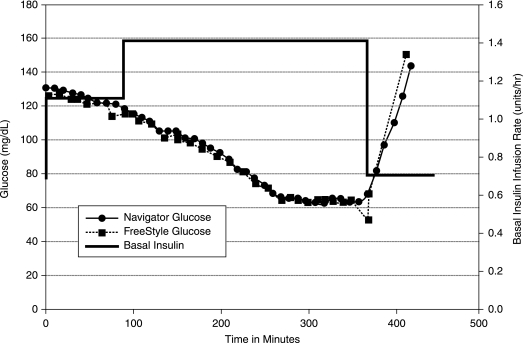
Twenty-two subjects with a mean age of 20 years (range 6–38 years) and a mean duration of diabetes of 12 years (range 2–24 years) completed both CRC admissions. Their mean body mass index was 22 kg/m2 (range 15–29 kg/m2). During the first CRC admission the basal rate was increased by a mean of 180% to induce hypoglycemia. During this admission, 18 of the 22 subjects (82%) reached a BG value of ≤60 mg/dL. The nadir glucose values for the four subjects not achieving a BG of <60 mg/dL were 61 mg/dL, 63 mg/dL, 66 mg/dL, and 91 mg/dL after 4–6 h of increasing basal rates up to 200% above their usual basal rate. Figure 1 is an example of glucose values from one study subject on the first admission. Her hypoglycemia was treated with 16 g of carbohydrate. During these admissions, the 30-min hypoglycemic prediction alarm that is integrated into the FreeStyle Navigator was set to 80 mg/dL and a 30-min projection horizon. This internal projection alarm failed to alarm in the first three subjects before their hypoglycemic threshold was reached. There after, only the LP or SP alarms were used to trigger pump suspension.
FIG. 1.
Graph of results from a first admission.
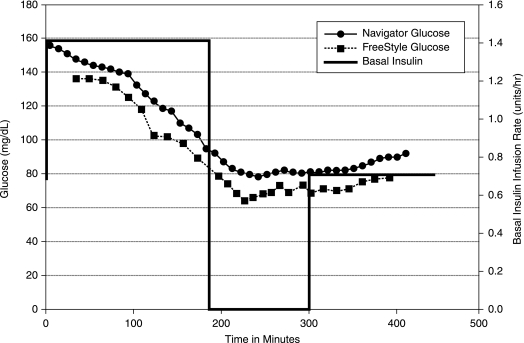
During the second admission, increases in the basal insulin infusion rate generated a predicted hypoglycemic alarm in 21 of 22 subjects. Figure 2 is an example of glucose values during the second admission from the same subject as in Figure 1. For the first 16 subjects, the alarm threshold was set to 80 mg/dL, and the projection horizon was set to 30 min. Of these subjects, the SP alarm was used with 15, and the LP alarm was used with one. Results are presented in Table 1. Nine of the 16 subjects (56%) had prevention of hypoglycemia, whereas the other seven had a BG level <60 mg/dL in the 90 min following pump suspension. One subject (clearly having T1D with three positive insulin autoantibodies at diagnosis, but with a body mass index of 29 kg/m2) was insulin resistant, and a predicted hypoglycemic alarm was unable to be generated despite a 200% increase in basal insulin rate (his lowest BG value was 98 mg/dL during his first admission and 78 mg/dL during his second admission). For the last five subjects studied, the LP alarm horizon was set to 45 min (with the same threshold of 80 mg/dL), and four out of the five subjects (80%) did not develop hypoglycemia following pump suspension. There were no SH events in any of the subjects.
FIG. 2.
Second admission with pump shutoff on projected alarm.
Table 1.
Effectiveness of Prediction Algorithms in Preventing Hypoglycemia (BG < 60 mg/dl) During the 90 Min of Pump Suspension
| Triggering alarm | Threshold (mg/dL) | Projection horizon | Number studied | Hypoglycemia prevented |
|---|---|---|---|---|
| SP | 80 | 30 min | 15 | 60% |
| LP | 80 | 30 min | 1 | 0% |
| LP | 80 | 45 min | 5 | 80% |
Following 90 min of pump suspension, there was no hyperglycemia (BG level >180 mg/dL) in the subsequent 2 h. The maximum BG level was 139 mg/dL (mean, 94 mg/dL; range, 72–139 mg/dL). Three subjects developed transient ketonemia (0.7–1.0 mmol/L) following pump suspension. This mild ketonemia occurred after 17–20 h of fasting and following 90 min of basal insulin suspension. Ketones resolved in all subjects with resumption of the basal insulin infusion and carbohydrate ingestion, and additional insulin was not required. No subject was symptomatic.
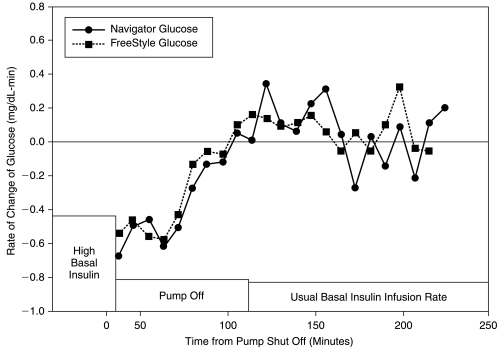
The mean duration of effective insulin action following pump suspension (time when the glucose rate of change reached 0 mg/dL-min) was 75 min based on venous BG readings and 80 min based on Navigator glucose readings (Fig. 3). The rate of change at the time of pump suspension was consistent with changes in the basal rate and was not as high as might be seen following an insulin bolus. The mean ± SD rate of change was −0.56 ± 0.44 mg/dL-min.
FIG. 3.
Rate of change of glucose levels following pump shutoff.
Discussion
For these pilot studies we initially used the “Projected Alarm” that is in the current version of the Navigator FreeStyle receiver with a threshold of 80 mg/dL and a projection horizon of 30 min. Using these settings, we did not generate a predictive alarm with our first three subjects. This is because the current model of the FreeStyle Navigator is set to provide a low frequency of false-positive alarms. Since this tuning of the alarm was not appropriate for our studies of preventing hypoglycemia by suspension of basal insulin infusion, we evaluated the use of two additional alarm algorithms. Using these alarms, we were still unable to prevent some subjects from decreasing to <60 mg/dL. With a projection horizon of 30 min and a glucose threshold of 80 mg/dL, pump suspension for 90 min prevented hypoglycemia 56% of the time. When the projection horizon was increased to 45 min, hypoglycemia was prevented 80% of the time.
It is clear that further development of algorithms to suspend insulin infusion is needed to prevent all episodes of hypoglycemia from occurring. Some additional modifications to future alarms could include: (1) consideration of the remaining “insulin-on-board” from both basal insulin and previous boluses of insulin; (2) using a longer prediction horizon so that the insulin infusion can be suspended earlier; (3) recalibration of the Navigator immediately before bed if BG levels are stable and showing greater than a 15% difference from the bedtime capillary BG readings; and (4) adding a user input to account for changes in insulin sensitivity. The latter might include vigorous activity earlier in the day. These were not taken into account in the current study. In our current studies we did have greater success in preventing hypoglycemia when we increased the alarm horizon to 45 min.
We were encouraged that we did not see rebound hyperglycemia in the 2 h following restoration of the usual basal infusion rates after pumps had been suspended for 90 min. These studies involved a prolonged fast for many subjects, from 9 p.m. until 4 p.m. the following day, and it was therefore not surprising that a few subjects developed mild ketonemia. This was transient and asymptomatic and resolved quickly once they received oral carbohydrates and insulin. Two of the subjects who did not experience hypoglycemia during the 90-min pump suspension did experience a BG <60 mg/dL in the ensuing 2 h. Thus, additional protective measures may be important.
One of the critical factors in determining when to suspend the insulin infusion to prevent hypoglycemia is the duration of action of basal insulin. Because only small amounts of insulin are given every few minutes, the pharmacodynamics of basal insulin action may be different when compared to a standard meal bolus. At the time of pump suspension, there was a negative rate of change of glucose levels, and this initial negative rate of change was maintained for about 30 min following pump suspension. Seventy-five minutes after pump suspension, the rate of change reached zero, indicating there was no longer sufficient insulin to lower BG levels. This time roughly correlates with the clinical finding that changes in basal insulin infusion rates need to occur about 1 h prior to the desired effect.
These pilot studies were conducted in a clinical research center during a daytime admission to determine the success of the hypoglycemic prediction algorithms in preventing hypoglycemia and to assess for rebound hyperglycemia after stopping basal insulin for 90 min. A larger study with proper sample size calculations now needs to be conducted in the home environment prior to applying similar algorithms to clinical practice. Some additional work also needs to be done on the hypoglycemic algorithm development to completely prevent hypoglycemic episodes from occurring. This may require initiating pump suspension 40–50 min before projected hypoglycemia or raising the alarm threshold above 80 mg/dL. There were no significant adverse events from suspending the basal insulin for 90 min. It remains to be determined if multiple episodes of pump suspension in a single night in the home environment would result in sustained hyperglycemia.
Acknowledgments
This research was supported by the Juvenile Diabetes Research Foundation. Abbott Diabetes Care provided the FreeStyle Navigator Continuous Glucose Monitoring Systems. Karmeen Kulkarni, M.S., R.D., B.C.-A.D.M., C.D.E., Kerstin Rebrin, M.D., Ph.D., Marc Taub, Ph.D., and Geoff V. McGarraugh from Abbott Diabetes Care provided technical and device support. The Clinical Research Center studies were supported in part by grants MO1 RR-00070 and RR000051 and 5 MO1 RR00069 from the National Center for Research Resources, National Institutes of Health.
Author Disclosure Statement
B.B. and H.P.C. have received research support from Abbott Diabetes Care in the form of devices and sensors for doing these studies. B.B. is also a consultant for Abbott Diabetes Care.
References
- 1.Wilson DM. Beck RW. Tamborlane WV. Dontchev MJ. Kollman C. Chase P. Fox LA. Ruedy KJ. Tsalikian E. Weinzimer SA DirecNet Study Group. The accuracy of the FreeStyle Navigator continuous glucose monitoring system in children with type 1 diabetes. Diabetes Care. 2007;30:59–64. doi: 10.2337/dc06-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weinzimer SA. Steil GM. Swan KL. Dziura J. Kurtz N. Tamborlane WV. Fully automated closed-loop insulin delivery versus semi-automated hybrid control in pediatric patients with type 1 diabetes using an artificial pancreas. Diabetes Care. 2008;31:934–939. doi: 10.2337/dc07-1967. [DOI] [PubMed] [Google Scholar]
- 3.Steil GM. Rebrin K. Darwin C. Hariri F. Saad MF. Feasibility of automating insulin delivery for the treatment of type 1 diabetes. Diabetes. 2006;55:3344–3350. doi: 10.2337/db06-0419. [DOI] [PubMed] [Google Scholar]
- 4.Cryer PE. Hypoglycemia is the limiting factor in the management of diabetes. Diabetes Metab Res Rev. 1999;15:42–46. doi: 10.1002/(sici)1520-7560(199901/02)15:1<42::aid-dmrr1>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 5.Diabetes Control and Complications Trial. Adverse events and their association with treatment regimens in the Diabetes Control and Complications Trial. Diabetes Care. 1995;18:1415–1427. doi: 10.2337/diacare.18.11.1415. [DOI] [PubMed] [Google Scholar]
- 6.Davis EA. Keating B. Byrne GC. Russell M. Jones TW. Hypoglycemia: incidence and clinical predictors in a large population-based sample of children and adolescents with IDDM. Diabetes Care. 1997;20:22–25. doi: 10.2337/diacare.20.1.22. [DOI] [PubMed] [Google Scholar]
- 7.Buckingham B. Block J. Burdick J. Kalajian A. Kollman C. Choy M. Wilson DM. Chase P Diabetes Research in Children Network. Response to nocturnal alarms using a real-time glucose sensor. Diabetes Technol Ther. 2005;7:440–447. doi: 10.1089/dia.2005.7.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heller S. Dead in bed. Diabet Med. 1999;16:782–785. [PubMed] [Google Scholar]
- 9.Nystrom L. Dahlqvist G. ‘Dead in bed’ in Norway. Diabet Med. 1996;13:495–496. doi: 10.1002/(SICI)1096-9136(199605)13:5<495::AID-DIA1119>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]