SUMMARY
The enzyme fatty-acid amide hydrolase (FAAH) catalyzes the in vivo degradation of the endocannabinoid anandamide, thus controlling its action at receptors. A novel FAAH inhibitor, AM3506, normalizes the elevated blood pressure and cardiac contractility of spontaneously hypertensive rats (SHR) without affecting these parameters in normotensive rats. These effects are due to blockade of FAAH and a corresponding rise in brain anandamide levels, resulting in CB1 receptor-mediated decrease in sympathetic tone. The supersensitivity of SHR to CB1 receptor-mediated cardiovascular depression is related to increased G-protein coupling of CB1 receptors. Importantly, AM3506 does not elicit hyperglycemia and insulin resistance seen with other FAAH inhibitors or in FAAH−/− mice, which is related to its inability to inhibit FAAH in the liver due to rapid hepatic uptake and metabolism. This unique activity profile offers improved therapeutic value in hypertension.
HIGHLIGHTS.
Unique pharmacological profile of novel fatty acid amide hydrolase inhibitor
Selectivity of AM3506 in FAAH inhibition in brain over liver in vivo
AM3506 as centrally acting antihypertensive agent with minimal cardiovascular effects under normotensive conditions and without adverse metabolic effects
INTRODUCTION
Endocannabinoids are polyunsaturated fatty acid amides such as arachidonoyl ethanolamide (anandamide) or esters such as 2-arachidonoyl glycerol (2-AG) that mobilize “on demand” and signal through two Gi/o protein-coupled cannabinoid receptors (Pacher, et al., 2006): CB1 receptors, highly expressed in the brain but also present at much lower yet functionally relevant concentrations in peripheral tissues, and CB2 receptors, expressed predominantly in immune and hematopoietic cells (Howlett, 2005).
The concentration of endocannabinoids at _target receptors is tightly controlled by enzymes that generate and eliminate them. Anandamide is preferentially degraded in vivo by fatty acid amide hydrolase (FAAH)(Cravatt, et al., 1996), whereas 2-AG is metabolized primarily by monoacylglyceride lipase (MAGL)(Dinh, et al., 2002), with additional involvement of αβ-hydrolase domain-containing 6 (ABHD6)(Marrs, et al., 2010). Pharmacological blockade or genetic ablation of FAAH in mice result in elevated tissue levels of anandamide, as well as CB1 receptor-mediated reductions in anxiety-like behavior (Kathuria, et al., 2003; Moreira, et al., 2008), pain sensation (Cravatt, et al., 2001) and enhanced hypotensive responsiveness to exogenous anandamide (Pacher, et al., 2005). The FAAH inhibitor URB597 was also found to normalize blood pressure in rats with various forms of hypertension by decreasing cardiac contractility and total peripheral resistance through a CB1 receptor-mediated mechanism (Batkai, et al., 2004). In humans, higher anandamide plasma levels have been linked to lower blood pressure in young males with a functional FAAH gene variant (Sarzani, et al., 2008) that had been found to result in reduced enzymatic activity (Chiang, et al., 2004). FAAH deficiency also promotes excessive energy storage and insulin resistance through a food intake-independent mechanism (Tourino, et al., 2010). Thus, a dynamic relationship exists between endocannabinoid tone and cardiovascular and metabolic functions.
Here we report the structure and pharmacological profile of a novel, highly potent and in vivo effective FAAH inhibitor, AM3506. AM3506 is more potent than URB597 in reducing blood pressure, heart rate and cardiac contractility in hypertensive animals through activation of CB1 receptors, which correlates with the blockade of FAAH activity and the consequent increase in tissue anandamide levels. The cardiovascular effects of AM3506 can be attributed to central CB1 receptor-mediated reduction in sympathetic tone, and the much greater responsiveness of hypertensive compared to normotensive animals is related to increased coupling of CB1 receptors to G proteins in the CNS of the former. Finally, unlike URB597 which blocks FAAH in both brain and liver and causes hyperglycemia in rats and glucose intolerance in obese mice, AM3506 does not block FAAH activity in liver and has no obvious effect on these glycemic parameters.
RESULTS
Structure of AM3506
AM3506 (Fig. 1E) is a hydroxyl substituted phenylalkylsulfonyl fluoride (Deutsch, et al., 1997) structurally related to the broad spectrum serine protease inhibitor, phenyl methyl sulfonyl fluoride (PMSF), but unrelated to the currently available in vivo effective FAAH inhibitors, such as the carbamate URB597 (Kathuria, et al., 2003) or the piperidine urea-based compounds (Ahn, et al., 2009).
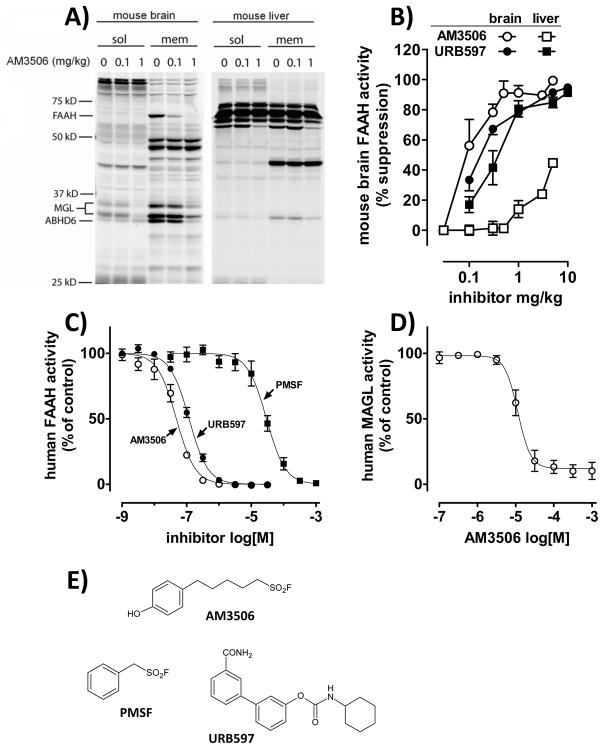
Figure 1.
Inhibition of FAAH activity by AM3506. A) Activity-Based Protein Profiling in mouse brain (left) and liver (right), soluble (sol) and membrane fractions (mem); B) Comparison of FAAH inhibition in mouse brain (○, ●) and liver (□, ■) by AM3506 (open symbols) and URB597 (filled symbols), as assessed by [3H]ethanolamine release from [3H]anandamide; C) Effect of inhibitors on human recombinant FAAH activity; D) Effect of AM3506 on human recombinant MAGL activity. Values in panels B–D are means±s.e.m. from 3–6 experiments in triplicates. E) Chemical structure of FAAH inhibitors.
FAAH Inhibitory Activity and _target Specificity of AM3506
The effectiveness and _target selectivity of AM3506 to inhibit FAAH activity was tested using several approaches. First, mice were treated with bolus i.p. doses of 0.1 or 1 mg/kg AM3506, and the activity of serine hydrolases in brain tissue obtained after 1 h was assayed using competitive activity-based protein profiling (Leung, et al., 2003). As illustrated in Fig. 1A, treatment of mice with 0.1 mg/kg AM3506 i.p. reduced brain FAAH activity with no change in the activity of any of a large number of other serine hydrolases whereas at 1 mg/kg, AM3506 completely blocked FAAH and partially inhibited MAGL and ABHD6. Using the same technique, AM3506 had similarly low ‘off-_target’ effects in the liver, with only MAGL and an unidentified, ~58kD protein, showing partial inhibition at the higher, 1 mg/kg dose. The effect of AM3506 on hepatic FAAH could not be assessed using this technique, due to the presence of prominent interfering proteins of similar molecular weight.
In the assay measuring the release of [3H]ethanolamine from [3H] anandamide (Fig. 1B), AM3506 caused dose-dependent inhibition of brain FAAH activity (ID50=0.10 mg/kg). Surprisingly, AM3506 was devoid of substantial FAAH inhibitory activity in liver of the same mice in doses up to 1 mg/kg. In contrast, the reference compound URB597 had similar FAAH inhibitory activity in liver (ID50=0.45 mg/kg) as in brain (ID50=0.22 mg/kg).
Using this same assay, both AM3506 and URB597 were fully effective orally and produced the same pattern of FAAH inhibition as after parenteral administration. Thus, 1 mg/kg AM3506 suppressed the enzyme in brain (by 90±0.4%), but had little effect on hepatic FAAH activity (by 13±2.6%; n=5), whereas 10 mg/kg URB597 equally suppressed FAAH activity in brain (by 86±2%) and liver (83±0.5%; n=5). The duration of FAAH inhibition was also tested. Following treatment with 1 mg/kg AM3506 i.p., FAAH was inhibited by >99% for up to 2 hours, ~90% at 12 hours and ~80% 24 hours in agreement with the covalent nature of its interaction with its _target, as reported earlier for fluorosulfonates, PMSF and some other fluorophosphonates (Seierstad and Breitenbucher, 2008).
The FAAH inhibitory activity of AM3506 and URB597 in vitro was also tested against human recombinant FAAH (Fig. 1C). In this assay, AM3506 was twice as potent as URB597 (IC50s of 48 nM and 118 nM, respectively), with PMSF being 3 orders of magnitude less potent. AM3506 weakly inhibited human recombinant MAGL with an IC50 of 11 μM (Fig. 1D), for a FAAH:MAGL potency ratio of >220-fold.
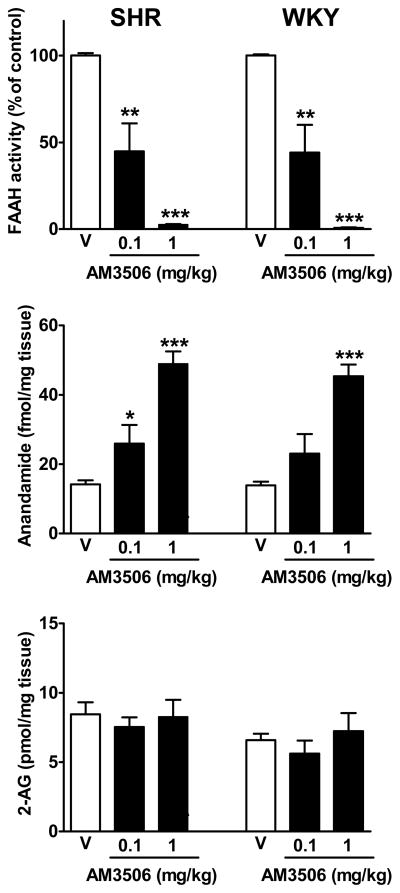
The assay based on the release of [3H]ethanolamine from [3H]anandamide was used to quantify FAAH activity in SHR and WKY brain (Fig. 2). Basal FAAH activity was similar in SHR (59.1±0.8) and WKY (62.6±1.1 fmol/mg/min, n=3–5), and was equally inhibited by AM3506, with ID50 values close to 0.1 mg/kg. The dose-dependent inhibition of FAAH by AM3506 was accompanied by parallel increases in brain anandamide levels, whereas 2-AG levels remained unaffected by AM3506 (Fig. 3). In a similar, ex vivo MAGL assay measuring the release of [3H]glycerol from 2-oleoyl [3H]glycerol in brain homogenates of mice treated 1 hour prior to sacrifice with vehicle vs. different i.p. doses of AM3506, no inhibition was observed at 0.3 and 1.0 mg/kg (−5.2±4.8% and −1.9±4.8%, respectively, n=4 in each group), and moderate inhibition was observed only after 3 mg/kg (−38.8±7.1%, n=4). This indicates >30-fold in vivo selectivity for FAAH over MAGL and also that the maximally effective in vivo dose of 1 mg/kg caused complete inhibition of FAAH with no inhibition of MAGL.
Figure 2.
Inverse relationship between FAAH activity and anandamide, but not 2-AG, levels in brain tissue is similar in SHR and WKY rats. Rats were treated with the indicated bolus i.p. doses of AM3506 and were sacrificed 1 h later to measure FAAH activity and anandamide and 2-AG levels in brain tissue. Values are means±s.e.m. from 5–6 experiments. *P<0.05, **P<0.01 and *** P<0.001, compared to vehicle (V).
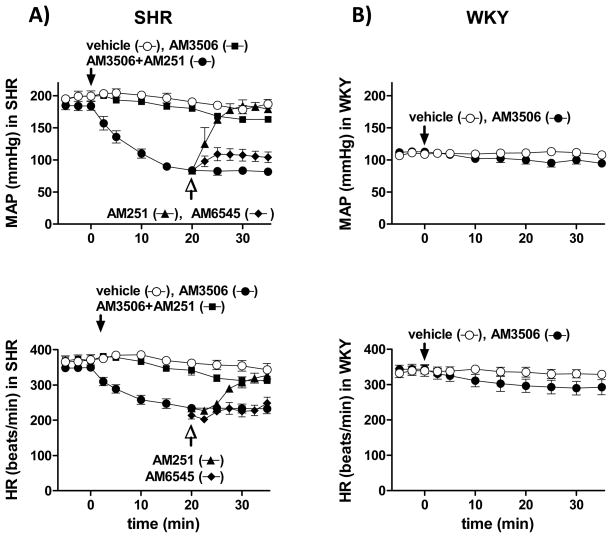
Figure 3.
Cardiovascular effects of AM3506 in anaesthetized SHR are mediated by CB1 receptors. Solid arrows indicate the i.v. injection to SHR (A) and WKY (B) of vehicle (○), 1 mg/kg AM3506 (●) and 1 mg/kg AM3506 following i.p. injection of 3 mg/kg AM251 60 min earlier (■). Open arrows indicate the i.v. injection of 3 mg/kg AM251 (▲) or 3 mg/kg AM6545 (◆) to a separate group of SHR, at the peak hypotensive response to AM3506. Values of MAP and HR are means±s.e.m. from 4–8 experiments.
AM3506 had low affinity for binding to CB1 (5.77μmol/L) and moderate affinity for CB2 receptors (192 nmol/L), and did not affect anandamide transport at concentrations up to 20 μmol/L.
Cardiovascular Effects of AM3506 are Mediated by Endogenous Anandamide Acting via CB1 Receptors
In SHR, AM3506 (1 mg/kg i.v.) elicited a profound and sustained decrease in MAP as well as marked bradycardia (Fig. 3A), whereas only slight changes in MAP and HR were observed in WKY (Fig. 3B). The effects of AM3506 in SHR were dose-dependent, with hypotensive and bradycardic ED50 values of 0.14 and 0.10 mg/kg, respectively, whereas URB597 was ~40 times less potent, with corresponding ED50 values of 5.2 and 4.0 mg/kg. The effects of AM3506 were prevented by pretreatment with the CB1 antagonist AM251, which also acutely reversed the fully developed effects (Fig. 3A). Similar acute reversal was elicited by the CB1 antagonist rimonabant (3 mg/kg, ΔMBP: +101±9 mmHg; ΔHR: +129±14 bpm), but not by a novel, peripherally restricted CB1 antagonist, AM6545 (Fig. 3A). In chronically cannulated, conscious SHR, AM3506 (0.5 mg/kg i.v.) reduced MAP and HR from 176 ±4 to 127±5 mmHg (P<0.01) and 355±6 to286±22 bpm (P<0.05, n=4), and the effects lasted up to an hour.
AM3506 (0.5 mg/kg i.v.) also caused hypotension and bradycardia in awake, chronically cannulated SHR. Peak decreases in MAP and HR were from 172 ± 4 to 128 ± 5 mmHg (P < 0.001, n = 5) and from 345 ± 10 to 290 ± 15 bpm (P < 0.05), respectively, and the effects lasted up to 60 minutes.
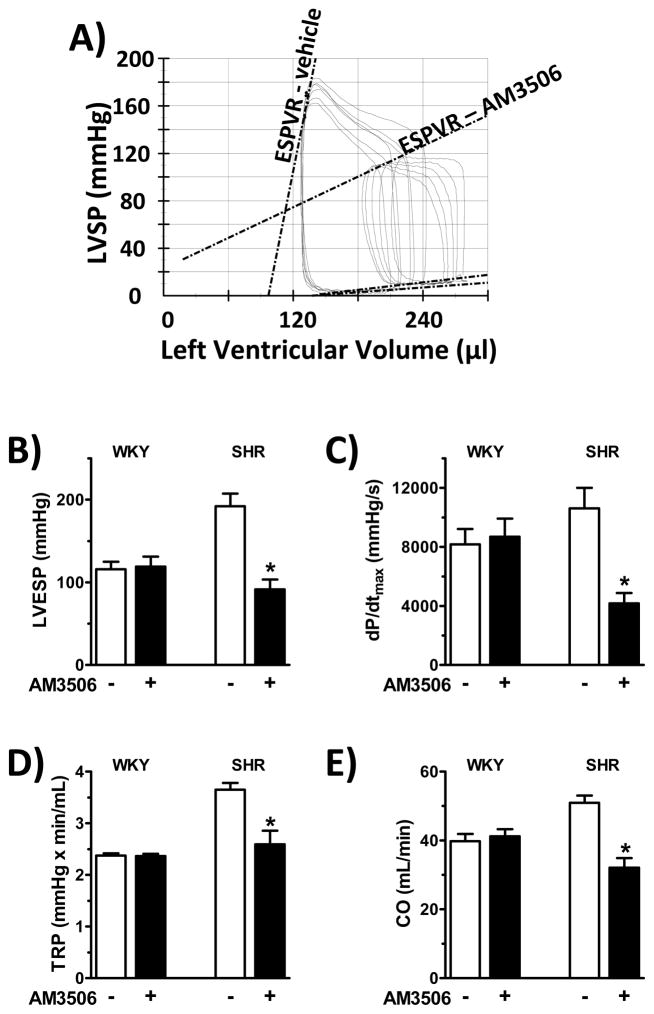
As shown in Fig. 4A, 1 mg/kg AM3506 caused a marked decrease in cardiac contractility in SHR, as assessed by the end-systolic pressure-volume relation (ESPVR), a load-independent indicator of systolic contractility (Kass, et al., 1987), as well as by decreases in left ventricular end-systolic pressure (LVESP) (Fig 4B) and dP/dtmax (Fig 4C), total peripheral resistance (TPR) (Fig 4D) and cardiac output (CO) (Fig 4E). No changes of these parameters were observed in normotensive WKY rats (Fig. 4B–E).
Figure 4.
Hemodynamic effects of AM3506 in anesthetized SHR and WKY rats. Anesthetized rats were instrumented with an intraventricular pressure/volume microcatheter system for measurement of A) end-systolic pressure-volume relations (ESPVR); B) left ventricular end-systolic pressure (LVESP); C) dP/dtmax; D) total peripheral resistance (TPR); and E) cardiac output (CO). Effects of vehicle (−, open columns) and 1 mg/kg AM3506 (+, filled columns) are shown. Panel A illustrates real-time pressure-volume loops recorded in a single SHR, whereas values in B to E are means±s.e.m. from 6 to 8 experiments. *P<0.05 compared to vehicle.
Cardiovascular Effects of AM3506 Depend on Autonomic Nervous Tone
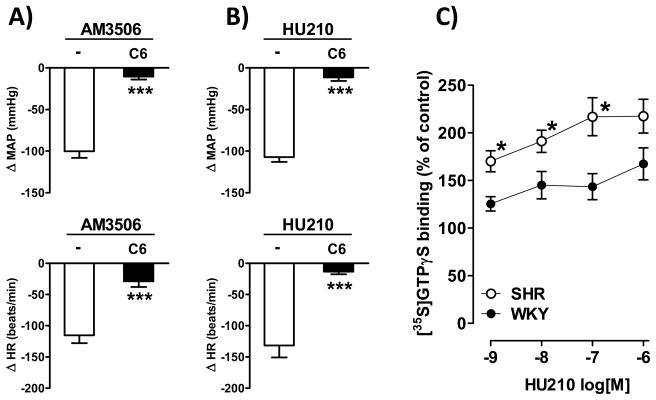
To test whether the effects of AM3506 are mediated via the autonomic nervous system, autonomic tone was disrupted by ganglionic blockade. Treatment of SHR with hexamethonium (C6, 20 mg/kg i.v) caused a profound drop in MAP and HR, which were then restored to the preexisting baseline by continuous infusion of vasopressin and isoproterenol, respectively. Blockade of autonomic tone was verified by the near-complete loss of reflex tachycardia or bradycardia in response to bolus injections of sodium nitroprusside or phenylephrine, respectively. In such animals, the hypotension and bradycardia elicited by 1 mg/kg AM3506 were reduced by 89.6±3.6% and 74.0±7.7%, respectively (Fig. 5A, n=5–13; P<0.001), indicating the dependence of these effects on intact autonomic tone. As a further indication that AM3506 decreases sympathetic tone, plasma norepinephrine levels in anesthetized SHR were reduced by 82±5%, from 311±41 to 53±12 pg/mL (n=5, P<0.01) 30 min after the i.v. administration of 1 mg/kg AM3506. In WKY, baseline plasma norepinephrine was lower than in SHR (198±11 pg/mL, P<0.05) and remained unaffected by AM3506 (167±35 pg/mL, P>0.2, n=3).
Figure 5.
Neuronal CB1 receptor coupling to G proteins is increased in hypertension. A bolus i.v. dose of 1 mg/kg AM3506 (A) or 1 μg/kg HU210 (B) was injected to anaesthetized control SHR (open columns) or SHR receiving hexamethonium+vasopressin+isoproterenol (C6; filled columns). Values are means±s.e.m. from 5–10 experiments, ***P<0.001 compared to control. C) Coupling efficiency of CB1 receptors in membrane preparations from whole brain of SHR and WKY rats was assessed by measuring HU210-induced increases in GTPγS labeling, as described in Methods. Values are means±s.e.m. from 4 experiments in triplicates. *P<0.05 compared to WKY rats.
Increased Cardiovascular Responsiveness of Hypertensive Rats to CB1 Receptor Activation
The greater depressor responses to AM3506 in SHR vs WKY despite similar degree of FAAH inhibition and rise in brain anandamide suggest increased _target-tissue responsiveness to CB1 receptor activation in SHR. Indeed, the cannabinoid agonist HU210 also elicited much greater hypotension in SHR than in WKY (Fig. 5B). In both strains, the effects of HU210 were abolished by AM251 or by ganglionic blockade, indicating the involvement of neuronal CB1 receptors.
The sympathoinhibitory effects of HU210 or AM3506 may be triggered in the CNS or at presynaptic CB1 receptors on postganglionic sympathetic nerve terminals in the heart and vasculature. To distinguish between these possibilities, we tested the cardiovascular effects of a non-brain penetrant CB1/CB2 mixed agonist, CB13 (Dziadulewicz, et al., 2007). The hypotensive effect of 0.3 mg/kg CB13 was similar in SHR and WKY (−40±6 vs −36±10 mmHg), suggesting that the increased hypotensive effectiveness of HU210 in SHR is due to a central mechanism.
Increased Efficiency of CB1 Receptor Coupling in SHR
The increased responsiveness of SHR to direct activation of CB1 receptors suggested increased expression and/or increased coupling efficiency of CB1 receptors. CB1 receptor density, as quantified by [3H]-rimonabant binding, was similar in WKY and SHR brain (1.58±0.16 vs 1.60±0.06 pmol/mg protein, respectively), with slightly higher affinity for the radioligand in SHR (0.71±0.09 nmol/L) than in WKY (1.92±0.53 nmol/L). However, CB1 receptor coupling efficiency, as deduced from HU210-stimulated GTPγS labeling, was dramatically increased in brain membrane preparations from SHR vs WKY (Fig. 5C).
Hepatic FAAH is not Inhibited by AM3506 Due to its Rapid Hepatic Metabolism
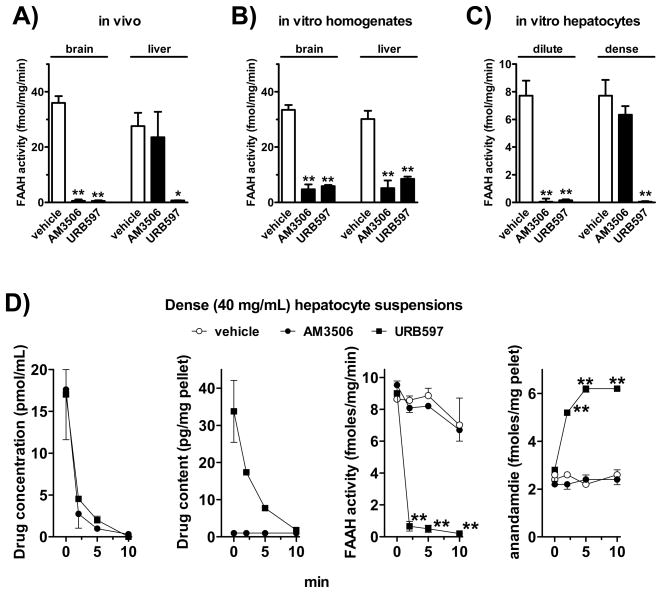
Following in vivo administration, a maximally effective dose of AM3506 (1 mg/kg i.p.) blocked FAAH activity in brain but not in liver, whereas an equimolar dose of URB597 (1.38 mg/kg i.p.) blocked FAAH activity equally in both tissues (Fig. 6A). In contrast, when the compounds were added in vitro to tissue homogenates at a concentration of 100 nM, they were both equally effective in blocking FAAH activity in brain and liver (Fig. 6B). This suggests that the ability of AM3506 to block FAAH is similar in brain and liver, but the uptake and metabolism of AM3506 by intact hepatocytes is rapid enough to prevent its access to FAAH.
Figure 6.
Selective resistance of hepatic FAAH to inhibition by AM3506 in vivo is related to rapid uptake and degradation of AM3506 by the liver. A) FAAH activity in brain and liver 1 h following in vivo treatment of mice with 1 mg/kg AM3506 or 1.38 mg/kg URB597. B) FAAH activity following in vitro incubation of brain or liver homogenates with 100 nM AM3506 or URB597. C) FAAH activity in cell pellets following incubation of dilute (1 mg cells/mL) or dense hepatocyte suspensions (40 mg cells/mL) with 100 nM AM3506 or URB597. D) Analysis of the fate and activity of AM3506 and URB597 in dense suspension of hepatocytes. Drugs or vehicle were added to hepatocyte suspensions at 0 min. At the indicated times, cells were rapidly centrifuged, and drug levels were determined in the cell-free supernatant (left) or pellet (2nd from left). The pellet was also used to determine FAAH activity (3d from left) and anandamide levels (right). Each point represents the means from 4–5 separate experiments. *P<0.05, **P<0.01 relative to corresponding 0 min value. For explanations, see text.
To further explore this possibility, we incubated hepatocyte suspensions of different cell density with a fixed concentration (100 nM) of AM3506 or URB597 versus vehicle for 30 min, and measured FAAH activity in the cell pellet (Fig. 6C). In a dilute suspension of hepatocytes (1 mg wet cell weight/mL medium), both AM3506 and URB597 nearly completely blocked FAAH, similar to what was observed in cell-free homogenates. When free drug was measured as the parent ion in the cell-free supernatant, 50–55% of the drug added at 0 min was still present for both AM3506 and URB597 at 10 min. In contrast, in dense suspensions of hepatocytes (40 mg/mL), URB597, but not AM3506 blocked FAAH activity, which replicates the findings in intact animals. Further analysis of such preparations indicated that both drugs were rapidly depleted from the medium (Fig. 6D, left panel). In the cell pellets, URB597 levels gradually declined over 10 min, whereas AM3506 levels were below threshold immediately after the addition of the drug and remained undetectable for the remaining incubation period (Fig. 6D, 2nd panel from left). In these experiments, the 0 min time point did include the time needed to separate the pellet (1 min of centrifugation at 0°C). FAAH activity was rapidly and nearly completely blocked in the URB597-exposed cells, whereas there was no measurable change in FAAH activity in AM3506-incubated cells (Fig. 6D, 3rd panel from left). Parallel to FAAH activity, anandamide levels in hepatocytes were markedly increased by URB597, but remained unchanged by AM3506 (Fig. 6D, right panel). The recovery of both drugs from cell-free medium remained constant from 0 to 30 min, so spontaneous degradation could not account for their time-dependent loss in the presence of hepatocytes.
Blockade of Hepatic FAAH by URB597 Results in Glucose Intolerance in Obese Mice
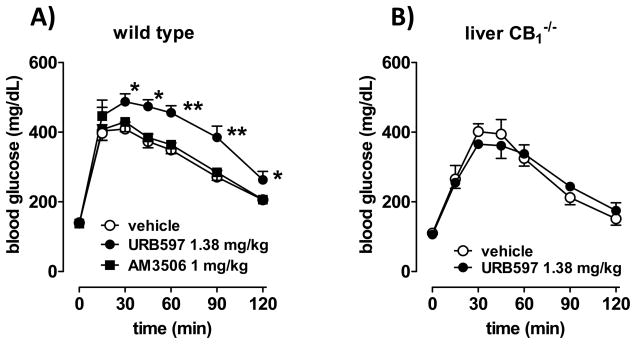
High-fat diet (HFD)-induced obesity (DIO) is associated with increased activity of the hepatic endocannabinoid/CB1 receptor system, which contributes to obesity-related glucose intolerance/insulin resistance (Osei-Hyiaman, et al., 2008). We tested whether acute treatment with FAAH inhibitors can influence glycemic control in DIO mice. As illustrated in Fig. 7A, treatment of male DIO mice with 1 mg/kg AM3506 did not affect glucose tolerance, as tested using the i.p. glucose tolerance test, whereas an equimolar dose of URB597 (1.38 mg/kg) resulted in a marked increase in glucose intolerance, even though it was much less effective in producing hypotension. Similar treatment with URB597 failed to affect glucose tolerance in liver-specific CB1−/− mice on HFD (Fig. 7B). Increasing the dose of URB597 to 10 mg/kg, which is equieffective with 1 mg/kg AM3506 in causing hypotension, caused the same degree of glucose intolerance in wild-type mice on HFD as the lower dose, and it remained ineffective in liver-specific CB1−/− mice on HFD (not shown). These findings indicate that blockade of FAAH in liver results in hepatic CB1 receptor-mediated increase in glucose intolerance.
Figure 7.
URB597, but not AM3506, increases glucose intolerance in mice with diet-induced obesity. A) Male mice kept on a high-fat diet for 14 weeks were subjected to an intraperitoneal glucose tolerance test (ipGTT) 60 min following the i.p. injection of vehicle, 1.38 mg/kg URB597 or 1 mg/kg AM3506, as described in Methods. Area under the curve (AUC, mg h/dL): 352±28 (vehicle), 570±45* (URB597), 411±35 (AM3506). *P<0.01 relative to value in vehicle treated mice; n=12–14 mice in each group. B) Liver-specific CB1 knockout (LCB1−/−) mice subjected to ipGTT following vehicle or URB597 treatment. AUC: 322±37 (vehicle) vs 337±14 (URB597), P>0.5, n=4.
URB597 (10 mg/kg i.p.) also increased non-fasting blood glucose in both SHR (from 104.0±7.4 to 144.0±9.6 mg/dL, n=4, P<0.05) and WKY (from 90.0±7.2 to 135.3±12.9 mg/dL, n=4, P<0.05) whereas AM3506, 1 mg/kg i.p., had no such effect (SHR: 97.8±5.6 to 96.8±2.9, n=6; WKY: 114.5±5.3 to 108.2±4.5, n=6). In the same rats, 10 mg/kg URB597 blocked hepatic FAAH activity by 93.1±0.3% in SHR and 92.6±0.2% in WKY, whereas 1 mg/kg AM3506 only marginally reduced it, by 11.1±0.4% in SHR and 6.7±0.2% in WKY (n=3–5).
DISCUSSION
Here we report the unique activity profile of a novel, highly potent and in vivo effective FAAH inhibitor, AM3506. Similar to another FAAH inhibitor, URB597, AM3506 reverses the increased blood pressure and cardiac contractility of hypertensive rats without causing similar effects in normotensive rats. These effects could be attributed to sympathoinhibition mediated indirectly by endogenous anandamide acting at CB1 receptors in the central nervous system. Unlike the other FAAH inhibitor, AM3506 does not inhibit FAAH activity in the liver and does not elicit hyperglycemia and insulin resistance seen after global inhibition or genetic ablation of FAAH, thus offering an improved therapeutic profile as a potential antihypertensive agent.
AM3506 is structurally unrelated to currently available FAAH inhibitors, and is a close structural analog of the serine protease inhibitor PMSF. The original observation that PMSF prolonged the half-life of anandamide and increased its apparent affinity for CB1 receptors in membrane preparations was the first evidence supporting the existence of an endogenous amidase being involved in the in vivo biodegradation of anandamide (Deutsch and Chin, 1993). Lead optimization efforts starting from AM374, an early generation FAAH inhibitor based on the PMSF structure (Deutsch, et al., 1997; Janero, et al., 2009; Lu, et al., 2006), led to AM3506, a sulfonyl fluoride analog with enhanced affinity and favorable oral bioavailability and brain penetrability. AM3506 is selective for FAAH over MAGL, the maximally effective in vivo dose of 1 mg/kg causing 100% inhibition of FAAH with no inhibition of MAGL, as verified using ex vivo enzyme activity assays.
Earlier observations indicated that the endocannabinoid system remains quiescent under normotensive conditions, but becomes activated in various experimental models of hypertension as a compensatory mechanism (Batkai, et al., 2004). This allows for the use of SHR as a model to study the cardiovascular consequences of modulating the activity of the endocannabinoid system (Batkai, et al., 2004). AM3506 caused strong cardiovascular depression in SHR and only modest hypotension and bradycardia in normotensive WKY rats. While these effects are similar to the earlier reported effects of the reference FAAH antagonist URB597 (Batkai, et al., 2004), they expand those findings in several ways.
First, ganglionic blockade combined with restoration of baseline vascular tone and heart rate resulted in a near complete loss of the hypotensive and bradycardic responses to AM3506 in SHR. These findings make it unlikely that modest increases in CB1 receptor expression in heart and aortic smooth muscle of SHR compared to WKY rats, as reported earlier (Batkai, et al., 2004), could account for the increased responsiveness of SHR to CB1 receptor activation, and indicate the dominant role of neuronal CB1 receptors. Reduction of sympathetic tone in SHR by AM3506 is further indicated by the marked reduction in plasma norepinephrine. This could result from a centrally triggered reduction in sympathetic outflow, or from activation of presynaptic CB1 receptors located at sympathetic nerve terminals in the heart and vasculature (Varga, et al., 1996). The former mechanism is favored, however, by the observation that the cardiovascular depressor response to AM3506 could be reversed by both rimonabant and AM251, brain-penetrant CB1 antagonists, but not by AM6545, a novel, peripherally restricted CB1 antagonist (Tam, et al., 2010). Furthermore, the moderate hypotensive effect of a peripherally restricted cannabinoid agonist was similar in SHR and WKY.
Second, there are both quantitative and qualitative differences between the FAAH inhibitory actions of AM3506 and URB597. AM3506 is more potent than URB597 in reducing blood pressure and heart rate as well as in inhibiting FAAH activity in brain, and its effects are much longer lasting. Unexpectedly, AM3506 failed to inhibit FAAH in the liver, whereas URB597 inhibited FAAH with similar potency and efficacy in brain and liver. A plausible reason for the lack of effect of AM3506 in the liver is its rapid uptake and metabolism by hepatocytes. Due to the covalent binding of AM3506 to its _target, it is not possible to quantitatively analyze its metabolic fate in intact cells. Instead, we used the approach of varying the ratio of liver tissue to drug in suspensions of isolated hepatocytes as a way explore the effect of cellular uptake and metabolism on the pharmacological action of a compound (Kunos, 1984). In dilute suspensions of hepatocytes, the capacity of the liver to eliminate AM3506 was not sufficient to prevent its access to and inhibition of FAAH. However, in a much more dense suspension of cells, which approximates the conditions in the intact liver, AM3506 was taken up and metabolized very rapidly, which prevented its ability to reach and block FAAH (Fig. 6D). URB597 was eliminated more slowly, and as a result it was able to fully block FAAH with the resulting increase in hepatic anandamide (Fig. 6D).
Formation of adducts of AM3506 with small molecules, as reported for PMSF (Fontana, et al., 1995), is unlikely to account for its lack of inhibition of FAAH in the liver, as AM3506 was able to fully block FAAH in the brain or in cell-free extracts of liver. Furthermore, interaction with ‘off-_target’ serine proteases in liver is also unlikely to explain the lack of FAAH inhibition, because AM3506 had fewer such _targets (Fig. 1A) than did URB597 (see Fig. 8 in (Ahn, et al., 2007), yet URB597 was able to block hepatic FAAH.
The lack of in vivo effect of AM3506 on hepatic FAAH turns out to be an advantageous feature, as the development of diet-induced fatty liver and insulin resistance have been linked to inhibition of FAAH activity in the liver and the resulting increase in hepatic anandamide levels (Osei-Hyiaman, et al., 2005; Osei-Hyiaman, et al., 2008; Tam, et al., 2010). The predisposition of FAAH-deficient mice to diet-induced hyperglycemia and insulin resistance (Tourino, et al., 2010) also supports this possibility. Indeed, URB597, but not AM3506, caused hyperglycemia in both SHR and WKY. Furthermore, URB597 even at a sub-hypotensive dose caused glucose intolerance in mice with diet-induced obesity, whereas an equimolar dose of AM3506, which elicited pronounced hypotension, caused no change in glucose tolerance. The absence of a similar effect of URB597 in hepatocyte-specific CB1 knockout mice indicates that this effect is likely mediated by anandamide acting via hepatic CB1 receptors (Fig. 7B).
Unlike in heart and aorta, CB1 receptor expression in the SHR brain is not increased. Instead, there was a marked increase in CB1 stimulated GTPγS binding in SHR compared to WKY brain tissue, indicating enhanced G protein activation. CB1 receptors are coupled to Gi/Go proteins, and upregulation of Giα protein isoforms has been documented in the heart and vasculature of rats with different forms of hypertension, including SHR (Ge, et al., 2006; Kost, et al., 1999; Marcil, et al., 1998; Marcil, et al., 1997). A similar mechanism in the brain may account for the enhanced cardiovascular responsiveness of hypertensive animals to CB1 receptor activation. Nevertheless, a small contribution of vascular and cardiac CB1 receptors is likely in view of the significant hypotensive response to the peripherally restricted CB receptor agonist, CB13. The fact that CB13 produced a moderate hypotensive effect in SHR and WKY also suggests that peripheral CB1 receptors are functional and may be subject for modulation in certain cardiovascular pathologies.
The location in the brain of CB1 receptors mediating cardiovascular depressor responses is unclear. Microinjection of anandamide or an anandamide uptake inhibitor into the brainstem nucleus of the solitary tract (NTS) of anesthetized normotensive rats was found to enhance the baroreflex inhibition of renal sympathetic nerve activity, an effect prevented by rimonabant, and increases in blood pressure resulted in enhanced anandamide content in the NTS (Seagard, et al., 2004). However, cannabinoids microinjected into the NTS caused less sympathoinhibition in SHR than in normotensive rats (Brozoski, et al., 2009), which is evidence against the NTS being the site of the antihypertensive effect of CB1 receptor activation. Another potential site is the rostral ventrolateral medulla, the location of premotor sympathetic neurons. It has been reported that microinjection of the synthetic cannabinoid agonist WIN55,212-2 into this site elicits hypotension in normotensive rats, whereas similar microinjection into the NTS had no effect on blood pressure (Niederhoffer, et al., 2003). The core central sympathetic network can also be regulated at hypothalamic nuclei, circumventricular organs and area postrema (Guyenet, 2006). Further studies are needed to identify the CNS location of CB1 receptors that mediate the enhanced cardiodepressor response to endogenous anandamide in SHR.
SIGNIFICANCE
The findings in this study provide an impetus to the use of FAAH inhibitors to treat hypertension. First, the absence of cardiovascular effects under normotensive conditions would minimize unwanted side effects, such as orthostatic hypotension. Second, FAAH inhibitors produce significant antianxiety effects in rodents (Kathuria, et al., 2003), which may provide added benefit in neurogenic hypertension. At the same time, behavioral effects that predict addictive potential, such as catalepsy, are not amplified by FAAH inhibition (Justinova, et al., 2008; Kathuria, et al., 2003), an important safety consideration. Third, a major component of the antihypertensive action of AM3506, similar to the previously reported effect of URB597 (Batkai, et al., 2004), is the marked reduction of the inappropriately increased cardiac contractility. This suggests that chronic inhibition of FAAH may not only normalize blood pressure but also mitigate the development of the associated cardiac hypertrophy, an independent risk factor for cardiovascular events and mortality. Finally, the distinctive pharmacological profile of AM3506 suggests a broader safety spectrum, especially with regard to absence of mechanism-based metabolic effects, which should make it attractive for potential therapeutic use as an antihypertensive in obese subjects.
EXPERIMENTAL PROCEDURES
Animals
Male 12–16 week-old spontaneously hypertensive rats (SHR) and age-matched Wistar Kyoto (WKY) rats were obtained from Charles River Laboratories; male C57Bl/6J mice were from Jackson Laboratories. Liver specific CB1−/− mice were generated as described (Osei-Hyiaman, et al., 2008). Experimental protocols were in accordance with the ethical guidelines for animal research and approved by the Institutional Animal Care and Use Committee of NIAAA.
General Surgical Procedures
Rats were anesthetized with isoflurane via a nose cone (induced with 3–4% and followed by 1.5% in pure oxygen). Adequacy of anesthesia was verified by the absence of tail-flick reflex. Animals were placed on a controlled heating pad and core body temperature, measured by a rectal probe, was maintained at 37°C. For experiments in conscious animals, rats were implanted with indwelling catheters in the femoral artery and femoral vein, as described earlier (Lake, et al., 1997), and allowed to recover. For other experiments, rats remained anesthetized and instrumented as described (Batkai, et al., 2004). Briefly, a left femoral artery was cannulated and the catheter connected to a pressure transducer (Abbott Park, IL) for monitoring mean arterial pressure (MAP). Heart rate (HR) was derived from the pressure wave. Signals were amplified through a 13–4615-71 Differentiator Plug-in Module (Brush-Gould) and recorded on PowerLab 4/30 data acquisition system (AD Instruments). Left femoral vein was cannulated for i.v. bolus injection of drugs in a volume of 0.25 mL/kg. In some experiments, the right femoral vein was prepared for drug infusion by means of a multichannel syringe pump (KD Scientific) and right carotid artery was catheterized for blood withdrawal. Mean arterial pressure (MAP) and heart rate (HR) were recorded. After 20 min stabilization, animals received a bolus intravenous (i.v.) dose of FAAH antagonist, HU210 or vehicle and mean arterial pressure (MAP) and heart rate (HR) were recorded. In some experiments, CB1 antagonist was injected i.p. 60 min before experiment or i.v. at the peak of the hypotensive response to AM3506.
Autonomic Blockade
Ganglionic blockade was achieved with hexamethonium, as described (Schreihofer, et al., 2007). Briefly, a bolus of 20 mg/kg hexamethonium was injected i.v., followed by a continuous maintenance infusion of 4 mg/kg/h. Vasopressin (0.04–0.4 IU/kg/h) and/or isoproterenol (0.3–0.5 μg/kg/h) were also infused to offset the hexamethonium-induced fall in basal MAP and HR, respectively. Blockade of the autonomic nervous system was verified using the phenylephrine/sodium nitroprusside sensitivity test (Schreihofer, et al., 2007). The baroreflex was quantified as a change HR/MAP ratio in response to phenylephrine or sodium nitroprusside.
Hemodynamic Analyses
Cardiac contractility was assessed in anaesthetized rats by a pressure-volume microcatheter system (Millar Instruments) as described (Batkai, et al., 2004; Pacher, et al., 2008). Briefly, a microtip pressure-volume catheter (SPR-838; Millar Instruments) was inserted into the right carotid artery and advanced into the left ventricle (LV) under pressure control. Signals were continuously recorded with an ARIA pressure-volume (PV) conductance system (Millar Instruments) coupled to a Powerlab/4SP A/D converter (AD Instruments), stored, and displayed on a computer. After stabilization for 20 minutes, AM3506 1 mg/kg or vehicle was injected i.v. and cardiac hemodynamic parameters were recorded 20 min later and computed using a cardiac PV analysis program (PVAN2.9; Millar). Maximal pressure developed by the ventricle at any given left ventricular volume was defined as the end-systolic pressure-volume relationship (ESPVR) and represented the inotropic state of the ventricle. Cardiac output (CO) was calculated and corrected according to in vitro and in vivo volume calibrations with PVAN2.9 and normalized to body weight (cardiac index). The maximal slope of systolic pressure increment (+dP/dtmax) was a time-derivative of left ventricular pressure used as a cardiac contractility parameter. Total peripheral resistance (TPR) was calculated as MAP/CO.
Catecholamine Assay
Blood was collected from left carotid artery before and after i.v. injection of 1 mg/kg AM506 and plasma norepinephrine levels were determined by liquid chromatography with electrochemical detection, as described (Eisenhofer, et al., 1986).
FAAH and MAGL activity
Antagonists were tested using inhibitor screening kits (Cayman Chemical Co.), based on the release of a fluorophore from a synthetic substrate by recombinant human FAAH and MAGL. AM3506 was also tested ex vivo using competitive Activity-Based Protein Profiling (Leung, et al., 2003) or the release of [3H]ethanolamine from [3H]anandamide for FAAH inhibition (Fowler, et al., 2000), or the release of [3H]glycerol from 2-oleoyl-[3H]glycerol in the presence of 1 μM URB597 for MAGL inhibitory activity (Dinh, et al., 2002). Drug or vehicle was injected i.p. or given by gavage and enzyme activity was determined in harvested tissues. In some experiments, FAAH activity was measured in brain and liver homogenates, adjusting the protein levels to yield equal amounts of FAAH in the assay (10 μg protein for brain and 3 μg for liver to compensate for the higher FAAH expression in liver).
Endocannabinoid Tissue Levels
Endocannabinoids were extracted and quantified using liquid chromatography/in-line mass spectrometry, as described (Batkai, et al., 2004). [2H4]anandamide was used as internal standard.
Isolation and treatment of hepatocytes
Mouse hepatocytes were isolated by in situ liver perfusion with Collagenase type I (Sigma-Aldrich) and Percoll (GE Healthcare) density gradient centrifugation as described (Osei-Hyiaman, et al., 2008). Hepatocytes were then re-suspended in DMEM medium, containing 10% fetal bovine serum and L-glutamine (Invitrogen). Concentrated (40 mg of wet cell weight per mL of medium) and dilute (1 mg/mL) hepatocyte suspensions were prepared and incubated with AM3506, URB597 (each 100 nM) or vehicle (DMSO; 1:1000v/v) at 37°C. Aliquots were removed at the indicated time points, quickly chilled on ice and centrifuged at 400 x g, 4°C for 5 min. Drugs were extracted from supernatant and hepatocyte pellets using the same protocol as for endocannabinoids.
Measurement of drug levels by LC/MS/MS
Drugs were quantified by LC-MS/MS using an Agilent 6410 triple quadrupole mass spectrometer (Agilent Technologies, USA) coupled with an Agilent 1200 LC system (Agilent Technologies, Germany). Liquid chromatographic separation was obtained using 2.5 μl injections of samples onto a Zorbax SB-C18 rapid resolution HT column (2.1mm×50 mm, 1.8 μm) from Agilent Technologies. The autosampler was set at 4°C and the column was maintained at 34°C during the analysis. Gradient elution mobile phases consisted of A (0.1% formic acid in water) and B (0.1% formic acid in methanol). Gradient elution (250 μL/min) began and was held at 10% B for the first 0.5 min, followed by a linear increase towards 85% B at 1 min; this was maintained until 12.5 min and then increased linearly to 100% B at 13 min; this was maintained until 14.5 min. At minute 16, the gradient changed linear to the initial setting which was maintained for 6 min. The mass spectrometer was set for ESI in positive ion mode. The source parameters were as follows; capillary voltage, 4000V; gas temperature, 350°C; drying gas, 10 L/min; nitrogen was used as the nebulizing gas with a pressure of 40 psig. Collision-induced dissociation (CID) was induced using nitrogen. Levels of each compound were analyzed by multiple reactions monitoring (MRM). Mass spectrometric conditions were optimized for AM3506, URB597, [2H4]AEA and AEA with injection of synthetic standards of each compound by using MassHunter Workstation Optimizer software (Agilent Technologies, USA). The molecular ion and fragments for each compound measured were as follows: m/z 247.1 → 107.0 and 247.1 → 77.1 for AM3506 (CID-energy: 12V and 52V, respectively), m/z 339.2 → 214.1 and 339.2 → 197.1 for URB597 (CID-energy: 8V and 20V, respectively), m/z 352.3 → 66.1 and 352.3 → 91 for [2H4]AEA (CID-energy: 12V and 56V, respectively) and m/z 348.3 → 62.1 and 348.3 → 91 for AEA (CID-energy: 12V and 48V, respectively). The acquisition and quantitation of analytes were achieved using MassHunter Workstation LC/QQQ Acquisition and MassHunter Workstation Quantitative Analysis softwares, respectively (Agilent Technologies, USA). Calibration curves were produced by using synthetic AEA, URB597 and AM3506. The amounts of AEA and AM3506 in the samples were determined by using linear regression of standard curves, whereas URB597 was determined by using quadratic regression of standard curve. Each time point represents hepatocytes pulled from 3–4 animals measured in duplicates. The limit of detection for AM3506 and URB597 was 1 pmol per injection.
CB1 and CB2 Receptor Binding and Anandamide Transport Assays
The affinity of AM3506 for CB1 and CB2 receptors was tested in radioligand displacement assays, using [3H]CP,55940 as the radioligand and membrane preparations from rat forebrain and spleen, respectively, as describe (Janero, et al., 2009; Lu, et al., 2006). The effect of AM3506 on anandamide transport was assessed by measuring its ability to inhibit [3H]anandamide uptake by cultured CCF-STTG1 astrocytoma cells, as described (Fegley, et al., 2004).
[35S]GTP S Binding
Basal and agonist-stimulated [35S]GTPγS binding was measured as described (Tam, et al., 2010).
Glucose Tolerance Test
Male C57Bl6/J mice, wild-type or LCB1−/−, were kept on a diet containing 60% of calories as fat (Research Diets Inc) for 14 weeks, by which time both strains have become fat due to increased adipose mass. Mice were then fasted overnight, followed by an i.p. injection of vehicle, 10 mg/kg URB597 or 1 mg/kg AM3506. Sixty minutes later the mice received 1.5 g/kg glucose i.p. and blood glucose levels were monitored for 2 hours, as described (Osei-Hyiaman, et al., 2008).
Drugs
URB597 was from Cayman; AM251, HU210 and CB13 were from Tocris Bioscience; rimonabant was provided by the NIDA Drug Supply program. [3H]anandamide and 2-oleoyl [3H]glycerol were from American Radiolabeled Chemicals. All other chemicals were from Sigma-Aldrich. AM3506 (5-(4-hydroxyphenyl)pentanesulfonyl fluoride) was synthesized in nine steps starting from commercially available 4-phenoxybutyl bromide and 4-anisaldehyde. Details of the synthesis along with a full structure-activity relationship study involving closely related analogs will be published elsewhere.
AM3506, URB597, CB13, rimonabant, AM251 and AM6545 were dissolved in DMSO, Tween 80 and saline (1:1:18). For gavage, AM3506 and URB597 were dissolved in DMSO and corn oil (1:19). HU210 was dissolved in DMSO and saline (1:19). All other drugs were dissolved in saline.
Data Analysis
Statistical analyses were performed using Prism 4 (GraphPad). T test for unpaired or paired data was used for comparison of values between two groups and one-way ANOVA followed by Dunnett’s test was used for comparison of multiple independent groups. When several variables were considered, two-way ANOVA with Bonferroni’s post-hoc test was applied. Differences were considered significant when P<0.05. Values were presented as means±s.e.m.).
Acknowledgments
We thank Prof. B. Lutz and Dr. G. Marsicano for providing CB1 floxed mice used to generate the liver-specific CB1−/− mice.
Source of Funding
This work was supported by funds from the intramural program of NIAAA.
Footnotes
Disclosure Statements: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahn K, Johnson DS, Fitzgerald LR, Liimatta M, Arendse A, Stevenson T, Lund ET, Nugent RA, Nomanbhoy TK, Alexander JP, et al. Novel mechanistic class of fatty acid amide hydrolase inhibitors with remarkable selectivity. Biochemistry. 2007;46:13019–13030. doi: 10.1021/bi701378g. [DOI] [PubMed] [Google Scholar]
- Ahn K, Johnson DS, Mileni M, Beidler D, Long JZ, McKinney MK, Weerapana E, Sadagopan N, Liimatta M, Smith SE, et al. Discovery and characterization of a highly selective FAAH inhibitor that reduces inflammatory pain. Chemistry & biology. 2009;16:411–420. doi: 10.1016/j.chembiol.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batkai S, Pacher P, Osei-Hyiaman D, Radaeva S, Liu J, Harvey-White J, Offertaler L, Mackie K, Rudd MA, Bukoski RD, et al. Endocannabinoids acting at cannabinoid-1 receptors regulate cardiovascular function in hypertension. Circulation. 2004;110:1996–2002. doi: 10.1161/01.CIR.0000143230.23252.D2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozoski DT, Dean C, Hopp FA, Hillard CJ, Seagard JL. Differential endocannabinoid regulation of baroreflex-evoked sympathoinhibition in normotensive versus hypertensive rats. Auton Neurosci. 2009;150:82–93. doi: 10.1016/j.autneu.2009.05.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang KP, Gerber AL, Sipe JC, Cravatt BF. Reduced cellular expression and activity of the P129T mutant of human fatty acid amide hydrolase: evidence for a link between defects in the endocannabinoid system and problem drug use. Human molecular genetics. 2004;13:2113–2119. doi: 10.1093/hmg/ddh216. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, Lichtman AH. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:9371–9376. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- Deutsch DG, Chin SA. Enzymatic synthesis and degradation of anandamide, a cannabinoid receptor agonist. Biochem Pharmacol. 1993;46:791–796. doi: 10.1016/0006-2952(93)90486-g. [DOI] [PubMed] [Google Scholar]
- Deutsch DG, Lin S, Hill WA, Morse KL, Salehani D, Arreaza G, Omeir RL, Makriyannis A. Fatty acid sulfonyl fluorides inhibit anandamide metabolism and bind to the cannabinoid receptor. Biochem Biophys Res Commun. 1997;231:217–221. doi: 10.1006/bbrc.1997.6072. [DOI] [PubMed] [Google Scholar]
- Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, Kathuria S, Piomelli D. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci U S A. 2002;99:10819–10824. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziadulewicz EK, Bevan SJ, Brain CT, Coote PR, Culshaw AJ, Davis AJ, Edwards LJ, Fisher AJ, Fox AJ, Gentry C, et al. Naphthalen-1-yl-(4-pentyloxynaphthalen-1-yl)methanone: a potent, orally bioavailable human CB1/CB2 dual agonist with antihyperalgesic properties and restricted central nervous system penetration. J Med Chem. 2007;50:3851–3856. doi: 10.1021/jm070317a. [DOI] [PubMed] [Google Scholar]
- Eisenhofer G, Goldstein DS, Stull R, Keiser HR, Sunderland T, Murphy DL, Kopin IJ. Simultaneous liquid-chromatographic determination of 3,4-dihydroxyphenylglycol, catecholamines, and 3,4-dihydroxyphenylalanine in plasma, and their responses to inhibition of monoamine oxidase. Clin Chem. 1986;32:2030–2033. [PubMed] [Google Scholar]
- Fegley D, Kathuria S, Mercier R, Li C, Goutopoulos A, Makriyannis A, Piomelli D. Anandamide transport is independent of fatty-acid amide hydrolase activity and is blocked by the hydrolysis-resistant inhibitor AM1172. Proc Natl Acad Sci U S A. 2004;101:8756–8761. doi: 10.1073/pnas.0400997101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana A, Di Marzo V, Cadas H, Piomelli D. Analysis of anandamide, an endogenous cannabinoid substance, and of other natural N-acylethanolamines. Prostaglandins Leukot Essent Fatty Acids. 1995;53:301–308. doi: 10.1016/0952-3278(95)90130-2. [DOI] [PubMed] [Google Scholar]
- Fowler CJ, Borjesson M, Tiger G. Differences in the pharmacological properties of rat and chicken brain fatty acid amidohydrolase. British journal of pharmacology. 2000;131:498–504. doi: 10.1038/sj.bjp.0703569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge C, Garcia R, Anand-Srivastava MB. Enhanced expression of Gialpha protein and adenylyl cyclase signaling in aortas from 1 kidney 1 clip hypertensive rats. Canadian journal of physiology and pharmacology. 2006;84:739–746. doi: 10.1139/y05-123. [DOI] [PubMed] [Google Scholar]
- Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- Howlett AC. Cannabinoid receptor signaling. Handb Exp Pharmacol. 2005:53–79. doi: 10.1007/3-540-26573-2_2. [DOI] [PubMed] [Google Scholar]
- Janero DR, Vadivel SK, Makriyannis A. Pharmacotherapeutic modulation of the endocannabinoid signalling system in psychiatric disorders: drug-discovery strategies. Int Rev Psychiatry. 2009;21:122–133. doi: 10.1080/09540260902782778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova Z, Mangieri RA, Bortolato M, Chefer SI, Mukhin AG, Clapper JR, King AR, Redhi GH, Yasar S, Piomelli D, et al. Fatty acid amide hydrolase inhibition heightens anandamide signaling without producing reinforcing effects in primates. Biological psychiatry. 2008;64:930–937. doi: 10.1016/j.biopsych.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass DA, Maughan WL, Guo ZM, Kono A, Sunagawa K, Sagawa K. Comparative influence of load versus inotropic states on indexes of ventricular contractility: experimental and theoretical analysis based on pressure-volume relationships. Circulation. 1987;76:1422–1436. doi: 10.1161/01.cir.76.6.1422. [DOI] [PubMed] [Google Scholar]
- Kathuria S, Gaetani S, Fegley D, Valino F, Duranti A, Tontini A, Mor M, Tarzia G, La Rana G, Calignano A, et al. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- Kost CK, Herzer WA, Li PJ, Jackson EK. Pertussis toxin-sensitive G-proteins and regulation of blood pressure in the spontaneously hypertensive rat. Clinical and experimental pharmacology & physiology. 1999;26:449–455. doi: 10.1046/j.1440-1681.1999.03058.x. [DOI] [PubMed] [Google Scholar]
- Kunos G. The hepatic alpha1-adrenoceptor. Trends Pharmacol Sci. 1984;5:380–383. [Google Scholar]
- Lake KD, Compton DR, Varga K, Martin BR, Kunos G. Cannabinoid-induced hypotension and bradycardia in rats mediated by CB1-like cannabinoid receptors. J Pharmacol Exp Ther. 1997;281:1030–1037. [PubMed] [Google Scholar]
- Leung D, Hardouin C, Boger DL, Cravatt BF. Discovering potent and selective reversible inhibitors of enzymes in complex proteomes. Nature biotechnology. 2003;21:687–691. doi: 10.1038/nbt826. [DOI] [PubMed] [Google Scholar]
- Lu D, Vemuri VK, Duclos RI, Jr, Makriyannis A. The cannabinergic system as a _target for anti-inflammatory therapies. Curr Top Med Chem. 2006;6:1401–1426. doi: 10.2174/15680266106061401. [DOI] [PubMed] [Google Scholar]
- Marcil J, de Champlain J, Anand-Srivastava MB. Overexpression of Gi-proteins precedes the development of DOCA-salt-induced hypertension: relationship with adenylyl cyclase. Cardiovascular research. 1998;39:492–505. doi: 10.1016/s0008-6363(98)00111-4. [DOI] [PubMed] [Google Scholar]
- Marcil J, Thibault C, Anand-Srivastava MB. Enhanced expression of Gi-protein precedes the development of blood pressure in spontaneously hypertensive rats. Journal of molecular and cellular cardiology. 1997;29:1009–1022. doi: 10.1006/jmcc.1996.0343. [DOI] [PubMed] [Google Scholar]
- Marrs WR, Blankman JL, Horne EA, Thomazeau A, Lin YH, Coy J, Bodor AL, Muccioli GG, Hu SS, Woodruff G, et al. The serine hydrolase ABHD6 controls the accumulation and efficacy of 2-AG at cannabinoid receptors. Nat Neurosci. 2010;13:951–957. doi: 10.1038/nn.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira FA, Kaiser N, Monory K, Lutz B. Reduced anxiety-like behaviour induced by genetic and pharmacological inhibition of the endocannabinoid-degrading enzyme fatty acid amide hydrolase (FAAH) is mediated by CB1 receptors. Neuropharmacology. 2008;54:141–150. doi: 10.1016/j.neuropharm.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Niederhoffer N, Schmid K, Szabo B. The peripheral sympathetic nervous system is the major _target of cannabinoids in eliciting cardiovascular depression. Naunyn-Schmiedeberg's archives of pharmacology. 2003;367:434–443. doi: 10.1007/s00210-003-0755-y. [DOI] [PubMed] [Google Scholar]
- Osei-Hyiaman D, DePetrillo M, Pacher P, Liu J, Radaeva S, Batkai S, Harvey-White J, Mackie K, Offertaler L, Wang L, et al. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J Clin Invest. 2005;115:1298–1305. doi: 10.1172/JCI23057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osei-Hyiaman D, Liu J, Zhou L, Godlewski G, Harvey-White J, Jeong WI, Batkai S, Marsicano G, Lutz B, Buettner C, et al. Hepatic CB(1) receptor is required for development of diet-induced steatosis, dyslipidemia, and insulin and leptin resistance in mice. J Clin Invest. 2008;118:3160–3169. doi: 10.1172/JCI34827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Batkai S, Kunos G. The endocannabinoid system as an emerging _target of pharmacotherapy. Pharmacol Rev. 2006;58:389–462. doi: 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Batkai S, Osei-Hyiaman D, Offertaler L, Liu J, Harvey-White J, Brassai A, Jarai Z, Cravatt BF, Kunos G. Hemodynamic profile, responsiveness to anandamide, and baroreflex sensitivity of mice lacking fatty acid amide hydrolase. American journal of physiology Heart and circulatory physiology. 2005;289:H533–541. doi: 10.1152/ajpheart.00107.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Nagayama T, Mukhopadhyay P, Batkai S, Kass DA. Measurement of cardiac function using pressure-volume conductance catheter technique in mice and rats. Nature protocols. 2008;3:1422–1434. doi: 10.1038/nprot.2008.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarzani R, Bordicchia M, Salvi F, Cola G, Franchi E, Battistoni I, Mancinelli L, Giovagnoli A, Dessi-Fulgheri P, Rappelli A. A human fatty acid amide hydrolase (FAAH) functional gene variant is associated with lower blood pressure in young males. American journal of hypertension. 2008;21:960–963. doi: 10.1038/ajh.2008.198. [DOI] [PubMed] [Google Scholar]
- Schreihofer AM, Mandel DA, Mobley SC, Stepp DW. Impairment of sympathetic baroreceptor reflexes in obese Zucker rats. American journal of physiology Heart and circulatory physiology. 2007;293:H2543–2549. doi: 10.1152/ajpheart.01201.2006. [DOI] [PubMed] [Google Scholar]
- Seagard JL, Dean C, Patel S, Rademacher DJ, Hopp FA, Schmeling WT, Hillard CJ. Anandamide content and interaction of endocannabinoid/GABA modulatory effects in the NTS on baroreflex-evoked sympathoinhibition. American journal of physiology Heart and circulatory physiology. 2004;286:H992–1000. doi: 10.1152/ajpheart.00870.2003. [DOI] [PubMed] [Google Scholar]
- Seierstad M, Breitenbucher JG. Discovery and development of fatty acid amide hydrolase (FAAH) inhibitors. J Med Chem. 2008;51:7327–7343. doi: 10.1021/jm800311k. [DOI] [PubMed] [Google Scholar]
- Tam J, Vemuri VK, Liu J, Batkai S, Mukhopadhyay B, Godlewski G, Osei-Hyiaman D, Ohnuma S, Ambudkar SV, Pickel J, et al. Peripheral CB1 cannabinoid receptor blockade improves cardiometabolic risk in mouse models of obesity. J Clin Invest. 2010;120:2953–2966. doi: 10.1172/JCI42551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourino C, Oveisi F, Lockney J, Piomelli D, Maldonado R. FAAH deficiency promotes energy storage and enhances the motivation for food. Int J Obes (Lond) 2010;34:557–568. doi: 10.1038/ijo.2009.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga K, Lake KD, Huangfu D, Guyenet PG, Kunos G. Mechanism of the hypotensive action of anandamide in anesthetized rats. Hypertension. 1996;28:682–686. doi: 10.1161/01.hyp.28.4.682. [DOI] [PubMed] [Google Scholar]