Abstract
The hypothalamic arcuate nucleus (ARCN) has been reported to play a significant role in cardiovascular regulation. It has been hypothesized that the ARCN may be one of the sites of cardiovascular actions of angiotensins (ANGs). Experiments were carried out in urethane-anesthetized, artificially ventilated, adult male Wistar rats. The ARCN was identified by microinjections of N-methyl-d-aspartic acid (NMDA; 10 mM). Microinjections (50 nl) of ANG-(1–12) (1 mM) into the ARCN elicited increases in mean arterial pressure (MAP), heart rate (HR), and greater splanchnic nerve activity (GSNA). The tachycardic responses to ANG-(1–12) were attenuated by bilateral vagotomy. The cardiovascular responses elicited by ANG-(1–12) were attenuated by microinjections of ANG II type 1 receptor (AT1R) antagonists but not ANG type 2 receptor (AT2R) antagonist. Combined inhibition of ANG-converting enzyme (ACE) and chymase in the ARCN abolished ANG-(1–12)-induced responses. Microinjections of ANG II (1 mM) into the ARCN also increased MAP and HR. Inhibition of ARCN by microinjections of muscimol (1 mM) attenuated the pressor and tachycardic responses to intravenously administered ANG-(1–12) and ANG II (300 pmol/kg each). These results indicated that 1) microinjections of ANG-(1–12) into the ARCN elicited increases in MAP, HR, and GSNA; 2) HR responses were mediated via both sympathetic and vagus nerves; 3) AT1Rs, but not AT2Rs, in the ARCN mediated ANG-(1–12)-induced responses; 4) both ACE and chymase were needed to convert ANG-(1–12) to ANG II in the ARCN; and 5) ARCN plays a role in mediating the cardiovascular responses to circulating ANGs.
Keywords: blood pressure, heart rate, microinjection, N-methyl-d-aspartic acid, sympathetic nerve activity
the hypothalamic arcuate nucleus (ARCN) may play a significant role in cardiovascular regulation (10, 38). Consistent with this notion, we (31) have recently reported that chemical stimulation of the ARCN elicited increases in mean arterial pressure (MAP), heart rate (HR), and sympathetic nerve activity (SNA). These reports have provided a basis for investigations on different neurotransmitters and neuromodulators in the ARCN that may play a role in the regulation of cardiovascular function in normal and pathological states.
A new endogenous angiotensin (ANG), ANG-(1–12), has recently been identified (30, 44). Intravenous administration of this peptide has been reported to elicit an immediate pressor response in the rat, and this effect was blocked by prior administration of an ANG-converting enzyme (ACE) inhibitor or an ANG II type 1 receptor (AT1R) antagonist (30). These data indicated that in the periphery, ANG-(1–12) exerts its actions via a rapid conversion to ANG II (30). High concentrations of ANG-(1–12) have been reported in the rat brain, and cells immunoreactive for ANG-(1–12) have been identified in the nucleus tractus solitarius (NTS) of the rat (1, 30). Bilateral microinjections of ANG-(1–12) into the NTS have been reported to elicit transient depressor responses and an attenuation of baroreflex sensitivity (1). Chronic immunoneutralization of endogenous brain ANG-(1–12) by intracerebroventricular injections of anti-ANG-(1–12) IgG in hypertensive rats have been reported to elicit antihypertensive effects (21). Several components of the renin-ANG system (RAS) have been identified in the ARCN. For example, angiotensinogen, ACE, and ANG receptors have been identified in the ARCN (13, 19, 22, 50). Based on the above-mentioned reports, we hypothesized that the ARCN may be one of the sites of cardiovascular actions of ANGs. To test our hypothesis, ANG-(1–12) was selected for this study because it has been reported to mediate its actions via its rapid conversion to ANG II (30). In selected experiments, ANG II was also included for a comparison with our ANG-(1–12)-induced cardiovascular responses.
MATERIALS AND METHODS
General procedures.
Experiments were done in adult male Wistar rats (Charles River Laboratories, Wilmington, MA) weighing 300–360 g (n = 105). All animals were housed under controlled conditions with a 12:12-h light-dark cycle. Food and water were available to the animals ad libitum. Experiments were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and with the approval of the Institutional Animal Care and Use Committee of this university.
The procedures used in this study have been described in detail elsewhere (31, 32). Briefly, rats were anesthetized with inhalation of isoflurane (2–3% in 100% O2), one of the veins was cannulated, and urethane (1.2–1.4 g/kg) was injected intravenously in eight to nine aliquots at 2-min intervals. Isoflurane inhalation was terminated as soon as urethane administration was completed. The absence of a pressor response and/or withdrawal of the limb in response to pinching of a hindpaw indicated that the rats were properly anesthetized. Rats were artificially ventilated, and end-tidal CO2 was maintained at 3.5–4.5%. Rectal temperature was maintained at 37.0 ± 0.5°C. Blood pressure (BP) and HR were recorded by standard techniques (31, 32).
Microinjections into the ARCN.
Rats were placed in a prone position in a stereotaxic instrument with a bite bar 11 mm below the interaural line. A hole (8–10 mm in diameter) was drilled in the midline at the junction of the two parietal bones caudal to the bregma. Microinjections were made through this hole on either side of the midline. Microinjections were made using multibarreled glass micropipettes (tip size: 20–40 μm). Each barrel was connected to a channel on a picospritzer. Two barrels always contained N-methyl-d-aspartic acid (NMDA) and artificial cerebrospinal fluid (aCSF). Depending on the experiment, the remaining barrels contained ANG-(1–12), ANG II, an ANG receptor antagonist, and ACE or chymase inhibitor. To avoid the sagittal sinus, the micropipette was placed at a level 2.56–3.80 mm caudal to the bregma, 0.8–0.9 mm lateral to the midline, at an angle (4°) and moved 9.8–10.2 mm ventral to the dura to reach the ARCN. Using this approach, the coordinates for the final site of microinjection in the ARCN were 2.56–3.80 mm caudal to the bregma, 0.1–0.3 mm lateral to the midline, and 9.8–10.2 mm deep from the dura. The coordinates used for different regions of the ARCN are mentioned in Table 2.
Table 2.
Cardiovascular effects of microinjections of ANG-(1–12) and ANG II at different levels of the ARCN
| ARCN Segments | n | Increase in MAP, mmHg | Increase in HR, beats/min |
|---|---|---|---|
| Effects of ANG-(1–12) (1 mM) | |||
| Rostral ARCN | 4 | 9.5 ± 0.6* | 9.5 ± 1.5* |
| Middle ARCN | 5 | 10.4 ± 0.6* | 12.8 ± 1.2* |
| Caudal ARCN | 5 | 10.5 ± 1.6* | 10.5 ± 2.6* |
| Effects of ANG II (1 mM) | |||
| Rostral ARCN | 5 | 13.8 ± 0.9† | 15.2 ± 2.8† |
| Middle ARCN | 5 | 14.8 ± 1.6† | 17.6 ± 1.7† |
Values are means ± SE. The ARCN (∼2 mm long in the rostrocaudal direction) was arbitrarily divided into three equal segments using the bregma as a reference point: the rostral segment (2.1–2.9 mm caudal), the middle segment (2.9–3.7 mm caudal), and the caudal segment (3.7–4.5 mm caudal). Microinjections were made into one site in each segment using the bregma as a reference point in the rostrocaudal direction, midline in the mediolateral direction and the dura in the dorsoventral direction. The coordinates are as follows: rostral ARCN (2.5 mm caudal, 0.1–0.3 mm lateral, and 9.8–10.2 mm deep); middle ARCN (3.3 mm caudal, 0.1–0.3 mm lateral, and 9.8–10.2 mm deep); and caudal ARCN (3.8 mm caudal, 0.1–0.3 mm lateral, 9.8–10.2 mm deep; the hypothalamic dorsomedial nucleus and hypothalamic ventromedial nucleus are not present at this level).
The differences in cardiovascular responses elicited by microinjections of ANG-(1–12) (1 mM) in the rostral ARCN, middle ARCN, and caudal ARCN were not significant (P > 0.05).
Cardiovascular responses elicited by ANG II (1 mM) in the rostral ARCN and middle ARCN were greater than those elicited by ANG-(1–12) (1 mM) at the same sites (P < 0.05).
Intravenous injections.
ANG-(1–12) and ANG II dissolved in 0.1 ml saline were injected into the cannula dwelling in the femoral vein and flushed with 0.2 ml saline, and MAP and HR were monitored. The duration of these intravenous injections was 4–6 s. Intravenous injections of saline were used as controls.
Nerve recording.
The greater splanchnic nerve (GSN) was selected for recording because it contains sympathetic fibers innervating major abdominal vascular beds and the kidney (3). The GSN was exposed retroperitoneally and sectioned as it joined the celiac ganglion, and whole nerve activity was recorded from the desheathed central end by standard techniques (41). At the end of the experiment, the nerves were sectioned centrally, and the remaining activity was considered to be the noise level, which was subtracted from the whole nerve activity.
Histology.
Microinjection sites in the ARCN were marked by diluted India ink (50 nl). Animals were perfused and fixed with 4% paraformaldehyde, and serial sections of the hypothalamus were cut (30 μm) and stained with cresyl violet using standard procedures (31, 32). Microinjection sites were identified using a standard atlas (36).
Drugs and chemicals.
The following drugs and chemicals were used: ANG-(1–12), ANG II, captopril (ACE inhibitor) (27), chymostatin (chymase inhibitor) (18), D-AP7 (NMDA receptor antagonist), losartan (AT1R antagonist), muscimol (GABAA receptor agonist), NMDA, PD-123319 (AT2R antagonist) (4), l-phenylephrine hydrochloride (PE), isoflurane, urethane, and ZD-7155 (AT1R antagonist) (35). All of the solutions for the microinjections were freshly prepared in aCSF (pH 7.4). Where applicable, the concentration of drugs refers to their salts. Sources of the drugs were as follows: ANG-(1–12) and ANG II (American Peptide, Sunnyvale, CA); captopril, chymostatin, losartan, and muscimol (Sigma-Aldrich Chemicals, St. Louis, MO); PD-123319 and ZD-7155 (Tocris-Cookson, Ellisville, MO); and isoflurane (Baxter Pharmaceutical Products, Deerfield, IL).
Statistical analyses.
Means and SEs were calculated for maximum changes in MAP and HR. One-way ANOVA followed by Tukey-Kramer's multiple-comparison test was applied to test differences in maximum changes in MAP and HR in different groups of rats. Desensitization to multiple injections of ANG-(1–12) and ANG II was tested by repeated-measures ANOVA followed by Tukey-Kramer's multiple-comparison test. Student's paired t-test was used in experiments where each animal served as its own control. Student's unpaired t-test was used to compare responses in different groups of rats. For the analysis of nerve activity, baseline value represented the average amplitude of integrated GSN activity (GSNA) during the 35-s period before the intravenous administration of PE or the microinjections of drugs into the ARCN. The maximum change in GSNA amplitude was expressed as the percent change from the baseline value. Mean values of the integrated nerve signals were compared using Student's paired t-test. In all cases, differences were considered significant at P < 0.05.
RESULTS
Baseline values for MAP and HR in urethane-anesthetized rats were 86.9 ± 1.5 mmHg and 383.1 ± 4.2 beats/min, respectively (n = 71).
Cardiovascular responses to microinjections of ANG-(1–12) into the ARCN.
The ARCN (∼2 mm long in the rostrocaudal direction) was arbitrarily divided into the following three equal segments (the coordinates mentioned for each segment indicate the level caudal to the bregma): the rostral segment (2.1–2.9 mm), middle segment (2.9–3.7 mm), and caudal segment (3.7–4.5 mm). In this and the other series of experiments, unless indicated otherwise, all microinjections into the ARCN were unilateral, their volume and duration were 50 nl and 5–10 s, respectively, and microinjections of aCSF (pH 7.4), used as controls, elicited no cardiovascular responses. The ARCN was always identified by microinjections of NMDA (10 mM), which elicited increases in MAP (19.4 ± 0.7 mmHg) and HR (83.0 ± 3.5 beats/min) (n = 71). The interval between the microinjections of NMDA and other agents was at least 2 min. The concentration response was first studied in the middle portion of ARCN; maximum cardiovascular responses were elicited by microinjections of a 1 mM concentration of ANG-(1–12) (Table 1). Microinjections of ANG-(1–12) (1 mM) at different rostrocaudal levels of the ARCN elicited similar cardiovascular responses (Table 2). Microinjections of ANG II (1 mM) into the ARCN elicited greater cardiovascular responses compared with the same concentration of ANG-(1–12) at the same levels (Table 2). The onset of cardiovascular responses to microinjections of ANG-(1–12) (1 mM) into the ARCN (11.2 ± 1.2 s) was significantly (P < 0.05) longer than the onset of action (8.3 ± 0.6 s) of microinjection of ANG II (1 mM) at the same site. The durations of cardiovascular responses to microinjections of ANG-(1–12) and ANG II (1 mM each) into the ARCN (9.2 ± 0.5 and 8.2 ± 0.5 min, respectively) and the peak effects of the same concentrations of these two peptides (2.3 ± 0.2 and 2.0 ± 0.1 min, respectively) were not significantly different (P > 0.05).
Table 1.
Concentration responses of microinjections of ANG-(1–12) into the middle ARCN
| Concentration of ANG-(1–12) | Increase in MAP, mmHg | Increase in HR, beats/min |
|---|---|---|
| 0.25 mM | 2.6 ± 0.9 | 3.2 ± 2.1 |
| 0.50 mM | 6.0 ± 1.0 | 6.0 ± 2.8 |
| 1.00 mM | 10.4 ± 0.6*§ | 12.8 ± 1.2‡§ |
| 2.00 mM | 10.8 ± 0.7†§ | 8.6 ± 3.4§ |
Values are means ± SE; n = 5. See Table 2 for the coordinates of the hypothalamic arcuate nucleus (ARCN). MAP, mean arterial pressure; HR, heart rate. *,† The pressor responses were significantly greater than those elicited by the 0.25 and 0.5 mM concentrations (P < 0.01).
The tachycardic response was significantly greater than the tachycardic responses elicited by 0.25 and 0.5 mM concentrations (P < 0.05).
The differences in pressor and tachycardic responses elicited by 1 and 2 mM concentrations were not significant (P > 0.05).
Bilateral microinjections of ANG-(1–12) into the middle ARCN elicited increases in MAP (12.8 ± 0.7 mmHg) and HR (17.6 ± 1.1 beats/min) (n = 5) that were significantly (P < 0.05) greater than the MAP (10.4 ± 0.6 mmHg) and HR (12.8 ± 1.2 beats/min) responses elicited by unilateral microinjections of this peptide into this nucleus.
Site specificity of ANG-(1–12)-induced responses in the ARCN.
We (31) have previously reported that microinjections of NMDA (10 mM) into the ARCN at different rostrocaudal levels (2.3–4.16 mm caudal to the bregma) and the dorsomedial hypothalamic nucleus (DMN; 3.6 caudal to bregma, 0.2 lateral to the midline, and 9.0 deep from the dura) elicited pressor and tachycardic responses. Other investigators (47) have reported an increase in SNA in response to microinjections of l-glutamate into the hypothalamic ventromedial nucleus (VMN). The possibility that DMN and VMN may have contributed to the cardiovascular responses elicited from the ARCN was excluded based on the following observations. As mentioned above, the increases in MAP and HR in response to microinjections of ANG-(1–12) into the caudal ARCN were not different from the responses elicited from middle and rostral levels of ARCN (Table 2). The DMN and VMN are not present at the caudal levels of ARCN, suggesting that these hypothalamic nuclei did not contribute to the cardiovascular responses elicited from the ARCN. Site specificity of the ANG-(1–12)-induced cardiovascular responses was also suggested by the following observations. Microinjections of either NMDA (10 mM) or ANG-(1–12) (1 mM) at more lateral sites (1 mm lateral to the midline) adjacent to the caudal ARCN elicited no cardiovascular responses. Microinjections of ANG-(1–12) (1 mM) into the third ventricle at a level 3.8 mm caudal to the bregma, midline, and 10 mm deep from the dura elicited small increases in MAP (4.0 ± 0.4 mmHg) and HR (5.2 ± 2.5 beats/min). However, in the same group of rats, microinjections of NMDA (10 mM, 50 nl) into the third ventricle at the same level elicited greater increases in MAP (13.5 ± 2.4 mmHg) and HR (78.3 ± 8.7 beats/min). The cardiovascular responses elicited from the third ventricle at this level were mediated via the ARCN bilaterally. This conclusion was based on the following observations. First, increases in MAP and HR were elicited by microinjections of NMDA (10 mM) into the third ventricle at a level 3.8 mm caudal to the bregma. At the same level, the ARCN was identified on each side using microinjections of NMDA (10 mM). After an interval of 20 min, D-AP7 (10 mM, 50 nl) was microinjected into the ARCN on each side. Subsequent microinjections of NMDA (10 mM) into the ARCN on each side, within 5 min, failed to elicit a cardiovascular response, indicating that NMDA receptors were blocked. At this time, repetition of microinjections of NMDA (10 mM) into the third ventricle elicited significantly smaller increases in MAP (5.0 ± 0.4 mmHg, P < 0.05) and HR (25.3 ± 4.9 beats/min, P < 0.01).
Reproducibility of ANG-(1–12)-induced responses in the ARCN.
The increases in MAP in response to three consecutive microinjections of ANG-(1–12) (1 mM) into the middle ARCN at 40-min intervals were 10.8 ± 1.0, 9.8 ± 1.0, and 10.0 ± 0.8 mmHg, respectively, and increases in HR were 9.2 ± 5.7, 12.2 ± 7.6, and 6.4 ± 2.6 beats/min, respectively (n = 5); these values were not statistically different (P > 0.05). Because no desensitization of responses was observed with repeated microinjections of ANG-(1–12) at 40-min intervals, this interval was chosen between microinjections of this peptide in all experiments.
Effect of bilateral vagotomy on ANG-(1–12)-induced responses in the ARCN.
Bilateral vagotomy did not alter the increases in MAP elicited by unilateral microinjections of either NMDA (10 mM) or ANG-(1–12) (1 mM) into the middle ARCN (Table 3). However, the increases in HR elicited by the same concentrations of NMDA and ANG-(1–12) at the same site were significantly reduced after vagotomy (Table 3).
Table 3.
Effect of different procedures or antagonists on the responses induced by microinjections of ANG-(1–12) into the middle ARCN
| ANG-(1–12)-Induced Increase in MAP, mmHg |
ANG-(1–12)-Induced Increase in HR, beats/min |
NMDA-Induced Increase in MAP, mmHg |
NMDA-Induced Increase in HR, beats/min |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Procedure or Injection of Antagonist Into the Middle ARCN | n | Before | After | Before | After | Before | After | Before | After |
| Vagotomy | 5 | 11.2 ± 1.2 | 10.4 ± 0.7 | 12.2 ± 1.9 | 4.4 ± 2.0* | 24.6 ± 4.4 | 22.4 ± 3.4 | 98.2 ± 15.3 | 40.8 ± 5.9† |
| ZD-7155 | 5 | 10.6 ± 1.3 | 4.2 ± 0.9‡ | 11.2 ± 4.3 | 3.4 ± 1.9* | 18.8 ± 1.3 | 20.0. ± 1.3 | 74.0 ± 12.7 | 70.6 ± 17.4 |
| Losartan | 8 | 10.9 ± 0.9 | 4.0 ± 0.9‡ | 13.9 ± 4.5 | 2.0 ± 1.0* | 20.5 ± 3.3 | 19.3 ± 2.1 | 91.1 ± 14.9 | 85.0 ± 14.3 |
| PD-123319 | 5 | 10.0 ± 0.4 | 9.4 ± 0.5 | 10.4 ± 7.9 | 8.4 ± 5.6 | 19.4 ± 2.0 | 19.2 ± 2.7 | 69.2 ± 6.7 | 62.4 ± 6.7 |
| Captopril | 5 | 10.8 ± 1.0 | 4.6 ± 0.9† | 13.4 ± 4.2 | 5.6 ± 3.0* | 20.6 ± 3.6 | 20.8 ± 2.7 | 89.8 ± 12.7 | 86.8 ± 8.4 |
| Chymostatin | 6 | 9.8 ± 0.7 | 5.0 ± 0.5† | 7.7 ± 2.5 | 2.3 ± 1.2* | 18.7 ± 1.4 | 20.8 ± 3.0 | 84.0 ± 6.2 | 68.2 ± 9.8 |
| Captopril + chymostatin | 5 | 9.6 ± 0.5 | 2.2 ± 0.2‡ | 12.2 ± 4.4 | 1.4 ± 1.4* | 17.0 ± 1.9 | 19.2 ± 3.0 | 74.0 ± 8.5 | 73.2 ± 9.5 |
Values are means ± SE. See Table 2 for the coordinates of the middle ARCN. The following concentrations of drugs were used: Ang-(1–12), 1 mM; N-methyl-d-aspartic acid (NMDA), 10 mM; ZD-7155, 2 mM; losartan, 10 mM; PD-123319, 50 mM; captopril, 200 mM; chymostatin, 10 mM. Significantly smaller responses compared with ANG-(1–12)-induced responses before the procedure or microinjection of the antagonist are indicated as follows:
P < 0.05,
P < 0.01, and
P < 0.001.
Effect of AT1R antagonists on ANG-(1–12)-induced responses in the ARCN.
Group data for the effects of two AT1R antagonists [ZD-7155 (2 mM) and losartan (10 mM)] on ANG-(1–12)-induced responses are shown in Table 3. The selection of the doses of ZD-7155 and losartan was based on our preliminary experiments and published reports (15, 42). Prior microinjections of ZD-7155 or losartan into the middle ARCN significantly attenuated the cardiovascular responses to subsequent microinjections (within 2 min) of ANG-(1–12) (1 mM). The ANG-(1–12)-induced increases in MAP and HR did not recover to the initial values within 60 min after the microinjection of either ZD-1755 or losartan into the ARCN. Microinjections of ZD-7155 or losartan did not alter the increases in MAP and HR elicited by microinjections of NMDA into the ARCN (Table 3). Unilateral or bilateral microinjections of the AT1R antagonists into the ARCN did not alter baseline BP or HR.
Effect of an AT2R antagonist on ANG-(1–12)-induced responses in the ARCN.
Microinjections of a selective AT2R antagonist [PD-123319 (50 mM)] into the middle ARCN did not alter the cardiovascular responses elicited by microinjections of ANG-(1–12) (1 mM) at the same site (Table 3). The dose of PD-123319 was selected from a previously published report (8).
Effect of captopril on ANG-(1–12)-induced responses in the ARCN.
Microinjections of captopril (200 mM) into the middle ARCN significantly reduced the increases in MAP and HR induced by subsequent microinjections (within 2 min) of ANG-(1–12) (1 mM) at the same site (Table 3). ANG-(1–12)-induced increases in MAP and HR did not completely recover to the initial values within 60 min of the microinjection of captopril into the ARCN. Microinjections of smaller doses of captopril (e.g., 50 mM) did not alter the responses to subsequent microinjections of ANG-(1–12) in the ARCN. Captopril (200 mM) did not alter the cardiovascular responses to microinjections of NMDA into the ARCN (Table 3). Unilateral microinjections of captopril alone into the ARCN did not elicit any cardiovascular responses. The dose of captopril (200 mM) used in our study to inhibit ACE was comparable with the dose used by others in microinjection studies (20, 45).
Effect of chymostatin on ANG-(1–12)-induced responses in the ARCN.
Microinjections of chymostatin (chymase inhibitor, 10 mM) (18) into the middle ARCN significantly attenuated the increases in MAP and HR induced by subsequent microinjections (within 2 min) of ANG-(1–12) (1 mM) at the same site (Table 3). Lower concentrations of chymostatin (2 and 5 mM) failed to alter the responses to ANG-(1–12) (1 mM). ANG-(1–12)-induced increases in MAP and HR did not completely recover to the initial values within 60 min of the microinjection of chymostatin into the ARCN. Chymostatin did not alter the cardiovascular responses to microinjections of NMDA into the ARCN (Table 3). Unilateral microinjections of chymostatin alone into the ARCN did not elicit any cardiovascular responses.
Effect of combined microinjections of captopril and chymostatin on ANG-(1–12)-induced responses in the ARCN.
Sequential microinjections of captopril (200 mM) and chymostatin (10 mM) (interval between the two microinjections was 2 min) elicited greater attenuation of ANG-(1–12)-induced cardiovascular responses in the middle ARCN compared with the attenuation elicited by either captopril or chymostatin alone (Table 3). Combined microinjections of captopril and chymostatin into the ARCN on one side did not alter baseline MAP and HR.
Effect of microinjections of ANG-(1–12) into the ARCN on SNA.
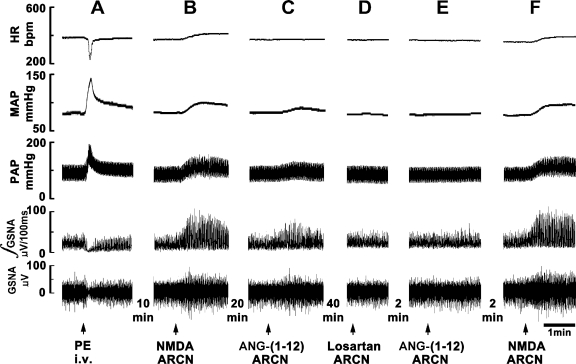
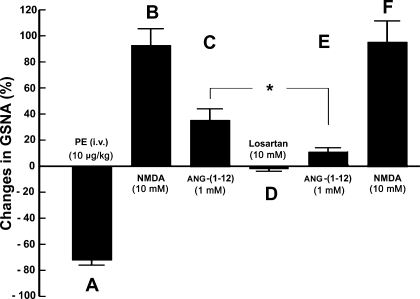
Figure 1 shows a typical recording of the effect of microinjections of ANG-(1–12) into the middle ARCN on efferent GSNA. A bolus injection of PE (10 μg/kg iv) increased MAP, which, in turn, elicited reflex bradycardia and inhibition of efferent GSNA lasting for 18.2 ± 3.1 s (Fig. 1A). Ten minutes later, when GSNA had recovered to baseline, a microinjection of NMDA (10 mM) into the ARCN elicited an increase in GSNA (Fig. 1B). After 20 min, microinjection of aCSF (50 nl) into the same ARCN site did not alter GSNA (not shown). Two minutes later, microinjection of ANG-(1–12) (1 mM) into the ARCN increased efferent GSNA (Fig. 1C). Forty minutes later, losartan (10 mM) was microinjected into the ARCN; no changes in GSNA were elicited (Fig. 1D). Two minutes later, ANG-(1–12) (1 mM) was again microinjected at the same site; the effect of ANG-(1–12) was blocked (Fig. 1E). Two minutes later, microinjection of NMDA (10 mM) continued to elicit an increase in efferent GSNA (Fig. 1F). Group data (n = 5) for this experiment are shown in Fig. 2. All changes in GSNA refer to comparisons with the basal nerve activity. An intravenous bolus injection of PE (10 μg/kg) decreased GSNA significantly (Fig. 2A). Ten minutes later, microinjections of NMDA (10 mM) into the ARCN elicited significant increases in GSNA (Fig. 2B). Twenty minutes later, microinjection of ANG-(1–12) (1 mM) at the same site elicited significant increases in GSNA (Fig. 2C). After 40 min, losartan (10 mM) was microinjected at the same site, and no significant changes in GSNA were elicited (Fig. 2D). Two minutes after the microinjection of losartan into the ARCN, the increases in GSNA (Fig. 2E) were significantly attenuated compared with the ANG-(1–12)-induced increases before the microinjection of losartan. Two minutes later, NMDA (10 mM) microinjected into the ARCN elicited an increase in GSNA (Fig. 2F), which was not significantly different from the NMDA-induced increase before the microinjection of losartan.
Fig. 1.
Effect of angiotensin (ANG)-(1–12) on sympathetic nerve activity. First trace, heart rate [HR; in beats/min (bpm)]; second trace, mean arterial pressure (MAP; in mmHg); third trace, pulmonary arterial pressure (PAP; in mmHg); fourth trace, integrated greater splanchic nerve activity (∫GSNA; in μV/100 ms); fifth trace, whole GSNA (in μV). A: reflex inhibition of GSNA elicited by a pressor response induced by phenylephrine (PE; 10 μg/kg iv) indicated that GSNA was barosensitive. B: 10 min later, microinjection of N-methyl-d-aspartic acid (NMDA; 10 mM) into the hypothalamic arcuate nucleus (ARCN) increased HR, MAP, PAP, ∫GSNA, and whole GSNA. C: 20 min later, microinjection of ANG-(1–12) (1 mM) into the ARCN increased HR, MAP, PAP, ∫GSNA, and whole GSNA. D: 40 min later, microinjection of losartan (10 mM) into the ARCN did not elicit any response. E: 2 min later, microinjection of ANG-(1–12) (1 mM) at the same site failed to elicit a response. F: 2 min later, microinjection of NMDA (10 mM) continued to elicit the usual cardiovascular responses.
Fig. 2.
Group data showing ANG-(1–12)-induced changes in GSNA (n = 5). A: an intravenous bolus injection of PE (10 μg/kg) significantly decreased GSNA (74.0 ± 2.1%, P < 0.01). B: 10 min later, microinjections of NMDA (10 mM) into the ARCN elicited significant (P < 0.01) increases in GSNA (94.2 ± 12.2%). C: 20 min later, microinjection of ANG-(1–12) (1 mM) at the same site elicited significant (P < 0.01) increases in GSNA (35.3 ± 8.8%). D: after 40 min, microinjection of losartan (10 mM) at the same site did not elicit significant changes in GSNA. E: 2 min after the microinjection of losartan into the ARCN, the increases in GSNA (11.1 ± 2.4%) were significantly attenuated (*P < 0.05) compared with the ANG-(1–12)-induced increases before the microinjection of losartan. F: 2 min later, NMDA (10 mM) microinjected into the ARCN elicited a significant increase (95.2 ± 18.6%, P < 0.01) in GSNA that was not significantly different (P > 0.05) from the NMDA-induced increase before the microinjection of losartan.
Effects of intravenously injected ANG-(1–12) and ANG II.
The dose responses of cardiovascular actions of intravenously administered ANG-(1–12) are shown in Table 4. Maximal increases in MAP were elicited by the 400 pmol/kg dose of ANG-(1–12). However, the increases in MAP elicited by 300 pmol/kg ANG-(1–12) were not significantly different from those elicited by 400 pmol/kg. The dose response of intravenous ANG-(1–12) in our study was similar to that reported by Nagata et al. (30). Repeated intravenous injections of ANG-(1–12) (300 pmol/kg) at 40-min intervals did not exhibit desensitization. The increases in MAP elicited by three consecutive injections of ANG-(1–12) were 27.5 ± 2.1, 28.8 ± 0.5, and 29.3 ± 2.4 mmHg, respectively. The increases in HR elicited by the same injections of ANG-(1–12) were 7.5 ± 1.0, 8.0 ± 3.2, and 8.3 ± 2.4 beats/min, respectively.
Table 4.
Cardiovascular effects of intravenous injections of ANG-(1–12) and ANG II
| n | Increase in MAP, mmHg | Increase in HR, beats/min | |
|---|---|---|---|
| Effects of ANG-(1–12) | |||
| ANG-(1–12) dose | |||
| 50 pmol/kg iv | 5 | 6.4 ± 0.8a | 5.0 ± 1.4 |
| 100 pmol/kg iv | 5 | 10.8 ± 0.8b | 6.0 ± 1.8 |
| 200 pmol/kg iv | 5 | 21.6 ± 2.5c | 6.6 ± 1.8 |
| 300 pmol/kg iv | 5 | 28.6 ± 3.7d | 9.6 ± 3.0 |
| 400 pmol/kg iv | 5 | 31.4 ± 5.1e | 11.2 ± 4.3 |
| Effects of ANG II | |||
| ANG II dose | |||
| 300 pmol/kg iv | 12 | 41.9 ± 2.4f | 12.1 ± 3.5 |
Values are means ± SE. c,d,eSignificantly greater (P < 0.05–0.001) than the increases in MAP in a. c,d,eThe differences in increases in MAP were not statistically significant (P > 0.05). b,cThe differences in increases in MAP were not statistically significant (P > 0.05). d,eSignificantly greater (P < 0.01) than the increases in MAP in b. d,fThe increases in MAP elicited by ANG II (300 pmol/kg) were significantly greater (P < 0.0001) than the increases in MAP elicited by ANG-(1–12) (300 pmol/kg). The differences in increases in HR elicited by different doses of ANG-(1–12) were not significant (P > 0.05). The increases in HR elicited by ANG II (300 pmol/kg) were not significantly different (P > 0.05) from HR increases elicited by ANG-(1–12) (300 pmol/kg).
Intravenous injections of ANG II (300 pmol/kg) also elicited increases in MAP (n = 12), which were significantly greater than the increases in MAP elicited by intravenous injections of the same dose of ANG-(1–12) (Table 4). However, there were no significant differences in the HR responses elicited by these two peptides (Table 4).
The onset and duration of the cardiovascular effects of ANG-(1–12) (300 pmol/kg iv) were 1.9 ± 0.2 s and 3.2 ± 0.3 min, respectively. The onset and duration of the cardiovascular effects of ANG II (300 pmol/kg iv) were 1.4 ± 0.2 s and 3.7 ± 0.3 min, respectively. The differences in the onset and duration of responses elicited by intravenous injections of ANG-(1–12) and ANG II were not statistically significant (P > 0.05).
Role of the ARCN in mediating cardiovascular responses to intravenously administered ANG-(1–12) and ANG II.
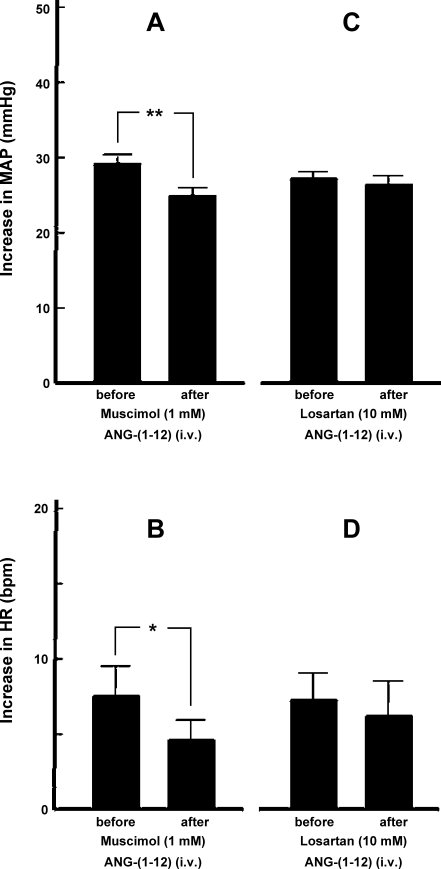
As mentioned above, the ARCN in the rat is ∼2 mm long. The diameter of the diffusion sphere of 100-nl microinjection has been estimated to be ∼1 mm (33). To ensure that most of the neurons in the ARCN were inhibited, two microinjections (100 nl each) of muscimol were made in the ARCN on each side, one rostrally (2.5 mm caudal to the bregma, 0.1–0.3 mm lateral to the midline, and 9.8–10.2 mm deep from the dura) and another caudally (3.6 mm caudal to the bregma, 0.1–0.3 mm lateral to the midline, and 9.8–10.2 mm deep from the dura) (n = 10). Bilateral inhibition of ARCN by muscimol did not alter basal MAP and HR. However, pressor (Fig. 3A) and tachycardic (Fig. 3B) responses to intravenously administered ANG-(1–12) (300 pmol/kg) were significantly attenuated after muscimol-induced bilateral inhibition of ARCN. Inhibition of the ARCN was confirmed by the lack of responses to microinjections of NMDA (10 mM). In another group of rats (n = 6), microinjections (100 nl) of losartan (10 mM) were made bilaterally into the ARCN as described for muscimol. Bilateral blockade of AT1Rs in the ARCN by losartan did not significantly alter pressor (Fig. 3C) and tachycardic (Fig. 3D) responses to intravenously administered ANG-(1–12) (300 pmol/kg). Basal MAP and HR were also not altered by bilateral blockade of AT1Rs in the ARCN by losartan.
Fig. 3.
Role of the ARCN in mediating cardiovascular responses to intravenous ANG-(1–12). A: the increases in MAP induced by intravenously injected ANG-(1–12) (300 pmol/kg) before and after bilateral inhibition of ARCN by muscimol (1 mM) were 29.5 ± 1.2 and 25.1 ± 1.1 mmHg, respectively. The pressor response after inhibition of the ARCN was significantly attenuated (n = 10, **P < 0.001). B: in the same group of rats, the increases in HR induced by intravenously injected ANG-(1–12) (300 pmol/kg) before and after bilateral inhibition of the ARCN by muscimol were 7.5 ± 2.0 and 4.6 ± 1.3 beats/min, respectively. The tachycardic response after the inhibition of ARCN was significantly attenuated (*P < 0.05). C: the increases in MAP induced by intravenously injected ANG-(1–12) (300 pmol/kg) before and after bilateral blockade of ANG II type 1 receptors (AT1Rs) in the ARCN by microinjections of losartan (10 mM) were 27.0 ± 1.9 and 26.3 ± 1.0 mmHg, respectively. The pressor responses were not significantly different (n = 6, P > 0.05). D: in the same group of rats, the increases in HR induced by intravenously injected ANG-(1–12) (300 pmol/kg) before and after bilateral blockade of AT1Rs in the ARCN by microinjections of losartan were 7.3 ± 1.8 and 6.2 ± 2.2 beats/min, respectively. The tachycardic responses were not significantly different (P > 0.05).
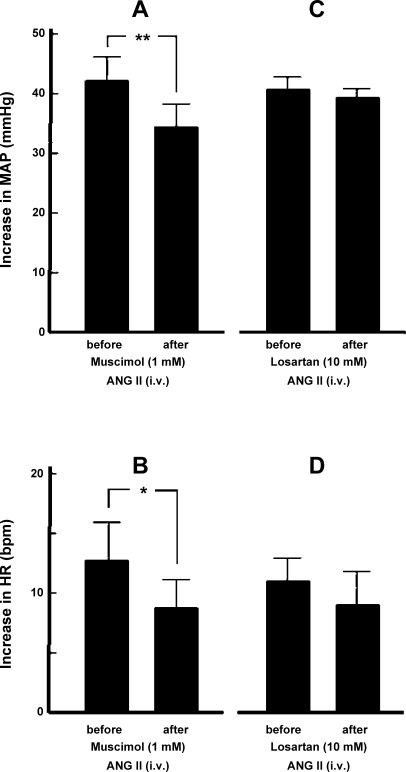
The role of ARCN in mediating responses to intravenous injections of ANG II was studied as follows. In one group of rats (n = 7), cardiovascular responses to intravenous injections of ANG II (300 pmol/kg) were determined before and after inhibition of the ARCN by muscimol. Bilateral inhibition of the ARCN by muscimol significantly attenuated pressor (Fig. 4A) and tachycardic (Fig. 4B) responses to intravenously administered ANG II. In another group of rats (n = 6), bilateral blockade of AT1Rs in the ARCN using losartan (10 mM) microinjections did not significantly alter pressor (Fig. 4C) and tachycardic (Fig. 4D) responses to intravenously administered ANG II.
Fig. 4.
Role of the ARCN in mediating cardiovascular responses to intravenous ANG II. A: the increases in MAP induced by intravenously injected ANG II (300 pmol/kg) before and after bilateral inhibition of the ARCN by muscimol (1 mM) were 42.4 ± 3.8 and 34.6 ± 3.6 mmHg, respectively. The pressor response after inhibition of the ARCN was significantly attenuated (n = 7, **P < 0.001). B: in the same group of rats, the increases in HR induced by intravenously injected ANG II (300 pmol/kg) before and after bilateral inhibition of the ARCN by muscimol were 12.9 ± 3.4 and 8.7 ± 2.5 beats/min, respectively. The tachycardic response after the inhibition of ARCN was significantly attenuated (*P < 0.05). C: the increases in MAP induced by intravenously injected ANG II (300 pmol/kg) before and after bilateral blockade of AT1Rs in the ARCN by microinjections of losartan (10 mM) were 41.0 ± 1.9 and 39.8 ± 1.5 mmHg, respectively. The pressor responses were not significantly different (n = 6, P > 0.05). D: in the same group of rats, the increases in HR induced by intravenously injected ANG II (300 pmol/kg) before and after bilateral blockade of AT1Rs in the ARCN by microinjections of losartan were 10.5 ± 2.5 and 9.0 ± 2.7 beats/min, respectively. The tachycardic responses were not significantly different (P > 0.05).
In these experiments, the interval between the two injections of ANG-(1–12) or ANG II was at least 40 min to avoid desensitization.
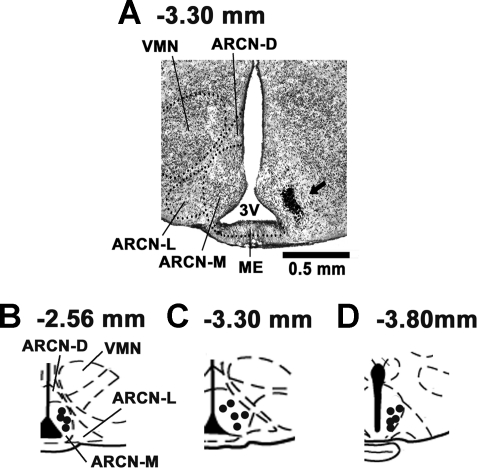
Histology.
A typical ARCN site marked with India ink (50 nl) is shown in Fig. 5A. Figure 5, B–D, shows composite diagrams of marked ARCN sites, which were located 2.56, 3.30, and 3.80 mm caudal to the bregma (n = 14).
Fig. 5.
Histological identification of microinjection sites. A: coronal section at a level 3.30 mm caudal to the bregma showing a microinjection site in the middle ARCN marked with India ink (50 nl, arrow). The center of the spot was 0.30 mm lateral to the midline and 10.1 mm deep from the dura. B–D: drawings of coronal sections 2.56 (B), 3.30 (C), and 3.80 (D) mm caudal to the bregma showing the ARCN microinjection sites as dark spots. Each spot represents a site in one animal. The microinjection sites were located in the ARCN, 0.1–0.3 mm lateral to the midline and 9.8–10.1 mm deep from the dura (n = 14). 3V, third ventricle; ARCN-L, ARCN-M, and ARCN-D, lateral, medial, and dorsal regions of the ARCN; ME, median eminence; VMN, hypothalamic ventromedial nucleus.
DISCUSSION
The major findings of the present study were as follows: 1) microinjections of ANG-(1–12) into the ARCN elicited increases in MAP, HR, and efferent GSNA; 2) these effects were mediated via the activation of AT1Rs, but not AT2Rs, in the ARCN; 3) ANG-(1–12)-induced cardiovascular responses were attenuated by prior microinjections of either captopril or chymostatin; 4) the combination of microinjections of captopril and chymostatin into the ARCN completely blocked the cardiovascular effects of ANG-(1–12); and 5) microinjections of ANG II into the ARCN increased MAP and HR. These observations suggested that the ARCN may be a new site of cardiovascular actions of ANGs.
The increases in MAP and HR elicited by microinjections of ANG-(1–12) into different segments of the ARCN were relatively small. There are no reports in the literature for a comparison of our results in the ARCN. However, increases in MAP and HR elicited by a related ANG [ANG II (1 mM)] into the hypothalamic paraventricular nucleus (PVN), which is known to play a significant role in central cardiovascular regulation, have also been reported to be small (7 ± 2 mmHg and 18 ± 6 beats/min, respectively) (48). It is possible that the pressor and tachycardic effects of ANG-(1–12) elicited from the ARCN, while small in normal states, may be exaggerated in pathological states, such as hypertension. Similar instances have been reported in other brain areas. For example, the pressor responses to microinjections of ANG II into the anterior hypothalamic area in normotensive Wistar-Kyoto rats are smaller than similar microinjections of ANG II into the same brain area of spontaneously hypertensive rats (24).
Stimulation of the DMN and VMN has been reported to increase MAP, HR, and SNA (11, 31, 47). The possibility that pressor and tachycardic responses elicited from the ARCN may be due to the spread of ANG-(1–12) or NMDA to the DMN and VMN was excluded because the cardiovascular responses elicited from the caudal ARCN, where the DMN and VMN are not present, were similar to those elicited from the middle and rostral ARCN. We (31) have previously excluded the possibility that NMDA microinjected into the ARCN may have spread to the DMN because the pressor and tachycardic responses to ARCN stimulation remained unaltered after the inhibition of the DMN by microinjections of muscimol. Although injections of NMDA into the third ventricle adjacent to the ARCN elicited increases in MAP and HR, these responses were mediated via the ARCN, where NMDA may have diffused. Injections of ANG-(1–12) into the same site in the third ventricle did not elicit a response. The differences in the responses elicited by ANG-(1–12) and NMDA in the third ventricle may be due to the differences in the diffusion properties of the two agents or density of corresponding receptors in the ARCN. The concentrations of NMDA (10 mM) and ANG-(1–12) (1 mM) that elicited pressor and tachycardic responses when microinjected (50 nl each) into the ARCN did not elicit detectable cardiovascular responses when injected intravenously, indicating that leakage of the drug, if any, from the microinjection site in the ARCN to the peripheral circulation was not responsible for the observed responses.
The responses of ANG-(1–12) were mediated via its conversion to ANG II. ACE has been reported to be involved in the conversion of ANG-(1–12) to ANG II in some regions of the brain (e.g., the NTS) (1). Chymase has been reported to be one of the alternate pathways by which ANG II is formed in various tissues (37). In the central nervous system, this pathway is involved in ANG II formation in the pituitary stalk and pineal gland (2). We have shown, for the first time, that chymase may be partially involved in the conversion of ANG-(1–12) to ANG II in the ARCN. Indeed, the effects of ANG-(1–12) were abolished when both enzymes (ACE and chymase) were inhibited simultaneously.
The responses to ANG-(1–12) were mediated via AT1Rs, and this observation was confirmed by using two AT1R antagonists. The blockade of ANG-(1–12)-induced responses could not be attributed to desensitization because the interval between the two doses of ANG-(1–12) was at least 40 min (desensitization to repeated injections of this ANG did not occur at this time interval).
Based on current knowledge regarding the role of the ARCN in cardiovascular regulation, the mechanism of increases in MAP, HR, and GSNA elicited by microinjections of ANG-(1–12) into the ARCN can be explained as follows (10, 31). Microinjections of ANG-(1–12) into the ARCN may excite the ARCN neurons involved in cardiovascular regulation. The mechanism of this excitation is not clear at this time and may involve pre- and/or postsynaptic effects of ANG-(1–12). Direct projections from the ARCN to the PVN, raphe nuclei, rostral ventrolateral medullary pressor area, and intermediolateral cell column of the thoracolumbar cord may mediate the increases in MAP, HR, and GSNA elicited by microinjections of ANG-(1–12) into the ARCN (6, 9, 25, 43). We (31) have previously shown that pressor responses elicited by microinjections of NMDA into the ARCN are mediated via the activation of ionotropic glutamate receptors (iGLURs) in the spinal cord. The tachycardic responses to microinjections of ANG-(1–12) may be mediated by both inhibition of vagal outflow to the heart and activation of spinal cord iGLURs, as previously reported for NMDA (31).
The ARCN includes different populations of neurons containing diverse neuroactive substances, including pro-opiomelanocortin (POMC), cocaine- and amphetamine-regulated transcript (CART), neuropeptide Y/agouti-related protein (NPY/AGRP), GABA, and glutamate (26). Although the functions of all phenotypes of ARCN neurons have not been established, it is known that POMC and CART neurons are involved in catabolic activity (decrease in food intake and increase in energy expenditure including an increase in SNA), whereas NPY/AGRP neurons are involved in anabolic activities (increase in food intake and decrease in energy expenditure, including a decrease in SNA). It may be speculated that microinjections of ANG-(1–12) may activate the ARCN neurons involved in catabolic activity (POMC/CART neurons) because an increase in GSNA was observed. The physiological or pathological role of ANG-(1–12) in the ARCN in energy expenditure remains to be studied.
The ventromedial ARCN has been reported to lack a blood-brain barrier (34). In our study, bilateral blockade of AT1Rs in the ARCN did not attenuate the cardiovascular responses to intravenously administered ANG-(1–12) or ANG II. This observation rules out the possibility that these ANGs cross the blood-brain barrier at the ARCN and elicit cardiovascular responses. However, bilateral inhibition of neurons in the ARCN by multiple injections of muscimol did attenuate the cardiovascular responses to intravenously injected ANG-(1–12) and ANG II. This observation suggested that the ARCN may play a role in mediating the increases in MAP and HR induced by intravenously administered ANGs. Consistent with this notion is a report (12) in which intravenous infusions of ANG II elicited an increase in Fos-related antigen immunoreactivity in the ARCN of rabbits. It is well known that circulating ANGs act on AT1Rs in the subfornical organ (SFO), which lacks a blood-brain barrier (14). Activation of neurons in the SFO could then activate neurons in the ARCN to elicit pressor and tachycardic responses. Because the SFO has been implicated in mediating the pressor responses of circulating ANG II (14) and we have shown that the pressor effects of intravenous ANG II and ANG-(1–12) are attenuated when the ARCN is inhibited by muscimol, it appears that direct or indirect projections from the SFO to the ARCN may exist. Indeed, direct projections from the SFO to the ARCN have been reported based on a retrograde tracing study (16). In this context, it may be noted that direct or indirect projections from the ARCN to the SFO have also been reported (39, 40). These inputs from the ARCN to the SFO may be involved in modulating the activity of SFO neurons that detect blood-borne signals from the depletion of intra- and extracellular fluid volumes. The transmitter at the terminals of direct projections from the SFO to the ARCN does not appear to be ANG because bilateral blockade of AT1Rs in the ARCN did not attenuate cardiovascular responses to intravenously administered ANGs. In these experiments, bilateral blockade of AT1Rs in the ARCN did not alter baseline BP and HR. There are no reports in the literature for a comparison of our results in the ARCN. Although unilateral or bilateral microinjections of an AT1R antagonist into the ARCN did not alter baseline BP and HR in normal rats, a decrease in these variables after AT1R blockade may be elicited in pathological states. Such a situation has been reported in the PVN, where bilateral microinjection of an AT1R antagonist had no significant effect on resting sympathetic activity in normal situations but significantly reduced sympathoexcitation induced by hyperosmolality or heart failure (7, 49).
Perspectives
It has been suggested that ANG-(1–12) may be formed directly from angiotensinogen in a renin-independent manner (44). Because high concentrations of angiotensinogen have been reported in the ARCN (22), this nucleus may be one of sites where ANG-(1–12) can be synthesized. ANG-(1–12) and/or ANG II may eventually emerge as important components of the RAS in the ARCN. The physiological and pathophysiological significance of ANGs in the ARCN can only be speculated at this time. In experimental animals, a high-fat diet has been shown to induce inflammatory signaling in the ARCN coincident with the onset of leptin resistance selectively in this brain region (29). In some pathological states (e.g., rats with heart failure), proinflammatory cytokines are increased in the hypothalamus and contribute to neurohumoral excitation by activating the brain RAS (23). Upregulation of the RAS in the brain has been reported in experimental and genetic models of hypertension (5, 17, 28, 46). This study provides the groundwork for future investigations on the role of ANG-(1–12) and ANG II in the ARCN in different pathological states such heart failure and hypertension, including obesity-induced hypertension.
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute Grants HL-024347 and HL-076248 (to H. N. Sapru).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Arnold AC, Isa K, Shaltout HA, Nautiyal M, Ferrario CM, Chappell MC, Diz DI. Angiotensin-(1–12) requires angiotensin converting enzyme and AT1 receptors for cardiovascular actions within the solitary tract nucleus. Am J Physiol Heart Circ Physiol 299: H763–H771, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baltatu O, Nishimura H, Hoffmann S, Stoltenburg G, Haulica ID, Lippoldt A, Ganten D, Urata H. High levels of human chymase expression in the pineal and pituitary glands. Brain Res 752: 269–278, 1997 [DOI] [PubMed] [Google Scholar]
- 3. Bell-Reuss E, Trevino DL, Gottschalk CW. Effect of renal sympathetic nerve stimulation on proximal water and sodium reabsorption. J Clin Invest 57: 1104–1107, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blankley CJ, Hodges JC, Klutchko SR, Himmelsbach RJ, Chucholowski A, Connolly CJ, Neergaard SJ, Van Nieuwenhze MS, Sebastian A, Quin J, III, Essenberg AD, Cohen DM. Synthesis and structure-activity relationships of a novel series of non-peptide angiotensin II receptor binding inhibitors specific for the AT2 subtype. J Med Chem 34: 3248–3260, 1991 [DOI] [PubMed] [Google Scholar]
- 5. Brooks VL. Interactions between angiotensin II and the sympathetic nervous system in the long-term control of arterial pressure. Clin Exp Pharmacol Physiol 24: 83–90, 1997 [DOI] [PubMed] [Google Scholar]
- 6. Cechetto DF, Saper CB. Neurochemical organization of the hypothalamic projection to spinal cord in the rat. J Comp Neurol 272: 579–604, 1988 [DOI] [PubMed] [Google Scholar]
- 7. Chen QH, Toney GM. AT1-receptor blockade in the hypothalamic PVN reduces central hyperosmolality-induced renal sympathoexcitation. Am J Physiol Regul Integr Comp Physiol 281: R1844–R1853, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Chen QH, Toney GM. Responses to GABA-A receptor blockade in the hypothalamic PVN are attenuated by local AT1 receptor antagonism. Am J Physiol Regul Integr Comp Physiol 285: R1231–R1239, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Ciriello J, McMurray JC, Babic T, de Oliveira CVR. Collateral axonal projections from hypothalamic hypocretin neurons to cardiovascular sites in nucleus ambiguus and nucleus tractus solitarius. Brain Res 991: 133–141, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Coote JH. The hypothalamus and cardiovascular regulation. In: Neural Mechanisms of Cardiovascular Regulation, edited by Dun NJ, Machado BH, Pilowsky PM. Boston, MA: Kluwer Academic, 2004, p. 117–146 [Google Scholar]
- 11. Dampney RA, Horiuchi J, Killinger S, Sheriff MJ, Tan PS, McDowall LM. Long-term regulation of arterial blood pressure by hypothalamic nuclei: some critical questions. Clin Exp Pharmacol Physiol 32: 419–425, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Davern PJ, Head GA. Fos-related antigen immunoreactivity after acute and chronic angiotensin II-induced hypertension in the rabbit brain. Hypertension 49: 1170–1177, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Donadio MV, Gomes CM, Sagae SC, Franci CR, Nselmo-Franci JA, Lucion AB, Sanvitto GL. Estradiol and progesterone modulation of angiotensin II receptors in the arcuate nucleus of ovariectomized and lactating rats. Brain Res 1083: 103–109, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Ferguson AV. Angiotensinergic regulation of autonomic and neuroendocrine outputs: critical roles for the subfornical organ and paraventricular nucleus. Neuroendocrinology 89: 370–376, 2009 [DOI] [PubMed] [Google Scholar]
- 15. Fontes MA, Martins Pinge MC, Naves V, Campagnole-Santos MJ, Lopes OU, Khosla MC, Santos RA. Cardiovascular effects produced by microinjection of angiotensins and angiotensin antagonists into the ventrolateral medulla of freely moving rats. Brain Res 750: 305–310, 1997 [DOI] [PubMed] [Google Scholar]
- 16. Gruber K, Rae-Degueurce A, Wilkin LD, Mitchell LD, Johnson AK. Forebrain and brainstem afferents to the arcuate nucleus in the rat: potential pathways for the modulation of hypophyseal secretions. Neurosci Lett 75:1–5, 1987 [DOI] [PubMed] [Google Scholar]
- 17. Gyurko R, Wielbo D, Phillips MI. Antisense inhibition of AT1 receptor mRNA and angiotensinogen mRNA in the brain of spontaneously hypertensive rats reduces hypertension of neurogenic origin. Regul Pept 49: 167–174, 1993 [DOI] [PubMed] [Google Scholar]
- 18. He S, Gaca MD, McEuen AR, Walls AF. Inhibitors of chymase as mast cell-stabilizing agents: contribution of chymase in the activation of human mast cells. J Pharmacol Exp Ther 291: 517–523, 1999 [PubMed] [Google Scholar]
- 19. Healy DP, Printz MP. Distribution of immunoreactive angiotensin II, angiotensin I, angiotensinogen and renin in the central nervous system of intact and nephrectomized rats. Hypertension 6: I130––I136., 1984 [DOI] [PubMed] [Google Scholar]
- 20. Hocht C, Gironacci MM, Mayer MA, Schuman M, Bertera FM, Taira CA. Involvement of angiotensin-(1–7) in the hypothalamic hypotensive effect of captopril in sinoaortic denervated rats. Regul Pept 146: 58–66, 2008 [DOI] [PubMed] [Google Scholar]
- 21. Isa K, Garcia-Espinosa MA, Arnold AC, Pirro NT, Tommasi EN, Ganten D, Chappell MC, Ferrario CM, Diz DI. Chronic immunoneutralization of brain angiotensin-(1–12) lowers blood pressure in transgenic (mRen2)27 hypertensive rats. Am J Physiol Regul Integr Comp Physiol 297: R111–R115, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Johren O, Inagami T, Saavedra JM. Localization of AT2 angiotensin II receptor gene expression in rat brain by in situ hybridization histochemistry. Brain Res Mol Brain Res 37: 192–200, 1996 [DOI] [PubMed] [Google Scholar]
- 23. Kang YM, Zhang ZH, Xue B, Weiss RM, Felder RB. Inhibition of brain proinflammatory cytokine synthesis reduces hypothalamic excitation in rats with ischemia-induced heart failure. Am J Physiol Heart Circ Physiol 295: H227–H236, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kubo T, Yamaguchi H, Tsujimura M, Hagiwara Y, Fukumori R. An angiotensin system in the anterior hypothalamic area anterior is involved in the maintenance of hypertension in spontaneously hypertensive rats. Brain Res Bull 52: 291–296, 2000 [DOI] [PubMed] [Google Scholar]
- 25. Li P, Tjen-ALooi SC, Guo ZL, Fu LW, Longhurst JC. Long-loop pathways in cardiovascular electroacupuncture responses. J Appl Physiol 106: 620–630, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Meister B. Neurotransmitters in key neurons of the hypothalamus that regulate feeding behavior and body weight. Physiol Behav 92: 263–271, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Migdalof BH, Antonaccio MJ, McKinstry DN, Singhvi SM, Lan SJ, Egli P, Kripalani KJ. Captopril: pharmacology, metabolism and disposition. Drug Metab Rev 15: 841–869, 1984 [DOI] [PubMed] [Google Scholar]
- 28. Morimoto S, Cassell MD, Beltz TG, Johnson AK, Davisson RL, Sigmund CD. Elevated blood pressure in transgenic mice with brain-specific expression of human angiotensinogen driven by the glial fibrillary acidic protein promoter. Circ Res 89: 365–372, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Munzberg H, Flier JS, Bjorbaek C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology 145: 4880–4889, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Nagata S, Kato J, Sasaki K, Minamino N, Eto T, Kitamura K. Isolation and identification of proangiotensin-12, a possible component of the renin-angiotensin system. Biochem Biophys Res Commun 350: 1026–1031, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Nakamura T, Bhatt S, Sapru HN. Cardiovascular responses to hypothalamic arcuate nucleus stimulation in the rat: role of sympathetic and vagal efferents. Hypertension 54: 1369–1375, 2009 [DOI] [PubMed] [Google Scholar]
- 32. Nakamura T, Kawabe K, Sapru HN. Cardiovascular responses to microinjections of urocortin 3 into the nucleus tractus solitarius of the rat. Am J Physiol Heart Circ Physiol 296: H325–H332, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nicholson C. Diffusion from an injected volume of a substance in brain tissue with arbitrary volume fraction and tortuosity. Brain Res 333: 325–329, 1985 [DOI] [PubMed] [Google Scholar]
- 34. Palkovits M. Stress-induced activation of neurons in the ventromedial arcuate nucleus: a blood-brain-CSF interface of the hypothalamus. Ann NY Acad Sci 1148: 57–63, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Patel D, Bohlke M, Phattanarudee S, Kabadi S, Maher TJ, Ally A. Cardiovascular responses and neurotransmitter changes during blockade of angiotensin II receptors within the ventrolateral medulla. Neurosci Res 60: 340–348, 2008 [DOI] [PubMed] [Google Scholar]
- 36. Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates (4th ed.). New York: Academic, 1986 [Google Scholar]
- 37. Prosser HC, Forster ME, Richards AM, Pemberton CJ. Cardiac chymase converts rat proangiotensin-12 (PA12) to angiotensin II: effects of PA12 upon cardiac haemodynamics. Cardiovasc Res 82: 40–50, 2009 [DOI] [PubMed] [Google Scholar]
- 38. Rahmouni K, Morgan DA. Hypothalamic arcuate nucleus mediates the sympathetic and arterial pressure responses to leptin. Hypertension 49: 647–652, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Rosas-Arellano MP, Solano-Flores LP, Ciriello J. Glutamate stimulation of arcuate nucleus inhibits responses of subfornical organ neurons to plasma hypernatremia and angiotensin II. Neurosci Lett 198:201–204, 1995 [DOI] [PubMed] [Google Scholar]
- 40. Rosas-Arellano MP, Solano-Flores LP, Ciriello J. Arcuate nucleus inputs onto subfornical organ neurons that respond to plasma hypernatremia and angiotensin II. Brain Res 707: 308–313, 1996 [DOI] [PubMed] [Google Scholar]
- 41. Sapru HN, Gonzalez ER, Krieger AJ. Greater splanchnic nerve activity in the rat. Brain Res Bull 8: 267–272, 1982 [DOI] [PubMed] [Google Scholar]
- 42. Sheriff MJ, Fontes MAP, Killinger S, Horiuchi J, Dampney RAL. Blockade of AT1 receptors in the rostral ventrolateral medulla increases sympathetic activity under hypoxic conditions. Am J Physiol Regul Integr Comp Physiol 290: R733–R740, 2006 [DOI] [PubMed] [Google Scholar]
- 43. Sim LJ, Joseph SA. Arcuate nucleus projections to brainstem regions which modulate nociception. J Chem Neuroanat 4: 97–109, 1991 [DOI] [PubMed] [Google Scholar]
- 44. Varagic J, Trask AJ, Jessup JA, Chappell MC, Ferrario CM. New angiotensins. J Mol Med 86: 663–671, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang HJ, Zhang F, Zhang Y, Gao XY, Wang W, Zhu GQ. AT1 receptor in paraventricular nucleus mediates the enhanced cardiac sympathetic afferent reflex in rats with chronic heart failure. Auton Neurosci 121:56–63, 2005 [DOI] [PubMed] [Google Scholar]
- 46. Yongue G, Angulo JA, McEwen BS, Myers MM. Brain and liver angiotensinogen messenger RNA in genetic hypertensive and normotensive rats. Hypertension 17: 485–491, 1991 [DOI] [PubMed] [Google Scholar]
- 47. Yoshimatsu H, Egawa M, Bray GA. Sympathetic nerve activity after discrete hypothalamic injections of l-glutamate. Brain Res 601: 121–128, 1993 [DOI] [PubMed] [Google Scholar]
- 48. Zheng H, Li Y, Wang W, Patel KP. Enhanced angiotensin-mediated excitation of renal sympathetic nerve activity within the paraventricular nucleus of anesthetized rats with heart failure. Am J Physiol Regul Integr Comp Physiol 297: R1364–R1374, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhu GQ, Lie G, Patel KM, Zucker IH, Wang W. ANG II in the paraventricular nucleus potentiates the cardiac sympathetic afferent reflex in rats with heart failure. J Appl Physiol 97: 1746–1754, 2004 [DOI] [PubMed] [Google Scholar]
- 50. Zini S, Masdehors P, Lenkei Z, Fournie-Zaluski MC, Roques BP, Corvol P, Llorens-Cortes C. Aminopeptidase A: distribution in rat brain nuclei and increased activity in spontaneously hypertensive rats. Neuroscience 78: 1187–1193, 1997 [DOI] [PubMed] [Google Scholar]