Abstract
Antimicrobial resistance is threatening the successful management of nosocomial infections worldwide. Despite the therapeutic limitations imposed by methicillin-resistant Staphylococcus aureus (MRSA), its clinical impact is still debated. The objective of this study was to estimate the excess mortality and length of hospital stay (LOS) associated with MRSA bloodstream infections (BSI) in European hospitals. Between July 2007 and June 2008, a multicenter, prospective, parallel matched-cohort study was carried out in 13 tertiary care hospitals in as many European countries. Cohort I consisted of patients with MRSA BSI and cohort II of patients with methicillin-susceptible S. aureus (MSSA) BSI. The patients in both cohorts were matched for LOS prior to the onset of BSI with patients free of the respective BSI. Cohort I consisted of 248 MRSA patients and 453 controls and cohort II of 618 MSSA patients and 1,170 controls. Compared to the controls, MRSA patients had higher 30-day mortality (adjusted odds ratio [aOR] = 4.4) and higher hospital mortality (adjusted hazard ratio [aHR] = 3.5). Their excess LOS was 9.2 days. MSSA patients also had higher 30-day (aOR = 2.4) and hospital (aHR = 3.1) mortality and an excess LOS of 8.6 days. When the outcomes from the two cohorts were compared, an effect attributable to methicillin resistance was found for 30-day mortality (OR = 1.8; P = 0.04), but not for hospital mortality (HR = 1.1; P = 0.63) or LOS (difference = 0.6 days; P = 0.96). Irrespective of methicillin susceptibility, S. aureus BSI has a significant impact on morbidity and mortality. In addition, MRSA BSI leads to a fatal outcome more frequently than MSSA BSI. Infection control efforts in hospitals should aim to contain infections caused by both resistant and susceptible S. aureus.
The emergence of resistant bacteria is a natural consequence of antibiotic use and complicates the treatment of infected patients. Staphylococcus aureus resistant to isoxazolyl penicillins (methicillin-resistant S. aureus [MRSA]) is one of the most frequent pathogens causing resistant infections in hospitals worldwide (14, 21, 33). The questions are whether and to what extent resistance affects survival and the duration of hospital admission in patients with bacterial infections. Previous studies compared patients with MRSA infections to those infected by methicillin-susceptible S. aureus (MSSA), using mortality as one of the main endpoints. New insights challenge this approach for a number of reasons.
Several studies have shown that patients with MRSA bloodstream infection (BSI) differ in many ways from those with MSSA BSI; they are older, have more comorbidities, and experience longer hospital admissions before the onset of infection (6, 22, 37). If these two groups of patients are compared directly, bias is introduced, compromising the validity of the results. Of all hospitalized patients at risk of acquiring MRSA BSI, the younger, relatively more healthy MSSA patients are selected as the control group, magnifying the possible impact of resistance (20). Moreover, time-dependent distortions are introduced, as patients staying in the hospital for a shorter period, like MSSA patients, have a smaller chance of acquiring MRSA BSI than patients hospitalized for longer periods, who for many reasons are more likely to die, thus leading to overestimation of the clinical impact of resistance (36).
It has also been shown that MRSA BSIs are not replacing infections caused by MSSA but adding to the existing burden (3, 15, 43). For this reason, MRSA-specific mortality needs to be measured against mortality in patients free of S. aureus BSI. A parallel matched-cohort design (23) allows this comparison. MRSA and MSSA patients are compared separately to controls who have chances to acquire an MRSA or MSSA BSI equal to those of the case patients to whom they are matched. At the same time, this design provides the means to determine the impact attributable to resistance per se and minimizes bias.
Different mortality outcomes are used in the literature. Some studies have focused on hospital mortality (10, 35, 37), while others determined mortality within a predefined interval (29, 32). Each outcome measure requires a distinct analytical approach, as well as correct interpretation. Since previous investigations often ignored this subtle distinction, subsequent analysis often resulted in flawed conclusions (31).
There is thus a need for more robust estimates of the impact of methicillin-resistant S. aureus BSI on unambiguously defined mortality and morbidity endpoints using the appropriate control groups and correct analytical methods. In the present study, the excess 30-day mortality, hospital mortality, and length of stay attributable to MRSA BSI were determined by collecting data from a large sample of European hospitals, using a parallel-cohort design and appropriate statistical approaches.
MATERIALS AND METHODS
Setting.
Thirteen tertiary care centers (WHO definition [2]) from as many European countries were selected from hospitals participating in the European Antimicrobial Resistance Surveillance System (EARSS) (12). For EARSS, the National Representatives are responsible for the selection of hospitals on the basis of geodemographic representativeness. EARSS requires that a minimum of 20% of hospitals participate per country. We are therefore well aware of the distribution of resistance in the participating countries and chose hospitals representative of the national level of methicillin resistance. The selected hospitals also showed good diagnostic accuracy, according to the results of the external quality assessment exercise carried out annually by EARSS.
Study design.
A prospective parallel matched-cohort design was chosen. Cohort I consisted of patients with MRSA BSI (MRSA cohort), cohort II consisted of patients with MSSA BSI (MSSA cohort), and both cohorts included controls free of S. aureus BSI. Any episode of MRSA or MSSA BSI in an adult patient (≥18 years old) was identified by daily laboratory liaison. Blood cultures were taken on clinical indication, and all hospital patients with a laboratory-confirmed diagnosis of S. aureus BSI were included. Susceptibility was determined by a cefoxitin or oxacillin screen test and confirmed by a PCR mecA test or a PBP2a agglutination test, according to consensus protocols published in the EARSS manual (11). The day of enrollment was defined as the date the first positive blood culture was obtained. Each patient with an S. aureus BSI was matched to two controls. Since taking into account the time dependency of nosocomial infections is more important than adjusting for other confounding factors (7, 42), controls were matched on the length of hospital stay (LOS) before enrollment of a patient with BSI (±3 days). Other matching variables were not included, because that would reduce the number of cases eligible for the study, thereby diminishing the representativeness of the cases for all patients with S. aureus bacteremia in the participating hospitals. If more than two patients were eligible as controls, the patients closest to the patient with S. aureus BSI in the ward registry were selected.
Data reporting and training of on-site investigators.
For each hospital, a dedicated on-site investigator was recruited. They were trained in consistent enrollment and data collection in two workshops, using anonymized patient records provided by one of the authors (P.G.D.). Patients were enrolled for 12 months between July 2007 and June 2008. Anonymized data were recorded using a Web-based and password-protected data submission tool hosted by the Netherlands National Institute of Public Health and the Environment. Built-in data checks secured data validity. To further ensure uniform application of study definitions, one author (M.E.A.D.K.) provided continuous help desk support. The data sources were patient records, the electronic hospital information system, the laboratory information system, and nursing notes. Postdischarge surveillance to determine survival 30 days after enrollment was carried out by telephone contact with patients or their general practitioners.
The preenrollment data recorded were transfer from a long-term care facility, nursing home, or another hospital; admission diagnosis; type of admission (emergency or elective); comorbidities in the Charlson Comorbidity Index (9); previous surgery; and frequent hospital exposure, defined as two or more hospital admissions in the previous 12 months. At enrollment, the following variables were recorded: demographical data (age and gender), the anatomical source of S. aureus BSI, the susceptibility profile of the causative pathogen, and poly- or monomicrobial bacteremia, as well as the presence of indwelling devices (tracheal tube, central venous catheter, arterial vascular access, peripheral vascular access, urinary catheter, tracheostomy, nasogastric tube, or wound drainage tube), where the presence of a tracheal tube was used as a surrogate marker for an intensive care unit (ICU) stay. The main outcomes were mortality 30 days after enrollment, hospital mortality, and LOS after enrollment.
Crude mortality was defined as the proportion of MRSA and MSSA patients who died. Attributable mortality was defined as the ratio between the risk of dying among patients with MRSA or MSSA BSI and that of their matched controls, taking into account matching and additional explanatory variables that influenced effect estimates by multivariate analysis. This was expressed as the odds ratio (OR) for 30-day mortality and the hazard ratio (HR) for hospital mortality.
This study complied with the Dutch patient confidentiality regulations and ethical standards (28) and was approved by local institutional ethical committees when required.
Statistical analysis.
Statistical analyses were performed using SAS 9.1 and R 2.8.1. Univariate comparisons of patients with S. aureus BSI (either MRSA or MSSA) and unexposed patients were performed using Cochran's Q statistic for categorical variables. For continuous variables, Friedman's rank sum test was used.
Analyses of outcomes (30-day or hospital mortality and LOS after enrollment) were performed separately for the MRSA and MSSA cohorts. All collected variables, other than LOS before infection (the matching criterion), that could influence the relationship between MRSA or MSSA and the outcome were tested for confounding in bivariate regression. Variables were included in multivariate analyses if they changed the effect estimate by more than 5%. Collinearity was assessed by generating a correlation coefficient matrix. A robust sandwich covariance matrix estimator was used to account for the matched design. The effect attributable to methicillin resistance was determined by the ratio of the adjusted effect measures for MRSA BSI and MSSA BSI from the parallel cohorts, and the confidence intervals were determined as described by Altman and Bland (4).
Thirty-day mortality and hospital mortality.
The effect of S. aureus BSI on mortality was determined by logistic regression for 30-day mortality and by Fine and Gray's extended Cox's regression for competing events for hospital mortality (13, 41). For hospital mortality, cumulative incidence graphs were created using a cause-specific hazard model in which both discharge alive and hospital mortality were included as competing endpoints (25, 30).
Length of hospital stay after enrollment.
A generalized linear model (GLM) with gamma distribution and log link function for positively skewed data was used to determine the impact of S. aureus BSI on LOS after enrollment (5, 16). The excess LOS was calculated by comparing the mean outcomes predicted by the multivariate model for all patients in each group. Confidence intervals for the difference in LOS in days were obtained by parametric bootstrapping.
Data heterogeneity.
To test for group effects at the hospital level, multilevel models for hierarchical data were used for logistic regression and the GLM models. Stratified analyses were used for the Fine and Gray model.
RESULTS
The participating hospitals reported 1,000 S. aureus bloodstream infections, 310 (31%) of which showed methicillin resistance (range, 7 to 65%) (Table 1). For 134 (13%) of the patients with BSI, no appropriate match (equal LOS before enrollment ±3 days) could be identified, and they were excluded. For 111 (13%) of the included patients with S. aureus BSI, only a single control could be matched. Therefore, the analyses were based on 248 patients with MRSA BSI matched to 453 controls and 618 patients with MSSA BSI matched to 1,170 controls.
TABLE 1.
Activity data for participating hospitals from July 2007 to June 2008
| Hospital no. | Country | No. of beds | No. of admissions | No. of bed days | No. of infecteda/controls | National % MRSAb | MRSA hospital mortality (%) |
|---|---|---|---|---|---|---|---|
| H1 | Austria | 2,137 | 99,761 | 657,268 | 50/99 | 8 | 50 |
| H2 | Belgium | 856 | 26,337 | 290,790 | 32/64 | 21 | 50 |
| H3 | Croatia | 1,724 | 63,804 | 479,528 | 72/143 | 35 | 43 |
| H4 | England | 1,210 | 104,680 | 292,030 | 117/229 | 31 | 44 |
| H5 | Germany | 1,234 | 56,193 | 391,258 | 81/126 | 19 | 30 |
| H6 | Greece | 949 | 44,214 | 293,632 | 56/66 | 41 | 25 |
| H7 | Ireland | 819 | 22,418 | 238,166 | 95/187 | 33 | 28 |
| H8 | Italy | 912 | 55,600 | 292,150 | 67/133 | 34 | 38 |
| H9 | Latvia | 1,029 | 46,343 | 307,006 | 54/108 | 13 | 33 |
| H10 | Malta | 835 | 48,504 | 252,488 | 80/151 | 56 | 29 |
| H11 | Romania | 1,109 | 72,739 | 427,666 | 37/70 | 33 | 52 |
| H12 | Scotland | 877 | 53,276 | 255,215 | 132/257 | 31 | 13 |
| H13 | Slovenia | 2,344 | 83,161 | 614,353 | 127/249 | 7 | 65 |
| Total | 16,035 | 777,030 | 4,791,550 | 1,000/1,882 | 30 | 36 |
Infected, number of first episodes of S. aureus BSI.
Proportion of MRSA according to EARSS 2008 (12).
The excluded patients had longer periods of hospitalization between admission and enrollment (P < 0.01), excluded MSSA patients also had a longer hospitalizations after enrollment (P < 0.01), and excluded MRSA patients had higher hospital mortality (P < 0.01). Otherwise, there were no significant differences.
Irrespective of methicillin susceptibility, the included patients with S. aureus BSI were more often male, more likely to have had frequent hospital exposure, more often had an emergency admission, had a higher number of comorbidities, frequently suffered from severe renal disease or diabetes with end organ damage, and had more indwelling devices than controls. Taking into account methicillin susceptibility, patients with MRSA BSI were older, more likely to have had two or more hospital admissions in the previous year, more often received antibiotics and more often had surgery before enrollment, had more comorbidities and higher prevalence of diabetes, and were more often exposed to indwelling devices at enrollment than patients with MSSA BSI. A similar difference in risk profiles was found for the controls in the MRSA versus the MSSA cohort. As expected, MRSA patients had a longer hospital stay before enrollment (median, 4 days; interquartile range [IQR], 0 to 12) than MSSA patients (median, 1 day; IQR, 0 to 6) (Table 2).
TABLE 2.
Characteristics of patients in the MRSA and MSSA cohort. P values correspond to Cochran's Q statistic or Friedman ranks sum test, whenever appropriate
| Characteristic | Valuea |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MRSA cohort |
MSSA cohort |
|||||||||
| BSI | N | Controls | N | P value | BSI | N | Controls | N | P value | |
| Female | 96 (39) | 248 | 230 (51) | 453 | <0.05 | 231 (37) | 618 | 543 (46) | 1,170 | <0.01 |
| Age (yr)b | 69 (58-77) | 248 | 67 (54-79) | 453 | 0.42 | 66 (53-76) | 618 | 67 (54-77) | 1,170 | 0.36 |
| Transfer from another institution | 38 (18) | 211 | 44 (11) | 391 | <0.05 | 87 (16) | 539 | 114 (11) | 1,021 | <0.05 |
| >2 hospital stays in previous yr | 80 (38) | 211 | 90 (23) | 391 | <0.01 | 131 (24) | 539 | 177 (17) | 1,021 | <0.01 |
| Emergency admission | 181 (73) | 248 | 283 (62) | 453 | <0.01 | 496 (80) | 618 | 784 (67) | 1,168 | <0.01 |
| Antibiotic therapy before enrollment | 148 (60) | 248 | 239 (53) | 453 | 0.27 | 195 (32) | 618 | 467 (40) | 1,170 | <0.01 |
| Surgery before enrollment | 60 (24) | 248 | 110 (24) | 453 | 0.10 | 106 (17) | 618 | 210 (18) | 1,170 | 0.65 |
| Length of stay before enrollmentb (days) | 4 (0-12) | 248 | 3 (1-11) | 453 | 0.06 | 1 (0-6) | 618 | 2 (0-6) | 1,170 | 0.07 |
| Admission diagnosis | ||||||||||
| Cardiovascular disease | 38 (15) | 248 | 81 (18) | 453 | 0.54 | 111 (18) | 618 | 266 (23) | 1,170 | <0.01 |
| Connective tissue disease | 4 (2) | 248 | 1 (0) | 453 | 12 (2) | 618 | 14 (1) | 1,170 | 0.50 | |
| Dermatological causes | 3 (1) | 248 | 6 (1) | 453 | 1.00 | 11 (2) | 618 | 16 (1) | 1,170 | 0.21 |
| Endocrine/metabolic causes | 8 (3) | 248 | 12 (3) | 453 | 0.75 | 13 (2) | 618 | 41 (4) | 1,170 | 0.16 |
| Gastrointestinal causes | 36 (15) | 248 | 79 (17) | 453 | <0.05 | 50 (8) | 618 | 155 (13) | 1,170 | <0.01 |
| Genitourinary causes | 28 (11) | 248 | 53 (12) | 453 | 0.96 | 42 (7) | 618 | 85 (7) | 1,170 | 0.25 |
| Gynecologic causes | 0 | 248 | 3 (1) | 453 | 2 (0) | 618 | 10 (1) | 1,170 | 0.26 | |
| Hematologic causes | 3 (1) | 248 | 19 (4) | 453 | <0.05 | 21 (3) | 618 | 24 (2) | 1,170 | 0.08 |
| Infectious disease | 55 (22) | 248 | 23 (5) | 453 | <0.01 | 149 (24) | 618 | 87 (7) | 1,170 | <0.01 |
| Neurological causes | 9 (4) | 248 | 39 (9) | 453 | <0.05 | 48 (8) | 618 | 103 (9) | 1,170 | 0.23 |
| Oncologic causes | 22 (9) | 248 | 43 (9) | 453 | 0.61 | 47 (8) | 618 | 91 (8) | 1,170 | 0.98 |
| Orthopedic causes | 8 (3) | 248 | 18 (4) | 453 | 0.10 | 26 (4) | 618 | 61 (5) | 1,170 | 0.38 |
| Pulmonary causes | 21 (8) | 248 | 32 (7) | 453 | 0.89 | 34 (6) | 618 | 108 (9) | 1,170 | 0.05 |
| Trauma | 5 (2) | 248 | 16 (4) | 453 | 0.07 | 29 (5) | 618 | 39 (3) | 1,170 | 0.28 |
| Undetermined | 8 (3) | 248 | 28 (6) | 453 | 0.19 | 23 (4) | 618 | 70 (6) | 1,170 | <0.05 |
| Charlson Comorbidity Index | ||||||||||
| Charlson scoreb | 3 (2-5) | 248 | 2 (1-3) | 453 | <0.01 | 2 (1-4) | 618 | 2 (0-3) | 1,170 | <0.01 |
| Myocardial infarct | 37 (15) | 248 | 40 (9) | 453 | <0.05 | 71 (11) | 618 | 125 (11) | 1,170 | 0.93 |
| Congestive heart failure | 57 (23) | 248 | 90 (20) | 453 | 0.54 | 103 (17) | 618 | 167 (14) | 1,170 | 0.14 |
| Cerebrovascular disease | 41 (17) | 248 | 48 (11) | 453 | <0.05 | 63 (10) | 618 | 139 (12) | 1,170 | 0.48 |
| Chronic pulmonary disease | 43 (17) | 248 | 59 (13) | 453 | 0.26 | 67 (11) | 618 | 155 (13) | 1,170 | 0.10 |
| Mild liver disease | 10 (4) | 248 | 22 (5) | 453 | 0.96 | 26 (4) | 618 | 30 (3) | 1,170 | 0.11 |
| Severe liver disease | 22 (9) | 248 | 20 (4) | 453 | 0.06 | 28 (5) | 618 | 34 (3) | 1,170 | <0.05 |
| Severe renal disease | 68 (27) | 248 | 76 (17) | 453 | <0.01 | 133 (22) | 618 | 157 (13) | 1,170 | <0.01 |
| Peripheral vascular disease | 35 (14) | 248 | 47 (10) | 453 | 0.45 | 67 (11) | 618 | 128 (11) | 1,170 | 0.30 |
| Connective tissue disease | 37 (15) | 248 | 40 (9) | 453 | 0.06 | 71 (11) | 618 | 125 (11) | 1,170 | 0.28 |
| Peptic ulcer | 12 (5) | 248 | 24 (5) | 453 | 0.90 | 31 (5) | 618 | 61 (5) | 1,170 | 0.26 |
| Diabetes | 82 (33) | 248 | 78 (17) | 453 | <0.01 | 125 (20) | 618 | 214 (18) | 1,170 | 0.84 |
| Diabetes with end organ damage | 33 (13) | 248 | 19 (4) | 453 | <0.01 | 44 (7) | 618 | 45 (4) | 1,170 | <0.01 |
| Hemiplegia/paraplegia | 20 (8) | 248 | 15 (3) | 453 | 0.06 | 21 (3) | 618 | 48 (4) | 1,170 | 0.25 |
| Cancer/leukemia | 47 (19) | 248 | 98 (22) | 453 | 0.47 | 113 (18) | 618 | 195 (17) | 1,170 | 0.76 |
| Metastatic solid tumor | 17 (7) | 248 | 36 (8) | 453 | 0.97 | 42 (7) | 618 | 61 (5) | 1,170 | 0.64 |
| AIDS | 0 (0) | 248 | 2 (0) | 453 | 4 (1) | 618 | 5 (0) | 1,170 | 0.72 | |
| Dementia | 12 (5) | 248 | 16 (4) | 453 | 0.88 | 19 (3) | 618 | 46 (4) | 1,170 | 0.72 |
| Indwelling device at enrollment | ||||||||||
| Intubation | 33 (13) | 248 | 41 (9) | 453 | 0.13 | 55 (9) | 618 | 53 (5) | 1,170 | <0.01 |
| Central venous catheter | 112 (46) | 246 | 108 (24) | 451 | <0.01 | 209 (34) | 617 | 146 (12) | 1,169 | <0.01 |
| Arterial vascular access | 40 (16) | 248 | 45 (10) | 453 | <0.05 | 71 (11) | 618 | 78 (7) | 1,170 | <0.01 |
| Peripheral vascular access | 181 (73) | 247 | 296 (67) | 444 | <0.05 | 448 (73) | 616 | 759 (65) | 1,162 | <0.01 |
| Urinary catheter | 126 (51) | 245 | 161 (36) | 451 | <0.01 | 215 (35) | 618 | 263 (23) | 1,165 | <0.01 |
| Tracheostomy | 9 (4) | 248 | 11 (2) | 453 | 0.48 | 19 (3) | 618 | 11 (1) | 1,170 | <0.01 |
| Nasogastric tube | 54 (22) | 247 | 60 (13) | 453 | <0.01 | 74 (12) | 618 | 71 (6) | 1,168 | <0.01 |
| Wound drainage tube | 41 (17) | 248 | 53 (12) | 450 | 0.08 | 43 (7) | 618 | 72 (6) | 1,169 | 0.90 |
| Characteristics of the BSI | ||||||||||
| Polymicrobial BSI | 22 (9) | 245 | 54 (9) | 607 | ||||||
| Hospital onset of BSI (>48 h) | 139 (56) | 248 | 275 (44) | 618 | ||||||
| Source | ||||||||||
| Bone/joint | 5 (2) | 248 | 22 (4) | 618 | ||||||
| CNSc foci | 0 | 248 | 12 (2) | 618 | ||||||
| Intervention | 2 (1) | 248 | 7 (1) | 618 | ||||||
| Ear-nose-throat | 6 (2) | 248 | 7 (1) | 618 | ||||||
| Intra-abdominal | 10 (4) | 248 | 11 (2) | 618 | ||||||
| Intravascular | 59 (24) | 248 | 156 (25) | 618 | ||||||
| Lower respiratory tract | 28 (11) | 248 | 63 (10) | 618 | ||||||
| Skin/soft tissue | 53 (21) | 248 | 126 (20) | 618 | ||||||
| Urinary-genital | 12 (5) | 248 | 22 (4) | 618 | ||||||
| Unknown | 73 (29) | 248 | 192 (31) | 618 | ||||||
| Outcome | ||||||||||
| Died in hospital | 85 (36) | 239 | 41 (9) | 446 | <0.01 | 138 (23) | 604 | 76 (7) | 1,166 | <0.01 |
| Died within 30 days after enrollment | 74 (31) | 242 | 36 (8) | 429 | <0.01 | 126 (22) | 585 | 83 (8) | 1,082 | <0.01 |
| Length of stay after enrollmentb (days) | 16 (6-32) | 240 | 7 (3-18) | 447 | <0.01 | 15 (7-26) | 604 | 8 (4-14) | 1,166 | <0.01 |
Percentages are in parentheses.
Median and interquartile range are shown.
CNS, central nervous system.
30-day mortality.
After the onset of MRSA BSI, 74 of the 242 (31%) exposed patients died within 30 days, whereas 36 of the 429 (8%) controls died within 30 days after enrollment. Most patients died in the hospital, but four (5%) of the MRSA patients and six (17%) of the controls died after discharge. Of 585 patients with MSSA BSI, 126 (22%) died, whereas out of 1,082 controls, 83 (8%) died within 30 days after enrollment. Six (5%) of the MSSA patients and 17 (20%) of the control patients died after discharge (Table 2).
Table 3 shows the results of the univariate and multivariate regression analyses. Since multilevel analysis showed that group effects at the hospital level did not influence the coefficients for S. aureus exposure, the numbers are based on regular logistic regression. Compared to controls without S. aureus BSI, exposure to MRSA BSI remained significantly associated with mortality after all potential confounders were adjusted for; the adjusted OR (aOR) for dying 30 days after enrollment was 4.4 (confidence interval [CI], 2.8 to 7.0). For patients exposed to MSSA BSI, the aOR for mortality 30 days after enrollment was 2.4 (CI, 1.7 to 3.3).
TABLE 3.
Impacts of MRSA and MSSA BSI on 30-day mortalitya
| Type of analysis | N | OR for effect measure (CI) | Effect measure and potential confounders in model |
|---|---|---|---|
| MRSA vs controls | |||
| Univariate | 661 | 4.8 (3.2-7.1) | BSI with MRSA |
| Multivariate | 533 | 4.4 (2.8-7.0) | BSI with MRSA, central venous catheter, peripheral vascular access, urinary catheter, >2 admissions in the previous yr, Charlson Comorbidity Index, and no. of indwelling devices |
| MSSA vs controls | |||
| Univariate | 1,614 | 3.3 (2.5-4.3) | BSI with MSSA |
| Multivariate | 1,590 | 2.4 (1.7-3.3) | BSI with MSSA, emergency admission, central venous catheter, urinary catheter, nasogastric tube, no. of indwelling devices |
| MRSA cohort vs MSSA cohort, comparison of multivariate effect estimates | 1.8 (1.04-3.2) | Methicillin resistance of S. aureus BSI |
Univariate and multivariate logistic regression and comparison of multivariate effect estimates from both cohorts.
Comparison of the aORs from the MSSA and MRSA cohorts showed an increased risk of death within 30 days, attributable to methicillin resistance, with an OR of 1.8 (CI, 1.04 to 3.2).
Hospital mortality.
Eighty-five of 239 (36%) MRSA patients died in the hospital, whereas only 41 of 446 control patients (9%) died during their hospital stay. Among patients with MSSA BSI, 138 of 604 (23%) died, whereas only 76 of 1,166 (7%) control patients died during their hospital stay (Table 2).
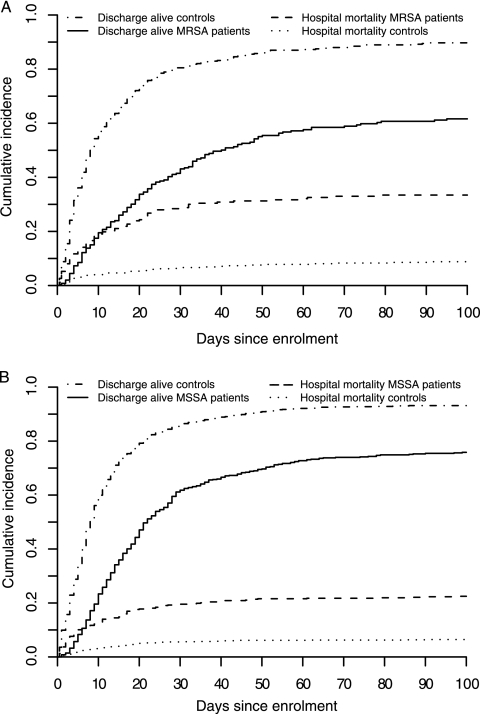
Figure 1 illustrates the dynamics of hospital mortality and discharge over time for both cohorts. Figure 1A shows that for patients with MRSA BSI, LOS till discharge alive varied widely, whereas most MRSA patients died in the hospital within 1.5 weeks after enrollment. Figure 1B reveals a similar pattern for MSSA patients, although a higher proportion of patients was discharged alive within a much shorter time interval.
FIG. 1.
Cumulative-incidence functions for the competing risks, discharge alive and hospital mortality, for patients with, and their matched controls without, S. aureus BSI. (A) MRSA BSI. (B) MSSA BSI.
Table 4 illustrates the impact of MRSA and MSSA BSI on hospital mortality using a subdistribution proportional hazards model. Since the stratified analysis did not show a group effect at the hospital level, the hospital level was not taken into account in these analyses. After all potential confounders were adjusted for, the hazard rate for dying in the hospital was 3.5 times higher for patients with MRSA BSI than for control patients. Patients with MSSA BSI had a 3.1-times-higher adjusted hazard rate for dying in the hospital than the matched control patients.
TABLE 4.
Impacts of MRSA and MSSA BSI on hospital mortalitya
| Type of analysis | N | HR for effect measure (CI) | Effect measure and potential confounders in model |
|---|---|---|---|
| MRSA vs controls | |||
| Univariate | 695 | 4.5 (3.2-6.3) | BSI with MRSA |
| Multivariate | 566 | 3.5 (2.4-5.2) | BSI with MRSA, no. of indwelling devices, Charlson Comorbidity Index, central venous catheter, urinary catheter, frequent hospital contact, diabetes |
| MSSA vs controls | |||
| Univariate | 1,787 | 3.8 (2.9-5.0) | BSI with MSSA |
| Multivariate | 1,765 | 3.1 (2.3-4.2) | BSI with MSSA, intubation, central venous catheter, urinary catheter, no. of indwelling devices |
| MRSA cohort vs MSSA cohort, comparison of multivariate effect estimates | 1.1 (0.7-1.8) | Methicillin resistance of S. aureus BSI |
Univariate and multivariate Fine and Gray proportional hazards regression and comparison of multivariate effect estimates from both cohorts.
Comparison of excess hospital mortality for patients with MRSA BSI and MSSA BSI resulted in an HR for hospital mortality of 1.1 (CI, 0.7 to 1.8) associated with methicillin resistance.
Length of stay after enrollment.
Patients with MRSA BSI stayed in the hospital for a median of 16 days (IQR, 6 to 32 days) after enrollment. Control patients had a median LOS of 7 days (IQR, 3 to 18 days) after enrollment (Table 2). The univariate regression model showed that MRSA patients stayed 1.6 times (CI, 1.4 to 2.0) longer from enrollment to discharge (alive or dead) than the matched control patients. (Table 5).
TABLE 5.
Impacts of MRSA and MSSA BSI on length of stay after enrollmenta
| Type of analysis | N | Ratio of median length of stay for effect measure (CI) | Extra length of stay in days for effect measure (CI) | Effect measure and potential confounders |
|---|---|---|---|---|
| MRSA vs controls | ||||
| Univariate | 669 | 1.6 (1.4-2.0) | 9.6 (5.7-13.8) | BSI with MRSA |
| Multivariate | 558 | 1.6 (1.3-2.0) | 9.2 (5.2-13.5) | BSI with MRSA, emergency admission, peripheral vascular access, frequent hospital contact, transfer from another institution, diabetes with end organ damage |
| MSSA vs controls | ||||
| Univariate | 1741 | 1.7 (1.5-1.9) | 8.4 (6.6-10.3) | BSI with MSSA |
| Multivariate | 1721 | 1.6 (1.5-1.8) | 8.6 (6.8-10.4) | BSI with MSSA, no. of indwelling devices |
| MRSA cohort vs MSSA cohort, comparison of multivariate effect estimates | 1.0 (0.8-1.3) | 0.6 (−3.7-5.3) | Methicillin resistance of S. aureus BSI |
Univariate and multivariate analysis (generalized linear model with gamma distribution and loglink function) and comparison of the multivariate effect estimates from both cohorts.
Patients with MSSA BSI stayed in the hospital for a median of 15 days (IQR, 7 to 26 days) after infection. Control patients had a median LOS of 8 days (IQR, 4 to 14 days) after enrollment (Table 2). The univariate regression model showed that MSSA patients stayed 1.7 times (IOR, 1.5 to 1.9 times) longer from enrollment to discharge than the matched control patients, as presented in Table 5.
Multilevel analysis showed that group effects at the hospital level did not change the coefficients for S. aureus BSI exposure, and therefore, the results presented are based on regular GLMs. After adjustment for potential confounders, MRSA patients stayed 1.6 times longer in the hospital after enrollment, which resulted in an excess length of stay of 9 days (IQR, 5 to 14 days). For the MSSA cohort, only the number of indwelling devices was an important confounder. The adjusted excess length of stay for patients with MSSA BSI was 1.6 times the length of stay of the matched controls, resulting in 9 excess days (IQR, 7 to 10 days).
Comparison of the excess lengths of stay for MRSA and MSSA patients showed that there was no difference in length of stay after enrollment (ratio, 1.0 [CI, 0.8 to 1.3]; excess days, 0.6 [CI, −4 to 5]) (Table 5).
DISCUSSION
Acknowledging the need for accurate and precise estimates of the clinical impact of methicillin resistance in S. aureus, we set out to determine the patient mortality at day 30 and the instantaneous risk of dying during the hospital stay, as well as the extra length of stay attributable to MRSA BSI, by conducting the largest prospective cohort study designed for this purpose. An effect attributable to methicillin resistance could be discerned only for mortality within 30 days, which was increased by 80% (OR, 1.8; CI, 1.04 to 3.2), but not for hospital mortality (HR, 1.1; CI, 0.7 to 1.8) or length of hospital stay (excess days, 0.6; CI, −3.7 to 5.3). Irrespective of the methicillin resistance trait, S. aureus BSI alone increased mortality, as well as LOS, and infected patients died 2 to 4 times more often within 30 days, the instantaneous probability for dying during the entire hospital admission was 3 to 4 times higher, and the patients stayed on average 9 days longer in the hospital than their controls. These findings were consistent among all participating hospitals.
Although mortality as a study endpoint seems straightforward, different measures can lead to different conclusions. It is important to realize that mortality within a predefined interval is a static measure indicating excess mortality within that interval (proportion), while hospital mortality is a dynamic measure that provides insight into the temporal dynamics of dying during the entire hospital stay (rate). Since mortality during hospital admission is easier to determine than mortality within a certain period of follow-up, hospital mortality is regularly used as a measure for the impact of resistance. For reasons of comparability, we included the same measure, despite the complexities related to time-to-event analyses and difficulties when interpreting the resulting hazard ratios. Although not statistically significant, we found that the mortality rate (deaths/hospital day) was higher for MRSA than for MSSA patients; at the same time, statistical tests confirmed that in absolute numbers, more MRSA than MSSA patients died within 30 days.
In comparison with previously published research, three important characteristics of this study stand out. The parallel matched-cohort design made it possible to match infected patients to controls with similar hospital exposure, thereby increasing he comparability of the risk profiles of infected and control patients and, as a consequence, diminishing time-dependent bias (40), as well as severity-of-illness bias (20). Furthermore, two explicitly defined mortality measures were included, of which hospital mortality was analyzed by appropriate analytical methods, taking into account the duration of hospital admission, as well as competing events, i.e., the fact that patients who are discharged alive will not die in the hospital and vice versa (31). Finally, the generalizability and precision of the estimates were improved by sampling from 777,030 patients treated in 13 tertiary care centers from as many different European countries for a total of 4.8 million bed days.
The possible limitations of this study include the potential distortion of results due to discrepancies between hospitals, such as variation in blood culture frequencies or local differences in clinical management. However, multilevel analyses, which were used to test for heterogeneity, indicated that differences between participating centers did not modify the results. Nevertheless, as with any observational study, residual bias or confounding can never be completely ruled out. Second, the nature of the matched-cohort design and our stringent matching criteria meant that we had to exclude exposed patients with excessive length of hospitalization before infection, because we were unable to find appropriate controls. These patients differed in a systematic manner from those enrolled, as they had a higher mortality (MRSA patients) or extra length of stay (MSSA patients). To impute the direction of impact of counterfactual controls is difficult, but due to the low number of excluded patients, the magnitude of the impact is expected to be small.
The differences in study design make this study unique and less amenable to a direct comparison with previously published results. Nonetheless, several studies lend support to our findings. A recent study focusing specifically on 30-day mortality but directly comparing MRSA and MSSA patients found a similarly increased ratio (OR, 2.2; CI, 1.0 to 5.0) (3). Three other studies (17, 19, 27) analyzing the impact of methicillin resistance on hospital mortality using time-to-event methods, but ignoring competing events, also found no impact on the instantaneous risk of dying. Finally, another study (6) supported our finding that patients with MRSA and MSSA infections have equal durations of hospitalization after enrollment. Others who made claims to the contrary ignored the influence of the duration of hospital exposure before infection (1, 8).
There are four intuitive explanations for the 80% excess mortality of MRSA patients over MSSA patients in our study: (i) increased vulnerability of the host, (ii) inappropriate empirical antibiotic treatment, (iii) delayed appropriate therapy, and (iv) inferior effectiveness of reserve antibiotics. In this prospective study, great efforts were undertaken to control for important host factors through matching for length of hospital admission prior to the onset of infection and adjusting for important confounders, like the severity of disease, making increased vulnerability an improbable explanation. Although it seems likely that administration of inappropriate therapy could lead to higher mortality in MRSA patients (18, 39), a recent systematic review (26) argues that this has never been adequately assessed, since detailed analyses that take into account timeliness and drug levels of empirical therapy are still lacking. Moreover, poor interrater agreement on the multiple factors that influence judgments about appropriateness make it difficult to measure (34). A priori, one would also assume that inappropriate empirical therapy should have had a different impact in each participating center due to distinct local prescribing practices. However, we found no evidence of heterogeneity between hospitals. The most likely reason for the increased mortality among MRSA patients is the delay in administration of appropriate therapy and the fact that conventional MRSA treatment, consisting of vancomycin, is not as effective as beta-lactams against MSSA (24, 38).
In conclusion, data from 13 tertiary care centers from different European countries showed that mortality and LOS attributable to S. aureus BSIs are significant. MSSA infections increased mortality more than 2-fold, and methicillin resistance contributed an additional 80% excess mortality at day 30 after infection. These results emphasize the clinical importance of invasive S. aureus infections but unequivocally underline the additional burden imposed by resistance, which not only aggravates the clinical outcome, but adds to the overall caseload of patients with S. aureus BSI. Ideally, interventions should be _targeted at prevention or improved management of both resistant and susceptible S. aureus BSI.
Acknowledgments
The BURDEN project was supported by DG-Sanco, Netherlands National Institute for Public Health and the Environment (RIVM), and University Medical Centre Groningen (UMCG).
P.G.D. has received support for research projects on the management of infection from Janssen-Cilag. He has been a member of an advisory board on antimicrobial resistance for Wyeth and an advisory board on new antibiotics for Johnson & Johnson. He has received speaker fees and support to attend meetings from Johnson & Johnson and Optimer Pharmaceuticals. All other authors have nothing to declare.
We are grateful to H. Boshuizen, J. van de Kassteele, and M. Schipper for their statistical support and B. van Benthem for additional epidemiological advice.
Footnotes
Published ahead of print on 10 January 2011.
REFERENCES
- 1.Abramson, M. A., and D. J. Sexton. 1999. Nosocomial methicillin-resistant and methicillin-susceptible Staphylococcus aureus primary bacteremia: at what costs? Infect. Control Hosp. Epidemiol. 20:408-411. [DOI] [PubMed] [Google Scholar]
- 2.Adam, T., D. B. Evans, and C. J. Murray. 2003. Econometric estimation of country-specific hospital costs. Cost. Eff. Resour. Alloc. 1:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allard, C., et al. 2008. Secular changes in incidence and mortality associated with Staphylococcus aureus bacteraemia in Quebec, Canada, 1991-2005. Clin. Microbiol. Infect. 14:421-428. [DOI] [PubMed] [Google Scholar]
- 4.Altman, D. G., and J. M. Bland. 2003. Interaction revisited: the difference between two estimates. BMJ 326:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basu, A., W. G. Manning, and J. Mullahy. 2004. Comparing alternative models: log vs Cox proportional hazard? Health Econ. 13:749-765. [DOI] [PubMed] [Google Scholar]
- 6.Ben-David, D., I. Novikov, and L. A. Mermel. 2009. Are there differences in hospital cost between patients with nosocomial methicillin-resistant Staphylococcus aureus bloodstream infection and those with methicillin-susceptible S. aureus bloodstream infection? Infect. Control Hosp. Epidemiol. 30:453-460. [DOI] [PubMed] [Google Scholar]
- 7.Beyersmann, J., T. Kneib, M. Schumacher, and P. Gastmeier. 2009. Nosocomial infection, length of stay, and time-dependent bias. Infect. Control Hosp. Epidemiol. 30:273-276. [DOI] [PubMed] [Google Scholar]
- 8.Brooklyn Antibiotic Task Force. 2002. The cost of antibiotic resistance: effect of resistance among Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, and Pseudmonas aeruginosa on length of hospital stay. Infect. Control Hosp. Epidemiol. 23:106-108. [DOI] [PubMed] [Google Scholar]
- 9.Charlson, M. E., P. Pompei, K. L. Ales, and C. R. MacKenzie. 1987. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J. Chronic Dis. 40:373-383. [DOI] [PubMed] [Google Scholar]
- 10.Cosgrove, S. E., et al. 2005. The impact of methicillin resistance in Staphylococcus aureus bacteremia on patient outcomes: mortality, length of stay, and hospital charges. Infect. Control Hosp. Epidemiol. 26:166-174. [DOI] [PubMed] [Google Scholar]
- 11.European Antimicrobial Resistance Surveillance System Management Team. 2005. EARSS Manual 2005. RIVM, Bilthoven, Netherlands.
- 12.European Antimicrobial Resistance Surveillance System Management Team, Advisory Board and National Representatives. 2009. EARSS annual report 2008. On-going surveillance of S. pneumoniae, S. aureus, E. coli, E. faecium, E. faecalis, K. pneumoniae, P. aeruginosa. RIVM, Bilthoven, Netherlands.
- 13.Fine, J. P., and R. J. Gray. 1999. A proportional hazards model for the subdistribution of a competing risk. J. Am. Stat. Assoc. 94:496-509. [Google Scholar]
- 14.Fluit, A. C., J. Verhoef, and F. J. Schmitz. 2001. Frequency of isolation and antimicrobial resistance of gram-negative and gram-positive bacteria from patients in intensive care units of 25 European university hospitals participating in the European arm of the SENTRY Antimicrobial Surveillance Program 1997-1998. Eur. J. Clin. Microbiol. Infect. Dis. 20:617-625. [DOI] [PubMed] [Google Scholar]
- 15.Gould, I. M. 2005. The clinical significance of methicillin-resistant Staphylococcus aureus. J. Hosp. Infect. 61:277-282. [DOI] [PubMed] [Google Scholar]
- 16.Graves, N., et al. 2007. Effect of healthcare-acquired infection on length of hospital stay and cost. Infect. Control Hosp. Epidemiol. 28:280-292. [DOI] [PubMed] [Google Scholar]
- 17.Guilarde, A. O., M. D. Turchi, C. M. Martelli, and M. G. Primo. 2006. Staphylococcus aureus bacteraemia: incidence, risk factors and predictors for death in a Brazilian teaching hospital. J. Hosp. Infect. 63:330-336. [DOI] [PubMed] [Google Scholar]
- 18.Harbarth, S., et al. 2003. Inappropriate initial antimicrobial therapy and its effect on survival in a clinical trial of immunomodulating therapy for severe sepsis. Am. J. Med. 115:529-535. [DOI] [PubMed] [Google Scholar]
- 19.Harbarth, S., O. Rutschmann, P. Sudre, and D. Pittet. 1998. Impact of methicillin resistance on the outcome of patients with bacteremia caused by Staphylococcus aureus. Arch. Intern. Med. 158:182-189. [DOI] [PubMed] [Google Scholar]
- 20.Harris, A. D., Y. Carmeli, M. H. Samore, K. S. Kaye, and E. Perencevich. 2005. Impact of severity of illness bias and control group misclassification bias in case-control studies of antimicrobial-resistant organisms. Infect. Control Hosp. Epidemiol. 26:342-345. [DOI] [PubMed] [Google Scholar]
- 21.Hidron, A. I., et al. 2008. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006-2007. Infect. Control Hosp. Epidemiol. 29:996-1011. [DOI] [PubMed] [Google Scholar]
- 22.Ibelings, M. M., and H. A. Bruining. 1998. Methicillin-resistant Staphylococcus aureus: acquisition and risk of death in patients in the intensive care unit. Eur. J. Surg. 164:411-418. [DOI] [PubMed] [Google Scholar]
- 23.Kaye, K. S., A. D. Harris, M. Samore, and Y. Carmeli. 2005. The case-case-control study design: addressing the limitations of risk factor studies for antimicrobial resistance. Infect. Control Hosp. Epidemiol. 26:346-351. [DOI] [PubMed] [Google Scholar]
- 24.Kim, S.-H., et al. 2008. Outcome of vancomycin treatment in patients with methicillin-susceptible Staphylococcus aureus bacteremia. Antimicrob. Agents Chemother. 52:192-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lau, B., S. R. Cole, and S. J. Gange. 2009. Competing risk regression models for epidemiologic data. Am. J. Epidemiol. 170:244-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGregor, J. C., et al. 2007. A systematic review of the methods used to assess the association between appropriate antibiotic therapy and mortality in bacteremic patients. Clin. Infect. Dis. 45:329-337. [DOI] [PubMed] [Google Scholar]
- 27.Melzer, M., S. J. Eykyn, W. R. Gransden, and S. Chinn. 2003. Is methicillin-resistant Staphylococcus aureus more virulent than methicillin-susceptible S. aureus? A comparative cohort study of British patients with nosocomial infection and bacteremia. Clin. Infect. Dis. 37:1453-1460. [DOI] [PubMed] [Google Scholar]
- 28.Ministerie van Volksgezondheid, Welzijn, en Sport. 2000. The Medical Research involving Human Subjects Act (WMO). International Publication Series Health, Welfare and Sport no. 2. The Hague, Netherlands.
- 29.Mylotte, J. M., and A. Tayara. 2000. Staphylococcus aureus bacteremia: predictors of 30-day mortality in a large cohort. Clin. Infect. Dis. 31:1170-1174. [DOI] [PubMed] [Google Scholar]
- 30.Putter, H., M. Fiocco, and R. B. Geskus. 2007. Tutorial in biostatistics: competing risks and multi-state models. Stat. Med. 26:2389-2430. [DOI] [PubMed] [Google Scholar]
- 31.Resche-Rigon, M., E. Azoulay, and S. Chevret. 2006. Evaluating mortality in intensive care units: contribution of competing risks analyses. Crit. Care. 10:R5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roghmann, M. C. 2000. Predicting methicillin resistance and the effect of inadequate empiric therapy on survival in patients with Staphylococcus aureus bacteremia. Arch. Intern. Med. 160:1001-1004. [DOI] [PubMed] [Google Scholar]
- 33.Rosenthal, V. D., et al. 2008. International Nosocomial Infection Control Consortium report, data summary for 2002-2007, issued January 2008. Am. J. Infect. Control 36:627-637. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz, D. N., et al. 2009. Lost in translation? Reliability of assessing inpatient antimicrobial appropriateness with use of computerized case vignettes. Infect. Control Hosp. Epidemiol. 30:163-171. [DOI] [PubMed] [Google Scholar]
- 35.Selvey, L. A., M. Whitby, and B. Johnson. 2000. Nosocomial methicillin-resistant Staphylococcus aureus bacteremia: is it any worse than nosocomial methicillin-sensitive Staphylococcus aureus bacteremia? Infect. Control Hosp. Epidemiol. 21:645-648. [DOI] [PubMed] [Google Scholar]
- 36.Shintani, A. K., et al. 2009. Immortal time bias in critical care research: application of time-varying Cox regression for observational cohort studies. Crit. Care Med. 37:2939-2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soriano, A., et al. 2000. Pathogenic significance of methicillin resistance for patients with Staphylococcus aureus bacteremia. Clin. Infect. Dis. 30:368-373. [DOI] [PubMed] [Google Scholar]
- 38.Stryjewski, M. E., et al. 2007. Use of vancomycin or first-generation cephalosporins for the treatment of hemodialysis-dependent patients with methicillin-susceptible Staphylococcus aureus bacteremia. Clin. Infect. Dis. 44:190-196. [DOI] [PubMed] [Google Scholar]
- 39.Teixeira, P. J. Z., R. Seligman, F. T. Hertz, D. B. Cruz, and J. M. G. Fachel. 2007. Inadequate treatment of ventilator-associated pneumonia: risk factors and impact on outcomes. J. Hosp. Infect. 65:361-367. [DOI] [PubMed] [Google Scholar]
- 40.Wolkewitz, M., J. Beyersmann, P. Gastmeier, and M. Schumacher. 2009. Efficient risk set sampling when a time-dependent exposure is present: matching for time to exposure versus exposure density sampling. Methods Inf. Med. 48:438-443. [DOI] [PubMed] [Google Scholar]
- 41.Wolkewitz, M., J. Beyersmann, P. Gastmeier, and M. Schumacher. 2009. Modeling the effect of time-dependent exposure on intensive care unit mortality. Intensive Care Med. 35:826-832. [DOI] [PubMed] [Google Scholar]
- 42.Wolkewitz, M., et al. 2008. Risk factors for the development of nosocomial pneumonia and mortality on intensive care units: application of competing risks models. Crit. Care 12:R44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wyllie, D. H., D. W. Crook, and T. E. Peto. 2006. Mortality after Staphylococcus aureus bacteraemia in two hospitals in Oxfordshire, 1997-2003: cohort study. BMJ 333:281. [DOI] [PMC free article] [PubMed] [Google Scholar]