Abstract
Objective
In lupus nephritis, glomerular injury correlates poorly with progression to renal failure. While the tubulointerstitium is also commonly involved, the importance of such involvement is not well defined. Therefore, we developed a simple method to assess the prognostic utility of measuring tubulointerstitial inflammation (TI).
Methods
Sixty-eight SLE patients with lupus nephritis were enrolled. Tubulointerstitial lymphocytic infiltrates were quantitated both by anti-CD45 antibody staining and standard histochemical staining. Follow-up data was obtained and survival analysis carried out to determine which histologic features were predictive of subsequent renal failure.
Results
By CD45 staining TI was a common pathological finding, with 72% of biopsies having moderate or severe involvement. The extent of TI correlated with serum creatinine, but not with dsDNA antibodies, serum C3, or glomerular inflammation. TI severity, but not glomerular injury, identified patients at greater risk for renal failure (p=0.02). A high NIH chronicity index also identified patients at risk for renal failure. However, when the glomerular and tubulointerstitial subcomponents of the NIH chronicity index were separated in a bivariate model, only tubulointerstitial chronicity provided prognostic information (HR 2.2, 95% C.I. 1.3, 3.6, p=0.002 vs. HR 1.0, 95% C.I. 0.7, 1.5. p=0.97 for glomerular chronicity).
Conclusion
TI identifies lupus nephritis patients at greatest risk for progression to renal failure. The immunological mechanisms underlying TI may provide novel _targets for therapeutic intervention.
Introduction
Lupus nephritis is the most common severe manifestation of systemic lupus erythematosus (SLE)(1, 2) and contributes significantly towards mortality in this disease (3-5). Up to 60% of SLE patients develop lupus nephritis, in many cases necessitating treatment with major immunosuppressive therapies such as cyclophosphamide or mycophenolate mofetil (6-8). Despite such aggressive treatments, many patients do not respond to therapy and progress to end stage renal disease (ESRD) (9-11).
Histologic features of renal biopsies often determine which patients receive cytotoxic therapies. Current pathological classification criteria of lupus nephritis stress the importance of glomerular injury. The 2003 International Society of Nephrology/Renal Pathology Society (ISN/RPS) lupus nephritis classification focuses exclusively on histologic changes of the glomerulus. Similarly, the NIH activity index quantifies the severity of lupus nephritis primarily based on glomerular changes (12). However, numerous studies indicate that the NIH activity index does not accurately identify patients at highest risk for subsequent renal failure (12-14) and that distinguishing proliferative glomerulonephritis from other types of lupus nephritis does not consistently predict adverse renal outcomes (11, 15, 16). These latter observations suggest that current histologic assessments may not identify those acute pathological processes most responsible for irreversible renal injury.
In view of the inadequacy of the NIH activity index in predicting poor renal outcomes, there is a need to identify prognostically meaningful acute pathologic processes on renal biopsies prior to the onset of irreversible damage. Presumably, patients with such findings would derive the greatest benefit from aggressive treatment and would be ideal study subjects in clinical trials. One candidate process is interstitial nephritis, which manifests as inflammatory infiltrates in the tubulointerstitium of the kidney. Some studies have suggested that interstitial nephritis and tubular atrophy correlated with elevated serum creatinine at the time of biopsy and with risk for subsequent renal failure (15, 17-21). However, these observations relied on non-specific histologic stains that likely underestimated the prevalence and severity of interstitial nephritis.
Recently we, and others, have demonstrated that B and T lymphocyte tubulointerstitial infiltration is common in lupus nephritis (22, 23). These B and T lymphocytes are often organized into structures reminiscent of those observed in secondary lymphoid organs. Furthermore, these structures appear to be functional, as they are associated with in situ lymphocyte expansion and antigen-driven selection. These observations suggest that in addition to the systemic autoimmune processes that have been implicated in glomerulonephritis, local in situ immune responses might be associated with interstitial nephritis.
To better define the role of interstitial nephritis in determining prognosis in lupus nephritis, we used semi-quantitative methods to describe the extent of tubulointerstitial inflammation by both specific immunohistochemical and standard histochemical staining for lymphocytes on lupus renal biopsies. This was then compared to other established lupus nephritis pathological scoring schema as well as clinical and demographic data, and then correlated with subsequent renal survival. These data indicate that interstitial inflammation and scarring identify lupus nephritis patients at high risk for progression to renal failure.
Materials and Methods
Subject inclusion and clinical data collection
This study was approved by the University of Chicago Medical Center Institutional Review Board. Subjects were eligible for inclusion if they had a renal biopsy consistent with lupus nephritis between 2001 and 2007 and sufficient material for analysis (≥six glomeruli and length of ≥0.5 cm). Subjects were excluded if they did not fulfill American College of Rheumatology (ACR) revised criteria for the classification of SLE (24) or had alternative diagnoses potentially causing glomerulonephritis. Of 70 initial subjects, 1 was excluded because ACR criteria were not fulfilled, and another excluded due to concurrent hepatitis C, resulting in 68 renal biopsies.
The clinical charts were reviewed for demographic data, medication history, degree of proteinuria and serum creatinine at time of biopsy and at most recent follow-up (or until renal failure ensued) and serological parameters at time of biopsy. Glomerular filtration rate (GFR) was estimated using the IDMS-traceable MDRD study equation in adults (25) and a modified IDMS-traceable Schwartz equation in children (26). Treatment at the time of renal biopsy was classified as major immunosuppression (prednisone or equivalent > 20 mg/day within one month of renal biopsy and/or cyclophosphamide, mycophenolate mofetil, azathioprine, or cyclosporine within three months of biopsy) versus minimal immunosuppression (prednisone or equivalent ≤ 20 mg/day within the month prior to biopsy and no major immunosuppressant as defined above within three months of biopsy.)
Renal outcome data was collected for survival analysis. End stage renal failure (ESRD) was defined as the initiation of chronic dialysis therapy or renal transplantation. The time until ESRD, or last known follow-up in the absence of renal failure, was collected by chart review.
Histologic assessments
Formalin-fixed, paraffin-embedded 2 μm sections were used for microscopic evaluation. For each biopsy, hematoxylin and eosin (H&E) stains and periodic acid-Schiff (PAS) stains were performed. Standard immunofluorescence microscopy using fluorescein isothiocyanate-conjugated antibodies for the following antigens: IgG, IgA, IgM, C3c, C1q, fibrinogen, κ and λ light chains, and albumin (DAKO, Carpinteria, CA) was applied and staining was semi-quantitatively scored from 0 to 4+. Standard electron microscopic procedures were applied using a Philips CM10 electron microscope. Interstitial inflammation was determined using monoclonal antibodies to CD45 (DAKO, Carpinteria, CA), a pan-leukocyte marker, on paraffin tissue sections. The percentage of positive cells for CD20 (DAKO), a pan-B cell marker, and anti-CD3 (Labvision, Fremont, CA), a pan-T cell marker, was also determined using the number of CD45+ cells as the denominator. Interstitial inflammation was also assessed by standard light microscopy, modified to exclude areas adjacent to tubular atrophy and interstitial fibrosis, (modified light microscopy or MLM). The extent of interstitial inflammation by each method was semiquantitatively scored without prior knowledge of the clinical outcomes from 0-4, which corresponded with 0%, <10%, 10-25%, 26-50% or >50% involvement of the tubulointerstitium.
The kidney biopsies were classified using the 2003 ISN/RPN revised lupus nephritis classification. Class III or Class IV lupus nephritis with concurrent Class V was subsumed under Class III or IV lupus nephritis. Subsequently, Class III and Class IV were combined into a proliferative nephritis category, and Class II and isolated Class V into a nonproliferative category for statistical analysis. The activity and chronicity indices were scored using the NIH system (12). To separate the effect of interstitial inflammation on the NIH activity index, a modified activity index which excluded the interstitial component was calculated by deducting the numeric score given for interstitial inflammation (0-3) from the total NIH activity index score, yielding a potential total score of 21. Similarly, the 12-point NIH chronicity index was separated into a glomerular chronicity score, and an interstitial chronicity score; each ranging between 0 and 6 (Table 1).
Table 1. National Institutes of Health version of activity and chronicity indices (12).
| Activity Index (24 points total) | Chronicity Index (12 points) | ||
|---|---|---|---|
| Glomerular activity | Glomerular chronicity | ||
| Cellular proliferation | 0-3 | Glomerular sclerosis | 0 - 3 |
| Fibrinoid necrosis, karyorrhexis* | (0-3) × 2 | Fibrous crescents | 0 - 3 |
| Cellular crescents* | (0-3) × 2 | Tubulointerstitial chronicity | |
| Hyaline thrombi, wire loops | 0-3 | Interstitial fibrosis | 0 - 3 |
| Leukocyte infiltration | 0-3 | Tubular atrophy | 0 - 3 |
| Tubulointerstitial activity | |||
| Mononuclear cell infiltration | 0-3 |
Fibrinoid necrosis and cellular crescents are weighted by a factor of 2.
Statistical Analysis
Data were analyzed using STATA 10.0 (StataCorp, College Station, Texas). Initially, cross-sectional analysis of interstitial nephritis in relation to a variety of demographic, clinical and histologic characteristics was performed. Univariate statistical analysis was performed using the Fisher's Exact test and the Wilcoxon Rank Sum test. Multivariate logistic regression using decreased GFR (< 60 ml/min/BSA) was used to examine the independence of demographic, clinical and histologic findings which were significant on univariate analysis. Degree of proteinuria at time of biopsy was included in the regression analysis because heavy proteinuria has been postulated as a cause of tubular disease (27, 28). Histologic variables in the regression analyses included the intensity of interstitial inflammation as quantified by CD45 staining; interstitial inflammation by MLM; and the chronicity index broken into its two subcomponents (tubulointerstitial chronicity score and glomerular chronicity score).
Survival analysis was carried out to determine the predictive value of each measure of interstitial inflammation. Three patients were censored from the survival analysis portion of the study due to lack of follow-up renal function and 6 patients were censored due to renal failure at time of biopsy, resulting in 59 subjects for the survival analysis. Renal survival analysis was performed by the Kaplan-Meier technique and the statistical difference between survival curves was assessed with the log rank test for equality of survivor functions, using time to ESRD as the event variable. The following group variables were used in separate sequential survival analyses: severe interstitial nephritis (binary variable), the 4-tier interstitial nephritis variable (log rank trend test), serum creatinine > 1.4 mg/dL, NIH activity index > 6, African-American race, NIH chronicity index > 3, glomerular chronicity index > 0, tubulointerstitial chronicity index > 0, LN class (proliferative vs. nonproliferative), pediatric age at biopsy, degree of proteinuria (>3.5 gms/24 hours), and the presence of dsDNA antibodies. A Cox Proportional Hazards analysis was used to determine if the tubulointerstitial chronicity score and glomerular chronicity score each independently contributed to risk of renal failure. This model consisted of two covariates, the tubulointerstitial chronicity score and glomerular chronicity score, with the dependent outcome variable being renal failure. Subsequently, this model was serially adjusted for degree of proteinuria and race to determine any effects upon these covariates. A p value of < 0.05 was considered statistically significant for the above analyses.
Results
Clinical Characteristics of Patient Cohort
We identified 68 subjects fulfilling ACR criteria for the classification of SLE (24) who had lupus nephritis on renal biopsy and had adequate stored tissue for histologic staining. The clinical characteristics of the population at time of biopsy are provided in Table 2. The population was predominantly African-American (81%) and female (85%). Median serum creatinine was 1.0 mg/dL (range 0.2-6.4), and median proteinuria was 2.5 gms/day (range 0-19.7). Sixty-five patients had data with which to estimate GFR. Fifty-five percent had Stage I or II chronic kidney disease (GFR > 60 ml/min/1.73m2), while 20% had Stage III, 11% Stage IV and 14% Stage V at time of biopsy. Seventy-five percent were receiving major immunosuppression within the month prior to biopsy. Fourteen subjects progressed to ESRD during the study, for a 24% rate of renal failure.
Table 2. Clinical and histological characteristics of subjects at the time of renal biopsy (n=68).
| Clinical Characteristics | |
|---|---|
| Sex, n (%) | |
| Female | 58 (85) |
| Male | 10 (15) |
| Ancestry, n (%) | |
| African-American | 55 (81) |
| Hispanic | 7 (10) |
| Caucasian | 6 (9) |
| Age, years | 31.1 ± 13.1 |
| Disease duration, months, median (range) | 36 (0-220) |
| Corticosteroid dosage, mg/day | 27 ± 25 |
| Major immunosuppression, n (%) | 50 (74.6) |
| Serum creatinine, mg/dL, median (range) | 1.0 (0.2-6.4) |
| Glomerular filtration rate > 60 ml/minute/BSA, n (%) | 36 (55.4) |
| Proteinuria, gm/24 hrs, median (range) | 2.5 (0-19.7) |
| Antinuclear antibody titer, median (range) | 1:2560 (1:40-1:2560) |
| Anti ds-DNA positive, n (%) | 55 (81) |
| Anti ds-DNA titer, median (range) | 1:160 (0 – 1:2560) |
| C3, mg/dL | 65 ± 32 |
| Histological Characteristics | |
| ISN/RPS lupus nephritis class, n (%) | |
| Class II | 3 (4) |
| Class III (± Class V) | 22 (32) |
| Class IV (± Class V) | 33 (49) |
| Class V | 10 (15) |
| NIH activity index | 6.6 ± 5.1 |
| NIH chronicity index | 2.7 ± 2.6 |
Values represent the mean ± SD, except where otherwise indicated. Anti-dsDNA = anti-double-stranded DNA; Major immunosuppression = prednisone or equivalent > 20 mg/day and/or major immunosuppressant.
The distribution of nephritis class by ISN/RPS classification criteria is shown in Table 2. Evaluation of biopsies using the NIH activity index revealed moderate activity (mean 6.6 ± 5.1, range 0-19). Scoring of biopsies using the NIH chronicity score revealed substantial scarring (mean 2.7 ± 2.6, range 0-10) at time of biopsy.
Tubulointerstitial inflammation was associated with a higher serum creatinine and decreased GFR at time of biopsy
To characterize the extent of active interstitial nephritis, paraffin sections from each biopsy were stained with antibodies to the pan-leukocyte marker CD45 and then quantified on a five-point scale (0-4) (Methods) (29). All biopsies had some interstitial infiltrates on CD45 staining. Six percent of biopsies were graded as 1, 22% as 2, 40% as 3, and 32% as 4. For most subsequent analyses, the interstitial activity scores were consolidated into a binary ranking of mild (0-2) and severe (3-4) interstitial nephritis.
Sections were also stained by H&E and PAS and analyzed by MLM. By MLM, 9% scored as 0, 32% as 1, 25% as 2, 15% as 3, and 19% as 4. Interstitial inflammation, as measured by CD45, correlated well with MLM (Spearman's rho 0.63, p< 0.0001).
Interstitial inflammation by CD45 staining correlated with renal function. The median serum creatinine was 1.4 mg/dL in the severe interstitial inflammation group and 0.7 mg/dL in the mild interstitial inflammation group (Wilcoxon rank sum test p=0.0001). Of 46 subjects with severe interstitial inflammation by CD45 staining, 19 had a normal GFR (< 60 ml/minute/BSA, 27 (41%), compared to 17/19 (89%) with normal GFR amongst mild interstitial inflammation subjects (Fisher's Exact test, p<0.001 for CD45 stain, Table 3). Similar findings were seen with MLM staining. Median serum creatinine was 2.2 mg/dL in subjects with severe interstitial inflammation, and 0.8 mg/dL in subjects with mild interstitial inflammation by MLM (p=0.004). Nineteen percent of subjects with severe interstitial inflammation by MLM had a normal GFR, while 73% with mild interstitial inflammation had a normal GFR (p<0.001). T cells predominated over B cells, with a mean of 66% versus 12% of interstitial cells. While neither B or T cell percentages correlated with GFR or serum creatinine, B cells were increased in specimens with intense interstitial infiltrates (mean CD20 cells 10% in low interstitial infiltrate subjects and 16% in high interstitial infiltrate subjects, p=0.015). The relative predominance of B cells by anti-CD20 or T cells by anti-CD3 did not correlate with GFR or serum creatinine (data not shown).
Table 3. Univariate analysis of severe tubulointerstitial inflammation (TI) by CD45 staining with relevant clinical and histological variables at time of renal biopsy (n=68).
| Selected Associations | Severe TI (n=49) |
Mild TI (n=19) |
p value |
|---|---|---|---|
| Clinical variables | |||
| Age, years | 31.2 | 30.8 | 0.90 |
| Disease duration, months (median) | 36 | 60 | 0.20 |
| African-American ancestry, % | 88 | 63 | 0.04 |
| Major immunosuppression, % | 71 | 84 | 0.36 |
| Serum creatinine, mg/dl (median) | 1.4 | 0.7 | 0.0001 |
| GFR > 60 ml/ minute/BSA, % | 41.3 | 89.5 | <.001 |
| Proteinuria, gm/24h (median) | 3.2 | 1.7 | 0.16 |
| dsDNA titer (median) | 320 | 80 | 0.22 |
| C3, mg/dL | 63 | 68 | 0.45 |
| Histological variables | |||
| ISN/RPS LN Class III and IV, % | 88 | 63 | 0.04 |
| NIH activity index | 7.5 | 4.4 | 0.01 |
| Modified activity index | 5.3 | 3.7 | 0.18 |
| NIH chronicity index | 3.3 | 1.3 | 0.003 |
| Glomerular chronicity score | 1.1 | 0.4 | 0.03 |
| Tubulointerstitial chronicity score | 2.1 | 0.8 | 0.001 |
Values represent mean unless otherwise indicated. Severe interstitial nephritis is defined as > 25% involvement of the renal interstitium. Major immunosuppression = prednisone ≥ 20 mg/day and/or major immunosuppressive agent. Modified activity index = NIH activity index with tubulointerstitial abnormalities removed. Glomerular chronicity score = chronicity score derived from the glomerular components in the NIH chronicity index. Tubulointerstitial chronicity score = chronicity score derived from the tubulointerstitial components of NIH chronicity index.
Surprisingly, severe interstitial nephritis by CD45 staining was not associated with accepted measures of lupus activity such as elevated dsDNA antibodies and low serum C3 (Table 3). While severe interstitial nephritis had a higher median level of proteinuria (3.2 grams/24 hours in severe interstitial nephritis versus 1.7 grams/24 hours in mild interstitial nephritis), this difference did not achieve statistical significance (p=0.16). Severe interstitial nephritis was weakly associated with African-American ancestry (p=0.04). Amongst African-American subjects, 78% had severe interstitial inflammation, compared to 46% amongst subjects with other ancestries.
There was a poor correlation between the magnitude of tubulointerstitial and glomerular involvement
ISN/RPS Class III or IV proliferative nephritis was somewhat more common in subjects with severe interstitial nephritis when analyzed by CD45 staining, occurring in 88% of the severe interstitial nephritis group and 63% of the mild interstitial nephritis group (p = 0.04, Table 3). However, among the 13 subjects with nonproliferative glomerular lesions, 6 (46%) had severe interstitial nephritis. Thus, severe interstitial nephritis was widespread regardless of ISN/RPS lupus nephritis class.
We then examined the relationships between interstitial nephritis by CD45 staining and the NIH activity index (Table 3). This index, although weighted towards glomerular pathology, awards 3 of 24 points based on the degree of interstitial inflammation. Therefore, it was not surprising that the interstitial activity score correlated with the NIH activity index (p=0.01). However, the modified NIH activity score, which excluded tubulointerstitial inflammation, did not correlate with the interstitial activity score (p=0.18), indicating that there is not a close correlation between the severity of pathology in the glomerulus and the interstitium.
Tubulointerstitial inflammation is associated with tubulointerstitial scarring
In contrast to the NIH activity index, a significant correlation was noted between the interstitial activity score by CD45 staining and the NIH chronicity index (Spearman's rho=0.38, p=0.002). However, the NIH chronicity index gives equal weight to scarring in the glomeruli and scarring in the tubulointerstitium. To determine which component of the NIH chronicity index correlated with the interstitial activity score, we calculated separate glomerular and interstitial chronicity subcomponent scores and compared these to the interstitial activity score. As can be seen in Table 3, the interstitial activity score, as assessed by CD45 staining, strongly correlated with the interstitial component of the NIH chronicity score (rho=.44, p=0.002) and with the total NIH chronicity score (rho=0.38, p=0.002). There was a weaker association between interstitial inflammation and the glomerular chronicity score (rho=0.27, p=0.02). However, in a multivariate analysis, severe interstitial inflammation correlated significantly with the interstitial (OR 1.79 CI 1.0, 3.1, p=0.035) but not glomerular chronicity scores (OR 1.32, CI 0.6, 3.1 p=0.53).
Interstitial scarring and inflammation independently were associated with low GFR
Because a variety of factors were associated with impaired GFR on univariate analysis, multivariate logistic regression was performed using the dependent variable of diminished GFR (< 60 ml/min/BSA) at time of renal biopsy. When both the interstitial chronicity and glomerular chronicity scores were used as covariates, the interstitial chronicity score was associated with low GFR (OR 2.4, 95% C.I. 1.4, 4.3, p=0.002), while the glomerular chronicity score was not (OR 1.3, 95% C.I. 0.6, 2.6, p=0.43). Subsequent adjustment of the model serially for degree of proteinuria, and glomerular crescents/necrosis did not significantly affect these associations. These data indicate that interstitial, and not glomerular, scarring is associated with renal dysfunction.
We then added severe interstitial inflammation by CD45 staining (grade 3-4) to the regression model containing interstitial and glomerular chronicity scores. These analysis indicated that interstitial inflammation was independently associated with a low GFR (OR 5.3, 95% C.I. 1.0, 28.9, p=0.05). These data suggest that at time of biopsy interstitial inflammation, independently of renal scarring, correlates with renal dysfunction.
Severity of interstitial nephritis, but not ISN/RPS lupus nephritis class or NIH activity index, predicts renal survival
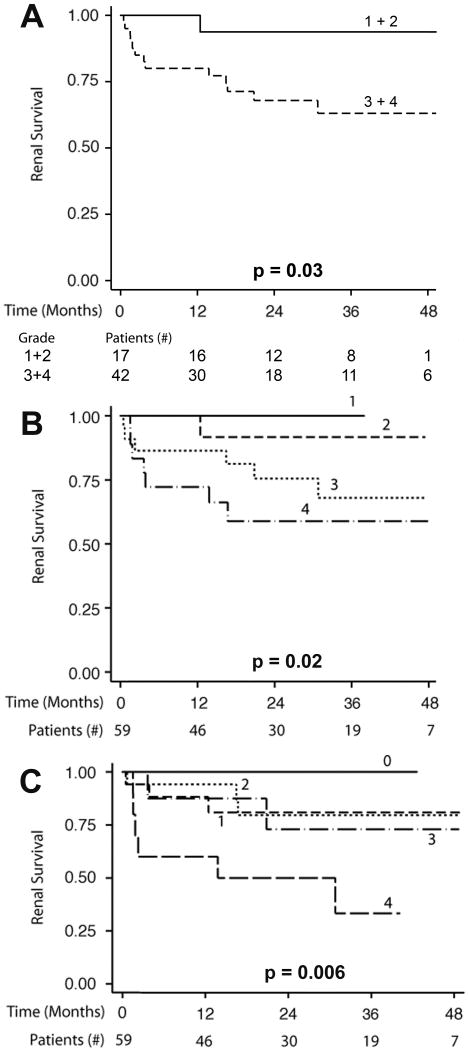
We next examined if classic histological measures, or the degree of interstitial nephritis on biopsy, identified those at risk for subsequent renal failure. As demonstrated in Figure 1A, approximately 37% of patients with severe interstitial nephritis by CD45 staining progressed to renal failure at 24 months compared to 15% of those with mild interstitial nephritis (p=0.03). The relationship between interstitial nephritis and subsequent renal failure was also apparent when interstitial nephritis was treated as a 4-tier variable (interstitial activity scores 1-4, Figure 1B). Those patients with little interstitial nephritis on renal biopsy (interstitial activity score 1) uniformly had an excellent prognosis while those with interstitial activity scores of 2 and 3 had relatively good and intermediate prognosis respectively. In contrast, nearly 40% of those with a score of 4 progressed to renal failure within 24 months. This incremental decline in renal survival was significant (p=0.02, Log-Rank Trend Test).
Figure 1. Interstitial nephritis severity predicts subsequent renal survival.
1A. Kaplan-Meier curves demonstrating renal survival in severe (grade 3-4) versus mild (grade 1-2) interstitial inflammation* by anti-CD45. 1B. Kaplan-Meier curves demonstrating renal survival in a 5 tier measure of interstitial inflammation* by anti-CD45 antibody. Of note, no subjects fell into grade 0; thus 4 tiers are present. 1C. Kaplan-Meier curves demonstrating renal survival in a 5 tier measure of interstitial inflammation* by modified conventional light microscopy (MLM) using H&E and PAS stain. The number of patients available for analysis in each subgroup over time is provided for Figure 1A, while the total numbers of subjects over time is provided below Figures 1B and 1C. Detailed subgroup numbers for Figure 1B and 1C are provided in Tables S1 and S2. P values were derived using either a log rank test (A) or a log rank trend test (B, C). *5 tier measure: none/grade 0=0%; minimal/grade 1= <10%, mild/grade 2= 10-25%, moderate/grade 3=26-50%; or severe/grade 4= >50%.
Because nonspecific inflammation may occur around areas of interstitial fibrosis, the survival analysis was repeated using modified light microscopy (MLM). This yielded similar results. The 5-tier interstitial activity score by MLM (interstitial activity scores 0-4, Figure 1C) also predicted renal failure risk (p=0.006, trend test). The group with an interstitial inflammation score of 4 had a much more grave renal prognosis (60% ESRD by 36 months) than the other MLM groups (scores 0-3). In contrast to CD45 staining, the other MLM groups (scores 1, 2, and 3) had survival curves that were overlapping and did not demonstrate a dose-response relationship between interstitial inflammation and ESRD (Figure 1B and 1C). These data suggest that CD45 staining better differentiates prognosis at intermediate levels of interstitial inflammation.
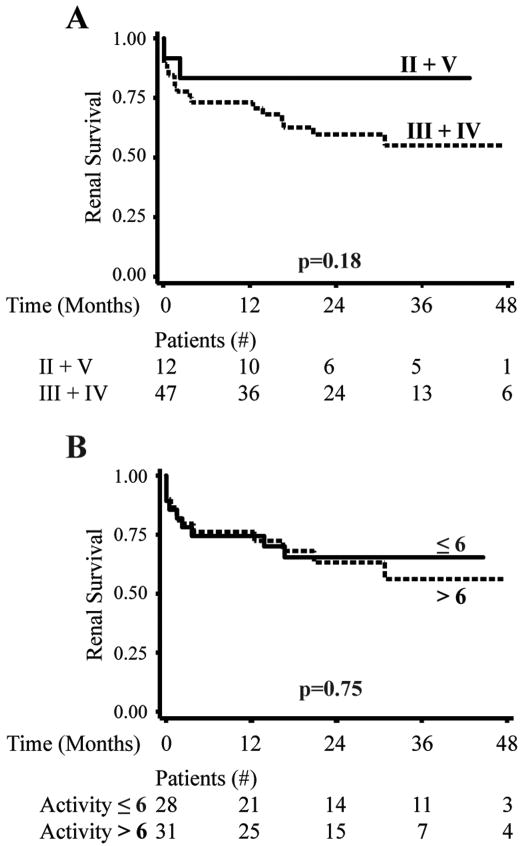
In contrast, the ISN/RPS lupus nephritis class and the NIH activity index had no statistically significant effect on subsequent renal survival (Figure 2, p=0.22 and p=0.80 respectively). Likewise, anti-dsDNA antibody titers, decreased C3, pediatric age and nephrotic range proteinuria were not useful in identifying patients at risk for subsequent renal failure (p all > 0.10). Therefore, among demographic data and measures of active inflammation, only the severity of interstitial nephritis provided prognostic information in our cohort and did so regardless of which histologic method was used.
Figure 2. ISN/RPS nephritis class and NIH activity index do not predict subsequent renal survival.
2A. Kaplan-Meier curves demonstrating subsequent renal survival in those with proliferative (III+IV) or non-proliferative (II+V) nephritis class on renal biopsy. 2B. Kaplan-Meier curves demonstrating renal survival in those with an activity index of > 6 or ≤ 6. The number of patients available for analysis at each time point is provided below each figure. P values were derived using a log rank test.
The prognostic utility of the NIH chronicity score is primarily driven by tubulointerstitial scarring
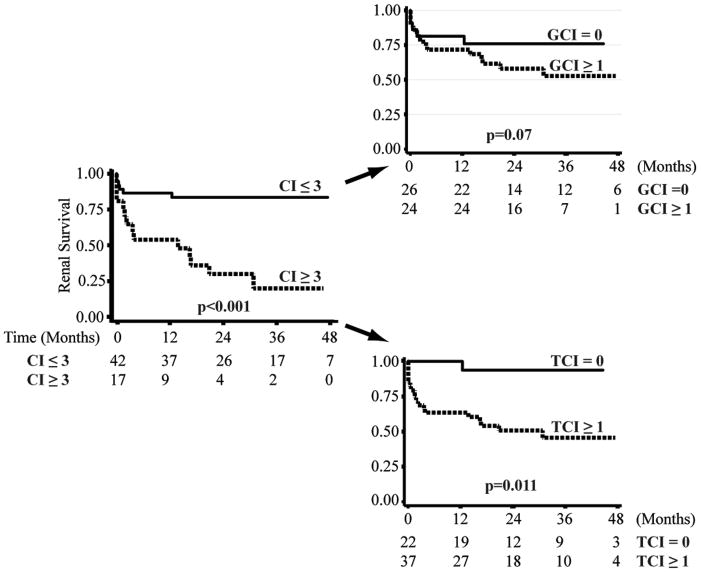
A higher NIH chronicity index is a well-established poor prognostic indicator in LN.(15, 30). Analysis of our cohort confirms these previous observations. Those with a NIH chronicity score of greater than 3 were much more likely to progress to renal failure (p<0.001). We then divided the NIH chronicity score into its glomerular and tubulointerstitial subcomponents to determine which was more predictive of subsequent renal survival (Figure 3). As demonstrated in Figure 3, we observed a somewhat more significant decline in renal survival with evidence of tubular atrophy or interstitial fibrosis (score > 0, p=0.011) compared to evidence of glomerular sclerosis or fibrous crescents (score > 0, p=0.07). The tubulointerstitial chronicity score (score > 0) did not correlate with the degree of proteinuria at biopsy (p=0.66, rank sum test), arguing against a causal role of chronic proteinuria in interstitial fibrosis. A multivariate renal survival analysis, reflected a strong independent association of the interstitial component of the chronicity index with renal failure (hazards ratio 2.20, 95% C.I. 1.3, 3.6, p=0.002) and no independent association of glomerular damage (hazards ratio 1.0, 95% C.I. 0.7, 1.5, p=0.97) with renal failure. The model was unaffected if adjusted for degree of proteinuria. These data indicate that the prognostic value of the NIH chronicity index is primarily due to those components that capture tubulointerstitial scarring.
Figure 3. The interstitial components of the NIH chronicity index predicts renal survival.
3A. Kaplan-Meier curves demonstrating renal survival in those with a NIH chronicity index (CI) of ≤3 or >3. The NIH activity index was then divided into the glomerular (GCI) and tubulointerstitial (TCI) components and Kaplan-Meier curves were then used to examine the association of each index with subsequent renal survival. The number of patients available for analysis at each time point is provided below each figure. P values were derived using a log rank test.
Discussion
In lupus nephritis, renal biopsies are commonly used to assess the extent of renal involvement, assign prognosis and to aid in making therapeutic decisions. However, current histologic measures of activity emphasize glomerular disease and perform poorly in identifying those patients at risk for subsequent renal failure. Herein, we demonstrate that one prognostically important process in lupus nephritis is interstitial inflammation. Active interstitial nephritis was the only inflammatory, and potentially reversible, marker of poor prognosis identified in this study. On a cross-sectional basis, interstitial nephritis was associated with the known poor prognostic markers of elevated serum creatinine at time of biopsy, low GFR, and elevated chronicity index scores. On renal survival analysis, increasing levels of interstitial nephritis (as assessed by CD45 staining) were associated with a progressively higher risk of progression to renal failure. In comparison, the ISN/RPS lupus nephritis classification was a poor predictor of prognosis and the glomerular-based NIH activity index provided no prognostic information. By successfully identifying inflammatory features that define prognosis, we might be able to identify high-risk patients prior to the onset of irreversible scarring, and develop novel therapeutic _targets to improve outcomes in this difficult disease.
It has been appreciated for some time that glomerular measures of disease activity do not accurately predict subsequent clinical course (11-16). Previous studies have noted that interstitial infiltrates had prognostic value (15, 19, 29). These studies also noted the lack of correlation of complement and dsDNA with interstitial disease in lupus nephritis (29), and found a greater prevalence of proliferative glomerulonephritis in biopsies with interstitial infiltrates (27), similar to what we have found in our cohort. Hill (29) found interstitial inflammation to be one of the strongest histologic correlates of serum creatinine. However, that study was cross-sectional in nature and did not adjust for severity of glomerular disease. Esdaile (15), in a detailed analysis of renal survivorship, examined tubulointerstitial inflammation only if interstitial fibrosis was already present. Yu et al. (21) recently found total tubulointerstitial inflammation measured by standard histochemical light microscopy to be predictive of renal failure in a Chinese cohort, but did not determine if tubulointerstitial inflammation in areas without fibrosis or atrophy was prognostically significant. Thus, our data is novel as it demonstrates that tubulointerstitial inflammation itself, whether measured in its entirety or confined to areas without atrophy or fibrosis, correlates with both renal function at biopsy and with renal survival. The severity of interstitial nephritis does not correlate with glomerular activity or longer disease duration, and is not significantly associated with degree of proteinuria, which has been postulated as the etiology of tubulointerstitial disease in lupus nephritis (27, 28). Therefore, the severity of interstitial nephritis provides meaningful prognostic information independent from and superior to measures of glomerular disease.
The lack of a correlation between the severity of glomerulonephritis and interstitial nephritis suggests that each are mediated by distinct processes. We observed cases in which very active glomerulonephritis was associated with little tubulointerstitial inflammation. Conversely, there are rare cases of lupus nephritis in which severe interstitial nephritis occurs in the absence of appreciable glomerulonephritis (31-33). Therefore, glomerulonephritis and interstitial nephritis can occur independently. Furthermore, recent studies indicate that severe tubulointerstitial inflammation is associated with local, in situ, immunological processes while glomerulonephritis is a consequence of systemic autoimmunity (23, 34). This dichotomy may explain why we did not observe significant correlations between interstitial nephritis and markers of systemic disease activity. These observations suggest that assessing interstitial inflammation captures an important, pathologic process that may be tissue-specific and independent of glomerular disease.
Our studies used immunohistochemistry with anti-CD45 antibodies and light microscopy to identify the tubulointerstitial inflammatory infiltrate. This is a reasonable approximation, as previous studies have demonstrated that the tubulointerstitial inflammatory infiltrate in lupus nephritis is composed primarily of lymphocytes (35-39). Historically, tubulointerstitial inflammation has been estimated using H&E and PAS stained sections. This method is adequate for identifying severe cases of interstitial inflammation, but appears less effective than immunohistochemistry in identifying patients at intermediate risk for progression to ESRD.
The importance of the tubulointerstitium is even more apparent when scarring is assessed. It is well established that the NIH chronicity index predicts progression to renal failure. However, our data indicate that the prognostic value of the chronicity index lies in those components that capture interstitial scarring. In other renal diseases, such as IgA nephropathy, interstitial scarring also identifies patients with poor prognoses (40, 41).
We were surprised to find that proteinuria did not significantly correlate with interstitial disease. Most likely this is due to the extent and variety of glomerular disease, in which some subjects had over 19 gms of proteinuria/24 hours. This may have drowned out the associated of interstitial disease and tubular proteinuria, which is often low-grade.
In our retrospective study, it was difficult to control for all factors that may affect renal survivorship. We have a high overall rate of renal failure in our study group (29.4%). However, this is within the range described previously in high-risk African-American lupus nephritis populations (9, 42). Our data is not adjusted for the effects of hypertension, which has been predictive of poor renal outcome in a previous study of lupus nephritis (15). However, hypertensive nephropathy is not associated with interstitial inflammation. We also did not control for the use of non-steroidal anti-inflammatory drugs (NSAIDs). The latter point is relevant as NSAIDs can induce interstitial nephritis. However, they do so infrequently (43) while interstitial disease was seen in a majority of our patients. We chose to use GFR as a surrogate measure of renal survivorship for part of our statistical analysis. While this is not an ideal measure of renal survival, it may also be less prone to bias, since patients in renal failure or who are referred primarily for renal biopsy are less likely to have follow-up data available and be included in this study. Thus, a GFR-based analysis allowed for a complementary sample with less concern for bias, for a cross-sectional analysis. The consistency of our findings between these two approaches further reinforces the strength of this data.
This was a single center study and therefore it is possible that unique genetic or local environmental factors contributed to the observed severity of interstitial nephritis. For example, the majority of our cohort was African-American and it is possible that interstitial inflammation is more severe in this population. This is intriguing, as African-American lupus nephritis is associated with a worse prognosis compared to other ancestral groups (9, 42, 44-46). Austin (47) observed an increased frequency of interstitial disease in African-American lupus nephritis patients. However, a larger, multicenter study will be needed to determine conclusively if the poor renal outcomes in African-American lupus nephritis patients are explained by an increased prevalence of interstitial nephritis in this population.
Regardless of the limitations inherent to a retrospective study, our findings highlight the importance of interstitial processes in determining prognosis in lupus nephritis. Our results indicate that simple immunohistochemistry provides a means of quantitatively assessing severity of tubulointerstitial inflammation and assigning risk for subsequent renal failure. As interstitial inflammation is potentially reversible, the presence of severe interstitial nephritis is an important histologic finding which may identify high risk patients who would benefit from aggressive or directed therapeutic interventions and may warrant more focus in drug development and clinical trials.
Supplementary Material
Figure 1S. Interstitial nephritis severity predicts subsequent renal survival. 1A. Kaplan-Meier curves demonstrating renal survival in a 5 tier measure of interstitial inflammation* by total conventional light microscopy (TLM) using H&E and PAS stain without subtracting areas with interstitial fibrosis and tubular atrophy. The number of patients available for analysis in each grade is provided. P values were derived using a log rank trend test. *5 tier measure: none/grade 0=0%; minimal/grade 1= <10%, mild/grade 2= 10-25%, moderate/grade 3=26-50%; or severe/grade 4= >50%.
Supplemental Table 1. Group Sizes for CD45 Grade, Figure 1B.
Supplemental Table 2. Group Sizes for MLM Grade, Figure 1C.
Acknowledgments
Supported by grants from the Lupus Research Institute (MRC) and National Institutes of Health (RO1-AR55646 and U19-AI082724, MRC)
References
- 1.Mok C, Tang SS. Incidence and predictors of renal disease in Chinese patients with systemic lupus erythematosus. Am J Med. 2004;117(10):791–5. doi: 10.1016/j.amjmed.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 2.Cervera R, Khamashta MA, Font J, Sebastiani GD, Gil A, Lavilla P, Mejia JC, Aydingtug AO, Chwalinska-Sadowska H, de Ramon E, Fernandez-Nebro A, Galeazzi M, Valen M, Mathieu A, Houssiau F, Caro N, Alba P, Ramos-Casals M, Ingelmo M, Hughes GR. European Working Party on Systemic Lupus Erythematosus. Morbidity and mortality in systemic lupus erythematosus during a 10-year period: a comparison of early and late manifestations in a cohort of 1,000 patients. Medicine (Baltimore) 2003;82(5):299–308. doi: 10.1097/01.md.0000091181.93122.55. [DOI] [PubMed] [Google Scholar]
- 3.Bernatsky S, Boivin JF, Joseph L, Manzi S, Ginzler E, Gladman DD, Urowitz M, Fortin PR, Petri M, Barr S, Gordon C, Bae SC, Isenberg D, Zoma A, Aranow C, Dooley MA, Nived O, Sturfelt G, Steinsson K, Alarcon G, Senecal JL, Zummer M, Hanly J, Ensworth S, Pope J, Edworthy S, Rahman A, Sibley J, El-Gabalawy H, McCarthy T, St Pierre Y, Clarke A, Ramsey-Goldman R. Mortality in systemic lupus erythematosus. Arthritis Rheum. 2006;54(8):2550–7. doi: 10.1002/art.21955. [DOI] [PubMed] [Google Scholar]
- 4.McLaughlin J, Bombardier C, Farewell VT, Gladman DD, Urowitz MB. Kidney biopsy in systemic lupus erythematosus. III. Survival analysis controlling for clinical and laboratory variables. Arthritis Rheum. 1994;37(4):559–67. doi: 10.1002/art.1780370417. [DOI] [PubMed] [Google Scholar]
- 5.Cook R, Gladman DD, Pericak D, Urositz MB. Prediction of short term mortality in systemic lupus erythematosus with time dependent measures of disease activity. J Rheumatol. 2000;27(8):1892–5. [PubMed] [Google Scholar]
- 6.Ginzler E, Dooley MA, Aranow C, Kim MY, Buyon J, Merrill JT, Petri, Gilkeson S, Wallace DJ, Weisman MH, Appel GB. Mycophenolate mofetil or intravenous cyclophosphamide for lupus nephritis. N Engl J Med. 2005;353(21):2219–28. doi: 10.1056/NEJMoa043731. [DOI] [PubMed] [Google Scholar]
- 7.Appel G, Contreras G, Dooley MA, Ginzler EM, Isenberg D, Jayne D, Li LS, Mysler E, Sanchez-Guerrero J, Solomons N, Wofsy D Aspreva Lupus Management Study Group. Mycophenolate mofetil versus cyclophosphamide for induction treatment of lupus nephritis. J Am Soc Nephrol. 2009;20(5):1103–12. doi: 10.1681/ASN.2008101028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flanc R, Roberts MA, Strippoli GF, Chadban SJ, Kerr PG, Atkins RC. Treatment for lupus nephritis. Cochrane Database Syst Rev. 2004;2004(1):CD0002922. doi: 10.1002/14651858.CD002922.pub2. [DOI] [PubMed] [Google Scholar]
- 9.Korbet S, Schwartz MM, Evans J, Lewis EJ. Severe lupus nephritis:racial differences in presentation and outcome. J Am Soc Nephrol. 2007;18:244–54. doi: 10.1681/ASN.2006090992. [DOI] [PubMed] [Google Scholar]
- 10.Donadio JJ, Hart GM, Bergstralh EJ, Holley KE. Prognostic determinants in lupus nephritis: a long-term clinicopathologic study. Lupus. 1995;4(2):109–15. doi: 10.1177/096120339500400206. [DOI] [PubMed] [Google Scholar]
- 11.Neumann K, Wallace DJ, Azen C, Nessim S, Fichman M, Metzger AL, Klinenberg JR. Lupus in the 1980s: III. Influence of clinical variables, biopsy, and treatment on the outcome in 150 patients with lupus nephritis seen at a single center. Semin Arthritis Rheum. 1995;25(1):47–55. doi: 10.1016/s0049-0172(95)80017-4. [DOI] [PubMed] [Google Scholar]
- 12.Austin Hr, Muenz LR, Joyce KM, Antonovych TA, Kullick ME, Klippel JH, Decker JL, Balow JE. Prognostic factors in lupus nephritis. Contribution of renal histologic data. Am J Med. 1983;75(3):382–91. doi: 10.1016/0002-9343(83)90338-8. [DOI] [PubMed] [Google Scholar]
- 13.Appel G, Cohen DJ, Pirani CL, Meltzer JI, Estes D. Long-term follow-up of patients with lupus nephritis. A study based on the classfication of the World Health Organization. Am J Med. 1987;83(5):877–85. doi: 10.1016/0002-9343(87)90645-0. [DOI] [PubMed] [Google Scholar]
- 14.Parichatikanond P, Francis ND, Malasit P, Laohapand T, Nimmannit S, Singchoovong L, Nilwarangkur S, Chrirawong P, Vanichakarn S. Lupus nephritis: clinicopathological study of 162 cases in Thailand. J Clin Pathol. 1986;39(2):160–6. doi: 10.1136/jcp.39.2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Esdaile J, Levinton C, Federgreen W, Hayslett JP, Kashgarian M. The clinical and renal biopsy predictors of Long-term outcome in Lupus Nephritis: A study of 87 patients and review of the literature. Quarterly Journal of Medicine, New Series. 1989;72(269):779–833. [PubMed] [Google Scholar]
- 16.Williams W, Sargeant LA, Smilkle M, Smith R, Edwards H, Shah D. The outcome of lupus nephritis in Jamaican patients. Am J Med Sci. 2007;334(6):426–30. doi: 10.1097/MAJ.0b013e3180de4997. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz M, Fennell JS, Lewis EJ. Pathologic changes in the renal tubule in systemic lupus erythematosus. Hum Pathol. 1982;13(6):534–47. doi: 10.1016/s0046-8177(82)80268-2. [DOI] [PubMed] [Google Scholar]
- 18.Bohle A, Grund KE, Mackensen S, Tolon M. Correlations between renal interstitium and level of serum creatinine - morphometric investigations of biopsies in perimembranous glomerulonephritis. Virchows Arch A Path Anat And Histol. 1977;373:13–22. doi: 10.1007/BF00432465. [DOI] [PubMed] [Google Scholar]
- 19.Park M, D'Agati V, Appel GB, Pirani CL. Tubulointerstitial disease in lupus nephritis: relationship to immune depositis, interestitial inflammation, glomerular changes, renal function, and prognosis. Nephron. 1986;44:309–19. doi: 10.1159/000184012. [DOI] [PubMed] [Google Scholar]
- 20.Arce-Salinas C, Villa AR, Martinez-Rueda JO, Munoz L, Cardiel MH, Alocer-Varela J, Alarcon-Segovia D. Factors associated with chronic renal failure in 121 patients with diffuse proliferative lupus nephritis: a case-control study. Lupus. 1995;4:197–203. doi: 10.1177/096120339500400306. [DOI] [PubMed] [Google Scholar]
- 21.Yu F, Wu LH, Tan Y, Li LH, Wang CL, et al. Tubulointerstitial lesions of patients with lupus nephritis classified by the 2003 international society of nephrology and renal pathology society system. Kidney Int. 2010 doi: 10.1038/ki.2010.13. advance online. [DOI] [PubMed] [Google Scholar]
- 22.Chang A, Henderson S, Liu N, Reddy R, Hsieh C, Utset T, Meehan S, Quigg R, Meffre E, Weigert M, Clark M. In Situ B cell-mediated immune responses and tubulointerstitial inflammation in human lupus nephritis. J Immunol. doi: 10.4049/jimmunol.1001983. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steinmetz O, Velden J, Kneissler U, Marx M, Klein A, Helmchen U, Stahl RAK, Panzer U. Analysis and classification of B cell infiltrates in lupus and ANCA-associated nephritis. Kidney Int. 2008;74:448–57. doi: 10.1038/ki.2008.191. [DOI] [PubMed] [Google Scholar]
- 24.Hochberg M. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus [letter] Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 25.Levey AS, Coresh J, Greene T, Stevens LS, Zhang Y, Hendriksen S, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–54. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–37. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Dell JR, Hays RC, Guggenheim SJ, Steigerwald JC. Tubulointerstitial renal disease in systemic lupus erythematosus. Arch Intern Med. 1985;145:1996–9. [PubMed] [Google Scholar]
- 28.Hill GS, Delahousse M, Nochy D, Mandet C, Bariety J. Proteinuria and tubulointerstitial lesions in lupus nephritis. Kidney Int. 2001;60:1893–903. doi: 10.1046/j.1523-1755.2001.00017.x. [DOI] [PubMed] [Google Scholar]
- 29.Hill G, Delahousse M, Nochy D, Tomkiewicz E, Remy P, Mignon F, Mery JP. A new morphologic index for the evaluation of renal biopsies in lupus nephritis. Kidney Int. 2000;58:1160–73. doi: 10.1046/j.1523-1755.2000.00272.x. [DOI] [PubMed] [Google Scholar]
- 30.Nossent H, Henzen-Logmans SC, Vroom TM, Berden JH, Swaak TJ. Contribution of renal biopsy data in predicting outcome in lupus nephritis. Analysis of 116 patients. Arthritis Rheum. 1990;33(7):970–7. doi: 10.1002/art.1780330708. [DOI] [PubMed] [Google Scholar]
- 31.Mori Y, Kishimoto N, Yamahara H, Kijima Y, Nose A, Uchiyama-Tanaka Y, Fukui M, Ktmura T, Tokoro T, Maaki H, Nagata T, Umeda Y, Nichikawa M, Iwasaka T. Predominant tubulointerstitial nephritis in a patient with systemic lupus nephritis. Clin Exp Nephrol. 2005;9:79–84. doi: 10.1007/s10157-004-0338-3. [DOI] [PubMed] [Google Scholar]
- 32.Omokawa A, Wakul H, Okuyama S, Togashi M, Ohtani H, Komatsuda A, Ichinohasama R, Sawada K. Predominant tubulointerstitial nephritis in a patient with systemic lupus erythematosis: phenotype of infiltrating cells. Clin Nephrol. 2008;69(5):436–44. doi: 10.5414/cnp69436. [DOI] [PubMed] [Google Scholar]
- 33.Gur H, Kopolovic Y, Gross J. Chronic predominant interstitial nephritis in a patient with systemic lupus erythematosus: a follow up of three years and review of the literature. Ann Rheum Dis. 1987;46:617–23. doi: 10.1136/ard.46.8.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang A, Henderson S, Brandt D, Liu N, Guttikonda R, Hsieh C, et al. In Situ B cell-mediated immune responses and tubulointerstitial inflammation in human lupus nephritis. J Immunol. doi: 10.4049/jimmunol.1001983. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alexopoulos E, Seron D, Hartley RB, Cameron JS. Lupus Nephritis: Correlation of interstitial cells with glomerular function. Kidney International. 1990;37:100–9. doi: 10.1038/ki.1990.14. [DOI] [PubMed] [Google Scholar]
- 36.Caligaris-Cappio F, Bergui L, Tesio L, Ziano R, Camussi G. HLA-Dr+ T cells of the Leu 3 (helper) type infiltrate the kidneys of patients with systemic lupus erythematosus. Clin Exp Immunol. 1985;59:185–9. [PMC free article] [PubMed] [Google Scholar]
- 37.D'Agati V, Appel GB, Estes D, Knowles DM, II, Pirani CL. Monoclonal antibody identification of infiltrating mononuclear leukocytes in lupus nephritis. Kidney Int. 1986;30:573–81. doi: 10.1038/ki.1986.223. [DOI] [PubMed] [Google Scholar]
- 38.Castiglione A, Bucci A, Fellin G, d'Amico G, Atkins RC. The relationship of infiltrating renal leucocytes to disease activity in lupus and cryoglobulinaemic glomerulonephritis. Nephron. 1988;50(1):14–23. doi: 10.1159/000185110. [DOI] [PubMed] [Google Scholar]
- 39.Boucher A, Droz D, Adafer E, Noel LH. Characterization of mononuclear cell subsets in renal cellualr infiltrates. Kidney Int. 1986;29:1043–9. doi: 10.1038/ki.1986.105. [DOI] [PubMed] [Google Scholar]
- 40.Coppo R, D'Amico G. Factors predicting progression of IgA nephropathies. J Nephrol. 2005;18(5):503–12. [PubMed] [Google Scholar]
- 41.Nath K. Tubulointerstitial changes as a major determinant in the progression of renal damage. Am J Kidney Dis. 1992;20(1):1–17. doi: 10.1016/s0272-6386(12)80312-x. [DOI] [PubMed] [Google Scholar]
- 42.Dooley M, Hogan S, Jennette C, Falk R. Cyclophosphamide therapy for lupus nephritis: poor renal survival in black Americans. Glomerular Disease Collaborative Network. Kidney Int. 1997;51(4):1188–95. doi: 10.1038/ki.1997.162. [DOI] [PubMed] [Google Scholar]
- 43.Kleinknecht D. Interstitial nephritis, the nephrotic syndrome, and chronic renal failure secondary to nonsteroidal anti-inflammatory drugs. Semin Nephrol. 1995;15(3):228–35. [PubMed] [Google Scholar]
- 44.Bakir A. The prognosis of lupus nephritis in African-Americans: a retrospective analysis. Am J Kidney Dis. 1994;24(2):159–71. doi: 10.1016/s0272-6386(12)80177-6. [DOI] [PubMed] [Google Scholar]
- 45.Lea J. Lupus nephritis in African Americans. Am J Med Sci. 2002;323(2):85–9. doi: 10.1097/00000441-200202000-00005. [DOI] [PubMed] [Google Scholar]
- 46.Contreras G, Lenz O, Pardo V, Borja E, Cely C, Iqbal K, Nahar N, de La Cuesta C, Hurtado A, Fornoni A, Beltran-Garcia L, Asif A, Young L, Diego J, Zachariah M, Smith-Norwood B. Outcomes in African Americans and Hispanics with lupus nephritis. Kidney Int. 2006;69:1846–51. doi: 10.1038/sj.ki.5000243. [DOI] [PubMed] [Google Scholar]
- 47.Austin H, Boumpas DT, Vaughan EM, Balow JE. Predicting renal outcomes in severe lupus nephritis: contributions of clinical and histologic data. Kidney Int. 1994;45:544–450. doi: 10.1038/ki.1994.70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 1S. Interstitial nephritis severity predicts subsequent renal survival. 1A. Kaplan-Meier curves demonstrating renal survival in a 5 tier measure of interstitial inflammation* by total conventional light microscopy (TLM) using H&E and PAS stain without subtracting areas with interstitial fibrosis and tubular atrophy. The number of patients available for analysis in each grade is provided. P values were derived using a log rank trend test. *5 tier measure: none/grade 0=0%; minimal/grade 1= <10%, mild/grade 2= 10-25%, moderate/grade 3=26-50%; or severe/grade 4= >50%.
Supplemental Table 1. Group Sizes for CD45 Grade, Figure 1B.
Supplemental Table 2. Group Sizes for MLM Grade, Figure 1C.