Abstract
The early steps in the biogenesis of secreted and membrane proteins occur in the lumen of the endoplasmic reticulum (ER), where resident proteins that make up the ER machinery assist in their folding, maturation, and complex assembly. Variation in the load of ER client proteins and in the function of the organelle's aforementioned machinery for coping with that load can lead to an imbalance between the two that is referred to as ER stress. This triggers a cellular response, mediated by highly conserved signaling pathways that collectively restore equilibrium to the protein-folding environment in the organelle by increasing the expression of genes that enhance nearly all aspects of ER function, and by transiently repressing the biosynthesis of new client proteins. Evidence accrued over the past 10 years suggests that ER stress and response to it influence the fate of mutant proteins that fold inefficiently, impact on the functionality of cells and tissues that cope with unusual loads of ER client proteins, and intersect with signaling pathways that influence inflammation and cancer biology. Here, we review some of the basic workings of unfolded protein response and relate them to processes that are of potential relevance to pulmonary disease.
Keywords: chaperones, protein folding, secretion, signal transduction, pulmonary disease
THE ROLE OF THE ENDOPLASMIC RETICULUM IN PROTEIN FOLDING AND THE PROBLEM OF ENDOPLASMIC RETICULUM STRESS
Secreted and membrane proteins are synthesized on ribosomes on the cytoplasmic face of the endoplasmic reticulum (ER) membrane, and translocate across the ER membrane in an unfolded state. In the ER lumen, they undergo chaperone-assisted folding, a variety of organelle-specific post-translational modifications, and often chaperone-assisted assembly into oligomeric structures. Once properly folded and assembled, most ER client proteins are packaged into vesicles that are transported to more distal sites in the secretory pathway. Proteins that fold inefficiently are retained in the ER, and are ultimately returned to the cytoplasm for proteasomal degradation, in a process known as ER-associated protein degradation (ERAD) (1).
The ER is endowed with a unique complement of resident proteins that carry out these functions, including enzymes for post-translational covalent modifications (e.g., N-linked glycosylation, disulfide bond formation), chaperones that assist in folding and assembly steps, translocation channels and transporters involved in transmembrane traffic, and various components involved in vesicular transport out of the ER. However, owing to changes in the flux of proteins through the secretory pathway, there are opportunities for mismatching between the capacity of the ER machinery and the load of client proteins it must handle. This potential is realized under physiological conditions when cells are stimulated to secrete more protein (e.g., as when nonsecretory B cells develop into secretory plasma cells). The problem is enhanced by mutations that may affect the ability of a client protein to fold, and by physiological or pathophysiological conditions, such as hypoxia, hypoglycemia, and other metabolic abnormalities that erode the functionality of the ER's protein-handling machinery.
The resulting imbalance between the ER machinery's capacity and the client protein load that it faces is referred to as ER stress, which we now know to be a pervasive feature of eukaryotic cells. Because so many critical processes in higher eukaryotes are dependent on intercellular communication, which, in turn, is dependent on efficient biogenesis of secreted and membrane proteins, the function of the ER and the problem of ER protein folding stress has emerged as a facet of cell biology with broad implications to human disease.
THE THREE ARMS OF THE UNFOLDED PROTEIN RESPONSE
Evidence that the threat of protein misfolding in the ER can elicit a rectifying cellular response was first obtained some 20 years ago with the observation that enforced high-level expression of misfolding-prone viral glycoproteins elicits the transcriptional up-regulation of the gene encoding the ER chaperone, binding immunoglobulin protein (BiP) (2). This appeared to represent an ER counterpart of the cytoplasmic heat-shock response, and was aptly named the unfolded protein response (UPR) (3). The first components of UPR were identified by genetic screens in yeast in the early 1990s, and, later that decade, metazoan-specific UPR signaling pathways were identified.
Inositol Requiring 1, a Component Conserved between Unicellular and Multicellular Eukaryotes
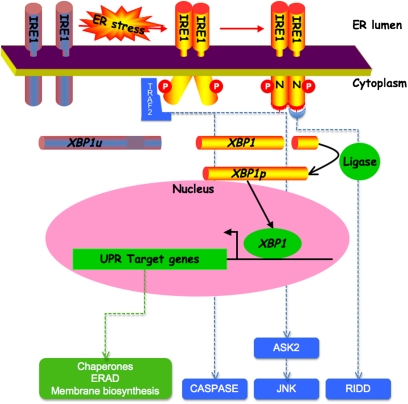
Signaling in this strand of the UPR is initiated by the product of the inositol requiring 1 (IRE1) IRE1 genes, the disruption of which in yeast uncouples ER stress from its downstream transcriptional response. IRE1 encodes a transmembrane ER-resident protein with an N-terminal domain residing in the ER lumen and a C-terminal domain that is exposed on the cytoplasmic side (4). The membrane topology and subcellular localization of IRE1 are well suited to transmit information on the state of the ER to the cell's interior. The lumenal domain senses ER stress conveying the signal across the ER membrane to the cytoplasmic domain, which broadcasts it to the nucleus, turning “on” UPR _target gene expression (Figure 1).
Figure 1.
Inositol requiring 1 (IRE1)-mediated signals in the unfolded protein response. Endoplasmic reticulum (ER) stress leads to dimerization of IRE1's lumenal domain driving trans-autophosphorylation of the cytoplasmic domain (“P”), which then enhances the affinity of IRE1 for nucleotide “N.” Nucleotide binding promotes stable dimerization of the cytoplasmic effector domain and unmasking of its catalytic activity (illustrated by the red bristles on the cytoplasmic domain), which is the cleavage (processing) of its _target mRNA, X-box binding protein 1 (XBP1). The two ends of the cleaved mRNA are joined together by a ligase. This converts the unprocessed (XBP1u) to the processed form of the mRNA (XBP1p), which encodes a potent transcription factor that activates unfolded protein response (UPR) _target genes. The UPR _target genes encode components of the ER protein handling machinery, such as chaperones and ER-associated protein degradation (ERAD) machinery and genes that promote membrane biogenesis. Phosphorylated IRE1 can also activate JUN N-terminal kinase and caspases by recruitment of tumor necrosis factor receptor associated factor 2 (TRAF2), and this may contribute to inflammation and cell death. Finally, activated IRE1 may promote degradation of membrane-bound mRNAs (regulated IRE1-dependent decay [RIDD]), and thus relieve the load on the ER by decreasing the influx of proteins into the ER. ASK2 = apoptosis signal regulating kinase 2.
IRE1's C-terminal, cytoplasmic, effector domain is a protein kinase, and undergoes autophosphorylation when activated by ER stress (5). However, unlike many other protein kinases that convey their signal by phosphorylating downstream _targets through a kinase relay, IRE1's kinase has no known substrates apart from other IRE1 molecules. Rather, the major effect of IRE1 autophosphorylation is to convert IRE1 from a low-affinity nucleotide-binding protein to a high-affinity nucleotide-binding protein. Nucleotide binding, in turn, stabilizes an active, back-to-back dimeric configuration of IRE1's effector cytoplasmic domain, which is observed in the recently obtained crystal structures of the yeast protein (6, 7).
The consequence of this rearrangement of IRE1's effector domain is to unmask a second catalytic activity, a sequence-specific endoRNase that cleaves a pre-existing mRNA (ATF/CREB 1 [Hac1] Hac1 in yeast and X-box binding protein 1 [XBP1] in mammals) in precisely two points, liberating a small RNA fragment (8). The two ends of the cleaved mRNA are later joined together by a ligase to generate a modified mRNA with a different coding region. The processed mRNA encodes a potent transcription factor that activates many genes in the UPR.
In addition to this ancient conserved signaling function, mammalian IRE1 has other effector functions. By recruitment of the adaptor, tumor necrosis factor receptor-associated factor 2 (TRAF2), it can contribute to activation of the JUN N-terminal kinase (9) and to caspase activation (10). More recently, IRE1 has been shown to possess sequence nonspecific RNase activity that results in promiscuous degradation of membrane-bound mRNAs in ER stressed cells (11, 12). The latter adaptation is expected to relieve the load on ER stressed cells by reducing the complement of available mRNAs that encoded secreted proteins. In addition to its effects on the expression of the machinery for coping directly with ER client proteins, the mammalian IRE1→XBP1 signaling pathway also influences lipid biosynthesis (13, 14) and autophagy (15, 16). Thus, IRE1 signaling is poised to affect diverse processes in the cell.
Activating Transcription Factor 6 and cAMP Responsive Element Binding Protein H, Transcription Factors Activated by Intramembrane Proteolysis in the Metazoan UPR
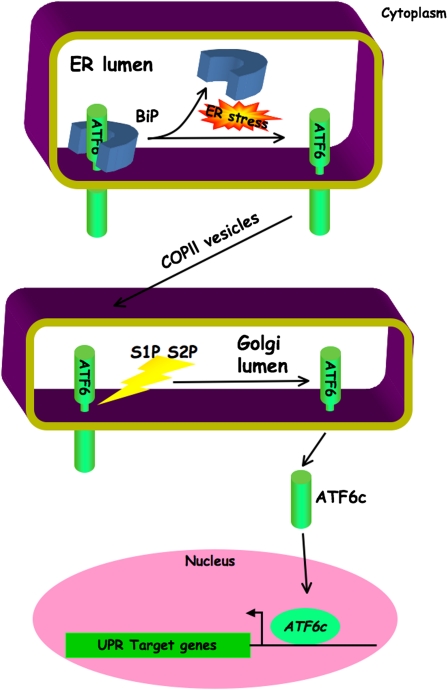
Activating transcription factor 6 (ATF6) is also an ER-localized transmembrane protein. However, ATF6 is inert in its membrane-bound form. Activation involves regulated intramembrane proteolysis, which liberates the cytoplasmic portion of ATF6 from the ER membrane under conditions of ER stress (17). Preceding this cleavage is the ER stress–dependent trafficking of ATF6 from the ER to the Golgi, where it encounters the same site 1 and site 2 proteases that process and activate the membrane bound transcription factor, sterol response element binding protein (SREBP), which regulates genes involved in sterol and fatty acid biosynthesis and assimilation (18) (Figure 2).
Figure 2.
Activating transcription factor 6 (ATF6) ATF6-mediated signals in the unfolded protein response. Binding of binding immunoglobulin protein (BiP) to the endoplasmic reticulum (ER) luminal domain of ATF6 under basal conditions retains full length ATF6 in the ER. During ER stress, sequestration of BiP by unfolded protein allows ATF6 molcules to progress via COPII vesicular transport to the Golgi. Cleavage of ATF6 by site 1 and site 2 proteases (S1P and S2P, respectively) liberates a soluble cytosolic fragment (ATF6c). This transactivates genes of the unfolded protein response (UPR). COPII = coat protein complex II.
There are two ATF6 genes, the functions of which are partially redundant, and this is reflected by the survival of mice with single mutations in either isoform and the embryonic lethality of the double mutant. Furthermore, ATF6 positively regulates many UPR _target genes (19), and ATF6 and XBP1 may have certain redundant functions, as they regulate related _target sequences (20), and the two proteins may also synergize to form a functional complex (21).
Like ATF6, cAMP responsive element binding protein H (CREBH) is also an ER-tethered transcription factor that is liberated from the ER membrane by intramembrane proteolysis after ER stress–mediated trafficking to the Golgi. However, unlike ATF6, CREBH does not appear to regulate classic UPR _target genes, but, rather, couples ER stress to activation of genes involved in the inflammatory response. As CREBH is preferentially expressed in the liver, this response is manifested in the induction of acute-phase response genes (22), exemplified by the hepcidin gene that regulates iron metabolism (23).
Protein Kinase-Like Endoplasmic Reticulum Kinase and Translational Control in the UPR
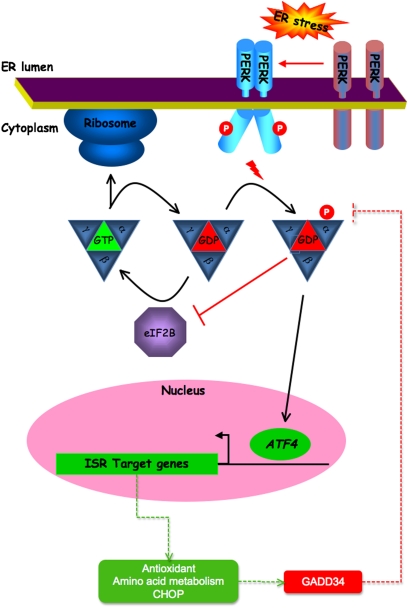
In addition to activated gene expression, ER stress results in a dramatic reduction in protein synthesis. Translational repression is an active process limiting the influx of client proteins into the stressed ER, and thus serves as a counterpart to the gene expression program, which increases the organelle's capacity to process client proteins. It was noted early on that translational repression by ER stress is mediated by phosphorylation of the α subunit of translation initiation factor (eIF) 2 on serine 51. This phosphorylation site is conserved in all eukaryotes and serves to regulate translation initiation in diverse stressful conditions. Trimeric eIF2, in complex with guanosine triphosphate (GTP), recruits the amino-acylated initiator, methionyl-transfer RNA, to the small ribosomal subunit, allowing translation initiation. Recognition of an AUG initiation codon on the mRNA leads to hydrolysis of GTP to GDP and dissociation of the ribosome–eIF2 complex. To participate in another round of translation initiation, the GDP bound to eIF2 must be exchanged to GTP. The enzyme catalyzing this exchange reaction (eIF2B) is inhibited by phosphorylated eIF2 (24) (Figure 3).
Figure 3.
Protein kinase R-like endoplasmic reticulum kinase (PERK)-mediated signals in the unfolded protein response. In unstressed conditions, α subunit of translation initiation factor (eIF) 2B–mediated guanine nucleotide exchange maintains the eIF2 complex in its GTP-bound (active) form, able to support protein translation by the ribosome. Endoplasmic reticulum (ER) stress leads to trans-autophosphorylation of PERK's cytoplasmic domain (“P”). This increases PERK's affinity for eIF2α, which is then phosphorylated on serine 51. Phosphorylated eIF2α binds avidly to eIF2B and inhibits further guanine nucleotide exchange. The net effect is reduced global protein translation. A “paradoxical” increase in activating transcription factor 4 (ATF4) translation allows transactivation of integrated stress response (ISR) genes. These include genes that encode antioxidant factors and proteins involved in amino acid sufficiency. Induction of the transcription factor, C/EBP homologous protein (CHOP) CHOP, in turn causes growth arrest and DNA damage 34 (GADD34) expression. When complexed with protein phosphatase 1, GADD34 dephosphorylates eIF2α, relieving its inhibitory effects on eIF2B, thus protein translation recovers, and a negative feedback loop is completed.
Distinct eIF2α kinases were known to be activated by distinct stress signals: protein kinase R (PKR) by double-stranded RNA in viral infection, and general control nonrepressed 2 (GCN2) by uncharged tRNAs in amino acid starvation. Protein kinase R-like endoplasmic reticulum kinase (PERK) is ER-resident transmembrane transfer protein conserved in metazoans (and absent from yeast) that couples ER stress to eIF2α phosphorylation, and is essential to translational regulation by ER stress (25). Cells lacking PERK are hypersensitive to ER stress (26) and, in mammals, PERK activity is especially important for preservation of cells engaged in high levels of secretion. Thus, humans with PERK mutations and mice lacking the gene develop diabetes mellitus and skeletal defects attributed to dysfunction of insulin- and collagen-secreting cells in the endocrine pancreas and bone, respectively (27).
In addition to limiting the load of client protein on the stressed ER (by inhibiting translation of most mRNA), eIF2α phosphorylation paradoxically activates translation of the transcription factor, ATF4, and thus contributes to the regulation of genes involved in amino acid transport and antioxidative stress responses (28). PERK signaling also up-regulates growth arrest and DNA damage 35 (GADD34), which encodes a phosphatase that dephosphorylates eIF2α and terminates signaling by PERK (29). The aforementioned PERK _target genes play an important role in maintaining translation by providing building blocks for secreted protein synthesis, and by promoting eIF2α dephosphorylation. Thus, PERK and eIF2α phosphorylation appears to have a dual role in the UPR. Early in the response, they protect the stress cell from ER overload, whereas, later, they promote conditions required for synthesis of secreted proteins (30). PERK signaling is also coupled to activation of the transcription factor, nuclear factor–κB (31, 32), but the physiological significance of that response remains to be determined.
Mechanisms for Sensing ER Stress
In the equilibrated ER, all three stress transducers—IRE1, ATF6, and PERK—are complexed to BiP, which binds on the lumenal side to their stress-sensing domains. These complexes are rapidly dissociated under conditions of ER stress. Furthermore, dissociation precedes activation of the stress transducers. BiP dissociation promotes oligomerization of IRE1 and PERK, which, in turn, leads to transautophosphorylation and activation of downstream signaling (33). It seems likely that BiP binding impedes a homo-oligomerization domain in the similarly structured lumenal domains of IRE1 and PERK.
BiP binding retains ATF6 in the ER, segregated from the proteases that release its transcription factor portion, as these are localized to a post-ER compartment. BiP dissociation liberates ATF6 to migrate to the protease-containing compartment, a migration that presumably takes place by vesicular transport (34). Thus, dispensable molecules of BiP, which are not engaged in chaperoning ER client proteins, actively repress signaling by all three known transducers of the UPR. This model posits that recognition of unfolded protein stress is performed by professional chaperones, and the stress transducers passively monitor the degree to which the latter are engaged by their clients.
The crystal structure of the lumenal domain of yeast IRE1 reveals a grove that traverses the dimer interface. Although empty in the crystal structure, its dimensions are well suited to bind the extended segments of unfolded proteins, and thus afford IRE1 the opportunity to engage and thereby sense unfolded proteins directly (35). A related direct mechanism for responding to changes in the ER-folding environment is suggested in the case of ATF6. However, rather than involving peptide binding, it appears to hinge on recognizing changes in the ER redox environment (36). The contributions made by the direct and indirect mechanisms to UPR activation remain to be determined.
ER STRESS IN LUNG PATHOLOGY
Smoking
Much of cigarette smoke's toxicity is mediated by direct oxidative damage, but recent findings suggest that ER stress may also play a role. When cultured airway epithelial cells are treated with cigarette smoke extracts, they exhibit a UPR with phosphorylation of eIF2α, attenuation of protein synthesis, and up-regulation of _target genes, BiP, C/EBP homologous protein (CHOP) CHOP, ATF4, and GADD34 (37–39). Similar responses are observed in vivo in the lungs of cigarette smoke–exposed mice (38), whereas proteome analysis shows UPR induction in human lung tissue from smokers (40). Overexpression of the ER chaperones, BiP and oxygen-regulated protein 150 kD (ORP150), in cultured bronchial epithelial cells protects them from smoke-induced apoptosis, supporting a role for ER stress in cigarette cytotoxicity (38). Precisely how smoke induces ER stress remains to be determined, but the protective effects of coadministered N-acetyl-cysteine or glutathione suggest that oxidation of an unknown _target(s) is likely to be important (37).
Inflammation
The existence of IRE1β, a gut- and lung-specific IRE1 isoform, argues that ER stress has unique consequences for mucosal tissues (41). So far, most studies have concentrated on the gut, but as the lung and gut share embryological origins, it seems likely that they will share similar challenges and adaptations. The IRE1β knockout mouse is prone to chemical-induced colitis (41), and the intestine-selective XBP 1–deleted mouse spontaneously develops inflammatory bowel disease (42). This mouse has 30% fewer intestinal goblet cells, which is of interest, because an unrelated screen for inflammatory bowel disease genes identified two mutations in the MUC2 mucin gene (43). These mucin mutations have also been shown to induce ER stress (43). Unlike humans, the unstressed mouse lung contains very few goblet cells, and so it remains to be seen how useful these murine models will be.
The XBP 1 mutant mouse also has impaired mucosal defense against Listeria monocytogenes with poorly bactericidal crypt secretions (42). XBP 1 signaling may be relevant during the response to other pathogens, because Streptomyces species secrete molecules that appear to inhibit XBP 1 mRNA splicing (44). Further studies are required to establish if these mediate a true UPR-directed virulence effect, which would support a central role for XBP 1 in the host defense against these pathogens.
ER stress also affects the acquired immune response. It is clear that the IRE1/XBP 1 pathway is crucial for differentiation programs that require expansion of the ER (e.g., differentiation of B lymphocytes into plasma cells) (8, 45). This requirement can be explained by the regulation of many lipid-synthetic genes by XBP 1 (13). In addition, XBP 1–dependent processes appear responsible for heightened inflammatory signaling in inflamed airway epithelium. When forced to express active XBP 1, bronchial epithelial cells show elevated bradykinin-induced IL-8 release (46). ER stress also affects antigen presentation by major histocompatibility complex (MHC) class I molecules. It reduces cell surface MHC class I expression by reducing translation of ER client peptides (47). When MHC class I expression is impaired by other means, tonic activation of the UPR ensues (48). This may represent a feedback relationship between ER stress signaling and antigen presentation.
Inflammation can also modify UPR signaling. Prior activation of the Toll-like receptor signaling pathways by lipopolysaccharide can selectively repress signaling via the ATF4–CHOP arm of the UPR (49). This may endow immunological cells with resistance to ER stress–induced death during sustained high secretory states, such as during a response to pathogen. Similarly, it has been shown that, during plasma cell differentiation, the PERK-dependent portion of the UPR can be suppressed, thus avoiding high levels of CHOP expression (50).
Surfactant Mutations and ER Stress
By modifying the impact of protein misfolding on cell function and survival, the UPR may affect the outcome of dominant diseases that affect the lung. In a minority of interstitial lung disease, causative mutations occur in the surfactant protein C (SP-C) gene. This encodes a large transmembrane proprotein that is proteolytically processed to the mature surfactant. One of its disease-causing mutants, a deletion of exon 4, fails to exit the ER, and induces ER stress (51–53). When expressed transiently in cultured cells, it accumulates as large, ubiquitinated inclusions, and inhibits normal proteasome function, ultimately killing the cell (51, 53, 54). Although it is possible to generate viable cell lines stably expressing this mutant, when these are infected with respiratory syncytial virus the cells accumulate high levels of mutant protein, activate the UPR, and show increased toxicity compared with wild-type SP-C–expressing cells (52). In a recent study, evidence of UPR activation (elevated BiP, endoplasmic reticulum degradation enhancer, mannosidase alpha-like 1 [EDEM] XBP 1 staining) was seen in a majority of interstitial lung disease cases, both with and without SP-C mutations (55). This may suggest an even wider relevance of ER stress in idiopathic pulmonary fibrosis.
Cancer
In a recent study, a majority of lung cancers showed evidence of UPR activation (37). ER processes consume much energy and, consequently, are sensitive to nutrient deprivation. Growing tissues use this to help match cellular proliferation rates with nutrient availability by linking UPR activation to cell cycle regulation. This may enable solid tumors to tolerate the hypoxia that frequently accompanies their high proliferative rates and limiting blood supply. Indeed, through the use of reporter mice, it has been possible to visualize ER stress within hypoxic microdomains of tumors (56). Consistent with this, when transformed PERK-deficient cells were injected into nude mice, they formed tumors less efficiently than their PERK wild-type counterparts, and displayed higher levels of tumor apoptosis (57).
The mechanism by which PERK inhibits the cell cycle remains poorly understood. Many studies have shown PERK-mediated G1 cell cycle arrest during ER stress (58–60). It has been proposed that this is mediated by loss of cyclin D1, due either to inhibition of its translation or its increased degradation (58–60). Other studies have implicated p53-dependent processes in the response to PERK activation (61–65). However, growth arrest is only attenuated, not abolished, in PERK−/− cells, suggesting the existence of PERK-independent regulation of the cell cycle (64).
The induction of ER stress may offer a _target for treating thoracic malignancy. Recent attempts to identify antimesothelioma therapies have demonstrated that proteasome inhibition with bortezomib can cause cell cycle arrest and death of cultured mesothelioma lines (66). The mechanism is not certain, but, because bortezomib induces the UPR in several cancer models, ER stress might plausibly be involved (67–69). In some cancers, bortezomib appears to _target hypoxic cells preferentially, perhaps because of their basal ER stress (70). The results of ongoing clinical trials are awaited.
CFTR
Cystic fibrosis (CF) is caused by CF transmembrane conductance regulator (CFTR) mutations that impede protein folding. High levels of ΔF508 CFTR expression, but not of wild-type protein, induce a UPR in cultured cells, but this may not fairly represent the lung, where expression levels are far lower (71). Nor is there evidence for a significant difference in the response to ER stress between mutant and wild-type CFTR human bronchial epithelial cells (72). However, rather than CFTR expression affecting ER stress, the clinically relevant relationship may be the converse. Recent data have suggested that ER stress affects CFTR expression. In cells treated with agents that induce the UPR, levels of mature CFTR protein are markedly diminished (73). This involves a selective reduction of genomic CFTR expression, an effect that is not seen with recombinantly expressed CFTR or with endogenous control genes (73). Repression of the CFTR promoter is achieved both by selective recruitment of ATF6 and by epigenetic changes, including altered DNA methylation and histone deacetylation. This is especially unfortunate, as CF mucopurelent secretions are sufficient to induce ER stress in human bronchial epithelial cells, suggesting that chronic airway sepsis may actually contribute to further impairment of CFTR expression in those cases where milder mutations allow some of the protein to reach the cell surface (46). This effect of ER stress on CFTR may also explain previous studies that have identified impaired CFTR expression and function in the upper airway of non-CF smokers (74). This had been attributed to oxidant effects alone, but might now be explained equally well as a response to smoke-induced ER stress. It is unclear if this contributes to the lung pathologies associated with smoking, but, if so, would provide a novel therapeutic _target.
CONCLUSIONS
The molecular cell biology of ER stress has been a font of discoveries for over 10 years. Many of the pathways that enable adaptation to ER protein misfolding are now well understood, although significant questions remain. Not least, how is the cell cycle regulated by the UPR, and how does ER stress kill cells? Nevertheless, ER stress clearly plays a role in many processes central to respiratory medicine, including the response to pathogens and, perhaps, the very toxicity of cigarette smoke. The stage is now set for the translation of ER stress biology into ER stress pulmonology.
Supported by grants from the Medical Research Council and Wellcome Trust.
Author Disclosure: S.J.M. received lecture fees from Boehringer Ingelheim (up to $1,000) and grant support from the Medical Research Council, United Kingdom (more than $100,001), Diabetes UK ($50,001–$100,000), and the British Lung Foundation (more than $100,001). D.R. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Ellgaard L, Helenius A. Quality control in the endoplasmic reticulum. Nat Rev Mol Cell Biol 2003;4:181–191. [DOI] [PubMed] [Google Scholar]
- 2.Kozutsumi Y, Segal M, Normington K, Gething MJ, Sambrook J. The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature 1988;332:462–464. [DOI] [PubMed] [Google Scholar]
- 3.Gething MJ, Sambrook J. Protein folding in the cell. Nature 1992;355:33–45. [DOI] [PubMed] [Google Scholar]
- 4.Urano F, Bertolotti A, Ron D. IRE1 and efferent signaling from the endoplasmic reticulum. J Cell Sci 2000;113:3697–3702. [DOI] [PubMed] [Google Scholar]
- 5.Shamu CE, Walter P. Oligomerization and phosphorylation of the IRE1p kinase during intracellular signaling from the endoplasmic reticulum to the nucleus. EMBO J 1996;15:3028–3039. [PMC free article] [PubMed] [Google Scholar]
- 6.Lee KPK, Dey M, Neculai D, Cao C, Dever TE, Sicheri F. Structure of the dual enzyme IRE1 reveals the basis for catalysis and regulation in non-conventional RNA splicing. Cell 2008;132:89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Korennykh AV, Egea PF, Korostelev AA, Finer-Moore J, Zhang C, Shokat KM, Stroud RM, Walter P. The unfolded protein response signals through high-order assembly of IRE1. Nature 2009;457:687–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iwakoshi NN, Lee AH, Vallabhajosyula P, Otipoby KL, Rajewsky K, Glimcher LH. Plasma cell differentiation and the unfolded protein response intersect at the transcription factor XBP-1. Nat Immunol 2003;4:321–329. [DOI] [PubMed] [Google Scholar]
- 9.Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding H, Ron D. Coupling of stress in the endoplasmic reticulum to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 2000;287:664–666. [DOI] [PubMed] [Google Scholar]
- 10.Yoneda T, Imaizumi K, Oono K, Yui D, Gomi F, Katayama T, Tohyama M. Activation of caspase-12, an endoplastic reticulum (ER) resident caspase, through tumor necrosis factor receptor–associated factor 2–dependent mechanism in response to the ER stress. J Biol Chem 2001;276:13935–13940. [DOI] [PubMed] [Google Scholar]
- 11.Hollien J, Weissman JS. Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science 2006;313:104–107. [DOI] [PubMed] [Google Scholar]
- 12.Hollien J, Lin JH, Li H, Stevens N, Walter P, Weissman JS. Regulated IRE1-dependent decay of messenger RNAs in mammalian cells. J Cell Biol 2009;186:323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sriburi R, Jackowski S, Mori K, Brewer JW. XBP1: a link between the unfolded protein response, lipid biosynthesis and biogenesis of the endoplasmic reticulum. J Cell Biol 2004;167:35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee AH, Glimcher LH. Intersection of the unfolded protein response and hepatic lipid metabolism. Cell Mol Life Sci 2009;66:2835–2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ogata M, Hino SI, Saito A, Morikawa K, Kondo S, Kanemoto S, Murakami T, Taniguchi M, Tanii I, Yoshinaga K, et al. Autophagy is activated for cell survival after ER stress. Mol Cell Biol 2006;26:9220–9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding W-X, Ni H-M, Gao W, Yoshinori T, Stolz DB, Ron D, Yin X-M. Linking of autophagy to ubiquitin–proteasome system is important for the regulation of endoplasmic reticulum stress and cell viability. Am J Pathol 2007;171:513–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haze K, Yoshida H, Yanagi H, Yura T, Mori K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol Biol Cell 1999;10:3787–3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ye J, Rawson RB, Komuro R, Chen X, Dave UP, Prywes R, Brown MS, Goldstein JL. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol Cell 2000;6:1355–1364. [DOI] [PubMed] [Google Scholar]
- 19.Wu J, Rutkowski DT, Dubois M, Swathirajan J, Saunders T, Wang J, Song B, Yau GD, Kaufman RJ. ATF6alpha optimizes long-term endoplasmic reticulum function to protect cells from chronic stress. Dev Cell 2007;13:351–364. [DOI] [PubMed] [Google Scholar]
- 20.Yoshida H, Haze K, Yanagi H, Yura T, Mori K. Identification of the cis-acting endoplasmic reticulum stress response element responsible for transcriptional induction of mammalian glucose–regulated proteins: involvement of basic leucine zipper transcription factors. J Biol Chem 1998;273:33741–33749. [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto K, Sato T, Matsui T, Sato M, Okada T, Yoshida H, Harada A, Mori K. Transcriptional induction of mammalian er quality control proteins is mediated by single or combined action of ATF6alpha and XBP1. Dev Cell 2007;13:365–376. [DOI] [PubMed] [Google Scholar]
- 22.Zhang K, Shen X, Wu J, Sakaki K, Saunders T, Rutkowski DT, Back SH, Kaufman RJ. Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell 2006;124:587–599. [DOI] [PubMed] [Google Scholar]
- 23.Vecchi C, Montosi G, Zhang K, Lamberti I, Duncan SA, Kaufman RJ, Pietrangelo A. ER stress controls iron metabolism through induction of hepcidin. Science 2009;325:877–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ron D, Harding H. eIF2a phosphorylation in cellular stress responses and disease. In: Sonenberg N, Hershey J, Mathews M, editors. Translational control. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2007. pp. 345–368.
- 25.Harding H, Zhang Y, Ron D. Translation and protein folding are coupled by an endoplasmic reticulum resident kinase. Nature 1999;397:271–274. [DOI] [PubMed] [Google Scholar]
- 26.Harding H, Zhang Y, Bertolotti A, Zeng H, Ron D. PERK is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell 2000;5:897–904. [DOI] [PubMed] [Google Scholar]
- 27.Harding H, Zeng H, Zhang Y, Jungreis R, Chung P, Plesken H, Sabatini D, Ron D. Diabetes mellitus and exocrine pancreatic dysfunction in PERK−/− mice reveals a role for translational control in survival of secretory cells. Mol Cell 2001;7:1153–1163. [DOI] [PubMed] [Google Scholar]
- 28.Harding HP, Calfon M, Urano F, Novoa I, Ron D. Transcriptional and translational control in the mammalian unfolded protein response. Annu Rev Cell Dev Biol 2002;18:575–599. [DOI] [PubMed] [Google Scholar]
- 29.Novoa I, Zhang Y, Zeng H, Jungreis R, Harding HP, Ron D. Stress-induced gene expression requires programmed recovery from translational repression. EMBO J 2003;22:1180–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, Jungreis R, Nagata K, Harding HP, Ron D. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev 2004;18:3066–3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang HY, Wek SA, McGrath BC, Scheuner D, Kaufman RJ, Cavener DR, Wek RC. Phosphorylation of the alpha subunit of eukaryotic initiation factor 2 is required for activation of NF-kappaB in response to diverse cellular stresses. Mol Cell Biol 2003;23:5651–5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deng J, Lu PD, Zhang Y, Scheuner D, Kaufman RJ, Sonenberg N, Harding HP, Ron D. Translational repression mediates activation of nuclear factor kappa B by phosphorylated translation initiation factor 2. Mol Cell Biol 2004;24:10161–10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bertolotti A, Zhang Y, Hendershot L, Harding H, Ron D. Dynamic interaction of BiP and the ER stress transducers in the unfolded protein response. Nat Cell Biol 2000;2:326–332. [DOI] [PubMed] [Google Scholar]
- 34.Shen J, Chen X, Hendershot L, Prywes R. Er stress regulation of atf6 localization by dissociation of BiP/Grp78 and unmasking of Golgi localization signals. Dev Cell 2002;3:99–111. [DOI] [PubMed] [Google Scholar]
- 35.Credle JJ, Finer-Moore JS, Papa FR, Stroud RM, Walter P. On the mechanism of sensing unfolded protein in the endoplasmic reticulum. Proc Natl Acad Sci USA 2005;102:18773–18784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nadanaka S, Okada T, Yoshida H, Mori K. Role of disulfide bridges formed in the luminal domain of ATF6 in sensing endoplasmic reticulum stress. Mol Cell Biol 2007;27:1027–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jorgensen E, Stinson A, Shan L, Yang J, Gietl D, Albino AP. Cigarette smoke induces endoplasmic reticulum stress and the unfolded protein response in normal and malignant human lung cells. BMC Cancer 2008;8:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tagawa Y, Hiramatsu N, Kasai A, Hayakawa K, Okamura M, Yao J, Kitamura M. Induction of apoptosis by cigarette smoke via ROS-dependent endoplasmic reticulum stress and CCAAT/enhancer-binding protein-homologous protein (CHOP). Free Radic Biol Med 2008;45:50–59. [DOI] [PubMed] [Google Scholar]
- 39.Hengstermann A, Muller T. Endoplasmic reticulum stress induced by aqueous extracts of cigarette smoke in 3T3 cells activates the unfolded-protein–response–dependent PERK pathway of cell survival. Free Radic Biol Med 2008;44:1097–1107. [DOI] [PubMed] [Google Scholar]
- 40.Kelsen SG, Duan X, Ji R, Perez O, Liu C, Merali S. Cigarette smoke induces an unfolded protein response in the human lung: a proteomic approach. Am J Respir Cell Mol Biol 2008;38:541–550. [DOI] [PubMed] [Google Scholar]
- 41.Bertolotti A, Wang X, Novoa I, Jungreis R, Schlessinger K, Cho JH, West AB, Ron D. Increased sensitivity to dextran sodium sulfate colitis in IRE1b deficient mice. J Clin Invest 2001;107:585–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaser A, Lee AH, Franke A, Glickman JN, Zeissig S, Tilg H, Nieuwenhuis EE, Higgins DE, Schreiber S, Glimcher LH, et al. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell 2008;134:743–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heazlewood CK, Cook MC, Eri R, Price GR, Tauro SB, Taupin D, Thornton DJ, Png CW, Crockford TL, Cornall RJ, et al. Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Med 2008;5:e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tashiro E, Hironiwa N, Kitagawa M, Futamura Y, Suzuki S, Nishio M, Imoto M. Trierixin, a novel inhibitor of ER stress–induced XBP1 activation from Streptomyces sp. 1. Taxonomy, fermentation, isolation and biological activities. J Antibiot (Tokyo) 2007;60:547–553. [DOI] [PubMed] [Google Scholar]
- 45.Gass JN, Gifford NM, Brewer JW. Activation of an unfolded protein response during differentiation of antibody-secreting b cells. J Biol Chem 2002;277:49047–49054. [DOI] [PubMed] [Google Scholar]
- 46.Martino ME, Olsen JC, Fulcher NB, Wolfgang MC, O'Neal WK, Ribeiro CM. Airway epithelial inflammation–induced endoplasmic reticulum Ca2+ store expansion is mediated by X-box binding protein–1. J Biol Chem 2009;284:14904–14913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Granados DP, Tanguay PL, Hardy MP, Caron E, de Verteuil D, Meloche S, Perreault C. ER stress affects processing of MHC class I–associated peptides. BMC Immunol 2009;10:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sabapathy K, Nam SY. Defective MHC class I antigen surface expression promotes cellular survival through elevated ER stress and modulation of p53 function. Cell Death Differ 2008;15:1364–1374. [DOI] [PubMed] [Google Scholar]
- 49.Woo CW, Cui D, Arellano J, Dorweiler B, Harding H, Fitzgerald KA, Ron D, Tabas I. Adaptive suppression of the ATF4-CHOP branch of the unfolded protein response by Toll-like receptor signalling. Nat Cell Biol 2009;11:1473–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ma Y, Shimizu Y, Mann MJ, Jin Y, Hendershot LM. Plasma cell differentiation initiates a limited ER stress response by specifically suppressing the PERK-dependent branch of the unfolded protein response. Cell Stress Chaperones 2010;15:281–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mulugeta S, Nguyen V, Russo SJ, Muniswamy M, Beers MF. A surfactant protein C precursor protein brichos domain mutation causes endoplasmic reticulum stress, proteasome dysfunction, and caspase 3 activation. Am J Respir Cell Mol Biol 2005;32:521–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bridges JP, Xu Y, Na CL, Wong HR, Weaver TE. Adaptation and increased susceptibility to infection associated with constitutive expression of misfolded SP-C. J Cell Biol 2006;172:395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mulugeta S, Maguire JA, Newitt JL, Russo SJ, Kotorashvili A, Beers MF. Misfolded brichos SP-C mutant proteins induce apoptosis via caspase-4– and cytochrome c–related mechanisms. Am J Physiol Lung Cell Mol Physiol 2007;293:L720–L729. [DOI] [PubMed] [Google Scholar]
- 54.Wang WJ, Mulugeta S, Russo SJ, Beers MF. Deletion of exon 4 from human surfactant protein C results in aggresome formation and generation of a dominant negative. J Cell Sci 2003;116:683–692. [DOI] [PubMed] [Google Scholar]
- 55.Lawson WE, Crossno PF, Polosukhin VV, Roldan J, Cheng DS, Lane KB, Blackwell TR, Xu C, Markin C, Ware LB, et al. Endoplasmic reticulum stress in alveolar epithelial cells is prominent in IPF: association with altered surfactant protein processing and herpesvirus infection. Am J Physiol Lung Cell Mol Physiol 2008;294:L1119–L1126. [DOI] [PubMed] [Google Scholar]
- 56.Spiotto MT, Banh A, Papandreou I, Cao H, Galvez MG, Gurtner GC, Denko NC, Le QT, Koong AC. Imaging the unfolded protein response in primary tumors reveals microenvironments with metabolic variations that predict tumor growth. Cancer Res 2010;70:78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bi M, Naczki C, Koritzinsky M, Fels D, Blais J, Hu N, Harding H, Novoa I, Varia M, Raleigh J, et al. ER stress–regulated translation increases tolerance to extreme hypoxia and promotes tumor growth. EMBO J 2005;24:3470–3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brewer JW, Hendershot LM, Sherr CJ, Diehl JA. Mammalian unfolded protein response inhibits cyclin D1 translation and cell-cycle progression. Proc Natl Acad Sci USA 1999;96:8505–8510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brewer JW, Diehl JA. PERK mediates cell-cycle exit during the mammalian unfolded protein response. Proc Natl Acad Sci USA 2000;97:12625–12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Raven JF, Baltzis D, Wang S, Mounir Z, Papadakis AI, Gao HQ, Koromilas AE. PKR and PKR-like endoplasmic reticulum kinase induce the proteasome-dependent degradation of cyclin D1 via a mechanism requiring eukaryotic initiation factor 2alpha phosphorylation. J Biol Chem 2008;283:3097–3108. [DOI] [PubMed] [Google Scholar]
- 61.Qu L, Huang S, Baltzis D, Rivas-Estilla AM, Pluquet O, Hatzoglou M, Koumenis C, Taya Y, Yoshimura A, Koromilas AE. Endoplasmic reticulum stress induces p53 cytoplasmic localization and prevents p53-dependent apoptosis by a pathway involving glycogen synthase kinase–3beta. Genes Dev 2004;18:261–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pluquet O, Qu LK, Baltzis D, Koromilas AE. Endoplasmic reticulum stress accelerates p53 degradation by the cooperative actions of HDM2 and glycogen synthase kinase 3beta. Mol Cell Biol 2005;25:9392–9405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li J, Lee B, Lee AS. Endoplasmic reticulum stress-induced apoptosis: multiple pathways and activation of p53–up-regulated modulator of apoptosis (PUMA) and NOXA by p53. J Biol Chem 2006;281:7260–7270. [DOI] [PubMed] [Google Scholar]
- 64.Zhang F, Hamanaka RB, Bobrovnikova-Marjon E, Gordan JD, Dai MS, Lu H, Simon MC, Diehl JA. Ribosomal stress couples the unfolded protein response to p53-dependent cell cycle arrest. J Biol Chem 2006;281:30036–30045. [DOI] [PubMed] [Google Scholar]
- 65.Baltzis D, Pluquet O, Papadakis AI, Kazemi S, Qu LK, Koromilas AE. The eIF2alpha kinases PERK and PKR activate glycogen synthase kinase 3 to promote the proteasomal degradation of p53. J Biol Chem 2007;282:31675–31687. [DOI] [PubMed] [Google Scholar]
- 66.Gordon GJ, Mani M, Maulik G, Mukhopadhyay L, Yeap BY, Kindler HL, Salgia R, Sugarbaker DJ, Bueno R. Preclinical studies of the proteasome inhibitor bortezomib in malignant pleural mesothelioma. Cancer Chemother Pharmacol 2008;61:549–558. [DOI] [PubMed] [Google Scholar]
- 67.Kardosh A, Golden EB, Pyrko P, Uddin J, Hofman FM, Chen TC, Louie SG, Petasis NA, Schonthal AH. Aggravated endoplasmic reticulum stress as a basis for enhanced glioblastoma cell killing by bortezomib in combination with celecoxib or its non-coxib analogue, 2,5-dimethyl-celecoxib. Cancer Res 2008;68:843–851. [DOI] [PubMed] [Google Scholar]
- 68.Obeng EA, Carlson LM, Gutman DM, Harrington WJ Jr, Lee KP, Boise LH. Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells. Blood 2006;107:4907–4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nawrocki ST, Carew JS, Dunner K Jr, Boise LH, Chiao PJ, Huang P, Abbruzzese JL, McConkey DJ. Bortezomib inhibits PKR-like endoplasmic reticulum (ER) kinase and induces apoptosis via ER stress in human pancreatic cancer cells. Cancer Res 2005;65:11510–11519. [DOI] [PubMed] [Google Scholar]
- 70.Fels DR, Ye J, Segan AT, Kridel SJ, Spiotto M, Olson M, Koong AC, Koumenis C. Preferential cytotoxicity of bortezomib toward hypoxic tumor cells via overactivation of endoplasmic reticulum stress pathways. Cancer Res 2008;68:9323–9330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bartoszewski R, Rab A, Jurkuvenaite A, Mazur M, Wakefield J, Collawn JF, Bebok Z. Activation of the unfolded protein response by deltaF508 CFTR. Am J Respir Cell Mol Biol 2008;39:448–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hybiske K, Fu Z, Schwarzer C, Tseng J, Do J, Huang N, Machen TE. Effects of cystic fibrosis transmembrane conductance regulator and deltaF508CFTR on inflammatory response, ER stress, and Ca2+ of airway epithelia. Am J Physiol Lung Cell Mol Physiol 2007;293:L1250–L1260. [DOI] [PubMed] [Google Scholar]
- 73.Rab A, Bartoszewski R, Jurkuvenaite A, Wakefield J, Collawn JF, Bebok Z. Endoplasmic reticulum stress and the unfolded protein response regulate genomic cystic fibrosis transmembrane conductance regulator expression. Am J Physiol Cell Physiol 2007;292:C756–C766. [DOI] [PubMed] [Google Scholar]
- 74.Cantin AM, Hanrahan JW, Bilodeau G, Ellis L, Dupuis A, Liao J, Zielenski J, Durie P. Cystic fibrosis transmembrane conductance regulator function is suppressed in cigarette smokers. Am J Respir Crit Care Med 2006;173:1139–1144. [DOI] [PubMed] [Google Scholar]