Abstract
In response to temporally orchestrated growth factor stimulation, developing neural stem/progenitor cells undergo extensive self-renewal and then generate neurons and astrocytes. Fetal neonatal leptin and insulin deficiency results in reduced hypothalamic axonal pathways regulating appetite, which may predispose to offspring hyperphagia and obesity. Neural development of the arcuate nucleus, a key _target of adiposity signals, leptin and insulin, is immature at birth. Hence, to explore proximate effects of leptin/insulin on hypothalamic development, we determined trophic and differentiation effects on neural stem/progenitor cells using a model of fetal hypothalamic neurospheres (NS). NS cultures were produced from embryonic d 20 fetal rats and passage 1 and passage 2 cells examined for proliferation and differentiation into neurons (neuronal nuclei, class IIIβ-tubulin, and doublecortin) and astrocytes (glial fibrillary acidic protein). Leptin-induced NS proliferation was significantly greater than that induced by insulin, although both effects were blocked by Notch, extracellular signal-regulated kinase, or signal transducer and activator of transcription 3 inhibition. Leptin preferentially induced neuronal, whereas insulin promoted astrocyte differentiation. Extracellular signal-regulated kinase inhibition suppressed both leptin and insulin-mediated differentiation, whereas signal transducer and activator of transcription inhibition only affected leptin-mediated responses. These findings demonstrate preferential and disparate differentiation paths induced by leptin and insulin. Altered fetal exposure to leptin or insulin, resulting from fetal growth restriction, macrosomia, or maternal diabetes, may potentially have marked effects on fetal brain development.
Obesity and its associated metabolic abnormalities represent a modern health crisis not only in the adults but notably also in children (1). The underpinning etiology is peripheral and central energy dysregulation. Although there are complex central mechanisms of energy homeostasis, appetite is primarily controlled by circuits of hypothalamic and hindbrain nuclei, which receive input from central and peripheral sources, including the brain, stomach, adipocytes (e.g. leptin), and pancreas (e.g. insulin). The arcuate nucleus (ARC), a key _target of appetite regulatory factors, contains populations of both orexigenic and anorexigenic neurons, which regulate food intake via downstream neuronal pathways (including paraventricular nucleus and hindbrain nucleus tractus solitarius) (2).
Orexigenic function must develop in utero in precocial species, to prepare for newborn life. In the rat, ARC anorexigenic and orexigenic neurons are identified as early as embryonic day (e)12.5 and e14.5(3), respectively, whereas ARC neural development continues through first 2 wk of postnatal life (4), and ARC projections are formed primarily during the second week of postnatal life (5–7). Recent studies demonstrate a critical neurotrophic role of leptin in the development of ARC pathways. In leptin-deficient (ob/ob) mice, these projection pathways are permanently disrupted, demonstrating axonal densities one-third to one-fourth that of controls (5). Mice that lack leptin signaling also show additional brain abnormalities and reduced brain weight, as well as altered expression of neuronal and glial proteins (8). Although less well studied, IGF-I (9, 10) and likely insulin (11) also have important neurotrophic effects. Similar to leptin, little or no insulin is produced in the brain (12). Thus, insulin enters brain regions in proportion with plasma levels, acting in areas with dense insulin receptors (IR) (e.g. ARC) (13, 14) to inhibit feeding. IR are coexpressed on both proopiomelanocortin as well as neuropeptide Y/agouti-related peptide neurons. Both insulin and leptin circulate at levels proportionate to fat mass.
Although reduced ARC axonal pathways resulting from leptin deficiency may contribute to appetite dysregulation, there may be a more proximate impact on the formation of a sufficient population of neurons within the ARC. In the mammalian brain, neurogenesis occurs in two major areas of the brain (dentate gyrus of the hippocampus and the periventricular zones of lateral and third ventricles). These sites represent the primary sources of neurons throughout fetal life and may remain active throughout adult life. During development, neural stem/progenitor cells (NPC) undergo extensive self-renewal and then generate neurons and astrocytes (15). Recent studies confirm that epigenetic gene regulation, in concert with temporally and stoichastically orchestrated extracellular signaling, prevents premature differentiation and ultimately assures the appropriate balance of neurons and astrocytes. However, an altered nutrient or neurotrophic environment may alter critical neurodevelopmental signaling. Consistent with this hypothesis, adult type II diabetic rats show increased proliferation but reduced survival of NPC (16) and NPC from hyperglycemic rats form smaller neurospheres (NS) that fail to respond to growth factors. Type I diabetic mice or streptozotocin-treated rats also have reduced neurogenesis (17). Notably, diabetic and/or obese animals have increased levels of insulin and leptin (18, 19).
Although obesity and its related diseases are the leading cause of death in Western society (20), there are markedly limited effective strategies for prevention or treatment. Our studies and others (21, 22) demonstrate that small for gestational age offspring exhibit reduced hypothalamic neural satiety pathways and dysregulated signaling, leading to programmed hyperphagia and adult obesity. Notably, small for gestational age newborns have reduced plasma leptin and insulin levels (23–25). In view of the demonstrated effects of leptin and perhaps insulin on neuronal pathway development, we sought to determine the trophic and differentiation effects on NPC during fetal development and to examine the specific signaling pathways. We used a model of hypothalamic NS cultured from e20 fetal rats.
Materials and Methods
Animals
Studies were approved by the Animal Research Committee of the Los Angeles Biomedical Research Institute at Harbor-University of California Los Angeles (LABioMed, Torrance, CA) and were in accordance with the American Association for Accreditation of Laboratory Care and National Institutes of Health guidelines. Four pregnant Sprague Dawley rats were used. Fetal brains were collected at e20 to integrate developmental neurogenesis and effective leptin/insulin signaling (26). Six fetal brains per litter were pooled.
Hypothalamic dissection
The brain was placed ventral side up. A coronal slice (∼4-mm thickness, which contains whole hypothalamus) was cut. The caudal margin of the slice was mamillary body, and rostral edge was optic chiasm. Then, semispheres adjunct to two sides of hypothalamus were cut, and finally, the dorsal part of the slice was cut off. The ventral part (∼2 mm), which is the hypothalamus, was used for NPC isolation.
Culture of hypothalamic NPC: NS
After Hanks' solution wash, the brains were transferred to DMEM/F12 medium, and hypothalami were minced and digested by 0.1% trypsin-EDTA (Invitrogen, Carlsbad, CA) plus 0.01% deoxyribonuclease (Sigma, St. Louis, MO) for 30 min at 37 C in CO2 incubator. Trypsin was inactivated with 10% fetal bovine serum and the digest filtered and centrifuged at 1000 rpm for 5 min. Cells were resuspended (∼5 × 104 cells/ml) in complete medium (CM) [neurobasal medium containing 1% anti-anti (Invitrogen), 2% B27 (catalog no. 17504-044; Invitrogen), 20 ng/ml fibroblast growth factor (FGF)2 (Sigma), 20 ng/ml epidermal growth factor (EGF) (Sigma), 1 μg/ml Heparin (Lilly, Indianapolis, IN), and 2.5 μg/ml l-glutamine (Invitrogen)], and 4 μg/ml insulin (because total absence of insulin has been shown to cause NPC cell death (27) with no leptin or IGF-I and were cultured for 8 d in CO2 incubator. FGF2 (10 ng/ml) was added to the culture every 3 d, and the NS at this stage represented passage (P)0. P0 NS were collected and disassociated into single cells by 0.1% trypsin-EDTA digestion at 37 C for 10 min. The disassociated cells were centrifuged at 1000 rpm for 5 min, reseeded in CM at same cell density as above, and represented P1 cells. P1 cells were used for treatment (see below) or after 8 d underwent further trypsinization and reseeding, representing P2 cells.
Induction of NPC differentiation
The following experiments on NPC proliferation and differentiation used only P1 and P2 cells. For the induction of differentiation, the disassociated cells were resuspended in differentiating medium (DM) (in absence of FGF2, EGF, and Heparin) and seeded in culture dishes precoated with 0.01% poly-l-lysine (Sigma).
Leptin and insulin treatments
For studying leptin and insulin effects, the NS or NPC were cultured in both CM and DM and treated with leptin (0, 20, and 40 ng/ml; Sigma) or insulin (0, 10, 20, and 40 μg/ml) every 48 h for 8 d. Subsequently, the cells were harvested for cell proliferation or protein expression analysis. Leptin and insulin concentrations used in the present study have been previously shown to be effective in inducing leptin-mediated effects in primary hypothalamic neuronal culture (26, 28) and insulin-mediated effects on brain slices (29).
Chemical inhibitors of Notch, extracellular signal-regulated kinase (ERK), and signal transducer and activator of transcription (STAT)3
NPC cultured in both CM and DM were treated every 48 h for 8 d with Notch inhibitor (γ-secretase inhibitor X, 2 μm; Sigma), ERK inhibitor (PD98059, 20 μg/ml; Chemicom, Billerica, MA) or STAT3 inhibitor (Tyrphostin AG490, 5 μm; Sigma) in presence or absence of leptin (40 ng/ml) or insulin (40 μg/ml). Cell proliferation or protein expression was determined.
NPC proliferation [3-(4,5-dimethylthiazol-2-yl)-diphenyl tetrazolium bromide (MTT) assay]
NPC proliferation rate was determined by the MTT (Sigma) colorimetric assay (30, 31). Disassociated NS cells in CM were seeded in 24-well-culture plate (1 ml/well). At d 8 of culture, 50 μl MTT solution (5 mg/ml in BPS) was added to each cell culture well and incubated for 1 h at 37 C in CO2 incubator. The cultured cells were harvested, and MTT reaction product formazan was extracted with 300 μl acidic isopropanol (isopropanol in 0.04 n HCl). The optical density of the formazan solution was measured on an ELISA plate reader (VICTOR 1420 Multilabel Counter, Perkin Elmer Wallac Inc., Gaithersburg, MD) at 570 nm. Cell proliferating index was expressed as value of OD 570 nm.
Western blot analysis
The disassociated NS cells in CM or DM were seeded in six-well plates. At d 8 of culture, the cells were harvested and dissolved in RIPA solution (Cell Signaling, Danvers, MA) with protease inhibitor cocktail (Thermo, Rockford, IL). After a brief sonication, the cell lysates were precleared by centrifugation (10,000 rpm) for 10 min at 4 C. Protein content of the cell lysates was determined by bicinchoninic acid Protein Assay kit (Thermo). Then the cell lysates were denatured in XT sample buffer (Bio-Rad, Hercules, CA) with XT Reducing Agent (Bio-Rad) at 100 C for 3 min, and 20 μg of proteins for each sample were used to run Critrion XT Precast Gel. After electro-transferring the fragmented proteins to Nitrocellulose Membrane (Bio-Rad), the blots were blocked for 1 h in Tris-buffered saline containing 0.05% Tween 20 (TBST) and 5% fat-free milk powder. Primary antibodies were diluted in blocking solution and incubated with blots overnight at 4 C. The blots were then washed in TBST for 10 min (three cycle washes) and incubated for 1 h in solution of secondary antibodies conjugated horseradish peroxidase (HRP) at room temperature. The secondary antibody included antimouse IgG-HRP (1:2000; Cell Signaling), antirabbit IgG-HRP (1:2000; Cell Signaling), and antigoat IgG-HRP (1:1000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA). After three cycle washes by TBST, blots were applied with SuperSignal West Pico Chemiluminescence Substrate (Pierce, Rockford, IL) to produce chemiluminescence, which was visualized by exposing blots to x-ray film (HyBlot CL Autoradiography Film; Denville Scientific, Inc., Metuchen, NJ). Densities of _target protein bands were determined by densitometer (Alpha Digidoc 1000; Alpha Innotech Corp., Santa Clara, CA) and normalized against glyceraldehyde-3-phosphate dehydrogenase loading controls. The primary antibodies used included: rabbit antinestin (1:5000; Sigma), rabbit anticlass IIIβ-tubulin (Tuj1) (1:5000; Sigma), rabbit antiglial fibrillary acidic protein (GFAP) (1:10,000; Dako North America Inc., Carpinteria, CA), rabbit anticleaved Notch1 (1:1000; Cell Signaling), rabbit antihairy and enhancer of split 1 (Hes1) (1:1000; Santa Cruz Biotechnology, Inc.), rabbit antiphosphorylated ERK1/2 (1:2000; Cell Signaling), mouse antiphosphorylated STAT3 (1:1000; Upstate, Waltham, MA), and mouse antiglyceraldehyde-3-phosphate dehydrogenase (1:10,000; Chemicon). The antibody concentrations were diluted according to manufacturer's recommendation. For Tuj1, GFAP, and nestin, range of dilutions were further undertaken to determine optimal dilution to reduce background and increase band intensity.
Immunostaining
For staining cultured cells, four separate NPC cultures were used. P0 disassociated NS cells in CM or DM were seeded in 12-well plates, in which round cover glasses (catalog no. 12-545-84 18CIR-1D; Fisher Scientific, Pittsburgh, PA) were preplaced. For each culture, P1 NPC were cultured in DM and were either untreated or treated with leptin (20 ng/ml) or insulin (20 μg/ml). At d 8, the cells were fixed in 4% paraformaldehyde in PBS for 30 min and were immunostained with rabbit antinestin (Sigma), rabbit anti-Tuj1 (1:500; Sigma), rabbit anti-GFAP (1:500; Dako), or 4′,6-diamidino-2-phenylindole (DAPI). Secondary antibodies were donkey antirabbit IgG-Alexa Fluor 488 (1:250) or donkey antimouse IgG-Alexa Fluor 568 (1:250). Images shown were taken at ×40. Approximately 3000 total cells (DAPI-stained nucleus) from four separate cultures were counted using ImageJ software. Stained images of Tuj1 or GFAP were superimposed on related DAPI image were. Cells with nuclei surrounded by Tuj1 or GFAP signals were counted as neurons or astrocytes, respectively. Thus, Tuj1 + DAPI and GFAP + DAPI positive cells were counted and are expressed as percentage of total DAPI cells. For negative control for NS immunostaining, the primary antibody against nestin, Tuj1, or GFAP was replaced by normal rabbit IgG. The rest of the procedure was identical to nestin/Tuj1/GFAP staining.
Data analysis
Differences between treatments were analyzed by ANOVA with Dunnett's post hoc test. Values (mean ± se) are presented as percentage of control. For clarity purposes, one representative blot is shown for protein data.
Results
NS growth and differentiation
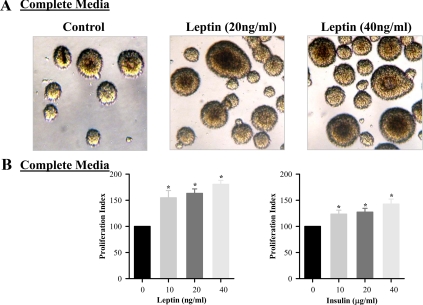
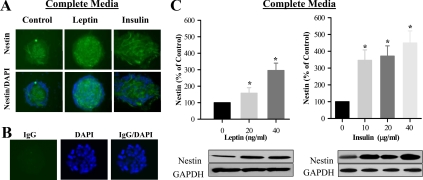
Fetal hypothalamic NS were consistently cultured in complete media and successfully produced P1 and P2 NS under controlled conditions. As demonstrated in Fig. 1, both leptin and insulin induced significant NS proliferation, in a dose-dependent response. For confirmation of NS proliferation, both control and leptin/insulin-stimulated NS were stained for DAPI (nucleus stained) and nestin, a neuroprogenitor cell marker and IgG (negative control) (Fig. 2, A and B). When measured by Western blot analysis, both leptin and insulin demonstrated increased nestin expression (Fig. 2C). Although the leptin-induced proliferation index was greater than that of insulin, insulin demonstrated a markedly increased level of nestin stimulation compared with leptin.
Fig. 1.
Leptin and insulin-stimulated hypothalamic NS proliferation. E20 hypothalamic NS were cultured in complete media. On second day of seeding, NS were treated with leptin/insulin every 48 h for 8 d. A, Live images (magnification, ×20) of leptin-treated NS. B, Cell proliferation rate of untreated and leptin/insulin-treated NS. Values are mean ± se; *, P < 0.05 vs. untreated NS.
Fig. 2.
Leptin and insulin-stimulated hypothalamic nestin expression. E20 hypothalamic NS were cultured in complete media. A, On second day of seeding, NS were treated with leptin (40 ng/ml) and insulin (40 μg/ml) every 48 h for 8 d. NS immunostained images (magnification, ×20) with DAPI (nuclear stain) and nestin (NS marker). B, Negative control for NS immunostaining. IgG (green color) and DAPI (blue). C, Protein expression of nestin (NS marker) in untreated and leptin/insulin-treated NS. Values are mean ± se; *, P < 0.05 vs. untreated NS. GAPDH, Glyceraldehyde-3-phosphate dehydrogenase.
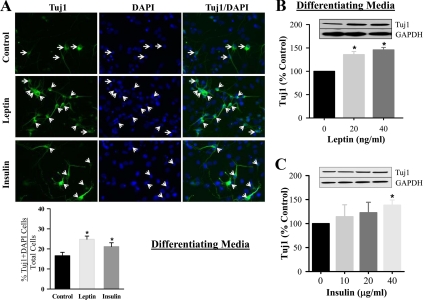
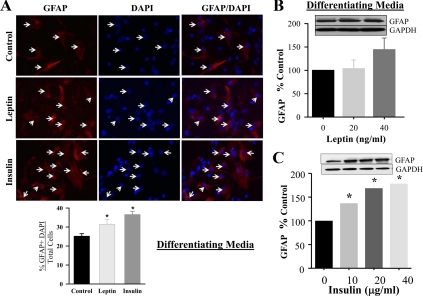
As evident by immunostaining, both leptin (20 ng/ml) and insulin (20 μg/ml) stimulated hypothalamic NS differentiation into neurons (Tuj1) and astrocytes (GFAP), when cultured in differentiation media, respectively (Figs. 3A and 4A). Due to potential limitation of precise quantitative assessment by immunostaining, we further confirmed the changes by determining protein expression using varying doses of leptin and insulin. The neuronal differentiation marker (Tuj1) demonstrated significant increases in response to increasing doses of leptin, although modest increases in response to insulin (Fig. 3, B and C). In contrast to neuronal differentiation, there was no significant increase in astrocyte GFAP expression in response to leptin, although insulin induced a significant increase in a dose-dependent fashion (Fig. 4, B and C).
Fig. 3.
Leptin and insulin-stimulated hypothalamic neuronal differentiation (Tuj1) from NPC. E20 hypothalamic NPC were cultured in differentiating media. For immunostaining (A), NPC were either untreated or treated with leptin (20 ng/ml) or insulin (20 μg/ml). At d 8, the cells were immunostained Tuj1 and DAPI. Negative control staining with normal IgG did not show any signal. Tuj1 with DAPI positive cells, as indicated by the arrows, were counted and expressed as percentage of total DAPI cells (DAPI-stained nucleus). On second day of seeding, NPC were treated with leptin (B) or insulin (C) every 48 h for 8 d. NPC were harvested and protein expression of neuronal marker, Tuj1 was determined. Values are mean ± se; *, P < 0.05 vs. untreated cells. GAPDH, Glyceraldehyde-3-phosphate dehydrogenase.
Fig. 4.
Leptin and insulin-stimulated hypothalamic astrocyte differentiation from NPC. E20 hypothalamic NPC were cultured in differentiating media. For immunostaining (A), NPC were either untreated or treated with leptin (20 ng/ml) or insulin (20 μg/ml). At d 8, the cells were immunostained GFAP and DAPI. Negative control staining with normal IgG did not show any signal. GFAP with DAPI positive cells, as indicated by the arrows, were counted and expressed as percentage of total DAPI cells (DAPI-stained nucleus). On second day of seeding, NPC were treated with leptin (B) or insulin (C) every 48 h for 8 d. NPC were harvested and protein expression of astrocyte marker, GFAP, was determined. Values are mean ± se; *, P < 0.05 vs. untreated NPC. GAPDH, Glyceraldehyde-3-phosphate dehydrogenase.
Determination of leptin and insulin signaling pathways
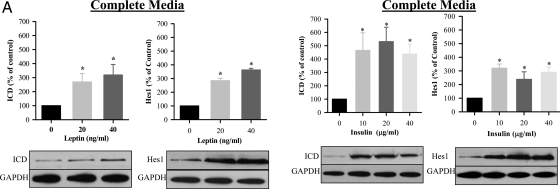
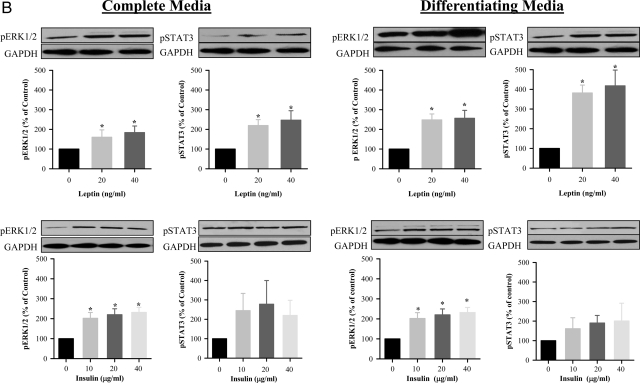
Both leptin and insulin induced Notch1 signaling, as evidenced by increased activation of the Notch1 intracellular domain (ICD) and Hes1 expression when cultured in complete (Fig. 5A) media. There was a relative absence of dose-response effects in complete media, with the lowest doses of both insulin and leptin demonstrating peak effects. There was no increase in Notch1 or Hes1 expression in differentiation media (data not shown). In contrast to the Notch1 pathway, leptin induced activation of the ERK and Janus Kinase/STAT pathway in both complete and differentiation media, as evidenced by increased pERK1/2 and pSTAT3 (Fig. 5B). Insulin induced pERK1/2 phosphorylation in complete media and differentiation media but had no significant effect on pSTAT3 (Fig. 5B).
Fig. 5.
A, Leptin and insulin-induced Notch1 signaling during NPC proliferation. E20 hypothalamic NPC were cultured in complete media. On second day of seeding, NPC were treated with leptin or insulin every 48 h for 8 d. NPC were harvested, and protein expression of Notch1 activation (cleaved Notch1, ICD) and Hes1 were determined. *, P < 0.05 vs. untreated NPC. B, Leptin-induced pERK and pSTAT3 signaling during NPC proliferation and differentiation. E20 hypothalamic NPC were cultured in complete or differentiating media. On second day of seeding, NPC were treated with leptin every 48 h for 8 d. NPC were harvested, and protein expression of pERK1/2 and pSTAT3 was determined. *, P < 0.05 vs. untreated NPC. GAPDH, Glyceraldehyde-3-phosphate dehydrogenase.
To investigate the role of these signaling pathways on NPC proliferation, differentiation, and signaling responses, we used selective Notch, ERK, and STAT3 inhibitors. As demonstrated in Table 1, leptin and insulin-mediated NS proliferation was inhibited by Notch, ERK, and STAT3 inhibitors. Notably, the Notch and ERK inhibitors suppressed control NS proliferation and reduced leptin and insulin-stimulated proliferation to below control levels. Although the STAT3 inhibitor had no effect on basal proliferation, it suppressed leptin and insulin-stimulated proliferation to control levels. Similar to the measure of proliferation index, the ERK inhibitor reduced both control and leptin/insulin-mediated hypothalamic nestin expression for NS cultured in complete media. The STAT inhibitor did not affect control nestin expression but significantly suppressed leptin/insulin-mediated nestin expression (Table 2). To further explore any cross talk between the signaling pathways, we measured the effects of PD98059 and AG490 on Notch activation. Neither the ERK nor STAT3 inhibitor affected leptin or insulin-induced Notch1 ICD or Hes1 (data not shown).
Table 1.
Effect of inhibitors on leptin/insulin-induced hypothalamic NPC proliferation
| Control | Inhibitor | Leptin | Inhibitor + leptin | Insulin | Inhibitor + insulin | |
|---|---|---|---|---|---|---|
| Notch inhibitor | 100 | 64.0 ± 5.2a | 158.3 ± 7.8a | 74.0 ± 7.9a,b | 132.1 ± 4.8a | 71.0 ± 5.8a,c |
| ERK inhibitor | 100 | 67.1 ± 3.1a | 153.3 ± 6.5a | 74.8 ± 4.8a,b | 125.5 ± 5.5a | 85.4 ± 1.7a,c |
| STAT3 inhibitor | 100 | 87.7 ± 3.5 | 152.1 ± 7.6a | 105.1 ± 8.8b | 129.7 ± 4.8a | 95.1 ± 5.5c |
E20 hypothalamic NPC were cultured in complete media. On second day of seeding, NPC were treated with inhibitors 1) Notch 1 (γ-secretase), 2) ERK (PD98059), or 3) STAT3 (AG490) in absence or presence of leptin (40 ng/ml) or insulin (40 μg/ml) every 48 h for 8 d. NPC were harvested and cell proliferation determined by MTT assay. Values are mean ± se.
P < 0.05 vs. control (untreated NPC).
P < 0.05 vs. leptin.
P < 0.05 vs. insulin.
Table 2.
Effect of inhibitors on leptin/insulin-induced hypothalamic nestin expression
| Control | Inhibitor | Leptin | Inhibitor + leptin | Insulin | Inhibitor + insulin | |
|---|---|---|---|---|---|---|
| ERK inhibitor | 100 | 66.5 + 4.8a | 195.8 + 9.5a | 153.2 + 10.5a,b | 240.9 + 19.4a | 128.8 + 12.9a,c |
| STAT3 inhibitor | 100 | 96.3 + 12.7 | 167.6 + 17.9a | 115.4 + 11.3b | 249.8 + 195.1a | 145.5 + 8.2a,c |
E20 hypothalamic NS were cultured in complete media. On second day of seeding, NPC were treated with ERK inhibitor, PD98059 in absence or presence of 1) 40 ng/ml leptin or 2) 40 μg/ml insulin every 48 h for 8 d. NS were harvested and protein expression of nestin (NS marker) determined. Values are mean ± se.
P < 0.05 vs. control (untreated NPC).
P < 0.05 vs. leptin.
P < 0.05 vs. insulin.
When examined in differentiation media, the ERK inhibitor had no effect on control neuronal differentiation or on leptin or insulin-induced neuronal marker expression. However, it completely blocked insulin-induced astrocyte differentiation (GFAP) (Table 3). In contrast, the STAT inhibitor significantly suppressed both control and leptin/insulin-induced Tuj1 neuronal marker expression, although there was no effect of the STAT inhibitor on control or insulin-induced astrocyte differentiation (Table 3). The effect of the Notch inhibitor on NS differentiation was not studied due to the lack of leptin or insulin-induced ICD/Hes1 activation in differentiation media.
Table 3.
Effect of inhibitors on leptin/insulin-induced hypothalamic neuronal and astrocyte differentiation from NPC
| Control | Inhibitor | Leptin | Inhibitor + leptin | Insulin | Inhibitor + insulin | |
|---|---|---|---|---|---|---|
| ERK inhibitor | ||||||
| Tuj1 | 100 | 99.8 + 7.4 | 141.8 + 8.3a | 138.9 + 7.6 | 129.1 + 9.6a | 120.7 + 7.1 |
| GFAP | 100 | 95.4 + 9.3 | 128.9 + 15.3 | 116.1 + 10.9 | 176.3 + 11.3a | 108.7 + 9.1c |
| STAT3 inhibitor | ||||||
| Tuj1 | 100 | 89.4 + 9.3a | 128.9 + 9.3a | 96.1 + 5.0b | 176.3 + 10.3a | 108.7 + 12.1c |
| GFAP | 100 | 117.3 + 9.6 | 131.7 + 18.2 | 126.2 + 13.3 | 185.9 + 11.6a | 171.9 + 13.4 |
E20 hypothalamic NPC were cultured in differentiating media. On second day of seeding, NPC were treated with ERK (PD98059) or STAT3 (AG490) inhibitor, in absence or presence of leptin (40 ng/ml) or insulin (40 μg/ml) every 48 h for 8 d. NPC were harvested and protein expression of neuronal (Tuj1) and astrocyte (GFAP) markers were determined. Values are mean ± se.
P < 0.05 vs. control (untreated NPC).
P < 0.05 vs. leptin.
P < 0.05 vs. insulin.
Discussion
The present study reports for the first time a model of embryonic hypothalamic NPC culture. Previous studies have demonstrated that the ARC is largely undeveloped and undifferentiated in newborn male and female rats. Specifically, there is a significant population of immature neuronal cell profiles still evident in 2- to 5-d-old rat pups, and there is progressive maturation of ARC through first 2 wk of postnatal life (4, 32). NPC cultures have been used to investigate the mechanism(s) regulating NPC renewal and multipotent differentiation in specific regions of the brain, such as olfactory bulb, hippocampus, and cortex from neonatal and adult rodents. NPC under tightly regulated spatiotemporal extrinsic and intrinsic cell factors determine the cell fate in a particular brain region at a specific developmental stage (33). Thus, area- and stage-specific regulation of NPC indicates that hypothalamic NPC culture is necessary to elucidate mechanisms regulating hypothalamic development by extracellular signals (e.g. growth factors, cytokines) and intracellular programs (e.g. transcriptional factors, epigenetic profiles).
Ingestive behavior is controlled by an interaction of diverse peripheral and central, endocrine and neuronal signals, which influence short- and long-term orexigenic and anorexigenic responses. Leptin, anadiposity signal, is the obesity (ob) gene product, a 16-kDa protein synthesized by adipocytes (34), stomach epithelia and glands (35, 36), and during pregnancy, by the placenta (37, 38). Leptin is released from adipocytes into the circulation and transported across the blood brain barrier (12, 13, 39) via the short form of the leptin receptor (ObRa) (14, 39). In the ARC nucleus, both neuropeptide Y and proopiomelanocortin neurons express ObRb (the long-form receptor), which has an intracellular signaling domain (40). Both insulin and leptin circulate at levels proportionate to fat mass. Because leptin levels are relatively insensitive to meal ingestion, leptin serves as a long-term appetite regulator. In contrast, insulin secretion increases rapidly after meals, acutely regulating ingestion (41).
In the adult, leptin and insulin mediate central anorexigenic signaling responses via different receptor molecules: leptin binds to ObRb activating the JAK-STAT3 pathway, resulting in phosphorylation of STAT3. Insulin binds to IR, primarily activating the phosphatidylinositol 3 kinase/serine-threonine protein kinase pathway (40). Recent evidence shows that leptin can act through components of the insulin signaling cascade, phosphatidylinositol 3 kinase, and can modify insulin-induced changes in gene expression in vitro and in vivo (42–46). In addition to signaling anorexigenic responses, leptin and insulin contribute importantly to neuronal development during fetal life and mediate their neurotrophic effects via the MAPK (ERK/MAPK) pathway, resulting in phosphorylation of ERK1/2(43).
Olfactory bulb, hippocampus, and cortex of neonatal and adult mice and rats are mainly used to isolate NPC for primary culture. Considering the complexity of the neural system, NPC in various brain areas at different developmental stages have to produce different cell types under a precise spatiotemporal control of extrinsic and intrinsic cell factors (33). Although rat hypothalamic NPC have been studied in vivo (47–50), the present model of hypothalamic NPC culture in vitro has not been previously reported.
As measured by the proliferation index and nestin expression, leptin and insulin demonstrated marked trophic effects on NS. Cells of ventricle zone in telecephalon and mesencephalon express ObRb mRNA at e12.5 (51), and ObRb receptor is coexpressed with neural stem cell marker nestin (52). Moreover, compared with the wild type, NPC of ob/ob embryonic cerebral cortex show impaired proliferating and differentiating potential in vivo and in vitro (52). Our studies have confirmed the expression of ObRb and IR on NS (data not shown), indicating the likely direct effect of these trophic factors. Notably, leptin-induced NS proliferation (DAPI) was significantly greater than that induced by insulin (despite the 1000-fold dose of insulin). Although normal human umbilical cord plasma leptin and insulin average approximately 6 and 0.5 ng/ml, respectively (53), the actual brain concentrations are not known. Unless there was greater cerebral transfer of insulin, these findings would suggest a greater physiologic role for leptin-mediated neurotrophic effects, compared with insulin.
Under differentiation conditions, leptin induced significant neuronal differentiation, as evidenced by increased expression of early and late neuronal markers. However, insulin exposure resulted in a nominal increase in early neuronal markers [doublecortin (DCX), Tuj1] and no significant increase in the late neuronal marker neuronal nuclei (NeuN). In contrast, insulin, although not leptin, significantly increased expression of the astrocyte marker, GFAP. To our knowledge, this is the first report of preferential and disparate differentiation paths induced by leptin and insulin. Thus, altered fetal exposure to leptin or insulin, resulting from fetal growth restriction, macrosomia, or maternal diabetes, may potentially have marked effects on fetal brain development.
To further explore the signaling pathways responsible for leptin and insulin-mediated proliferation/differentiation, we studied Notch1, ERK, and STAT3 pathways. The Notch signaling pathway of cell-cell communication contributes to the fate of NPC. Interaction of the Notch receptor with its ligands (e.g. Jagged) (54) releases the Notch ICD, which translocates to the nucleus, complexes with transcription factor CSL (CBF1/RBP-Jk, Suppressor of Hairless, Lag-1) and induces expression of Hes1(55), thus maintaining or increasing the number of NPC. Consistent with known Notch mechanisms, leptin and insulin induced ICD and Hes1 expression only in complete media conditions in which NPC proliferate. Thus, Notch is not activated under conditions of differentiation. Leptin stimulated phosphorylation of ERK1/2 and STAT3 in both complete and differentiation media, indicating activation of both signaling pathways, whereas insulin significantly activated only the ERK pathway, with a nonsignificant trend toward increased pSTAT3. Consistent with pathway responses, Notch, ERK, and STAT3 inhibition suppressed leptin and insulin-mediated proliferation. These results indicate these three pathways each contribute essential factors to NS proliferation. Because the STAT3 inhibitor suppressed insulin-mediated proliferation index and nestin expression, and insulin induced a trend toward increased pSTAT3, these findings are consistent with some contribution of the STAT pathway to insulin-mediated effects (56). Both the Notch and ERK inhibitor suppressed control proliferation, indicating that FGF2 and EGF within the complete media likely stimulated basal proliferation, and this is mediated, in part, via Notch and ERK, although not the STAT pathway.
In regards to differentiation, the ERK inhibitor suppressed leptin-mediated NeuN expression but did not alter leptin-mediated DCX or Tuj1. The suppression of NeuN indicates that an effective dose of PD98059 was used but suggests that early neuronal differentiation may occur via alternative (e.g. STAT) pathways or may be less sensitive to ERK inhibition. As insulin had minimal stimulation of neuronal markers, the lack of effect of the ERK inhibitor on insulin-stimulated neuronal DCX, Tuj1, and NeuN was expected. However, the ERK inhibitor demonstrated a marked inhibition of insulin-stimulated astrocyte (GFAP) differentiation. Consistent with STAT representing the primary leptin signaling pathway, the STAT inhibitor suppressed leptin-mediated neuronal (Tuj1, NeuN) differentiation but did not affect insulin-induced neuronal or astrocyte effects.
The results of the present study demonstrate important trophic and differentiation properties of leptin and insulin. Although insulin signaling occurs primarily via the ERK pathway, leptin acts via both the ERK and STAT pathways. Considering the significant fetal plasma levels and the abilities to cross the blood brain barrier, both leptin and insulin likely represent important neurotrophic and neurodifferentiation factors. Under conditions of macrosomia associated with maternal diabetes, fetal plasma leptin and insulin levels are elevated. Conversely, fetal growth restriction is associated with reduced plasma leptin and insulin levels. Because leptin preferentially stimulates neuronal differentiation and insulin preferentially astrocyte differentiation, altered plasma concentrations at critical times may induce subtle or gross neurologic abnormalities. Certainly, the treatment of maternal gestational diabetes with agents that enhance fetal insulin effects (e.g. metformin, glyburide) (57, 58) may have unforeseen effects on fetal neurodevelopment. Future studies will examine the effects of an altered leptin/insulin environment on the proliferation and differentiation properties of hypothalamic NS.
Acknowledgments
We acknowledge Linda Day and Stacy Behare for their technical assistance.
This work was supported by National Institute of Health Grants R01HD054751, R01DK081756, and R03HD060241.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ARC
- Arcuate nucleus
- CM
- complete medium
- DAPI
- 4′,6-diamidino-2-phenylindole
- DCX
- doublecortin
- DM
- differentiating medium
- e
- embryonic day
- EGF
- epidermal growth factor
- ERK
- extracellular signal-regulated kinase
- FGF
- fibroblast growth factor
- GFAP
- glial fibrillary acidic protein
- Hes1
- hairy and enhancer of split 1
- HRP
- horseradish peroxidase
- ICD
- intracellular domain
- IR
- insulin receptor
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-diphenyl tetrazolium bromide
- NeuN
- neuronal nuclei
- NPC
- neural stem/progenitor cell
- NS
- neurosphere
- P
- passage
- STAT
- signal transducer and activator of transcription
- TBST
- Tris-buffered saline containing 0.05% Tween 20
- Tuj1
- class IIIβ-tubulin.
References
- 1. Dietz WH. 1998. Health consequences of obesity in youth: childhood predictors of adult disease. Pediatrics 101:518–525 [PubMed] [Google Scholar]
- 2. Blevins JE, Schwartz MW, Baskin DG. 2002. Peptide signals regulating food intake and energy homeostasis. Can J Physiol Pharmacol 80:396–406 [DOI] [PubMed] [Google Scholar]
- 3. Kagotani Y, Hashimoto T, Tsuruo Y, Kawano H, Daikoku S, Chihara K. 1989. Development of the neuronal system containing neuropeptide Y in the rat hypothalamus. Int J Dev Neurosci 7:359–374 [DOI] [PubMed] [Google Scholar]
- 4. Walsh RJ, Brawer JR, Naftolin F. 1982. Early postnatal development of the arcuate nucleus in normal and sexually reversed male and female rats. J Anat 135:733–744 [PMC free article] [PubMed] [Google Scholar]
- 5. Bouret SG, Draper SJ, Simerly RB. 2004. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science 304:108–110 [DOI] [PubMed] [Google Scholar]
- 6. Bouret SG, Simerly RB. 2006. Developmental programming of hypothalamic feeding circuits. Clin Genet 70:295–301 [DOI] [PubMed] [Google Scholar]
- 7. Ahima RS, Prabakaran D, Flier JS. 1998. Postnatal leptin surge and regulation of circadian rhythm of leptin by feeding. Implications for energy homeostasis and neuroendocrine function. J Clin Invest 101:1020–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bouret SG, Simerly RB. 2004. Minireview: leptin and development of hypothalamic feeding circuits. Endocrinology 145:2621–2626 [DOI] [PubMed] [Google Scholar]
- 9. Torres-Aleman I, Naftolin F, Robbins RJ. 1990. Trophic effects of insulin-like growth factor-I on fetal rat hypothalamic cells in culture. Neuroscience 35:601–608 [DOI] [PubMed] [Google Scholar]
- 10. Duenas M, Torres-Aleman I, Naftolin F, Garcia-Segura LM. 1996. Interaction of insulin-like growth factor-I and estradiol signaling pathways on hypothalamic neuronal differentiation. Neuroscience 74:531–539 [DOI] [PubMed] [Google Scholar]
- 11. Castillo-Quan JI. 2009. Rosiglitazone effects to ameliorate Alzheimer's disease pathogenic features: insulin signaling and neurotrophic factors. J Neuropsychiatry Clin Neurosci 21:347–348 [DOI] [PubMed] [Google Scholar]
- 12. Caro JF, Kolaczynski JW, Nyce MR, Ohannesian JP, Opentanova I, Goldman WH, Lynn RB, Zhang PL, Sinha MK, Considine RV. 1996. Decreased cerebrospinal-fluid/serum leptin ratio in obesity: a possible mechanism for leptin resistance. Lancet 348:159–161 [DOI] [PubMed] [Google Scholar]
- 13. Banks WA, Kastin AJ, Huang W, Jaspan JB, Maness LM. 1996. Leptin enters the brain by a saturable system independent of insulin. Peptides 17:305–311 [DOI] [PubMed] [Google Scholar]
- 14. Lee GH, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, Friedman JM. 1996. Abnormal splicing of the leptin receptor in diabetic mice. Nature 379:632–635 [DOI] [PubMed] [Google Scholar]
- 15. Miller FD, Gauthier AS. 2007. Timing is everything: making neurons versus glia in the developing cortex. Neuron 54:357–369 [DOI] [PubMed] [Google Scholar]
- 16. Lang BT, Yan Y, Dempsey RJ, Vemuganti R. 2009. Impaired neurogenesis in adult type-2 diabetic rats. Brain Res 1258:25–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Beauquis J, Saravia F, Coulaud J, Roig P, Dardenne M, Homo-Delarche F, De Nicola A. 2008. Prominently decreased hippocampal neurogenesis in a spontaneous model of type 1 diabetes, the nonobese diabetic mouse. Exp Neurol 210:359–367 [DOI] [PubMed] [Google Scholar]
- 18. Desai M, Gayle D, Babu J, Ross MG. 2007. The timing of nutrient restriction during rat pregnancy/lactation alters metabolic syndrome phenotype. Am J Obstet Gynecol 196:555–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lin X, Chavez MR, Bruch RC, Kilroy GE, Simmons LA, Lin L, Braymer HD, Bray GA, York DA. 1998. The effects of a high fat diet on leptin mRNA, serum leptin and the response to leptin are not altered in a rat strain susceptible to high fat diet-induced obesity. J Nutr 128:1606–1613 [DOI] [PubMed] [Google Scholar]
- 20. Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, Qizilbash N, Collins R, Peto R. 2009. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet 373:1083–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Delahaye F, Breton C, Risold PY, Enache M, Dutriez-Casteloot I, Laborie C, Lesage J, Vieau D. 2008. Maternal perinatal undernutrition drastically reduces postnatal leptin surge and affects the development of arcuate nucleus proopiomelanocortin neurons in neonatal male rat pups. Endocrinology 149:470–475 [DOI] [PubMed] [Google Scholar]
- 22. Coupé B, Amarger V, Grit I, Benani A, Parnet P. 2010. Nutritional programming affects hypothalamic organization and early response to leptin. Endocrinology 151:702–713 [DOI] [PubMed] [Google Scholar]
- 23. Desai M, Gayle D, Babu J, Ross MG. 2005. Programmed obesity in intrauterine growth-restricted newborns: modulation by newborn nutrition. Am J Physiol Regul Integr Comp Physiol 288:R91–R96 [DOI] [PubMed] [Google Scholar]
- 24. Desai M, Gayle D, Han G, Ross MG. 2007. Programmed hyperphagia due to reduced anorexigenic mechanisms in intrauterine growth-restricted offspring. Reprod Sci 14:329–337 [DOI] [PubMed] [Google Scholar]
- 25. Franco-Sena AB, Goldani MZ, Tavares do Carmo MG, Velásquez-Melendez G, Kac G. 2010. Low leptin concentration in the first gestational trimester is associated with being born small for gestational age: prospective study in Rio de Janeiro, Brazil. Neonatology 97:291–298 [DOI] [PubMed] [Google Scholar]
- 26. Carlo AS, Pyrski M, Loudes C, Faivre-Baumann A, Epelbaum J, Williams LM, Meyerhof W. 2007. Leptin sensitivity in the developing rat hypothalamus. Endocrinology 148:6073–6082 [DOI] [PubMed] [Google Scholar]
- 27. Yu SW, Baek SH, Brennan RT, Bradley CJ, Park SK, Lee YS, Jun EJ, Lookingland KJ, Kim EK, Lee H, Goudreau JL, Kim SW. 2008. Autophagic death of adult hippocampal neural stem cells following insulin withdrawal. Stem Cells 26:2602–2610 [DOI] [PubMed] [Google Scholar]
- 28. Lee CC, Huang CC, Wu MY, Hsu KS. 2005. Insulin stimulates postsynaptic density-95 protein translation via the phosphoinositide 3-kinase-Akt-mammalian _target of rapamycin signaling pathway. J Biol Chem 280:18543–18550 [DOI] [PubMed] [Google Scholar]
- 29. Montessuit C, Papageorgiou I, Lerch R. 2008. Nuclear receptor agonists improve insulin responsiveness in cultured cardiomyocytes through enhanced signaling and preserved cytoskeletal architecture. Endocrinology 149:1064–1074 [DOI] [PubMed] [Google Scholar]
- 30. Mosmann T. 1983. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63 [DOI] [PubMed] [Google Scholar]
- 31. Deckwerth TL, Johnson EM., Jr 1993. Temporal analysis of events associated with programmed cell death (apoptosis) of sympathetic neurons deprived of nerve growth factor. J Cell Biol 123:1207–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Walsh RJ, Brawer JR. 1979. Cytology of the arcuate nucleus in newborn male and female rats. J Anat 128:121–133 [PMC free article] [PubMed] [Google Scholar]
- 33. Falk S, Sommer L. 2009. Stage- and area-specific control of stem cells in the developing nervous system. Curr Opin Genet Dev 19:454–460 [DOI] [PubMed] [Google Scholar]
- 34. Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. 1994. Positional cloning of the mouse obese gene and its human homologue. Nature 372:425–432 [DOI] [PubMed] [Google Scholar]
- 35. Oliver P, Picó C, De Matteis R, Cinti S, Palou A. 2002. Perinatal expression of leptin in rat stomach. Dev Dyn 223:148–154 [DOI] [PubMed] [Google Scholar]
- 36. Cinti S, Matteis RD, Picó C, Ceresi E, Obrador A, Maffeis C, Oliver J, Palou A. 2000. Secretory granules of endocrine and chief cells of human stomach mucosa contain leptin. Int J Obes Relat Metab Disord 24:789–793 [DOI] [PubMed] [Google Scholar]
- 37. Hoggard N, Hunter L, Duncan JS, Williams LM, Trayhurn P, Mercer JG. 1997. Leptin and leptin receptor mRNA and protein expression in the murine fetus and placenta. Proc Natl Acad Sci USA 94:11073–11078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Masuzaki H, Ogawa Y, Sagawa N, Hosoda K, Matsumoto T, Mise H, Nishimura H, Yoshimasa Y, Tanaka I, Mori T, Nakao K. 1997. Nonadipose tissue production of leptin: leptin as a novel placenta-derived hormone in humans. Nat Med 3:1029–1033 [DOI] [PubMed] [Google Scholar]
- 39. Jéquier E. 2002. Leptin signaling, adiposity, and energy balance. Ann NY Acad Sci 967:379–388 [DOI] [PubMed] [Google Scholar]
- 40. Porte D, Jr, Baskin DG, Schwartz MW. 2002. Leptin and insulin action in the central nervous system. Nutr Rev 60:S20–S29 [DOI] [PubMed] [Google Scholar]
- 41. Havel PJ. 2001. Peripheral signals conveying metabolic information to the brain: short-term and long-term regulation of food intake and energy homeostasis. Exp Biol Med 226:963–977 [DOI] [PubMed] [Google Scholar]
- 42. Kohno D, Nakata M, Maekawa F, Fujiwara K, Maejima Y, Kuramochi M, Shimazaki T, Okano H, Onaka T, Yada T. 2007. Leptin suppresses ghrelin-induced activation of neuropeptide Y neurons in the arcuate nucleus via phosphatidylinositol 3-kinase- and phosphodiesterase 3-mediated pathway. Endocrinology 148:2251–2263 [DOI] [PubMed] [Google Scholar]
- 43. Cui H, Cai F, Belsham DD. 2006. Leptin signaling in neurotensin neurons involves STAT, MAP kinases ERK1/2, and p38 through c-Fos and ATF1. FASEB J 20:2654–2656 [DOI] [PubMed] [Google Scholar]
- 44. Niswender KD, Morton GJ, Stearns WH, Rhodes CJ, Myers MG, Jr, Schwartz MW. 2001. Intracellular signalling. Key enzyme in leptin-induced anorexia. Nature 413:794–795 [DOI] [PubMed] [Google Scholar]
- 45. Morton GJ, Gelling RW, Niswender KD, Morrison CD, Rhodes CJ, Schwartz MW. 2005. Leptin regulates insulin sensitivity via phosphatidylinositol-3-OH kinase signaling in mediobasal hypothalamic neurons. Cell Metab 2:411–420 [DOI] [PubMed] [Google Scholar]
- 46. Morrison CD, Morton GJ, Niswender KD, Gelling RW, Schwartz MW. 2005. Leptin inhibits hypothalamic Npy and Agrp gene expression via a mechanism that requires phosphatidylinositol 3-OH-kinase signaling. Am J Physiol Endocrinol Metab 289:E1051–E1057 [DOI] [PubMed] [Google Scholar]
- 47. Kokoeva MV, Yin H, Flier JS. 2005. Neurogenesis in the hypothalamus of adult mice: potential role in energy balance. Science 310:679–683 [DOI] [PubMed] [Google Scholar]
- 48. Pencea V, Bingaman KD, Wiegand SJ, Luskin MB. 2001. Infusion of brain-derived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamus. J Neurosci 21:6706–6717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Xu Y, Tamamaki N, Noda T, Kimura K, Itokazu Y, Matsumoto N, Dezawa M, Ide C. 2005. Neurogenesis in the ependymal layer of the adult rat 3rd ventricle. Exp Neurol 192:251–264 [DOI] [PubMed] [Google Scholar]
- 50. Oya S, Yoshikawa G, Takai K, Tanaka J, Higashiyama S, Saito N, Kirino T, Kawahara N. 2008. Region-specific proliferative response of neural progenitors to exogenous stimulation by growth factors following ischemia. Neuro Rep 19:805–809 [DOI] [PubMed] [Google Scholar]
- 51. Udagawa J, Hatta T, Naora H, Otani H. 2000. Expression of the long form of leptin receptor (Ob-Rb) mRNA in the brain of mouse embryos and newborn mice. Brain Res 868:251–258 [DOI] [PubMed] [Google Scholar]
- 52. Udagawa J, Hashimoto R, Suzuki H, Hatta T, Sotomaru Y, Hioki K, Kagohashi Y, Nomura T, Minami Y, Otani H. 2006. The role of leptin in the development of the cerebral cortex in mouse embryos. Endocrinology 147:647–658 [DOI] [PubMed] [Google Scholar]
- 53. Tsai PJ, Yu CH, Hsu SP, Lee YH, Chiou CH, Hsu YW, Ho SC, Chu CH. 2004. Cord plasma concentrations of adiponectin and leptin in healthy term neonates: positive correlation with birthweight and neonatal adiposity. Clin Endocrinol 61:88–93 [DOI] [PubMed] [Google Scholar]
- 54. Yoon K, Gaiano N. 2005. Notch signaling in the mammalian central nervous system: insights from mouse mutants. Nat Neurosci 8:709–715 [DOI] [PubMed] [Google Scholar]
- 55. Iso T, Kedes L, Hamamori Y. 2003. HES and HERP families: multiple effectors of the Notch signaling pathway. J Cell Physiol 194:237–255 [DOI] [PubMed] [Google Scholar]
- 56. Carvalheira JB, Torsoni MA, Ueno M, Amaral ME, Araújo EP, Velloso LA, Gontijo JA, Saad MJ. 2005. Cross-talk between the insulin and leptin signaling systems in rat hypothalamus. Obes Res 13:48–57 [DOI] [PubMed] [Google Scholar]
- 57. Garcia-Bournissen F, Feig DS, Koren G. 2003. Maternal-fetal transport of hypoglycaemic drugs. Clin Pharmacokinet 42:303–313 [DOI] [PubMed] [Google Scholar]
- 58. Klieger C, Pollex E, Koren G. 2008. Treating the mother–protecting the unborn: the safety of hypoglycemic drugs in pregnancy. J Matern Fetal Neonatal Med 21:191–196 [DOI] [PubMed] [Google Scholar]