Abstract
Insertion and removal of AMPA receptors from the synaptic membrane underlie dynamic tuning of synaptic transmission and enduring changes in synaptic strength. Preclinical addiction research suggests that AMPA receptor trafficking plays an important role in nucleus accumbens (NAc) neuroplasticity underlying the compulsive and persistent quality of drug-seeking. Considering the parallels between drug addiction and compulsive eating, plus the supranormal reward properties of sucrose, and the role of dieting as a risk factor in development of binge pathology, the present study used a biochemical subcellular fractionation approach to determine whether brief intake of a 10% sucrose solution increases synaptic delivery of AMPA receptors in NAc of chronically food-restricted (FR) relative to ad libitum fed (AL) rats. FR, alone, produced a small but significant increase in synaptic expression of AMPA receptors. This may contribute to NAc integrative mechanisms that mediate the enhanced behavioral responsiveness of FR subjects to phasic reward stimuli, including food and drugs. Brief intake of sucrose increased GluR1 in the PSD, regardless of dietary condition, though the net effect was greater in FR than AL subjects. A marked increase in GluR2 was also observed, but only in FR rats. This set of results suggests that in FR subjects, sucrose may have primarily increased delivery of GluR1/GluR2 heteromers to the PSD, while in AL subjects sucrose increased delivery of GluR2-lacking channels. The functional consequences of these possible differences in subunit composition of trafficked AMPA receptors between diet groups remain to be determined. Nevertheless, the present set of results suggest a promising new avenue to pursue in the effort to understand synaptic plasticity involved in adaptive and pathological food-directed behavior, and the mechanistic basis of severe dieting as a risk factor for the latter.
Keywords: AMPA receptors, AMPA receptor trafficking, nucleus accumbens, sucrose, reward, food restriction
INTRODUCTION
Changes in AMPA receptor abundance in the synaptic membrane mediate dynamic tuning of synaptic transmission as well as enduring forms of synaptic plasticity. Activity-dependent regulation of postsynaptic receptor mobility, involving lateral diffusion of AMPA receptors between extrasynaptic and synaptic membrane, can alter synaptic transmission within seconds (Choquet, 2010), while trafficking of cytoplasmic AMPA receptors to the postsynaptic membrane, which takes place across minutes or tens of minutes, is a fundamental mechanism of experience-dependent behavioral plasticity (Kessels and Malinow, 2009). Recent preclinical addiction research has demonstrated that increased synaptic expression of GluR1-containing AMPA receptors in nucleus accumbens (NAc) is a mechanistic underpinning of behavioral sensitization and persistent drug-seeking (for review: Wolf and Ferrario, 2010). The involvement of AMPA receptor trafficking in behavioral modifications relating to the pursuit of natural rewards has received considerably less attention, although studies conducted in GluR1 knockout mice (Mead and Stephens, 2003) and mice with _targeted mutations of the GluR1 Ser831 and Ser845 phosphorylation sites (Crombag et al., 2008) have revealed deficits in the ability of food-paired cues to control operant responding.
Most recently, we observed that brief intake of a highly palatable sucrose solution rapidly and transiently increased GluR1 abundance in the postsynaptic density at asymmetric synapses in nucleus accumbens (NAc) core (Tukey et al., 2011). Further, in a study aimed at comparing GluR1 phosphorylation and function in ad libitum fed (AL) and chronically food-restricted (FR) rats, we observed that administration of a D-1 dopamine (DA) receptor agonist, or brief intake of 10% sucrose solution, increased phosphorylation of the AMPA receptor GluR1 subunit on Ser845 in NAc; the response to D-1 agonist was greater in FR than in AL rats, and the response to sucrose was exclusively observed in FR rats (Carr et al., 2010). The functional significance of this result was supported by observation that a polyamine antagonist of GluR2-lacking Ca2+-permeable AMPA receptors, microinjected in NAc shell, decreased the rewarding effect of D-1 receptor stimulation preferentially in FR, relative to AL, rats. Considering that GluR1 phosphorylation on Ser845 mobilizes receptors to extrasynaptic sites and primes them for synaptic insertion (Man et al., 2007; Gao et al., 2006; Oh et al., 2006), these results raise the possibility that FR upregulates DA-dependent AMPA receptor trafficking in NAc. If so, this could represent a neuroadaptation that promotes incentive learning and food acquisition during periods of negative energy balance and adipose depletion in the wild. However, if FR is self-imposed, rather than a consequence of food scarcity, and the environment in which it occurs includes access to drugs and energy-dense foods with supranormal reward properties, this mechanism might confer a heightened risk of developing maladaptive reward-directed behavior. Severe dieting is, in fact, an established risk factor for binge pathology (Stice et al., 2008), and FR with periodic access to highly palatable food leads to the emergence of binge eating in animal models (Hagan and Moss, 1997; Avena et al., 2008). Moreover, associations between FR, binge pathology, and substance abuse have been documented in both clinical and general populations (e.g., Krahn et al., 1992; Pisetsky et al., 2008).
As a first step toward investigating the role of FR-induced upregulation of synaptic plasticity in adaptive and pathological reward-directed behavior, the present study examined whether brief intake of sucrose increases AMPA receptor abundance in the synaptosomal and postsynaptic density fractions of NAc in AL and FR rats.
METHODS
Subjects
Subjects were male Sprague-Dawley rats initially weighing 350–400 grams. Animals were individually housed in clear plastic cages with bedding and maintained under a 12-h light/dark cycle, with lights on at 0700 h. Half of the subjects had ad libitum (AL) access to pelleted Purina rat chow and half were maintained on a FR regimen in which daily meals were delivered one hour before dark onset and consisted of 10 g of chow (~ 40% of AL intake). The regimen was maintained until subjects sustained a 20% decrease in body weight (~ 2 weeks). Daily feeding was titrated to clamp body weight at this value for the duration of the experiment. Experimental training and testing were initiated after body weight of the FR group had been stabilized for one week at the new value. All rats had ad libitum access to tap water available through a port in the rear wall of the home cage. Experimental procedures were approved by the New York University School of Medicine Institutional Animal Care and Use Committee and were performed in accordance with the “Principles of Laboratory Animal Care” (NIH publication number 85-23). All efforts were made to minimize animal suffering, to reduce the number of animals used and to utilize alternatives to in vivo techniques.
Behavioral training
Rats were individually housed and transported from the central animal facility to the laboratory where their cages, with inverted wire tops, were placed on a rack in a dark quiet room. After acclimating for 30-min, a graded drinking tube containing either 10% sucrose or tap water was inserted through the wire top and animals were given 30-min to sample and ingest the fluid. Over the next two weeks eight additional training sessions were conducted with the period of fluid access decreasing such that in the final five sessions, FR rats had access for 5-min and AL rats for 8-min. Pilot testing had revealed that this protocol yields essentially identical sucrose intake volumes of about 16 ml in the two diet groups. The tenth session was a terminal session in which animals were sacrificed immediately following their period of fluid access. In this terminal session, subjects with sucrose access ingested volumes ranging from 12 to 20 ml and mean intake of the two diet groups remained equivalent at about 16 ml. Intake of subjects with access to water was negligible.
In a follow-up experiment, aimed at determining whether AMPA receptor changes were dependent upon sucrose being ingested immediately prior to sacrifice, three groups of FR subjects were trained; one with water and two with sucrose as above. However, one sucrose-trained group was sacrificed 24 hrs rather than immediately after the final episode of sucrose ingestion.
Tissue preparation and western blotting
Whole cell homogenates
Rats were briefly exposed to CO2, decapitated by guillotine, and NAc dissected from fresh brain on ice. NAc of two or four rats per treatment condition were pooled for fractionation. Protease inhibitor cocktail and PMSF were added to 0.32 M sucrose solution containing 1mM NaHCO3, 1mM MgCl2 and 0.5 mM CaCl2 (Solution A). Brain tissue was rinsed, homogenized and subsequently diluted to 10% weight/volume in Solution A. After being well mixed, 50 μl of the whole cell homogenates were stored at −80 °C until use.
Synaptosomal fraction
The remaining homogenate was centrifuged at 2,000 g for 10 min, after which intact cells and nuclei formed a pellet at the bottom of the tube. The supernatant was saved and the pellet was resuspended in Solution A. The homogenate was again centrifuged at 1,400 g for 10 min. The supernatant was collected and combined with the previously collected supernatant. They were centrifuged together at 1,400 g for 10 min and then at 13,800 g for 30 min. The pellet was collected and homogenized in 0.32 M sucrose solution containing 1 mM of NaHCO3, protease inhibitor cocktail and PMSF (Solution B). This homogenate was placed on a sucrose gradient and centrifuged for 2 h at 82,500 g. The synaptosomal layer was collected from the interface of the 1 M and 1.2 M sucrose layers. The sample was then resuspended in Solution B and centrifuged at 82,500 g for 45 min. After centrifugation, the upper liquid was discarded and the pellet was resuspended in a solution of 25 mM Tris, pH 7.4. Half of the resuspended sample (adding SDS, final concentration: 2%) was stored as the synaptosomal membrane at −80 °C until use.
Postsynaptic density (PSD) fraction
An equal volume solution containing 1% Triton X-100, 0.32 M sucrose and 12 mM Tris, pH 8.1, was added to the remaining resuspended sample, the mixture was then rocked at 4 °C for 15 min, followed by centrifugation at 13,800 g for 30 minutes. After centrifugation, the upper liquid was discarded and the pellet was resuspended in a solution of 25 mM Tris, pH 7.4 with 2% SDS and stored at −80 °C until use.
Western blotting
Proteins were separated by electrophoresis on precast 4–12% sodium dodecyl sulfate polyacrylamide gels (Lonza, Rockland, ME). Dual-colored protein standard molecular weight markers (Bio-Rad, Hercules, CA) were loaded to assure complete electrophoretic transfer and estimate the size of bands of interest. Proteins were electrophoretically transferred to nitrocellulose membranes and blocked for 60 minutes with 5% nonfat milk in phosphate buffered saline with 0.05% Tween-20 (PBST) with shaking at room temperature. The blots were cut at the 70 kDa mark. The upper halves of the blots were incubated with primary antibodies for _target proteins and the lower halves of the blots were incubated with primary antibody for the protein loading control, α-tubulin by shaking overnight at 4 °C (in 3% nonfat milk/PBST).
Primary antibodies used included mouse monoclonal anti-GluR1 (1: 1,500; MAB2263, Millipore, Temecula, CA), rabbit polyclonal anti-GluR2 (1: 1,000; PA1-4659, Thermo Scientific, Rockford, IL), rabbit polyclonal anti-PSD95 (1: 1,000; AB9708, Millipore, Temecula, CA) and mouse monoclonal anti-α-tubulin (1: 5,000; T6199, Sigma-Aldrich, St. Louis, MO). Blots were washed 3×8 min in PBST, incubated with the appropriate secondary antibodies (1: 15,000) for 1 h at room temperature (in 3% nonfat milk/PBST), washed 2×8 min in PBST and 1X8 min in PBS, then treated with West Pico enhanced chemiluminescence (ECL) reagent (Pierce, Rockford, IL, USA) and exposed to film to visualize bands of interest.
Data analysis
For the main experiment, three cohorts of subjects were prepared, each with an equal number of rats assigned to the four treatment conditions. In each cohort, NAc from two or four subjects per treatment condition were pooled. Consequently, a total of 64 subjects were tested and generated six samples per treatment condition. All Western analyses were run in triplicate and results were averaged to yield each data point for a sample.
For the follow-up experiment, eight subjects were assigned to the water intake treatment condition, and twenty four were assigned to the sucrose intake treatment condition with twelve sacrificed at each of the two time points. NAc from four subjects per treatment condition were pooled.
Immunoblots were analyzed using NIH Image J software. Results were analyzed by 2-way ANOVA, with significant interaction effects followed by pair-wise comparisons of interest using the error term from the ANOVA in the denominator of a t-statistic.
RESULTS
GluR1
As displayed in Figure 1 (left panel), levels of GluR 1 in the whole cell preparation of NAc were not affected by chronic FR (F1,20=2.69, p >.10), or sucrose intake (F1,20=0.24), suggesting no change in synthesis or degradation of GluR1 protein. However, in the NAc synaptosomal fraction (middle panel), FR was observed to increase levels of GluR1 (F1,20=8.34, p <.01), without a significant effect of sucrose intake (F1,20=2.56, p =.12), and no interaction between diet and sucrose (F1,20=1.12). Consequently, it appears that chronic FR increases trafficking of GluR1-containing AMPA receptors from the intracellular compartment to the synaptic membrane. While results suggest a greater effect in FR subjects that ingested sucrose relative to those that had access to water, this difference was not significant. In the NAc postsynaptic density fraction (right panel), both FR (F1,20=12.35, p <.01) and sucrose intake (F1,20=20.6, p <.001) increased levels of GluR1, suggesting that that each of these factors increased delivery of GluR1-containing AMPA receptors to the PSD, leading to the greatest net effect in FR rats that ingested sucrose.
Figure 1.
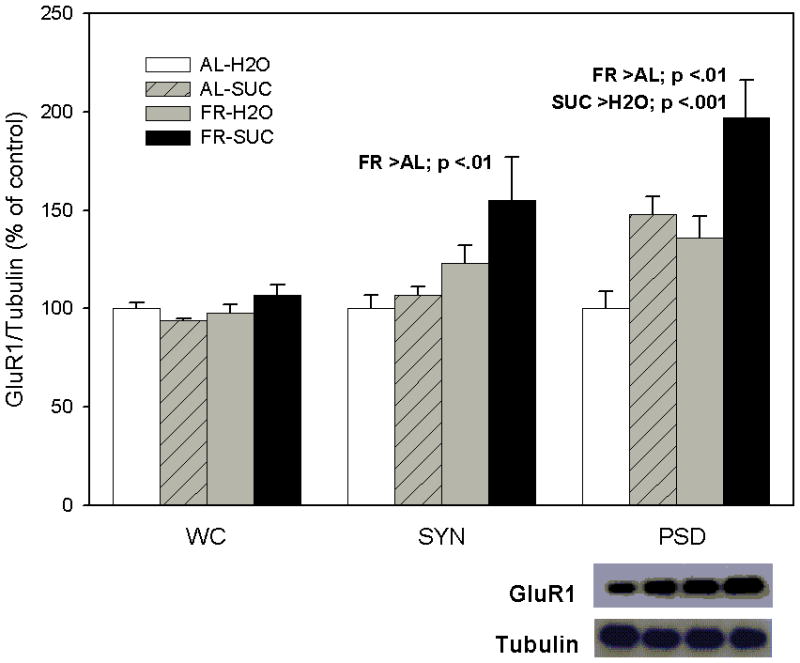
Effect of food restriction (FR) and brief intake of 10% sucrose (SUC) on GluR1 abundance in nucleus accumbens. Immediately following intake, brains were harvested and a biochemical subcellular fractionation method was used to obtain synaptosomal (SYN) and postsynaptic density (PSD) fractions from the whole cell (WC) preparation. Each fraction was immunoblotted with anti-GluR1 and anti-α-tubulin antibodies. Following densitometry, intensities of bands corresponding to GluR1 for each sample were divided by the intensities of the corresponding α-tubulin bands. Results (mean ± s.e.m.) are expressed in comparison to the normalized control, defined as the ad libitum (AL) fed group with brief access to H2O. Significant differences obtained within each fraction are indicated above the corresponding bar graph and a representative immunoblot for the PSD is included (bottom, right).
GluR2
As displayed in Figure 2 (left panel) levels of GluR2 in the whole cell preparation of NAc were not affected by FR (F1,20=1.22) or sucrose intake (F1,20=2.6, p>.10), suggesting that, as with GluR1, neither treatment altered synthesis or degradation of GluR2 protein. However, in the NAc synaptosomal fraction, FR was observed to increase levels of GluR2 (F1,20=8.22, p <.01), and sucrose intake produced a trend in the direction of increasing GluR2 levels (F1,20=3.26, p =.08). Overall, the pattern of results obtained in the synaptosomal fraction for GluR1 (Figure 1) and GluR2 (Figure 2) appear very similar. In the NAc postsynaptic density fraction (right panel), a significant interaction between diet and sucrose (F1,17=10.1, p <.01) followed by pair-wise comparison of cell means revealed that only the combination of FR and sucrose intake led to increased levels of GluR2 (all p<.001), suggesting that GluR2-containing AMPA receptors are trafficked to the PSD exclusively in FR rats that have ingested sucrose.
Figure 2.
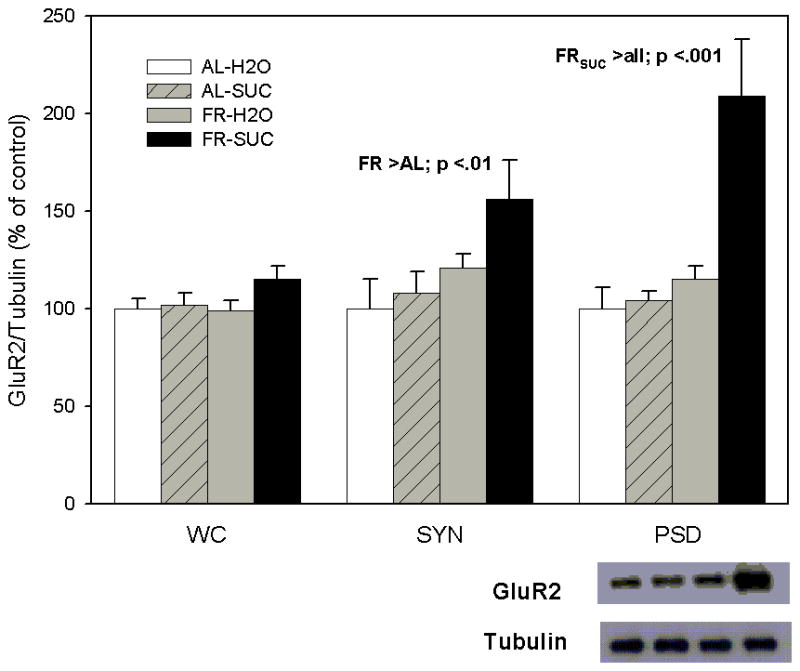
Effect of food restriction and brief intake of 10% sucrose on GluR2 abundance in nucleus accumbens. Immediately following intake, brains were harvested, whole cell (WC), synaptosomal (SYN) and postsynaptic density (PSD) fractions were separated, and each was immunoblotted with anti-GluR-2 and anti-α-tubulin antibodies. Following densitometry, intensities of bands corresponding to GluR2 for each sample were divided by the intensities of the corresponding α-tubulin bands. Results (mean ± s.e.m.) are expressed in comparison to the normalized control, defined as the ad libitum fed group with brief access to H2O. Significant differences obtained within each fraction are indicated above the corresponding bar graph and a representative immunoblot for the PSD is included (bottom, right).
PSD95
As displayed in Figure 3, levels of PSD95 did not vary across treatment conditions in any of the three NAc fractions tested.
Figure 3.
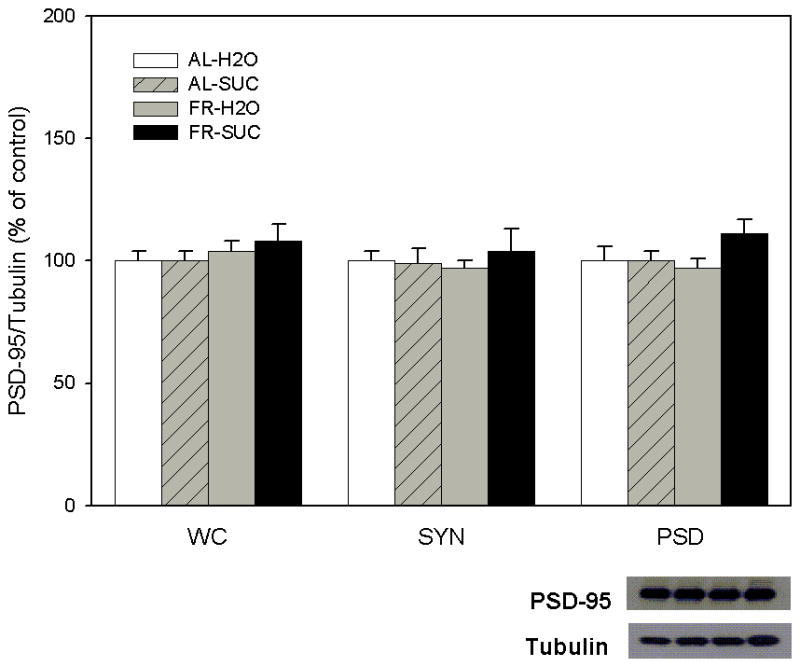
Effect of food restriction and brief intake of 10% sucrose on PSD95 abundance in nucleus accumbens. Immediately following intake, brains were harvested, whole cell (WC), synaptosomal (SYN) and postsynaptic density (PSD) fractions were separated, and each was immunoblotted with anti-PSD95 and anti-α-tubulin antibodies. Following densitometry, intensities of bands corresponding to PSD95 for each sample were divided by the intensities of the corresponding α-tubulin bands. Results (mean ± s.e.m.) are expressed in comparison to the normalized control, defined as the ad libitum fed group with brief access to H2O. Neither diet nor intake treatment altered levels of PSD95. A representative immunoblot for the PSD is included (bottom, right).
Prior sucrose
The increased levels of GluR1 and GluR2 in the PSD of FR rats following sucrose intake, relative to FR rats which ingested water, appeared similar, whether NAc was sampled immediately or twenty four hours following the final episode of sucrose ingestion (Table 1).
Table 1.
GluR1 and GluR2 in PSD of FR subjects (% control)
| GluR1 | Sucrose | Prior Sucrose |
|---|---|---|
| Sample 1 (n=4) | 110% | 135% |
| Sample 2 (n=4 | 155% | 118% |
| Sample 3 (n=4) | 180% | 170% |
| GluR2 | ||
| Sample 1 (n=4) | 133% | 178% |
| Sample 2 (n=4 | 130% | 130% |
| Sample 3 (n=4) | 140% | 110% |
DISCUSSION
The present results indicate that both FR and brief intake of sucrose affect synaptic abundance of AMPA receptors in NAc. FR, alone, increased GluR1 and GluR2 in synaptosomes with a trend in the direction of greater effects in FR subjects that ingested sucrose relative to those that had access to water. Recent quantitative co-immunoprecipitation studies have indicated that the vast majority of GluR1 in NAc is physically associated with GluR2 (Reimers et al., 2011). Further, assessment of AMPA receptor assembly state in NAc indicated that most GluR2 that is not associated with GluR1 is present in dimers or monomers, probably representing partially assembled receptors (Reimers et al., 2011). Consequently, it is likely that the present results reflect increased synaptic levels of heteromeric GluR1/GluR2 in FR subjects.
In the postsynaptic density, FR also increased the abundance of GluR1 but without a concomitant increase in GluR2. This result suggests that a GluR2-lacking channel has been inserted which, based on the work of Reimers and coworkers (2011), would likely reflect insertion of homomeric GluR1 or heteromeric GluR1/GluR3, both of which are minor receptor populations in the NAc. It bears mention that the increases being attributed to FR were observed in subjects that had access to, but ingested little or no, water and are presumed to reflect the basal state of FR subjects. The changes were small relative to the effects of sucrose (see below), but represent a potentially important new finding. The FR-induced increase in GluR1 was particularly prominent in the PSD relative to the synaptosomal fraction which may explain why, in a prior study limited to measurement in the synaptosomal fraction, we observed no difference between AL and FR rats (Carr et al., 2010). However, it may also be of importance that the duration of FR in the present study was twice that of the former (i.e., ~ 6 weeks vs 3 weeks).
To date, the effort to understand NAc integrative mechanisms that account for the enhanced behavioral responsiveness of FR subjects to phasic reward stimuli, including food, drugs, and associated cues, has been focused on dopamine release (Pothos et al., 1995; Cadoni et al., 2003), reuptake (Zhen et al., 2006) and receptor function (Carr et al., 2003; Thanos et al., 2008). GluR2-lacking AMPA receptors are Ca2+ -permeable, and even a small increase in the PSD would be expected to increase the excitability of medium spiny neurons (MSN), priming them to fire in response to glutamate-coded signals. In addition to the well characterized glutamatergic inputs to NAc from amygdala, hippocampus, and prefrontal cortex, relating to motivationally significant cues, contexts and executive controls, respectively, it is now established that glutamate is co-released with DA by mesoaccumbens neurons that fire in response to primary reward stimuli (Stuber et al., 2010; Tecuapetla et al., 2010). Increased synaptic expression of AMPA receptors in NAc of FR subjects, as is also seen in subjects withdrawn from cocaine, may enhance behavioral responsiveness to salient environmental stimuli (e.g., Carelli and Ijames, 2001); in the case of cocaine withdrawal, one consequence is vulnerability to cue-induced relapse (Conrad et al., 2008), while in the case of FR the consequence would presumably be a facilitation of foraging, food approach and acquisition. One caveat, is that increased reward-directed behavior may require that increased MSN excitability be limited to the D-1 DA receptor-expressing population which has been shown, using optogenetic stimulation, to exist in an opponent relationship with D-2 receptor expressing MSNs in regulating cocaine reward and locomotor behavior (Lobo et al., 2010).
Brief intake of a highly palatable sucrose solution increased GluR1 levels in the PSD, across diet conditions, though the net effect was greater in FR than AL rats. This observation is consistent with and extends a result of our recent study in which brief intake of sucrose by AL rats was shown to increase NAc GluR1 abundance in the PSD (Tukey et al, 2011). However, in the present study a marked increase in GluR2 levels was also observed, but only in FR rats. This set of results would suggest that in FR subjects, sucrose intake increased delivery of GluR1/GluR2 heteromers to the PSD. Importantly, the stable levels of PSD95 across diet and intake conditions supports the AMPA receptor specificity of the changes observed in this study.
GluR1/GluR2 heteromers are known to be delivered to synapses in an activity dependent manner during plasticity (Shi et al., 2001) and, in VTA-PFC co-cultures, this response has been shown to rapidly follow D-1 DA receptor stimulation and display NMDA and AMPA receptor-dependence (Gao and Wolf, 2007). Considering the latter results, the present finding in FR subjects is compatible with our prior observations of increased D-1 DA receptor function in NAc of FR rats and downstream increases in phosphorylation of NMDA NR1 (Haberny and Carr, 2005) and AMPAR GluR1 subunits (Carr et al., 2010). Synaptic insertion of GluR1/GluR2 heteromers may not, however, be expected to increase neuronal excitability in the same manner as GluR2-lacking AMPA receptors because the latter have high Ca2+ permeability and channel conductance and the former do not. Yet, the Na+ conductance of GluR1/GluR2 heteromers will contribute to depolarization and it is not possible to tell from the present data how much the overall currents increase when GluR2 or GluR1 is added. Further, functionally significant synaptic potentiation has been observed in conjunction with a stimulus-induced increase in synaptic GluR1 and GluR2; following a single associative learning trial, abundance of GluR1 and GluR2 protein in hippocampal synaptosomes rapidly increased and was associated with an enhancement of the field excitatory postsynaptic potential in CA1 (Whitlock et al, 2006).
Although present results indicate delivery of AMPA receptors to the PSD in response to sucrose intake, it is not likely that the resultant increase in MSN excitability mediates or invigorates the consummatory response itself. Numerous findings support the hypothesis that suppression of MSN activity releases the consummatory act. For example, sucrose intake correlates with a decrease in firing rate of NAc neurons (Roitman et al., 2005), neurons in NAc shell and core display long-lasting inhibition during sucrose-licking (Taha and Fields, 2006), and pharmacological inhibiton of NAc shell, using microinjection of AMPA receptor antagonist or GABA-A agonist, elicits voracious eating (Kelley et al, 2005). On the other hand, it is of interest that GluR2, but not GluR1, knockout mice display a deficit in place preference conditioning reinforced by food but not cocaine (Mead et al., 2005). Thus, the increased PSD GluR1 under basal conditions in FR subjects may serve to enhance responsiveness to glutamate-coded signals that guide food-seeking and procurement, while the delivery of GluR1/GluR2 during sucrose consumption may play an important role in reward-related learning. However, understanding the functional implication of increased GluR1 in the PSD of FR rats under basal conditions and increased GluR1 and, prominently, GluR2 during sucrose intake will, at the least, require clarification of whether these changes are differentially localized to NAc shell versus core as well as D-1 versus D-2 DA receptor-expressing MSNs (Lobo et al., 2010; Nicola, 2007).
The finding that subjects sacrificed 24-hrs after the final episode of sucrose ingestion display increased synaptic expression of GluR1 and GluR2 suggests that sucrose-induced delivery of AMPA receptors to the PSD in FR subjects persists for at least twenty four hours after the most recent episode of sucrose intake. In future studies, it will be important to determine the extent of this persistence and, if lengthy, whether it reverses upon the return of subjects from FR to AL feeding conditions. It will also be important to assess whether persistence is a cumulative effect of the nine prior episodes of sucrose intake over the preceding ~2 weeks or whether a single episode can induce both an immediate and enduring increase in AMPA receptors in the PSD.
Detection of a sucrose-induced increase in AMPAR abundance in the PSD fraction obtained from large tissue samples of NAc suggests that the response is relatively widespread throughout the NAc. This is similar to observations made in NAc of subjects withdrawn from chronic cocaine (Boudreau and Wolf, 2005). Wolf and Ferrario (2010) have considered how an apparent global response may lead to the specific behavioral result of an enduring AMPA receptor-dependent vulnerability to reinstatement of cocaine-seeking. They suggest that the global change may serve an enabling function, with other context-specific synaptic inputs selecting and strengthening the neuronal ensemble(s) that drives a specific form of reward-directed behavior. It is therefore possible that a widespread delivery of AMPA receptors to the PSD, induced by sucrose intake, facilitates a more enduring modification of select neural circuits that bind the sucrose reward to context, cues, and behavioral responses.
Both human neuroimaging studies and animal models have provided evidence of behavioral and neurophysiological parallels between drug addiction and compulsive eating. Mechanistically, the evidence to date has emphasized the involvement of striatal D-2 DA receptor downregulation (Wang et al., 2004; Johnson and Kenny, 2010). The present results suggest a new avenue to pursue in the effort to understand the development of maladaptive eating behavior, and the mechanistic basis of severe dieting as a risk factor. Because insertion and removal of AMPA receptors from the neuronal membrane underlie changes in synaptic strength (Barry and Ziff, 2002), and modulation of neuronal ensembles in NAc may underlie shifts in the hierarchical ordering of goal-directed behaviors (Deadwyler et al., 2004), episodes of “breakthrough” palatability-driven eating during self-imposed FR may induce neuroplastic changes that undercut the dieter’s goal of resisting energy-dense foods and, in some cases, engender a disposition to binge. This proposal does have some anchoring in the established clinical efficacy of topiramate in the treatment of binge eating disorder (Arbaizar et al., 2008); topiramate decreases phosphorylation of GluR1 on Ser845, decreases Ca2+ influx through AMPA receptors, and presumably interferes with synaptic insertion (Angehagen et al., 2005). Among the numerous critically important questions to be addressed going forward are (i) whether enduring synaptic strengthening occurs in discrete neuronal ensembles, (ii) whether episodes of sucrose intake during FR lead to altered sucrose-directed behavior following return to AL feeding, (iii) whether there is, in fact, a difference in the subunit composition of AMPA receptors delivered to the PSD in response to sucrose in FR versus AL subjects, and (iv) whether a mechanistic parallel, involving AMPAR trafficking, exists between potentiating effects of FR on drug- and food-directed behavior.
Acknowledgments
Supported by DA003956 (KDC), MH067229 (EBZ), T32 DA007254 (X-X P) from NIH, a NARSAD Independent Investigtor Award (KDC), and a seed grant in the Center of Excellence on Addiction from the New York University Langone Medical Center.
References
- Angehagen M, Ronnback L, Hansson E, Ben-Menachem E. Topiramate reduces AMPA-induced Ca(2+) transients and inhibits GluR1 subunit phosphorylation in astrocytes from primary cultures. J Neurochem. 2005;94:1124–1130. doi: 10.1111/j.1471-4159.2005.03259.x. [DOI] [PubMed] [Google Scholar]
- Arbaizar B, Gomez-Acebo I, Llorca J. Efficacy of topiramate in bulimia nervosa and binge-eating disorder: a systematic review. 2008;30:471–475. doi: 10.1016/j.genhosppsych.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: Behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev. 2008;32:20–39. doi: 10.1016/j.neubiorev.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry MR, Ziff EB. Receptor trafficking and the plasticity of excitatory synapses. Curr Opin Neurobiol. 2002;12:279–286. doi: 10.1016/s0959-4388(02)00329-x. [DOI] [PubMed] [Google Scholar]
- Boudreau AC, Wolf ME. Behavioral sensitization to cocaine associated with increased AMPA receptor surface expression in the nucleus accumbens. J Neurosci. 2005;25:9144–9151. doi: 10.1523/JNEUROSCI.2252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadoni C, Solinas M, Valentini V, Di Chiara G. Selective psychostimulant sensitization by food restriction: differential changes in accumbens shell and core dopamine. Eur J Neurosci. 2003;18:2326–2334. doi: 10.1046/j.1460-9568.2003.02941.x. [DOI] [PubMed] [Google Scholar]
- Carelli RM, Ijames SG. Selective activation of accumbens neurons by cocaine- associated stimuli during a water/cocaine multiple schedule. Brain Res. 2001;907:156–161. doi: 10.1016/s0006-8993(01)02604-x. [DOI] [PubMed] [Google Scholar]
- Carr KD, Chau LS, Cabeza de Vaca S, Gustafson K, Stouffer M, Tukey D, Restituito S, Ziff E. AMPA receptor subunit GluR1 downstream of D-1 dopamine receptor stimulation in nucleus accumbens shell mediates increased drug reward magnitude in food-restricted rats. Neurosci. 2010;165:1074–1086. doi: 10.1016/j.neuroscience.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr KD, Tsimberg Y, Berman Y, Yamamoto N. Evidence of increased dopamine receptor signaling in food-restricted rats. Neurosci. 2003;119:1157–1167. doi: 10.1016/s0306-4522(03)00227-6. [DOI] [PubMed] [Google Scholar]
- Choquet D. Fast AMPAR trafficking for a high-frequency synaptic transmission. Eur J Neurosci. 2010;32:250–260. doi: 10.1111/j.1460-9568.2010.07350.x. [DOI] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng L-J, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Sutton JM, Takamiya K, Holland PC, Gallagher M, Huganir RL. A role for alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid GluR1 phosphorylation in the modulatory effects of appetitive reward cues on goal-directed behavior. Eur J Neurosci. 2008;27:3284–91. doi: 10.1111/j.1460-9568.2008.06299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deadwyler SA, Hayashizaki S, Cheer J, Hampson RE. Reward, memory and substance abuse: functional neuronal circuits in the nucleus accumbens. Neurosci Biobehav Rev. 2004;27:703–11. doi: 10.1016/j.neubiorev.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Derkach VA, Oh MC, Guire ES, Soderling TR. Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nature Rev Neurosci. 2007;8:101–113. doi: 10.1038/nrn2055. [DOI] [PubMed] [Google Scholar]
- Gao C, Sun X, Wolf ME. Activation of D1 dopamine receptors increases surface expression of AMPA receptors and facilitates their synaptic incorporation in cultured hippocampal neurons. J Neurochem. 2006;98:1664–1677. doi: 10.1111/j.1471-4159.2006.03999.x. [DOI] [PubMed] [Google Scholar]
- Gao C, Wolf ME. Dopamine alters AMPA receptor synaptic expression and subunit composition in dopamine neurons of the ventral tegmental area cultured with prefrontal cortex neurons. J Neurosci. 2007;27:14275–14285. doi: 10.1523/JNEUROSCI.2925-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberny SL, Carr KD. Food restriction increases NMDA receptor-mediated CaMK II and NMDA receptor/ERK 1/2-mediated CREB phosphorylation in nucleus accumbens upon D-1 dopamine receptor stimulation in rats. Neurosci. 2005;132:1035–1043. doi: 10.1016/j.neuroscience.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Hagan MM, Moss DE. Persistence of binge-eating patterns after a history of restriction with intermittent bouts of refeeding on palatable food in rats: implications for bulimia nervosa. Int J Eating Disorders. 1997;22:411–420. doi: 10.1002/(sici)1098-108x(199712)22:4<411::aid-eat6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci. 2010;13:635–641. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol Behav. 2005;86:773–795. doi: 10.1016/j.physbeh.2005.08.066. [DOI] [PubMed] [Google Scholar]
- Kessels HW, Malinow R. Synaptic AMPA receptor plasticity and behavior. Neuron. 2009;61:340–350. doi: 10.1016/j.neuron.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krahn D, Kurth C, Demitrack M, Drewnowski A. The relationship of dieting severity and bulimic behaviors to alcohol and other drug use in young women. J Substance Abuse. 1992;4:341–353. doi: 10.1016/0899-3289(92)90041-u. [DOI] [PubMed] [Google Scholar]
- Lobo MK, Covington HE, 3rd, Chaudhury D, Friedman AK, Sun H, Damez-Werno D, Dietz DM, Zaman S, Koo JW, Kennedy PJ, Mouzon E, Mogri M, Neve RL, Deisseroth K, Han MH, Nestler EJ. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science. 2010;330:385–390. doi: 10.1126/science.1188472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man HY, Sekine-Aizawa Y, Huganir RL. Regulation of {alpha}-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor trafficking through PKA phosphorylation of the Glu receptor 1 subunit. Proc Natl Acad Sci. 2007;104:3579–3584. doi: 10.1073/pnas.0611698104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead AN, Brown G, Le Merrer J, Stephens DN. Effects of deletion of gria 1 or gria 2 genes encoding glutamatergic AMPA-receptor subunits on place preference conditioning in mice. Psychopharmacol. 2005;179:164–171. doi: 10.1007/s00213-004-2071-8. [DOI] [PubMed] [Google Scholar]
- Mead AN, Stephens DN. Selective disruption of stimulus-reward learning in glutamate receptor gria1 knock-out mice. J Neurosci. 2003;23:1041–1048. doi: 10.1523/JNEUROSCI.23-03-01041.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola SM. The nucleus accumbens as part of a basal ganglia action selection circuit. Psychopharmacol. 2007;191:521–550. doi: 10.1007/s00213-006-0510-4. [DOI] [PubMed] [Google Scholar]
- Oh MC, Derkach VA, Guire ES, Soderling TR. Extrasynaptic membrane trafficking regulated by GluR1 serine 845 phosphorylation primes AMPA receptors for long-term potentiation. J Biol Chem. 2006;281:752–758. doi: 10.1074/jbc.M509677200. [DOI] [PubMed] [Google Scholar]
- Pisetsky EM, Chao YM, Dierker LC, May AM, Striegel-Moore RH. Disordered eating and substance use in high-school students: results from the Youth Risk Behavior Surveillance System. Int J Eat Disord. 2008;41:464–70. doi: 10.1002/eat.20520. [DOI] [PubMed] [Google Scholar]
- Pothos EN, Creese I, Hoebel BG. Restricted eating with weight loss selectively decreases extracellular dopamine in the nucleus accumbens and alters dopamine response to amphetamine, morphine, and food intake. J Neurosci. 1995;15:6640–6650. doi: 10.1523/JNEUROSCI.15-10-06640.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimers JM, Milovanovic M, Wolf ME. Quantitative analysis of AMPA receptor subunit composition in addiction-related brain regions. Brain Res. 2011;1367:223–233. doi: 10.1016/j.brainres.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitman MF, Wheeler RA, Carelli RM. Nucleus accumbens neurons are innately tuned for rewarding and aversive taste stimuli, encode their predictors, and are linked to motor output. Neuron. 2005;45:587–597. doi: 10.1016/j.neuron.2004.12.055. [DOI] [PubMed] [Google Scholar]
- Shi S-H, Hayashi Y, Esteban JA, Malinow R. Subunit-specific rules governing AMPA receptor trafficking to synapses to hippocampal pyramidal neurons. Cell. 2001;105:331–343. doi: 10.1016/s0092-8674(01)00321-x. [DOI] [PubMed] [Google Scholar]
- Stice E, Davis K, Miller NP, Marti NC. Fasting increases risk for onset of binge eating and bulimic pathology: A 5-year prospective study. J Abnorm Psychol. 2008;117:941–946. doi: 10.1037/a0013644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Hnasko TS, Britt JP, Edwards RH, Bonci A. Dopaminergic terminals in the nucleus accumbens but not the dorsal striatum corelease glutamate. J Neurosci. 2010;30:8229–8233. doi: 10.1523/JNEUROSCI.1754-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taha SA, Fields HL. Inhibitions of nucleus accumbens neurons encode a gating signal for reward-directed behavior. J Neurosci. 2006;26:217–222. doi: 10.1523/JNEUROSCI.3227-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecuapetla F, Patel JC, Xenias H, English D, Tadros I, Shah F, Berlin J, Deisseroth K, Rice ME, Tepper JM, Koos T. Glutamatergic signaling by mesolimbic dopamine neurons in the nucleus accumbens. J Neurosci. 2010;30:7105–7110. doi: 10.1523/JNEUROSCI.0265-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos PK, Michaelides M, Piyis YK, Wang G-J, Volkow ND. Food restriction markedly increases dopamine D2 receptor (D2R) in a rat model of obesity as assessed with in-vivo μPET imaging ([11C] raclopride) and in-vitro ([3H] spiperone) autoradiography. Synapse. 2008;62:50–61. doi: 10.1002/syn.20468. [DOI] [PubMed] [Google Scholar]
- Tukey DA, Ferreira JM, Antoine SO, Ninan, Cabeza de Vaca S, Hartner DT, Goffer Y, Guarini CB, Marzan DE, Mahajan SS, Carr KD, Aoki C, Ziff EB. Consumption of sucrose, a natural reward, induces GluR1 trafficking and hyperactivity. 2011 (under review) [Google Scholar]
- Wang GJ, Volkow ND, Thanos PK, Fowler JS. Similarity between obesity and drug addiction as assessed by neurofunctional imaging: a concept review. J Addict Dis. 2004;23:39–53. doi: 10.1300/J069v23n03_04. [DOI] [PubMed] [Google Scholar]
- Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- Wolf ME, Ferrario CR. AMPA receptor plasticity in the nucleus accumbens after repeated exposure to cocaine. Neurosci Biobehav Rev. 2010 Jan 28; doi: 10.1016/j.neubiorev.2010.01.013. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen J, Reith MEA, Carr KD. Chronic food restriction and dopamine transporter function in rat striatum. Brain Res. 2006;1082:98–101. doi: 10.1016/j.brainres.2006.01.094. [DOI] [PubMed] [Google Scholar]


