Abstract
Acetaminophen/paracetamol-induced liver failure—which is induced by the binding of reactive metabolites to mitochondrial proteins and their disruption—is exacerbated by fasting. As fasting promotes SIRT3-mediated mitochondrial-protein deacetylation and acetaminophen metabolites bind to lysine residues, we investigated whether deacetylation predisposes mice to toxic metabolite-mediated disruption of mitochondrial proteins. We show that mitochondrial deacetylase SIRT3−/− mice are protected from acetaminophen hepatotoxicity, that mitochondrial aldehyde dehydrogenase 2 is a direct SIRT3 substrate, and that its deacetylation increases acetaminophen toxic-metabolite binding and enzyme inactivation. Thus, protein deacetylation enhances xenobiotic liver injury by modulating the binding of a toxic metabolite to mitochondrial proteins.
Keywords: SIRT3, acetaminophen liver failure, ALDH2, mitochondrial protein acetylation, N-acetyl-p-benzoquinoneimine
Introduction
The acetylation of protein lysine residues is a common post-translational modification (Kim et al, 2006; Zhao et al, 2010). In the liver, mitochondrial proteins show nutrient-level-dependent lysine acetylation and deacetylation (Kim et al, 2006; Schwer et al, 2009), and the functional consequences of this being explored. The mitochondrial-enriched deacetylase SIRT3 promotes protection against redox and nutrient-excess stressors (Yang et al, 2007; Sundaresan et al, 2008; Bao et al, 2010; Tao et al, 2010), and SIRT3-dependent reduction in redox-stress is augmented by fasting or caloric restriction (Someya et al, 2010; Tao et al, 2010).
In the light of these nutrient-restricted SIRT3 antioxidant effects, an interesting paradox exists: fasting or caloric restriction exacerbate redox-stress-dependent toxicity of the analgesic agent acetaminophen (N-acetyl-p-aminophenol or APAP; Whitcomb & Block, 1994; Fernando & Ariyananda, 2009). APAP is the most common drug-induced cause of acute liver failure (Hutson, 2010); the APAP toxic metabolite, N-acetyl-p-benzoquinoneimine (NAPQI) depletes hepatic glutathione in a dose-dependent manner (James et al, 2003; Jaeschke & Bajt, 2006). Unconjugated NAPQI then binds to cysteine residues causing oxidative stress, mitochondrial disruption and hepatotoxicity (Ruepp et al, 2002; Kon et al, 2004). Although there is N-acetylcysteine therapy, mortality from APAP-induced liver failure is not uncommon (Lai et al, 2006) and alternative therapies are being investigated (North et al, 2010). Although NAPQI also binds to hepatic-protein lysine residues (Zhou et al, 1996), the functional significance of this interaction is unknown. As lysine acetylation might partly function by competing with alternative post-translational modifications, _targeting the same residue (Yang & Seto, 2008), the paradox regarding fasting and redox stress in the context of acetaminophen-induced liver injury (AILI) might be due, in part, to protein lysine residue deacetylation-mediated susceptibility to NAPQI binding.
We hypothesized that if protein acetylation allosterically inhibits NAPQI binding, SIRT3-mediated deacetylation might exacerbate hepatotoxicity, despite the protective role of SIRT3 against redox-stress.
Results
The absence of SIRT3 protects against AILI
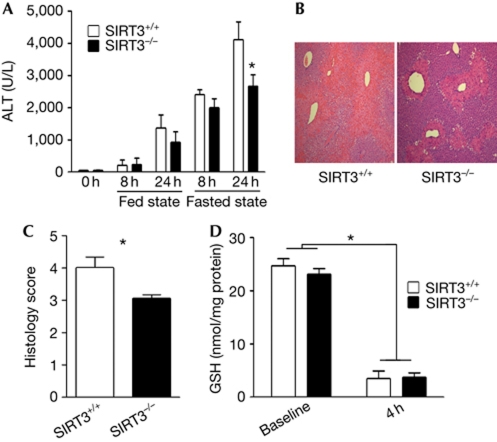
We administered a toxic dose of APAP to SIRT3+/+ and SIRT3−/− mice in fed and fasted conditions. Baseline serum alanine transaminase (ALT) levels were indistinguishable between genotypes, and similar ALT release was seen in fed mice, irrespective of their genotype (Fig 1A). As expected, fasting increased susceptibility to AILI (Fig 1A). However, in contrast to the fed mice, the fasted SIRT3−/− mice showed less hepatotoxicity, as shown by ALT release and less centrilobular coagulative necrosis—compared with the SIRT3 competent mice—in response to APAP administration (Fig 1A–C). This difference in fasting hepatotoxic susceptibility between the SIRT3+/+ and SIRT3−/− mice is not because of the differential rate of depletion of glutathione, as the levels of glutathione are similar following the overnight fast in the two genotypes, and are similarly depleted 4 h after APAP administration (Fig 1D).
Figure 1.
SIRT3−/− mice are less susceptible to acetaminophen-induced liver injury than SIRT3+/+ mice. (A) Fed and overnight-fasted mice received a bolus of N-acetyl-p-aminophenol (APAP), and serum alanine transaminase (ALT) activity was measured at baseline, 8 and 24 h (P<0.05, n⩾7 mice per group). (B) Liver histology with haematoxylin and eosin stain was evaluated at 24 h after APAP in fasted mice. Five mice were analysed per group and a representative result from each group is shown. (C) Blinded quantitation of the degree of necrosis; higher scores reflect increased injury. The scoring system is described in the supplementary information online. (D) Reduced glutathione (GSH) levels 4 h after the administration of either saline or APAP in mice that had been fasted overnight (n=6 per group). Asterisk indicates a P value <0.02, compared with the respective controls.
Identification of putative _targets for SIRT3-mediated AILI
To identify new mitochondrial-protein acetylation _targets that might orchestrate the protective effects against AILI, we screened for candidate proteins that could potentially mediate this effect, by comparing hepatic mitochondrial-protein acetylation profiles between fasted SIRT3−/− and SIRT3+/+ mice. Two-dimensional gel and immunoblot analysis with an antibody recognizing acetylated lysine were used. Differentially acetylated spots were isolated, and sequenced by tandem mass spectrometry (MS/MS). We identified 17 proteins that reproducibly showed increased lysine acetylation in the SIRT3−/− compared with SIRT3+/+ mice (supplementary Table S1 online; supplementary Fig S1 online).
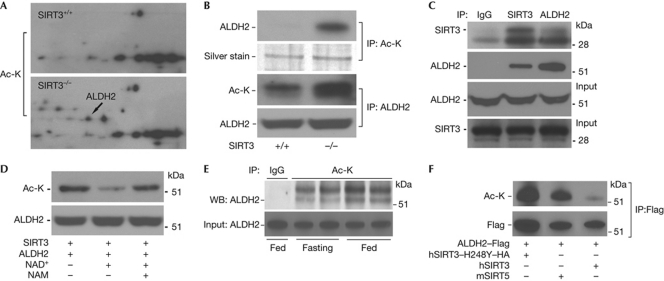
We selected aldehyde dehydrogenase 2 (ALDH2) as the index protein, because ALDH2 is a known _target of NAPQI (Landin et al, 1996; Qiu et al, 1998), NAPQI binding is associated with reduced ALDH2 activity (Landin et al, 1996) and this dehydrogenase functions to oxidize and detoxify aldehydes, including lipid peroxidation products such as trans-4-hydroxy-2-nonenal (4-HNE; Doorn et al, 2006). Increased acetylation of ALDH2 in SIRT3−/− liver mitochondria was evident (Fig 2A). To confirm that ALDH2 was differentially acetylated, immunoprecipitation studies were performed using liver mitochondrial-protein lysates. Immunoprecipitation with antibodies against ALDH2 and acetylated-lysine residues with reciprocal immunoblot analysis showed that ALDH2 was more-robustly acetylated in SIRT3−/− mice (Fig 2B). This change in acetylation was associated with a direct interaction between SIRT3 and ALDH2 by bidirectional immunoprecipitation and immunoblot analysis using antibodies against SIRT3 and ALDH2 (lysates from SIRT+/+ mice; Fig 2C). We also demonstrate in vitro deacetylation of ALDH2 in the presence of SIRT3 and NAD+ and the preservation of acetylation in the presence of the sirtuin deacetylase inhibitor nicotinamide (Fig 2D). Furthermore, we confirmed that fasting increased hepatic ALDH2 deacetylation in SIRT3-competent mice (Fig 2E), and that the SIRT5 mitochondrial deacetylase does not _target ALDH2 for deacetylation (Fig 2F).
Figure 2.
ALDH2 is a functional _target of SIRT3. (A) Liver mitochondrial proteins isolated from SIRT3+/+ and SIRT3−/− mice were separated by IEF/SDS two-dimensional gel electrophoresis and probed with acetylated-lysine (Ac-K) antibody. The experiment was repeated three times and representative blots are shown. (B) Liver mitochondrial proteins from SIRT3+/+ and SIRT3−/− mice immunoprecipitated with antibodies against Ac-K and ALDH2, with inverse immunoblot analysis to assess the degree of ALDH2 acetylation relative to the levels of hepatic SIRT3. Silver staining of the gel showed similar input of Ac-K immunoprecipitation samples. The ALDH2 immunoblot analysis showed equal input of the samples precipitated by ALDH2 for Ac-K immunoblot analysis. The experiment was performed three times and representative blots from a single experiment are shown. (C) Co-immunoprecipitation with antibodies against SIRT3 and ALDH2, with dual antibody immunoblot analysis to examine the interaction between SIRT3 and ALDH2 in protein extracted from wild-type mouse liver. The experiment was performed three times and representative blots from a single experiment are shown. (D) Mitochondrial protein was isolated from wild-type mouse liver. SIRT3 and ALDH2 proteins were immunoprecipitated with SIRT3 and ALDH2 antibodies and incubated in an in vitro deacetylation buffer in the presence or absence of NAD+ and/or NAM. The reaction products were probed with Ac-K antibody to determine the levels of ALDH2 acetylation in response to conditions facilitating or inhibiting protein deacetylation. The experiment was performed three times and representative blots from a single experiment are shown. (E) Level of acetylation of liver ALDH2 in the fed and fasted state in SIRT3+/+ mice. The Ac-K antibody was used to immunoprecipitate mitochondrial protein, and immunoblot analysis was performed with an antibody _targeting ALDH2. The band immediately above the 51 kDa marker is ALDH2. Four mice per group were analysed and a representative blot showing two animals from each group is shown. (F) Following co-transfection of Flag-tagged ALDH2 and the listed sirtuin deacetylases, the exogenous ALDH2 was immunoprecipitated using a Flag antibody. Precipitant was run on gel and immunoblotted for the Flag-tag and for Ac-K, to demonstrate the extent of ALDH2 deacetylation. (A–D,F) Experiment were performed three times and representative blots are shown. ALDH2, aldehyde dehydrogenase 2; IEF, isoelectric focusing; IgG, immunoglobulin G; IP, immunoprecipitation; NAD, nicotinamide adenine dinucleotide; NAM, nicotinamide; WB, western blot.
ALDH2 activity in SIRT3−/− mice after APAP administration
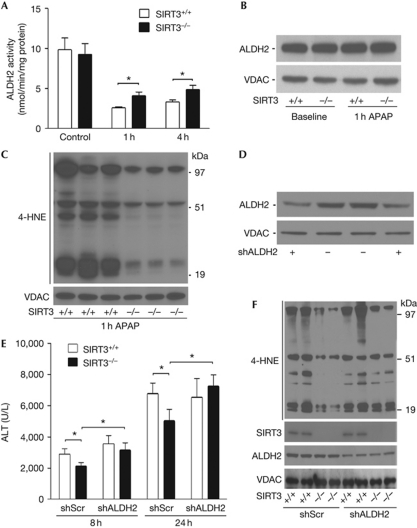
As baseline ALDH2 activity was indistinguishable between genotypes (Fig 3A), we evaluated whether functional effects of ALDH2 acetylation could be revealed by measuring enzyme activity and consequences of enzymatic action in response to APAP toxicity in fasted mice. A reduction in mitochondrial ALDH2 activity at 1 and 4 h after APAP administration was evident in both genotypes, although the relative activity was sustained at approximately 40% higher levels in SIRT3−/− mitochondria at both of these time points (Fig 3A). This difference in activity was probably not due to changes in protein degradation, as steady-state ALDH2 levels were not diminished in response to APAP (Fig 3B). To evaluate the functional consequences of altered ALDH2 activity, we measured levels of lipid peroxidation adduct-binding to mitochondrial proteins. The baseline levels of 4-HNE in liver mitochondria were similar (supplementary Fig S2 online). By contrast, 1 h after APAP exposure, the SIRT3−/− mitochondrial proteins showed lower 4-HNE-adduct levels compared with SIRT3+/+ mice (Fig 3C). As disruption of mitochondrial respiration is an early indicator of APAP-induced hepatotoxicity (Donnelly et al, 1994), we quantified mitochondrial respiration in response to APAP and showed that oxidative metabolism was preserved to a greater extent in the SIRT3−/− compared with SIRT3+/+ mice (supplementary Fig S3 online).
Figure 3.
ALDH2 function is relatively preserved in fasted SIRT3−/− mice in response to APAP, and it is required to reduce acetaminophen-induced liver injury in SIRT3−/− mice. (A) Mitochondrial ALDH2 activity was measured by the change in absorbance at 340 nm, in hepatic mitochondrial protein extracted at baseline, 1 and 4 h after APAP administration in SIRT3+/+ and SIRT3−/− mice (n=6 per group). (B) Relative protein levels of hepatic ALDH2 were evaluated by immunoblot analysis at baseline and 1 h after APAP administration comparing SIRT3+/+ and SIRT3−/− mice. Liver tissue from four mice from each genotype was analysed at the two time points and a representative blot is shown. (C) 4-HNE adducts bound to liver mitochondrial protein 1 h after the administration of APAP. Liver tissue from six mice in each group were analysed and a representative blot showing three samples from each genotype are shown. (D) Immunoblot analysis of liver tissue showing ALDH2 levels following infection with shRNA _targeted against ALDH2 compared with scrambled (Scr) control shRNA. Four mice from each group were analysed and two representative samples of each shRNA knockdown are shown. (E) Five days after administration of the scrambled compared with ALDH2 shRNA lentiviral particles, mice were treated with APAP and ALT levels were measured at 8 and 24 h, respectively (n>10 per group). (F) 4-HNE adducts bound to liver mitochondrial protein 1 h after the administration of APAP in mice following knockdown of ALDH2. The re-emergence of 4-HNE adducts in the SIRT3−/− mice following ALDH2 knockdown are highlighted by the arrowheads on the right side of the blot. Four mice from each group were analysed and two representative samples of each shRNA knockdown are shown. Asterisk indicates a P value <0.03, compared with the respective controls. ALDH2, aldehyde dehydrogenase 2; ALT, serum alanine transaminase; APAP, N-acetyl-p-aminophenol; shRNA, short-hairpin RNA; VDAC, voltage-dependent anion channel; 4-HNE, trans-4-hydroxy-2-nonenal.
The depletion of ALDH2 counters AILI resistance
The functional requirement of ALDH2 activity in resisting APAP toxicity in SIRT3−/− mice was then interrogated using lentiviral short-hairpin RNA (shRNA). Different shRNA viral particles _targeting ALDH2 or scrambled controls were tested in murine liver cells and construct sh5–ALDH2 showed the most robust knockdown (supplementary Fig S4A online). Five days after tail-vein injection with this shALDH2 and scrambled control lentiviral particles, liver extracts showed an approximately 50% reduction in ALDH2 protein levels compared with scrambled shRNA-infected mice (Fig 3D). APAP toxicity studies were performed in control and ALDH2 shRNA-_targeted SIRT3+/+ and SIRT3−/− mice. The introduction of lentiviral particles increased liver injury in both strains compared with toxicity in the absence of virus (compare Fig 3E with Fig 1A). The specific knockdown of ALDH2 in SIRT3−/− mice increased liver injury in response to APAP, with greater elevations in ALT levels at 8 and 24 h (Fig 3E). AILI susceptibility was not, however, changed in SIRT3+/+ mice (Fig 3E), indicating that the disruption of ALDH2 function by AILI in wild-type mice cannot be perturbed further by the shRNA depletion of ALDH2. The enhanced liver injury following ALDH2 knockdown in the SIRT3−/− mice was reflected by increased levels of 4-HNE adducts binding to mitochondrial proteins (Fig 3F) and by exacerbation of centrilobular necrosis (supplementary Fig S4B online).
ALDH2 acetylation modulates NAPQI binding
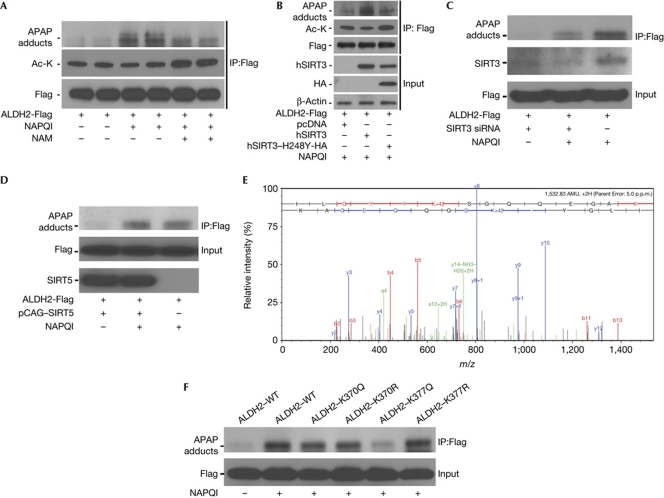
The possibility that a reduction in ALDH2 deacetylation could maintain enzymatic activity in response to toxic doses of acetaminophen has not been previously recognized. As binding of the APAP reactive metabolite, NAPQI, to ALDH2 has previously been associated with lower ALDH2 activity (Landin et al, 1996), we investigated whether inhibition of sirtuin activity could modulate NAPQI binding. Exogenous ALDH2 was expressed in murine liver cells and exposed to NAPQI in the presence or absence of the sirtuin inhibitor nicotinamide. The amount of NAPQI bound to ALDH2 was reduced by inhibition of sirtuin deacetylase functioning, with a concomitant increase in ALDH2 protein acetylation (Fig 4A). In parallel, binding of NAPQI to ALDH2 was increased after overexpression of deacetylase-competent SIRT3, and attenuated in the presence of the deacetylase-mutant SIRT3, with reduced ALDH2 acetylation correlating to the degree of NAPQI binding (Fig 4B). In parallel, the small-interfering RNA knockdown of SIRT3 in these hepatocytes diminished NAPQI binding to ALDH2 (Fig 4C) and the overexpression of SIRT5 did not alter NAPQI binding to ALDH2 (Fig 4D).
Figure 4.
The retention of ALDH2 acetylation after SIRT3 inactivation reduces NAPQI adduct binding to ALDH2. (A) Hepa-1c1c7 cells were transfected with pCMV6-Flag control vector or pCMV6–ALDH2-Flag. After 48 h, NAPQI (200 μM) and either nicotinamide (5 mM) or vehicle were administered. After 1 h incubation, the Flag-tagged ALDH2 construct was extracted by immunoprecipitation. The immunoprecipitate was run on SDS–polyacrylamide gel electrophoresis gel and immunoblot analysis was performed with antibodies against APAP adducts, acetylated lysine and the Flag-tag. This experiment was repeated three times in duplicate, and a representative experiment is shown. (B) Similar studies were performed with the additional introduction of either wild-type or deacetylase-mutant SIRT3 construct. This experiment was repeated three times and a representative experiment is shown. (C) Levels of NAPQI binding following SIRT3 depletion by siRNA. This experiment was repeated three times and a representative experiment is shown. (D) The overexpression of SIRT5 does not modify NAPQI adduct binding. Duplicate sample experiments were repeated three times to confirm the reproducibility of interactions. This experiment was repeated three times and a representative experiment is shown. (E) ALDH2 K377 containing peptide ILGYIKSGQQEGAK (precursor m/z 767.4210, 5 p.p.m. mass error) was detected to be acetylated in SIRT3−/− mice mitochondria by liquid chromatography MS/MS after acetyl-lysine antibody enrichment. Shifts in fragment masses confirmed that K377 was the acetylation site within the peptide. Using a label-free quantitation approach, the acetylation level of K377 was shown to increase 2.5-fold in SIRT3−/− mice, compared with SIRT3+/+ mice. This experiment was repeated twice and a representative spectrum of the peptide containing K377 is shown. (F) Mutagenesis studies showing that the substitution of basic lysine (K) with a non-polar glutamine (Q) to mimic acetylation diminishes NAPQI binding when substituted on K377, but not on K370. The arginine (R) substitution that mimics the deacetylated state does not alter NAPQI binding. This experiment was repeated three times and a representative experiment is shown. To prevent the luminescent emission from the secondary antibody binding to the IgG heavy chain from overwhelming the signal of the APAP adducts binding to ALDH2 (>52 kDa), the IgG signal was blocked below approximately 50 kDa. The residual signal at the bottom of the adduct gels in panels A–D and F reflect this intervention. ALDH2, aldehyde dehydrogenase 2; APAP, N-acetyl-p-aminophenol; HA, haemagglutinin; IgG, immunoglobulin G; IP, immunoprecipitation; MS, mass spectrometry; NAM, nicotinamide; NAPQI, N-acetyl-p-benzoquinoneimine; siRNA, small-interfering RNA; WT, wild type.
Deacetylation of ALDH2 Lys377 enhances NAPQI binding
To confirm that the acetyl status of lysine residues modifies NAPQI binding to ALDH2, we identified ALDH2 lysine residues that were deacetylated by SIRT3. Liquid chromatography-MS/MS sequencing of acetylated lysine antibody immuno-captured trypsin-digested peptides (Kim et al, 2006) from SIRT3+/+ and SIRT3−/− mice mitochondria identified Lys370 and Lys377 as acetylation sites that might be operational in this allosteric modification of NAPQI binding (Fig 4E). Mutational studies were performed to mimic deacetylation (Lys to Arg) and acetylation (Lys to Gln), and showed that the ALDH2 Lys377 is a principal lysine residue modifying NAPQI binding (Fig 4F).
Discussion
The predominant approach to ameliorate toxic effects of AILI has focused on antioxidant pathways on the basis of NAPQI binding to cysteine residues (Nelson, 1990; Qiu et al, 1998). Even though NAPQI binds to lysine residues (Zhou et al, 1996), the functional consequences of this interaction have, to our knowledge, not been explored. This study investigates whether occupancy of lysine by acetyl groups modifies NAPQI binding, and assesses whether lysine acetylation ameliorates APAP hepatotoxic pathophysiology. Our findings support the hypothesis that maintenance of lysine acetylation attenuates competing metabolites _targeting the same residue (Yang & Seto, 2008), and advances this to include retardation of NAPQI binding.
The temporal sequence of APAP-induced toxicity is well established; adduct binding and glutathione depletion occur within the first few hours, followed by the release of liver enzymes, and cell death over the next 8–48 h (Roberts et al, 1991; Hinson et al, 2004). The role of fasting-induced SIRT3 lysine residue deacetylation in increasing NAPQI binding to ALDH2 in the early phase of hepatotoxicity after an acute toxic dose of APAP is shown in this study. The consequences of this toxicity are illustrated by the exacerbation of liver injury 24 h later in wild-type compared with SIRT3-null mice.
These data do not dismiss the role of glutathione depletion in APAP toxicity, as evidenced by greater injury in both genotypes when comparing fasted with fed mice. Instead this study expands our understanding of the pathophysiology of APAP toxicity by uncovering a new functional role for ALDH2 in attenuating acetaminophen hepatotoxicity, and identifying the role of acetylation in altering NAPQI binding to mitochondrial proteins. Also, although the knockdown of ALDH2 nullifies the ameliorative effects of SIRT3 deficiency in this pathophysiology, our data cannot exclude the role of the other SIRT3 mitochondrial _targets identified in this (supplementary Table S1 online) and other studies. Characterization of these additional SIRT3 _targets in this toxicity warrants further investigation. Interestingly, reactive metabolites of halothane, which are also hepatotoxic, similarly bind to lysine residues (Zhou et al, 1996; Bourdi et al, 2001). Whether the concept of toxic metabolite binding to lysine has broader implications in xenobiotic-mediated hepatic injury should also be explored.
Finally, this study has not only identified ALDH2 as a new SIRT3 _target, but also shows that the allosteric binding of the acetaminophen toxic metabolite to ALDH2 is controlled by the acetylation status of this dehydrogenase. This nutrient-dependent function of SIRT3 identifies a unique mediator of stress-susceptibility in response to a xenobiotic metabolite, that is distinct from the antioxidant effect of SIRT3, previously shown in the activation of manganese superoxide dismutase by deacetylation (Tao et al, 2010).
In conclusion, the SIRT3-dependent acetylation status of mitochondrial proteins modulates susceptibility to AILI. Mitochondrial ALDH2 is identified as a new functional substrate of SIRT3 deacetylation that has an important detoxifying role in modulating APAP toxicity, and the acetylation of ALDH2 at position Lys377 modifies the amount of NAPQI binding. The manipulation of mitochondrial protein acetylation is a new concept to investigate in the search for strategies to reduce acetaminophen hepatotoxicity.
Methods
Detailed experimental procedures are described in the supplementary information online.
Animal studies. The inbred C57BL/6 SIRT3+/− mice were studied and a heterozygous breeding scheme was used. APAP was administered in a single toxic dose (350 mg/kg, by intraperitoneal injection) to either fed or overnight-fasted mice. All animal experiments were approved by the NHLBI Animal Care and Use Committee.
Lentiviral shRNA preparation and mice injection. Lentiviruses were produced encoding a non-_targeting control sequence or constructs encoding mouse ALDH2 shRNA sequences. Mice were subjected to tail-vein injections with control or shRNA lentivirus (1.2 × 1011 particles). ALDH2 knockdown was confirmed and APAP was subsequently administered to fasting mice. ALT measurement and liver histology were then analysed.
NAPQI-binding studies. pCMV6–ALDH2–Flag plasmid was transfected into Hepa-1c1c7 cells. To evaluate the role of SIRT3 deacetylase, the wild type (hSIRT3–Flag), the deacetylase catalytically inactive constructs (hSIRT3–H248Y–HA) or mSIRT5–3xFlag were overexpressed. Cells were then treated with NAPQI (200 μM) and nicotinamide (5 mM) or vehicle control. Immunoprecipitation using a Flag antibody to extract the ALDH2 was performed and run on SDS–polyacrylamide gel electrophoresis gels. APAP metabolite binding to ALDH2 was detected by immunoblot analysis with an antibody to NAPQI protein adducts.
Statistical analysis. Differences between data groups were evaluated for significance using the two-tailed Student's t-test. Multiple comparison analysis was performed using analysis of variance. Statistical analysis was performed using Graphpad Prism, and data are expressed as mean±s.e.m. P<0.05 is considered to be statistically significant.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
We thank F.W. Alt and H.-L. Cheng from Harvard Medical School, USA, for their generous gift of the C57BL/6 strain of SIRT3+/− mice. We thank T. Finkel (NHLBI) and B.J. Song (National Institute of Alcohol Abuse and Alcoholism) for helpful discussions, and the NHLBI Laboratory of Animal Medicine and Surgery core facility for assistance with murine tail-vein injection of lentivirus shRNA. This research was funded by NHLBI Division of Intramural Research.
Author contributions: Z.L., M.B., M.G., L.R.P. and M.N.S. designed the study. Z.L., M.B., J.H.L., A.M.A. and Y.C. performed the experiments. D.B.L. contributed key reagents. Z.L., M.B., A.M.A. and Y.C. analysed the data. Z.L., M.B., D.B.L., M.G., L.R.P. and M.N.S. wrote the manuscript.
Footnotes
The authors declare that they have no conflict of interest.
References
- Bao J, Scott I, Lu Z, Pang L, Dimond CC, Gius D, Sack MN (2010) SIRT3 is regulated by nutrient excess and modulates hepatic susceptibility to lipotoxicity. Free Radic Biol Med 49: 1230–1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdi M, Amouzadeh HR, Rushmore TH, Martin JL, Pohl LR (2001) Halothane-induced liver injury in outbred guinea pigs: role of trifluoroacetylated protein adducts in animal susceptibility. Chem Res Toxicol 14: 362–370 [DOI] [PubMed] [Google Scholar]
- Donnelly PJ, Walker RM, Racz WJ (1994) Inhibition of mitochondrial respiration in vivo is an early event in acetaminophen-induced hepatotoxicity. Arch Toxicol 68: 110–118 [DOI] [PubMed] [Google Scholar]
- Doorn JA, Hurley TD, Petersen DR (2006) Inhibition of human mitochondrial aldehyde dehydrogenase by 4-hydroxynon-2-enal and 4-oxonon-2-enal. Chem Res Toxicol 19: 102–110 [DOI] [PubMed] [Google Scholar]
- Fernando WK, Ariyananda PL (2009) Paracetamol poisoning below toxic level causing liver damage in a fasting adult. Ceylon Med J 54: 16–17 [DOI] [PubMed] [Google Scholar]
- Hinson JA, Reid AB, McCullough SS, James LP (2004) Acetaminophen-induced hepatotoxicity: role of metabolic activation, reactive oxygen/nitrogen species, and mitochondrial permeability transition. Drug Metab Rev 36: 805–822 [DOI] [PubMed] [Google Scholar]
- Hutson S (2010) Painkiller concerns grow ahead of new guidelines. Nat Med 16: 10. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Bajt ML (2006) Intracellular signaling mechanisms of acetaminophen-induced liver cell death. Toxicol Sci 89: 31–41 [DOI] [PubMed] [Google Scholar]
- James LP, Mayeux PR, Hinson JA (2003) Acetaminophen-induced hepatotoxicity. Drug Metab Dispos 31: 1499–1506 [DOI] [PubMed] [Google Scholar]
- Kim SC et al. (2006) Substrate and functional diversity of lysine acetylation revealed by a proteomics survey. Mol Cell 23: 607–618 [DOI] [PubMed] [Google Scholar]
- Kon K, Kim JS, Jaeschke H, Lemasters JJ (2004) Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology 40: 1170–1179 [DOI] [PubMed] [Google Scholar]
- Lai MW, Klein-Schwartz W, Rodgers GC, Abrams JY, Haber DA, Bronstein AC, Wruk KM (2006) 2005 Annual Report of the American Association of Poison Control Centers’ national poisoning and exposure database. Clin Toxicol (Phila) 44: 803–932 [DOI] [PubMed] [Google Scholar]
- Landin JS, Cohen SD, Khairallah EA (1996) Identification of a 54-kDa mitochondrial acetaminophen-binding protein as aldehyde dehydrogenase. Toxicol Appl Pharmacol 141: 299–307 [DOI] [PubMed] [Google Scholar]
- Nelson SD (1990) Molecular mechanisms of the hepatotoxicity caused by acetaminophen. Semin Liver Dis 10: 267–278 [DOI] [PubMed] [Google Scholar]
- North TE, Babu IR, Vedder LM, Lord AM, Wishnok JS, Tannenbaum SR, Zon LI, Goessling W (2010) PGE2-regulated wnt signaling and N-acetylcysteine are synergistically hepatoprotective in zebrafish acetaminophen injury. Proc Natl Acad Sci USA 107: 17315–17320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y, Benet LZ, Burlingame AL (1998) Identification of the hepatic protein _targets of reactive metabolites of acetaminophen in vivo in mice using two-dimensional gel electrophoresis and mass spectrometry. J Biol Chem 273: 17940–17953 [DOI] [PubMed] [Google Scholar]
- Roberts DW, Bucci TJ, Benson RW, Warbritton AR, McRae TA, Pumford NR, Hinson JA (1991) Immunohistochemical localization and quantification of the 3-(cystein-S-yl)-acetaminophen protein adduct in acetaminophen hepatotoxicity. Am J Pathol 138: 359–371 [PMC free article] [PubMed] [Google Scholar]
- Ruepp SU, Tonge RP, Shaw J, Wallis N, Pognan F (2002) Genomics and proteomics analysis of acetaminophen toxicity in mouse liver. Toxicol Sci 65: 135–150 [DOI] [PubMed] [Google Scholar]
- Schwer B, Eckersdorff M, Li Y, Silva JC, Fermin D, Kurtev MV, Giallourakis C, Comb MJ, Alt FW, Lombard DB (2009) Calorie restriction alters mitochondrial protein acetylation. Aging Cell 8: 604–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Someya S, Yu W, Hallows WC, Xu J, Vann JM, Leeuwenburgh C, Tanokura M, Denu JM, Prolla TA (2010) Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell 143: 802–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaresan NR, Samant SA, Pillai VB, Rajamohan SB, Gupta MP (2008) SIRT3 is a stress-responsive deacetylase in cardiomyocytes that protects cells from stress-mediated cell death by deacetylation of Ku70. Mol Cell Biol 28: 6384–6401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao R et al. (2010) Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol Cell 40: 893–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitcomb DC, Block GD (1994) Association of acetaminophen hepatotoxicity with fasting and ethanol use. JAMA 272: 1845–1850 [DOI] [PubMed] [Google Scholar]
- Yang H et al. (2007) Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival. Cell 130: 1095–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XJ, Seto E (2008) Lysine acetylation: codified crosstalk with other posttranslational modifications. Mol Cell 31: 449–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S et al. (2010) Regulation of cellular metabolism by protein lysine acetylation. Science 327: 1000–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, McKenzie BA, Eccleston ED Jr, Srivastava SP, Chen N, Erickson RR, Holtzman JL (1996) The covalent binding of [14C]acetaminophen to mouse hepatic microsomal proteins: the specific binding to calreticulin and the two forms of the thiol:protein disulfide oxidoreductases. Chem Res Toxicol 9: 1176–1182 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.