Abstract
The consequence of chronic protein misfolding is the basis of many human diseases. To combat the deleterious effects of accumulated protein damage, all cells possess robust quality-control systems, specifically molecular chaperones and clearance machineries, that sense and respond to protein misfolding. However, for many protein conformational diseases, it is unclear why this quality-control system does not efficiently counter protein aggregation. Previous findings that the heat shock response in Caenorhabditis elegans is regulated by thermosensory neurons led us to consider whether neuronal activity could also be responsible for the inadequate response of an organism to chronic protein misfolding. Here we show, in animals expressing polyglutamine expansion proteins and mutant SOD-1G93A in intestinal or muscle cells, that the nervous system does indeed control the cellular response to misfolded proteins. Whereas polyglutamine expansion-expressing animals with WT thermosensory neurons readily express protein aggregates, leading to cellular dysfunction without concomitant up-regulation of molecular chaperones, modulation of the nervous system results in chaperone up-regulation that suppresses aggregation and toxicity. The neuronal signal is transmitted through calcium-activated dense core vesicle neurosecretion. Cell-nonautonomous control of chaperone expression by the thermosensory neurons allows C. elegans to respond differently to acute stress such as heat shock, and chronic stress caused by the expression of misfolded proteins, suggesting that neuronal signaling determines the course of cellular proteotoxicity.
Keywords: neuronal control, proteostasis, stress response, Hsp70, small heat shock proteins
The inability to maintain protein quality control has detrimental effects on cell physiology and function, and is the basis of diseases of protein conformation, including Huntington disease, Parkinson disease, Alzheimer's disease, cancer, and type II diabetes, in which cells accumulate misfolded and aggregated proteins (1, 2). To inhibit aggregation and toxicity, cells and organisms have evolved multiple stress responsive networks that detect and respond to the accumulation of damaged proteins (3–8). Central to these protective measures is the heat shock (HS) response (HSR) and HS transcription factor HSF-1. HSF-1 regulates the inducible expression of HS proteins (HSPs), many of which are molecular chaperones that protect the proteome by enhancing folding, suppressing misfolding, and _targeting damaged proteins for degradation (8). The current hypothesis for the induction of chaperones is that elevated levels of misfolded and damaged proteins caused by environmental insults or errors in protein biogenesis titrate the molecular chaperones away from HSF-1 and toward association with misfolded proteins, resulting in the derepression and activation of HSF-1 (9, 10).
Despite the evidence that protein misfolding triggers HSF-1 (9, 10), the up-regulation of chaperones and activation of HSF-1 is infrequently observed in animal models of protein aggregation and tissues from affected humans (11–16). Instead, the protein quality control system appears to be impaired as a result of the titration of chaperones, disruption of proteasomal activity (17–19), modification of HSF-1 and its regulators, or sequestration of important cofactors by aggregation-prone proteins (17–21). However, disruption of proteostasis by other means, such as genetic knockdown or treatment with small-molecule inhibitors of proteasome or chaperone function, activates HSF-1, increasing the levels of protective HSPs and restoring proteostasis (3, 22). Similarly, the purposeful overexpression of specific chaperones or the expression of constitutively active HSF-1 can inhibit protein aggregation and toxicity in multiple cell and animal models (23–28). Therefore, the absence of a consistent HSR in diseases of protein conformation is unexpected.
Previous studies in the metazoan Caenorhabditis elegans have shown that the HSR is regulated by the AFD thermosensory neurons and AIY interneurons (29). Neuronal control provides a means by which the acute response to stress is under organismal control. In this study, we examine whether neuronal activity also regulates the cellular response to chronic expression of misfolded proteins.
Results
Thermosensory Neuronal Mutants Suppress Aggregation of Polyglutamine Expansion Proteins Expressed in Intestinal Cells of C. elegans.
The role of neuronal signaling on chronic protein misfolding in C. elegans was examined by using the gcy-8 (30) and ttx-3 (31) loss-of-function mutations that specifically affect the AFD thermosensory neurons and AIY interneurons, respectively. GCY-8 is a guanylyl cyclase expressed only in the two AFD neurons (30), and TTX-3 is an LIM-homeobox transcription factor expressed in the AIY interneurons (31). We monitored misfolding by using the in vivo folding reporter Q44::YFP, which consists of 44 glutamine residues fused to YFP under the control of the intestine-specific vha-6 promoter (32). Q44::YFP has been shown to aggregate in an age-dependent manner, impairing cellular function (32). Our previous data that gcy-8 and ttx-3 mutants are deficient in the HSR (29) led us to expect that mutations in the thermosensory circuitry would exacerbate polyglutamine expansion (polyQ) protein aggregation.
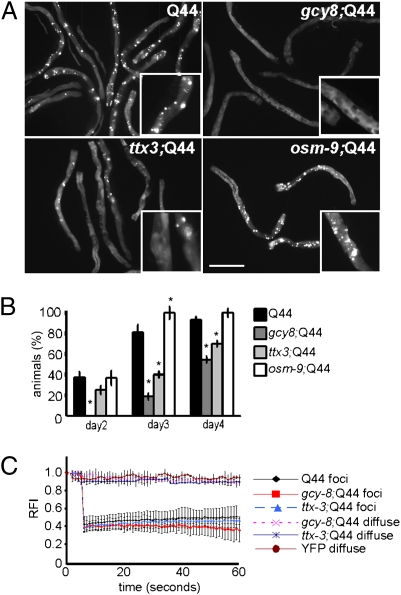
Instead, expression of Q44 in the background of gcy-8 or ttx-3 mutations led to a reduction in the onset and extent of polyQ aggregation in intestinal cells (Fig. 1 A–C and Fig. S1A). Whereas 40% of adult day 2 animals expressing Q44::YFP accumulate immobile polyQ aggregates (Materials and Methods), none of the gcy-8; Q44 animals, and only 25% of the ttx-3; Q44 animals of comparable age, had aggregates (Fig. 1B). This suppression of aggregation persisted on day 3 of adulthood with less than half the gcy-8; Q44 and ttx-3; Q44 animals with aggregates, and the remaining animals containing fewer than 20 aggregates (Fig. 1 A and B and Fig. S1A), compared with 80% of WT Q44 animals with more than 20 aggregates (Fig. 1 A and B and Fig. S1A). The reduction in polyQ aggregation in the gcy-8 and ttx-3 animals was not to caused by decreased expression of polyQ mRNA and protein levels relative to WT animals (Fig. S1 B and C). Fluorescence recovery after photobleaching (FRAP) was used to demonstrate that the aggregates, in all animals, were immobile and not exchangeable with the surrounding diffusible polyQ::YFP (Fig. 1 B and C). In the thermosensory mutant animals that expressed fewer polyQ aggregates, the diffuse polyQ protein in intestinal cells of gcy-8; Q44 and ttx-3; Q44 animals was soluble, with properties similar to YFP alone (Fig. 1C). Thus, disruption of thermosensory neuronal activity suppressed aggregation and increased soluble polyQ in intestinal cells. This effect was not a consequence of general inhibition of sensory neuronal activity, as disruption of the chemosensory neurons ASH, ADL, and ASE in the osm-9 mutation (33) (Fig. 1 A and B and Fig. S1A) or ocr-2 mutation (33) (Fig. S2 A–C) did not affect polyQ aggregation.
Fig. 1.
Thermosensory neurons regulate polyQ aggregation in C. elegans intestine. (A) Q44 aggregation in the intestine of day 3 adult animals with WT thermosensory neurons (Q44), gcy-8 mutation (gcy-8;Q44), ttx-3 mutation (ttx-3;Q44), and osm-9 mutation (osm-9;Q44). (Scale bar: 100 μm.) Inset: Representative areas chosen for photobleaching. (B) Percentage of animals with visible aggregates in Q44, gcy-8;Q44, ttx-3;Q44, and osm-9;Q44 animals on days 2 to 4 of adulthood. Error bars indicate SE (*P < 0.01). (C) FRAP measurements on 10 fluorescent aggregates (foci) and 10 to 20 regions that did not contain aggregates (diffuse) in WT Q44, gcy-8;Q44, and ttx-3;Q44 day 3 adult animals. YFP alone used as a control. Error bars indicate SD.
Thermosensory Neuronal Mutants Suppress Aggregation and Toxicity of Multiple Folding Reporters in C. elegans.
These results led us to ask whether the beneficial effects of thermosensory mutations on polyQ aggregation was limited to the intestinal cells or extended to metastable proteins expressed in other tissues of C. elegans. To address this, we examined the effect of the gcy-8 and ttx-3 mutations on three additional models for protein misfolding corresponding to polyQ aggregation in muscle cells (14, 34), expression of ALS-associated mutant SOD-1G93A (35), and an endogenous metastable temperature-sensitive isoform of paramyosin (36).
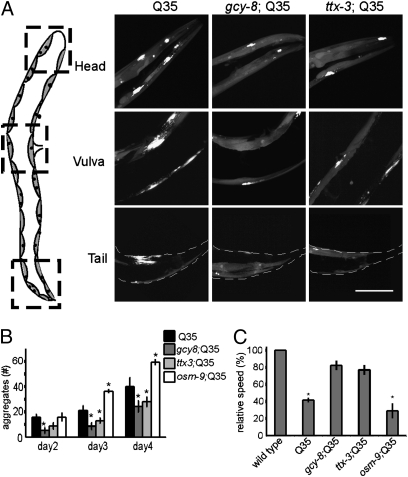
For each protein, we observed that gcy-8 and ttx-3 mutations suppressed misfolding and associated toxicity (Figs. 2 and 3 and Fig. S3). Q35::YFP expressed in body wall muscle cells exhibits age-dependent aggregation (Fig. 2 A and B), which was markedly suppressed in gcy-8 and ttx-3 animals, as determined by morphological criteria (Fig. 2 A and B) and FRAP analysis (Fig. S3A). Suppression of polyQ aggregation in the gcy-8 and ttx-3 backgrounds was not caused by decreased expression of Q35 mRNA or protein (Fig. S3 B and C) and was specific to disruption of the thermosensory circuitry, as RNAi-mediated down-regulation of other gene products expressed by the AFD neurons also led to the suppression of aggregation (Fig. S3D), whereas the osm-9 (Fig. 2B) and ocr-2 (Fig. S2D) mutations did not.
Fig. 2.
Thermosensory neurons regulate polyQ aggregation in C. elegans body wall muscle cells. (A) Collapsed Z-sections of representative regions of the body wall muscle in day 3 adult animals with WT thermosensory neurons (Q35), gcy-8 mutation (gcy-8;Q35), and ttx-3 mutation (ttx-3;Q35). The numbers of aggregates in the head region would be counted as 6, 3, and 2 in the Q35, gcy-8;Q35, and ttx-3;Q35 respectively. (Scale bar: 20 μm.) (B) The average number of aggregates per animal in Q35, gcy-8;Q35, ttx-3;Q35, and osm-9;Q35 as animals age. (C) The average motility of Q35, gcy-8;Q35, ttx-3;Q35, and osm-9;Q35 day 4 animals normalized to WT animals not expressing polyQ. Error bars indicate SE (*P < 0.01).
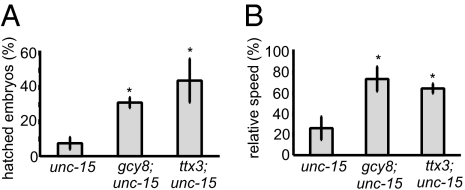
Fig. 3.
Thermosensory mutations suppress the toxicity caused by the misfolding of paramyosin. (A) The percentage of embryos that hatched when unc-15(ts), gcy-8;unc-15(ts), and ttx-3;unc-15(ts) embryos were transferred at the comma stage from 15 °C to 25 °C. (B) The motility of unc-15(ts), gcy-8;unc-15(ts), and ttx-3;unc-15(ts) relative to WT animals 2 d after being transferred from 15 °C to 25 °C. Error bars indicate SE (*P < 0.01).
Expression of Q35 in muscle cells causes a 60% reduction in motility of day 4 adult animals (14, 34) relative to age-matched nontransgenic animals (Fig. 2C). However, in gcy-8 and ttx-3 animals expressing Q35, the motility rates were restored close to WT levels (Fig. 2C), whereas the osm-9 mutation showed no effect (Fig. 2C). This protective role of the gcy-8 and ttx-3 mutations also extended to another disease-related aggregation prone protein, mutant SOD-1G93A (35). Expression of mutant SOD-1G93A in the background of a WT thermosensory circuitry causes developmental delays such that only 30% of animals develop past the second larval (L2) stage at 25 °C. In the thermosensory mutant background, nearly all SOD-1G93A–expressing animals developed past L2 stage (Fig. S3E).
In addition to suppressing the misfolding and toxicity of exogenous aggregation-prone proteins, the thermosensory mutations also suppressed the proteotoxicity associated with misfolding of a metastable temperature-sensitive isoform of paramyosin, unc-15(ts) (36). At the restrictive temperature (25 °C), paramyosin (ts) misfolds and aggregates, disrupting muscle structure and function (36). Consequently, only approximately 10% of unc-15(ts) embryos develop beyond the comma stage (Fig. 3A) (37), with adult animals showing approximately 75% reduction in motility relative to WT animals (Fig. 3B and Movie S1). In the presence of gcy-8 and ttx-3 mutations, the toxicity caused by the misfolding of paramyosin unc-15(ts) (Fig. 3) was substantially reduced. Approximately 30% of gcy-8;unc-15(ts) and approximately 40% of ttx-3;unc-15(ts) embryos hatched (Fig. 3A), and motility of adult gcy-8;unc-15(ts) and ttx-3;unc-15(ts) animals was restored to near that of WT animals (Fig. 3B and Movie S1).
These results demonstrate that modulation of thermosensory neuronal activity suppressed protein misfolding and related cellular dysfunction as a result of the expression of multiple metastable proteins in separate tissues. This suggests that the protein folding environment was affected globally by cell-nonautonomous control by the thermosensory neuronal circuitry.
Thermosensory Circuitry Modulates Protein Misfolding Through Dense Core Vesicle-Dependent Neurosecretion.
To investigate how the gcy-8 mutation influenced aggregation and toxicity, we examined whether the loss of downstream signaling pathways would recapitulate the global suppression of protein misfolding seen in the gcy-8 animals. The thermosensory mutations not only suppressed aggregation in muscle cells but also affected intestinal tissue that is not innervated, suggesting that neuronal signaling could occur through secreted molecules. Therefore, we tested whether inhibition of one of the major modes of neurosecretion, the dense core vesicle (DCV) release through calcium-activated protein secretion (CAPS) (38, 39), also suppressed polyQ aggregation in intestinal and muscle cells.
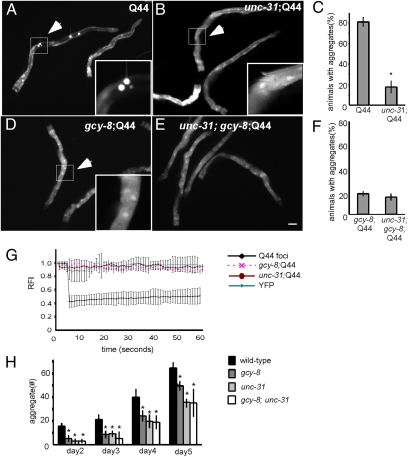
Deletion of the single C. elegans orthologue of CAPS, unc-31 (38, 39), and the subsequent loss of DCV-dependent neurosecretion resulted in suppression of Q44::YFP aggregation in the intestine (Fig. 4 A–C) and Q35::YFP aggregation in muscle cells (Fig. 4H), and the appearance of soluble protein by FRAP analysis (Fig. 4G). Moreover, polyQ aggregation in the gcy-8; unc-31 double mutants was indistinguishable from either of the single gcy-8 or unc-31 mutants alone (Fig. 4 D–F and H), suggesting that gcy-8 and unc-31 are in the same genetic pathway. These results showed that the AFD neurons modulate aggregation through suppression of DCV-dependent neurosecretion and suggested that these pathways may exert a net-inhibitory influence on the protein quality-control machinery of nonneuronal cells.
Fig. 4.
Thermosensory circuitry modulates protein misfolding through DCV-dependent neurosecretion. Q44 aggregation in the intestine of day 3 adult animals with (A) WT thermosensory neurons, (B) unc-31 mutation, (C) gcy-8 mutation, and (D) gcy-8;unc-31 double mutation. Inset: Representative areas chosen for photobleaching. (Scale bar: 100 μm.) (E) Percentage of Q44, unc31;Q44, gcy-8;Q44, and gcy-8;unc-31;Q44 day 3 adult animals with visible aggregates. (F) FRAP data following photobleaching of areas similar to those indicated in the inset. YFP alone used as a control. (G) The average number of aggregates per animal in Q35, gcy-8;Q35, unc-31;Q35, and gcy-8;unc-31;Q35 as animals age. Error bars in E and G indicate SE; error bars in F indicate SD (*P < 0.01). (H) The average number of aggregates per animal in wild-type, gcy-8;Q44, unc-31;Q44, and gcy-8;unc-31; Q44 animals as animals age.
Suppression of Proteotoxicity in Thermosensory Mutants Caused by HSF-1–Dependent Up-Regulation of Chaperones.
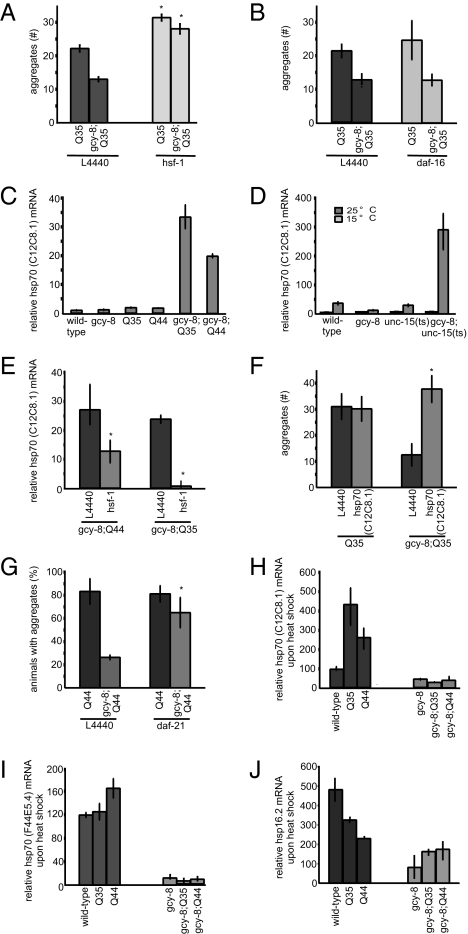
Protein quality control in C. elegans is regulated at the molecular level by HSF-1 (34, 40). Reducing the levels of hsf-1 by RNAi-induced knockdown restored aggregation in the gcy-8 animals to that of WT animals (Fig. 5A and Fig. S4B). Likewise, the suppression of polyQ aggregation in unc-31 mutant animals was HSF-1–dependent (Fig. S4C). RNAi-induced knockdown of DAF-16 did not revert the suppression of polyQ aggregation in the gcy-8 (Fig. 5B and Fig. S4B) or unc-31 animals (Fig. S4C), suggesting that the effects of the neuronal mutations on protein aggregation was dependent on HSF-1.
Fig. 5.
Thermosensory neurons regulate chaperones expression upon protein misfolding. The average number of aggregates per 3-d adult animals with WT thermosensory neurons or gcy-8 mutation grown on control bacteria (L4440) and upon RNAi-induced knockdown of (A) hsf-1 or (B) daf-16. (C) mRNA levels of hsp70 (C12C8.1) in day 3 adult animals quantified by quantitative RT-PCR (qRT-PCR). (D) mRNA levels of hsp70 (C12C8.1) quantified by qRT-PCR in animals without (15 °C) and with (25 °C) misfolded paramyosin. Values at 15 °C are 0.8 to 1.2, 0.8 to 1.4, 0.6 to 1.0, and 0.6 to 0.7 for WT, gcy-8, unc-15(ts), and gcy-8;unc-15(ts), respectively. (E) mRNA levels of hsp70 (C12C8.1) in 3 d adult gcy-8;Q35 and gcy-8;Q44 animals on control bacteria (L4440), and upon RNAi-induced knockdown of hsf-1. (F) Number of Q35 aggregates in day 3 adult Q35 and gcy-8;Q35 animals on control bacteria (L4440) and upon RNAi-induced knockdown of hsp70 (C12C8.1). (G) Percentage of day 3 adult Q44 animals containing aggregates in Q44 and gcy-8;Q44 animals on control bacteria (L4440) and upon RNAi-induced knockdown of hsp90 (daf-21). (H) mRNA levels of hsp70 (C12C8.1), (I) hsp70 (F44E5.4), and (J) small HSP (hsp16.2) quantified by qRT-PCR in day 3 adult animals expressing polyQ proteins following HS. Values in C–E were normalized to WT. Values in H–J were normalized to basal chaperone levels in the absence of HS. Actin was used as an internal control. Error bars in D–F indicate SE (*P < 0.01).
We next examined whether chaperone levels were up-regulated in the thermosensory mutants expressing aggregation-prone proteins. Mutations affecting the thermosensory neurons alone do not alter the basal levels of chaperones (Fig. 5 and Fig. S5). Expression levels of the constitutive HSP70 (hsp-1), the inducible HSP70s (C12C8.1 and F44E5.4), the inducible small HSP (hsp16.2), and HSP90 (daf-21) were similar for gcy-8 and WT animals (Fig. 5 and Fig. S5). In addition, as seen in other model systems the aggregation of polyQ did not up-regulate chaperone expression (Fig. 5C and Fig. S5 A–E). However, the expression of polyQ in the gcy-8 animals but not in other chemosensory mutants resulted in the up-regulation of each of these HSP genes (Fig. 5C and Figs. S2E and S5 A–E).
Similar results were obtained with regard to the misfolding of paramyosin. At the permissive temperature of 15 °C, the basal levels of inducible HSP70s (C12C8.1 and F44E5.4) in WT and gcy-8 animals were similar regardless of whether unc-15(ts) was expressed. The misfolding of paramyosin (ts) at the restrictive temperature of 25 °C in animals with WT thermosensory neurons did not cause the up-regulation of hsp70 (C12C8.1 and F44E5.4) mRNA: chaperone levels in the unc-15(ts) animals were similar to animals that expressed the WT isoform of paramyosin (Fig. 5D and Fig. S5F). In contrast, the misfolding of paramyosin in gcy-8 mutant animals resulted in up-regulation of the inducible cytoplasmic HSP70s (Fig. 5D and Fig. S5F).
We confirmed that the up-regulation of chaperones in gcy-8 mutant animals expressing misfolded proteins was dependent on HSF-1 (Fig. 5E). Moreover, up-regulation of chaperones was responsible for the suppression of aggregation in the thermosensory mutant animals (Fig. 5 F and G). RNAi-induced knockdown of hsp70 (C12C8.1) levels in the gcy-8;Q35 animals prevented the gcy-8–dependent rescue of aggregation (Fig. 5F). We observed no effect in Q35 animals with WT thermosensory function, presumably as hsp70 (C12C8.1) was not induced. Similarly, knockdown of hsp90 (daf-21) in the gcy-8;Q44 animals blocked the suppression of aggregation observed in these animals (Fig. 5G).
Thus, the chronic expression of aggregation-prone proteins in C. elegans tissues does not result in the up-regulation of chaperones. Disruption of neuronal activity restored HSF-1–dependent chaperone up-regulation upon chronic expression of misfolded proteins, and suppressed aggregation and toxicity.
Thermosensory Neuronal Function Allows C. elegans to Distinguish Between Acute Temperature Stress and Chronic Stress of Protein Misfolding.
The up-regulation of chaperones in thermosensory mutant animals and the rescue of aggregation and toxicity presented a conundrum, as we had previously observed that these mutations in the neuronal circuitry dampened the HSR. We reasoned that this may be because a short, acute HS that causes a rapid and transient change in protein folding dynamics is not recognized in the same manner as a prolonged chronic accumulation of misfolded proteins. The ability of the thermosensory neurons of C. elegans to detect and respond to temperature change and regulate proteostasis may allow for distinct organismal responses to these stresses.
As shown previously, aggregation of polyQ in C. elegans does not up-regulate chaperones in animals with a WT neuronal circuitry (Fig. 5 and Fig. S5). However, exposure of Q35 and Q44 animals with WT thermosensory neurons to HS (34 °C for 15 min) induced the expression of HSP genes (Fig. 5 H–J), whereas gcy-8;Q35 and gcy-8; Q44 animals showed no further increase in chaperone expression beyond basal levels (Fig. 5 H–J). This indicates that the expression of aggregation-prone proteins within the cells of C. elegans does not compromise the HSR. Rather, a WT thermosensory neuron compromises the response to chronic protein aggregation. To verify that the activity of thermosensory neurons distinguished between acute and chronic stress, we exposed WT and gcy-8 mutant animals to chronic heat stress. We predicted that gcy-8 animals, although deficient for chaperone induction upon acute HS, would induce chaperone expression when exposed to the chronic stress. This was indeed the case: even through gcy-8 animals did not respond to a transient HS (Fig. S6A), chaperone induction was observed upon exposure to repeated or prolonged HS (Fig. S6 B and C).
These data show that the thermosensory neurons inhibit chaperone up-regulation upon chronic stress and the occurrence of misfolded proteins, but permit HSP up-regulation upon acute HS, allowing the animal to respond differently to acute and chronic proteotoxic conditions.
Discussion
The nervous system of the metazoan C. elegans exerts an inhibitory, cell-nonautonomous control over the organismal response to protein misfolding. This inhibitory effect of the AFD neurons on the somatic cell HSR can be relieved by down-regulating the thermosensory neurons or by inhibiting CAPS-mediated DCV neurosecretion. In WT animals, this signal inhibits HSF-1 activity in intestinal and muscle cells even when challenged by the chronic expression of misfolded proteins. We propose that neuronal control of the HSR serves to modulate the organismal response to protein aggregation and to distinguish between acute HS and chronic accumulation of protein damage. Animals with WT thermosensory neurons express basal levels of chaperones despite the chronic accumulation of misfolded proteins, while retaining their ability to respond to acute heat stress. Our results show that mutations in thermosensory neuronal signaling invert this response such that chaperone induction in gcy-8 mutant animals now occurs when misfolded proteins are chronically expressed, but is dampened in response to acute HS. We hypothesize that thermosensory neurons serve as a homeostatic switch for the control of chaperone expression in C. elegans, allowing tissues within the organism to maintain optimal levels of chaperones for normal function and yet respond to transient exposures to environmental stress by up-regulating chaperones (Fig. S7). This would be important because chronic activation of HSF-1 and elevated HSP levels could interfere with growth and cell cycle progression (41, 42) and increase susceptibility to cellular transformation (43). Thus, the tight control over chaperone levels within individual cells would be all the more important for metazoan physiology. Indeed, chaperone levels are not maintained in excess (36), but rather are finely tuned to specific cellular requirements. In support of the role of the AFD neurons as a homeostatic switch over chaperone expression, it was very recently shown that HSF-1 in peripheral tissues modulates AFD thermosensory neuronal activity, providing a means of feedback regulation essential for any homeostatic control system (44).
Our results also show that the AFD thermosensory neurons control protein folding through Ca2+-dependent DCV secretion. As in other organisms, CAPS-dependent DCV release in C. elegans is responsible for peptidergic signaling through the secretion of neuropeptides, insulin-like peptides, and biogenic amines (39). The link between insulin signaling and the HSR is well established: HSF-1 activity in C. elegans is essential for lifespan enhancement by modulation of the insulin signaling pathway. Moreover, many of these peptides appear in the pseudocoelom of the animal following neurosecretion, where they would be expected to interact with somatic tissues. Thus, characterization of the molecule(s) released by the DCVs under physiological conditions, and upon acute heat stress, may help elucidate the signaling pathways by which the AFD neuron exerts its effects on protein folding homeostasis in nonneuronal cells.
The centralized control of HSF-1 activity described here has features in common with the systemic inhibition of acute inflammation in mammals (45, 46). Both the inflammatory response and the HSR are protective under a controlled regimen, but are detrimental if unchecked. In the case of the immune response, the systemic inhibition of acute inflammation by the central nervous system, together with local amplification by the peripheral nervous system, allows the response to be localized to regions of necessity, and subsequently terminated to restore homeostasis (45, 46). A similar hierarchical control of the HSR and HSF-1 activity could be envisioned whereby stress signaling networks between different tissues and the nervous system regulate the organismal response to different stress conditions.
The demonstration in C. elegans that the stress of chronic misfolding can be regulated by neuronal cell-nonautonomous control may have broader relevance to proteotoxicity in human disease. Cell-nonautonomous effects of afferent sensory and cortical projections on the toxicity and degeneration of the motor neuron has been described recently in mouse models of human ALS, spinal muscular atrophy, and Huntington disease (47–49). If protein folding homeostasis in diverse tissues of other metazoans is under the regulation of sensory neuronal modalities as we have described for C. elegans, this would suggest that another method for treatment of human conformational diseases could involve modulation of neurosensory systems.
Materials and Methods
C. elegans Strains and Growth Conditions.
The C. elegans strains and methods of cultivation are listed in SI Materials and Methods.
Scoring of Aggregates.
Aggregates were scored every 24 h post-L4, using a Leica fluorescent stereomicroscope (MZFLIII) with the EYFP filter set (excitation, 510/20; emission, 560/40). Aggregates were recognized visually, based on experience from FRAP regarding which foci did not recover following photobleaching. Aggregates too close to be resolved under the stereomicroscope, or smaller satellite aggregates scattered near a large aggregate were accounted as one. This system was consistently maintained for all strains. The number of aggregates in each worm was scored blind, by independent investigators and yielded very similar results. To quantify Q44 aggregation in the intestine, animals were binned into pools containing 0, 1 to 20, and greater than 20 aggregates. To quantify Q35 aggregation in the body wall muscle cells, all visible aggregates in approximately 100 animals, corresponding to more than three biological samples, were counted over a period of 4 d.
RNA Extraction and Quantitative RT-PCR.
mRNA was prepared by using TRIzol extraction as previously published and detailed in SI Materials and Methods.
RNAi Experiments.
RNAi experiments were conducted according to standard protocols and the experimental protocol, and RNAi constructs are detailed in SI Materials and Methods. RNAi against the AFD genes was conducted in the sensitized strain rrf-3 strain expressing Q35, which accumulated slightly lower numbers of aggregates.
Embryonic Lethality Experiments.
Toxicity in the SOD-1G93A animals was measured by transferring 10 L4 WT G93A and gcy-8 G93A larvae from 20 °C to 25 °C, allowing them to lay eggs for 24 to 36 h, counting total embryos, and calculating the percentage of larvae that grew to L3 stage. The experiment was repeated three or four times.
Embryonic lethality in unc-15 (ts) animals was measured by allowing approximately 10 unc-15(ts), gcy-8;unc-15(ts), or ttx-3;unc-15(ts) animals to lay eggs at 15 °C, and 24 h later, transferring 20 to 50 embryos at the comma cell stage onto fresh plates at 25 °C. The number of larvae that developed into L2 larvae after 24 to 48 h were scored. Experiments were repeated at least three times.
Motility Assays.
The motility of N2, gcy-8, Q35, gcy-8;Q35, ttx-3;Q35, unc-15(ts), gcy-8;unc-15(ts), ttx-3;unc-15(ts) was assayed upon transferring 10 animals to a new 6-cm plate seeded uniformly with Op50 equilibrated to room temperature. Movies of crawling animals were recorded with a Leica MZ10 microscope and Hamamatsu C10600-10B (Orca-R2) camera by using SimplePCI software (Leica) at 2 × 2 binning and 5 frames/s, and analyzed by using ImageJ as described in SI Materials and Methods. Each sample of 10 animals was measured only once. At least three samples of 10 animals were considered one biological sample, and each report of motility involved three to five biological samples.
FRAP.
A detailed description of FRAP methodology, the basis for selection of the aggregates, and how many aggregates were analyzed is included in SI Materials and Methods.
SDS/PAGE Gels and Western Blots for Examining Protein Levels.
PolyQ protein expression was measured by boiling 10 adult day 3 animals in SDS sample buffer for 15 min, and conducting a standard Western analysis. Antibodies used are listed in SI Materials and Methods. No cleavage of YFP from the polyQ-expressing polypeptide was observed.
HS.
For acute HS, 10 L4 animals (N2, Q35, Q44, gcy-8, gcy-8;Q35, gcy-8;Q44) were moved onto a fresh plate from a population, allowed to mature into day 3 adults and heat-shocked in a water bath for 15 min at 34 °C according to previously published methods. We ensured that the polyQ-expressing animals had accumulated aggregates before HS. For prolonged chronic HS, 10 L4 animals were exposed to a constant temperature of 28 °C for 24 h. Repeated acute HSs were delivered by three cycles of exposure to 34 °C for 15 min interspersed by 15 min recovery at 20 °C.
Supplementary Material
Acknowledgments
We thank members of the R.I.M. laboratory for discussions and comments, Dr. Jesper Pedersen for help with the motility assays, and Catarina Silva for help with quantifying aggregates. We acknowledge the C. elegans Genetics Center, supported by a grant from the National Institutes of General Medicine and the International Consortium, for providing C. elegans strains. We thank Dr. I. Mori for the gcy-8(oy44) strains and Dr. Janet Richmond for the unc-31(e928) strain. This study was supported by National Institutes of Health grants (through the National Institute of General Medical Sciences, National Institute on Aging, and National Institute of Neurological Disorders and Stroke) (to R.I.M.), Huntington's Disease Society of America Coalition for the Cure (R.I.M.), and the Daniel F. and Ada L. Rice Foundation (R.I.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1106557108/-/DCSupplemental.
References
- 1.Soto C, Estrada LD. Protein misfolding and neurodegeneration. Arch Neurol. 2008;65:184–189. doi: 10.1001/archneurol.2007.56. [DOI] [PubMed] [Google Scholar]
- 2.Carrell RW. Cell toxicity and conformational disease. Trends Cell Biol. 2005;15:574–580. doi: 10.1016/j.tcb.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science. 2008;319:916–919. doi: 10.1126/science.1141448. [DOI] [PubMed] [Google Scholar]
- 4.Sherman MY, Goldberg AL. Cellular defenses against unfolded proteins: A cell biologist thinks about neurodegenerative diseases. Neuron. 2001;29:15–32. doi: 10.1016/s0896-6273(01)00177-5. [DOI] [PubMed] [Google Scholar]
- 5.Hartl FU, Hayer-Hartl M. Converging concepts of protein folding in vitro and in vivo. Nat Struct Mol Biol. 2009;16:574–581. doi: 10.1038/nsmb.1591. [DOI] [PubMed] [Google Scholar]
- 6.Jonikas MC, et al. Comprehensive characterization of genes required for protein folding in the endoplasmic reticulum. Science. 2009;323:1693–1697. doi: 10.1126/science.1167983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- 8.Akerfelt M, Morimoto RI, Sistonen L. Heat shock factors: Integrators of cell stress, development and lifespan. Nat Rev Mol Cell Biol. 2010;11:545–555. doi: 10.1038/nrm2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Voellmy R, Boellmann F. Chaperone regulation of the heat shock protein response. Adv Exp Med Biol. 2007;594:89–99. doi: 10.1007/978-0-387-39975-1_9. [DOI] [PubMed] [Google Scholar]
- 10.Morimoto RI. Regulation of the heat shock transcriptional response: Cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 1998;12:3788–3796. doi: 10.1101/gad.12.24.3788. [DOI] [PubMed] [Google Scholar]
- 11.Zourlidou A, et al. Hsp27 overexpression in the R6/2 mouse model of Huntington's disease: Chronic neurodegeneration does not induce Hsp27 activation. Hum Mol Genet. 2007;16:1078–1090. doi: 10.1093/hmg/ddm057. [DOI] [PubMed] [Google Scholar]
- 12.Hay DG, et al. Progressive decrease in chaperone protein levels in a mouse model of Huntington's disease and induction of stress proteins as a therapeutic approach. Hum Mol Genet. 2004;13:1389–1405. doi: 10.1093/hmg/ddh144. [DOI] [PubMed] [Google Scholar]
- 13.Muchowski PJ. Protein misfolding, amyloid formation, and neurodegeneration: A critical role for molecular chaperones? Neuron. 2002;35:9–12. doi: 10.1016/s0896-6273(02)00761-4. [DOI] [PubMed] [Google Scholar]
- 14.Satyal SH, et al. Polyglutamine aggregates alter protein folding homeostasis in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2000;97:5750–5755. doi: 10.1073/pnas.100107297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vleminckx V, et al. Upregulation of HSP27 in a transgenic model of ALS. J Neuropathol Exp Neurol. 2002;61:968–974. doi: 10.1093/jnen/61.11.968. [DOI] [PubMed] [Google Scholar]
- 16.Ferraiuolo L, et al. Microarray analysis of the cellular pathways involved in the adaptation to and progression of motor neuron injury in the SOD1 G93A mouse model of familial ALS. J Neurosci. 2007;27:9201–9219. doi: 10.1523/JNEUROSCI.1470-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bence NF, Sampat RM, Kopito RR. Impairment of the ubiquitin-proteasome system by protein aggregation. Science. 2001;292:1552–1555. doi: 10.1126/science.292.5521.1552. [DOI] [PubMed] [Google Scholar]
- 18.Stenoien DL, et al. Polyglutamine-expanded androgen receptors form aggregates that sequester heat shock proteins, proteasome components and SRC-1, and are suppressed by the HDJ-2 chaperone. Hum Mol Genet. 1999;8:731–741. doi: 10.1093/hmg/8.5.731. [DOI] [PubMed] [Google Scholar]
- 19.Kim S, Nollen EA, Kitagawa K, Bindokas VP, Morimoto RI. Polyglutamine protein aggregates are dynamic. Nat Cell Biol. 2002;4:826–831. doi: 10.1038/ncb863. [DOI] [PubMed] [Google Scholar]
- 20.Olzscha H, et al. Amyloid-like aggregates sequester numerous metastable proteins with essential cellular functions. Cell. 2011;144:67–78. doi: 10.1016/j.cell.2010.11.050. [DOI] [PubMed] [Google Scholar]
- 21.Tatzelt J, Voellmy R, Welch WJ. Abnormalities in stress proteins in prion diseases. Cell Mol Neurobiol. 1998;18:721–729. doi: 10.1023/A:1020646321841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bush KT, Goldberg AL, Nigam SK. Proteasome inhibition leads to a heat-shock response, induction of endoplasmic reticulum chaperones, and thermotolerance. J Biol Chem. 1997;272:9086–9092. doi: 10.1074/jbc.272.14.9086. [DOI] [PubMed] [Google Scholar]
- 23.Fujimoto M, et al. Active HSF1 significantly suppresses polyglutamine aggregate formation in cellular and mouse models. J Biol Chem. 2005;280:34908–34916. doi: 10.1074/jbc.M506288200. [DOI] [PubMed] [Google Scholar]
- 24.Auluck PK, Chan HY, Trojanowski JQ, Lee VM, Bonini NM. Chaperone suppression of alpha-synuclein toxicity in a Drosophila model for Parkinson's disease. Science. 2002;295:865–868. doi: 10.1126/science.1067389. [DOI] [PubMed] [Google Scholar]
- 25.Warrick JM, et al. Suppression of polyglutamine-mediated neurodegeneration in Drosophila by the molecular chaperone HSP70. Nat Genet. 1999;23:425–428. doi: 10.1038/70532. [DOI] [PubMed] [Google Scholar]
- 26.Cummings CJ, et al. Over-expression of inducible HSP70 chaperone suppresses neuropathology and improves motor function in SCA1 mice. Hum Mol Genet. 2001;10:1511–1518. doi: 10.1093/hmg/10.14.1511. [DOI] [PubMed] [Google Scholar]
- 27.Fernandez-Funez P, et al. Identification of genes that modify ataxin-1-induced neurodegeneration. Nature. 2000;408:101–106. doi: 10.1038/35040584. [DOI] [PubMed] [Google Scholar]
- 28.Muchowski PJ, et al. Hsp70 and hsp40 chaperones can inhibit self-assembly of polyglutamine proteins into amyloid-like fibrils. Proc Natl Acad Sci USA. 2000;97:7841–7846. doi: 10.1073/pnas.140202897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prahlad V, Cornelius T, Morimoto RI. Regulation of the cellular heat shock response in Caenorhabditis elegans by thermosensory neurons. Science. 2008;320:811–814. doi: 10.1126/science.1156093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inada H, et al. Identification of guanylyl cyclases that function in thermosensory neurons of Caenorhabditis elegans. Genetics. 2006;172:2239–2252. doi: 10.1534/genetics.105.050013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hobert O, et al. Regulation of interneuron function in the C. elegans thermoregulatory pathway by the ttx-3 LIM homeobox gene. Neuron. 1997;19:345–357. doi: 10.1016/s0896-6273(00)80944-7. [DOI] [PubMed] [Google Scholar]
- 32.Mohri-Shiomi A, Garsin DA. Insulin signaling and the heat shock response modulate protein homeostasis in the Caenorhabditis elegans intestine during infection. J Biol Chem. 2008;283:194–201. doi: 10.1074/jbc.M707956200. [DOI] [PubMed] [Google Scholar]
- 33.Kahn-Kirby AH, Bargmann CI. TRP channels in C. elegans. Annu Rev Physiol. 2006;68:719–736. doi: 10.1146/annurev.physiol.68.040204.100715. [DOI] [PubMed] [Google Scholar]
- 34.Morley JF, Brignull HR, Weyers JJ, Morimoto RI. The threshold for polyglutamine-expansion protein aggregation and cellular toxicity is dynamic and influenced by aging in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2002;99:10417–10422. doi: 10.1073/pnas.152161099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gidalevitz T, Krupinski T, Garcia S, Morimoto RI. Destabilizing protein polymorphisms in the genetic background direct phenotypic expression of mutant SOD1 toxicity. PLoS Genet. 2009;5:e1000399. doi: 10.1371/journal.pgen.1000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gidalevitz T, Ben-Zvi A, Ho KH, Brignull HR, Morimoto RI. Progressive disruption of cellular protein folding in models of polyglutamine diseases. Science. 2006;311:1471–1474. doi: 10.1126/science.1124514. [DOI] [PubMed] [Google Scholar]
- 37.Gengyo-Ando K, Kagawa H. Single charge change on the helical surface of the paramyosin rod dramatically disrupts thick filament assembly in Caenorhabditis elegans. J Mol Biol. 1991;219:429–441. doi: 10.1016/0022-2836(91)90184-8. [DOI] [PubMed] [Google Scholar]
- 38.Hammarlund M, Watanabe S, Schuske K, Jorgensen EM. CAPS and syntaxin dock dense core vesicles to the plasma membrane in neurons. J Cell Biol. 2008;180:483–491. doi: 10.1083/jcb.200708018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Speese S, et al. UNC-31 (CAPS) is required for dense-core vesicle but not synaptic vesicle exocytosis in Caenorhabditis elegans. J Neurosci. 2007;27:6150–6162. doi: 10.1523/JNEUROSCI.1466-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morley JF, Morimoto RI. Regulation of longevity in Caenorhabditis elegans by heat shock factor and molecular chaperones. Mol Biol Cell. 2004;15:657–664. doi: 10.1091/mbc.E03-07-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feder JH, Rossi JM, Solomon J, Solomon N, Lindquist S. The consequences of expressing hsp70 in Drosophila cells at normal temperatures. Genes Dev. 1992;6:1402–1413. doi: 10.1101/gad.6.8.1402. [DOI] [PubMed] [Google Scholar]
- 42.Akerfelt M, Morimoto RI, Sistonen L. Heat shock factors: Integrators of cell stress, development and lifespan. Nat Rev Mol Cell Biol. 2010;11:545–555. doi: 10.1038/nrm2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dai C, Whitesell L, Rogers AB, Lindquist S. Heat shock factor 1 is a powerful multifaceted modifier of carcinogenesis. Cell. 2007;130:1005–1018. doi: 10.1016/j.cell.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sugi T, Nishida Y, Mori I. Regulation of behavioral plasticity by systemic temperature signaling in Caenorhabditis elegans. Nat Neurosci. 2011 doi: 10.1038/nn.2854. 10.1038/nn.2854. [DOI] [PubMed] [Google Scholar]
- 45.Sternberg EM. Neural regulation of innate immunity: A coordinated nonspecific host response to pathogens. Nat Rev Immunol. 2006;6:318–328. doi: 10.1038/nri1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 47.Spampanato J, Gu X, Yang XW, Mody I. Progressive synaptic pathology of motor cortical neurons in a BAC transgenic mouse model of Huntington's disease. Neuroscience. 2008;157:606–620. doi: 10.1016/j.neuroscience.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mentis GZ, et al. Early functional impairment of sensory-motor connectivity in a mouse model of spinal muscular atrophy. Neuron. 2011;69:453–467. doi: 10.1016/j.neuron.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ilieva HS, et al. Mutant dynein (Loa) triggers proprioceptive axon loss that extends survival only in the SOD1 ALS model with highest motor neuron death. Proc Natl Acad Sci USA. 2008;105:12599–12604. doi: 10.1073/pnas.0805422105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.