Abstract
Background
Our ability to measure the cognitive components of complex decision-making across species has greatly facilitated our understanding of its neurobiological mechanisms. One task in particular, reversal learning, has proven valuable in assessing the inhibitory processes that are central to executive control. Reversal learning measures the ability to actively suppress reward-related responding and to disengage from ongoing behavior, phenomena that are biologically and descriptively related to impulsivity and compulsivity. Consequently, reversal learning could index vulnerability for disorders characterized by impulsivity, such as proclivity for initial substance abuse, as well as the compulsive aspects of dependence.
Objective
Though we describe common variants and similar tasks, we pay particular attention to discrimination reversal learning, its supporting neural circuitry, neuropharmacology and genetic determinants. We also review the utility of this task in measuring impulsivity and compulsivity in addictions.
Methods
We restrict our review to instrumental, reward-related reversal learning studies, as they are most germane to addiction.
Conclusion
The research reviewed here suggests that discrimination reversal learning may be used as a diagnostic tool for investigating the neural mechanisms that mediate impulsive and compulsive aspects of pathological reward-seeking and –taking behaviors. Two interrelated mechanisms are posited for the neuroadaptations in addiction that often translate to poor reversal learning: frontocorticostriatal circuitry dysregulation and poor dopamine (D2 receptor) modulation of this circuitry. These data suggest new approaches to _targeting inhibitory control mechanisms in addictions.
Keywords: orbitofrontal cortex, striatum, decision-making, dopamine, serotonin, psychostimulant, methamphetamine, noradrenaline, response control
Organisms, human and non-human alike, must make a multitude of second-by-second decisions about how to adaptively respond to the environment in order to optimize gains and minimize losses associated with their behaviors. These decisions are colored by their innate drives, reinforcement history, and cognitive expectations. The selection of appropriate action involves a balance of processes that permit hasty action in the absence of deliberative consideration (i.e., impulsive responses and habits) and those that involve a slow set of cognitions necessary for optimized response selection in a complex, conditional and/or changing world. By virtue of repetition, and embedded in a long-term reinforcement rule, there are certain behaviors (e.g., stimulus-response habits) that require little cognitive effort to be elicited and/or emitted (Balleine and O’Doherty, 2010). For controlled decision-making processes to be optimized, individuals must be able to exert inhibitory control over these rapid, automatic response systems, when appropriate (Roberts and Wallis, 2000, Aron et al., 2004, Eagle et al., 2008).
Our understanding of the role for inhibitory control in human decision-making processes has been greatly advanced through the study of cognitively-guided behavior of laboratory animals. The power of this experimental approach depends heavily on our ability to accurately measure a latent process of interest using a behavioral task (i.e., construct validity), as well as whether we can use an array of tasks to measure a unitary construct in humans and laboratory animals (i.e., predictive or translational validity). For relatively simple behavioral phenomena (e.g., fear conditioning), it is easy to see how these criteria can be satisfied, but for processes like decision-making and its associated executive functions, the challenge is more substantial. Fortunately, studies of a phenomenon called reversal learning, in a variety of species, have been critical in uncovering both the brain circuitry that support flexible choices during decision-making, as well as identifying relevant neurochemical and genomic determinants.
Discrimination reversal learning involves repeated pairing of an action (digging in a bowl, physically interacting with a lever or touchscreen, or displacing an object) with an outcome (e.g. provision of a food reward). In these contexts, subjects can learn about reward contingencies through the sensory properties of cues that predict reward availability and the actions required to procure that reward. Operationally, the subject first learns that discriminative stimuli carry information about whether a particular response instrumentally generates a reward (e.g., dig in a bowl scented with aroma A but not B; press the left, but not right, lever; or touch visual stimulus A on a screen, but not B to obtain food). Over the course of training, subjects become proficient at providing discriminated behavior, consistent with the associative rules. The rules learned can be deterministic or probabilistic.
Typically, after reaching a learning criterion for accuracy on this discrimination problem, the reversal phase is implemented, and the reward contingencies are reversed. At reversal, the trained response no longer results in reward, though it remains at least temporarily dominant (prepotent) because of the initial training history. For this reason, reversal learning (unlike the initial stage of discrimination learning or acquisition) emphasizes the need for the subject to effortfully withhold the initially-trained response and, instead, emit those responses it previously learned to be useless. Reversal learning is therefore thought to measure flexibility of response, referred to in the literature using an array of terminology: “cognitive flexibility,” “behavioral flexibility,” “cognitive control,” “inhibitory control,” “impulse control,” “response inhibition,” and “behavioral inhibition,” to name a few. The ability to adapt to changes in reward contingency during reversal learning relies on a circumscribed neural circuitry (Fineberg et al., 2010, Chudasama, 2011), an orchestrated balance of neurotransmitters (Robbins, 2005, Dalley et al., 2008, Flagel et al., 2011) and genes (Laughlin et al., 2011).
The study of individual differences in flexible responding during reversal learning is potentially relevant to our understanding of normal human behavior and temperament. For example, individual differences in the propensity to make quick, poorly-considered choices may underlie dimensions of impulsive temperament and personality. Evidence shows that inflexible responding in reversal learning is genetically related to impulsivity (Franken et al., 2008, Crews and Boettiger, 2009, Romer et al., 2009, Fineberg et al., 2010). This poses the interesting possibility of employing the reversal learning paradigm to quantify impulsive behavior across species and to index biological vulnerability for disorders characterized by extreme impulsivity, such as attention-deficit-hyperactivity disorder, or ADHD (Itami and Uno, 2002), impulse control disorders, and the propensity for the initiation of illicit substance use (Brewer and Potenza, 2008, Winstanley et al., 2010).
Another conceptualization of reversal learning emphasizes the notion that the difficulty to disengage from ongoing behavior after a contingency shift reflects a compulsive or habitual response tendency. This suggests that the task measures a set of processes related to automatized behavior, which may also be germane to psychiatric disorders, like addiction. In essence, drug dependence involves the compulsion to consume an illicit substance and a loss of control over intake, despite negative consequences (DSM-IV, American Psychiatric Association). Therefore, reversal learning abilities in subjects may be informative of both impulsive and compulsive aspects of a variety of forms of psychopathology.
In this review, we first describe common variants of reversal learning and identify shared and unique characteristics relative to other commonly-used tasks in animal behavioral neuroscience research to measure impulsive or compulsive behavior, specifically instrumental extinction and go/no-go tasks. We also review the neural circuitry of reversal learning and its supporting neuropharmacology and genetic determinants. We then relate reversal learning to the impulsive and compulsive aspects of addiction.
Discrimination reversal learning
The literature on the neuroanatomy and neuropharmacology of reversal learning is vast, particularly considering there are many different task variants and implementations. Because of an interest in using these phenotypes to better understand impulsive and compulsive reward seeking (addictions), we restrict this review to instrumental, reward-related reversal learning, as opposed to tasks that involve reversal of Pavlovian rules or tasks that primarily involve aversively-motivated learning. Additionally, we limit our discussion to tasks that emphasize updating of behavior in response to changes in reinforcement contingencies, as opposed to shifts in attentional focus or strategy.
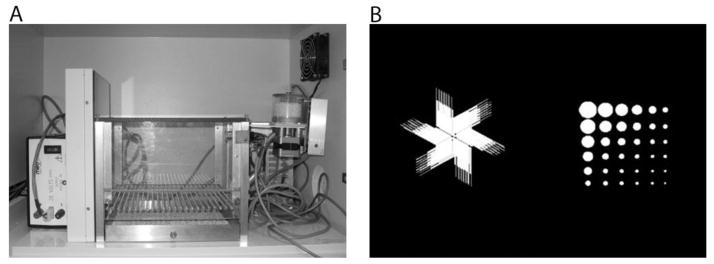
There are many kinds of reversal tasks studied; each varying according to the sensory modality of the discrimination, the concurrent or sequential nature of stimulus presentation and the type of response made. Examples include reversal of: concurrent or sequential odor discriminations (McAlonan and Brown, 2003, Schoenbaum et al., 2003), spatial discriminations in maze contexts (Jentsch and Taylor, 2001, Bannerman et al., 2003), operant discriminations (El-Ghundi et al., 2003, Boulougouris et al., 2007, Laughlin et al., 2011) and visual discriminations. The latter frequently employ touchscreen response methodology (such as that shown in Figure 1A) and are emergent paradigms in both mice (Bussey et al., 2001, Izquierdo et al., 2006, Brigman et al., 2010b, Barkus et al., 2011, Bissonette and Powell, 2011) and rats (Chudasama and Robbins, 2003, Izquierdo et al., 2010), being similar to methods used in monkeys (Dias et al., 1996, Rudebeck et al., 2008) and humans (Robbins et al., 1998). Most discrimination reversal studies administer two stimuli (or objects) that the subject is required to discriminate (Figure 1B). Often, post hoc analyses are used to assess the kinds of errors committed during reversal learning: stages of learning (below chance, at chance, and above chance performance; Jones and Mishkin, 1972), trial-by-trial rewarded and unrewarded choices (Rudebeck and Murray, 2008), as well as perseveration indices representing consecutive or “correction” errors over the total number of errors committed (Chudasama and Robbins, 2003, Izquierdo et al., 2006). Other methods have incorporated the use of three or more concurrent items to dissect error type within the task itself (Lee et al., 2007, Seu et al., 2009).
Figure 1.
An example of the apparatus and stimuli used in touchscreen-based operant methods for visual discrimination reversal learning. A: An operant chamber is modified to accommodate a touchscreen (this setup is used for testing mice). Animals are required to nosepoke the touchscreen on one end of the chamber, and procure a pellet on the opposite side of the chamber. B: Equiluminant stimuli used for discrimination and reversal learning. Adapted from Izquierdo et al. 2006 Behav Brain Res.
As noted above, the relationships between actions and outcomes in reversal learning tasks can be either deterministic (fully predictive) or probabilistic. In most animal studies, deterministic rules are used, though probabilistic rules are commonly used in human subjects in order to slow down the rate of initial and reversal learning. This represents an important dimension of discrepancy, though recent studies in rats have begun to use probabilistic rules (Bari et al., 2010) and other experiments in human subjects have used deterministic rules (Ghahremani et al., 2010), helping to bridge the gap.
A final factor of relevance in considering implementations of reversal learning tasks relates to whether subjects learn one reversal or multiple (serial) reversals. If administered serially, the task encourages an automatized switching tendency, possible rule learning, acquisition of a reversal learning “set,” as well as prospective planning for anticipated reward contingencies (Murray and Gaffan, 2006). These task details theoretically correspond to different underlying neural mechanisms.
Comparison with related tasks
Reversal learning procedures are just one of an array of tasks designed to measure aspects of impulsive actions and choices. Tests of impulsive choice, like delay and effort discounting, juxtapose the possibility of procuring an immediate or low-effort reward of small magnitude or a delayed or effortful, albeit larger, reward. When compared to reversal learning, the effects of lesions and pharmacological manipulations on these tasks appear to vary more frequently with the methods used. Due to space limitations, we restrict our comparison of reversal learning with more qualitatively similar tests of inhibitory control (that also have the capability of measuring impulsive action): namely, instrumental extinction and go/no-go.
Reversal learning, tests of instrumental extinction, and go/no-go tasks all implement dynamic adjustments in the occurrence of reward and measure functions related to response inhibition or inhibitory control. During reversal learning, subjects must suppress one response while engaging actively in another to obtain reward; therefore, there remains a strong motivational impulse to respond post reversal. In extinction, however, the subject may simply inhibit the conditional response altogether, reflecting the importance of conserving energy when actions no longer result in reward. Therefore, inhibition of response in reversal and extinction exists in different motivational contexts. Reversal learning may reflect selective response inhibition, as opposed to more general behavioral inhibition mechanisms.
In the go/no-go task, the subject is also required to inhibit a response in the presence of a discriminative stimulus. These procedures are conditional discriminations in the sense that the subject must respond to “go” cues (e.g., nosepoke an aperture in the presence of a green light) and inhibit response to the “no-go” cues (e.g., withhold nosepoke in the presence of a red light). Usually these two types of trials are presented randomly throughout the testing session, with go cues being more frequent in order to form the prepotent tendency to respond strongly. One main difference between go/no-go and reversal learning is the presentation of the discriminative stimuli or cues: in the go/no-go task they are presented serially whereas in reversal learning they are usually presented concurrently. Consequently, the “no-go” trials are more similar to instrumental extinction trials, supporting general behavioral or response inhibition mechanisms rather than eliciting a more selective reallocation and control of response. Additionally, in the go/no-go task, withholding a response on “no-go” trials may be rewarded, whereas in reversal learning only an instrumental response yields reward.
Though we do not describe in detail the underlying neural substrates of instrumental extinction and go/no-go in this review, there is much overlap with those supporting reversal learning (described below), consonant with their shared features.
Neural circuitry of reversal learning
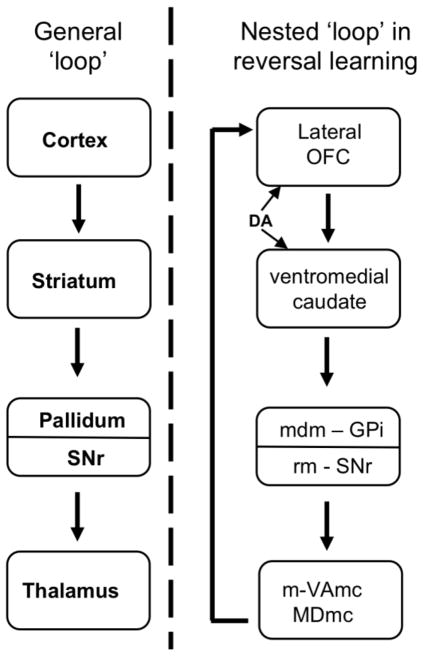
Reversal learning recruits frontocorticostriatothalamic loops often implicated in psychiatric and neurological disorders (Haber, 2003) (Figure 2). Studies of the effects of discrete brain lesions on reversal learning have traditionally placed greater emphasis on determining the neural substrates of reversal, and not initial discrimination, learning. Such reports of “reversal-specific” effects across many species have greatly contributed to both the predictive validity of the task itself as a measure of inhibitory control, as well as its discriminant validity (e.g., its utility as a diagnostic tool).
Figure 2.
Frontocorticostriatal circuitry in discrimination reversal learning. Adapted from Chudasama and Robbins, 2006 Biol Psychol. Left of the dotted line: A general loop of connectivity. Frontal cortex and its connections with different levels of the striatum are rerouted back to itself by the thalamus. Right of the dotted line: One of several nested anatomical loops within the general loop, this one specifically implicated in discrimination reversal learning. Connections between orbitofrontal cortex (OFC) and striatum are modulated by dopamine (DA). Additional abbreviations: GPi, internal segment of globus pallidus; SNr, substantia nigra pars reticulata; VAmc, ventralis anterior pars magnocelullaris; MDmc, medialis dorsalis pars magnocellularis; m, medial; rm, rostromedial; mdm, medial dorsomedial.
Orbitofrontal Cortex
If we restrict our description of the circuitry to that which supports two-stimuli odor or visual discrimination reversal learning, the literature is quite consistent across species. Mice (Bissonette et al., 2008), rats (Chudasama and Robbins, 2003, McAlonan and Brown, 2003) and monkeys (Jones and Mishkin, 1972, Dias et al., 1996, Izquierdo et al., 2004) with lesions that include the orbitofrontal cortex (OFC) exhibit normal acquisition of the initial discrimination but are impaired at reversal, often exhibiting perseverative responding to the previously rewarded stimulus (Rudebeck and Murray, 2008). Conversely, medial and dorsal subregions of the frontal cortex are not critical to discrimination reversal learning in mice, rats, or monkeys (Birrell and Brown, 2000, Boulougouris et al., 2007, Bissonette et al., 2008, Rudebeck et al., 2008). Instead, the medial wall of the frontal cortex in the rodent brain (to include prelimbic and infralimbic cortices) appears to be more important in task and strategy switching (Ragozzino et al., 1999a, Ragozzino et al., 1999b, Floresco et al., 2008, Ghods-Sharifi et al., 2008, Rich and Shapiro, 2009), which have been described as serving more of a working memory, action-monitoring function (Ragozzino et al., 1998, Delatour and Gisquet-Verrier, 1999, 2000, Gisquet-Verrier and Delatour, 2006). Similar results have been obtained in monkey studies (Dias et al., 1996, Rudebeck et al., 2008), with a recent report showing that a subregion within the OFC, Walker’s area 14, may be most critical in learning to inhibit responses to a previously rewarded stimulus (Rudebeck and Murray, 2011). Importantly, behavioral and neuroimaging studies in human subjects with OFC damage (Fellows and Farah, 2003, Hornak et al., 2004), patients diagnosed with OCD (Remijnse et al., 2006) and healthy controls (O’Doherty et al., 2001, Ghahremani et al., 2010), all show that this region is important for accurate performance across a variety of reversal learning paradigms. In sum, the functional localization of discrimination reversal learning within the frontal cortex is well-preserved across species (Chudasama, 2011).
Striatum
The idea that areas of the striatum may also mediate “frontocortical”-like processes was proposed and supported by empirical evidence as early as the late 1960s (Divac et al., 1967, Winocur and Mills, 1969, Winocur and Eskes, 1998). The anatomical interconnectivity (Fig. 2) may account for some functional overlap in reversal learning: lesions of the medial striatum, like damage limited to the OFC, produce perseverative responding on reversal learning in rats (Castane et al., 2010) and in marmoset monkeys (Clarke et al., 2008, Man et al., 2008). Additionally, mediodorsal thalamus, heavily interconnected with OFC, is a contributor to accurate performance during reversal learning (Chudasama et al., 2001), yet its circumscribed contribution has not been fully identified.
Medial temporal lobe structures
Severe discrimination and reversal learning impairments are found following rhinal cortex, specifically perirhinal cortex, probably due to this region’s essential role in object recognition and object identification (Murray and Richmond, 2001). Damage restricted to the hippocampus also results in object reversal learning impairments in monkeys (Murray et al., 1998); yet in rodents, hippocampal recruitment appears to depend on the spatial nature of the reversal task (Morellini et al., 2010). Certain regions of the medial temporal lobe, though substantially involved in discrimination learning and in forming stimulus-reward associations generally, are not critically important to reversal learning per se. For example, selective lesions of the amygdala do not disrupt reversal learning (Izquierdo and Murray, 2007, Stalnaker et al., 2007) and even facilitate reversal learning (Stalnaker et al., 2007) and instrumental extinction (Izquierdo and Murray, 2005). Thus, medial temporal lobe structures are critical to associative learning processes involving reward, yet frontocorticostriatal circuitry is most reliably implicated in support of reversal-specific learning.
Neurotransmitter Mechanisms in Reversal Learning
An extensive literature exists on the pharmacological regulation of reversal learning abilities in birds, rodents and non-human and human primates. Some of this work involves the use of pharmacological agents that produce wide-ranging non-specific impairments in cognitive and executive dysfunction. This section describes the modulatory role for monoamine systems that, while not proposed to exert selective actions on reversal abilities, do nevertheless exert more constrained influence on inhibitory control mechanisms, rather than working more generally on a broad array of cognitive and/or executive functions.
Serotonin
Some of the first evidence linking serotonin mechanisms to discrimination reversal came from studies of the effects of ondansetron – a centrally active 5-HT3 receptor antagonist – on object discrimination acquisition and reversal in monkeys. In both marmosets and rhesus monkeys, ondansetron improved performance, but this effect was noted for both initial acquisition and reversal learning, meaning that the pharmacological effect could not be localized to the inhibitory control processes measured in reversal (Barnes et al., 1990, Domeney et al., 1991, Arnsten et al., 1997).
Reductions in serotonin concentrations produced by dietary deficiencies in tryptophan have largely found no effect of low indoleamine transmission on reversal learning (Murphy et al., 2002, Evers et al., 2005a, Evers et al., 2005b, van der Plasse and Feenstra, 2008). On the other hand, toxin-mediated depletions of serotonin (which potentially produce larger magnitude reductions in transmitter) have often been reported to affect reversal learning in a behaviorally selective manner. Focal destruction of serotonin terminals in the OFC impairs the ability to update behavior during reversal in marmosets (Clarke et al., 2004, Clarke et al., 2005, Clarke et al., 2007). Similar findings have been reported after cortical serotonin depletions in rats (Masaki et al., 2006, Lapiz-Bluhm et al., 2009) yet this manipulation appears to have no effect on reversal learning in mice, even though chronic fluoxetine treatment produces a reversal enhancement in this species (Brigman et al., 2010). Very little study has been focused on the receptor mechanisms within prefrontal cortex that mediate effects on reversal learning, though 5-HT2 subtype receptors appear to be plausible candidates (Boulougouris et al., 2008b, Boulougouris and Robbins, 2010).
Additional evidence relating serotonin to reversal learning derives from genetic findings in laboratory animals. In rhesus monkeys, variation in the non-coding regions of the gene encoding the serotonin transporter (that inactivates synaptic serotonin) is linked to reversal learning (Izquierdo et al., 2007, Vallender et al., 2009, Jedema et al., 2010); since the studies conducted so far linked independent, non-segregating variants with reversal learning, the relationship between this gene and reversal appears to be a robust finding. Experimental studies in mice and rats (involving genetic or pharmacological inhibition of the serotonin transporter) support these findings, as well (Brigman et al., 2010, Lapiz-Bluhm et al., 2009; Nonkes et al., 2011). The influence of genetic variation in the serotonin transporter on reversal learning probably depends on an interaction of factors, including stress (Graybeal et al. 2011).
Unfortunately, much remains unknown about the details of the control of reversal learning by central serotonin systems. The types of manipulations conducted so far (dietary and pharmacological depletions, reductions in reuptake) are not specific enough to uncover the cellular and molecular mechanisms that mediate these influences and that are relevant to treatment of disorders linked with reversal learning problems. These findings are summarized in Table 1.
Table 1.
Summary of evidence for serotonergic modulation of reversal learning.
| Species | Manipulation | Task Conditions | Effect | Reference |
|---|---|---|---|---|
| Mouse | Serotonin transporter knockout, serotonin transporter inhibitor, and serotonin depletion | Stimuli: visual Response: touchscreen |
Low transporter function enhances reversal learning while serotonin depletion has no effect on reversal learning | (Brigman et al., 2010) |
| Rat | Cortical serotonin depletion | Stimuli: olfactory Response: digging in medium |
Depletion impairs reversal learning | (Lapiz-Bluhm et al., 2009) |
| Stimuli: visual Response: nosepoke |
(Masaki et al., 2006) | |||
| Serotonin transporter knockout | Stimuli: auditory Response: magazine approach |
Low transporter function enhances reversal learning | (Nonkes et al., 2011) | |
| 5HT2A/C receptor antagonists | Stimuli: spatial Response: lever press |
5HT2A antagonists impair, while 5HT2C antagonists improve, reversal learning; 5HT2C antagonist effects mediated in OFC | (Boulougouris et al., 2008b; Boulougouris and Robbins, 2010) | |
| New world monkey | Serotonin depletion in OFC | Stimuli: visual Response: touchscreen |
Loss of serotonin in OFC produces perserverative deficit in reversal learning | (Clarke et al., 2004; Clarke et al., 2005; Clarke et al., 2007) |
| Old world monkey | 5-HT3 receptor antagonists | Stimuli: objects Response: foraging in a WGTA |
5-HT receptor antagonists enhance both acquisition and reversal learning | (Domeney et al., 1991; Arnsten et al., 1997) |
| Genetic variation in the serotonin transporter gene | Stimuli: objects Response: foraging in a WGTA |
Rare variants in the serotonin transporter gene are associated with poorer reversal learning | (Izquierdo et al., 2007; Vallender et al., 2009) | |
| Stimuli: visual Response: touchscreen |
Subjects carrying the short allele show improved reversal learning | (Jedema et al., 2010) | ||
| Normal human subjects | Tryptophan depletion | Stimuli: visual Response: touchscreen |
No measured effect | (Murphy et al., 2002; Evers et al., 2005a; Evers et al., 2005b) |
WGTA, Wisconsin General Testing Apparatus; OFC, orbitofrontal cortex
Dopamine
Though depletion of dopamine in OFC does not impair reversal learning the way that serotonin depletion does (Clarke et al., 2007), there are clear data linking dopaminergic systems to reversal learning. A recent study found that depletion of dopamine, but not serotonin, in the medial caudate nucleus results in a non-perseverative impairment on reversal learning in marmosets (Clarke et al., 2011).
Pharmacological studies, on the other hand, have provided convincing evidence for dopaminergic modulation of reversal learning. Using an object discrimination task, Ridley and colleagues reported that both indirect dopamine agonists and dopamine receptor antagonists, d-amphetamine and haloperidol, respectively, produced behaviorally-specific problems with reversal (Ridley et al., 1981a, Ridley et al., 1981b). While both selectively impaired performance in reversal, amphetamine increased perseverative errors, and haloperidol produced “non-perseverative” responding. Haloperidol was found to block the effect of amphetamine (Ridley et al., 1981b), showing that amphetamine-induced perseveration was due to increased dopamine output produced by the drug (as opposed to an action on another monoamine system).
Studies of the effects of amphetamine, or its analogues, on reversal learning in rodent models have generated variable results, including impairments (Idris et al., 2005, McLean et al., 2010), no effect (Wilpizeski and Hamilton, 1964) or improvements (Kulig and Calhoun, 1972, Weiner and Feldon, 1986); the variability in types of reversal used (spatial vs. visual; appetitively-reinforced vs. escape-reinforced; Pavlovian vs. instrumental) and the inconsistency in doses and routes of administration make a direct resolution of these disparate findings challenging.
The fact that haloperidol blocked the effects of amphetamine, while producing an impairment itself (Ridley et al., 1981b, Idris et al., 2005), raises the hypothesis that over or under stimulation of dopamine D2-like receptor function causes impairments in reversal learning, and over the years, this hypothesis has received remarkable support. In rats, activation or antagonism of D2-like receptors (D2 and D3 receptors, in particular) affects reversal learning (Idris et al., 2005, Boulougouris et al., 2008a), and the particularly important role of the D2 receptor gene has been confirmed through the study of dopamine D2 receptor gene knockout mice (Kruzich and Grandy, 2004, Kruzich et al., 2006, De Steno and Schmauss, 2009). In non-human primates, activation or antagonism of D2 and D3 receptors disrupts reversal learning (Smith et al., 1999, Lee et al., 2007), with similar effects in human subjects (Mehta et al., 2001). It is important to note that not all these studies agree on the symmetrical effects of agonists and antagonists, with some showing effects of one, alone with no effects of the other; however, these discrepancies are almost certainly related to variation in doses administered and the difficulty of the reversal problem being solved. These studies do not imply that dopamine D1 receptors are unrelated to reversal learning; indeed, systemic treatment with the D1 agonist SKF81297 results in a selective early reversal learning impairments in mice, leaving visual discrimination learning unaffected (Izquierdo et al., 2006).
Beyond the behavioral pharmacological data, recent studies in a variety of species indicate that individual differences in reversal learning are related to D2-mediated dopamine transmission, with low receptor availability predicting poor reversal in otherwise normal mice (Laughlin et al., 2011), non-human primates (Groman et al., 2011) and humans (Jocham et al., 2009). Additionally, the reversal learning deficits in patients with psychostimulant addiction, a condition associated with low D2 receptor availability (Volkow et al., 2001, Lee et al., 2009), are reversed by administration of a D2-like receptor agonist (Ersche et al., 2011), suggesting that the treatment implications of this dopamine-mediated effect on reversal learning are strong. These findings are summarized in Table 2.
Table 2.
Summary of evidence for dopaminergic modulation of reversal learning.
| Species | Manipulation | Task Conditions | Effect | Reference |
|---|---|---|---|---|
| Mouse | Dopamine D2 receptor gene knockout | Stimuli: odor Response: digging in medium |
Impairs reversal learning, with mixed evidence of effects on discrimination acquisition | (Kruzich and Grandy, 2004; Kruzich et al., 2006; De Steno and Schmauss, 2009) |
| Dopamine D2 receptor antagonist | Impairs reversal learning, with no effect on acquisition | (De Steno and Schmauss, 2009) | ||
| Dopamine D1 agonist | Stimuli: visual Response: touchscreen |
Impairs early reversal learning, but not acquisition | (Izquierdo et al., 2006) | |
| Strain differences | Stimuli: spatial Response: nose poke aperture |
D2 receptor levels positively correlates with reversal learning competency; no relationship to acquisition | (Laughlin et al., 2011) | |
| Rat | Dopamine D2/D3 receptor agonist/antagonists | Stimuli: spatial Response: lever press |
Agonist, but not antagonist, impairs reversal learning | (Boulougouris et al., 2008a) |
| Dopamine releaser and dopamine D2 antagonists | Dopamine releaser impairs reversal learning; effect is blocked by D2 antagonist | (Idris et al., 2005) | ||
| New world monkey | Dopamine D3-preferring agonist and D2/D3 antagonists | Stimuli: objects Response: foraging in a WGTA |
D3-preferring agonist impaired performance; dopamine D2/D3 antagonists had no effect on their own but one (raclopride) prevented agonist effects | (Smith et al., 1999) |
| Dopamine releaser and dopamine D2 antagonists | Dopamine releaser and D2 antagonist impair reversal learning; amphetamine effect is blocked by D2 antagonist | (Ridley et al., 1981a; Ridley et al., 1981b) | ||
| Dopamine depletions in OFC | Stimuli: visual Response: touchscreen |
No effect on reversal learning | (Clarke et al., 2007) | |
| Old world monkey | Dopamine D1 and D2 receptor antagonists | Stimuli: visual Response: foraging in a WGTA |
D2, but not D1, receptor antagonists selectively impair reversal; D2 effects but not new learning, conditions confined to reversal, but not new learning, conditions. | (Lee et al., 2007) |
| Individual differences | D2-like receptor availability correlates positively with reversal learning competency, with no measured relationship to acquisition or retention | (Groman et al., 2011) | ||
| Normal human subjects | Dopamine D2 receptor agonist | Stimuli: visual Response: touchscreen |
D2 receptor agonists impair reversal learning, particularly in subjects with higher numbers of dopamine D2 receptors | (Mehta et al., 2001; Cools et al., 2009) |
| Genetic variation in the DRD2 gene | DRD2 alleles associated with low receptor expression predict poor reversal learning | (Jocham et al., 2009) | ||
| Stimulant-dependent humans | Dopamine D2 receptor agonist | Stimuli: visual Response: touchscreen |
Dopamine D2 receptor agonists enhance reversal learning | (Ersche et al., 2011) |
WGTA, Wisconsin General Testing Apparatus; OFC, orbitofrontal cortex.
Together, these data collectively support the notion that dopamine D2 receptors are major players in coordinating effective behavioral flexibility during reversal learning and that low receptor complement/function is associated with poor inhibitory control in this task.
Noradrenaline
Increases in synaptic noradrenaline levels elicited by selective reuptake inhibitors or alpha-2 adrenergic autoreceptors are sufficient to trigger improvements in reversal learning (Lapiz and Morilak, 2006, Lapiz et al., 2007, Seu and Jentsch, 2009, Seu et al., 2009). Though it is tempting to speculate that this effect is mediated by increased noradrenaline output into OFC, this remains untested. Consequently, it remains possible that noradrenergic mechanisms in another cortical or subcortical region are mediating this effect or that these mechanisms are secondary to increases in dopamine output in the frontal cortex (Millan et al., 2000, Bymaster et al., 2002). This is an area worthy of much more systematic study.
Reversal learning and addiction
Evenden (1999) described impulsivity as an all-encompassing term for “actions that are poorly conceived, prematurely expressed, unduly risky, or inappropriate to the situation and that often result in undesirable outcomes.” The term compulsivity, on the other hand, denotes a state of being compelled to behavior as if “driven to perform” them, despite one’s own volition, (DSM-IV, American Psychiatric Association). Though impulsivity and compulsivity are each multidimensional constructs relevant to behavioral addictions, they may both be considered endpoint exemplars of maladaptive decision-making; impulsivity clustered at the initiation of behavior and compulsivity most prevalent at the cessation of behavior. Similarly, they can each be positioned along a single, unitary dimension of cognitive rigidity, both characterized by frontocorticostriatal imbalance or dysfunction (Dalley et al., 2011). An important question is whether impulsive and compulsive behaviors, measured using reversal learning tasks, represent predisposing processes to addictions or whether they instead reflect sequelae arising from neuroadaptations caused by drug experience.
Poor inhibitory control: Cause and consequence?
Addiction and cognitive rigidity are related in a complex circuitous relationship that is not yet fully understood (Schoenbaum and Shaham, 2008). The observation that substance abusers exhibit inhibitory control deficits such as increased perseveration in reversal learning (Ersche et al., 2008) and risky decision-making may indicate pre-morbid vulnerability factors for addiction, direct consequences of long-term drug intake, or a combination of these (Verdejo-Garcia et al., 2008). Animal studies have substantiated both views: that exposure to addictive drugs causes cognitive deficits (Jentsch et al., 1997, Jentsch et al., 2002, Shoblock et al., 2003, Schoenbaum et al., 2004, Kantak et al., 2005) and that individual variation in inhibitory control influences addiction vulnerability (Dalley et al., 2007, Belin et al., 2008, Diergaarde et al., 2008, Perry and Carroll, 2008).
Daily exposure to cocaine over a 2-week period produces persistent deficits in reversal learning (Jentsch et al., 2002). This is strong evidence for deficits of inhibitory control following prolonged stimulant exposure; a similar long-lasting impairment has been found in rodent models of cocaine or methamphetamine administration, using a range of reversal-like tasks (Shoblock et al., 2003, Schoenbaum et al., 2004, Kantak et al., 2005, Schoenbaum and Setlow, 2005). Additionally, even brief exposure to methamphetamine results in selective impairments on a wide range of reversal tasks in rats: spatial reversal (White et al., 2009), response reversal (Cheng et al., 2007), and discrimination reversal learning (Izquierdo et al., 2010). The learning impairment observed in the latter study was reversal-specific, leaving initial discrimination learning, as well as attentional set shifting, unaffected. Follow-up studies in our lab confirm that impaired reversal learning is just one example of cognitive rigidity after relatively brief exposures to methamphetamine that do not produce any significant, measured dopaminergic neurotoxicity (Kosheleff et al., 2011).
Rats that, in turn, exhibit poor inhibitory control (assessed prior to any experience with drug) have been shown to acquire cocaine or nicotine self-administration faster and to exhibit greater overall intake and diminished extinction of the drug-taking response (Dalley et al., 2007, Belin et al., 2008, Diergaarde et al., 2008). While the precise relationship between poor inhibitory control and addiction liability is still unknown, this difference in propensity may be due to the fact that impulsive individuals are more sensitive to the acute stimulus effects of the drugs (Perkins et al., 2008). Additionally, inhibiting prepotent responses may be a phenotype that contributes to a more rapid change in drug-taking behavior from a “goal-directed” pattern of use to a more compulsive pattern (Groman et al., 2008). This relationship has not yet been shown for reversal learning measures, specifically.
A putative mechanism for the loss of control of drug use involves frontocorticostriatal adaptations after protracted and perhaps even brief exposure to drug. For example, imaging studies in humans reveal an increasing involvement of ventral-to-dorsal striatum with increasing severity of stimulant craving and habit (Volkow et al., 2006). This may represent the neurophysiological correlate to increased automaticity and habitized behavior (Takahashi et al., 2007) measured in reversal learning. Plasticity within frontocorticostriatal circuitry resulting from drug exposure could contribute to the transition from recreational use to addiction (Everitt et al., 2007). With more striatal control in addiction, there is a concomitant decrease in frontocortical (e.g. OFC) involvement in inhibitory control, and consequently decreased control over use of the drug.
The neurochemical mechanisms by which pre-morbid differences in inhibitory control function affect addiction liability are unknown, but recent studies suggest that low D2-like receptor function is a potential mechanistic determinant (Dalley et al., 2007, Zald et al., 2008, Ersche et al., 2011, Groman and Jentsch, in press). These results suggest that low D2-like receptor function is a molecular convergence point for pre-morbid genetic factors influencing impulsivity and for chronic stimulant-drug induced deficits of inhibitory control. This underscores the possibility that pre-morbid differences in inhibitory control affect vulnerability, and that addiction further impairs inhibitory control, as well as that a common molecular alteration may mediate the relationship between both associations.
Summary
Reversal learning is impaired in individuals affected by addictions and we have conceptually linked it to both the impulsive and compulsive aspects of drug-seeking and –taking. Though many questions still remain unanswered (see Questions for Future Research), this review has described the phenomena of reversal learning, underscored its relevance for understanding impulse control disorders and addictions, and defined what is known about the underlying biological determination of inhibitory control processes measured in these tasks. The literature reviewed here suggests that discrimination reversal learning may continue to be used and further developed as a diagnostic tool for pathology typified by poor inhibitory control. Preclinical and clinical research point to two interrelated neuroadaptations in addiction related to poor reversal learning: frontocorticostriatal circuitry dysregulation and poor dopamine (D2 receptor) modulation of this circuitry. If new therapeutics were to mitigate or ameliorate these adaptations, they have the potential to enhance the chance of abstinence and reduce the risk of relapse in addiction.
Questions for Future Research
The role for frontocorticostriatal circuits and dopamine D2 receptors are central to the mechanisms mediating inhibitory control abilities, yet little is known about genetic factors that code for individual differences in reversal learning. Candidate gene studies have confirmed roles for the serotonin and dopamine systems (Kruzich and Grandy, 2004, Izquierdo et al., 2007, De Steno and Schmauss, 2009, Jocham et al., 2009, Vallender et al., 2009, Brigman et al., 2010), and whole genome strategies have been initiated in an attempt to localize major effect loci in novel molecular systems (Laughlin et al., 2011). Because these genes theoretically also represent liability factors for disorders associated with extreme variations in impulsivity, they are crucial _targets of future research.
It remains unclear whether effective pharmacological treatment of inhibitory control problems will translate into a clinically-meaningful benefit. To test this, such a pharmacological treatment is required. In theory, cognitive-enhancing therapeutics could ameliorate problematic inhibitory control, and help with drug abstinence. One such study showed that impairments in reversal could be rescued by subchronic citalopram treatment (Lapiz-Bluhm et al., 2009). To date, atomoxetine, and other noradrenaline reuptake inhibitors, remain some of the best-characterized tools (Lapiz et al., 2007, Seu and Jentsch, 2009, Seu et al., 2009), yet their ability to modulate problematic drug use remains undemonstrated.
Acknowledgments
This work was supported by Public Health Service grants 1SC2MH087974 (Izquierdo), the NIH MBRS program at CSULA (grant R25 GM61331) and by the Consortium for Neuropsychiatric Phenomics at UCLA (UL1-DE019580 and RL1-MH083270). Additional support was derived from PHS grants P20-DA022539 and P50-MH077248 (Jentsch).
Footnotes
Disclosure/Conflicts of Interest: There is nothing to disclose, nor are there any conflicts of interest.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. APA; Washington DC: 1994. [Google Scholar]
- Arnsten AF, Lin CH, Van Dyck CH, Stanhope KJ. The effects of 5-HT3 receptor antagonists on cognitive performance in aged monkeys. Neurobiol Aging. 1997;18:21–28. doi: 10.1016/s0197-4580(96)00162-5. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Balleine BW, O’Doherty JP. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology. 2010;35:48–69. doi: 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerman DM, Deacon RM, Seeburg PH, Rawlins JN. GluR-A-Deficient mice display normal acquisition of a hippocampus-dependent spatial reference memory task but are impaired during spatial reversal. Behav Neurosci. 2003;117:866–870. doi: 10.1037/0735-7044.117.4.866. [DOI] [PubMed] [Google Scholar]
- Bari A, Theobald DE, Caprioli D, Mar AC, Aidoo-Micah A, Dalley JW, Robbins TW. Serotonin modulates sensitivity to reward and negative feedback in a probabilistic reversal learning task in rats. Neuropsychopharmacology. 2010;35:1290–1301. doi: 10.1038/npp.2009.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkus C, Feyder M, Graybeal C, Wright T, Wiedholz L, Izquierdo A, Kiselycznyk C, Schmitt W, Sanderson DJ, Rawlins JN, Saksida LM, Bussey TJ, Sprengel R, Bannerman D, Holmes A. Do GluA1 knockout mice exhibit behavioral abnormalities relevant to the negative or cognitive symptoms of schizophrenia and schizoaffective disorder? Neuropharmacology. 2011 doi: 10.1016/j.neuropharm.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes JM, Costall B, Coughlan J, Domeney AM, Gerrard PA, Kelly ME, Naylor RJ, Onaivi ES, Tomkins DM, Tyers MB. The effects of ondansetron, a 5-HT3 receptor antagonist, on cognition in rodents and primates. Pharmacol Biochem Behav. 1990;35:955–962. doi: 10.1016/0091-3057(90)90385-u. [DOI] [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320:1352–1355. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonette GB, Martins GJ, Franz TM, Harper ES, Schoenbaum G, Powell EM. Double dissociation of the effects of medial and orbital prefrontal cortical lesions on attentional and affective shifts in mice. J Neurosci. 2008;28:11124–11130. doi: 10.1523/JNEUROSCI.2820-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonette GB, Powell EM. Reversal learning and attentional set-shifting in mice. Neuropharmacology. 2011 doi: 10.1016/j.neuropharm.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulougouris V, Castane A, Robbins TW. Dopamine D2/D3 receptor agonist quinpirole impairs spatial reversal learning in rats: investigation of D3 receptor involvement in persistent behavior. Psychopharmacology (Berl) 2008a;202:611–620. doi: 10.1007/s00213-008-1341-2. [DOI] [PubMed] [Google Scholar]
- Boulougouris V, Dalley JW, Robbins TW. Effects of orbitofrontal, infralimbic and prelimbic cortical lesions on serial spatial reversal learning in the rat. Behav Brain Res. 2007;179:219–228. doi: 10.1016/j.bbr.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Boulougouris V, Glennon JC, Robbins TW. Dissociable effects of selective 5-HT2A and 5-HT2C receptor antagonists on serial spatial reversal learning in rats. Neuropsychopharmacology. 2008b;33:2007–2019. doi: 10.1038/sj.npp.1301584. [DOI] [PubMed] [Google Scholar]
- Boulougouris V, Robbins TW. Enhancement of spatial reversal learning by 5-HT2C receptor antagonism is neuroanatomically specific. J Neurosci. 2010;30:930–938. doi: 10.1523/JNEUROSCI.4312-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JA, Potenza MN. The neurobiology and genetics of impulse control disorders: relationships to drug addictions. Biochem Pharmacol. 2008;75:63–75. doi: 10.1016/j.bcp.2007.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigman JL, Mathur P, Harvey-White J, Izquierdo A, Saksida LM, Bussey TJ, Fox S, Deneris E, Murphy DL, Holmes A. Pharmacological or genetic inactivation of the serotonin transporter improves reversal learning in mice. Cereb Cortex. 2010;20:1955–1963. doi: 10.1093/cercor/bhp266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM, Rothblat LA. Discrimination of computer-graphic stimuli by mice: a method for the behavioral characterization of transgenic and gene-knockout models. Behav Neurosci. 2001;115:957–960. doi: 10.1037//0735-7044.115.4.957. [DOI] [PubMed] [Google Scholar]
- Bymaster FP, Katner JS, Nelson DL, Hemrick-Luecke SK, Threlkeld PG, Heiligenstein JH, Morin SM, Gehlert DR, Perry KW. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27:699–711. doi: 10.1016/S0893-133X(02)00346-9. [DOI] [PubMed] [Google Scholar]
- Castane A, Theobald DE, Robbins TW. Selective lesions of the dorsomedial striatum impair serial spatial reversal learning in rats. Behav Brain Res. 2010;210:74–83. doi: 10.1016/j.bbr.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng RK, Etchegaray M, Meck WH. Impairments in timing, temporal memory, and reversal learning linked to neurotoxic regimens of methamphetamine intoxication. Brain Res. 2007;1186:255–266. doi: 10.1016/j.brainres.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Chudasama Y. Animal models of prefrontal-executive function. Behav Neurosci. 2011;125:327–343. doi: 10.1037/a0023766. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Bussey TJ, Muir JL. Effects of selective thalamic and prelimbic cortex lesions on two types of visual discrimination and reversal learning. Eur J Neurosci. 2001;14:1009–1020. doi: 10.1046/j.0953-816x.2001.01607.x. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Dissociable contributions of the orbitofrontal and infralimbic cortex to pavlovian autoshaping and discrimination reversal learning: further evidence for the functional heterogeneity of the rodent frontal cortex. J Neurosci. 2003;23:8771–8780. doi: 10.1523/JNEUROSCI.23-25-08771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Functions of frontostriatal systems in cognition: Comparative neuropsychopharmacological studies in rats, monkeys and humans. Biol Psychol. 2006;73:19–38. doi: 10.1016/j.biopsycho.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Clarke HF, Dalley JW, Crofts HS, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion. Science. 2004;304:878–880. doi: 10.1126/science.1094987. [DOI] [PubMed] [Google Scholar]
- Clarke HF, Hill GJ, Robbins TW, Roberts AC. Dopamine, but not serotonin, regulates reversal learning in the marmoset caudate nucleus. J Neurosci. 2011;31:4290–4297. doi: 10.1523/JNEUROSCI.5066-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke HF, Robbins TW, Roberts AC. Lesions of the medial striatum in monkeys produce perseverative impairments during reversal learning similar to those produced by lesions of the orbitofrontal cortex. J Neurosci. 2008;28:10972–10982. doi: 10.1523/JNEUROSCI.1521-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke HF, Walker SC, Crofts HS, Dalley JW, Robbins TW, Roberts AC. Prefrontal serotonin depletion affects reversal learning but not attentional set shifting. J Neurosci. 2005;25:532–538. doi: 10.1523/JNEUROSCI.3690-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke HF, Walker SC, Dalley JW, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion is behaviorally and neurochemically specific. Cereb Cortex. 2007;17:18–27. doi: 10.1093/cercor/bhj120. [DOI] [PubMed] [Google Scholar]
- Cools R, Frank MJ, Gibbs SE, Miyakawa A, Jagust W, Esposito DM. Striatal dopamine predicts outcome-specific reversal learning and its sensitivity to dopaminergic drug administration. J Neurosci. 2009;29:1538–1543. doi: 10.1523/JNEUROSCI.4467-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Boettiger CA. Impulsivity, frontal lobes and risk for addiction. Pharmacol Biochem Behav. 2009;93:237–247. doi: 10.1016/j.pbb.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011;69:680–694. doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, Pena Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Mar AC, Economidou D, Robbins TW. Neurobehavioral mechanisms of impulsivity: fronto-striatal systems and functional neurochemistry. Pharmacol Biochem Behav. 2008;90:250–260. doi: 10.1016/j.pbb.2007.12.021. [DOI] [PubMed] [Google Scholar]
- De Steno DA, Schmauss C. A role for dopamine D2 receptors in reversal learning. Neuroscience. 2009;162:118–127. doi: 10.1016/j.neuroscience.2009.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delatour B, Gisquet-Verrier P. Lesions of the prelimbic-infralimbic cortices in rats do not disrupt response selection processes but induce delay-dependent deficits: evidence for a role in working memory? Behav Neurosci. 1999;113:941–955. doi: 10.1037//0735-7044.113.5.941. [DOI] [PubMed] [Google Scholar]
- Delatour B, Gisquet-Verrier P. Functional role of rat prelimbic-infralimbic cortices in spatial memory: evidence for their involvement in attention and behavioural flexibility. Behav Brain Res. 2000;109:113–128. doi: 10.1016/s0166-4328(99)00168-0. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Primate analogue of the Wisconsin Card Sorting Test: effects of excitotoxic lesions of the prefrontal cortex in the marmoset. Behav Neurosci. 1996;110:872–886. doi: 10.1037//0735-7044.110.5.872. [DOI] [PubMed] [Google Scholar]
- Diergaarde L, Pattij T, Poortvliet I, Hogenboom F, de Vries W, Schoffelmeer AN, De Vries TJ. Impulsive choice and impulsive action predict vulnerability to distinct stages of nicotine seeking in rats. Biol Psychiatry. 2008;63:301–308. doi: 10.1016/j.biopsych.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Divac I, Rosvold HE, Szwarcbart MK. Behavioral effects of selective ablation of the caudate nucleus. J Comp Physiol Psychol. 1967;63:184–190. doi: 10.1037/h0024348. [DOI] [PubMed] [Google Scholar]
- Domeney AM, Costall B, Gerrard PA, Jones DN, Naylor RJ, Tyers MB. The effect of ondansetron on cognitive performance in the marmoset. Pharmacol Biochem Behav. 1991;38:169–175. doi: 10.1016/0091-3057(91)90606-3. [DOI] [PubMed] [Google Scholar]
- Eagle DM, Bari A, Robbins TW. The neuropsychopharmacology of action inhibition: cross-species translation of the stop-signal and go/no-go tasks. Psychopharmacology (Berl) 2008;199:439–456. doi: 10.1007/s00213-008-1127-6. [DOI] [PubMed] [Google Scholar]
- El-Ghundi M, O’Dowd BF, Erclik M, George SR. Attenuation of sucrose reinforcement in dopamine D1 receptor deficient mice. Eur J Neurosci. 2003;17:851–862. doi: 10.1046/j.1460-9568.2003.02496.x. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Roiser JP, Abbott S, Craig KJ, Muller U, Suckling J, Ooi C, Shabbir SS, Clark L, Sahakian BJ, Fineberg NA, Merlo-Pich EV, Robbins TW, Bullmore ET. Response perseveration in stimulant dependence is associated with striatal dysfunction and can be ameliorated by a d(2/3) receptor agonist. Biol Psychiatry. 2011;70:754–762. doi: 10.1016/j.biopsych.2011.06.033. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Roiser JP, Robbins TW, Sahakian BJ. Chronic cocaine but not chronic amphetamine use is associated with perseverative responding in humans. Psychopharmacology (Berl) 2008;197:421–431. doi: 10.1007/s00213-007-1051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenden JL. Varieties of impulsivity. Psychopharmacology (Berl) 1999;146:348–361. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Hutcheson DM, Ersche KD, Pelloux Y, Dalley JW, Robbins TW. The orbital prefrontal cortex and drug addiction in laboratory animals and humans. Ann N Y Acad Sci. 2007;1121:576–597. doi: 10.1196/annals.1401.022. [DOI] [PubMed] [Google Scholar]
- Evers EA, Cools R, Clark L, van der Veen FM, Jolles J, Sahakian BJ, Robbins TW. Serotonergic modulation of prefrontal cortex during negative feedback in probabilistic reversal learning. Neuropsychopharmacology. 2005a;30:1138–1147. doi: 10.1038/sj.npp.1300663. [DOI] [PubMed] [Google Scholar]
- Evers EA, Tillie DE, van der Veen FM, Lieben CK, Jolles J, Deutz NE, Schmitt JA. Effects of a novel method of acute tryptophan depletion on plasma tryptophan and cognitive performance in healthy volunteers. Psychopharmacology (Berl) 2005b;178:92–99. doi: 10.1007/s00213-004-2141-y. [DOI] [PubMed] [Google Scholar]
- Fellows LK, Farah MJ. Ventromedial frontal cortex mediates affective shifting in humans: evidence from a reversal learning paradigm. Brain. 2003;126:1830–1837. doi: 10.1093/brain/awg180. [DOI] [PubMed] [Google Scholar]
- Fineberg NA, Potenza MN, Chamberlain SR, Berlin HA, Menzies L, Bechara A, Sahakian BJ, Robbins TW, Bullmore ET, Hollander E. Probing compulsive and impulsive behaviors, from animal models to endophenotypes: a narrative review. Neuropsychopharmacology. 2010;35:591–604. doi: 10.1038/npp.2009.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, Akers CA, Clinton SM, Phillips PE, Akil H. A selective role for dopamine in stimulus-reward learning. Nature. 2011;469:53–57. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Block AE, Tse MT. Inactivation of the medial prefrontal cortex of the rat impairs strategy set-shifting, but not reversal learning, using a novel, automated procedure. Behav Brain Res. 2008;190:85–96. doi: 10.1016/j.bbr.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Franken IH, van Strien JW, Nijs I, Muris P. Impulsivity is associated with behavioral decision-making deficits. Psychiatry Res. 2008;158:155–163. doi: 10.1016/j.psychres.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Ghahremani DG, Monterosso J, Jentsch JD, Bilder RM, Poldrack RA. Neural components underlying behavioral flexibility in human reversal learning. Cereb Cortex. 2010;20:1843–1852. doi: 10.1093/cercor/bhp247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghods-Sharifi S, Haluk DM, Floresco SB. Differential effects of inactivation of the orbitofrontal cortex on strategy set-shifting and reversal learning. Neurobiol Learn Mem. 2008;89:567–573. doi: 10.1016/j.nlm.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Gisquet-Verrier P, Delatour B. The role of the rat prelimbic/infralimbic cortex in working memory: not involved in the short-term maintenance but in monitoring and processing functions. Neuroscience. 2006;141:585–596. doi: 10.1016/j.neuroscience.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Graybeal C, Feyder M, Schulman E, Saksida LM, Bussey TJ, Brigman JL, Holmes A. Paradoxical reversal learning enhancement by stress or prefrontal cortical damage: rescue with BDNF. Nature Neurosci. doi: 10.1038/nn.2954. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groman SM, James AS, Jentsch JD. Poor response inhibition: At the nexus between substance abuse and attention deficit/hyperactivity disorder. Neurosci Biobehav Rev. 2008 doi: 10.1016/j.neubiorev.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groman SM, Jentsch JD. Cognitive control and the dopamine D2-like receptor: A dimensional understanding of addiction. Depress Anxiety. doi: 10.1002/da.20897. in press. [DOI] [PubMed] [Google Scholar]
- Groman SM, Lee B, London ED, Mandelkern MA, James AS, Feiler K, Rivera R, Dahlbom M, Sossi V, Vandervoort E, Jentsch JD. Dorsal striatal D2-like receptor availability covaries with sensitivity to positive reinforcement during discrimination learning. J Neurosci. 2011;31:7291–7299. doi: 10.1523/JNEUROSCI.0363-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN. The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat. 2003;26:317–330. doi: 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Hornak J, O’Doherty J, Bramham J, Rolls ET, Morris RG, Bullock PR, Polkey CE. Reward-related reversal learning after surgical excisions in orbito-frontal or dorsolateral prefrontal cortex in humans. J Cogn Neurosci. 2004;16:463–478. doi: 10.1162/089892904322926791. [DOI] [PubMed] [Google Scholar]
- Idris NF, Repeto P, Neill JC, Large CH. Investigation of the effects of lamotrigine and clozapine in improving reversal-learning impairments induced by acute phencyclidine and D-amphetamine in the rat. Psychopharmacology (Berl) 2005;179:336–348. doi: 10.1007/s00213-004-2058-5. [DOI] [PubMed] [Google Scholar]
- Itami S, Uno H. Orbitofrontal cortex dysfunction in attention-deficit hyperactivity disorder revealed by reversal and extinction tasks. Neuroreport. 2002;13:2453–2457. doi: 10.1097/00001756-200212200-00016. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Belcher AM, Scott L, Cazares VA, Chen J, O’Dell SJ, Malvaez M, Wu T, Marshall JF. Reversal-specific learning impairments after a binge regimen of methamphetamine in rats: possible involvement of striatal dopamine. Neuropsychopharmacology. 2010;35:505–514. doi: 10.1038/npp.2009.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Murray EA. Opposing effects of amygdala and orbital prefrontal cortex lesions on the extinction of instrumental responding in macaque monkeys. Eur J Neurosci. 2005;22:2341–2346. doi: 10.1111/j.1460-9568.2005.04434.x. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Murray EA. Selective bilateral amygdala lesions in rhesus monkeys fail to disrupt object reversal learning. J Neurosci. 2007;27:1054–1062. doi: 10.1523/JNEUROSCI.3616-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Newman TK, Higley JD, Murray EA. Genetic modulation of cognitive flexibility and socioemotional behavior in rhesus monkeys. Proc Natl Acad Sci U S A. 2007;104:14128–14133. doi: 10.1073/pnas.0706583104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Suda RK, Murray EA. Bilateral orbital prefrontal cortex lesions in rhesus monkeys disrupt choices guided by both reward value and reward contingency. J Neurosci. 2004;24:7540–7548. doi: 10.1523/JNEUROSCI.1921-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Wiedholz LM, Millstein RA, Yang RJ, Bussey TJ, Saksida LM, Holmes A. Genetic and dopaminergic modulation of reversal learning in a touchscreen-based operant procedure for mice. Behav Brain Res. 2006;171:181–188. doi: 10.1016/j.bbr.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Jedema HP, Gianaros PJ, Greer PJ, Kerr DD, Liu S, Higley JD, Suomi SJ, Olsen AS, Porter JN, Lopresti BJ, Hariri AR, Bradberry CW. Cognitive impact of genetic variation of the serotonin transporter in primates is associated with differences in brain morphology rather than serotonin neurotransmission. Mol Psychiatry. 2010;15:512–522. 446. doi: 10.1038/mp.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Olausson P, De La Garza R, 2nd, Taylor JR. Impairments of reversal learning and response perseveration after repeated, intermittent cocaine administrations to monkeys. Neuropsychopharmacology. 2002;26:183–190. doi: 10.1016/S0893-133X(01)00355-4. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Redmond DE, Jr, Elsworth JD, Taylor JR, Youngren KD, Roth RH. Enduring cognitive deficits and cortical dopamine dysfunction in monkeys after long-term administration of phencyclidine. Science. 1997;277:953–955. doi: 10.1126/science.277.5328.953. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impaired inhibition of conditioned responses produced by subchronic administration of phencyclidine to rats. Neuropsychopharmacology. 2001;24:66–74. doi: 10.1016/S0893-133X(00)00174-3. [DOI] [PubMed] [Google Scholar]
- Jocham G, Klein TA, Neumann J, von Cramon DY, Reuter M, Ullsperger M. Dopamine DRD2 polymorphism alters reversal learning and associated neural activity. J Neurosci. 2009;29:3695–3704. doi: 10.1523/JNEUROSCI.5195-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B, Mishkin M. Limbic lesions and the problem of stimulus--reinforcement associations. Exp Neurol. 1972;36:362–377. doi: 10.1016/0014-4886(72)90030-1. [DOI] [PubMed] [Google Scholar]
- Kantak KM, Udo T, Ugalde F, Luzzo C, Di Pietro N, Eichenbaum HB. Influence of cocaine self-administration on learning related to prefrontal cortex or hippocampus functioning in rats. Psychopharmacology (Berl) 2005;181:227–236. doi: 10.1007/s00213-005-2243-1. [DOI] [PubMed] [Google Scholar]
- Kosheleff AR, Grimes M, O’Dell SJ, Marshall JF, Izquierdo A. Work aversion and associated changes in dopamine and serotonin transporter after methamphetamine exposure in rats. Psychopharmacology (Berl) 2011 doi: 10.1007/s00213-011-2367-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruzich PJ, Grandy DK. Dopamine D2 receptors mediate two-odor discrimination and reversal learning in C57BL/6 mice. BMC Neurosci. 2004;5:12. doi: 10.1186/1471-2202-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruzich PJ, Mitchell SH, Younkin A, Grandy DK. Dopamine D2 receptors mediate reversal learning in male C57BL/6J mice. Cogn Affect Behav Neurosci. 2006;6:86–90. doi: 10.3758/cabn.6.1.86. [DOI] [PubMed] [Google Scholar]
- Kulig BM, Calhoun WH. Enhancement of successive discrimination reversal learning by methamphetamine. Psychopharmacologia. 1972;27:233–240. doi: 10.1007/BF00422803. [DOI] [PubMed] [Google Scholar]
- Lapiz MD, Bondi CO, Morilak DA. Chronic treatment with desipramine improves cognitive performance of rats in an attentional set-shifting test. Neuropsychopharmacology. 2007;32:1000–1010. doi: 10.1038/sj.npp.1301235. [DOI] [PubMed] [Google Scholar]
- Lapiz MD, Morilak DA. Noradrenergic modulation of cognitive function in rat medial prefrontal cortex as measured by attentional set shifting capability. Neuroscience. 2006;137:1039–1049. doi: 10.1016/j.neuroscience.2005.09.031. [DOI] [PubMed] [Google Scholar]
- Lapiz-Bluhm MD, Soto-Pina AE, Hensler JG, Morilak DA. Chronic intermittent cold stress and serotonin depletion induce deficits of reversal learning in an attentional set-shifting test in rats. Psychopharmacology (Berl) 2009;202:329–341. doi: 10.1007/s00213-008-1224-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin RE, Grant TL, Williams RW, Jentsch JD. Genetic dissection of behavioral flexibility: reversal learning in mice. Biol Psychiatry. 2011;69:1109–1116. doi: 10.1016/j.biopsych.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Groman S, London ED, Jentsch JD. Dopamine D2/D3 receptors play a specific role in the reversal of a learned visual discrimination in monkeys. Neuropsychopharmacology. 2007;32:2125–2134. doi: 10.1038/sj.npp.1301337. [DOI] [PubMed] [Google Scholar]
- Lee B, London ED, Poldrack RA, Farahi J, Nacca A, Monterosso JR, Mumford JA, Bokarius AV, Dahlbom M, Mukherjee J, Bilder RM, Brody AL, Mandelkern MA. Striatal dopamine d2/d3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. J Neurosci. 2009;29:14734–14740. doi: 10.1523/JNEUROSCI.3765-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man MS, Clarke HF, Roberts AC. The Role of the Orbitofrontal Cortex and Medial Striatum in the Regulation of Prepotent Responses to Food Rewards. Cereb Cortex. 2008 doi: 10.1093/cercor/bhn137. [DOI] [PubMed] [Google Scholar]
- Masaki D, Yokoyama C, Kinoshita S, Tsuchida H, Nakatomi Y, Yoshimoto K, Fukui K. Relationship between limbic and cortical 5-HT neurotransmission and acquisition and reversal learning in a go/no-go task in rats. Psychopharmacology (Berl) 2006;189:249–258. doi: 10.1007/s00213-006-0559-0. [DOI] [PubMed] [Google Scholar]
- McAlonan K, Brown VJ. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav Brain Res. 2003;146:97–103. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- McLean SL, Neill JC, Idris NF, Marston HM, Wong EH, Shahid M. Effects of asenapine, olanzapine, and risperidone on psychotomimetic-induced reversal-learning deficits in the rat. Behav Brain Res. 2010;214:240–247. doi: 10.1016/j.bbr.2010.05.043. [DOI] [PubMed] [Google Scholar]
- Mehta MA, Swainson R, Ogilvie AD, Sahakian J, Robbins TW. Improved short-term spatial memory but impaired reversal learning following the dopamine D(2) agonist bromocriptine in human volunteers. Psychopharmacology (Berl) 2001;159:10–20. doi: 10.1007/s002130100851. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Lejeune F, Gobert A, Brocco M, Auclair A, Bosc C, Rivet JM, Lacoste JM, Cordi A, Dekeyne A. S18616, a highly potent spiroimidazoline agonist at alpha(2)-adrenoceptors: II. Influence on monoaminergic transmission, motor function, and anxiety in comparison with dexmedetomidine and clonidine. J Pharmacol Exp Ther. 2000;295:1206–1222. [PubMed] [Google Scholar]
- Morellini F, Sivukhina E, Stoenica L, Oulianova E, Bukalo O, Jakovcevski I, Dityatev A, Irintchev A, Schachner M. Improved reversal learning and working memory and enhanced reactivity to novelty in mice with enhanced GABAergic innervation in the dentate gyrus. Cereb Cortex. 2010;20:2712–2727. doi: 10.1093/cercor/bhq017. [DOI] [PubMed] [Google Scholar]
- Murphy FC, Smith KA, Cowen PJ, Robbins TW, Sahakian BJ. The effects of tryptophan depletion on cognitive and affective processing in healthy volunteers. Psychopharmacology (Berl) 2002;163:42–53. doi: 10.1007/s00213-002-1128-9. [DOI] [PubMed] [Google Scholar]
- Murray EA, Baxter MG, Gaffan D. Monkeys with rhinal cortex damage or neurotoxic hippocampal lesions are impaired on spatial scene learning and object reversals. Behav Neurosci. 1998;112:1291–1303. doi: 10.1037//0735-7044.112.6.1291. [DOI] [PubMed] [Google Scholar]
- Murray EA, Gaffan D. Prospective memory in the formation of learning sets by rhesus monkeys (Macaca mulatta) J Exp Psychol Anim Behav Process. 2006;32:87–90. doi: 10.1037/0097-7403.32.1.87. [DOI] [PubMed] [Google Scholar]
- Murray EA, Richmond BJ. Role of perirhinal cortex in object perception, memory, and associations. Curr Opin Neurobiol. 2001;11:188–193. doi: 10.1016/s0959-4388(00)00195-1. [DOI] [PubMed] [Google Scholar]
- Nonkes LJ, Maes JH, Homberg J. Improved cognitive flexibility in serotonin transporter knockout rats is unchanged following chronic cocaine self-administration. Addict Biol. 2011 doi: 10.1111/j.1369-1600.2011.00351.x. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Lerman C, Coddington SB, Jetton C, Karelitz JL, Scott JA, Wilson AS. Initial nicotine sensitivity in humans as a function of impulsivity. Psychopharmacology (Berl) 2008;200:529–544. doi: 10.1007/s00213-008-1231-7. [DOI] [PubMed] [Google Scholar]
- Perry JL, Carroll ME. The role of impulsive behavior in drug abuse. Psychopharmacology (Berl) 2008;200:1–26. doi: 10.1007/s00213-008-1173-0. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Adams S, Kesner RP. Differential involvement of the dorsal anterior cingulate and prelimbic-infralimbic areas of the rodent prefrontal cortex in spatial working memory. Behav Neurosci. 1998;112:293–303. doi: 10.1037//0735-7044.112.2.293. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Detrick S, Kesner RP. Involvement of the prelimbic-infralimbic areas of the rodent prefrontal cortex in behavioral flexibility for place and response learning. J Neurosci. 1999a;19:4585–4594. doi: 10.1523/JNEUROSCI.19-11-04585.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Wilcox C, Raso M, Kesner RP. Involvement of rodent prefrontal cortex subregions in strategy switching. Behav Neurosci. 1999b;113:32–41. doi: 10.1037//0735-7044.113.1.32. [DOI] [PubMed] [Google Scholar]
- Remijnse PL, Nielen MM, van Balkom AJ, Cath DC, van Oppen P, Uylings HB, Veltman DJ. Reduced orbitofrontal-striatal activity on a reversal learning task in obsessive-compulsive disorder. Arch Gen Psychiatry. 2006;63:1225–1236. doi: 10.1001/archpsyc.63.11.1225. [DOI] [PubMed] [Google Scholar]
- Rich EL, Shapiro M. Rat prefrontal cortical neurons selectively code strategy switches. J Neurosci. 2009;29:7208–7219. doi: 10.1523/JNEUROSCI.6068-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley RM, Baker HF, Haystead TA. Perseverative behaviour after amphetamine; dissociation of response tendency from reward association. Psychopharmacology (Berl) 1981a;75:283–286. doi: 10.1007/BF00432439. [DOI] [PubMed] [Google Scholar]
- Ridley RM, Haystead TA, Baker HF. An analysis of visual object reversal learning in the marmoset after amphetamine and haloperidol. Pharmacol Biochem Behav. 1981b;14:345–351. doi: 10.1016/0091-3057(81)90401-9. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Chemistry of the mind: neurochemical modulation of prefrontal cortical function. J Comp Neurol. 2005;493:140–146. doi: 10.1002/cne.20717. [DOI] [PubMed] [Google Scholar]
- Robbins TW, James M, Owen AM, Sahakian BJ, Lawrence AD, McInnes L, Rabbitt PM. A study of performance on tests from the CANTAB battery sensitive to frontal lobe dysfunction in a large sample of normal volunteers: implications for theories of executive functioning and cognitive aging. Cambridge Neuropsychological Test Automated Battery. J Int Neuropsychol Soc. 1998;4:474–490. doi: 10.1017/s1355617798455073. [DOI] [PubMed] [Google Scholar]
- Roberts AC, Wallis JD. Inhibitory control and affective processing in the prefrontal cortex: neuropsychological studies in the common marmoset. Cereb Cortex. 2000;10:252–262. doi: 10.1093/cercor/10.3.252. [DOI] [PubMed] [Google Scholar]
- Romer D, Betancourt L, Giannetta JM, Brodsky NL, Farah M, Hurt H. Executive cognitive functions and impulsivity as correlates of risk taking and problem behavior in preadolescents. Neuropsychologia. 2009;47:2916–2926. doi: 10.1016/j.neuropsychologia.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Behrens TE, Kennerley SW, Baxter MG, Buckley MJ, Walton ME, Rushworth MF. Frontal cortex subregions play distinct roles in choices between actions and stimuli. J Neurosci. 2008;28:13775–13785. doi: 10.1523/JNEUROSCI.3541-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Murray EA. Amygdala and orbitofrontal cortex lesions differentially influence choices during object reversal learning. J Neurosci. 2008;28:8338–8343. doi: 10.1523/JNEUROSCI.2272-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Murray EA. Dissociable effects of subtotal lesions within the macaque orbital prefrontal cortex on reward-guided behavior. J Neurosci. 2011;31:10569–10578. doi: 10.1523/JNEUROSCI.0091-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Saddoris MP, Ramus SJ, Shaham Y, Setlow B. Cocaine-experienced rats exhibit learning deficits in a task sensitive to orbitofrontal cortex lesions. Eur J Neurosci. 2004;19:1997–2002. doi: 10.1111/j.1460-9568.2004.03274.x. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B. Cocaine makes actions insensitive to outcomes but not extinction: implications for altered orbitofrontal-amygdalar function. Cereb Cortex. 2005;15:1162–1169. doi: 10.1093/cercor/bhh216. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B, Nugent SL, Saddoris MP, Gallagher M. Lesions of orbitofrontal cortex and basolateral amygdala complex disrupt acquisition of odor-guided discriminations and reversals. Learn Mem. 2003;10:129–140. doi: 10.1101/lm.55203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Shaham Y. The role of orbitofrontal cortex in drug addiction: a review of preclinical studies. Biol Psychiatry. 2008;63:256–262. doi: 10.1016/j.biopsych.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seu E, Jentsch JD. Effect of acute and repeated treatment with desipramine or methylphenidate on serial reversal learning in rats. Neuropharmacology. 2009;57:665–672. doi: 10.1016/j.neuropharm.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seu E, Lang A, Rivera RJ, Jentsch JD. Inhibition of the norepinephrine transporter improves behavioral flexibility in rats and monkeys. Psychopharmacology (Berl) 2009;202:505–519. doi: 10.1007/s00213-008-1250-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoblock JR, Maisonneuve IM, Glick SD. Differences between d-methamphetamine and d-amphetamine in rats: working memory, tolerance, and extinction. Psychopharmacology (Berl) 2003;170:150–156. doi: 10.1007/s00213-003-1522-y. [DOI] [PubMed] [Google Scholar]
- Smith AG, Neill JC, Costall B. The dopamine D3/D2 receptor agonist 7-OH-DPAT induces cognitive impairment in the marmoset. Pharmacol Biochem Behav. 1999;63:201–211. doi: 10.1016/s0091-3057(98)00230-5. [DOI] [PubMed] [Google Scholar]
- Stalnaker TA, Franz TM, Singh T, Schoenbaum G. Basolateral amygdala lesions abolish orbitofrontal-dependent reversal impairments. Neuron. 2007;54:51–58. doi: 10.1016/j.neuron.2007.02.014. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Roesch MR, Stalnaker TA, Schoenbaum G. Cocaine exposure shifts the balance of associative encoding from ventral to dorsolateral striatum. Front Integr Neurosci. 2007;1 doi: 10.3389/neuro.07.011.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallender EJ, Lynch L, Novak MA, Miller GM. Polymorphisms in the 3′ UTR of the serotonin transporter are associated with cognitive flexibility in rhesus macaques. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:467–475. doi: 10.1002/ajmg.b.30835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Plasse G, Feenstra MG. Serial reversal learning and acute tryptophan depletion. Behav Brain Res. 2008;186:23–31. doi: 10.1016/j.bbr.2007.07.017. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Lawrence AJ, Clark L. Impulsivity as a vulnerability marker for substance-use disorders: review of findings from high-risk research, problem gamblers and genetic association studies. Neurosci Biobehav Rev. 2008;32:777–810. doi: 10.1016/j.neubiorev.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, Gifford A, Wong C, Pappas N. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner I, Feldon J. Reversal and nonreversal shifts under amphetamine. Psychopharmacology (Berl) 1986;89:355–359. doi: 10.1007/BF00174374. [DOI] [PubMed] [Google Scholar]
- White IM, Minamoto T, Odell JR, Mayhorn J, White W. Brief exposure to methamphetamine (METH) and phencyclidine (PCP) during late development leads to long-term learning deficits in rats. Brain Res. 2009;1266:72–86. doi: 10.1016/j.brainres.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilpizeski CR, Hamilton CL. Drug-induced decrement in spatial reversal learning in the white rat. Psychopharmacologia. 1964;6:475–479. doi: 10.1007/BF00429574. [DOI] [PubMed] [Google Scholar]
- Winocur G, Eskes G. Prefrontal cortex and caudate nucleus in conditional associative learning: dissociated effects of selective brain lesions in rats. Behav Neurosci. 1998;112:89–101. doi: 10.1037//0735-7044.112.1.89. [DOI] [PubMed] [Google Scholar]
- Winocur G, Mills JA. Effects of caudate lesions on avoidance behavior in rats. J Comp Physiol Psychol. 1969;68:552–557. doi: 10.1037/h0027645. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Olausson P, Taylor JR, Jentsch JD. Insight into the relationship between impulsivity and substance abuse from studies using animal models. Alcohol Clin Exp Res. 2010;34:1306–1318. doi: 10.1111/j.1530-0277.2010.01215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zald DH, Cowan RL, Riccardi P, Baldwin RM, Ansari MS, Li R, Shelby ES, Smith CE, McHugo M, Kessler RM. Midbrain dopamine receptor availability is inversely associated with novelty-seeking traits in humans. J Neurosci. 2008;28:14372–14378. doi: 10.1523/JNEUROSCI.2423-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]