Abstract
Shorter survival in the elderly has been associated with deterioration of the immune system and also with functional disability. To analyze the relationship between functional and immune impairment in older individuals, we studied 100 elderly who lived in a nursing home, were age matched, and grouped according to their functional status. We characterized cell subpopulations by flow cytometry, quantified TREC by RT–PCR, and measured the T-cell proliferation and activation response (IFN-γ by ELISPOT, CD69) against anti-CD3 and CMV. Specific antibody titers against influenza virus and CMV were determined by ELISA. Individuals with worse functional status had significantly higher levels of NK cells and fewer B cells. These poorly functioning elders also had a significantly lower proportion of CD4+ T cells, increased CD8+ T cells, and a decreased CD4/CD8 ratio. TREC levels in CD4+ T cells were significantly lower in individuals with a high disability. Lower TREC levels correlated with a lower frequency of naïve T-cell subpopulations (CD45RA+CCR7+) and higher percentages of effector cells (CD45RA−CCR7−). The functionally impaired group had lower anti-CD3 responses, but gradually increased responses against CMV. Similarly, the higher CMV titers were found in elderly with worse functional status. On the contrary, the functional response in vivo, and the titer of antibodies generated after vaccination against influenza virus, was higher in individuals with better performance status. In summary, we concluded that the functional decline of elderly individuals was clearly associated with the aging of their immune system, and the intensity of the response to CMV.
Keywords: Immunosenescence, Functional status, T lymphocytes, Differentiation, CMV
Introduction
Older people suffer from age-associated changes in the immune system, including decreased immune function, increased incidence and severity of infections, development of autoimmune phenomena, and cancer (DelaRosa et al. 2006; Prelog 2006). These defective immune responses are also manifested in a reduced ability of vaccines and infections to induce immunological memory, and a lower incidence of acute rejection in elderly transplant patients (Bradley et al. 2001; Weinberger et al. 2008). The aging process seems to alter both branches of the immune system, innate and adaptive in different ways: the innate immunity seems to be better preserved globally (Dace and Apte 2008; Le Garff-Tavernier et al. 2010), while the adaptive immune response exhibits profound age-dependent modifications (Haynes and Maue 2009). Lower T-cell counts can be partially explained by thymic involution which decreases output of naïve T cells and reduces the numbers of T cells in peripheral blood and lymphoid tissues (Aspinall and Andrew 2000; Linton et al. 2005). In support, studies characterizing the T-cell receptor excision circles (TREC) (Ribeiro and Perelson 2007) showed that the frequency of the TREC declines exponentially with age (Naylor et al. 2005). The elderly accumulate highly differentiated T cells. Mature T cells have a reduced susceptibility to apoptosis, and oligoclonal expansions against CMV and other chronical antigens are evident (Clambey et al. 2005; Cao et al. 2009). Since CMV can reactivate promptly after periods of immunosuppression, a substantial proportion of the immune repertoire may be required to control its replication. Studies have associated the changes in the number of lymphocytes expressing activation markers both with age and with CMV antibody titer (Looney et al. 1999). The expansion of these functional T cells may contribute to anti-CMV surveillance, but these T cells may also exert pathogenic effects upon cells and tissues in close proximity via recently suggested molecular mechanisms (Bolovan-Fritts et al. 2007; Qiu et al. 2008). Moreover, they may contribute to the inflammation of unknown origin that occurs during aging (Ferrucci et al. 2005), as well as the pathogenesis and progression of inflammatory diseases (Soderberg-Naucler 2006).
In agreement, an immune risk profile (IRP) was initially identified in the Swedish OCTO immune longitudinal study using a cluster analysis approach (Ferguson et al. 1995). A higher 2-year mortality occurred in a population of very old Swedish individuals who had a high frequency of CD8 T cells, a low frequency of CD4 cells, and a poor proliferative response to Con A. The inverted CD4/CD8 ratio was the sole marker significantly associated with the IRP (Wikby et al. 1998). Subsequently, cytomegalovirus (CMV) infection has been shown to exert a major impact on the immunosenescence process (Hadrup et al. 2006).
Functional disability is an important health indicator in the elderly, and jeopardizes quality of life, causes heavy social impact with long-term institutionalization, and increases use of medical care (Guralnik et al. 1996). The main risk factors for functional disability in the elderly were low sociocultural level, chronic diseases, immune disorders, body mass index above 25, cognitive impairment, depression, and sedentary lifestyle. The Barthel index (BI) was developed to assess disability in patients with neuromuscular and musculoskeletal conditions that required inpatient rehabilitation (Mahoney and Barthel 1965; Sainsbury et al. 2005). Extensive literature has evaluated the predictors of functional decline in samples of elderly people (age, cognitive status, etc.), but few studies have tried to find a physiological cause for this deterioration (Ishizaki et al. 2004).
Both the impairment of functional capacity (Wilkinson and Sainsbury 1998) and the deterioration of the immune system (Wayne et al. 1990) have been associated with increased morbidity and mortality. To further explore this relationship, the BI was used to group our elders, who had similar ages and diseases, into four groups with different functional capabilities. We conducted an observational cross-sectional study that evaluated and compared the state of the immune system at both phenotypic and functional levels in the groups. We described, for the first time, a direct relationship between the functional status of the elderly and their immune system. We observed a poorer functional status in elderly groups with reduced immune capabilities and with increased humoral and cellular response to CMV.
Materials and methods
Study population
One hundred elderly (76 women and 24 men) living at the Santa Teresa nursing home (Oviedo, Spain) were enrolled in the study. Blood for the hematological and immunological analyses were drawn from the 100 individuals. All subjects received a physical examination and answered standardized questionnaires to assess clinical history, current disease, and medication. Inclusion criteria were elders who were older than 69 years and who were classified according to the Barthel index as a measure of their functional status. Exclusion criteria were conditions with possible influence on the immune system such as recent or current infection, inflammation, autoimmune or malignant disease, malnutrition, abnormal laboratory data (hemoglobin <12 mg/dL, leukopenia <3,500 cells/μL, neutropenia <1,500 cells/μL, leukocytosis >15,000 cells/μL, platelets <105 cells/μL, and PCR >5 mg/dL), and pharmacological interference (steroids, nonsteroidal anti-inflammatory agents, and immunosuppressive drugs). Informed consent was obtained from the elders prior to participation in the study. The study was approved by the Hospital Central de Asturias (Oviedo, Spain) ethics committee.
The functional abilities of the subjects were assessed by using the Barthel Index of Activities of Daily Living (BI) (Mahoney and Barthel 1965). Each person was evaluated at 10 tasks that measured daily functioning for various activities of daily living and mobility. The highest BI score (100) meant that the person needed no assistance with any part of the tasks. The BI scores were used to divide the elderly into four groups from total independence to total dependence: group 0 = 100 (n = 25), group 1 = 95–80 (n = 25), group 2 = 75–40 (n = 27), and group 3 = 35–0 (n = 23), as previously described (Saxena et al. 2006; Supervia et al. 2008).
Hematological analysis and immunological phenotyping
The hematological parameters were determined by a Sysmex XT-2000i (Syxmex, Hamburg-Norderstedt, Germany). Cytometric studies were acquired and analyzed in the FACSCalibur Cytometer using CellQuest software (BD Biosciences, San José, CA, USA). CaliBRITE Beads (BD Biosciences) were used to adjust instrument settings, set fluorescence compensation, and check instrument sensitivity. Surface staining of EDTA peripheral blood was performed with Multiset CD3-FITC/CD16+56-PE/CD45-PerCP/CD19-APC Reagent, anti-CD4 (APC), anti-CD8 (PE), anti-CD8 (PerCP), anti-CD45RA (FITC), anti-CD27 (PE) (Immunostep, Salamanca, Spain), anti-CD4 (PerCP), anti-CD28 (PerCP), anti-CCR7 (Alexa Fluor 647), anti-CD3 (FITC), anti-CD45RO (FITC), anti-CD25 (APC) (BD Biosciences), anti-NKG2D (PE), and anti-CD127 (PE) (eBioscience, San Diego, CA, USA). One hundred microliters of whole blood from elderly were stained with different combinations of labeled monoclonal antibodies for 20 min at room temperature. Samples were red blood lysed with FACS Lysing Solution (BD Biosciences), washed in PBS, and analyzed with CellQuest software in the FACSCalibur Cytometer. Appropriate isotype control mAbs were used for marker settings.
To analyze the proliferation status of CD4+ and CD8+ T cells, peripheral blood mononuclear cells (PBMC) were isolated by centrifugation on Ficoll-Hypaque gradients (Lymphoprep; Nycomed, Oslo, Norway). CD4+ and CD8+ T cells from elderly were isolated with magnetic beads (Myltenyi Biotec GmbH, Bergisch Gladbach, Germany). Cells were lysed and fixed with Fixation/Permeabilization Solution (eBioscience), permeabilized with Permeabilizing Buffer (eBioscience), and stained with anti-Ki-67-PE (eBioscience) for 30 min at room temperature. Cells were washed and resuspended in 1% paraformaldehyde until FACS analysis. Frequencies of positive cells for Ki-67 from groups 0 and 1 (n = 10) and groups 2 and 3 (n = 10) were compared.
TRECs quantification
The DNA of isolated CD4+ (purity > 90%) from PBMCs was extracted using a QIAamp DNA Mini Kit (Qiagen GmbH, Hilden, Germany) according to the manufacturer’s instructions. Quantification of signal-joint (sj) TREC was performed by using SYBR Green real-time quantitative PCR and an iCycler thermocycler (Bio-Rad; Life Science Research Group, Hercules, CA, USA). The sequences of the utilized primers were the following: forward primer 5′-CCATGCTGACACCTCTGGTT-3′, reverse primer 5′-TCGTGAGAACG GTGAATGAAG-3′. As an internal control measurement to normalize for input DNA, the Cα constant region that remains present on TCR genes despite rearrangement processes was amplified in every sample tested (forward primer 5′-CCTGATCCTCTTGTCCCACAG-3′, reverse primer 5′-GGATTT AGAGTCTCTCAGCTGGTACA-3′). Thermal cycling conditions began with 50°C for 2 min and 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. Experimental samples were run in duplicate and the replicate average value recorded as the sample result.
Cytomegalovirus (CMV) and influenza virus serology
Immunoglobulin G levels of CMV-specific antibodies were determined by enzyme-linked immunosorbent assay Vir-ELISA Anti-CMV-IgG (Viro-Immun Labor-Diagnostika GmbH, Oberursel, Germany) according to the manufacturer’s specifications. Patient samples were quantified and interpreted by means of the calculation of the ratio (cutoff index = OD value of sample/cut-off value), whereby a ratio of 1.0 is equivalent to the cut-off value. Cutoff indexes >1.1 were considered positive, and the result of this ratio is a semi-quantitative titer.
Anti-influenza virus antibodies in serum obtained from elderly individuals were measured by ELISA as previously described (Ohishi et al. 2002), with some modifications (Kang et al. 2004). The OD values of individual samples were compared against a calibration curve made by the OD values of serial dilutions of the same internal control serum from a healthy vaccinated young person throughout the experiments. Because antibody titer decreased significantly with time since vaccination, we normalized this value by dividing the titer by the elapsed time since immunization.
Activation studies
The activation of heparinized whole blood by anti-CD3 or CMV antigens was assessed by surface staining with anti-CD69 (eBioscience). Briefly, heparinized whole blood (250 μL) was stimulated with soluble anti-CD3 (10 ng/mL) (eBioscience) or with a CMV supernatant (104 PFU/mL) in 15 mL conical polypropylene tubes for 18 h at 37°C and 5% carbon dioxide. CMV-infected cell lysate was prepared by infecting human embryonic lung fibroblasts with the AD169 CMV strain, and viral titers in the supernatant were determined by standard plaque assays. The virus was inactivated by five repeated freeze–thaw cycles. The cells were also stained with anti-CD4 and anti-CD8 mononuclear antibodies.
Proliferation cultures
PBMCs were resuspended in PBS at a final concentration of 5–10 × 106 cells/mL and incubated with 1.5 μM CFSE (Invitrogen, Paisley, Scotland, UK) for 10 min at 37°C, washed with RPMI 1640 medium containing 2 × 103 M l-glutamine and Hepes (BioWhitaker, Verviers, Belgium) twice, and cultured at 1 × 106 cells/mL in the presence of soluble anti-CD3 (10 ng/mL) and CMV extract (104 PFU/mL). The proliferative responses of CD4+ and CD8+ T cells were analyzed on day 7 by FACSCalibur after staining with anti-CD4 and anti-CD8.
ELISPOT assay
PBMCs were resuspended to 2 × 106/mL in RPMI 1640 medium containing 2 × 10−3 M l-glutamine and Hepes and supplemented with 10% FCS (ICN Flow, Costa Mesa, CA, USA) and antibiotics. PBMC were stimulated in triplicate with anti-CD3 (10 ng/mL) and with the same extract of CMV used in the activation and proliferation cultures. PBMC (100 μL, 1.5 × 105/well) were placed into each well of a 96-well filter plate (Millipore, Billerica, MA, USA) that was coated with anti-IFN-γ Ab (BD Biosciences), and the cells were cultured for 20 h. IFN-γ captured by the plate-bound Ab was detected by biotinylated anti-IFN-γ Ab (BD Biosciences), followed by streptavidin-conjugated alkaline phosphatase (BD Biosciences). Spots were developed using the red color-forming substrate 3-amino-9-ethylcarbazole (BD Biosciences). Spots were counted with the ELISPOT reader system ELR02 (Autoimmun Diagnostika GmbH, Straβberg, Germany). A sample of cultured cells was stained with anti-CD3 (FITC) and this percentage of CD3+ cells was used to calculate the number of T cells in each well of the assay.
Statistical analysis
Two groups were compared with the Mann–Whitney U non-parametric method for data that were not normally distributed, or with the Student’s t test. More than two groups were compared by using the non-parametric Kruskal–Wallis test or by ANOVA analysis for data that were normally distributed. Results were expressed as median and range or mean and standard deviation. In some graphs, mean and standard error of the mean were displayed. Correlations between variables were assessed by the nonparametric Spearman test (ρ). The χ2 test was used to compare dichotomous variables and multiple linear regression was used in multivariate analysis. Analyses were performed using the SPSS 15.0 statistical software package program (SPSS Inc. Chicago, IL, USA) and p values of 0.05 or less were considered significant.
Results
Demographic and hematologic characteristics of the study population
The characteristics of the 100 individuals enrolled in the study are shown in Table 1. People were placed into four groups according to their BI. The female/male ratio in each group was 2.1:1 in group 0, 4.0:1 in group 1, 2.7:1 in group 2, and 2.8:1 in group 3. There were no significant differences in the age of donors belonging to the four groups. The average number of drugs taken by members included in the study and the most frequent pathologies associated with each group were listed.
Table 1.
Characteristics of the subjects participating in the study according to their BI
| Group 0 | Group 1 | Group 2 | Group 3 | |
|---|---|---|---|---|
| (n = 24) | (n = 26) | (n = 27) | (n = 23) | |
| Age (years) | ||||
| Mean ± SE | 85.7 ± 1.2 | 86.0 ± 0.9 | 87.0 ± 1.1 | 88.2 ± 1.3 |
| Range | (75–97) | (77–94) | (74–97) | (69–96) |
| No. of subjects investigated | ||||
| Women | 16 | 22 | 20 | 17 |
| Men | 8 | 4 | 7 | 6 |
| Mean number of drugs | 4.2 | 6.3 | 6.9 | 5.9 |
| Pathologies | ||||
| Cognitive impairment | 0 | 1 | 7 | 9 |
| Dementia | 1 | 2 | 2 | 4 |
| Osteoporosis | 4 | 5 | 8 | 2 |
| Arterial hypertension | 9 | 13 | 11 | 5 |
| COPD | 3 | 2 | 2 | 3 |
| Osteoarthritis | 7 | 3 | 8 | 3 |
| Depression | 4 | 4 | 4 | 3 |
| Heart failure | 1 | 1 | 2 | 2 |
| Ischemic heart disease | 1 | 3 | 2 | 0 |
| Dyslipidemia | 3 | 4 | 7 | 2 |
| Diabetes | 2 | 5 | 3 | 6 |
COPD chronic obstructive pulmonary disease
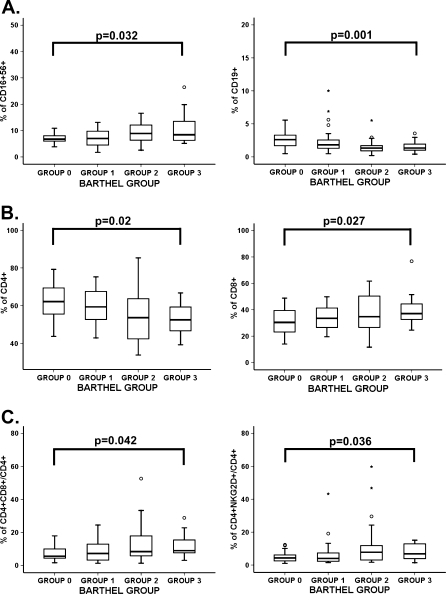
Blood cell counts and an immune phenotype of majority populations were performed in all individuals included in the study. The groups did not show significant differences in absolute numbers or percentages in the blood cell counts (Table 2). In contrast, the groups with worse functional status showed a gradual higher percentage of NK cells (CD16+56+) and reduction of B cells (CD19+) (Fig. 1a). Although the differences in T lymphocytes between the four groups were not significant, the populations of CD4+ and CD8+ T lymphocytes were significantly different between groups (ANOVA test, p = 0.02 and p = 0.027, respectively) (Fig. 1b). Elders in group 3 had the lowest percentage of CD4+ and the highest percentage of CD8+ T cells. The four groups presented similar frequencies of CD4+ regulatory T cells (data not shown).
Table 2.
Hematology values of studied subjects
| Group 0 | Group 1 | Group 2 | Group 3 | p value between groupsa | |
|---|---|---|---|---|---|
| (n = 24) | (n = 26) | (n = 27) | (n = 23) | ||
| RBCs (106/μL) | 4.4 (3.5–5.2) | 4.4 (3.1–5.4) | 4.3 (2.2–5.7) | 4.2 (2.0–4.8) | ns |
| Hemoglobin (g/dL) | 13.4 (9.9–16.4) | 13.0 (10.4–17.3) | 13.2 (9.5–17.3) | 12.3 (7.9–16.2) | ns |
| Hematocrit (%) | 40.6 (31.1–48.9) | 39.3 (29.1–48.1) | 40.2 (18.0–51.4) | 38.0 (23.5–46.9) | ns |
| MCV (fL) | 90.7 (86.2–108.5) | 90.1 (73.8–101.5) | 92.2 (85.5–103.8) | 92.7 (69.6–119.3) | ns |
| Platelets (103/μL) | 200.5 (129.0–270.0) | 229.5 (132.0–571.0) | 218.3 (130.0–334.0) | 226.1 (140.0–402.0) | ns |
| WBCs (103/μL) | 5.9 (3.1–8.2) | 6.7 (3.8–11.9) | 6.1 (3.5–11.8) | 6.5 (3.2–9.5) | ns |
| Neutrophils (103/μL) | 3.4 (1.8–5.3) | 4.0 (1.4–7.7) | 3.6 (1.9–6.7) | 3.8 (1.6–7.2) | ns |
| Monocytes (103/μL) | 0.48 (0.29–0.77) | 0.49 (0.24–3.5) | 0.49 (0.28–1.0) | 0.44 (0.05–0.86) | ns |
| Lymphocytes (103/μL) | 1.77 (0.87–2.81) | 1.77 (0.85–3.37) | 1.82 (0.88–5.01) | 1.94 (0.65–3.44) | ns |
aCalculated using the Mann–Whitney U non-parametric test among the four BI groups
Fig. 1.
Immune phenotype in peripheral blood from elderly according to their BI group. Elders were stratified according to their BI (group 0 BI = 100, group 1 BI = 95–80, group 2 BI = 75–40, and group 3 BI = 35–0). The number of donors in each group was group 0 = 24, group 1 = 26, group 2 = 27, and group 3 = 23. Whole blood from elderly individuals was stained with different antibody combinations and analyzed by flow cytometry (105 cells acquired in each experiment). Outlier values were represented by circles and extreme values by stars, calculated by adding 1.5 and 3 times the IR to the 75th percentile, respectively. The ANOVA test (when data were normally distributed) and Kruskal–Wallis non-parametric methods (when data were not normally distributed) were used to compare frequencies between groups. p values are depicted in the panels. a Percentages of CD16+56+ and CD19+ cells with respect to the total CD45+ cells were compared between the four groups of elderly. Staining was performed with “Multiset CD3-FITC/CD16+56-PE/CD45-PerCP/CD19-APC” and frequencies of CD16+56 and CD19+ cells in gated CD45+ subsets were analyzed. b Percentages of CD4+ and CD8+ cells were analyzed with respect to the total CD45+CD3+ and were compared between the four groups. Staining was performed with anti-CD3-FITC, anti-CD4-APC, and anti-CD8-PerCP to gate CD4+ and CD8+ T cells. c Percentages of CD4+ T cells expressing CD8 and expressing NKG2D in peripheral blood from elderly. Whole blood was stained with anti-CD3-FITC, anti-NKG2D-PE, anti-CD8-PerCP, and anti-CD4-APC. Frequencies of NKG2D+ cells in gated CD3+CD4+ lymphocytes were quantified
Loss of CD28 is a typical feature of senescence in T cells (Borthwick et al. 1996). CD28 expression on CD8+ T cells did not differ significantly between groups, whereas the CD4+CD28− T-cell population showed a tendency to increase with worse functional status (data not shown). The double positive CD4+CD8+ and CD4+NKG2D+ are two other subsets of T cells that have been related to aging (Pawelec 1995; Colombatti et al. 1998; Alonso-Arias et al. 2009). The proportion of CD4+CD8+ and CD4+NKG2D+ in the peripheral blood increased in elders with worse functional status (Fig. 1c). Donors in groups 2 and 3 had significantly higher levels than those in group 0. In summary, these results demonstrated that many of the age-related changes in immune parameters were dependent on the functional status of the elderly.
IRP parameters and functional status
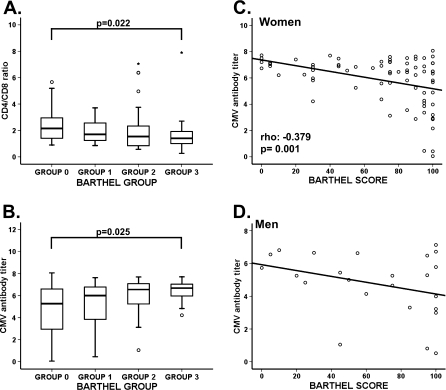
Parameters that define the IRP are the inverted ratio CD4/CD8 (ratio < 1.0) and CMV infection (Olsson et al. 2000; Hadrup et al. 2006). Low CD4+ numbers and elevated CD8+ T cells were associated with a worse functional status. Accordingly, the CD4/CD8 ratio were significantly different between groups (Kruskal–Wallis non-parametric test, p = 0.022) (Fig. 2a). The proportion of elders with an inverted ratio also showed a gradual and significant increase with reduced functional status (χ2 test, p = 0.041) (Table 3).
Fig. 2.
CD4/CD8 ratio, anti-CMV antibody titer from elderly of the four BI groups, and correlation between the CMV antibody titer and the BI score in women and men. The two most important parameters that define the immune risk profile (IRP) are the inverted CD4/CD8 ratio and CMV infection. a CD4/CD8 ratios were analyzed and compared between the four groups. Staining was performed with anti-CD3-FITC, anti-CD4-APC, and anti-CD8-PerCP to gate CD4+ and CD8+ T cells. CD4/CD8 ratio less than 1.0 was used to identify individuals with an IRP. b Serum anti-CMV antibody titer was measured by ELISA and compared between the BI groups. Patient samples are quantified and interpreted by means of the calculation of the ratio (cut-off index = OD value of sample/cut-off value), whereby a ratio of 1.0 is equivalent to the cut-off value. Cut-off index >1.1 were considered positive and the result of this ratio is a semi-quantitative titer. Outlier and extreme values are represented by circles and extreme values by stars, calculated by adding 1.5 and 3 times the IR to the 75th percentile, respectively. The Kruskal–Wallis non-parametric method was used to compare frequencies between groups and p values are depicted in the panels. c Correlation between CMV antibody titer and the BI score in women. d Relationship between CMV antibody titer and BI score in men. A non-parametric Spearman test was applied to calculate the correlations, p value, and coefficient of correlation which are listed in the lower left hand corner
Table 3.
IRP parameters in the BI groups
| Group 0 | Group 1 | Group 2 | Group 3 | p value between groups | |
|---|---|---|---|---|---|
| (n = 24) | (n = 26) | (n = 27) | (n = 23) | ||
| Inverted CD4/CD8 ratio | |||||
| Ratio < 1.0 | 2 | 4 | 10 | 8 | |
| (%) | (8.3%) | (15.4%) | (37.0) | (34.8%) | 0.041a |
| CMV serology | |||||
| Negative | 4 | 1 | 2 | 1 | |
| (%) | (16%) | (4%) | (7.4%) | (4.4%) | nsa |
aCalculated using χ2 test between all groups
The relationship between CMV infection and functional status was also analyzed. Although the largest percentage of CMV-seronegative elders was in group 0 (16%), no significant differences between groups were observed (Table 3). However, we detected a gradual increase in CMV antibody titer concomitant with deteriorating functional status (Fig. 2b).
We have also examined the relationship between the median BI score and CMV serological status. The median BI score was not significantly related to CMV serological status: the median in seronegative individuals was 95 points (IR = 53.75 points) and in CMV seropositive was 75 points (IR = 50 points). However, comparison of the CMV antibody titer with the BI score across all individuals revealed a negative correlation (Spearman Rho test; rho −0.346; p = 0.0005). Only CMV serological status was independently associated with the BI score in a multivariate analysis (multiple linear regression, p < 0.001) with age and gender as confounding factors.
Comparison between the BI score and CMV titer separately in men and women showed that the correlation remained significant among women (Spearman Rho test; rho −0.379; p = 0.001) (Fig. 2c) but not among men (Fig. 2d). The lack of correlation in men may be due to the low number of male volunteers in the study. In fact, there is a clear trend, which likely would be significant with a higher number of volunteers.
T-cell differentiation subsets
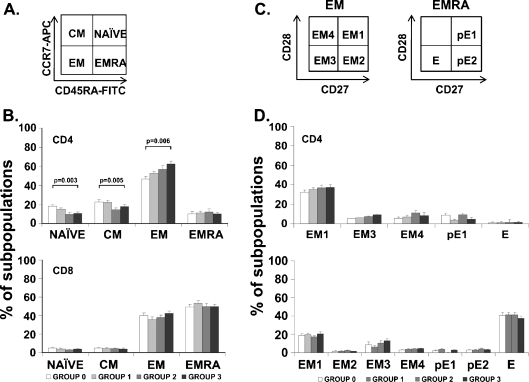
One of the most widely accepted models in immunosenescence is that the T-cell compartment is progressively deteriorating with advancing age. T cells can be separated into functionally different populations using combinations of cell surface markers such as the tyrosine phosphatase isoform CD45RA and the chemokine receptor CCR7. With these markers, we subdivided the T cells into naïve (NAÏVE; CD45RA+CCR7+), central memory (CM; CD45RA−CCR7+), effector memory (EM; CD45RA−CCR7−), and effector memory RA (EMRA; CD45RA+CCR7−) (Sallusto et al. 1999). To detect an association between functional status and the differentiation degree of T-cell subsets, we compared the distribution of the distinct T-cell subpopulations in young and elderly individuals, as shown by representative samples (Fig. 3a). The analysis of these subsets of CD4+ T cells revealed that the functional deterioration in elderly was related to the reduced population of undifferentiated subsets (Fig. 3b). In fact, the frequency of both the NAÏVE CD4+ cells and the CM populations in elders from groups 2 and 3 were significantly decreased compared to those from groups 0 and 1. On the contrary, the more differentiated subset EM had a significant and progressive increase from group 0 to group 3. Although the proportions of EMRA cells increased with age, they were not significantly different between the groups (Fig. 3b). CD8+ T cells also did not differ significantly among groups: the level of the four populations was similar in all groups. Most CD8+ T cells were in the EM and EMRA subsets, which were the last stages of differentiation (Fig. 3b).
Fig. 3.
Distribution of CD4+ and CD8+ T cells into naïve, central memory, effector memory and effector memory RA, and distribution of EM and EMRA in CD4+ and CD8+ T cells into subsets defined by CD28 and CD27 expression. Expression of CD45RA, CCR7, CD27, and CD28 was analyzed by flow cytometry in isolated CD4+ and CD8+ T cells from the four BI groups of elders. a Schematic model of the T-cell differentiation subsets accordingly to CD45RA and CCR7 expression. Whole blood was stained with anti-CD45RA-FICT, anti-CD8-PE, anti-CD4-PerCP, and anti-CCR7-APC, and 105 cells were acquired in each experiment. b Histograms represent percentage of cells in each subset (NAÏVE, CM, EM, and EMRA) in the four groups of elderly accordingly to their functional status (group 0—white bars, group 1—light gray bars, group 2—dark gray bars, group 3—black bars). Significant differences between subsets are indicated (ANOVA or Kruskal–Wallis non-parametric method). Each bar in the histograms represented the mean ± SEM. c Representative dot plots of the subsets defined by CD27 and CD28 expression for individuals in each group. EM T cells can be divided into EM1 (CD27+CD28+), EM2 (CD27+CD28null, only in CD8+ T cells), EM3 (CD27nullCD28null), and EM4 (CD27nullCD28+). Similarly, EMRA can be divided into pE1 (CD27+CD28+) and pE2 (CD27+CD28null, only in CD8 T cells) and E (CD27nullCD28null). d Percentage of cells in each subset in the four groups of elderly accordingly to their functional status (group 0—white bars, group 1—light gray bars, group 2—dark gray bars, group 3—black bars). Bars in the histograms represented the mean ± SEM
EM and EMRA are heterogeneous populations, and the staining of two additional markers, CD27 and CD28, has proven useful in identifying less differentiated (CD27+ and/or CD28+) or more differentiated (CD27nullCD28null) cells (Koch et al. 2008) (Fig. 3c). Differentiating CD4+ T cells lose expression of CD27 first and subsequently lose CD28 in a later phase (Amyes et al. 2003; van Leeuwen et al. 2004). In contrast, CD8+ T cells lose expression of CD28 first and then CD27 (Gamadia et al. 2003). Despite the existence of a different degree of differentiation defined by the expression of CCR7 and CD45RA markers, we found no significant differences in CD27 and CD28 expression between groups (Fig. 3d).
Taken together, these results indicated that a higher differentiation degree of CD4+ T cells, but not CD8+ T cells, was related to worse functional status. Thus, the maturation stage and number of past episodes of activation and cell cycling of CD4+ T cells differentiated elders with different motor abilities.
TREC quantification and basal proliferation
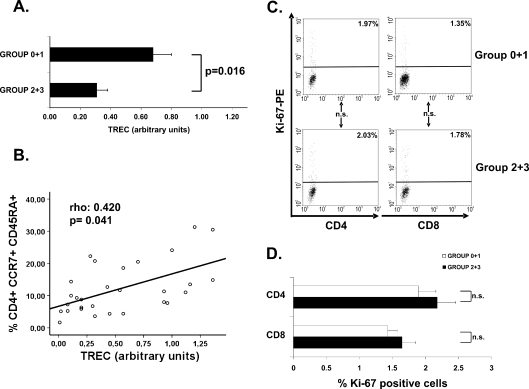
To further examine the differences found in the differentiated status of CD4+ subsets, we assessed the replicative history of these cells by quantifying the content of TREC in CD4+ T cells belonging to the four elder groups. TREC is a traceable molecular marker produced in newly naïve T cells; the content of TREC in peripheral T cells is an indicator of the number of divisions that the cell has undergone (Douek et al. 1998). Since sample volumes were insufficient to perform CD4+ T-cell isolation in all donors and differences were not found between groups 0 and 1 and between groups 2 and 3, we grouped samples: group 0 + 1 and group 2 + 3. The analysis of the groups displayed significant differences in TREC content. The TREC was lower in CD4+ T cells from elders with worse functional status and more differentiated subsets (Mann–Whitney U test, p = 0.016) (Fig. 4a).
Fig. 4.
TREC content in CD4+ T cells, its correlation with the NAÏVE subset and Ki-67 quantification. a The TREC content was measured in CD4+ T cells from elders belonging to group 0 + 1 (n = 14) and group 2 + 3 (n = 15). CD4+ population was isolated by magnetic bead separation and the TREC copy number was determined by real-time PCR. Experiments were conducted in duplicate and bars represented results from the grouped elders (mean ± SEM). b Relationship between TREC content and NAÏVE (CD4+CCR7+CD45RA+) subset in the four groups of elderly was analyzed. The correlations, p value, and coefficient of correlation were calculated by using the non-parametric Spearman test and are listed in the upper left hand corner. c The quantification of Ki-67 was performed in CD4+ and CD8+ T cells from elders belonging to group 0 + 1 (n = 10) and group 2 + 3 (n = 10). CD4+ and CD8+ populations were isolated by magnetic bead separation and the Ki-67 quantification was determined by intracellular staining and flow cytometry. Representative dot plots show the frequency of Ki-67 expression in CD4+ and CD8+ subsets from elderly with different functional status. Percentage of positive cells in each subpopulation in these representative experiments are expressed in the upper right corner. Appropriate isotype control mAbs were used for marker settings. d Histograms summarize the percentage of positive cells for Ki-67 (mean ± SEM). The Student’s t test method was used to compare frequencies between groups
Next, to determine whether the lower TREC number observed in elders with worse functional capabilities correlated to a lower frequency of naïve CD4+ T cells, we compared the TREC content to levels of naïve CD4+ T cells and detected a positive correlation (Spearman Rho test; rho 0.421; p = 0.041) (Fig. 4b). The elders with a lower TREC content had a worse functional status and lower frequency of naïve CD4+ T cells. Thus, cells from the elderly groups 2 and 3 have undergone more cell divisions since they migrated from the thymus than cells from groups 0 and 1.
To further examine the proliferative capacity, we measured the level of the cellular marker for proliferation Ki-67 on CD4+ and CD8+ populations isolated from 10 elderly in groups 0 and 1 and from 10 volunteers from groups 2 and 3 (Fig. 4c). No significant differences in Ki-67 levels on both cell populations between the two groups of elderly were detected, and thus the basal proliferation did not significantly differ between the groups (Fig. 4d).
Functional immunoresponse in vitro
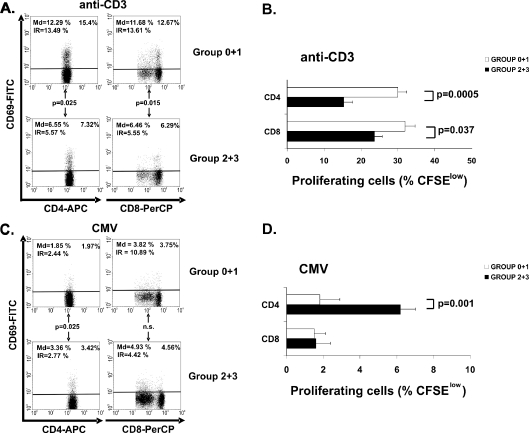
To evaluate whether phenotypic changes that were observed with the deteriorating functional ability were also associated with the reduced immune responsiveness associated with aging in previous reports, we measured the activation capability by comparing CD69 expression in CD4+ and CD8+ T cells after anti-CD3 stimulation. Elders with better functional status (group 0 + 1) showed a higher CD69 expression level in both CD4+ and CD8+ T cells than group 2 + 3 did (Student’s t test, p = 0.025 and p = 0.015, respectively) (Fig. 5a). Group 0 + 1 also exhibited a higher anti-CD3 proliferative response in the CD4+ and CD8+ T-cell subsets than group 2 + 3 (Student’s t test, p = 0.0005 and p = 0.037, respectively) (Fig. 5b).
Fig. 5.
CD69 expression and proliferative capacity of CD4+ and CD8+ depending on the functional capacity. Whole blood from the BI groups of elders (group 0 + 1, n = 12 and group 2 + 3, n = 14) was stimulated for 18 h and expression of CD69 in CD4+ and CD8+ T cells was evaluated by flow cytometry. Proliferative capacity of CD4+ and CD8+ T cells subsets was also evaluated in the two groups (group 0 + 1, n = 19 and group 2 + 3, n = 16) by labeling the PBMC with CFSE. Cells were stained and 1 × 105 cells were acquired per experiment. a Representative dot plots showing the frequency of CD69 expression in CD4+ and CD8+ subset from elderly with different functional status. Cells were stimulated using anti-CD3 (10 ng/mL). Percentage of positive cells in each subpopulation in this representative experiment is expressed in the upper right corner and summarized results from all donors (median and IR) were also expressed in dot plots. b Proliferative capacity of CD4+ and CD8+ T cells subsets in response to anti-CD3. PBMC were labeled with CFSE (1.5 μM) and cultured in presence of anti-CD3 (10 ng/mL) for 5 days. Percentage of dividing CD4+ and CD8+ T cells is represented. Bars represent results from the grouped elders (mean ± SEM). c Expression of CD69 into de CD4+ and CD8+ T cell subset was analyzed in the same way as in Fig. 5a in response to a CMV supernatant (104 PFU/mL). d Proliferative capacity of CD4+ and CD8+ T-cell subsets in response to the CMV supernatant. Bars represent resulted from the grouped elders (mean ± SEM). The Student’s t test (when data were normally distributed) and Mann–Whitney non-parametric (when data were not normally distributed) methods were used to compare frequencies between groups. p values are depicted in the panels
CMV antibody titer may reflect the number of previous CMV reactivations. Since elders who had the highest levels of CMV antibodies also mounted the worse response to anti-CD3, we postulated that episodes of viral activation could reflect this deficient cellular immune response or, by the contrary, could boost CMV-specific T-cell responses. The cellular response against CMV was measured by stimulating whole-blood cultures with CMV antigens. The magnitude of the CD4+ T-cell immune response to CMV was significantly higher in the elders with the worst functional status, as detected by CD69 expression (Mann–Whitney U test, p = 0.025) (Fig. 5c) and by specific proliferation (Mann–Whitney U test, p = 0.001) (Fig. 5d). Differences in CD8+ T-cell activation were not found (Fig. 5c), and proliferative responses in both groups were very limited (Fig. 5d).
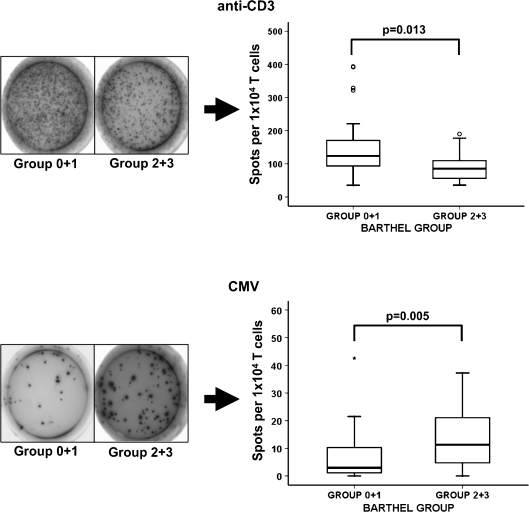
Accordingly to the above results, the frequency of IFN-γ production by anti-CD3 stimulated T cells in group 0 + 1 was 123.8 (IR = 85.6) and in group 2 + 3 was 85.8 (IR = 61.1) (Fig. 6; Mann–Whitney U test, p = 0.013). The median frequency of CMV stimulated group 0 + 1 T cells was 2.9 (IR = 9.2) and that from group 2 + 3 was 11.3 (IR = 17.05) (Mann–Whitney U test, p = 0.005). These results indicated that despite an impaired cellular immune response, elders with worse functional status presented the highest activation, proliferation, and IFN-γ production against CMV antigens.
Fig. 6.
IFN-γ production in response to anti-CD3 and to CMV antigens. Production of IFN-γ was measured in the two groups of elders (group 0 + 1, n = 28 and group 2 + 3, n = 21) by ELISPOT assay. PBMCs from elderly were stimulated with anti-CD3 (10 ng/mL) or CMV extracts (104 PFU/mL) for 20 h at 37°C/5% CO2. An example of the spots generated in response to anti-CD3 and to CMV is represented for both groups. The mean numbers of antigen-specific spot forming cells after background subtraction of control wells with no antigen were plotted. Experiments were conducted in triplicate. Outlier values are represented by circles and extreme values by stars, calculated by adding 1.5 and 3 times the IR to the 75th percentile, respectively. The Student’s t test (when data were normally distributed) and Mann–Whitney non-parametric (when data were not normally distributed) methods were used to compare frequencies between groups and p values are depicted in the panels
Response to vaccination and functional capacity
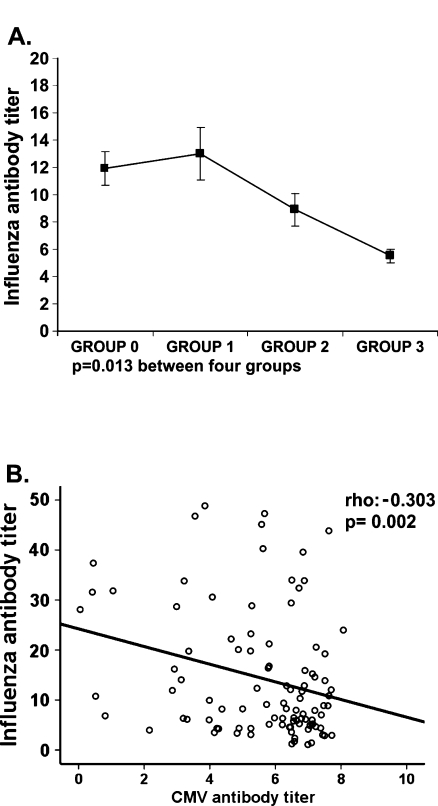
Next, to determine whether the lower immune response observed in vitro in elders with worse functional capabilities correlated to poor ability to respond to immunization in vivo, we measured the specific antibodies produced against influenza virus vaccination in the four groups of elders. The production of specific antibodies to vaccine was significantly lower in the group 3 than in the other groups (Fig. 7a). Comparison of the CMV antibody titer with influenza vaccination response revealed a negative correlation (Spearman Rho test; rho −0.303; p = 0.002) (Fig. 7b).
Fig. 7.
Response to influenza virus vaccination and its correlation to CMV titer. Influenza antibodies titer was quantified by ELISA in the serum of the elders after vaccination. a The Kruskal–Wallis non-parametric method was used to compare the influenza antibody titer between the four groups. b Relationship between influenza and CMV antibody titer in the four groups of elderly was analyzed. A non-parametric Spearman test was applied to calculate the correlations, p value and coefficient of correlation are listed in the upper right hand corner
Therefore, we concluded that those elders with a higher CMV antibody titer had worse functional status and may indicate a causal effect between CMV reactivation and reduced immune responses.
Discussion
In this study, we have demonstrated for the first time a clear association between functional decline of elderly individuals and aging of their immune system. We found significant differences in the distribution and differentiation state of cell subpopulations, in the cellular response in vitro, and in the in vivo ability for immunization. Furthermore, the elderly with worse functional capacity had the higher anti-CMV titer and T-cell response to CMV than the elderly with better functional status. In summary, we demonstrated a relationship between the intensity of the response to CMV, the immune system status, and the functional ability of older people.
Although several aspects of the innate immune response are affected by normal human aging, we only found significant changes in NK cells. As is well known, the number of NK cells increase with age (Le Garff-Tavernier et al. 2010). Elderly donors with worse functional status have a higher NK cell percentage than elderly with better status. NK cells play a role in the recognition and regulation of virally infected cells (Bottino et al. 2006) and they are particularly important in immunosurveillance against CMV (Lopez-Botet et al. 2004). In agreement, we found that older people with worse functional status had a higher antibody titer against CMV. Possibly, NK cells are increased in order to fight against this herpesvirus.
The factors that influenced the IRP include the CD4/CD8 ratio and CMV seropositivity (Olsson et al. 2000; Hadrup et al. 2006). However, CMV antibody titer may also be important factor in the time of survival since a high CMV antibody titer is related to a reduced survival time (Strandberg et al. 2009; Roberts et al. 2010). Elders in our study with poor functional status had a lower CD4/CD8 ratio and had a higher CMV antibody titer compared to the other groups. CMV infection caused a significant decrease in the CD4/CD8 ratio in elderly individuals, because it increased CD8+ T-cell count and reduced CD4+ T-cell numbers (Chidrawar et al. 2009). Here, subjects with the worst IRP occurred in groups with worse functional status, but it did not associate with individuals’ age.
As people age, differentiated cells (EM and EMRA) of the immune system accumulate and the less-differentiated immune cells (Naïve and CM) decline in frequency. These changes could be due to several mechanisms, but a factor with more influence is CMV infection. Elders in the BI groups with the highest CMV antibody titers also had more differentiated subpopulations of CD4+ T cells, but no differences were detected in the CD8+ T cells. The two subsets undergo the same principal phenotypic shifts, but the rate at which they occur or accumulate with age was different. CD4+ T cells were more resistant to phenotypic and functional changes with aging than CD8+ T cells (Czesnikiewicz-Guzik et al. 2008).
One of these phenotypic changes which appeared in old age is the lack of the costimulatory molecule CD28 (Fagnoni et al. 1996). The population of CD8+CD28null is a majority, and we found an increasing trend in the population of CD4+CD28null among the elderly with poorer functional status. We have recently found that expression of NKG2D+ in the CD4+CD28null T cells may be a better marker of cellular senescence (Alonso-Arias et al. 2011). In fact, the proportion of CD4+NKG2D+ significantly increased in the groups of elders with worse functional status. Similarly, double-positive CD4+CD8+ T cells (Pawelec 1995), which generally contain a high proportion of memory and differentiated cells, were also increased in groups with worse functional status. CD4+NKG2D+ and CD4+CD8+ subsets have been associated with CMV infection; there was a significant correlation between their expansion and CMV infection and viral reactivation (Saez-Borderias et al. 2006; Alonso-Arias et al. 2009).
Naïve T cells, which are needed to protect against new pathogens, are reduced in the elderly by several interrelated events: involution of the thymus, decline of naïve T cells, reduction in T-cell repertoire diversity, and accumulation of memory T cells that are specific for persisting pathogens (Nikolich-Zugich 2008). One way to verify the differentiation status of T cells is to measure TREC. We observed that CD4+ T cells in elders in worse functional status had a lower TREC content. This corroborates the findings that more differentiated cells have undergone more division cycles, and therefore they have a low TREC content. The lack of significant differences in the Ki-67 quantitation may occur because the cells of the subjects in this study have a very low turnover due to their advanced age and this very low cellular turnover may limit the number of cells that would show differences between the groups.
The ability to activate via TCR stimulation was significantly lower in groups with worse functional status in both CD4+ and CD8+ T-cell subsets. Highly differentiated T cells lose their ability to proliferate in response to stimulation (Appay et al. 2002). In contrast, CD4-specific response against CMV was increased in our groups with worse functional capacity. In an analogous manner, Vescovini et al. recently reported that groups of elderly individuals with cognitive impairment and poor functional capabilities had significantly higher anti-CMV IgG titers and higher CD4+ T cells specific for CMV, although they used different criteria to score their patients and their subjects (Vescovini et al. 2010) and we have also analyzed a larger number of variables which have never been correlated before to functional status in elderly. The cell repertoire limitation and the high degree of differentiation caused by CMV infection could play a major role in the reduced anti-CD3 response of T cells in the elderly with worse functional status. The importance of the CMV-specific CD4+ T-cell response in elders has been less studied than the constriction of the CD8+ T-cell repertoire. We found differences in the response to CMV in the CD4+ T-cell subset, but not in CD8+ T cells. CMV-specific CD4+ T cells typically display a memory phenotype (Fletcher et al. 2005), and this subset is indeed increased in elderly CMV-seropositive donors at the expense of the less-differentiated populations. The individuals with worse functional status presented a greater number of CMV-specific cells, and the high frequency of anti-CMV may occur from a large number of virus reactivations or an enhanced response against the virus.
The most profound clinical impact of age on the immune system concerned the response of the elderly to vaccination. In our study, the percentage of B cells is significantly decreased in the groups of elderly with the worse functional capacity. This may partly explain the poorer response to vaccination against influenza virus in the BI groups 2 and 3. CD4+CD28null are deficient in providing help to B cells, and their accumulation of CD4+CD28null T cells could provide one mechanism for impaired humoral responses in the elderly. Despite the aforementioned, we believe that the increase in the CMV titer and therefore the increase in the percentage of cells involved in the response against this herpesvirus is, maybe, the greatest limitation in the response to vaccination. In fact, we found a negative correlation between the CMV titer and the response to the vaccine influenza virus: the worst responses to vaccination were observed in the groups with the more compromised functional capacity.
Since the deterioration of the physical and functional status in elders may be directly related to the state of the immune system, "slowing" the deterioration of the immune system could possibly improve the quality of life of elders. Findings from cross-sectional studies mostly show enhanced immunity in physically active elders compared to sedentary older adults (Simpson and Guy 2009). Another field of action would be the regeneration of the T-cell population since in principle it is one of the most affected subsets by the aging process.
To our knowledge, this is the first time that a study correlates a poorer motor ability in old age and compromised immune system, both in the cellular response in vitro and response to immunization in vivo. The functional status in older people may be influenced by the state of their immune system or vice versa. All immunological parameters we studied are more impaired in the elderly with worse functional status, but these elders had a higher antibody titer and a higher response to CMV infection. Therefore, we plan to study these parameters in more detail using more subjects in each group in the future. Further studies will help us to develop new strategies to slow or reverse age-associated immune dysfunction.
Acknowledgments
We thank Isabel Cuevas Pérez, José María Díaz Pérez, and Ramona Julia Fernández García for their excellent technical assistance. This work was supported by Red de Investigación Renal (REDinREN), Spanish grant FIS PI080566 from Instituto “Carlos III” (European FEDER founds) and PEST 08-05 from FICYT.
References
- Alonso-Arias R, Lopez-Vazquez A, Diaz-Pena R, Sampere A, Tricas L, Asensi V, Rodrigo L, Lopez-Larrea C. CD8dim and NKG2D expression defines related subsets of CD4+ T cells in HIV-infected patients with worse prognostic factors. J Acquir Immune Defic Syndr. 2009;51:390–398. doi: 10.1097/FTD.0b013e3181679015. [DOI] [PubMed] [Google Scholar]
- Alonso-Arias R, Moro-Garcia MA, Lopez-Vazquez A, Rodrigo L, Baltar J, Garcia FM, Jaurrieta JJ, Lopez-Larrea C (2011) NKG2D expression in CD4+ T lymphocytes as a marker of senescence in the aged immune system. Age (Dordr) [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- Amyes E, Hatton C, Montamat-Sicotte D, Gudgeon N, Rickinson AB, McMichael AJ, Callan MF. Characterization of the CD4+ T cell response to Epstein–Barr virus during primary and persistent infection. J Exp Med. 2003;198:903–911. doi: 10.1084/jem.20022058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appay V, Dunbar PR, Callan M, Klenerman P, Gillespie GM, Papagno L, Ogg GS, King A, Lechner F, Spina CA, Little S, Havlir DV, Richman DD, Gruener N, Pape G, Waters A, Easterbrook P, Salio M, Cerundolo V, McMichael AJ, Rowland-Jones SL. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med. 2002;8:379–385. doi: 10.1038/nm0402-379. [DOI] [PubMed] [Google Scholar]
- Aspinall R, Andrew D. Thymic involution in aging. J Clin Immunol. 2000;20:250–256. doi: 10.1023/A:1006611518223. [DOI] [PubMed] [Google Scholar]
- Bolovan-Fritts CA, Trout RN, Spector SA. High T-cell response to human cytomegalovirus induces chemokine-mediated endothelial cell damage. Blood. 2007;110:1857–1863. doi: 10.1182/blood-2007-03-078881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borthwick NJ, Bofill M, Hassan I, Panayiotidis P, Janossy G, Salmon M, Akbar AN. Factors that influence activated CD8+ T-cell apoptosis in patients with acute herpesvirus infections: loss of costimulatory molecules CD28, CD5 and CD6 but relative maintenance of Bax and Bcl-X expression. Immunology. 1996;88:508–515. [PMC free article] [PubMed] [Google Scholar]
- Bottino C, Moretta L, Moretta A. NK cell activating receptors and tumor recognition in humans. Curr Top Microbiol Immunol. 2006;298:175–182. doi: 10.1007/3-540-27743-9_9. [DOI] [PubMed] [Google Scholar]
- Bradley BA, Takemoto S, Gjertson D, Reed E, Cecka M. Elderly transplant recipients may require less immunosuppression. Transplant Proc. 2001;33:1115–1116. doi: 10.1016/S0041-1345(00)02453-2. [DOI] [PubMed] [Google Scholar]
- Cao JN, Gollapudi S, Sharman EH, Jia Z, Gupta S. Age-related alterations of gene expression patterns in human CD8+ T cells. Aging Cell. 2009;9:19–31. doi: 10.1111/j.1474-9726.2009.00534.x. [DOI] [PubMed] [Google Scholar]
- Chidrawar S, Khan N, Wei W, McLarnon A, Smith N, Nayak L, Moss P. Cytomegalovirus-seropositivity has a profound influence on the magnitude of major lymphoid subsets within healthy individuals. Clin Exp Immunol. 2009;155:423–432. doi: 10.1111/j.1365-2249.2008.03785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clambey ET, Dyk LF, Kappler JW, Marrack P. Non-malignant clonal expansions of CD8+ memory T cells in aged individuals. Immunol Rev. 2005;205:170–189. doi: 10.1111/j.0105-2896.2005.00265.x. [DOI] [PubMed] [Google Scholar]
- Colombatti A, Doliana R, Schiappacassi M, Argentini C, Tonutti E, Feruglio C, Sala P. Age-related persistent clonal expansions of CD28(−) cells: phenotypic and molecular TCR analysis reveals both CD4(+) and CD4(+)CD8(+) cells with identical CDR3 sequences. Clin Immunol Immunopathol. 1998;89:61–70. doi: 10.1006/clin.1998.4580. [DOI] [PubMed] [Google Scholar]
- Czesnikiewicz-Guzik M, Lee WW, Cui D, Hiruma Y, Lamar DL, Yang ZZ, Ouslander JG, Weyand CM, Goronzy JJ. T cell subset-specific susceptibility to aging. Clin Immunol. 2008;127:107–118. doi: 10.1016/j.clim.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dace DS, Apte RS. Effect of senescence on macrophage polarization and angiogenesis. Rejuvenation Res. 2008;11:177–185. doi: 10.1089/rej.2007.0614. [DOI] [PubMed] [Google Scholar]
- DelaRosa O, Pawelec G, Peralbo E, Wikby A, Mariani E, Mocchegiani E, Tarazona R, Solana R. Immunological biomarkers of ageing in man: changes in both innate and adaptive immunity are associated with health and longevity. Biogerontology. 2006;7:471–481. doi: 10.1007/s10522-006-9062-6. [DOI] [PubMed] [Google Scholar]
- Douek DC, McFarland RD, Keiser PH, Gage EA, Massey JM, Haynes BF, Polis MA, Haase AT, Feinberg MB, Sullivan JL, Jamieson BD, Zack JA, Picker LJ, Koup RA. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396:690–695. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- Fagnoni FF, Vescovini R, Mazzola M, Bologna G, Nigro E, Lavagetto G, Franceschi C, Passeri M, Sansoni P. Expansion of cytotoxic CD8+ CD28− T cells in healthy ageing people, including centenarians. Immunology. 1996;88:501–507. doi: 10.1046/j.1365-2567.1996.d01-689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson FG, Wikby A, Maxson P, Olsson J, Johansson B. Immune parameters in a longitudinal study of a very old population of Swedish people: a comparison between survivors and nonsurvivors. J Gerontol A Biol Sci Med Sci. 1995;50:B378–B382. doi: 10.1093/gerona/50A.6.B378. [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Corsi A, Lauretani F, Bandinelli S, Bartali B, Taub DD, Guralnik JM, Longo DL. The origins of age-related proinflammatory state. Blood. 2005;105:2294–2299. doi: 10.1182/blood-2004-07-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher JM, Vukmanovic-Stejic M, Dunne PJ, Birch KE, Cook JE, Jackson SE, Salmon M, Rustin MH, Akbar AN. Cytomegalovirus-specific CD4+ T cells in healthy carriers are continuously driven to replicative exhaustion. J Immunol. 2005;175:8218–8225. doi: 10.4049/jimmunol.175.12.8218. [DOI] [PubMed] [Google Scholar]
- Gamadia LE, Remmerswaal EB, Weel JF, Bemelman F, Lier RA, Berge IJ. Primary immune responses to human CMV: a critical role for IFN-gamma-producing CD4+ T cells in protection against CMV disease. Blood. 2003;101:2686–2692. doi: 10.1182/blood-2002-08-2502. [DOI] [PubMed] [Google Scholar]
- Guralnik JM, Fried LP, Salive ME. Disability as a public health outcome in the aging population. Annu Rev Public Health. 1996;17:25–46. doi: 10.1146/annurev.pu.17.050196.000325. [DOI] [PubMed] [Google Scholar]
- Hadrup SR, Strindhall J, Kollgaard T, Seremet T, Johansson B, Pawelec G, thor Straten P, Wikby A. Longitudinal studies of clonally expanded CD8 T cells reveal a repertoire shrinkage predicting mortality and an increased number of dysfunctional cytomegalovirus-specific T cells in the very elderly. J Immunol. 2006;176:2645–2653. doi: 10.4049/jimmunol.176.4.2645. [DOI] [PubMed] [Google Scholar]
- Haynes L, Maue AC. Effects of aging on T cell function. Curr Opin Immunol. 2009;21:414–417. doi: 10.1016/j.coi.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaki T, Kai I, Kobayashi Y, Matsuyama Y, Imanaka Y. The effect of aging on functional decline among older Japanese living in a community: a 5-year longitudinal data analysis. Aging Clin Exp Res. 2004;16:233–239. doi: 10.1007/BF03327389. [DOI] [PubMed] [Google Scholar]
- Kang I, Hong MS, Nolasco H, Park SH, Dan JM, Choi JY, Craft J. Age-associated change in the frequency of memory CD4+ T cells impairs long term CD4+ T cell responses to influenza vaccine. J Immunol. 2004;173:673–681. doi: 10.4049/jimmunol.173.1.673. [DOI] [PubMed] [Google Scholar]
- Koch S, Larbi A, Derhovanessian E, Ozcelik D, Naumova E, Pawelec G. Multiparameter flow cytometric analysis of CD4 and CD8 T cell subsets in young and old people. Immun Ageing. 2008;5:6. doi: 10.1186/1742-4933-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garff-Tavernier M, Beziat V, Decocq J, Siguret V, Gandjbakhch F, Pautas E, Debre P, Merle-Beral H, Vieillard V. Human NK cells display major phenotypic and functional changes over the life span. Aging Cell. 2010;9:527–535. doi: 10.1111/j.1474-9726.2010.00584.x. [DOI] [PubMed] [Google Scholar]
- Linton PJ, Li SP, Zhang Y, Bautista B, Huynh Q, Trinh T. Intrinsic versus environmental influences on T-cell responses in aging. Immunol Rev. 2005;205:207–219. doi: 10.1111/j.0105-2896.2005.00266.x. [DOI] [PubMed] [Google Scholar]
- Looney RJ, Falsey A, Campbell D, Torres A, Kolassa J, Brower C, McCann R, Menegus M, McCormick K, Frampton M, Hall W, Abraham GN. Role of cytomegalovirus in the T cell changes seen in elderly individuals. Clin Immunol. 1999;90:213–219. doi: 10.1006/clim.1998.4638. [DOI] [PubMed] [Google Scholar]
- Lopez-Botet M, Angulo A, Guma M. Natural killer cell receptors for major histocompatibility complex class I and related molecules in cytomegalovirus infection. Tissue Antigens. 2004;63:195–203. doi: 10.1111/j.1399-0039.2004.00210.x. [DOI] [PubMed] [Google Scholar]
- Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Md State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
- Naylor K, Li G, Vallejo AN, Lee WW, Koetz K, Bryl E, Witkowski J, Fulbright J, Weyand CM, Goronzy JJ. The influence of age on T cell generation and TCR diversity. J Immunol. 2005;174:7446–7452. doi: 10.4049/jimmunol.174.11.7446. [DOI] [PubMed] [Google Scholar]
- Nikolich-Zugich J. Ageing and life-long maintenance of T-cell subsets in the face of latent persistent infections. Nat Rev Immunol. 2008;8:512–522. doi: 10.1038/nri2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohishi K, Ninomiya A, Kida H, Park CH, Maruyama T, Arai T, Katsumata E, Tobayama T, Boltunov AN, Khuraskin LS, Miyazaki N. Serological evidence of transmission of human influenza A and B viruses to Caspian seals (Phoca caspica) Microbiol Immunol. 2002;46:639–644. doi: 10.1111/j.1348-0421.2002.tb02746.x. [DOI] [PubMed] [Google Scholar]
- Olsson J, Wikby A, Johansson B, Lofgren S, Nilsson BO, Ferguson FG. Age-related change in peripheral blood T-lymphocyte subpopulations and cytomegalovirus infection in the very old: the Swedish longitudinal OCTO immune study. Mech Ageing Dev. 2000;121:187–201. doi: 10.1016/S0047-6374(00)00210-4. [DOI] [PubMed] [Google Scholar]
- Pawelec G. Molecular and cell biological studies of ageing and their application to considerations of T lymphocyte immunosenescence. Mech Ageing Dev. 1995;79:1–32. doi: 10.1016/0047-6374(94)01549-2. [DOI] [PubMed] [Google Scholar]
- Prelog M. Aging of the immune system: a risk factor for autoimmunity? Autoimmun Rev. 2006;5:136–139. doi: 10.1016/j.autrev.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Qiu H, Straat K, Rahbar A, Wan M, Soderberg-Naucler C, Haeggstrom JZ. Human CMV infection induces 5-lipoxygenase expression and leukotriene B4 production in vascular smooth muscle cells. J Exp Med. 2008;205:19–24. doi: 10.1084/jem.20070201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro RM, Perelson AS. Determining thymic output quantitatively: using models to interpret experimental T-cell receptor excision circle (TREC) data. Immunol Rev. 2007;216:21–34. doi: 10.1111/j.1600-065X.2006.00493.x. [DOI] [PubMed] [Google Scholar]
- Roberts ET, Haan MN, Dowd JB, Aiello AE. Cytomegalovirus antibody levels, inflammation, and mortality among elderly Latinos over 9 years of follow-up. Am J Epidemiol. 2010;172:363–371. doi: 10.1093/aje/kwq177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez-Borderias A, Guma M, Angulo A, Bellosillo B, Pende D, Lopez-Botet M. Expression and function of NKG2D in CD4+ T cells specific for human cytomegalovirus. Eur J Immunol. 2006;36:3198–3206. doi: 10.1002/eji.200636682. [DOI] [PubMed] [Google Scholar]
- Sainsbury A, Seebass G, Bansal A, Young JB. Reliability of the Barthel Index when used with older people. Age Ageing. 2005;34:228–232. doi: 10.1093/ageing/afi063. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- Saxena SK, Ng TP, Yong D, Fong NP, Gerald K. Total direct cost, length of hospital stay, institutional discharges and their determinants from rehabilitation settings in stroke patients. Acta Neurol Scand. 2006;114:307–314. doi: 10.1111/j.1600-0404.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- Simpson RJ, Guy K. Coupling aging immunity with a sedentary lifestyle: has the damage already been done?—a mini-review. Gerontology. 2009;56:449–458. doi: 10.1159/000270905. [DOI] [PubMed] [Google Scholar]
- Soderberg-Naucler C. Does cytomegalovirus play a causative role in the development of various inflammatory diseases and cancer? J Intern Med. 2006;259:219–246. doi: 10.1111/j.1365-2796.2006.01618.x. [DOI] [PubMed] [Google Scholar]
- Strandberg TE, Pitkala KH, Tilvis RS. Cytomegalovirus antibody level and mortality among community-dwelling older adults with stable cardiovascular disease. JAMA. 2009;301:380–382. doi: 10.1001/jama.2009.4. [DOI] [PubMed] [Google Scholar]
- Supervia A, Aranda D, Marquez MA, Aguirre A, Skaf E, Gutierrez J. Predicting length of hospitalisation of elderly patients, using the Barthel Index. Age Ageing. 2008;37:339–342. doi: 10.1093/ageing/afn049. [DOI] [PubMed] [Google Scholar]
- Leeuwen EM, Remmerswaal EB, Vossen MT, Rowshani AT, Wertheim-van Dillen PM, Lier RA, Berge IJ. Emergence of a CD4+CD28− granzyme B+, cytomegalovirus-specific T cell subset after recovery of primary cytomegalovirus infection. J Immunol. 2004;173:1834–1841. doi: 10.4049/jimmunol.173.3.1834. [DOI] [PubMed] [Google Scholar]
- Vescovini R, Biasini C, Telera AR, Basaglia M, Stella A, Magalini F, Bucci L, Monti D, Lazzarotto T, Dal Monte P, Pedrazzoni M, Medici MC, Chezzi C, Franceschi C, Fagnoni FF, Sansoni P. Intense antiextracellular adaptive immune response to human cytomegalovirus in very old subjects with impaired health and cognitive and functional status. J Immunol. 2010;184:3242–3249. doi: 10.4049/jimmunol.0902890. [DOI] [PubMed] [Google Scholar]
- Wayne SJ, Rhyne RL, Garry PJ, Goodwin JS. Cell-mediated immunity as a predictor of morbidity and mortality in subjects over 60. J Gerontol. 1990;45:M45–M48. doi: 10.1093/geronj/45.2.m45. [DOI] [PubMed] [Google Scholar]
- Weinberger B, Herndler-Brandstetter D, Schwanninger A, Weiskopf D, Grubeck-Loebenstein B. Biology of immune responses to vaccines in elderly persons. Clin Infect Dis. 2008;46:1078–1084. doi: 10.1086/529197. [DOI] [PubMed] [Google Scholar]
- Wikby A, Maxson P, Olsson J, Johansson B, Ferguson FG. Changes in CD8 and CD4 lymphocyte subsets. T cell proliferation responses and non-survival in the very old: the Swedish longitudinal OCTO-immune study. Mech Ageing Dev. 1998;102:187–198. doi: 10.1016/S0047-6374(97)00151-6. [DOI] [PubMed] [Google Scholar]
- Wilkinson TJ, Sainsbury R. The association between mortality, morbidity and age in New Zealand's oldest old. Int J Aging Hum Dev. 1998;46:333–343. doi: 10.2190/9TE4-JCB5-4C8T-PFK9. [DOI] [PubMed] [Google Scholar]