Abstract
The periaqueductal gray (PAG) is part of a descending pain modulatory system that, when activated, produces widespread and profound antinociception. Microinjection of either opioids or cannabinoids into the PAG elicits antinociception. Moreover, microinjection of the cannabinoid 1 (CB1) receptor agonist HU-210 into the PAG enhances the antinociceptive effect of subsequent morphine injections, indicating a direct relationship between these two systems. The objective of this study was to characterize the distribution of CB1 receptors in the dorsolateral and ventrolateral PAG in relationship to mu-opioid peptide (MOP) receptors. Immunocytochemical analysis revealed extensive and diffuse CB1 receptor labeling in the PAG, 60% of which was found in somatodendritic profiles. CB1 and MOP receptor immunolabeling were co-localized in 32% of fluorescent Nissl-stained cells that were analyzed. Eight percent (8%) of PAG neurons that were MOP receptor-immunoreactive received CB1 receptor-immunoreactive appositions. Ultrastructural analysis confirmed the presence CB1 receptor-immunoreactive somata, dendrites and axon terminals in the PAG. These results indicate that behavioral interactions between cannabinoids and opioids may be the result of cellular adaptations within PAG neurons co-expressing CB1 and MOP receptors.
Keywords: periaqueductal gray, pain, antinociception, descending modulation
1. INTRODUCTION
Opioids and cannabinoids have been shown to have analgesic properties. Although the analgesic efficacy of cannabinoids is less substantial than opioids, recent experimental data suggest that opioids and cannabinoids may interact in therapeutically beneficial ways. Systemic co-administration of cannabinoid and opioid agonists results in synergistic antinociception (Cichewicz and McCarthy, 2003, Tham et al., 2005, Roberts et al., 2006, Smith et al., 2007), and systemic pre-treatment or co-administration of cannabinoids attenuates the development of morphine tolerance (Cichewicz and Welch, 2003, Smith et al., 2007, Wilson et al., 2008).
A number of findings indicate that the periaqueductal gray (PAG) contributes to the enhanced antinociception produced by co-administration of opioids and cannabinoids. Microinjection of either cannabinoids or opioids into the PAG produces antinociception (Jensen and Yaksh, 1986, Martin et al., 1995, Lichtman et al., 1996, Morgan et al., 1998, Finn et al., 2003), and microinjection of a cannabinoid 1 (CB1) receptor agonist into the ventrolateral PAG enhances subsequent morphine antinociception (Wilson et al., 2008). These effects could be produced by co-localization of receptors on the same neurons. Autoradiography, in situ hybridization, and immunohistochemistry studies confirm that CB1 and mu-opioid peptide (MOP) receptors are located in the PAG (Mailleux and Vanderhaeghen, 1992, Kalyuzhny et al., 1996, Tsou et al., 1998, Commons et al., 2000, Abrams et al., 2011). CB1 and MOP receptors are both G-protein coupled receptors that interact primarily with Gi/Go (Connor and Christie, 1999, Howlett et al., 2002). Activation of both CB1 and MOP receptors inhibits adenylyl cyclase, which results in decreased production and accumulation of cyclic AMP (cAMP, (Howlett, 1985, Childers et al., 1992, Meng and Johansen, 2004). It is important to note that adenylyl cyclase upregulation also has been implicated in the development of morphine tolerance (Ingram et al., 1998, Bohn et al., 2000). In addition, mitogen-activated protein kinase (MAPK) cascades are modulated by both CB1 and MOP receptor activation (Howlett, 2005, Asensio et al., 2006). Because extracellular-related kinase (ERK)1/2 appears to counter morphine tolerance in the PAG (Macey et al., 2009), this signaling cascade could be an important convergence point for opioid/cannabinoid interactions in the PAG.
The aforementioned molecular interactions are dependent upon the co-localization of the CB1 and MOP receptors on PAG neurons. CB1 and MOP receptors are each found in somatodendritic profiles in the PAG (Tsou et al., 1998, Commons et al., 2000). In the dorsal horn of the spinal cord, both receptor types co-localize in dendrites (Pugh et al., 1996, Abrams et al., 2011). However, to our knowledge, CB1 and MOP receptor co-localization has not been demonstrated in PAG neurons. It is possible that cannabinoid/opioid interactions occur between neurons where the receptors are not co-localized on the same cell, but interact synaptically. Endocannabinoids are retrograde messengers, with CB1 receptors located on presynaptic terminals (Wilson and Nicoll, 2001, Alger, 2002, Wilson and Nicoll, 2002). Thus, CB1-receptive terminals contacting MOP receptor-ir cells could be alternative sites of opioid/cannabinoid interaction. The present anatomical studies were designed to test these hypotheses by determining the cellular substrates and interactions between CB1 and MOP receptors within the PAG that could support the functional interactions.
CB1 receptor agonists produce greater antinociception when injected into the dorsolateral PAG compared to the ventrolateral PAG (Martin et al., 1995), whereas the antinociception produced by microinjection of morphine into the dorsal and lateral regions of the PAG is associated with severe flight responses (Morgan et al., 1998). Furthermore, the dorsolateral PAG is specialized to produce cannabinoid-mediated stress-induced analgesia (Hohmann et al., 2005). These functional differences in subregions of the PAG could have anatomical underpinnings, which this study will elucidate by comparing the distribution of CB1 and MOP receptors in these regions.
Previous reports have shown that chronic drug administration can induce changes in the density of the CB1 and MOP receptors in several sites in the central nervous system. In reward-related brain areas, there is a reciprocal up-regulation of CB1 and MOP receptors after chronic drug exposure (Fattore et al., 2007). Similarly, chronic morphine administration increases the density of both CB1 and CB2 receptors in the dorsal horn of the spinal cord (Fattore et al., 2007, Morgan and Christie, 2011). These effects have not been studied in the PAG. The final objective of this study is to determine whether changes in receptor density underlie sensitized morphine responses after chronic cannabinoid administration.
The behavioral and anatomical data cited above suggest that CB1 and MOP receptors have direct interactions in the PAG. The current study was undertaken to characterize CB1 receptor distribution in the PAG, and address the following questions: 1) Are CB1 and MOP receptors co-localized on the same neurons? 2) Do CB1 receptor-labeled presynaptic terminals make contact with MOP receptor-expressing cells? 3) Are there differences in the amount or pattern of CB1 receptor labeling in the dorsolateral compared to the ventrolateral PAG? and 4) Does chronic drug administration cause an up-regulation of receptor density in the PAG?
2. EXPERIMENTAL PROCEDURES
2.1. Subjects
Experiments were performed in male Sprague-Dawley rats (220–280g, Harlan, Livermore, CA), housed three to a cage. Animals were kept on a reverse light-dark cycle with food and water available ad libitum. Experiments were conducted in accordance with the care and use guidelines of the International Association for the Study of Pain. The Institutional Care and Use Committee of Washington State University approved this research.
2.2. Perfusion and tissue preparation for immunocytochemistry
Rats were given a lethal dose of sodium pentobarbital (150 mg/kg, i.p.), and perfused transcardially through the ascending aorta with 10 mL of heparinized saline (1000 U/mL), followed by 50 mL of 3.8% acrolein in 2% paraformaldehyde, followed by 200 mL of 2% paraformaldehyde in 0.1 M phosphate buffer (PB, pH 7.4) (Aicher et al., 2003, Hegarty et al., 2010). Immediately following perfusion, brains were removed, and blocks of tissue containing the PAG were placed in fixative for 30 min and then transferred to 0.1 M PB. Blocks of tissue were sectioned coronally on a vibrating microtome (Leica Microsystems, Inc., Buffalo Grove, IL) at 40 μm. Prior to immunocytochemical processing, free-floating sections were placed in 1% NaBH4 (Sigma-Aldrich; St. Louis, MO) for 30 min to bind remaining free aldehydes and increase the antigenicity of acrolein-perfused tissues (Hegarty et al., 2010).
2.3. Experiment 1: Distribution of CB1 and MOP receptors in the PAG
2.3.1. Dual-label immunofluorescent labeling
Dual-label immunofluorescent studies were performed as previously described (Hegarty et al., 2010). Tissue sections from four naïve rats were incubated in 0.5% bovine serum albumin (BSA; Sigma) in 0.1 M Tris-buffered saline (TS) for 30 minutes to reduce nonspecific binding followed by incubation in a primary antibody cocktail made in 0.1% BSA and 0.25% Triton X-100 (Sigma) in 0.1 M TS for 2 nights at 4 °C with continuous agitation. The primary antibody cocktail was made up of a polyclonal rabbit anti-CB1 receptor antibody (1:1000; gift from Dr. Ken Mackie), directed against a synthetic peptide of the last 73 amino acid residues (residues 401-473) of the intracellular C-terminus of the rat CB1 receptor (Hajos et al., 2000, Wager-Miller et al., 2002, Suarez et al., 2010), and a guinea pig anti-mu opioid receptor (MOR-1), antibody (1:5000; Millipore, Billerica, MA), directed against a synthetic peptide of amino acid residues 384-398 of the C-terminus of the rat MOP receptor (manufacturer’s technical specifications). Tissue sections were rinsed and then incubated in a cocktail of fluorescent secondary antibodies made in 0.1% BSA in 0.1 M TS for 2 hours at room temperature protected from light. In order to visualize the CB1 receptor, a donkey anti-rabbit antibody conjugated to Alexa Fluor 488 (1:800; Invitrogen, Carlsbad, CA) was used. We used a donkey anti-guinea pig antibody conjugated with Cy5 (Jackson ImmunoResearch, West Grove, PA) to visualize the MOP receptor primary antibody. This combination of secondary antibodies was chosen to ensure that the fluorophores would be spectrally distinct. Tissue sections were rinsed again and then incubated in NeuroTrace® 530/615 red fluorescent Nissl stain (NT, 1:250; Invitrogen) made in 0.1 M PB for 20 minutes light-protected at room temperature according to manufacturer’s instructions. After profuse rinsing in 0.1 M PB, sections were mounted on gelatin-coated slides, coverslipped with ProLong Gold™ antifade reagent (Invitrogen), sealed and stored at −20 °C to preserve labeling.
Previous studies have demonstrated that the rabbit anti-CB1 receptor antibody recognizes its antigen in rat cerebellum tissue using Western blotting (Suarez et al., 2008) and that CB1 receptor labeling is absent in cerebellar and hippocampal tissue from CB1 receptor knockout mice (Suarez et al., 2008, Suarez et al., 2010). CB1 receptor immunoreactivity was abolished by incubating drug-naïve rat PAG tissue in a cocktail that contained the CB1 receptor antibody and the antigenic peptide corresponding to the last 73 amino acid residues (residues 401-473) of the intracellular C-terminus of the rat CB1 receptor (Figure 1B, D).
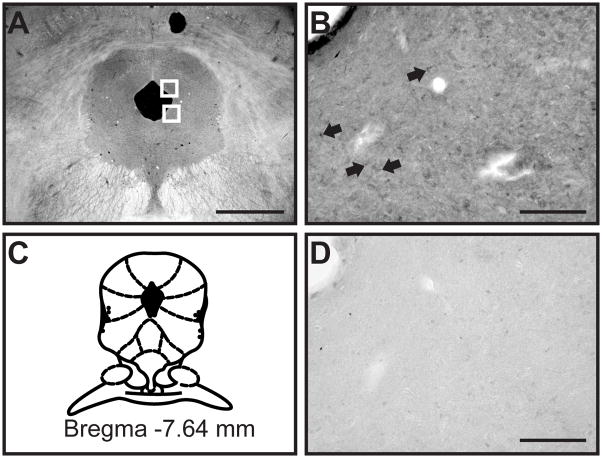
Figure 1.
Light microscopy shows diffuse CB1 receptor labeling in ventrolateral and dorsolateral PAG. (A) In a darkfield image of the PAG, white boxes delineate the areas of the ventrolateral and dorsolateral PAG that were imaged for confocal and electron microscopic analysis. (B) CB1 receptor labeling was diffuse in the PAG, with some punctate labeling found in cells (black arrows). (C) This representative diagram of the PAG is modified from the digital atlas of Paxinos and Watson (Paxinos and Watson, 2005) and is reproduced here with permission from the publisher. (D) CB1 receptor labeling was abolished after preadsorption with the antigenic control peptide. Scale bar in (A) = 1 mm; Scale bars in B and D = 100 μm.
The specificity of the secondary antibodies was confirmed by omitting the primary antibody or by incubating the tissue with one primary antibody (e.g. rabbit anti-CB1 receptor) followed by the wrong secondary antibody (Cy5-conjugated donkey anti-guinea pig). There was no immunolabeling in any of the omission or mismatch combinations tested.
2.3.2. Confocal microscopy and data analysis
Fluorescent labeling in PAG sections was viewed using a Zeiss LSM 510 META confocal microscope outfitted with an Argon/2 laser and 2 HeNe lasers (Carl Zeiss MicroImaging, Thornwood, NY). The ventrolateral and dorsolateral regions from one PAG section per animal were assessed for the presence of CB1 and MOP receptor labeling and NT staining. Anatomical landmarks such as the size and shape of the cerebral aqueduct were used to select PAG sections that were approximately −7.6 mm from bregma (Figure 1A, C). Tissue was sampled in close proximity to the aqueduct to ensure that light, confocal, and electron microscope experiments were all conducted in nearly identical regions of the ventrolateral and dorsolateral PAG (Figure 1A, white boxes). The ventrolateral and dorsolateral regions of PAG from each rat were individually imaged with a Plan-Neofluar 40x/1.3 NA oil immersion objective using a single pass, multi-tracking format to minimize overlap. In each region, Z-stacks were bounded by the vertical extent of antibody labeling and NT staining. Zeiss ZEN software was used to assign color profiles to fluorescence images and analysis (see below). Confocal micrographs used for publication were taken at a higher magnification (63x/1.4 NA) and are projections of three (Figure 2) or four (Figure 4) 0.4 μm thick optical sections and adjusted for optimal brightness and contrast using Zeiss ZEN software.
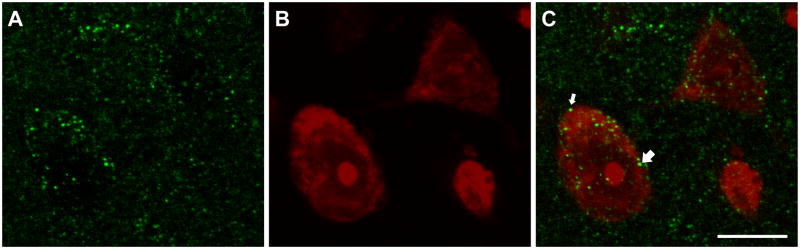
Figure 2.
Confocal micrographs show immunofluorescent labeling of CB1 receptors (A, green) and NeuroTrace staining (B, red) of cells in the ventrolateral PAG. (C). An overlay image shows CB1 receptor-labeled cells receiving appositions from CB1 receptor-labeled varicosities (arrows). Confocal micrographs are a projection of 3 consecutive and overlapping 0.4 μm optical sections composing a 1.2 μm thick Z stack. Scale bar = 10 μm for panels A – C.
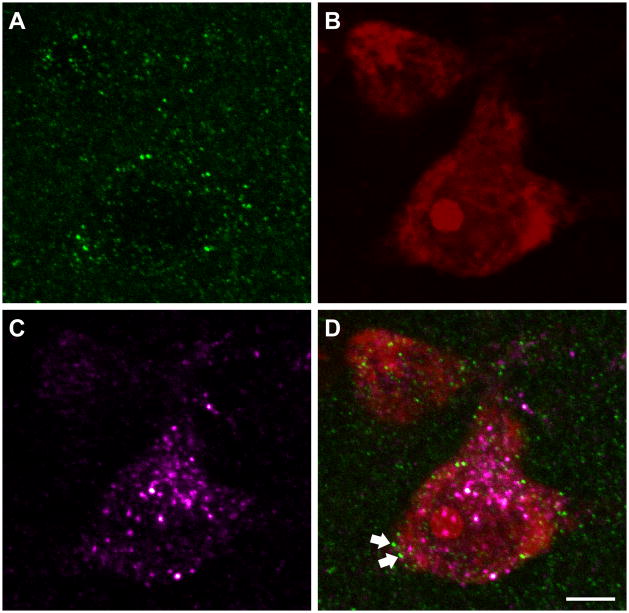
Figure 4.
Confocal micrographs of CB1 receptor labeling (A, green), NeuroTrace (B, red) and MOR labeling (C, magenta) in PAG. (D) The overlay image shows a NeuroTrace-stained cell that is CB1 receptor-ir and MOR-ir and is receiving appositions from CB1 receptor-labeled varicosities (arrows). Confocal micrographs are a projection of 4 consecutive and overlapping 0.4 μm optical sections composing a 1.6 μm thick Z stack. Scale bar = 5 μm for panels A – D.
Images from four animals were analyzed to determine the distribution of CB1 receptor and MOR immunoreactivity in the rat ventrolateral and dorsolateral PAG. Image analysis was confined to those optical sections that contained CB1 receptor and MOR labeling as well as NT staining. First, with the channels used to detect CB1 receptor and MOR immunofluorescent labeling turned off, each 0.5 μm optical section was examined for cells stained with NT, which were then numbered. Cells were considered to be stained with NT if the entire cell body and nucleus were visible in at least two consecutive 0.5 μm thick optical sections. A total of 421 (217 ventrolateral, 204 dorsolateral) NT cells were examined in the PAG. After identifying NT-stained cells, the channels used to detect CB1 receptor and MOR immunolabeling were turned on individually and each identified cell throughout the Z-stack was analyzed for intracellular CB1 receptor and/or MOR immunolabeling. A cell was scored as CB1 receptor-immunoreactive (-ir) or MOR-ir if labeling was present within the boundaries of the NT-stained cell, in at least two consecutive 0.5 μm thick optical sections. NT-stained cells that were CB1 receptor-ir or MOR-ir or that co-expressed the CB1 receptor and MOR were further analyzed for appositions with CB1 receptor-ir axonal varicosities. Varicosities were defined as approximately circular punctate profiles that were present in at least 2 consecutive optical sections and were similar to those analyzed in our previous studies (Bailey et al., 2006, Hegarty et al., 2010). If a varicosity was outside and directly adjacent to an NT-stained cell in two consecutive optical sections, it was considered an apposition. Analysis of the intracellular CB1 receptor and MOR immunoreactivity content of NT-stained cells as well as the CB1 receptor appositional analysis was assessed by two independent and blinded observers.
2.3.3. Immunogold labeling for electron microscopy
Tissue sections to be used for electron microscopic analysis of CB1 receptor distribution in PAG were processed using colloidal gold immunocytochemical methods similar to those described previously (Aicher et al., 1997, Hegarty et al., 2007). The freeze-thaw method in which the PAG tissue sections were briefly immersed into liquid chlorodifluoromethane (Freon) followed by liquid nitrogen was used to increase antibody penetration of the tissue. Tissue sections were then blocked in 0.5% BSA in 0.1 M TS followed by incubation in the primary antibody solution consisting of the polyclonal rabbit anti-CB1 receptor antibody (1:250) in 0.1% BSA in 0.1 M TS for 2 nights at 4°C with continuous agitation. Following rinses, tissue sections were incubated in colloidal gold-conjugated goat anti-rabbit secondary antibody (1:50; Electron Microscopy Sciences (EMS), Hatfield, PA) for 2 hours at room temperature. Tissue sections were then rinsed and fixed in 2% electron microscopy (EM) grade glutaraldehyde. Silver intensification of the gold particles was performed using the Silver Enhancement Kit for Light and Electron Microscopy (Ted Pella, Redding, CA). Tissue sections were then osmicated in 1% osmium tetroxide for 15 min, rinsed in 0.1 M PB, dehydrated through ethanols and propylene oxide and then incubated overnight in a 1:1 mixture of propylene oxide and EMBed 812 (EMS). The following day, tissue sections were incubated in EMBed 812 for 2 hours and then embedded between two sheets of Aclar flurohalocarbon plastic film (Ted Pella). The embedded tissue was placed in a 60°C oven for 2 nights. Regions of ventrolateral and dorsolateral PAG that contained CB1 receptor labeling were glued onto Beem capsules (Ted Pella) and sectioned on an ultramicrotome (Leica Microsystems, Inc.) at 75 nm. Ultrathin sections were then collected onto 400 mesh copper grids (EMS) and counterstained with uranyl acetate and lead citrate.
2.3.4. Electron microscopy and data analysis
Ultrastructural analysis was conducted on plastic-embedded sections of dorsolateral and ventrolateral PAG from two animals. Electron microscopic image collection was performed on a Tecnai 12 electron microscope (FEI, Hillsboro, OR) interfaced to a digital camera and associated software (Advanced Microscopy Techniques, Danvers, MA). Ultrathin sections were examined and chosen based on the optimal preservation of morphological details and maximal detection of the immunogold labeling (Peters, 1991). Sections were selected from an area just below the surface of the tissue, at the tissue/plastic interface, where the penetration of the antibody was optimal in order to avoid underdetection of the immunogold antigen (Chan et al., 1990, Aicher et al., 2003). Images were assessed for: (1) the type of profile (i.e., perikarya, dendrites, terminals, axons or glia) labeled with immunogold, (2) the minimum cross-sectional diameter of immunogold-labeled profiles and (3) the type of synaptic input to labeled perikarya or dendrites (symmetric or asymmetric) or the type of synapse formed by labeled terminals (Peters, 1991). In order for a profile to be considered positive for immunogold labeling, there had to be at least two gold particles in dendritic, axonal, axon terminal and somatic profiles with a minimum cross-sectional diameter under 1.0 μm, at least three gold particles in profiles between 1.1 and 2.0 μm and at least four gold particles in profiles over 2.1 μm. Two or more gold particles had to be present in glial profiles to be considered gold-labeled. In small, unmyelinated axons with a minimum cross-sectional diameter of 0.5 μm or less, the criterion was at least one gold particle associated with the plasmalemmal surface (Garzon et al., 1999, Aicher et al., 2000, Aicher et al., 2003, Hegarty et al., 2007). Immunogold-labeled profiles were further analyzed for the distribution of gold particles on the plasmalemmal surface compared to intracellular membrane sites (Drake et al., 2005). All micrographs analyzed were initially calibrated to their respective scale bars. Nearly identical square micron areas of tissue per area of PAG per rat were examined. The total area of tissue analyzed from the ventrolateral and dorsolateral regions of two animals was 6290 μm2 (3145 μm2 per region).
2.4. Experiment 2: Effects of chronic drug administration on CB1 receptor density
2.4.1. Chronic drug administration
Rats received subcutaneous injections of either the cannabinoid receptor agonist (9)-tetrahydrocannabinol (THC) (a gift from the National Institute on Drug Abuse (NIDA),10 mg/kg, N = 3), morphine sulfate, (NIDA, N = 3) or an equivalent volume of vehicle (N = 3). Injections were given twice per day (0900 and 1600) for three consecutive days. All drugs were dissolved in a vehicle of 50% ethanol, 50% dimethyl sulfoxide (DMSO). Nociception was assessed using the hot plate test. The hot plate test measured the latency for the animal to lick a hind paw when placed on a 52.5°C surface. The rat was immediately removed from the apparatus following a response or after 40 seconds if no response occurred. Hot plate calibration at this temperature produced baseline latencies of 8.7 to 11.4 seconds. Baseline latencies were obtained immediately before the animal received its first injection of THC or morphine sulfate on Day 1. Tolerance to the antinociceptive effect of THC and morphine was assessed 30 minutes after the final injection on Day 3. Animals were perfused for tissue collection on Day 4, 18 h after the final injection.
2.4.2. Light Microscopy
Immunocytochemistry for light microscopic peroxidase detection of the CB1 receptor in the PAG was performed as previously described (Hegarty et al., 2007). Following incubation in 0.5% BSA in 0.1 M TS for 30 minutes, PAG sections were incubated in the rabbit anti-CB1 receptor (1:1000) primary antibody with 0.1% BSA, 0.25% Triton X-100 (Sigma) in 0.1 M TS for 2 nights at 4°C with continuous agitation. Bound CB1 receptor primary antibody was visualized by incubating the PAG tissue sections in biotinylated goat anti-rabbit IgG secondary antibody (1:400, Vector Laboratories, Burlingame, CA) followed by incubation in Avidin-Biotin (Elite Vectastain ABC kit; Vector Laboratories) and diaminobenzidine-hydrogen peroxide (DAB-H2O2) solutions. Trios of animals were processed through identical immunocytochemical conditions to ensure comparable labeling conditions. Tissue punctures through the ventral edge of vibratome sections were used to distinguish drug pretreatment conditions, and tissue sections from the trio of animals were combined during all antibody incubations. Tissue sections were mounted on slides, serially dehydrated in increasing ethanol concentrations and xylenes, and coverslipped using DPX mounting medium (Sigma). One section per animal at the midpoint of PAG (approximately −7.6 mm from bregma, N = 3 rats in each condition) was identified based on the size and shape of the cerebral aqueduct, and images of both the dorsolateral and ventrolateral PAG were taken from each section using an Olympus BX51 microscope equipped with a DP71 camera (Olympus America, Center Valley, PA) and associated software. MetaMorph imaging software (Molecular Devices Inc., Sunnyvale, CA) was used to measure the density of peroxidase labeling in each image. Briefly, a blinded observer drew four identical regions of interest (ROI) onto each image: one background ROI in the cerebral aqueduct and three ROIs in the PAG. The density of peroxidase labeling from the background ROI was subtracted from the average density from the three ROIs drawn on the PAG. ROIs of the same dimensions were used for each image and they were situated in similar areas in the fields of view. Tissue samples were imaged in close proximity to the aqueduct (Figure 1A) to ensure that the aqueduct ROI (background) could be subtracted from the other ROIs.
2.4.3. Statistical analyses
In confocal experiments, t-tests (SigmaStat, Systat Software, Inc., San Jose, CA) were used to compare the number of CB1 receptor and MOR co-localizations and appositions between the dorsolateral and ventrolateral PAG. In electron microscope experiments, Mann-Whitney Rank Sum tests (SigmaStat) were used to compare the numbers of total CB1 receptor-ir profiles as well as the numbers of dendrites, axon terminals, axons, glia and soma between the dorsolateral and ventrolateral PAG. In chronic drug treatment experiments, a two-way ANOVA with post-hoc Tukey test (SPSS) was used to compare changes in drug potency between the first and last injections. One way ANOVAs were run on the raw densities (SigmaStat) to analyze drug-induced changes in CB1 receptor density in both vlPAG and dlPAG.
3. RESULTS
3.1. Experiment 1: Distribution of CB1 and Mu-opioid receptors in the PAG
3.1.1. CB1 receptor immunolabeling in the PAG
CB1 receptor immunoreactivity in the ventrolateral and dorsolateral PAG is diffuse and extensive (Figure 1B). CB1 receptor immunofluorescence appears to be a mix of somatodendritic and presynaptic structures. The majority (62%) of Neurotrace (NT)-stained cell bodies analyzed contain CB1 receptor immunofluorescence (N = 261, Figure 2), however there is also a great deal of CB1 receptor immunoreactivity outside of cell bodies (Figure 2), which is consistent with the axonal and astrocytic labeling seen in other CNS sites (Tsou et al., 1998, Abramoff, 2004, Mukhopadhyay et al., 2010). A subset of NT-stained cells also received appositions from CB1 receptor-immunoreactive (-ir) structures, including CB1 receptor-ir cells (N = 21, 5%, Figure 2C, arrows) and cells that were not CB1 receptor-ir (N = 5, 1%).
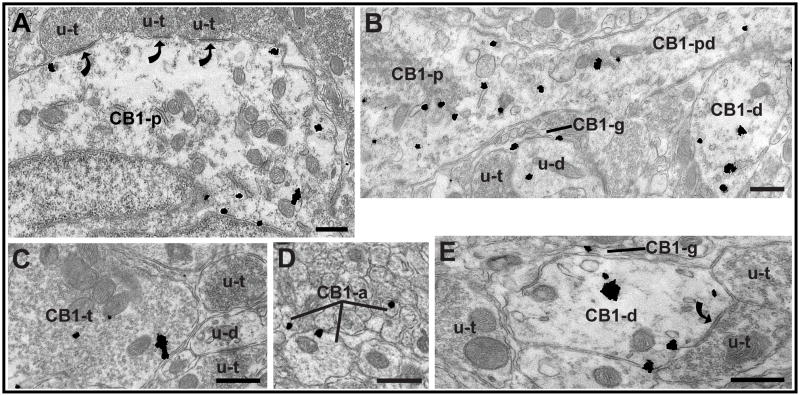
Electron microscopy was used to more clearly determine the subcellular distribution of CB1 receptor-ir in the PAG. Consistent with the immunofluorescence experiments, immunogold labeling was found in somatodendritic (Figure 3A, B, E) and presynaptic (Figure 3C, D) structures, as well as in astrocytic glia (Figure 3B, E). There were no significant differences between the dorsolateral and ventrolateral PAG in the total number of CB1 receptor-labeled profiles found, nor in the numbers of CB1 receptor-labeled dendrites, terminals, axons, glia and soma, so the data were pooled. Of the 527 CB1 receptor-gold labeled profiles examined from dorsolateral and ventrolateral PAG, 58% were postsynaptic, 27% were presynaptic, and 15% were glial, which is consistent with the large proportion of somatodendritic CB1 receptor immunoreactivity seen in other CNS sites (Scavone et al., 2010). In CB1 receptor-ir axon terminals, 87% of the gold particles were cytoplasmic, while the remaining 13% were plasmalemmal, which is consistent with CB1 receptor immunoreactivity in the dorsal horn of the spinal cord (Pugh et al., 1996). A similar cytoplasmic/plasmalemmal ratio was seen in postsynaptic PAG profiles, where 87% of gold particles were cytoplasmic. The most common synaptic interaction observed throughout the ventrolateral PAG and dorsolateral PAG was CB1 receptor-ir dendrites contacted by unlabeled terminals (Figure 3E). Of those synaptic interactions, the majority (76%) of them were symmetric synaptic specializations. CB1 receptor-ir terminals were also observed (Figure 3C), and of the few CB1 receptor-ir terminals that formed distinguishable synapses (5 out of 49), most (4 out of 5) were symmetric. Most CB1 receptor-ir terminals form simple appositions with other neuronal profiles in the plane of section observed.
Figure 3.
Electron microscopy shows CB1 receptor immunogold particles in both presynaptic and postsynaptic profiles. (A) A CB1 receptor-ir perikaryon receives symmetric synaptic input (curved arrows) from unlabeled terminals (u-t). (B) A proximal CB1 receptor-ir dendrite (CB1-pd) extends from a CB1 receptor-ir perikaryon (CB1-p). Another CB1 receptor-ir dendrite (CB1-d) is also visible in the image. A CB1 receptor-ir glial process (CB1-g) surrounds an unlabeled terminal (u-t) and an unlabeled dendrite (u-d). Although the unlabeled dendrite (u-d) contains one gold particle, it did not meet our criteria as being immunogold-labeled and was therefore considered to be unlabeled. (C) A CB1 receptor-ir terminal (CB1-t) is surrounded by thin astrocytic processes and is near unlabeled terminals (u-t) and a small unlabeled dendrite (u-d). (D) CB1 receptor-ir unmyelinated axons (CB1-a), each with one gold particle present on the plasmalemmal surface, are present among unlabeled unmyelinated axons. (E) A CB1-ir glial process (CB1-g), with one gold particle on the plasmalemmal surface, surrounds a CB1 receptor-ir dendrite (CB1-d) that is receiving symmetric synaptic input (curved arrow) from an unlabeled terminal (u-t). Another unlabeled terminal forms a simple apposition with the dendrite. Scale bar for all panels = 0.5 μm.
3.1.2. Relationship of CB1 and MOP receptors in the PAG
Figure 4 demonstrates the mostly somatodendritic pattern of MOP receptor immunoreactivity in the PAG. Of all NT-stained cells that were examined, 28% were immunoreactive for the MOP receptor alone (N = 116), while 32% of all NT-stained cells contained both MOP and CB1 receptor immunolabeling (N = 134, Figure 4). A subset of MOP receptor-ir cells received appositions from CB1 receptor-ir profiles, which are presumed to be presynaptic elements such as axon terminals (N = 35, 8%). Many NT-stained cells in the PAG that received CB1 receptor-ir appositions were cells that were immunoreactive for both CB1 and MOP receptors (N = 20, 33%, Figure 4). It should also be noted that 44 of the 421 NT-stained cells examined (10%) were not immunoreactive for either MOP or CB1 receptors.
3.1.3. PAG sub-regional MOP/CB1 receptor distribution
Table 1 shows the results of CB1 and MOP receptor immunofluorescent labeling in the dorsolateral and ventrolateral PAG. Immunolabeling was highly consistent between regions and among animals. The dorsolateral and ventrolateral PAG were similar in the number of cells expressing both CB1 and MOP receptor immunoreactivity (t(6) = 0.266, p = 0.79), and CB1 receptor appositions onto all NT-stained cells t(6) = 0.311, p = 0.76).
Table 1.
MOP and CB1 receptor distribution in the dorsolateral and ventrolateral PAG
| NT-labeled | MOR1 only | CB1 only | Co-localized | MOR1 total | CB1 total | Unlabeled | |
|---|---|---|---|---|---|---|---|
| dlPAG | 204 | 60 | 57 | 65 | 125 | 122 | 22 |
| vlPAG | 217 | 56 | 70 | 69 | 125 | 139 | 22 |
3.2. Experiment 2: Effects of chronic drug administration on CB1 receptor density
Previous studies have demonstrated that chronic opioid exposure can enhance CB1 receptor density in reward-related brain regions (Fattore et al., 2007), however this effect has never been examined in the PAG. Antinociceptive tests confirmed that chronic THC or morphine administration caused tolerance to the antinociceptive effect of both drugs, as measured by the hot plate (Table 2, F(1,6) = 22.69, p = 0.003, SPSS). PAG tissue from these animals was assessed for changes in the density of the CB1 receptor immunolabeling. Neither chronic morphine nor THC treatment had a statistically significant effect on the density of CB1 receptor peroxidase labeling in either the ventrolateral (F(2,8) = 0.052, p = 0.95) or dorsolateral (F(2,8) = 0.024, p = 0.98) PAG (Figure 5).
Table 2.
Antinociceptive tolerance to repeated THC (10mg/kg) and morphine (5mg/kg) injections
| Average hot plate latencies (s) | ||
|---|---|---|
| Injection 1 | Injection 6 | |
| Vehicle | 11.5 | 8.5 |
| THC | 23.5 | 9.1 |
| Morphine | 27.6 | 10.7 |
Figure 5.
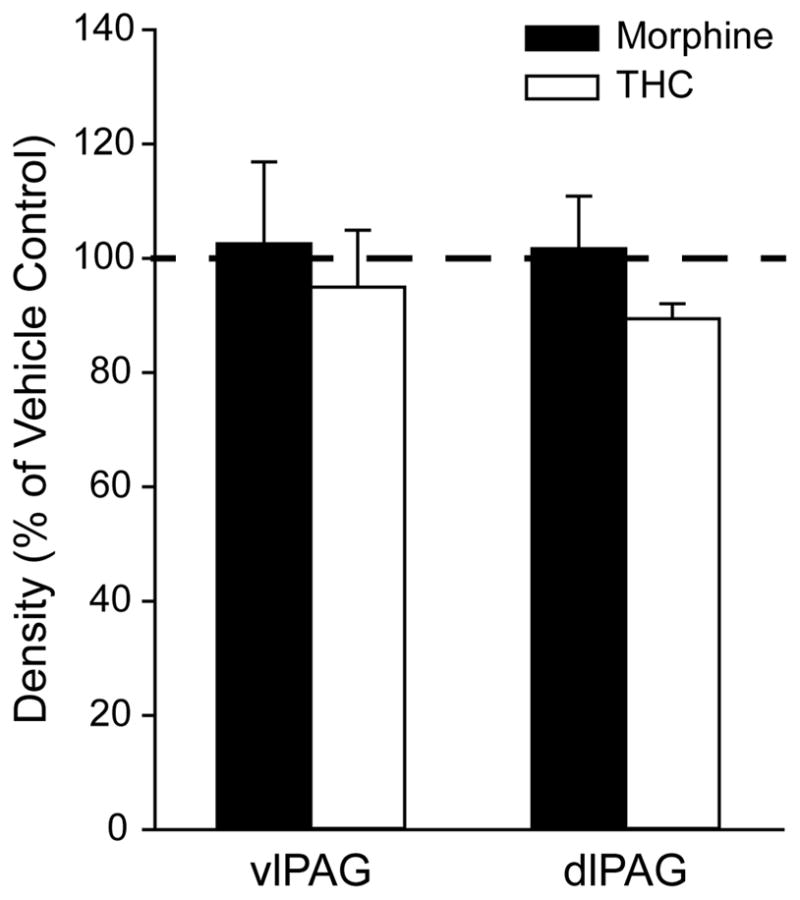
Density of CB1 receptor immunoperoxidase labeling in ventrolateral (vl) and dorsolateral (dl) PAG from animals chronically treated with morphine or THC. The densities of CB1 receptor labeling in morphine- and THC-treated rats are expressed as a percent of the density measured in vehicle control rats (Dotted line = 100%). Error bars represent the standard error of the mean of the sample (n = 3). Chronic morphine or THC treatment had no significant effect on the density of CB1 receptor immunoperoxidase labeling in either the vlPAG or dlPAG.
4. DISCUSSION
CB1 receptor distribution is diffuse and extensive in both the dorsolateral and ventrolateral sub-regions of the PAG, with the majority of CB1 receptor immunoreactivity in somatodendritic profiles. CB1 and MOP receptors were frequently co-localized in the PAG, suggesting that intracellular adaptations in these cells may underlie the behavioral interactions of opioids and cannabinoids. We also found that CB1 receptor-ir profiles (likely to be presynaptic) were often apposed to cells expressing MOP receptors, and electron microscopic studies confirmed the presence of CB1 receptor-ir axon terminals in the PAG. Finally, CB1 receptor immunolabeling in both the dorsolateral and ventrolateral PAG was unchanged by chronic treatment with morphine or THC.
The CB1 receptor is one of the most ubiquitously expressed G-protein coupled receptors in the central nervous system, and the results of this study confirm this widespread distribution. Our results in PAG were consistent with previous reports that demonstrated somatodendritic (Abrams et al., 2011), axonal/terminal (Tsou et al., 1998), and astrocytic (Abramoff, 2004, Mukhopadhyay et al., 2010) CB1 receptor immunolabeling in the CNS. Although cannabinoids are well known as retrograde messengers (Hohmann et al., 2005, Welch, 2009), the large number of somatodendritic profiles we observed suggests that the CB1 receptor may have additional functions in the PAG. The prevalence of symmetrical synapses onto CB1 receptor-ir somatodendritic profiles also provides an anatomical substrate for cannabinoid/GABAergic interactions in the PAG.
Cannabinoid/opioid interaction has been demonstrated in various behavioral responses and across many administration modalities (Ayhan et al., 1979, Cichewicz et al., 2005, De Laurentiis et al., 2010, Hegarty et al., 2010). Systemically co-administered opioids and cannabinoids produce potentiated antinociception (Rubino et al., 1997, Cichewicz and McCarthy, 2003). In the PAG, co-administration of cannabinoids and opioids does not produce acute antinociceptive synergy, however, pre-treatment microinjections of cannabinoids create a sensitized morphine antinociceptive response and attenuates the development of morphine tolerance (Wilson et al., 2008).
This study is the first to demonstrate the high degree of co-localization of the CB1 and MOP receptors in a brain area that is critically involved in descending modulation of nociception, as well as opioid tolerance and dependence. These results are consistent with previous findings that CB1 and MOP receptors co-localize in somatodendritic profiles in the dorsal horn of the spinal cord (Pugh et al., 1996). The co-localization of these receptors indicates that behavioral enhancement of antinociception may be at least partially explained by intracellular signaling changes within these neurons. Because low-dose combination of opioids and cannabinoids does not alter receptor-mediated G-protein activation (Smith et al., 2007), antinociceptive enhancement between opioids and cannabinoids is more likely to occur as a result of altered downstream signaling such as cAMP or mitogen-activated protein kinases (MAPK) (Smith et al., 1998, Finn et al., 2004, Macey et al., 2009).
Our results also demonstrate the presynaptic localization of CB1 receptors on axons and axon terminals in the dorsolateral and ventrolateral PAG, which is consistent with previous reports of CB1 receptor distribution (Pugh et al., 1996, Tsou et al., 1998, Scavone et al., 2010). Furthermore, our results show that some MOR-ir cells receive appositions from CB1 receptor-ir profiles, which are presumed to be presynaptic. Networks consisting of MOP-ir cells receiving inputs from CB1 receptor-ir axon terminals could thus undergo synaptic adaptations that underlie behavioral interactions between cannabinoids and opioids after chronic drug treatment.
The results of this study indicate that the extent and pattern of CB1 receptor distribution is uniform across PAG sub-regions. The relationship of the CB1 receptor to the MOP receptor is similarly homogeneous, with a high degree of consistency of co-localization and appositions between the dorsolateral and ventrolateral PAG. The anatomical homogeneity of these sub-regions is somewhat surprising, given that cannabinoids produce better antinociception in the dorsolateral PAG than the ventrolateral PAG (Lichtman et al., 1996), Thus, PAG sub-regional differences in antinociceptive potency (Martin et al., 1995) and flight responses (Morgan et al., 1998) seem unrelated to the pattern or extent of CB1 receptor distribution in the PAG. The differential behavior driven by these two sub-regions is more likely the result of differential outputs from these regions (Cameron et al., 1995).
In the spinal cord and reward-related brain regions, chronic opioid exposure increases the density of the CB1 receptor (Fattore et al., 2007, Morgan and Christie, 2011). The PAG however, does not demonstrate this characteristic, as neither chronic opioid nor cannabinoid exposure altered the density of CB1 receptor immunolabeling in the dorsolateral or ventrolateral PAG. These results suggest that the up-regulation of CB1 receptors after chronic morphine exposure may be region-specific, or dependent upon the drug administration method. Thus, antinociceptive enhancement or tolerance prevention between cannabinoids and opioids seems unrelated to receptor density in the PAG.
Cannabinoids and opioids interact in a number of ways that could be therapeutically beneficial. The results of this study confirm the co-localization of the CB1 receptor and MOP receptor in the PAG, which is critically involved in the development of opioid tolerance. The potential for cannabinoids to enhance morphine antinociception and attenuate morphine tolerance warrants the investigation of these mechanisms at the cellular and molecular level.
Highlights.
CB1 receptor expression is diffuse and extensive in the periaqueductal gray (PAG).
The mu-opioid peptide (MOP) and CB1 receptors co-localize in many PAG neurons.
CB1 labeling was mostly somatodendritic, but CB1 axon terminals were also detected.
The pattern and extent of CB1 labeling was similar in the dlPAG and vlPAG.
Chronic morphine or THC administration had no effect on CB1 receptor density.
Acknowledgments
The authors would like to thank Dr. Ken Mackie for his generous gift of the rabbit anti-CB1 receptor antibody. The authors would also like to thank Sam Hermes and James Huang for their technical assistance.
ROLE OF THE FUNDING SOURCE
This work was supported by grants from the NIH: DA026591 (A.R.W. and M.M.M), DE012640 (S.A.A. and D.M.H), P30 NS061800 (S.A.A), RR016858 (confocal microscope), DA011322 (Dr. Ken Mackie for CB1 receptor antibody production and purification). The electron microscopy was made possible by an instrumentation grant from the M.J. Murdock Charitable Trust.
ABBREVIATIONS
- PAG
periaqueductal gray
- MOP
mu-opioid peptide
- CB1
cannabinoid 1
- cAMP
cyclic AMP
- MAPK
mitogen-activated protein kinase
- ERK
extracellular-related kinase
- PB
phosphate buffer
- TS
Tris-buffered saline
- BSA
bovine serum albumin
- NT
NeuroTrace
- -ir
immunoreactive
- ROI
region of interest
- EMS
Electron Microscopy Sciences
- THC
(9)-tetrahydrocannabinol
- DMSO
dimethyl sulfoxide
- NIDA
National Institute on Drug Abuse
Footnotes
Submission declaration
The work described in this manuscript has not been published previously (except as part of a published academic thesis), and it is not under consideration for publication elsewhere. Its publication is approved by all authors and tacitly by the responsible authorities where the work was carried out. If accepted, it will not be published elsewhere including electronically in the same form, in English or in any other language, without the written consent of the copyright-holder.
Contributors
The contributions of each author are as follows:
Wilson-Poe, A.R.: Responsible for experimental conception, data collection, data analysis and interpretation, first draft of manuscript, and final draft of manuscript. Morgan, M.M.: Supervision and manuscript revisions. Aicher, S.A.: Supervision, facilities and materials, and manuscript revisions. Hegarty, D.M.: Training and assistance with data collection and analysis (including statistical analysis), constructing figures, and manuscript construction and revisions. All authors have approved the final article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
M.M. Morgan, Email: mmmorgan@vancouver.wsu.edu.
D.M. Hegarty, Email: aichers@ohsu.edu.
References
- Abramoff MD, Magalhaes PJ, Ram SJ. Image Processing with ImageJ. Biophotonics International. 2004;11:36–42. [Google Scholar]
- Abrams DI, Couey P, Shade SB, Kelly ME, Benowitz NL. Cannabinoid-opioid interaction in chronic pain. Clin Pharmacol Ther. 2011;90:844–851. doi: 10.1038/clpt.2011.188. [DOI] [PubMed] [Google Scholar]
- Aicher SA, Mitchell JL, Swanson KC, Zadina JE. Endomorphin-2 axon terminals contact mu-opioid receptor-containing dendrites in trigeminal dorsal horn. Brain Res. 2003;977:190–198. doi: 10.1016/s0006-8993(03)02678-7. [DOI] [PubMed] [Google Scholar]
- Aicher SA, Punnoose A, Goldberg A. mu-Opioid receptors often colocalize with the substance P receptor (NK1) in the trigeminal dorsal horn. J Neurosci. 2000;20:4345–4354. doi: 10.1523/JNEUROSCI.20-11-04345.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aicher SA, Sharma S, Cheng PY, Pickel VM. The N-methyl-D-aspartate (NMDA) receptor is postsynaptic to substance P-containing axon terminals in the rat superficial dorsal horn. Brain Res. 1997;772:71–81. doi: 10.1016/s0006-8993(97)00637-9. [DOI] [PubMed] [Google Scholar]
- Alger BE. Retrograde signaling in the regulation of synaptic transmission: focus on endocannabinoids. Prog Neurobiol. 2002;68:247–286. doi: 10.1016/s0301-0082(02)00080-1. [DOI] [PubMed] [Google Scholar]
- Asensio VJ, Miralles A, Garcia-Sevilla JA. Stimulation of mitogen-activated protein kinase kinases (MEK1/2) by mu-, delta- and kappa-opioid receptor agonists in the rat brain: regulation by chronic morphine and opioid withdrawal. Eur J Pharmacol. 2006;539:49–56. doi: 10.1016/j.ejphar.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Ayhan IH, Kaymakcalan S, Tulunay FC. Interaction between delta 9-tetrahydrocannabinol and morphine on the motor activity of mice. Psychopharmacology (Berl) 1979;63:169–172. doi: 10.1007/BF00429697. [DOI] [PubMed] [Google Scholar]
- Bailey TW, Hermes SM, Andresen MC, Aicher SA. Cranial visceral afferent pathways through the nucleus of the solitary tract to caudal ventrolateral medulla or paraventricular hypothalamus: _target-specific synaptic reliability and convergence patterns. J Neurosci. 2006;26:11893–11902. doi: 10.1523/JNEUROSCI.2044-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn LM, Gainetdinov RR, Lin FT, Lefkowitz RJ, Caron MG. Mu-opioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nature. 2000;408:720–723. doi: 10.1038/35047086. [DOI] [PubMed] [Google Scholar]
- Cameron AA, Khan IA, Westlund KN, Willis WD. The efferent projections of the periaqueductal gray in the rat: a Phaseolus vulgaris-leucoagglutinin study. II. Descending projections. J Comp Neurol. 1995;351:585–601. doi: 10.1002/cne.903510408. [DOI] [PubMed] [Google Scholar]
- Chan J, Aoki C, Pickel VM. Optimization of differential immunogold-silver and peroxidase labeling with maintenance of ultrastructure in brain sections before plastic embedding. J Neurosci Methods. 1990;33:113–127. doi: 10.1016/0165-0270(90)90015-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childers SR, Fleming L, Konkoy C, Marckel D, Pacheco M, Sexton T, Ward S. Opioid and cannabinoid receptor inhibition of adenylyl cyclase in brain. Ann N Y Acad Sci. 1992;654:33–51. doi: 10.1111/j.1749-6632.1992.tb25954.x. [DOI] [PubMed] [Google Scholar]
- Cichewicz DL, McCarthy EA. Antinociceptive synergy between delta(9)-tetrahydrocannabinol and opioids after oral administration. J Pharmacol Exp Ther. 2003;304:1010–1015. doi: 10.1124/jpet.102.045575. [DOI] [PubMed] [Google Scholar]
- Cichewicz DL, Welch SP. Modulation of oral morphine antinociceptive tolerance and naloxone-precipitated withdrawal signs by oral Delta 9-tetrahydrocannabinol. J Pharmacol Exp Ther. 2003;305:812–817. doi: 10.1124/jpet.102.046870. [DOI] [PubMed] [Google Scholar]
- Cichewicz DL, Welch SP, Smith FL. Enhancement of transdermal fentanyl and buprenorphine antinociception by transdermal delta9-tetrahydrocannabinol. Eur J Pharmacol. 2005;525:74–82. doi: 10.1016/j.ejphar.2005.09.039. [DOI] [PubMed] [Google Scholar]
- Commons KG, Aicher SA, Kow LM, Pfaff DW. Presynaptic and postsynaptic relations of mu-opioid receptors to gamma-aminobutyric acid-immunoreactive and medullary-projecting periaqueductal gray neurons. J Comp Neurol. 2000;419:532–542. doi: 10.1002/(sici)1096-9861(20000417)419:4<532::aid-cne8>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Connor M, Christie MD. Opioid receptor signalling mechanisms. Clin Exp Pharmacol Physiol. 1999;26:493–499. doi: 10.1046/j.1440-1681.1999.03049.x. [DOI] [PubMed] [Google Scholar]
- De Laurentiis A, Fernandez-Solari J, Mohn C, Burdet B, Zorrilla Zubilete MA, Rettori V. The hypothalamic endocannabinoid system participates in the secretion of oxytocin and tumor necrosis factor-alpha induced by lipopolysaccharide. J Neuroimmunol. 2010;221:32–41. doi: 10.1016/j.jneuroim.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Drake CT, Aicher SA, Montalmant FL, Milner TA. Redistribution of mu-opioid receptors in C1 adrenergic neurons following chronic administration of morphine. Exp Neurol. 2005;196:365–372. doi: 10.1016/j.expneurol.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Fattore L, Vigano D, Fadda P, Rubino T, Fratta W, Parolaro D. Bidirectional regulation of mu-opioid and CB1-cannabinoid receptor in rats self-administering heroin or WIN 55,212-2. Eur J Neurosci. 2007;25:2191–2200. doi: 10.1111/j.1460-9568.2007.05470.x. [DOI] [PubMed] [Google Scholar]
- Finn DP, Beckett SR, Roe CH, Madjd A, Fone KC, Kendall DA, Marsden CA, Chapman V. Effects of coadministration of cannabinoids and morphine on nociceptive behaviour, brain monoamines and HPA axis activity in a rat model of persistent pain. Eur J Neurosci. 2004;19:678–686. doi: 10.1111/j.0953-816x.2004.03177.x. [DOI] [PubMed] [Google Scholar]
- Finn DP, Jhaveri MD, Beckett SR, Roe CH, Kendall DA, Marsden CA, Chapman V. Effects of direct periaqueductal grey administration of a cannabinoid receptor agonist on nociceptive and aversive responses in rats. Neuropharmacology. 2003;45:594–604. doi: 10.1016/s0028-3908(03)00235-1. [DOI] [PubMed] [Google Scholar]
- Garzon M, Vaughan RA, Uhl GR, Kuhar MJ, Pickel VM. Cholinergic axon terminals in the ventral tegmental area _target a subpopulation of neurons expressing low levels of the dopamine transporter. J Comp Neurol. 1999;410:197–210. doi: 10.1002/(sici)1096-9861(19990726)410:2<197::aid-cne3>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Hajos N, Katona I, Naiem SS, MacKie K, Ledent C, Mody I, Freund TF. Cannabinoids inhibit hippocampal GABAergic transmission and network oscillations. Eur J Neurosci. 2000;12:3239–3249. doi: 10.1046/j.1460-9568.2000.00217.x. [DOI] [PubMed] [Google Scholar]
- Hegarty DM, Mitchell JL, Swanson KC, Aicher SA. Kainate receptors are primarily postsynaptic to SP-containing axon terminals in the trigeminal dorsal horn. Brain Res. 2007;1184:149–159. doi: 10.1016/j.brainres.2007.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegarty DM, Tonsfeldt K, Hermes SM, Helfand H, Aicher SA. Differential localization of vesicular glutamate transporters and peptides in corneal afferents to trigeminal nucleus caudalis. J Comp Neurol. 2010;518:3557–3569. doi: 10.1002/cne.22414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann AG, Suplita RL, Bolton NM, Neely MH, Fegley D, Mangieri R, Krey JF, Walker JM, Holmes PV, Crystal JD, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. An endocannabinoid mechanism for stress-induced analgesia. Nature. 2005;435:1108–1112. doi: 10.1038/nature03658. [DOI] [PubMed] [Google Scholar]
- Howlett AC. Cannabinoid inhibition of adenylate cyclase. Biochemistry of the response in neuroblastoma cell membranes. Mol Pharmacol. 1985;27:429–436. [PubMed] [Google Scholar]
- Howlett AC. Cannabinoid receptor signaling. Handb Exp Pharmacol. 2005:53–79. doi: 10.1007/3-540-26573-2_2. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, Felder CC, Herkenham M, Mackie K, Martin BR, Mechoulam R, Pertwee RG. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- Ingram SL, Vaughan CW, Bagley EE, Connor M, Christie MJ. Enhanced opioid efficacy in opioid dependence is caused by an altered signal transduction pathway. J Neurosci. 1998;18:10269–10276. doi: 10.1523/JNEUROSCI.18-24-10269.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen TS, Yaksh TL. Comparison of antinociceptive action of morphine in the periaqueductal gray, medial and paramedial medulla in rat. Brain Res. 1986;363:99–113. doi: 10.1016/0006-8993(86)90662-1. [DOI] [PubMed] [Google Scholar]
- Kalyuzhny AE, Arvidsson U, Wu W, Wessendorf MW. mu-Opioid and delta-opioid receptors are expressed in brainstem antinociceptive circuits: studies using immunocytochemistry and retrograde tract-tracing. J Neurosci. 1996;16:6490–6503. doi: 10.1523/JNEUROSCI.16-20-06490.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman AH, Cook SA, Martin BR. Investigation of brain sites mediating cannabinoid-induced antinociception in rats: evidence supporting periaqueductal gray involvement. J Pharmacol Exp Ther. 1996;276:585–593. [PubMed] [Google Scholar]
- Macey TA, Bobeck EN, Hegarty DM, Aicher SA, Ingram SL, Morgan MM. Extracellular signal-regulated kinase 1/2 activation counteracts morphine tolerance in the periaqueductal gray of the rat. J Pharmacol Exp Ther. 2009;331:412–418. doi: 10.1124/jpet.109.152157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailleux P, Vanderhaeghen JJ. Distribution of neuronal cannabinoid receptor in the adult rat brain: a comparative receptor binding radioautography and in situ hybridization histochemistry. Neuroscience. 1992;48:655–668. doi: 10.1016/0306-4522(92)90409-u. [DOI] [PubMed] [Google Scholar]
- Martin WJ, Patrick SL, Coffin PO, Tsou K, Walker JM. An examination of the central sites of action of cannabinoid-induced antinociception in the rat. Life Sci. 1995;56:2103–2109. doi: 10.1016/0024-3205(95)00195-c. [DOI] [PubMed] [Google Scholar]
- Meng ID, Johansen JP. Antinociception and modulation of rostral ventromedial medulla neuronal activity by local microinfusion of a cannabinoid receptor agonist. Neuroscience. 2004;124:685–693. doi: 10.1016/j.neuroscience.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Morgan MM, Christie MJ. Analysis of opioid efficacy, tolerance, addiction and dependence from cell culture to human. Br J Pharmacol. 2011;164:1322–1334. doi: 10.1111/j.1476-5381.2011.01335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MM, Whitney PK, Gold MS. Immobility and flight associated with antinociception produced by activation of the ventral and lateral/dorsal regions of the rat periaqueductal gray. Brain Res. 1998;804:159–166. doi: 10.1016/s0006-8993(98)00669-6. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay P, Pan H, Rajesh M, Batkai S, Patel V, Harvey-White J, Mukhopadhyay B, Hasko G, Gao B, Mackie K, Pacher P. CB1 cannabinoid receptors promote oxidative/nitrosative stress, inflammation and cell death in a murine nephropathy model. Br J Pharmacol. 2010;160:657–668. doi: 10.1111/j.1476-5381.2010.00769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson SJ. The rat brain, in stereotaxic coordinates. Sydney: Academic Press; 2005. [Google Scholar]
- Peters A, Palay SL, Webster HD. The Fine Structure of the Nervous System: Neurons and Their Supporting Cells. New York: Oxford University Press; 1991. [Google Scholar]
- Pugh G, Jr, Smith PB, Dombrowski DS, Welch SP. The role of endogenous opioids in enhancing the antinociception produced by the combination of delta 9-tetrahydrocannabinol and morphine in the spinal cord. J Pharmacol Exp Ther. 1996;279:608–616. [PubMed] [Google Scholar]
- Roberts JD, Gennings C, Shih M. Synergistic affective analgesic interaction between delta-9-tetrahydrocannabinol and morphine. Eur J Pharmacol. 2006;530:54–58. doi: 10.1016/j.ejphar.2005.11.036. [DOI] [PubMed] [Google Scholar]
- Rubino T, Tizzoni L, Vigano D, Massi P, Parolaro D. Modulation of rat brain cannabinoid receptors after chronic morphine treatment. Neuroreport. 1997;8:3219–3223. doi: 10.1097/00001756-199710200-00007. [DOI] [PubMed] [Google Scholar]
- Scavone JL, Mackie K, Van Bockstaele EJ. Characterization of cannabinoid-1 receptors in the locus coeruleus: relationship with mu-opioid receptors. Brain Res. 2010;1312:18–31. doi: 10.1016/j.brainres.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith FL, Cichewicz D, Martin ZL, Welch SP. The enhancement of morphine antinociception in mice by delta9-tetrahydrocannabinol. Pharmacol Biochem Behav. 1998;60:559–566. doi: 10.1016/s0091-3057(98)00012-4. [DOI] [PubMed] [Google Scholar]
- Smith PA, Selley DE, Sim-Selley LJ, Welch SP. Low dose combination of morphine and delta9-tetrahydrocannabinol circumvents antinociceptive tolerance and apparent desensitization of receptors. Eur J Pharmacol. 2007;571:129–137. doi: 10.1016/j.ejphar.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez J, Bermudez-Silva FJ, Mackie K, Ledent C, Zimmer A, Cravatt BF, de Fonseca FR. Immunohistochemical description of the endogenous cannabinoid system in the rat cerebellum and functionally related nuclei. J Comp Neurol. 2008;509:400–421. doi: 10.1002/cne.21774. [DOI] [PubMed] [Google Scholar]
- Suarez J, Romero-Zerbo SY, Rivera P, Bermudez-Silva FJ, Perez J, De Fonseca FR, Fernandez-Llebrez P. Endocannabinoid system in the adult rat circumventricular areas: an immunohistochemical study. J Comp Neurol. 2010;518:3065–3085. doi: 10.1002/cne.22382. [DOI] [PubMed] [Google Scholar]
- Tham SM, Angus JA, Tudor EM, Wright CE. Synergistic and additive interactions of the cannabinoid agonist CP55,940 with mu opioid receptor and alpha2-adrenoceptor agonists in acute pain models in mice. Br J Pharmacol. 2005;144:875–884. doi: 10.1038/sj.bjp.0706045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- Wager-Miller J, Westenbroek R, Mackie K. Dimerization of G protein-coupled receptors: CB1 cannabinoid receptors as an example. Chem Phys Lipids. 2002;121:83–89. doi: 10.1016/s0009-3084(02)00151-2. [DOI] [PubMed] [Google Scholar]
- Welch SP. Interaction of the cannabinoid and opioid systems in the modulation of nociception. Int Rev Psychiatry. 2009;21:143–151. doi: 10.1080/09540260902782794. [DOI] [PubMed] [Google Scholar]
- Wilson A, Maher L, Morgan M. Repeated cannabinoid injections into the rat periaqueductal gray enhance subsequent morphine antinociception. Neuropharmacology. 2008;55:1219–1225. doi: 10.1016/j.neuropharm.2008.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endocannabinoid signaling in the brain. Science. 2002;296:678–682. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]