Abstract
A prospective clinical cohort study was established to investigate the humoral immune response in middle ear fluids (MEF) and serum against bacterial surface proteins in children suffering from recurrent acute otitis media (rAOM) and chronic otitis media with effusion (COME), using Luminex xMAP technology. The association between the humoral immune response and the presence of Moraxella catarrhalis and Streptococcus pneumoniae in the nasopharynx and middle ear was also studied. The levels of antigen-specific IgG, IgA, and IgM showed extensive interindividual variation. No significant differences in anti-M. catarrhalis and anti-S. pneumoniae serum and MEF median fluorescence intensity (MFI) values (anti-M. catarrhalis and antipneumococcal IgG levels) were observed between the rAOM or COME groups for all antigens tested. No significant differences were observed for M. catarrhalis and S. pneumoniae colonization and serum IgG levels against the Moraxella and pneumococcal antigens. Similar to the antibody response in serum, no significant differences in IgG, IgA, and IgM levels in MEF were observed for all M. catarrhalis and S. pneumoniae antigens between OM M. catarrhalis- or S. pneumoniae-positive and OM M. catarrhalis- or S. pneumonia-negative children suffering from either rAOM or COME. Finally, results indicated a strong correlation between antigen-specific serum and MEF IgG levels. We observed no significant in vivo expressed anti-M. catarrhalis or anti-S. pneumoniae humoral immune responses using a range of putative vaccine candidate proteins. Other factors, such as Eustachian tube dysfunction, viral load, and genetic and environmental factors, may play a more important role in the pathogenesis of OM and in particular in the development of rAOM or COME.
INTRODUCTION
Otitis media (OM) is an important upper respiratory tract disease of early childhood and the primary reason for young children to visit a physician. The disease has a considerable negative impact on the quality of life during childhood and causes much concern to parents. OM encompasses a spectrum of conditions, including acute otitis media (AOM) and otitis media with effusion (OME), with approximately 80% of children having experienced an episode of AOM by the age of 3 years. Up to one-third of these children will have experienced recurrent infections, with many of these episodes being facilitated by a bacterial infection (3, 37). In fact, bacteria may be isolated from the middle ear fluid (MEF) of approximately 80% of children with AOM and 30 to 50% of chronic middle ear effusions obtained from children presenting with OME (12). In many countries, OM is a common reason to prescribe antibiotics or to undergo surgery for the insertion of ventilation tubes, resulting in a significant burden on health care systems (21, 25, 29). This means that the direct costs associated with OM are substantial (2) and that the prevention of OM disease via alternative methods such as vaccination offers a promising approach to reduce the burden of OM disease and its economic consequences.
Traditionally, Streptococcus pneumoniae has been reported to be the predominant bacterial species cultured in AOM disease, followed by Haemophilus influenzae and Moraxella catarrhalis. However, H. influenzae tends to predominate in OME disease, followed to a lesser extent by S. pneumoniae and M. catarrhalis (7, 9, 32). Further, although these common OM-related bacterial species may be cultured from the middle ear of children during OM episodes, either as single pathogens or as cocultures (28), research has also shown the importance of (frequently culture-negative) bacterial biofilm formation in the development of middle ear disease (22). Finally, the introduction of a conjugated heptavalent pneumococcal vaccine (PCV7) for use in children in the community has resulted in a significant reduction in the overall proportion of S. pneumoniae isolates and vaccine serotypes observed in AOM. Indeed, the success of vaccination against S. pneumoniae means that H. influenzae is now becoming the predominant pathogen isolated from children suffering from persistent AOM disease (6, 10).
Children are frequently colonized with bacterial pathogens at an early age, and the pattern of nasopharyngeal colonization is an important determinant for OM disease (15, 16). Further, research has also indicated that, as well as the presence of particular bacterial species, both the adaptive and innate immune systems, Eustachian tube dysfunction, viral load, and genetic and environmental factors all may be involved in the pathogenesis of OM (19, 23, 30, 31, 33, 38).
The recent recognition of M. catarrhalis as an important human pathogen has stimulated active investigation into the molecular mechanisms of its pathogenesis. An essential step in colonization and infection is bacterial adherence to the mucosal epithelium of the respiratory tract. A growing number of adhesins have been identified in M. catarrhalis, and most of these proteins are highly conserved and immunogenic and express distinct epitopes on the bacterial surface. This means that they may be suitable as potential M. catarrhalis vaccine candidates (27). However, relatively little is known regarding the development of the natural humoral immune response to these potential vaccine candidates in children. As yet, no licensed vaccine has been marketed against M. catarrhalis, and to date, none of the putative vaccine candidates so far described in the literature have actually progressed to clinical trials.
On the other hand, vaccination against S. pneumoniae infection is already established, for example, via the introduction of the PCV7 vaccine. PCV7 was primarily used to prevent invasive pneumococcal disease (meningitis and other pneumococcal infections such as pneumonia) in children, with the introduction of PCV7 having led to a noticeable reduction in the incidence of S. pneumoniae vaccine strains in the etiology of AOM (13). However, an increase in the carriage of nonvaccine serotypes has been reported, as well as a consequent increase in invasive disease by these nonvaccine serotypes, which could reduce or even negate the benefits initially obtained through vaccination with PCV7 (11, 26). In fact, S. pneumoniae serotype replacement and subsequent vaccine failure in PCV7-vaccinated children have become serious concerns in recent years, with most of these problems assigned to the serotype-specific nature of the PCV7 vaccine. Currently, several pneumococcal surface proteins, either alone or in combinations, have been suggested as putative vaccine candidates (1, 13, 20) and could serve as more effective vaccines than those currently available, providing broad coverage against most pneumococcal serotypes. However, similar to the situation faced by vaccine candidates of M. catarrhalis, relatively little is known about the development of the humoral immune response to S. pneumoniae protein-based vaccine candidates in children.
With this study, we provide insights into the anti-M. catarrhalis and antipneumococcal humoral immune response in a cohort comprising Dutch children exhibiting recurrent (recurrent acute otitis media [rAOM]) and chronic (chronic otitis media with effusion [COME]) episodes of OM. Currently, there is a common perception that rAOM tends to be caused by recurrent episodes of AOM that are associated with bacteria and/or viral infections. In contrast, COME is generally considered a sterile inflammation. Studying the immune response and the accompanying pathogens associated with rAOM and COME will allow us to better distinguish the types of bacterial and immune factors that are involved in the pathogenesis of rAOM and COME and lead to a better understanding of the pathogenesis of these 2 clinical presentations of OM disease.
MATERIALS AND METHODS
Study cohort.
This study was performed as part of a prospective clinical cohort study set up at Radboud University Nijmegen Medical Centre (RUNMC), Nijmegen, The Netherlands, to determine the immune response to putative vaccine candidates of M. catarrhalis and S. pneumoniae in children suffering from OM disease.
Patients were enrolled in two hospitals in Nijmegen, The Netherlands, from 1 April 2008 to 1 July 2009, with ages ranging from birth up to 5 years of age, and all suffering from rAOM or COME (for which tympanostomy tube insertion was indicated). Recurrent OM was defined as 3 or more episodes of AOM in the last 6 months or 4 episodes in the last 12 months. The COME patient population consisted of children who experienced a period of persistent OM lasting longer than 3 months. Diagnosis was made by an otolaryngologist based upon signs, symptoms, otoscopy, and audiometry including tympanometry. Patient characteristics and risk factors were identified using a questionnaire. Permission was obtained from the Committee on Research Involving Human Subjects in January 2008 (CMO 2007/239; international trial register number NCT00847756).
Clinical materials and detection of bacterial pathogens.
Middle ear fluid, a nasopharyngeal swab, and serum were collected during surgery. Nasopharyngeal swabs were cultured according to standard laboratory procedures in order to determine the presence of M. catarrhalis and S. pneumoniae. Quantitative real-time PCR (qPCR) was performed in order to determine the presence of M. catarrhalis and S. pneumoniae in MEF (K. Stol, S. Verhaegh, K. Graamans, J. Engel, P. Sturm, W. Melchers, J. Meis, A. Warris, J. Hays, and P. Hermans, submitted for publication).
Moraxella catarrhalis antigens.
The previously described M. catarrhalis recombinant proteins used in this study comprised ubiquitous surface proteins A (UspA1557-704 [amino acids (aa) 557 to 704 of UspA1] and UspA2165-318), two fragments of Moraxella immunoglobulin D-binding protein (MID764-913 and MID962-1200), human erythrocyte agglutinin (Hag385-863), M. catarrhalis hemagglutinin-like proteins (MhaB and MhaC), and M. catarrhalis adherence protein (McaP51-333). Orf238 and Orf296 are hypothetical proteins that share homology with lipoprotein family A proteins and with a M. osloensis disulfide isomerase gene virulence factor, respectively. These 10 recombinant proteins (from 8 different OMPs) represented the majority of published M. catarrhalis immunogenic proteins discovered at the time that the study was initiated and are derived from the M. catarrhalis reference strains Bc5 (UspA1557-704, UspA2165-318, MID764-913, and MID962-1200) and O35E (MhaB, MhaC, McaP51-333, and Hag385-863) (5, 24, 34, 35).
Streptococcus pneumoniae antigens.
The previously described S. pneumoniae recombinant proteins used in this study comprised choline binding protein A (PspC/CbpA); α-enolase (Eno); hyaluronidase (Hyl); immunoglobulin A1 (IgA-1) protease; neuraminidase (NanA); pneumolysin (PLY); a double mutant of pneumolysin (PdbD); putative protease maturation protein A (PpmA); pneumococcal surface adhesin A (PsaA); pneumococcal surface protein A (PspA); the pneumococcal histidine triad (Pht) proteins (SP1003 [PhtD] and BVH-3 [PhtE]); streptococcal lipoprotein rotamase A (SlrA); S. pneumoniae proteins SP0189 (hypothetical protein), SP0376 (response regulator, intracellular location), and SP1651 (thiol peroxidase, intracellular location); and Pilus A (8, 20).
Multiplex M. catarrhalis and S. pneumoniae antibody assay.
Recombinant proteins were coupled to carboxylated SeroMAP beads that were developed for serological application, as detailed by Verkaik et al. (39). Uncoupled beads were used as a negative control to determine nonspecific antibody binding. If nonspecific binding was observed, then the median fluorescence intensity (MFI) values obtained from this nonspecific binding were subtracted from the antigen-specific results.
The Luminex multiplex procedure was performed as described previously (39). Briefly, after validation of the assay (achieved by comparison of human pooled serum [HPS] MFI values obtained using the multiplex assay with HPS MFI values obtained using the singleplex assays), the different antigen-coupled microspheres were mixed to a working concentration of 4,000 beads per color per well. Serum samples were diluted 1:100 in phosphate-buffered saline containing 1% bovine serum albumin (PBS-BN) for measurement of antigen-specific IgG. Fifty microliters per diluted sample was incubated with the microspheres in a 96-well filter microtiter plate (Millipore) for 35 min at room temperature on a Thermomixer plate shaker (Eppendorf). The plate was washed twice with assay buffer (PBS-BN) and aspirated using a vacuum manifold. The microspheres were suspended in 50 μl of assay buffer, and 50 μl of a 1:200 dilution of R-phycoerythrin (RPE)-conjugated AffiniPure goat anti-human IgG (Jackson Immuno Research) was added in separate wells. The plate was incubated for 35 min at room temperature and washed. The microspheres were suspended in 100 μl of assay buffer. Measurements were performed on the Luminex 100 instrument (BMD) using Luminex IS software (version 2.2). Tests were performed in duplicate, and the MFI values, reflecting quantitative antibody levels, were averaged. The coefficient of variation was calculated for each serum sample and averaged per protein and antibody isotype. The procedure for MEF was identical to that outlined above, except that IgA and IgM were also measured. Briefly, MEF samples were diluted 1:100 in PBS-BN for measurement of antigen-specific IgG and 1:50 for measurement of IgA and IgM. Fifty microliters of a 1:100 dilution of RPE-conjugated AffiniPure goat anti-human IgG and IgA and 50 μl of a 1:200 dilution of RPE-conjugated donkey anti-human IgM were then added. For assay validation, MFI values obtained from pooled MEF (PMEF) using the multiplex assay were compared to MFI values obtained from PMEF obtained using the singleplex assay.
Statistical analysis.
Statistical analyses were performed using SPSS PASW Statistics version 17. Correlations between antigen-specific IgG in serum and MEF were assessed using Spearman's correlation coefficient. The Mann-Whitney U test was used to compare anti-M. catarrhalis and antipneumococcal immunoglobulin (Ig) levels between children diagnosed with rAOM and COME and to compare differences in Ig levels between colonized and noncolonized children. The Bonferroni correction was applied to correct for multiple testing. A P value of ≤0.0006 was considered to be statistically significant.
RESULTS
Correlation between anti-M. catarrhalis and antipneumococcal IgG levels in serum and MEF.
To determine the correlation between the levels of anti-Moraxella and antipneumococcal IgG in serum compared to in MEF, samples from 111 children who donated both blood and MEF at tympanostomy surgery were included. The mean IgG levels (reflected by MFI values) in these samples were calculated for each protein (Fig. 1). IgG levels in serum showed a strong correlation to IgG levels in MEF for both M. catarrhalis (R2 = 0.97) as well as S. pneumoniae (R2 = 0.89).
Fig 1.
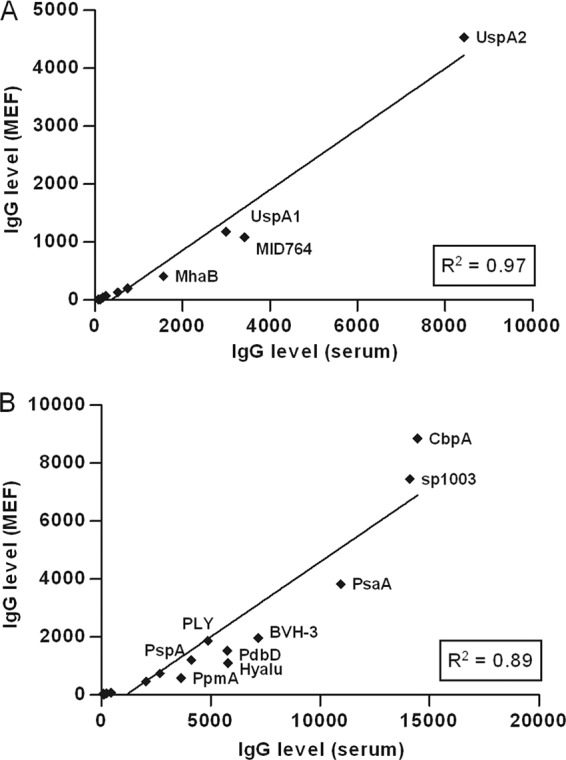
Correlation between IgG levels in serum and MEF. Mean IgG levels in serum and MEF were used to calculate the correlation statistics for each respective M. catarrhalis (A) or S. pneumoniae (B) protein (Spearman's correlation coefficient, R2 = 0.97 and 0.89).
Dynamics of the anti-M. catarrhalis and antipneumococcal antibody response in children with recurrent and chronic otitis media disease.
A total of 156 children were included in the analysis of the antibody response to M. catarrhalis and S. pneumoniae proteins in serum. Forty-two children were diagnosed with rAOM, whereas 114 children were diagnosed with COME. No significant differences in anti-M. catarrhalis and S. pneumoniae serum MFI values (anti-M. catarrhalis and antipneumococcal IgG levels) were observed between the rAOM or COME groups for all antigens tested.
A total of 121 children were included in the analysis of the antibody response to M. catarrhalis and S. pneumoniae proteins in MEF, with 25 children and 96 children diagnosed with rAOM and COME, respectively. No significant differences in anti-M. catarrhalis and antipneumococcal MEF IgG, IgA, and IgM levels were found for all proteins tested.
Association between bacterial colonization and infection and anti-M. catarrhalis and antipneumococcal antibody levels in children with rAOM and COME.
A total of 156 children were included in the analysis of the antibody IgG response to M. catarrhalis and S. pneumoniae antigens in serum. Forty-two children were diagnosed with rAOM, whereas 114 children were diagnosed with COME. No significant difference was observed for the presence of M. catarrhalis and S. pneumoniae and serum IgG levels against the Moraxella and pneumococcal antigens.
A total of 117 children were included in the analysis of the antibody response to M. catarrhalis and S. pneumoniae proteins in MEF, including 23 children suffering from rAOM and 94 children suffering from COME. Similar to the antibody response in serum, no significant differences in IgG, IgA, and IgM levels in MEF were observed for all M. catarrhalis and S. pneumoniae antigens between OM M. catarrhalis- or S. pneumonia-positive and OM M. catarrhalis- or S. pneumonia-negative children suffering from either rAOM or COME.
Figure 2 shows an example of the results obtained; specifically, the figure shows a comparison of antipneumococcal IgA-1 protease IgA levels in COME-positive children in the presence and absence of S. pneumoniae in the middle ear.
Fig 2.
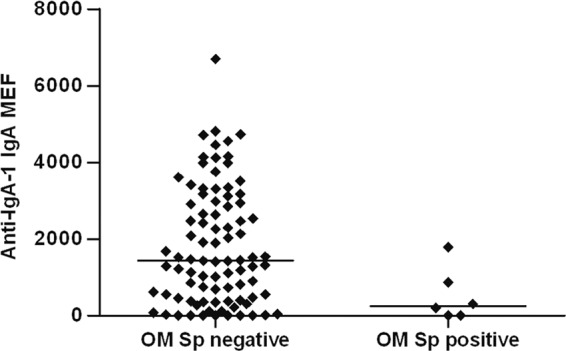
Example of the results obtained; specifically, the figure shows a comparison of antipneumococcal IgA-1 protease IgA levels in COME-positive children in the absence and presence of S. pneumoniae in the middle ear.
DISCUSSION
Studies have shown that M. catarrhalis and S. pneumoniae colonize the nasopharynx soon after birth, and frequent colonization with M. catarrhalis and S. pneumoniae has been reported to increase the risk of OM (15). The adaptive immune system and the innate immune system are also important factors in the pathogenesis of OM (31). The immune response to pathogens develops rapidly during the first few years of life, as children become exposed to an increasing number of microbial species and strains. The high incidence and high rate of spontaneous recovery from OM suggests that recovery is a natural phenomenon and part of the gradual maturation of the child's immune system, though a defective or immature antibody response to OM pathogens may explain the increased susceptibility of some children to OM (36).
Differences in the presence/absence and levels of pathogen-specific IgG and IgM at particular body sites may be one of the mechanisms by which OM pathogens are able to facilitate the development of OM disease. In this study, no significant differences in anti-M. catarrhalis and anti-S. pneumoniae serum and MEF IgG levels were observed between the rAOM or COME groups for all antigens tested, suggesting that differences in IgG levels between body sites do not play a significant role in the development of rAOM or COME disease. Interestingly, however, a strong correlation was observed between IgG antibody levels in serum and IgG antibody levels in MEF, for both M. catarrhalis and pneumococcal proteins for the whole study cohort. This is an interesting finding, as it indicates that serum antibody levels are predictive of the presence of local (middle ear) IgG antibody (14, 17) and raises the possibility that the measurement of OM pathogen-specific IgG antibodies in the middle ear may be achieved by simply measuring OM pathogen-specific IgG antibody levels in serum. At this moment in time, however, it is not known whether OM pathogen-specific IgG is actually produced locally in the middle ear or transudes into the middle ear from the general circulation (4, 14). From our results, it appears more likely that OM pathogen-specific IgG actually transudes into the middle ear rather than being locally produced, where it potentially provides some protection against OM disease. No significant differences in anti-M. catarrhalis and anti-S. pneumoniae MEF IgM levels were observed for either the rAOM or COME cohort in this study.
The local production of secretory antibody is an important immunological defense at epithelial surfaces, including the surfaces of the upper respiratory tract and middle ear. Secretory IgA has been shown to inhibit S. pneumoniae adherence and reduce nasopharyngeal bacterial colonization (18), and it is therefore possible that children with recurrent OM might lack OM pathogen-specific secretory IgA. In this study, the MFI levels of antigen-specific IgA showed extensive interindividual variability over time, with IgA levels to all M. catarrhalis and S. pneumoniae OMPs being relatively low throughout the study period. However, though not significant, S. pneumoniae-negative/COME-positive children showed higher MEF IgA levels against the pneumococcal IgA-1 protease protein than S. pneumoniae-positive/COME-positive children. In fact, many mucosal pathogens, including S. pneumoniae, express an IgA-1 protease that cleaves IgA molecules, thereby circumventing the protective effects of IgA production (40). Our results suggest that IgA antibody directed against anti-IgA-1 protease may have a restorative effect by helping to neutralize the effect of secreted anti-IgA-1 protease, thereby facilitating the binding of intact IgA to bacterial cells and promoting pathogen recognition and clearance by the immune response. However, it should be noted that the possibility exists that the differences in antibody levels observed in S. pneumonia-positive and -negative COME MEFs are simply a product of antibody binding to S. pneumoniae bacterial cells, thereby making the antibodies inaccessible to measurement using our Luminex assay. Further research will be required in order to determine whether IgA-1 protease antibodies actually provide protection in patients suffering from OM disease.
Studying the immune response and pathogens associated with rAOM and COME disease indicated that COME is not in fact a sterile inflammation as is generally considered the case. Importantly, investigation into the immune response to potential vaccine candidates of M. catarrhalis and S. pneumoniae indicated that there may be a lack of a characteristic immune response profiles that will allow clinicians to distinguish between rAOM and COME. Our data correlate with the findings of Stol et al. (submitted), who investigated bacterial colonization and infection in the nasopharynx and middle ear of the same prospective cohort of children and found no clear-cut differences in the microbial flora present in middle ear fluids obtained during rAOM and COME disease.
In summary, the levels of antigen-specific serum IgG and MEF IgG/IgM/IgA showed extensive interindividual variation in our rAOM and COME cohorts of children, with no significant differences between the 2 groups. Both rAOM and COME children possessed a range of antibodies (IgG, IgA, and IgM) against a variety of novel M. catarrhalis and S. pneumoniae recombinant proteins, and there were no distinguishing immune response profiles observed between the 2 groups. Factors such as Eustachian tube dysfunction, viral load, and genetic and environmental factors may play a more important role in the pathogenesis of rAOM and COME disease than the humoral immune response to OM-associated bacterial pathogens.
ACKNOWLEDGMENTS
We thank all of the children and parents who participated in this study. We are grateful to the staff of the departments ORL and Medical Microbiology of the Canisius Wilhelmina Hospital and the Radboud University Nijmegen Medical Centre for their commitment to the study. We thank Birgitta Henriques-Normark and Timothy Mitchell for kindly supplying the S. pneumoniae proteins and Nelianne J. Verkaik and Willem J. van Wamel for valuable discussions.
This work was supported by a European Union FP6 project grant (OMVac 037653).
Footnotes
Published ahead of print 25 April 2012
REFERENCES
- 1. American Academy of Pediatrics 2000. Committee on Infectious Diseases. Policy statement: recommendations for the prevention of pneumococcal infections, including the use of pneumococcal conjugate vaccine (Prevnar), pneumococcal polysaccharide vaccine, and antibiotic prophylaxis. Pediatrics 106: 362– 366 [DOI] [PubMed] [Google Scholar]
- 2. American Academy of Pediatrics Subcommittee on Management of Acute Otitis Media 2004. Diagnosis and management of acute otitis media. Pediatrics 113: 1451– 1465 [DOI] [PubMed] [Google Scholar]
- 3. Arguedas A, Kvaerner K, Liese J, Schilder AG, Pelton SI. 2010. Otitis media across nine countries: disease burden and management. Int. J. Pediatr. Otorhinolaryngol. 74: 1419– 1424 [DOI] [PubMed] [Google Scholar]
- 4. Bakaletz LO, Holmes KA. 1997. Evidence for transudation of specific antibody into the middle ears of parenterally immunized chinchillas after an upper respiratory tract infection with adenovirus. Clin. Diagn. Lab. Immunol. 4: 223– 225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Balder R, Hassel J, Lipski S, Lafontaine ER. 2007. Moraxella catarrhalis strain O35E expresses two filamentous hemagglutinin-like proteins that mediate adherence to human epithelial cells. Infect. Immun. 75: 2765– 2775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Block SL, et al. 2004. Community-wide vaccination with the heptavalent pneumococcal conjugate significantly alters the microbiology of acute otitis media. Pediatr. Infect. Dis. J. 23: 829– 833 [DOI] [PubMed] [Google Scholar]
- 7. Bluestone CD, Stephenson JS, Martin LM. 1992. Ten-year review of otitis media pathogens. Pediatr. Infect. Dis. J. 11: S7– S11 [DOI] [PubMed] [Google Scholar]
- 8. Bogaert D, Hermans PW, Adrian PV, Rumke HC, de Groot R. 2004. Pneumococcal vaccines: an update on current strategies. Vaccine 22: 2209– 2220 [DOI] [PubMed] [Google Scholar]
- 9. Broides A, Dagan R, Greenberg D, Givon-Lavi N, Leibovitz E. 2009. Acute otitis media caused by Moraxella catarrhalis: epidemiologic and clinical characteristics. Clin. Infect. Dis. 49: 1641– 1647 [DOI] [PubMed] [Google Scholar]
- 10. Casey JR, Pichichero ME. 2004. Changes in frequency and pathogens causing acute otitis media in 1995–2003. Pediatr. Infect. Dis. J. 23: 824– 828 [DOI] [PubMed] [Google Scholar]
- 11. Chibuk TK, Robinson JL, Hartfield DS. 2010. Pediatric complicated pneumonia and pneumococcal serotype replacement: trends in hospitalized children pre and post introduction of routine vaccination with pneumococcal conjugate vaccine (PCV7). Eur. J. Pediatr. 169: 1123– 1128 [DOI] [PubMed] [Google Scholar]
- 12. De Baere T, Vaneechoutte M, Deschaght P, Huyghe J, Dhooge I. 2010. The prevalence of middle ear pathogens in the outer ear canal and the nasopharyngeal cavity of healthy young adults. Clin. Microbiol. Infect. 16: 1031– 1035 [DOI] [PubMed] [Google Scholar]
- 13. De Wals P, Black S, Borrow R, Pearce D. 2009. Modeling the impact of a new vaccine on pneumococcal and nontypable Haemophilus influenzae diseases: a new simulation model. Clin. Ther. 31: 2152– 2169 [DOI] [PubMed] [Google Scholar]
- 14. Faden H, et al. 1989. Otitis media in children: local immune response to nontypeable Haemophilus influenzae. Infect. Immun. 57: 3555– 3559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Faden H, et al. 1997. Relationship between nasopharyngeal colonization and the development of otitis media in children. Tonawanda/Williamsville Pediatrics. J. Infect. Dis. 175: 1440– 1445 [DOI] [PubMed] [Google Scholar]
- 16. Faden H, et al. 1991. Nasopharyngeal flora in the first three years of life in normal and otitis-prone children. Ann. Otol. Rhinol. Laryngol. 100: 612– 615 [DOI] [PubMed] [Google Scholar]
- 17. Faden HS. 1997. Immunology of the middle ear: role of local and systemic antibodies in clearance of viruses and bacteria. Ann. N. Y. Acad. Sci. 830: 49– 60 [DOI] [PubMed] [Google Scholar]
- 18. Fukuyama Y, et al. 2010. Secretory-IgA antibodies play an important role in the immunity to Streptococcus pneumoniae. J. Immunol. 185: 1755– 1762 [DOI] [PubMed] [Google Scholar]
- 19. Garcia-Rodriguez JA, Fresnadillo Martinez MJ. 2002. Dynamics of nasopharyngeal colonization by potential respiratory pathogens. J. Antimicrob. Chemother. 50 Suppl S2: 59– 73 [DOI] [PubMed] [Google Scholar]
- 20. Giefing C, et al. 2008. Discovery of a novel class of highly conserved vaccine antigens using genomic scale antigenic fingerprinting of pneumococcus with human antibodies. J. Exp. Med. 205: 117– 131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Grevers G, First International Roundtable ENT Meeting Group 2010. Challenges in reducing the burden of otitis media disease: an ENT perspective on improving management and prospects for prevention. Int. J. Pediatr. Otorhinolaryngol. 74: 572– 577 [DOI] [PubMed] [Google Scholar]
- 22. Hall-Stoodley L, et al. 2006. Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media. JAMA 296: 202– 211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Labout JA, et al. 2008. Factors associated with pneumococcal carriage in healthy Dutch infants: the Generation R Study. J. Pediatr. 153: 771– 776 [DOI] [PubMed] [Google Scholar]
- 24. LaFontaine ER, et al. 2009. Identification of domains of the Hag/MID surface protein recognized by systemic and mucosal antibodies in adults with chronic obstructive pulmonary disease following clearance of Moraxella catarrhalis. Clin. Vaccine Immunol. 16: 653– 659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Leibovitz E. 2003. Acute otitis media in pediatric medicine: current issues in epidemiology, diagnosis, and management. Paediatr. Drugs 5 Suppl 1: 1– 12 [PubMed] [Google Scholar]
- 26. Melegaro A, et al. 2010. Dynamic models of pneumococcal carriage and the impact of the heptavalent pneumococcal conjugate vaccine on invasive pneumococcal disease. BMC Infect. Dis. 10: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Murphy TF, Parameswaran GI. 2009. Moraxella catarrhalis, a human respiratory tract pathogen. Clin. Infect. Dis. 49: 124– 131 [DOI] [PubMed] [Google Scholar]
- 28. Pettigrew MM, Gent JF, Revai K, Patel JA, Chonmaitree T. 2008. Microbial interactions during upper respiratory tract infections. Emerg. Infect. Dis. 14: 1584– 1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Plasschaert AI, Rovers MM, Schilder AG, Verheij TJ, Hak E. 2006. Trends in doctor consultations, antibiotic prescription, and specialist referrals for otitis media in children: 1995–2003. Pediatrics 117: 1879– 1886 [DOI] [PubMed] [Google Scholar]
- 30. Post C. 2011. Genetics of otitis media. Adv. Otorhinolaryngol. 70: 135– 140 [DOI] [PubMed] [Google Scholar]
- 31. Rovers MM, Schilder AG, Zielhuis GA, Rosenfeld RM. 2004. Otitis media. Lancet 363: 465– 473 [DOI] [PubMed] [Google Scholar]
- 32. Ruuskanen O, Heikkinen T. 1994. Otitis media: etiology and diagnosis. Pediatr. Infect. Dis. J. 13: S23– S26 [PubMed] [Google Scholar]
- 33. Rye MS, et al. 2011. Unraveling the genetics of otitis media: from mouse to human and back again. Mamm. Genome. 22: 66– 82 [DOI] [PubMed] [Google Scholar]
- 34. Tan TT, Christensen JJ, Dziegiel MH, Forsgren A, Riesbeck K. 2006. Comparison of the serological responses to Moraxella catarrhalis immunoglobulin D-binding outer membrane protein and the ubiquitous surface proteins A1 and A2. Infect. Immun. 74: 6377– 6386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Timpe JM, Holm MM, Vanlerberg SL, Basrur V, Lafontaine ER. 2003. Identification of a Moraxella catarrhalis outer membrane protein exhibiting both adhesin and lipolytic activities. Infect. Immun. 71: 4341– 4350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Veenhoven R, et al. 2004. Immunoglobulins in otitis-prone children. Pediatr. Res. 55: 159– 162 [DOI] [PubMed] [Google Scholar]
- 37. Vergison A, et al. 2010. Otitis media and its consequences: beyond the earache. Lancet Infect. Dis. 10: 195– 203 [DOI] [PubMed] [Google Scholar]
- 38. Verhaegh SJ, et al. 2010. Determinants of Moraxella catarrhalis colonization in healthy Dutch children during the first 14 months of life. Clin. Microbiol. Infect. 16: 992– 997 [DOI] [PubMed] [Google Scholar]
- 39. Verkaik N, Brouwer E, Hooijkaas H, van Belkum A, van Wamel W. 2008. Comparison of carboxylated and Penta-His microspheres for semi-quantitative measurement of antibody responses to His-tagged proteins. J. Immunol. Methods 335: 121– 125 [DOI] [PubMed] [Google Scholar]
- 40. Weiser JN, et al. 2003. Antibody-enhanced pneumococcal adherence requires IgA1 protease. Proc. Natl. Acad. Sci. U. S. A. 100: 4215– 4220 [DOI] [PMC free article] [PubMed] [Google Scholar]


