Abstract
Dental pulp stem cells (DPSCs) possess immunoregulatory properties, but the underlying mechanism is not fully understood. Here we showed that DPSCs were capable of inducing activated T-cell apoptosis in vitro and ameliorating inflammatory-related tissue injuries when systemically infused into a murine colitis model. Mechanistically, DPSC-induced immunoregulation was associated with the expression of Fas ligand (FasL), a transmembrane protein that plays an important role in inducing the Fas apoptotic pathway. Knockdown of FasL expression by siRNA in DPSCs reduced their capacity to induce T-cell apoptosis in vitro and abolished their therapeutic effects in mice with colitis. However, the expression level of FasL did not affect either DPSC proliferation rate or multipotent differentiation potential. In summary, FasL governs the immunoregulatory property of DPSCs in the context of inducing T-cell apoptosis.
Keywords: dental pulp stem cells, immunomodulation, Fas ligand, T-cells, apoptosis, differentiation
Introduction
Mesenchymal stem cells (MSCs) are multipotent stem cells capable of differentiating into a variety of cell types, including osteoblasts, adipocytes, chondrocytes, and neural cells (Bianco et al., 2001). In addition to differentiating into multiple cell types, MSCs are capable of regulating immune cells, such as T-cells, B-cells, dendritic cells (DC), and natural killer (NK) cells (Ren et al., 2008; Yamaza et al., 2010, 2011; Zhao et al., 2010), a characteristic which has provided a foundation for the clinical use of MSCs to treat a variety of autoimmune diseases (Le Blanc et al., 2008; Sun et al., 2009). The orofacial region contains several distinctive MSC populations, including dental pulp stem cells (DPSC), stem cells from human exfoliated deciduous teeth (SHED), periodontal ligament stem cells (PDLSC), stem cells from root apical papilla (SCAP), follicle stem/progenitor cells, and gingival stem cells (GMSC) (Gronthos et al., 2000; Miura et al., 2003; Yamaza et al., 2010, 2011; Seo et al., 2004). These orofacial-tissue-derived MSCs also possess an immunomodulatory property (Wada et al., 2009; Zhang et al., 2009; Ding et al., 2010; Yamaza et al., 2010). To determine whether DPSCs can serve as a cell source for immune therapy, we used DPSCs to treat Dextran Sulfate Sodium (DSS)-induced mouse colitis.
Fas ligand (FasL or CD95L) is a type-II transmembrane protein that belongs to the tumor necrosis factor (TNF) family. Binding with Fas receptor, FasL induces a capase-mediated apoptotic process in many cell types. Recently, our group revealed that MSC-mediated immune therapies may be associated with the expression of FasL, which induces T-cell apoptosis to trigger immune tolerance (Akiyama et al., 2012). It has recently been reported that the Fas-FasL pathway plays an important role in dental follicle cells and cementoblast-induced apoptosis of ameloblast-lineage, Hertwig’s epithelial root sheath (HERS), and epithelial rests of Malassez (ERM) cells during tooth development (Lee et al., 2012). However, it is unknown whether DPSCs express FasL. In this study, we show that DPSC-mediated immune therapy is associated with the expression of FasL.
Materials & Methods
Animals
C57BL6J strain, female Sprague Dawley rats were purchased from the Jackson Lab (Bar Harbor, ME, USA). Female immunocompromised mice (Beige nude/nude XIDIII) were purchased from Harlan (Indianapolis, IN, USA). These animal experiments were performed under the institutionally approved protocols for the use of animal research (University of Southern California #10941, 11141, and 11327).
Antibodies and Reagents
All antibodies and reagents used in this study are described in the Appendix.
Cell Cultures
The DPSCs from rat and human were cultured as described in the Appendix. BMMSC culture was described previously (Yamaza et al., 2011).
Colony-forming Units-Fibroblastic (CFU-F) Assay
CFU-F assay was performed as described previously (Yamaza et al., 2011) and in the Appendix.
Proliferation Assay
The proliferation rates of subconfluent cultures of DPSCs and BMMSCS were assessed by bromodeoxyuridine (BrdU, Invitrogen, Carlsbad, CA, USA) incorporation for 24 hrs with the use of a Zymed BrdU staining kit (Invitrogen), as described previously (Yamaza et al., 2011)
Population Doublings
The PD assay was repeated with 3 independent isolated cells for each experimental group. The detailed methods are described in the Appendix.
Flow Cytometric Analysis
P1 MSCs were stained for cell-surface markers and analyzed by flow cytometry. MSC surface markers CD90, CD29, CD73, and CD106 were selected to verify DPSCs. To exclude the hematological origin, we also examined expression levels of hematological markers CD34, CD45, and CD49, and macrophage marker CD11b. Detailed methods are described in the Appendix.
Immunofluorescence Staining
Immunofluorescence staining was performed as described in the Appendix.
FasL siRNA
For knockdown FasL expression, 0.5 x 106 rat or human DPSCs were seeded in a 6-well culture plate. FasL siRNA kits (Ambion, NY and Santa Cruz, CA, USA) were used to treat rat DPSCs and human DPSCs, respectively, according to the manufacturers’ protocols.
Western Blot Analysis
Western blot was performed as described in the Appendix.
Co-culture of DPSCs with Activated Splenocytes
Co-culture of DPSCs with activated splenocytes was performed as described in the Appendix.
Dextran Sulfate Sodium (DSS)-induced Mouse Colitis and Treatment with DPSCs
Acute colitis was induced in C57BL/6 mice. The detailed methods were as described previously (Gonzalez-Rey et al., 2009; Zhang et al., 2009) and as in the Appendix. P1 DPSCs were infused into mice with colitis (n = 5 per group) intravenously at day 3 after they were fed with DSS water, with a dose of 1 x 105 cells/10 g body weight in 100 μL PBS according to our previous report (Yamaza et al., 2010). All mice were harvested at day 10 after being fed DSS water and analyzed as previously described (Alex et al., 2009). The results are representative of 3 independent experiments.
Multi-lineage Differentiation Assay
For in vitro differentiation assay, P1 MSCs were cultured under osteogenic and adipogenic conditions as described in the Appendix. For in vivo osteogenic assay, details are described in the Appendix.
Statistics
SPSS 13.0 was used to perform statistical analysis. Significance was assessed by independent two-tailed Student’s t test or analysis of variance (ANOVA). P values less than 0.05 were considered significant.
Results
Isolation and Characterization of Rat DPSCs
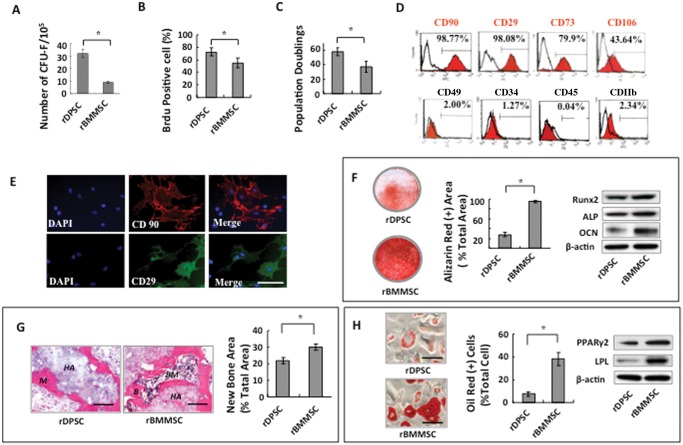
To isolate rat DPSCs (rDPSCs), we generated single-cell suspensions by enzyme digestion of rat pulp tissue and plated digested cells at a low density. rDPSCs were capable of forming adherent clonogenic colony clusters originating from a single attached cell. These single colonies were similar to primary cultured rat bone marrow MSCs (rBMMSCs), but rDPSCs generated significantly higher numbers of CFU-F than rBMMSCs (Fig. 1A). The rDPSCs showed elevated cell proliferation rate and self-renewal capability, as determined by BrdU incorporation and population doubling assays, respectively, when compared with rBMMSCs (Figs. 1B, 1C). Flow cytometric analysis showed that the cultured cells are positive for mesenchymal stem cell surface markers (CD90, CD29, CD73, and CD106) and negative for hematological markers (CD34, CD45/CD49) and macrophage marker (CD11b) (Fig. 1D). Immunocytochemistry staining confirmed that rDPSCs responded positively to CD90 and CD29 antibodies (Fig. 1E). Under osteogenic culture conditions, rDPSCs showed reduced capability to form mineralized nodules, as assessed by Alizarin Red staining and the expression of osteoblastic markers Runx2, ALP, and OCN, as assessed by Western blot analysis, when compared with rBMMSCs (Fig. 1F). After 8 wks of subcutaneous transplantation into immunocompromised mice with HA/TCP as a carrier, rDPSCs formed less mineralized tissue when compared with rBMMSCs (Fig. 1G). Next, we examined the adipogenic differentiation potential of rDPSCs and found that rDPSCs were able to develop into Oil Red O-positive adipocytes after 4 wks of adipogenic-inductive cultures. Western blot analysis confirmed that rDPSCs showed up-regulation of the adipocyte-specific transcripts PPARγ2 and LPL after adipogenic induction (Fig. 1H).
Figure 1.
Characterization of rDPSCs. (A) rDPSCs generated markedly higher numbers of CFU-F than did rBMMSCs. (B) rDPSCs showed an increased proliferation rate in comparison with that of rBMMSCs, as determined by BrdU incorporation assay. (C) rDPSCs showed more elevated population doublings than did rBMMSCs. (D) Flow cytometric analysis showed that rDPSCs expressed surface molecules CD90, CD29, CD73, and CD106. (E) Immunocytochemistry staining further confirmed that rDPSCs expressed CD90 and CD29. (F) Alizarin Red staining showed that rDPSCs had a lower capacity than rBMMSCs to form mineralized nodules after culture in osteoinductive conditions for 4 wks. Western blot analysis confirmed that rDPSCs expressed elevated levels of the osteogenic genes ALP, Runx2, and OCN in the osteoinductive group. (G) rDPSCs were capable of forming mineralized tissue when transplanted subcutaneously into immunocompromised mice with hydroxyapatite tricalcium phosphate (HA/TCP) as a carrier. rDPSCs formed less mineralized tissue when compared with rBMMSCs. B = bone, M = mineralized tissue, BM = bone marrow, H = HA/TCP carrier. (H) Oil Red O staining showed that rDPSCs had reduced capacity to differentiate into adipocytes compared with rBMMSCs at 2 wks post-adipogenic induction. Western blot analysis confirmed that adipogenic induction up-regulated expression of the adipogenic genes PPARγ-2 and LPL.
FasL is Required for DPSC-based Immunoregulation
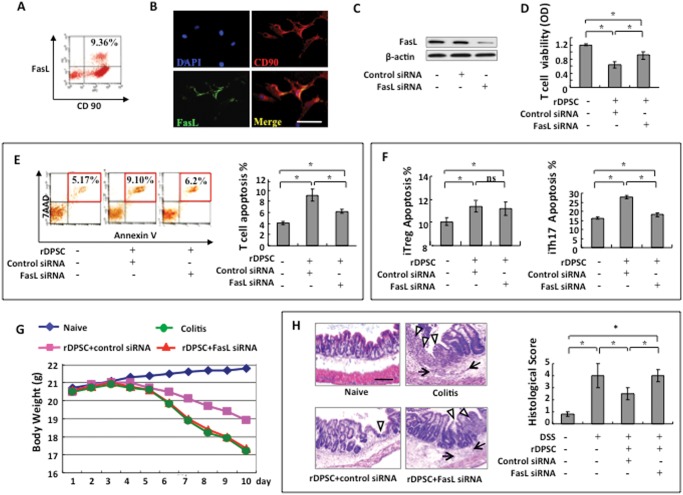
Since FasL plays an important role in BMMSC-induced immune tolerance (Ren et al., 2008; Yamaza et al., 2010, 2011; Zhao et al., 2010), we hypothesized that FasL would contribute to the immunoregulatory function of DPSCs. To test this hypothesis, we first showed, by flow cytometry, that ~9.36% rDPSCs co-expressed FasL with the mesenchymal marker CD90 (Fig. 2A). We further confirmed, by immunocytochemical staining, that rDPSCs co-expressed FasL and CD90 (Fig. 2B). To examine whether FasL contributes to the immunoregulatory properties of rDPSCs, we used the siRNA approach to knock down FasL expression (Fig. 2C). Then we showed that FasL knockdown resulted in a reduced capacity to inhibit T-cell viability in vitro when compared with the control siRNA group (Fig. 2D). Flow cytometric analysis confirmed that FasL knockdown abrogated rDPSC-induced T-cell apoptosis (Fig. 2E). Moreover, we revealed that FasL knockdown reduced rDPSC-mediated inhibition of iTh17 in an in vitro co-culture system when compared with the control siRNA group, whereas FasL knockdown had no significant effect on iTreg (Fig. 2F).
Figure 2.
FasL is required for rDPSC-based immunomodulation. (A) Flow cytometric analysis showed that ~9.63% of cultured rDPSCs at passage 3 co-expressed FasL with CD90. (B) Immunofluorescence confirmed that rDPSCs express FasL. (C) Western blot analysis showed that FasL siRNA could knock down FasL expression in rDPSCs. (D, E) When co-cultured with T-cells, FasL-knockdown rDPSCs showed a reduced capacity to inhibit T-cell viability. Apoptosis assay confirmed that FasL-knockdown rDPSCs showed a reduced capacity to induce 7AAD/Annexin-positive apoptotic T-cells when compared with the control siRNA group. (F) FasL-knockdown rDPSCs showed a reduced capacity to induce iTh17 cell apoptosis compared with the control siRNA group. No significant difference was observed in the induction of iTreg apoptosis between the rDPSC FasL-siRNA group and the rDPSC control siRNA group. (G) Systemic infusion of control rDPSCs (control siRNA) protected mice from colitis-induced body weight loss. (H) HE staining showed the infiltration of inflammatory cells (arrow) in the colon, with destruction of the epithelial layer (triangles) in mice with colitis. rDPSC, but not FasL siRNA rDPSC, transplantation rescued the disease phenotype in the colon and reduced the histological activity index. *p < 0.05. Bar length = 20 µm.
To examine the in vivo immunomodulatory properties of rDPSCs, we generated a colitis model in C57BL/6 mice by oral administration of 3% DSS for 7 days (Zhang et al., 2009). Overall elevation of colitis scores was based on the presence of sustained weight loss and bloody diarrhea/loose feces (Fig. 2G), with severe colonic transmural inflammation, increased wall thickness, localized inflammatory cell infiltration, and epithelial ulceration (Fig. 2H). We found that the systemic infusion of rDPSCs, but not FasL-knockdown rDPSCs by siRNA, protected mice from colitis-related tissue injuries and reduced overall disease severity compared with the colitis group, along with a marked rescue of body weight (Fig. 2G) and decrease in histological score (Fig. 2H). Also, the rDPSC treatment group significantly ameliorated colonic transmural inflammation, decreased wall thickness, suppressed epithelial ulceration, and restored normal intestinal architecture. In contrast, the FasL-knockdown rDPSC group failed to ameliorate histological phenotypes of colitis (Fig. 2H). Analysis of these data suggests that FasL is required for rDPSC-mediated immune therapy in the colitis mouse model.
To determine whether FasL plays an important role in the immunomodulation of human DPSCs (hDPSCs), we isolated hDPSCs from human molars and showed that these hDPSCs expressed the MSC-associated markers CD90, CD29, CD73, and CD105 (Fig. 3A). Also, hDPSCs expressed FasL, as determined by flow cytometry (Fig. 3B). Knockdown of FasL expression in hDPSCs by siRNA (Fig. 3C) reduced their capacity to inhibit T-cell proliferation and induce T-cell apoptosis in vitro compared with the control group (Figs. 3D, 3E).
Figure 3.
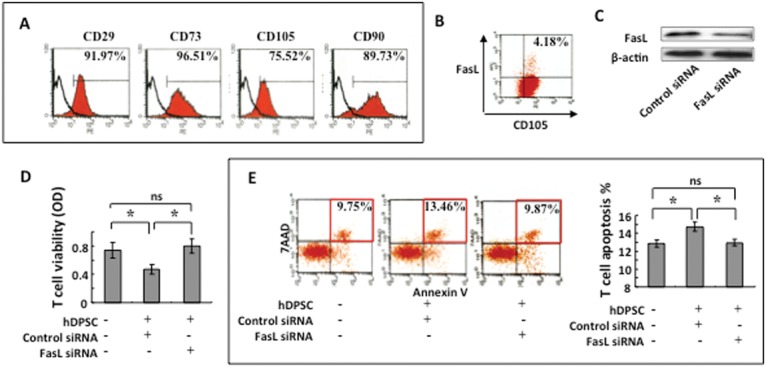
Human DPSCs express FasL to induce T-cell apoptosis. (A) Flow cytometric analysis showed that human DPSCs (hDPSCs) expressed CD29, CD73, CD105, and CD90. (B) Flow cytometric analysis showed that cultured hDPSCs co-expressed CD105 with FasL. (C) Western blot analysis showed that siRNA reduced FasL expression in hDPSCs. (D) When co-cultured with T-cells, FasL-knockdown hDPSCs showed a reduced capacity to inhibit T-cell viability. (E) Apoptosis assay confirmed that FasL-knockdown hDPSCs showed a reduced capacity to induce 7AAD/Annexin-positive apoptotic T-cells when compared with the siRNA-negative control group. *p < 0.05. Bar length = 20 µm.
FasL Expression Has No Impact on DPSC Differentiation
Since FasL expression is important for DPSC-based immunomodulation, it was interesting to determine whether FasL expression was associated with stem cell properties. We used the siRNA approach to knock down FasL expression in DPSCs and found that reduced FasL expression failed to influence DPSC proliferation and differentiation. When FasL expression was knocked down by siRNA, the proliferation rate, as assessed by BrdU incorporation, showed no marked alteration (Figs. 4A). Moreover, expression levels of CD90, CD29, CD73, CD106, CD49, CD34, CD45, and CD11b showed no significant difference between FasL-knockdown and control rDPSCs (Fig. 4B). Western blot analysis confirmed that the expression of CD90 was not changed by FasL knockdown (Fig. 4C). Since DPSCs can differentiate into multiple cell types, such as odontoblasts and adipocytes, we further determined whether FasL knockdown affected their multipotent differentiation capacity. We found that FasL knockdown failed to alter the osteogenic differentiation capacity of rDPSCs, as indicated by Alizarin Red staining, to show mineralized nodule formation, and by Western blot analysis, to show expression of the osteo/odontogenic genes Runx2, ALP, and OCN (Fig. 4D). We next subcutaneously implanted rDPSCs into immunocompromised mice, using HA/TCP as a carrier. This in vivo implantation assay showed that FasL knockdown did not affect the tissue regeneration capacity of rDPSCs (Fig. 4E). Additionally, FasL knockdown was unable to alter adipogenic differentiation of rDPSCs, as indicated by the number of Oil Red O–positive cells and expression of the adipogenic genes PPARγ2 and LPL (Fig. 4F).
Figure 4.
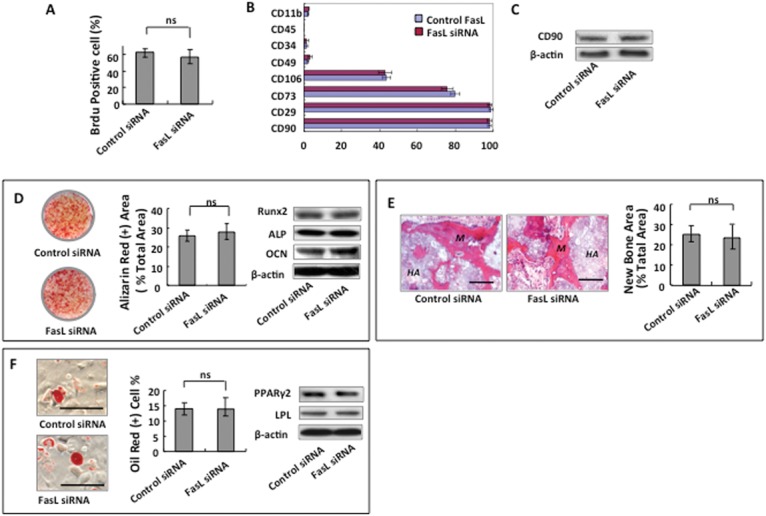
FasL expression has no impact on rDPSC proliferation and differentiation. (A) Knockdown FasL expression by siRNA did not affect rDPSC proliferation, as assessed by BrdU labeling assay. (B) FasL-knockdown by siRNA failed to alter the expression of surface molecules CD29, CD90, CD73, CD106, CD49, CD34, CD45, and CD11b in rDPSCs. (C) Western blot analysis confirmed that knockdown FasL expression by siRNA did not change the expression of CD90 in rDPSCs. (D) Osteogenic differentiation capacity of rDPSCs was not altered by FasL knockdown, as indicated by Alizarin Red staining, to show mineralized nodule formation and by Western blot analysis to show expression levels of the osteogenic genes ALP, Runx2, and OCN. (E) In vivo implantation assay showed that FasL knockdown rDPSCs formed an amount of mineralized tissue similar to that observed in control rDPSCs. (F) FasL knockdown was unable to alter adipogenic differentiation capacity, as indicated by the number of Oil Red O–positive cells and expression of the PPARγ2 and LPL.
Discussion
It has been reported that some connective-tissue-derived cells, such as adipocytes and fibroblasts, shared similar immunomodulatory properties (Halberg et al., 2008; Cappellesso-Fleury et al., 2010). However, when compared with somatic cells, MSCs are generally considered to be less immunogenic because of low expression levels of major histocompatibility complex (MHC) class I molecules and negativity for MHC class II cells (Le Blanc et al., 2003; Morandi et al., 2008). MSCs have been proven to possess immunomodulatory properties and have been used clinically to treat autoimmune diseases, such as graft-vs.-host disease (GVHD), systemic lupus erythematosus (SLE), and systemic sclerosis (SS) (Le Blanc et al., 2008; Sun et al., 2009). Given the fact that MSC/osteoblast lineage constitutes an important niche for hematopoietic stem cells (HSCs), it is reasonable to postulate that MSCs may interact with T-cells in a cell-cell-contact manner. The binding of membrane-bound FasL and Fas is an important cell death pathway which mediates apoptosis in several cell types (Mazar et al., 2009; O’Reilly et al., 2009). It has been reported that BMMSCs express FasL to induce T-cell apoptosis through the FasL/Fas pathway (Akiyama et al., 2012). In this study, we found that DPSCs also expressed FasL; thus, we hypothesized that DPSCs might induce T-cell apoptosis and ameliorate diseased phenotypes in mice with colitis.
Although DPSCs share several characteristics with BMMSCs, DPSCs show reduced osteogenic and adipogenic potentials (Gronthos et al., 2000). Such differences may be partly due to the fact that DPSCs derive from neural crest cells, whereas BMMSCs originate from the mesoderm (Musina et al., 2006; Janebodin et al., 2011).
The DSS-induced ulcerative colitis mouse model is a well-established autoimmune disease model, which is characterized by dysfunction of innate and adaptive immunity, resulting in colonic mucosal injury to the distal small intestine (Zhang et al., 2009). To evaluate dextran sulfate sodium-induced colitis objectively, we performed histological analysis according to well-established methods (Akiyama et al., 2012). The results are representative of 3 independent experiments. To confirm the role of FasL in DPSC-mediated immunoregulation in vivo, we used this animal model to demonstrate that infusion of rat DPSCs would ameliorate inflammatory-related colonic tissue injury, which is similar to recent studies with human adipose-derived and gingival-derived MSCs (Gonzalez-Rey et al., 2009; Zhang et al., 2009). The therapeutic mechanism may lie in the reduced colonic infiltration of inflammatory cells and down-regulation of inflammatory cytokines, as indicated by analysis of our histological data. Other mechanisms may involve the increased infiltration of regulatory T-cells and the expression of anti-inflammatory cytokine IL-10 at the colonic sites (Zhang et al., 2009). Interestingly, FasL knockdown in DPSCs resulted in a reduced capacity to ameliorate colitis phenotypes, indicating that FasL is required for DPSC-mediated immunoregulation. Moreover, in vitro co-culture assays demonstrated that DPSCs induced apoptosis of iTh17, but not iTreg, suggesting that such FasL-mediated apoptosis was specific for T-helper cells, but not for Tregs, a finding which was supported by our previous study on bone marrow MSCs (Akiyama et al., 2012). Additionally, FasL knockdown was unable to alter the multipotent differentiation capacity of DPSCs, suggesting that the role of FasL may be limited to controlling interaction between DPSCs and immune cells.
Our group found that bone marrow MSCs secrete Fas-mediated monocyte chemotactic protein 1 (MCP-1) to recruit activated T-cells and then induce T-cell apoptosis. The apoptotic T-cells subsequently trigger macrophages to produce high levels of TGFβ, which, in turn, leads to the up-regulation of CD4+CD25+Foxp3+ Tregs to induce ultimate immune tolerance (Akiyama et al., 2012). Also, several studies have indicated that soluble factors, such as prostaglandin E2 (PGE2), nitric oxide (NO), indoleamine 2,3-dioxygenase (IDO), and hepatic growth factor (HGF), also contributed to MSC-mediated immunosuppression (Ren et al., 2008; Spaggiari et al., 2008). Therefore, it is necessary to further elucidate the detailed mechanism by which systemically infused DPSCs regulate the immune system. The fact that FasL does not affect DPSC proliferation and differentiation suggests that expression of FasL in DPSCs contributes only to their ability to interact with immune cells.
In summary, we have demonstrated that DPSCs express FasL and then use FasL to induce T-cell apoptosis. DPSC-mediated immune therapy in colitis mice is associated with FasL-induced T-cell apoptosis.
Footnotes
This work was supported by grants from the US National Institute of Dental and Craniofacial Research, National Institutes of Health, Department of Health and Human Services (R01DE017449 and R01DE019932 to S.S.), from the National Natural Science Foundation of China (No. 81020108019 to Y.J. and S.S.), and from the National Basic Research Program (973 Program) of China (No. 2011CB964700 to Y.J.).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Akiyama K, Chen C, Wang D, Xu X, Qu C, Yamaza T, et al. (2012). Mesenchymal-stem-cell-induced immunoregulation involves FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell 10:544-555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alex P, Zachos NC, Nguyen T, Gonzales L, Chen TE, Conklin LS, et al. (2009). Distinct cytokine patterns identified from multiplex profiles of murine DSS and TNBS-induced colitis. Inflamm Bowel Dis 15:341-352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco P, Riminucci M, Gronthos S, Robey PG. (2001). Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells 19:180-192 [DOI] [PubMed] [Google Scholar]
- Cappellesso-Fleury S, Puissant-Lubrano B, Apoil PA, Titeux M, Winterton P, Casteilla L, et al. (2010). Human fibroblasts share immunosuppressive properties with bone marrow mesenchymal stem cells. J Clin Immunol 30:607-619 [DOI] [PubMed] [Google Scholar]
- Ding G, Liu Y, An Y, Zhang C, Shi S, Wang W, et al. (2010). Suppression of T cell proliferation by root apical papilla stem cells in vitro. Cells Tissues Organs 191:357-364 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Rey E, Anderson P, Gonzalez MA, Rico L, Buscher D, Delgado M. (2009). Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut 58:929-939 [DOI] [PubMed] [Google Scholar]
- Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. (2000). Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA 97:13625-13630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberg N, Wernstedt I, Scherer PE. (2008). The adipocyte as an endocrine cell. Endocrinol Metab Clin North Am 37:753-768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janebodin K, Horst OV, Ieronimakis N, Balasundaram G, Reesukumal K, Pratumvinit B, et al. (2011). Isolation and characterization of neural crest-derived stem cells from dental pulp of neonatal mice. PLoS One 6:e27526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Blanc K, Tammik L, Sundberg B, Haynesworth SE, Ringdén O. (2003). Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol 57:11-20 [DOI] [PubMed] [Google Scholar]
- Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, et al. (2008). Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet 371:1579-1586 [DOI] [PubMed] [Google Scholar]
- Lee JH, Lee DS, Nam H, Lee G, Seo BM, Cho YS, et al. (2012). Dental follicle cells and cementoblasts induce apoptosis of ameloblast-lineage and Hertwig’s epithelial root sheath/epithelial rests of Malassez cells through the Fas-Fas ligand pathway. Eur J Oral Sci 120:29-37 [DOI] [PubMed] [Google Scholar]
- Mazar J, Thomas M, Bezrukov L, Chanturia A, Pekkurnaz G, Yin S, et al. (2009). Cytotoxicity mediated by the Fas ligand (FasL)-activated apoptotic pathway in stem cells. J Biol Chem 284:22022-22028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, et al. (2003). SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci USA 100:5807-5812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morandi F, Raffaghello L, Bianchi G, Meloni F, Salis A, Millo E, et al. (2008). Immunogenicity of human mesenchymal stem cells in HLA-class I-restricted T-cell responses against viral or tumorassociated antigens. Stem Cells 26:1275-1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musina RA, Bekchanova ES, Belyavskii AV, Sukhikh GT. (2006). Differentiation potential of mesenchymal stem cells of different origin. Bull Exp Biol Med 141:147-151 [DOI] [PubMed] [Google Scholar]
- O’Reilly LA, Tai L, Lee L, Kruse EA, Grabow S, Fairlie WD, et al. (2009). Membrane-bound Fas ligand only is essential for Fas-induced apoptosis. Nature 461:659-663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, et al. (2008). Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2:141-150 [DOI] [PubMed] [Google Scholar]
- Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, et al. (2004). Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 364:149-155 [DOI] [PubMed] [Google Scholar]
- Spaggiari GM, Capobianco A, Abdelrazik H, Becchetti F, Mingari MC, Moretta L. (2008). Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood 111:1327-1333 [DOI] [PubMed] [Google Scholar]
- Sun L, Akiyama K, Zhang H, Yamaza T, Hou Y, Zhao S, et al. (2009). Mesenchymal stem cell transplantation reverses multiorgan dysfunction in systemic lupus erythematosus mice and humans. Stem Cells 27:1421-1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada N, Menicanin D, Shi S, Bartold PM, Gronthos S. (2009). Immunomodulatory properties of human periodontal ligament stem cells. J Cell Physiol 219:667-676 [DOI] [PubMed] [Google Scholar]
- Yamaza T, Kentaro A, Chen C, Liu Y, Shi Y, Gronthos S, et al. (2010). Immunomodulatory properties of stem cells from human exfoliated deciduous teeth. Stem Cell Res Ther 1:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaza T, Ren G, Akiyama K, Chen C, Shi Y, Shi S. (2011). Mouse mandible contains distinctive mesenchymal stem cells. J Dent Res 90:317-324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Shi S, Liu Y, Uyanne J, Shi Y, Le AD. (2009). Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. J Immunol 183:7787-7798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Wehner R, Bornhauser M, Wassmuth R, Bachmann M, Schmitz M. (2010). Immunomodulatory properties of mesenchymal stromal cells and their therapeutic consequences for immune-mediated disorders. Stem Cells Dev 19:607-614 [DOI] [PubMed] [Google Scholar]