Abstract
Allosteric modulation of G-protein–coupled receptors represents a key goal of current pharmacology. In particular, endogenous allosteric modulators might represent important _targets of interventions aimed at maximizing therapeutic efficacy and reducing side effects of drugs. Here we show that the anti-inflammatory lipid lipoxin A4 is an endogenous allosteric enhancer of the CB1 cannabinoid receptor. Lipoxin A4 was detected in brain tissues, did not compete for the orthosteric binding site of the CB1 receptor (vs. 3H-SR141716A), and did not alter endocannabinoid metabolism (as opposed to URB597 and MAFP), but it enhanced affinity of anandamide at the CB1 receptor, thereby potentiating the effects of this endocannabinoid both in vitro and in vivo. In addition, lipoxin A4 displayed a CB1 receptor-dependent protective effect against β-amyloid (1–40)-induced spatial memory impairment in mice. The discovery of lipoxins as a class of endogenous allosteric modulators of CB1 receptors may foster the therapeutic exploitation of the endocannabinoid system, in particular for the treatment of neurodegenerative disorders.
Keywords: allosteric modulation, psychopharmacology, GPCR, inflammation, neuroprotection
The endocannabinoid system, comprising metabotropic cannabinoid receptors (CB1 and CB2), endogenous lipid ligands (endocannabinoids), and enzymes responsible for their synthesis and degradation, is a key regulator of neuronal function, being proposed as a therapeutic _target for several diseases (1). Activation of CB1 receptors reduces cAMP levels, inhibits voltage-dependent Ca2+ channels, and activates inward-rectifying K+ channels, resulting in reduced neuronal excitability and presynaptic inhibition of neurotransmitter release (1, 2). The efficacy of endogenous CB1 agonists varies according to the nature of the molecule (3). The endocannabinoid anandamide (AEA) is considered a partial agonist, whereas 2-arachidonoylglycerol (2-AG) is a full agonist inducing maximal responses (4, 5). At least three other endocannabinoids are known (noladin ether, virodhamine, and N-arachidonoyl dopamine) (1, 2). Each endocannabinoid has different affinities, efficacies, and sometimes distinct effects at the CB1 receptor, which could also be cell type-dependent (1, 2). Cannabinoids have many effects on laboratory animals, but the prominent ones are known as the cannabinoid tetrad: analgesia, catalepsy, hypolocomotion, and hypothermia.
The selectivity of CB1 agonists may be explained by multiple binding sites in the CB1 receptor (6), in agreement with the current view of metabotropic receptors as dynamic macromolecules, rather than mere on/off switches of a transduction system (7). Allosteric modulators bind to additional site(s) on the receptor influencing the affinity and/or efficacy of endogenous molecules binding to the orthosteric or primary site (the orthosteric site is defined as the binding site for known endogenous ligand) (8). Two synthetic compounds, Org27596 and Org29647, enhance the affinity and reduce the efficacy of CB1 agonists, suggesting the existence of an allosteric binding site at CB1 receptor (9). However, the existence of endogenous allosteric cannabinoid modulators has not yet been proved.
Synthetic and metabolic pathways of eicosanoids impact endocannabinoid levels, suggesting functional relationships among endocannabinoids, prostaglandins (10), and lipoxins (11). Lipoxin A4 (LXA4), the most studied endogenous lipoxin (12), is largely involved in immune system regulation and is linked to resolution of inflammation (13). The metabotropic ALX receptor (also called FPRL-1) is responsible for the immunological effects of LXA4 and is expressed in peripheral organs, but has negligible occurrence in the central nervous system (CNS) (14). Nevertheless, LXA4 is released in brain tissues during ischemia (15), suggesting the presence of non-ALX receptor _targets in the brain.
Brain effects of LXA4 include modulation of slow wave sleep (16), neuronal signaling (via PKCγ) (17, 18), and plasticity (19) through unknown mechanisms. Interestingly, these effects are similar to those of the endocannabinoid AEA (20, 21). We previously showed that intracerebroventricular (i.c.v.) injections of the aspirin-triggered LXA4 (15-Epi-LXA4) induce cannabinoid-like catalepsy in mice, which was prevented by the CB1 antagonist SR141716A and not by an ALX antagonist. Altogether, these findings suggest that LXA4 could have CB1 receptor-dependent effects in the brain (22). Here we report that LXA4 not only binds to CB1 receptors to exert cannabimimetic effects in the brain, but does so by allosterically enhancing AEA signaling. This may have important implications for the therapeutic exploitation of the endocannabinoid system.
Results
LXA4 Displays Cannabimimetic Effects in the Brain.
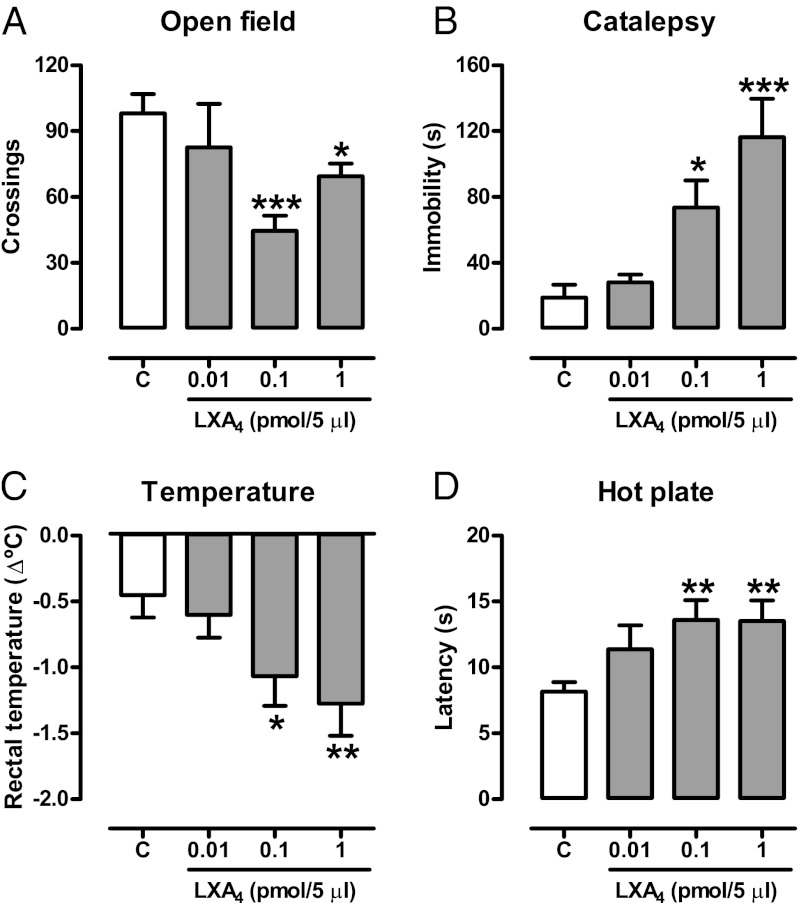
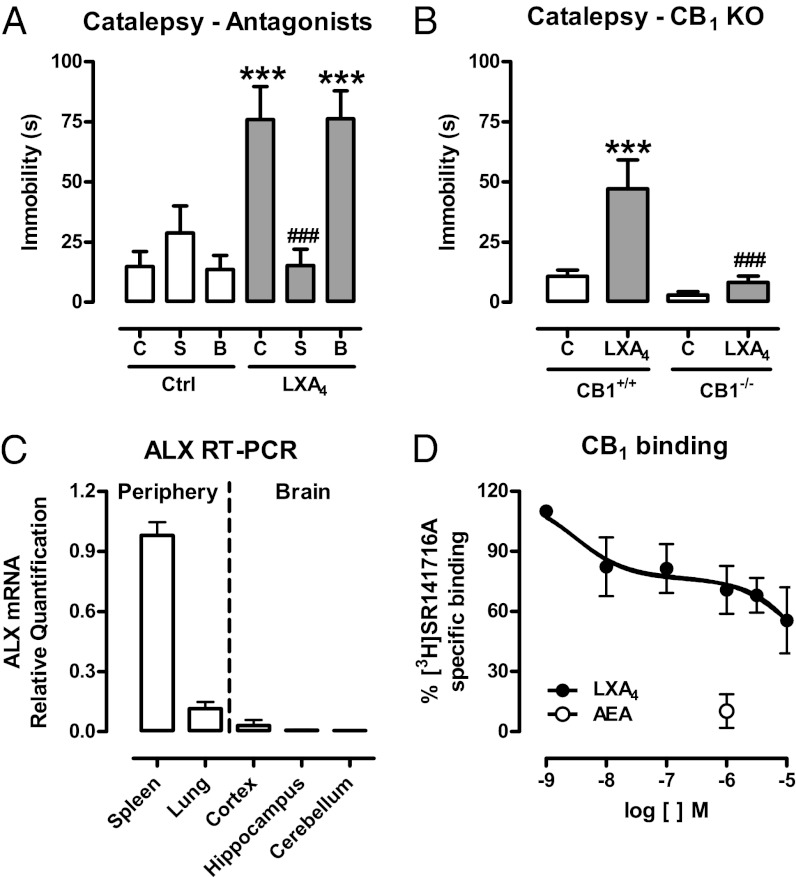
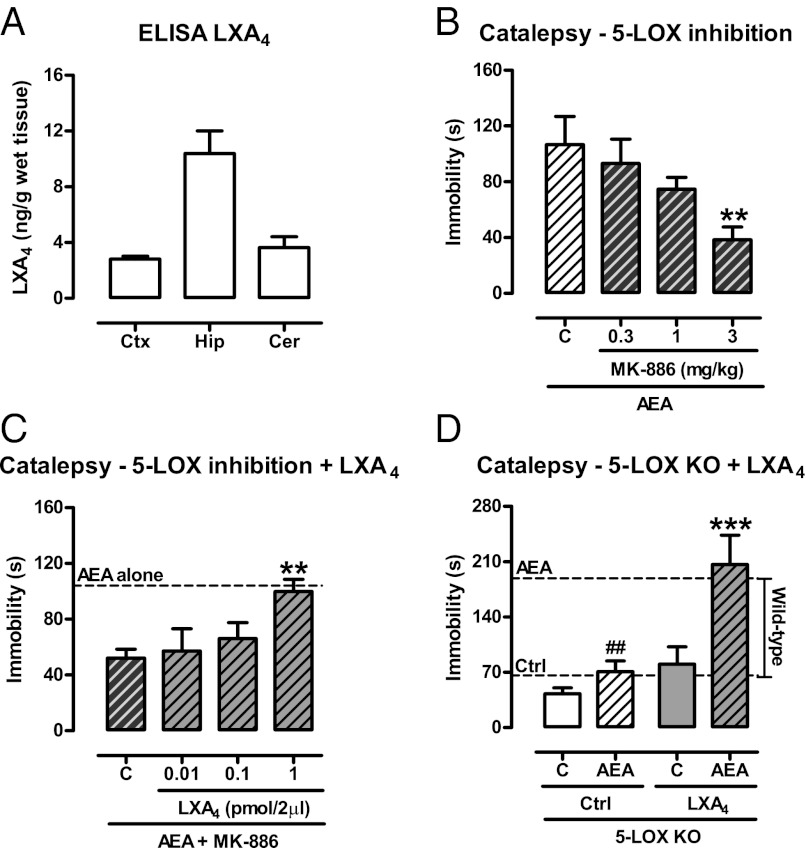
The cannabinoid “tetrad” represents a prototypic signature of cannabinoid effects (23). Brain injection of LXA4 (1 pmol/5 µL, i.c.v.) induced the full spectrum of tetrad cannabinoid effects in mice (Fig. 1). These effects were prevented by the CB1 receptor antagonist SR141716A (1 mg/kg, i.p.), but not by the ALX receptors antagonist Boc-2 (10 µg/kg, i.p.) (Fig. 2A; Figs. S1 and S2) and were absent in CB1-KO mice (Fig. 2B). Real-time PCR quantification revealed that the ALX receptor is present in negligible amounts in the mouse brain, compared with spleen and lung tissues (Fig. 2C). To determine whether LXA4 binds directly to the CB1 receptor, we measured the displacement of the CB1-selective antagonist [3H]SR141716A (0.5 nM) by LXA4 using mouse brain membranes. LXA4 partially displaced [3H]SR141716A binding at concentrations up to 10 µM, reaching a maximum of 40% displacement (Fig. 2D). Also, LXA4 did not change the amount of cAMP accumulated in CB1 receptor-transfected HEK cells (Fig. 3D). These findings apparently conflicted with the in vivo results, in which i.c.v. administration of LXA4 (2–200 nM) potently mimicked endocannabinoid actions. Therefore, a typical agonistic activation of the CB1 orthosteric site by LXA4 did not seem to be the likely mechanism supporting LXA4’s cannabimimetic effects in vivo.
Fig. 1.
LXA4 displays cannabimimetic effects in the brain. (A–D) Lipoxin A4 (LXA4 0.01–1 pmol/5 µL, i.c.v.) or control (C) was injected in Swiss mice 5 min before the cannabinoid tetrad test (locomotion, catalepsy, body temperature, nociception). The treatment reduced the number of crossings in the open field [F(3,43) = 4.56, P = 0.007, n = 9–14/group], increased the immobility time in the bar catalepsy test [F(3,24) = 9.07, P = 0.0003, n = 7/group], reduced body temperature [F(3,43) = 3.49, P = 0.02, n = 11–12/group], and increased the nociceptive latency in the hot plate [F(3,43) = 3.18, P < 0.03, n = 11–12/group]. Data are mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 vs. control (Duncan’s post hoc).
Fig. 2.
Role of CB1 cannabinoid receptor on LXA4-induced catalepsy. (A) The CB1 antagonist SR141716A (S; 1 mg/kg, i.p.), the ALX antagonist BOC-2 (B; 10 µg/kg, i.p.) or control (C) was injected 50 min before lipoxin A4 (LXA4, 1 pmol/5 µL, i.c.v.) or control (Ctrl) and tested in the catalepsy test 5 min later. LXA4 induced catalepsy, which was prevented by the CB1 antagonist (pretreatment vs. treatment) [F(2,42) = 10.07, P = 0.0003, n = 8/group]. (B) A selected dose of LXA4 (1 pmol/5 µL, i.c.v.) or control (C) was injected in CB1 knockout (CB1−/−) or wild-type mice (CB1+/+) 5 min before the bar catalepsy test. LXA4 induced catalepsy in CB1+/+, but not in CB1−/−mice (genotype vs. treatment) [F(1,21) = 4.75, P = 0.04, n = 6–7/group]. (C) Real-time PCR confirmed the negligible expression of ALX receptors in the brain. Spleen and lung tissues were used as positive controls for ALX mRNA (n = 4/group). (D) Competitive binding of LXA4 (1 nM–10 µM) against the CB1-selective radiolabeled antagonist [3H]SR141716A (0.5 nM) in mouse brain membranes revealed very low affinity of LXA4 for the CB1 receptors (Ki > 10 µM, n = 4/group). AEA (1 µM) was used as a positive control and inhibited nearly 90% of [3H]SR141716A binding. Binding curves were generated by nonlinear regression (curve fitting). Data are represented as mean ± SEM. ***P < 0.001 vs. Control; ###P < 0.001 vs. LXA4 + C (A) or vs. LXA4 in CB1+/+ mice (B) (Duncan’s post hoc).
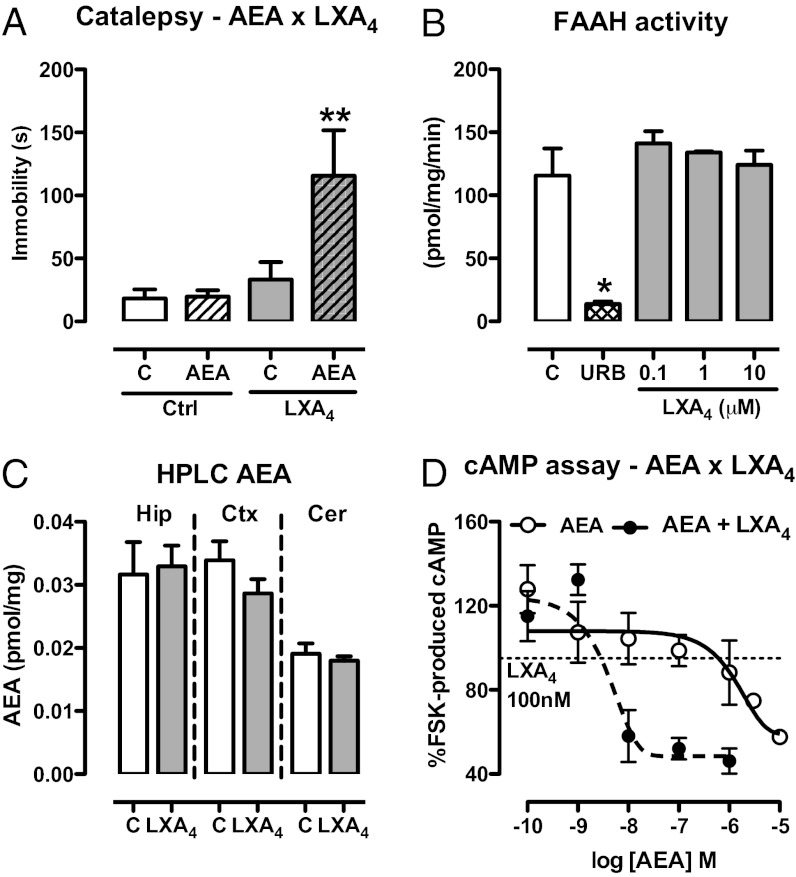
Fig. 3.
LXA4 interacts positively with the endocannabinoid AEA. (A) Selected pre-effective dose of AEA (10 pmol/2 µL, i.c.v.) was coinjected with LXA4 (0.01 pmol/2 µL, i.c.v.) 5 min before the bar catalepsy test. LXA4 interacted with AEA [F(3,29) = 4.98, P = 0.007, n = 8–9/group]. (B) Activity of the AEA-degrading enzyme FAAH was measured in the presence of LXA4 (100 nM–10 µM) using [14C]AEA (1.8 µM) as substrate. The FAAH inhibitor URB597 (URB, 50 nM) was used as positive control. LXA4 did not interfere with FAAH activity [F(3,7) = 0.73, P = 0.58, n = 3/group], as opposed to the positive control URB597 (t = 4.70, P < 0.05). (C) AEA levels in brain tissues were assessed by HPLC-MS 5 min after injection of LXA4 (1 pmol/2 µL, i.c.v.) or control (C). There were no signs of treatment-related alterations of endocannabinoid content in the hippocampus (Hip), cortex (Ctx), or cerebellum (Cer) (n = 6/group). (D) cAMP production in response to FSK stimulation was investigated in HEK cells transfected with mouse CB1 receptors. Cells were incubated with AEA (0.1 nM–10 μM) or AEA + LXA4 (100 nM), stimulated for 10 min with FSK for evaluation of the intracellular content of cAMP (∼386 times potency increase in presence of LXA4; EC50: 1,547 × 4 nM). The results of the cAMP assay were normalized by the FSK group. Efficacy curves were generated by nonlinear regression (curve fitting). Data are represented as mean ± SEM. *P < 0.05, **P < 0.01 vs. control (Duncan’s post hoc).
LXA4 Potentiates AEA Effects.
To understand the high potency of LXA4 despite its relatively low affinity to CB1 receptors, we investigated the interaction between LXA4 and endocannabinoids in vivo. Subeffective doses of AEA and 2-AG (10 and 1 pmol, respectively; Fig. S2 C and D) were coinjected i.c.v. with a subeffective dose of LXA4 in mice that were tested for catalepsy. Interestingly, LXA4 potentiated the cataleptic effect of AEA (Fig. 3A), but not the one of 2-AG (Fig. S3A) or significantly of CP55940 (Fig. S4). LXA4 might potentiate the effect of AEA by inhibiting endocannabinoid degradation. However, LXA4 did not alter the activity of the main AEA-degrading enzyme fatty acid amide hydrolase (FAAH; Fig. 3B), nor of the main 2-AG–degrading enzyme monoacylglycerol lipase (Fig. S3B). Consistently, i.c.v. administration of LXA4 did not alter brain levels of AEA (Fig. 3C) or of 2-AG (Fig. S3C). The interaction between LXA4 and AEA was further confirmed in HEK cells transfected with mouse CB1 receptors. LXA4 increased the potency of AEA in decreasing forskolin (FSK)-induced cAMP levels by ∼386 times (EC50 AEA 1,547 nM × EC50 AEA + LXA4 4 nM; Fig. 3D) at a subeffective concentration (100 nM; Fig. S3D). LXA4 did not influence cAMP levels in concentrations ranging from 0.1 nM to 1 μM (Fig. S3D). LXA4 slightly potentiated 2-AG–induced inhibition of FSK-induced cAMP accumulation (EC50 2-AG 147 nM; EC50 2-AG + LXA4 0.1 nM) in HEK-CB1 cells, but apparently reduced 2-AG efficacy in half (Fig. S3D). Interestingly, the AEA–LXA4 interaction was not observed in a GTPγS assay (Fig. S5).
LXA4 Is a Positive Allosteric Modulator of CB1 Receptors.
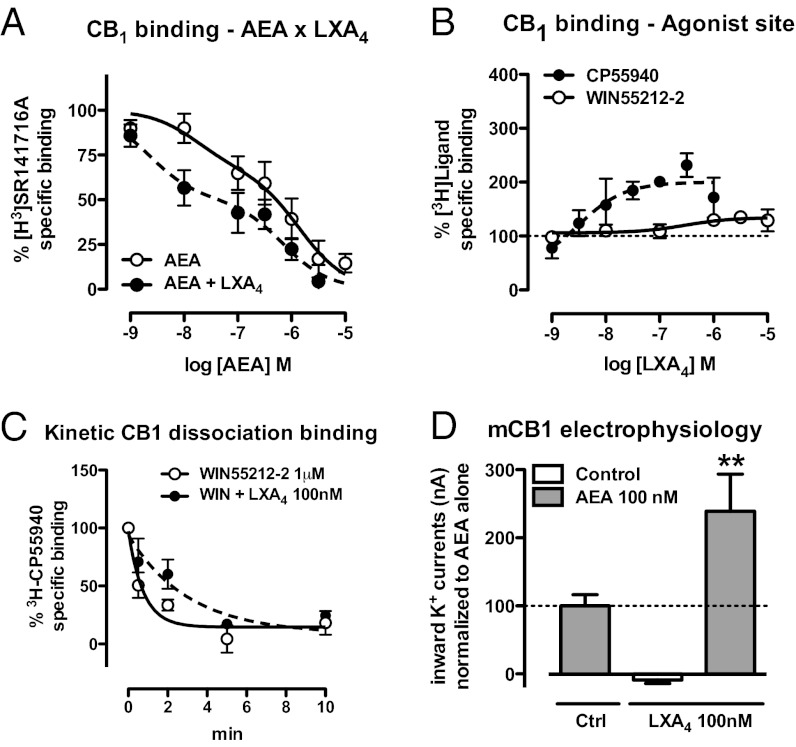
LXA4 might directly modulate AEA interaction with CB1 receptors. Therefore, we investigated whether [3H]SR141716A displacement by AEA would be affected by a subeffective concentration of LXA4 (100 nM). The binding curve of AEA (1 nM–10 µM) was displaced to the left by LXA4, suggesting enhancement of AEA-CB1 affinity by LXA4 (Fig. 4A). The best fitting of the AEA curve supported a two-site interaction, with a clearer LXA4 effect at the high-affinity binding site (IC50 17 vs. 2 nM) compared with the low-affinity binding site (IC50 1,409 vs. 692 nM) (Fig. 4A). Using radiolabeled cannabinoid agonists (0.5 nM [3H]CP55940 and 0.5 nM [3H]WIN55212-2), and performing the binding assays with increasing concentrations of LXA4, confirmed that LXA4 enhances the affinity of these ligands to CB1 receptors. LXA4 enhanced 100% of [3H]CP55940 binding (Fig. 4B) and nearly 30% of [3H]WIN55212-2 binding (Fig. 4B), suggesting a functional selectivity in the LXA4 effects (Fig. 4B). Kinetic dissociation-binding assays allow evaluating the influence of a given substance on the dissociation kinetics of a preformed orthosteric ligand–receptor complex. As the dissociation kinetic is not altered if the interacting ligands recognize the same binding site, this assay is considered the method of choice for measuring allosteric modulation (9). The binding of [3H]CP55940 was displaced by an excessive amount of WIN55,212 and followed over time. Addition of LXA4 slowed down the agonist dissociation rate (k) (Control = 1.3 ± 0.48, r2 = 0.74 vs. k LXA4 0.33 ± 0.13, r2 = 0.71, P < 0.05; Fig. 4C), which is consistent with the notion that LXA4 increases the affinity of the CB1 receptor via an allosteric mechanism.
Fig. 4.
Positive allosteric modulation of CB1 receptors by LXA4. (A) Competitive binding of AEA (1 nM–10 µM) against the CB1-selective radiolabeled antagonist [3H]SR141716A (0.5 nM) in mouse brain membranes performed in the presence or absence of LXA4 (100 nM). LXA4 increased the affinity of AEA for the CB1 cannabinoid receptors (n = 9–10/group). (B) Competitive binding of LXA4 (1 nM–10 µM) against the cannabinoid agonists [3H]CP55914 and [3H]WIN55212-2 in mouse brain membranes. LXA4 increases twofold the affinity of [3H]CP55914 to CB1 receptors (n = 4–5/group). (C) Kinetic dissociation binding showing the displacement of the CB1-ligand [3H]CP55914 (0.5 nM) by an excess of the agonist WIN55212-2 (1 µM) over time. The decrease in dissociation rate (k Control = 1.3 ± 0.48, r2 = 0.74 vs. k LXA4 0.33 ± 0.13, r2 = 0.71, P < 0.05) confirms that LXA4 increases the affinity to the CB1 receptor via an allosteric mechanism. (D) Electrophysiological recording of Xenopus oocytes expressing mouse CB1 receptors and K+ channels was performed to confirm that the AEA–LXA4 (100 nM) interaction occurs in fact at the CB1 receptor protein (n = 4–9/group). LXA4 increases twofold the potency of AEA to generate CB1-dependent K+ currents in the oocytes [F(2,17) = 7.26, P = 0.04]. Binding curves generated by nonlinear regression (curve fitting). Data are represented as mean ± SEM. **P < 0.01 vs. AEA 100 nM (Duncan’s post hoc).
Allosteric modulation may be achieved by the binding of a compound to an allosteric site in a given receptor, but it can also indirectly result from protein–protein interactions, in either case changing the pharmacological properties of the main receptor (24). The fact that cells transfected with mouse CB1 showed enhanced AEA-induced cAMP inhibition with coapplication of LXA4, an effect not observed in nontransfected cells, suggests that the cannabinoid receptor is the site of the allosteric modulation of the CB1 receptor. To investigate the dynamic action of LXA4 at the CB1 receptor, Xenopus laevis oocytes were injected with mouse CB1 receptor and two GIRK subunit cDNAs to study the electrophysiological interactions between LXA4 and AEA. AEA (100 nM) potently increased inward K+ currents measured in the oocytes. LXA4 (100 nM) alone had no effect on these currents, but it strongly potentiated the effect of AEA (Fig. 4D). Thus, LXA4 potentiates the effects of AEA at the level of the CB1 receptor protein. Altogether, our results strongly suggest that LXA4 is an endogenous allosteric modulator of the CB1 receptor that specifically enhances AEA signaling.
LXA4 Contributes to AEA in Vivo Effects.
Exogenous LXA4 causes in vivo and in vitro effects consistent with a positive allosteric modulation of the CB1 receptor. As LXA4 is an endogenous compound present at significant levels in the hippocampus, cortex, and cerebellum (Fig. 5A), we tested whether endogenous levels of LXA4 in the brain would influence AEA effects in vivo. LXA4 synthesis was reduced by the administration of the 5-lipoxygenase (LOX) inhibitor MK-886 (0.3–3 mg/kg, i.p.) before the i.c.v. injection of an effective dose of AEA (Fig. 5B). 5-LOX inhibition dose-dependently reduced the cataleptic effect of AEA up to roughly 50% (Fig. 5B). The MK-886 effect was reverted by exogenous i.c.v. LXA4, suggesting that LXA4 is the 5-LOX derivative that contributes to AEA effects in the brain (Fig. 5C). Very similar results were obtained in 5-LOX KO mice, which show decreased capacity to produce LXA4 (25, 26). The effect of AEA is strongly reduced in the 5-LOX KO mice, and the coadministration of LXA4 fully rescued the phenotype (Fig. 5D). Thus, endogenous LXA4 in the brain is necessary for the full effect of AEA.
Fig. 5.
Endogenous LXA4 contributes to endocannabinoid signaling. (A) Presence of LXA4 in the mouse brain assessed by ELISA in the cortex (Ctx), hippocampus (Hip), and cerebellum (Cer) (n = 5/group). (B) Inhibition of the LXA4-synthesizing enzyme 5-LOX by MK-886 (0.3–3 mg/kg, i.p.) reduced the effects of AEA (200 pmol/2 µL, i.c.v.) in the bar catalepsy test [F(3,24) = 3.38, P = 0.04, n = 6–8/group]. (C and D) Supplementation of LXA4 (0.01–1 pmol/2 µL, i.c.v.) restored the “normal” phenotype under 5-LOX inhibition (MK-886 3 mg/kg, i.p.) [F(3,19) = 3.27, P = 0.04, n = 5–6/group] or in the 5-LOX knockout mice (pretreatment vs. treatment) [F(1,17) = 4.96, P = 0.04, n = 5–6/group], indicating that the endogenous levels of LXA4 contribute to endocannabinoid function. Dashed lines represent the mean of groups treated with AEA (200 pmol/2 µL, i.c.v.) or control, as indicated. Data are represented as mean ± SEM. **P < 0.01; ***P < 0.001 vs. Control; ##P < 0.01 vs. AEA wild-type mice (dashed lines) (Duncan’s post hoc).
Neuroprotection Against β-Amyloid (1–40)-Induced Memory Impairments.
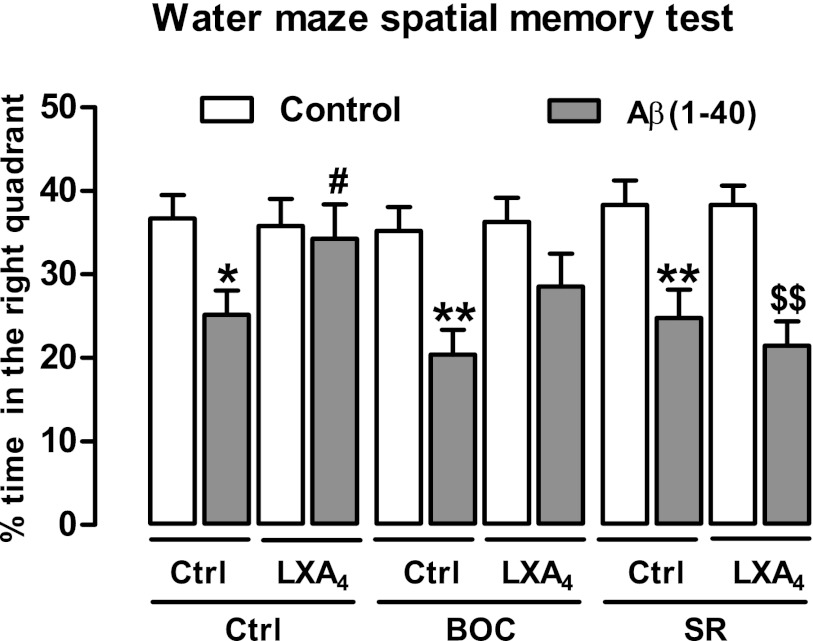
To test for potential therapeutic application of LXA4 as an allosteric enhancer of CB1 receptors, we investigated the effects of LXA4 in the β-amyloid (1–40)-induced spatial memory impairments in the water maze test, which is sensitive to endocannabinoid modulation (27, 28). It is already known that AEA is endogenously released in the first week after β-amyloid (1–40) i.c.v. injection (28). Therefore, LXA4 (1 pmol/2 µL) was coinjected i.c.v. with β-amyloid (1–40) (400 pmol/2 µL, i.c.v.) 7 d before training of spatial memory retention in the water maze (Fig. 6 and Fig. S6). β-Amyloid (1–40) impaired spatial memory formation, which was prevented by coinjection of LXA4 (Fig. 6). LXA4 effects were only mildly inhibited by Boc-2 (BOC, 10 µg/kg, i.p.), but they were fully prevented by SR141716A (SR, 1 mg/kg, i.p.), showing that LXA4-induced neuroprotection depends on CB1 cannabinoid receptors.
Fig. 6.
LXA4 protects against β-amyloid (1–40)-induced memory impairment in a CB1-dependent fashion. Coinjection with LXA4 (1 pmol/5 µL, i.c.v.) prevents the β-amyloid (1–40) [Aβ (1–40)-induced; 400 pmol/2 µL, i.c.v.] spatial memory impairment in the water maze test observed 7 d later [F(2,88) = 4.82, P = 0.01, n = 7–10/group]. Neuroprotective effects of LXA4 were prevented by the CB1 cannabinoid receptor antagonist SR141716A (SR; 1 mg/kg, i.p.) and only partially by the ALX lipoxin receptor antagonist BOC-2 (BOC 10 µg/kg, i.p.) The figure indicates the percentage of time spent in the _target quadrant, where the hidden platform was previously located (see Fig. S6 for water maze training and administration schedule). Data are represented as mean ± SEM. *P < 0.05, **P < 0.01 vs. Ctrl-Ctrl (white bars); #P < 0.05 vs. Aβ (1–40)-Ctrl; $$P < 0.01 vs. Aβ (1–40) + LXA4 (Duncan’s post hoc).
Discussion
The present data show that the endogenous eicosanoid LXA4 is an allosteric enhancer of CB1 receptor signaling in the brain. The data do not preclude, nor reduce the importance of the extensive work done on LXA4 effects in the periphery, showing that LXA4 contributes to inflammation resolution (29). Rather, our data may impact those studies by suggesting a _target for LXA4 that may contribute to its therapeutic effects. For example, a convincing mechanism for LXA4-induced analgesia was still an open question, and here we show that LXA4 is a potent CB1 receptor-dependent central analgesic in vivo. The interaction between cannabinoids and lipoxins was suggested earlier in a study showing that the nonpsychotropic cannabinoid ajulemic acid induces the release of LXA4 (30). In addition to our previous study showing that aspirin-triggered LXA4 enhances AEA effects (22), here we show that endogenous LXA4 contributes to CB1-mediated effects as a positive allosteric modulator, with physiological relevance for endocannabinoid-dependent regulation of brain functions and potential therapeutic utility.
Allosteric modulation of CB1 receptor was originally described using synthetic compounds (9). The “Org” compounds (Org27596 and Org29647) and PSNCBAM-1 (31) enhance affinity and reduce efficacy of cannabinoid agonists acting at the orthosteric site of CB1 receptors (9). These compounds have ligand-dependent effects, as they increase the affinity of [3H]CP55940 but decrease the affinity of [3H]SR141716A (9). Therefore, these compounds were considered as interesting negative regulators of (endo)cannabinoids, with potential therapeutically important regional and temporal selectivity (32). LXA4 differs from the previously described compounds because (i) it promotes enhancement, rather than reduction of CB1-mediated effects; (ii) it has apparent functional selectivity for AEA over 2-AG in vivo and in vitro; and (iii) it is physiologically present in the brain. The impact of LXA4 on endocannabinoid affinity is more evident in the high-affinity binding site in a two-site interaction model, likely suggesting an increase of affinity toward the activated conformational state (R*) of CB1 (9). The allosteric nature of the LXA4–CB1 interaction was confirmed with a dissociation-binding assay, where the dissociation kinetics of a preformed orthosteric ligand–receptor complex is evaluated. Therefore, our interpretation of the current results is that LXA4 likely helps in stabilizing the pair formed by AEA and CB1 receptors in a given conformation that favors AEA efficacy. For unknown reasons, the conformation stabilized by LXA4 does not favor 2-AG as well. Considering the agonists tested in the present study, we may suggest that LXA4 potentiates AEA = CP > WIN > 2-AG, although this phenomenon of LXA4-induced functional selectivity certainly deserves further characterization.
Our findings may have an impact on the current interpretation of the role of AEA/2-AG as endocannabinoids. AEA has been described as an endocannabinoid (33), but lately it has been regarded as an endovanilloid, displaying higher affinity for TRPV1 than for CB1 receptors under certain conditions (34). Furthermore, AEA is only a partial agonist, whereas 2-AG is a full agonist of the CB1 receptor (4, 35), suggesting that 2-AG is the “true” endocannabinoid in the CNS to which the retrograde messenger-mediated neuroplasticity can be attributed (36). According to the current results, there is an enhancement of AEA affinity/potency in the presence of LXA4, which could help bring AEA back to the status of a “true” endocannabinoid, potentially reducing the efficacy of 2-AG effects. Thus, our data strongly suggest the existence of a functional selectivity between AEA and 2-AG, which is in good agreement with recent data proposing that these two molecules may mediate substantially distinct physiological effects and regulate each other (37–39).
We found that FSK-stimulated cAMP production is more strongly and more efficiently suppressed by the costimulation with AEA and LXA4 compared with AEA alone. On the other hand, the costimulation with AEA and LXA4 did result, unexpectedly, in a decrease in G-protein binding in the GTPγS-binding assay. At the moment, the reasons for this apparent discrepancy are not known. One possible methodological explanation may reside in the fact that the GTPγS assay is known to be biased toward the measurement of Gi/o activation and is less efficient in determining receptor coupling to Gs or Gq proteins (40). The CB1 receptor is able to couple to all three types of G proteins and, although Gi/o is the most prominent one (23), coupling to Gs (41) and Gq protein (42) has also been reported. Thus, the costimulation of AEA with LXA4 might reduce the coupling of the CB1 receptor to Gs, which is more difficult to detect using GTPγS assays (40). In addition, Gi protein-independent effects of CB1 were recently proposed (43), which could also explain the different results obtained with cAMP and GTPγS assays. Notably, our results imply that nearly half of what we understand to be “pure” AEA effects are LXA4-dependent, which may lead to further investigation of physiological and therapeutic effects previously attributed exclusively to AEA (44). Another interesting link may be suggested by the report that an unknown LOX derivative would be partially responsible for TRPV1-mediated AEA effects in the isolated bronchus (45). If this holds true for the CNS, the corelease of AEA with that “unknown entity” or with LXA4 could be a kind of molecular switch driving AEA affinity toward TRPV1 or CB1 receptors. Thus, it will be very interesting to test the role of the “affinity switch” of lipoxins in endovanilloid and cannabinoid signaling.
Moreover, knowing that ajulemic acid induces the release of LXA4 acting as a proresolving mediator in inflammation (30); that LXA4 increases the affinity of AEA for the CB1 receptors (this study); and that certain metabolites of AEA degradation by lipoxygenases retain affinity for the CB1 receptor (46) or reduce AEA metabolism by FAAH (47), we may hypothesize that LOX derivatives such as LXA4 might participate in a positive feedback loop sustaining endocannabinoid tonus under certain conditions, for example, brain inflammation (48), epilepsy (49, 50), or aging (51). This may be an interesting explanation for the observed neuroprotection against β-amyloid (1–40)-induced memory impairment, which is regarded as an important component of Alzheimer’s disease pathophysiology (52). A recent report showed that the degradation of the endocannabinoid 2-AG by monoacyl glycerol lipase (MAGL) generates COX-derived neuroinflammatory eicosanoids that negatively impact the development of symptoms in a Parkinson’s disease mouse model (10). This may suggest two different pathways of responses after neuronal injury. On one hand, a LOX–AEA–FAAH pathway may contribute to inflammation resolution, whereas a COX-2–AG–MAGL pathway may worsen the neuroinflammation process.
Pharmacological blockade or genetic inactivation of the LXA4-synthesizing enzyme 5-LOX decreases AEA effects in vivo, strongly suggesting that the lipoxin acts as an endogenous modulator of AEA signaling. Our data show that LXA4 exerts an endogenous role in AEA-related signaling, as shown by pharmacological inhibition or genetic deletion of the synthesizing enzyme 5-LOX. An alternative interpretation is that a LOX-derived molecule resulting from AEA metabolism could underlie the observed effect (53). Interestingly, 5-LOX inhibition abolishes long-term potentiation induction in the hippocampus (19), and recent data suggest a role for the CB1 receptor in this phenomenon (20). Thus, it is possible that endogenous LXA4 enhancement of AEA signaling might participate in long-term synaptic plasticity. Notably, previous studies describing CNS-related LOX functions did not associate these effects with any known receptor (14, 15, 17). Therefore, by linking LXA4 to enhancement of CB1 receptor function, the present study might provide a potential mechanism to make those observations.
Although the LXA4-driven functional selectivity for endocannabinoids needs further characterization, our data clearly show that LXA4 is necessary for some cannabinoid effects of AEA through a likely allosteric modulation mechanism at the CB1 receptor. These results add a player in brain endocannabinoid signaling, which might help in clarifying unsolved issues in the field, such as the differential mechanism of action of different endocannabinoids (54). Moreover, the endogenous coagonist nature of LXA4 for AEA actions at CB1 receptors might pave the way to developing therapeutic concepts to exploit the potentialities of the endocannabinoid system in brain diseases.
Materials and Methods
A complete description of materials and methods is provided in SI Materials and Methods.
Experiments were conducted in Swiss albino mice, inbred C57BL/6 mice, CB1 knockouts (CB1−/−) and controls (CB+/+), and 5-LOX knockouts. Behavioral tests included tetrad test screening for cannabinoid effects and water maze spatial memory task for long-term memory. Ligand affinity was studied by competitive and dissociation binding assays using cannabinoid ligands ([3H]SR141716A, [3H]CP55914 and [3H]WIN55212-2) in mouse whole-brain membranes. Endocannabinoid metabolism was studied by enzymatic activity of FAAH and MAGL using [14C]AEA and [3H]2-AG in brain homogenates and quantification of AEA and 2-AG levels by HPLC. In vitro functional assay of FSK-induced cAMP accumulation in CB1-transfected HEK293T cells and investigation of G protein–CB1 receptor interaction by agonist-stimulated [35S]GTPγS binding were conducted. Immunodetection of LXA4 levels in the brain using an ELISA kit and real-time PCR for ALX receptors. Electrophysiology (voltage-clamp) in Xenopus oocytes containing CB1 receptor linked G-protein–gated K+ channels (Kir 3.1 and Kir 3.4).
Supplementary Material
Acknowledgments
F.A.P., M.Z.P.G., and R.N.T. wish to thank Gabriel Soares de Matos for help with transfections and Dr. Newton G. de Castro and Dr. Giles A. Rae for helpful scientific discussions. This work was supported by the German research foundation Deutsche Forschungsgemeinschaft (Grant FOR926 to B.L.); Institut National de la Santé et de la Recherche Médicale (G.M.), Aquitaine Region (G.M.), European Research Council (ERC-2010-StG-260515, to G.M.), and Fondation pour la Recherche Médicale (G.M.); Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) (M.Z.P.G.); PRONEX (R.N.T.), Fundação de Amparo à Pesquisa e Inovação do Estado de Santa Catarina (FAPESC) (R.N.T. and J.B.C.); and Conselho Nacional de Desenvolvimento Científico e Tecnológico-CNPq (R.N.T., J.F., and J.B.C.). F.A.P. received a doctoral fellowship from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1202906109/-/DCSupplemental.
See Commentary on page 20781.
References
- 1.Pacher P, Bátkai S, Kunos G. The endocannabinoid system as an emerging _target of pharmacotherapy. Pharmacol Rev. 2006;58(3):389–462. doi: 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howlett AC, et al. Cannabinoid physiology and pharmacology: 30 years of progress. Neuropharmacology. 2004;47(Suppl 1):345–358. doi: 10.1016/j.neuropharm.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 3.Glass M, Northup JK. Agonist selective regulation of G proteins by cannabinoid CB(1) and CB(2) receptors. Mol Pharmacol. 1999;56(6):1362–1369. doi: 10.1124/mol.56.6.1362. [DOI] [PubMed] [Google Scholar]
- 4.Gonsiorek W, et al. Endocannabinoid 2-arachidonyl glycerol is a full agonist through human type 2 cannabinoid receptor: Antagonism by anandamide. Mol Pharmacol. 2000;57(5):1045–1050. [PubMed] [Google Scholar]
- 5.Hillard CJ, et al. Synthesis and characterization of potent and selective agonists of the neuronal cannabinoid receptor (CB1) J Pharmacol Exp Ther. 1999;289(3):1427–1433. [PubMed] [Google Scholar]
- 6.Reggio PH. Pharmacophores for ligand recognition and activation/inactivation of the cannabinoid receptors. Curr Pharm Des. 2003;9(20):1607–1633. doi: 10.2174/1381612033454577. [DOI] [PubMed] [Google Scholar]
- 7.Kenakin T. Functional selectivity through protean and biased agonism: Who steers the ship? Mol Pharmacol. 2007;72(6):1393–1401. doi: 10.1124/mol.107.040352. [DOI] [PubMed] [Google Scholar]
- 8.Neubig RR, Spedding M, Kenakin T, Christopoulos A. International Union of Pharmacology Committee on Receptor Nomenclature and Drug Classification International Union of Pharmacology Committee on Receptor Nomenclature and Drug Classification. XXXVIII. Update on terms and symbols in quantitative pharmacology. Pharmacol Rev. 2003;55(4):597–606. doi: 10.1124/pr.55.4.4. [DOI] [PubMed] [Google Scholar]
- 9.Price MR, et al. Allosteric modulation of the cannabinoid CB1 receptor. Mol Pharmacol. 2005;68(5):1484–1495. doi: 10.1124/mol.105.016162. [DOI] [PubMed] [Google Scholar]
- 10.Nomura DK, et al. Endocannabinoid hydrolysis generates brain prostaglandins that promote neuroinflammation. Science. 2011;334(6057):809–813. doi: 10.1126/science.1209200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kozak KR, Marnett LJ. Oxidative metabolism of endocannabinoids. Prostaglandins Leukot Essent Fatty Acids. 2002;66(2–3):211–220. doi: 10.1054/plef.2001.0359. [DOI] [PubMed] [Google Scholar]
- 12.Serhan CN, Hamberg M, Samuelsson B. Lipoxins: Novel series of biologically active compounds formed from arachidonic acid in human leukocytes. Proc Natl Acad Sci USA. 1984;81(17):5335–5339. doi: 10.1073/pnas.81.17.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McMahon B, Mitchell S, Brady HR, Godson C. Lipoxins: Revelations on resolution. Trends Pharmacol Sci. 2001;22(8):391–395. doi: 10.1016/s0165-6147(00)01771-5. [DOI] [PubMed] [Google Scholar]
- 14.Chiang N, Takano T, Arita M, Watanabe S, Serhan CN. A novel rat lipoxin A4 receptor that is conserved in structure and function. Br J Pharmacol. 2003;139(1):89–98. doi: 10.1038/sj.bjp.0705220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim SJ. Formation of lipoxins by alveolar macrophages. Biochem Biophys Res Commun. 1988;150(2):870–876. doi: 10.1016/0006-291x(88)90473-1. [DOI] [PubMed] [Google Scholar]
- 16.Sri Kantha S, et al. Effects of prostaglandin D2, lipoxins and leukotrienes on sleep and brain temperature of rats. Prostaglandins Leukot Essent Fatty Acids. 1994;51(2):87–93. doi: 10.1016/0952-3278(94)90083-3. [DOI] [PubMed] [Google Scholar]
- 17.Shearman MS, et al. Isolation of protein kinase C subspecies from a preparation of human T lymphocytes. FEBS Lett. 1988;234(2):387–391. doi: 10.1016/0014-5793(88)80122-4. [DOI] [PubMed] [Google Scholar]
- 18.Shearman MS, Naor Z, Sekiguchi K, Kishimoto A, Nishizuka Y. Selective activation of the gamma-subspecies of protein kinase C from bovine cerebellum by arachidonic acid and its lipoxygenase metabolites. FEBS Lett. 1989;243(2):177–182. doi: 10.1016/0014-5793(89)80125-5. [DOI] [PubMed] [Google Scholar]
- 19.Williams JH, Bliss TV. Induction but not maintenance of calcium-induced long-term potentiation in dentate gyrus and area CA1 of the hippocampal slice is blocked by nordihydroguaiaretic acid. Neurosci Lett. 1988;88(1):81–85. doi: 10.1016/0304-3940(88)90319-9. [DOI] [PubMed] [Google Scholar]
- 20.de Oliveira Alvares L, et al. AM251, a selective antagonist of the CB1 receptor, inhibits the induction of long-term potentiation and induces retrograde amnesia in rats. Brain Res. 2006;1075(1):60–67. doi: 10.1016/j.brainres.2005.11.101. [DOI] [PubMed] [Google Scholar]
- 21.Murillo-Rodríguez E, et al. Anandamide modulates sleep and memory in rats. Brain Res. 1998;812(1–2):270–274. doi: 10.1016/s0006-8993(98)00969-x. [DOI] [PubMed] [Google Scholar]
- 22.Pamplona FA, Menezes-de-Lima O, Jr, Takahashi RN. Aspirin-triggered lipoxin induces CB1-dependent catalepsy in mice. Neurosci Lett. 2010;470(1):33–37. doi: 10.1016/j.neulet.2009.12.050. [DOI] [PubMed] [Google Scholar]
- 23.Howlett AC, et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54(2):161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- 24.Christopoulos A, Kenakin T. G protein-coupled receptor allosterism and complexing. Pharmacol Rev. 2002;54(2):323–374. doi: 10.1124/pr.54.2.323. [DOI] [PubMed] [Google Scholar]
- 25.Aliberti J, Serhan C, Sher A. Parasite-induced lipoxin A4 is an endogenous regulator of IL-12 production and immunopathology in Toxoplasma gondii infection. J Exp Med. 2002;196(9):1253–1262. doi: 10.1084/jem.20021183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bafica A, et al. Host control of Mycobacterium tuberculosis is regulated by 5-lipoxygenase-dependent lipoxin production. J Clin Invest. 2005;115(6):1601–1606. doi: 10.1172/JCI23949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramírez BG, Blázquez C, Gómez del Pulgar T, Guzmán M, de Ceballos ML. Prevention of Alzheimer’s disease pathology by cannabinoids: Neuroprotection mediated by blockade of microglial activation. J Neurosci. 2005;25(8):1904–1913. doi: 10.1523/JNEUROSCI.4540-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Stelt M, et al. Endocannabinoids and beta-amyloid-induced neurotoxicity in vivo: Effect of pharmacological elevation of endocannabinoid levels. Cell Mol Life Sci. 2006;63(12):1410–1424. doi: 10.1007/s00018-006-6037-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: Dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8(5):349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zurier RB, et al. Ajulemic acid, a synthetic cannabinoid, increases formation of the endogenous proresolving and anti-inflammatory eicosanoid, lipoxin A4. FASEB J. 2009;23(5):1503–1509. doi: 10.1096/fj.08-118323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horswill JG, et al. PSNCBAM-1, a novel allosteric antagonist at cannabinoid CB1 receptors with hypophagic effects in rats. Br J Pharmacol. 2007;152(5):805–814. doi: 10.1038/sj.bjp.0707347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kenakin TP. ’7TM receptor allostery: Putting numbers to shapeshifting proteins. Trends Pharmacol Sci. 2009;30(9):460–469. doi: 10.1016/j.tips.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 33.Devane WA, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 34.Di Marzo V, Bisogno T, De Petrocellis L. Anandamide: Some like it hot. Trends Pharmacol Sci. 2001;22(7):346–349. doi: 10.1016/s0165-6147(00)01712-0. [DOI] [PubMed] [Google Scholar]
- 35.Savinainen JR, Järvinen T, Laine K, Laitinen JT. Despite substantial degradation, 2-arachidonoylglycerol is a potent full efficacy agonist mediating CB(1) receptor-dependent G-protein activation in rat cerebellar membranes. Br J Pharmacol. 2001;134(3):664–672. doi: 10.1038/sj.bjp.0704297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tanimura A, et al. The endocannabinoid 2-arachidonoylglycerol produced by diacylglycerol lipase alpha mediates retrograde suppression of synaptic transmission. Neuron. 2010;65(3):320–327. doi: 10.1016/j.neuron.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 37.Darmani NA, et al. Cisplatin increases brain 2-arachidonoylglycerol (2-AG) and concomitantly reduces intestinal 2-AG and anandamide levels in the least shrew. Neuropharmacology. 2005;49(4):502–513. doi: 10.1016/j.neuropharm.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 38.Maccarrone M, et al. Anandamide inhibits metabolism and physiological actions of 2-arachidonoylglycerol in the striatum. Nat Neurosci. 2008;11(2):152–159. doi: 10.1038/nn2042. [DOI] [PubMed] [Google Scholar]
- 39.Makara JK, et al. Selective inhibition of 2-AG hydrolysis enhances endocannabinoid signaling in hippocampus. Nat Neurosci. 2005;8(9):1139–1141. doi: 10.1038/nn1521. [DOI] [PubMed] [Google Scholar]
- 40.Milligan G. Principles: Extending the utility of [35S]GTP gamma S binding assays. Trends Pharmacol Sci. 2003;24(2):87–90. doi: 10.1016/s0165-6147(02)00027-5. [DOI] [PubMed] [Google Scholar]
- 41.Glass M, Felder CC. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors augments cAMP accumulation in striatal neurons: Evidence for a Gs linkage to the CB1 receptor. J Neurosci. 1997;17(14):5327–5333. doi: 10.1523/JNEUROSCI.17-14-05327.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lauckner JE, Hille B, Mackie K. The cannabinoid agonist WIN55,212-2 increases intracellular calcium via CB1 receptor coupling to Gq/11 G proteins. Proc Natl Acad Sci USA. 2005;102(52):19144–19149. doi: 10.1073/pnas.0509588102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahn KH, Mahmoud MM, Kendall DA. Allosteric modulator ORG27569 induces CB1 cannabinoid receptor high affinity agonist binding state, receptor internalization, and Gi protein-independent ERK1/2 kinase activation. J Biol Chem. 2012;287(15):12070–12082. doi: 10.1074/jbc.M111.316463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Russo R, et al. The fatty acid amide hydrolase inhibitor URB597 (cyclohexylcarbamic acid 3′-carbamoylbiphenyl-3-yl ester) reduces neuropathic pain after oral administration in mice. J Pharmacol Exp Ther. 2007;322(1):236–242. doi: 10.1124/jpet.107.119941. [DOI] [PubMed] [Google Scholar]
- 45.Craib SJ, Ellington HC, Pertwee RG, Ross RA. A possible role of lipoxygenase in the activation of vanilloid receptors by anandamide in the guinea-pig bronchus. Br J Pharmacol. 2001;134(1):30–37. doi: 10.1038/sj.bjp.0704223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Edgemond WS, Hillard CJ, Falck JR, Kearn CS, Campbell WB. Human platelets and polymorphonuclear leukocytes synthesize oxygenated derivatives of arachidonylethanolamide (anandamide): Their affinities for cannabinoid receptors and pathways of inactivation. Mol Pharmacol. 1998;54(1):180–188. doi: 10.1124/mol.54.1.180. [DOI] [PubMed] [Google Scholar]
- 47.van der Stelt M, et al. Oxygenated metabolites of anandamide and 2-arachidonoylglycerol: Conformational analysis and interaction with cannabinoid receptors, membrane transporter, and fatty acid amide hydrolase. J Med Chem. 2002;45(17):3709–3720. doi: 10.1021/jm020818q. [DOI] [PubMed] [Google Scholar]
- 48.Eljaschewitsch E, et al. The endocannabinoid anandamide protects neurons during CNS inflammation by induction of MKP-1 in microglial cells. Neuron. 2006;49(1):67–79. doi: 10.1016/j.neuron.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 49.Marsicano G, et al. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science. 2003;302(5642):84–88. doi: 10.1126/science.1088208. [DOI] [PubMed] [Google Scholar]
- 50.Monory K, et al. The endocannabinoid system controls key epileptogenic circuits in the hippocampus. Neuron. 2006;51(4):455–466. doi: 10.1016/j.neuron.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Albayram O, et al. Role of CB1 cannabinoid receptors on GABAergic neurons in brain aging. Proc Natl Acad Sci USA. 2011;108(27):11256–11261. doi: 10.1073/pnas.1016442108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Caputo CB, Salama AI. The amyloid proteins of Alzheimer’s disease as potential _targets for drug therapy. Neurobiol Aging. 1989;10(5):451–461. doi: 10.1016/0197-4580(89)90096-1. [DOI] [PubMed] [Google Scholar]
- 53.Pamplona FA, Takahashi RN. Psychopharmacology of the endocannabinoids: Far beyond anandamide. J Psychopharmacol. 2012;26(1):7–22. doi: 10.1177/0269881111405357. [DOI] [PubMed] [Google Scholar]
- 54.Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci. 2003;4(11):873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.