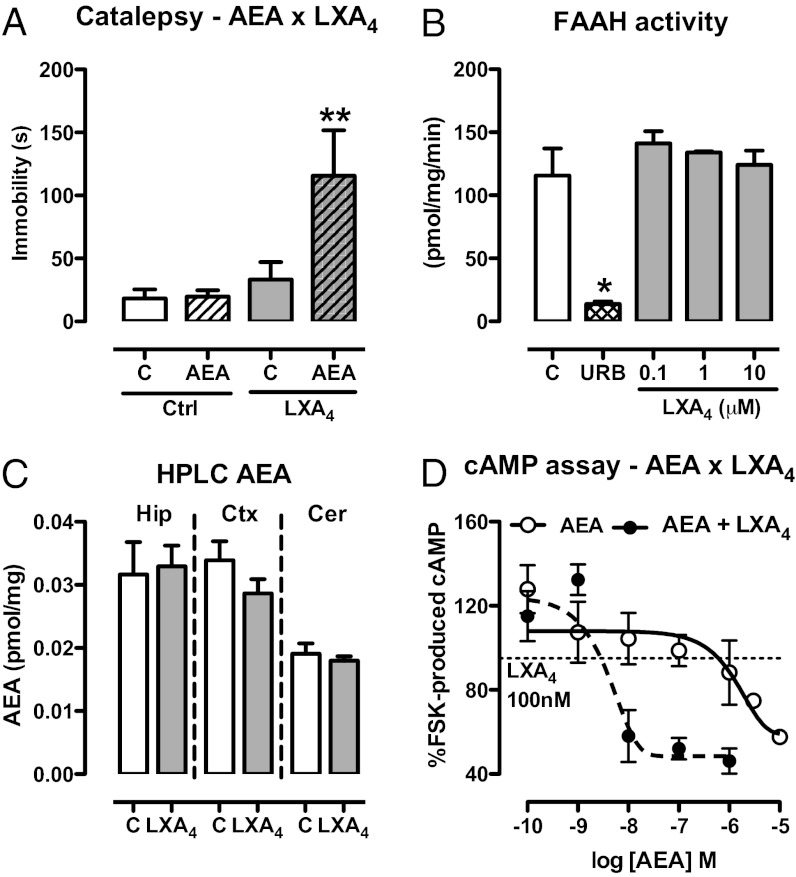
Fig. 3.
LXA4 interacts positively with the endocannabinoid AEA. (A) Selected pre-effective dose of AEA (10 pmol/2 µL, i.c.v.) was coinjected with LXA4 (0.01 pmol/2 µL, i.c.v.) 5 min before the bar catalepsy test. LXA4 interacted with AEA [F(3,29) = 4.98, P = 0.007, n = 8–9/group]. (B) Activity of the AEA-degrading enzyme FAAH was measured in the presence of LXA4 (100 nM–10 µM) using [14C]AEA (1.8 µM) as substrate. The FAAH inhibitor URB597 (URB, 50 nM) was used as positive control. LXA4 did not interfere with FAAH activity [F(3,7) = 0.73, P = 0.58, n = 3/group], as opposed to the positive control URB597 (t = 4.70, P < 0.05). (C) AEA levels in brain tissues were assessed by HPLC-MS 5 min after injection of LXA4 (1 pmol/2 µL, i.c.v.) or control (C). There were no signs of treatment-related alterations of endocannabinoid content in the hippocampus (Hip), cortex (Ctx), or cerebellum (Cer) (n = 6/group). (D) cAMP production in response to FSK stimulation was investigated in HEK cells transfected with mouse CB1 receptors. Cells were incubated with AEA (0.1 nM–10 μM) or AEA + LXA4 (100 nM), stimulated for 10 min with FSK for evaluation of the intracellular content of cAMP (∼386 times potency increase in presence of LXA4; EC50: 1,547 × 4 nM). The results of the cAMP assay were normalized by the FSK group. Efficacy curves were generated by nonlinear regression (curve fitting). Data are represented as mean ± SEM. *P < 0.05, **P < 0.01 vs. control (Duncan’s post hoc).