Summary
A remarkable up-regulation of neurogenesis through increased proliferation of neural stem/progenitor cells (NSCs) is a well-known plasticity displayed by the young dentate gyrus (DG) following brain injury. To ascertain whether this plasticity is preserved during aging, we quantified DG neurogenesis in the young adult, middle-aged and aged F344 rats after kainic acid induced hippocampal injury. Measurement of new cells that are added to the dentate granule cell layer (GCL) between post-injury days 4 and 15 using 5′-bromodeoxyuridine labeling revealed an increased addition of new cells in the young DG but not in the middle-aged and aged DG. Quantification of newly born neurons using doublecortin immunostaining also demonstrated a similar trend. Furthermore, the extent of ectopic migration of new neurons into the dentate hilus was dramatically increased in the young DG but was unaltered in the middle-aged and aged DG. However, there was no change in neuronal fate-choice decision of newly born cells following injury in all age groups. Similarly, comparable fractions of new cells that are added to the GCL after injury exhibited 5-month survival and expressed the mature neuronal marker NeuN, regardless of age or injury at the time of their birth. Thus, hippocampal injury does not adequately stimulate NSCs in the middle-aged and aged DG, resulting in no changes in neurogenesis after injury. Interestingly, rates of both neuronal fate-choice decision and long-term survival of newly born cells remain stable with injury in all age groups. These results underscore that the ability of the DG to increase neurogenesis after injury is lost as early as middle age.
Keywords: 5′-bromodeoxyuridine, adult neurogenesis, aging, dentate gyrus, dentate neurogenesis, doublecortin, kainic acid, neural stem cells, rat, stem cell differentiation, stem cell proliferation
Introduction
New dentate granule cells, generated through division of the neural stem/progenitor cells (NSCs) located in the subgranular zone (SGZ) of the dentate gyrus (DG), are added to the dentate granule cell layer (GCL) throughout life in virtually all mammals including humans (Altman & Das, 1965; Kuhn et al., 1996; Gould et al., 1997, 1999a; Eriksson et al., 1998; Kornack & Rakic, 1999). The newly added neurons become functionally mature by 1–2 months (Zhao et al., 2006; Ge et al., 2007; Toni et al., 2007) and contribute to hippocampal-dependent learning and memory functions and mood (Gould et al., 1999b; van Praag et al., 1999, 2002, 2005; Feng et al., 2001; Shors et al., 2001, 2002; Drapeau et al., 2003, 2007; Santarelli et al., 2003; Bruel-Jungerman et al., 2005; Aimone et al., 2006; Leuner et al., 2006; Kee et al., 2007). However, the extent of dentate neurogenesis depends on many extrinsic and intrinsic factors. For instance, increased neurogenesis is observed with exposure to enriched environment (Kempermann et al., 1998, 2002; Nilsson et al., 1999) and physical exercise (van Praag et al., 1999, 2005), increased levels of serotonin (Gould, 1999) and NSC proliferation factors such as brain-derived neurotrophic factor (BDNF; Lee et al., 2002; Sairanen et al., 2005), fibroblast growth factor-2 (FGF-2; Yoshimura et al., 2001; Jin et al., 2003; Rai et al., 2007), insulin-like growth factor-1 (IGF-1; Lichtenwalner et al. 2001), and vascular endothelial growth factor (VEGF; Jin et al., 2002; Sun et al., 2003, 2006). Likewise, considerably diminished neurogenesis is observed with increased levels of stress hormones (Montaron et al., 2003; Mirescu et al., 2004), damage to vascular niches through irradiation (Monje et al., 2003), exposure to drugs of abuse (He et al., 2005) and environmental toxicants (Gilbert et al., 2007), brain inflammation (Ekdahl et al., 2003; Monje et al., 2003), and aging (Kuhn et al., 1996; Cameron & McKay, 1999; Nacher et al., 2003; Rao et al., 2005, 2006a).
Dentate neurogenesis in the young brain shows remarkable plasticity to injury through considerable up-regulation in the production of new neurons. The examples include considerable increases in the dentate neurogenesis observed in the young brain after ischemia, stroke or hypoxic injury (Liu et al., 1998; Jin et al., 2001, 2004) and acute seizures or status epilepticus induced through excitotoxins or cholinomimetic chemicals (Bengzon et al., 1997; Parent et al., 1997, 2006; Gray & Sundstrom, 1998; Hattiangady et al., 2004; Gong et al., 2007). Although the precise benefits or adverse effects of increased neurogenesis after brain injury are still being studied (Parent, 2003; Jessberger et al., 2007; Scharfman & Gray, 2007; Shetty & Hattiangady, 2007), it is generally believed that this plasticity is useful for reducing impairments in cognitive functions after brain injury (Kleindienst et al., 2005; Sun et al., 2007). However, it is unknown whether aging alters the response of NSCs in the DG to brain injury, as most studies on changes in dentate neurogenesis following injury were performed using young animals. Studies on neurogenesis during normal aging demonstrate that the number of NSCs remains stable during the course of aging but NSCs in the middle-aged and aged hippocampus exhibit dramatically diminished proliferation (Rao et al., 2006a; Tang et al., 2007; Hattiangady & Shetty, 2008). This is likely because of changes in their microenvironment such as the reduced concentration of NSC mitogenic factors BDNF, FGF-2, IGF-1, and VEGF (Hattiangady et al., 2005; Shetty et al., 2005a), changes in the relationship between vascular niches and NSCs (Hattiangady & Shetty, 2008) and alterations in receptors mediating the actions of mitogenic factors (Chadashvili & Peterson, 2006). Moreover, studies on post-lesion plasticity indicate that aging considerably diminishes the synaptic reorganization that occurs in response to injury in the hippocampus. This is evidenced by the dampened sprouting and synaptogenesis of hippocampal commissural associational axons after entorhinal cortex lesions (Woods et al., 1998), diminished sprouting response of dentate mossy fibers and subdued up-regulation of various neurotrophic factors to kainic acid (KA)-induced injury (Shetty & Turner, 1999a; Shetty et al., 2004). Considering these, and because the aged population is generally more vulnerable to brain injury resulting from stroke, seizures and head injury (LaRoche & Helmers, 2003; Bernal & Peterson, 2004), it is important to examine age-related changes in the response of dentate neurogenesis to injury.
We rigorously examined whether the ability of the DG to increase neurogenesis in response to hippocampal injury is maintained during aging. We quantified and compared the extent of dentate neurogenesis in three different age groups (young adult, middle-aged and aged) of Fischer 344 (F344) rats following a hippocampal injury induced through a unilateral intracerebroventricular (ICV) administration of the excitotoxin KA. Specifically, we measured new cells and neurons added to the SGZ-GCL of hippocampi ipsilateral to KA administration between post-injury days 4 and 15 in all three age groups. For this, we employed 5′-bromodeoxyuridine (BrdU) labeling of newly born cells, immunostaining for the early neuronal marker doublecortin (DCX), dual immunostaining for BrdU and DCX, and optical fractionator cell-counting method. Additionally, using BrdU cell counts and confocal microscopic analyses of cells positive for BrdU and the neuron-specific nuclear antigen (NeuN), we ascertained whether the long-term (5 months) survival of new cells and neurons added to the GCL shortly after injury depends on the age of the hippocampus at the time of their birth.
Results
Behavioral observations and hippocampal cytoarchitecture after a unilateral ICV KA Administration
All animals were observed for 4 h after a unilateral ICV KA administration for identifying any age-related differences in behavioral activity following ICV KA injections. Behavioral changes suggestive of motor seizures, normally observed after intraperitoneal injections of KA in non-anesthetized rats (Rao et al., 2006c), were not seen in any of the rats receiving ICV KA injections under anesthesia. This is consistent with earlier observations that ICV KA administration, particularly at the low dose used in this study, does not produce motor seizures but consistently leads to hippocampal neurodegeneration (Shetty & Turner, 1999a,b; Shetty et al., 2003, 2004, 2005b). Thus, there were no age-related differences in the behavioral activity of animals as they come out of anesthesia following ICV KA administration.
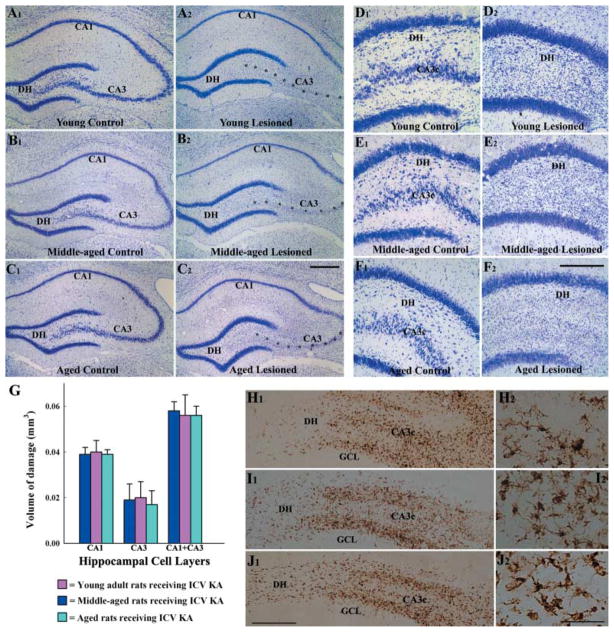
In all age groups, unilateral ICV KA, however, induced neurodegeneration in hippocampus ipsilateral to the KA administration (Fig. 1). Analyses of the extent of hippocampal lesion size through examination of serial sections stained for Nissl revealed extensive loss of CA3 pyramidal neurons in hippocampus ipsilateral to the KA administration in all age groups of rats (Fig. 1 [A2, B2, C2]), in comparison to hippocampus of age-matched naïve rats (Fig. 1 [A1, B1, C1]). This encompassed most of the CA3 cell layer except for a smaller zone adjacent to the CA2 cell layer, where larger pyramidal neurons of CA3a subregion were preserved. The CA1 pyramidal cell layer was mostly spared in the anterior half of the hippocampus but fractions of CA1 pyramidal cells exhibited degeneration in the posterior half of the hippocampus in all groups. There was no apparent loss of neurons in the dentate granule cell layer but dentate hilus (DH) exhibited considerable loss of neurons in all age groups of rats receiving KA (Fig. 1 [D2, E2, F2]), in comparison to age-matched naïve rats (Fig. 1 [D1, E1, F1]). Quantification of the extent of hippocampal injury revealed that the volume of damage to both CA3 and CA1 cell layers following ICV KA administration is comparable across the three age groups of rats (Fig. 1 [G]). In hippocampus contralateral to the KA administration, the cell layers remained intact in all age groups (data not illustrated). Because of this, analyses of injury-induced neurogenesis in this study were performed for hippocampi ipsilateral to the KA administration in all age groups. The hippocampus ipsilateral to the KA administration is referred to as ‘the injured hippocampus’ in every age group.
Fig 1.
Pattern of hippocampal neurodegeneration at 16 days after an unilateral intracerebroventricular (ICV) kainic acid (KA) administration, visualized by Nissl staining. Left panel: A1, B1, and C1 illustrate examples of the hippocampus from naïve young adult (A1), middle-aged (B1) and aged (C1) rats, whereas A2, B2, and C2 show examples of the hippocampus ipsilateral to the ICV KA administration from age-matched KA-treated young adult (A2), middle-aged (B2) and aged (C2) rats. Note an extensive loss of CA3 pyramidal neurons (indicated by asterisks) in all three age groups. Right panel: D1, E1, and F1 illustrate examples of the dentate gyrus from naïve young adult (A1), middle-aged (B1) and aged (C1) rats, whereas D2, E2, and F2 show examples of the dentate gyrus ipsilateral to the ICV KA administration from age-matched KA-treated young adult (D2), middle-aged (E2) and aged (F2) rats. Note an extensive loss of neurons in the dentate hilus (DH) in all three age groups. Scale bar: A1–C2, 400 μm; D1–F2, 200 μm. The bar chart (G) illustrates the volume of damage in CA1 and CA3 cell layers of different age groups of rats after ICV KA administration. H1–J2 show the distribution of ED-1 immunopositive activated microglial cells in the dentate gyrus at 16 days after the KA administration in the three age groups of animals. Note that the distribution and density of ED-1 immunopositive activated microglial cells in the DH and the CA3c subregion is comparable in the young adult (H1), middle-aged (I1), and aged (J1) rats. H2, I2 and J2 are magnified views of regions from H1, I1 and J1 demonstrating the morphology of ED-1 immunopositive activated microglial cells in the three age groups. The morphology appears similar across the three age groups. GCL, granule cell layer; scale bar: H1, I1, J1 = 250 μm; H2, I2, J2 = 50 μm.
A similar pattern of injury in the hippocampus of different age groups of rats was also confirmed by the analyses of the extent of hippocampal inflammation at 16 days after ICV KA administration using ED-1 immunostaining (Fig. 1 [H1, I1, J1]). A high density of ED-1+ activated microglial cells was observed in areas of hippocampal neurodegeneration in all rats receiving ICV KA regardless of the age of the animal at the time of KA administration (Fig. 1 [H1–J2]). Thus, the degree of hippocampal inflammation following KA-induced injury, as assessed by ED-1 immunostaining appeared similar across the three age groups.
Age-related changes in the extent of addition of new cells to SGZ-GCL after KA-induced injury
The age-related changes in the extent of addition of new cells to the SGZ-GCL following hippocampal injury were assessed through BrdU immunostaining of hippocampal sections from animals belonging to all groups killed at 24 h after the last of 12 daily BrdU injections (Fig. 2). In KA-treated animals, the BrdU injections were administered during the post-injury days 4–15. In all groups, BrdU immunostaining revealed newly generated cells in the GCL and the SGZ throughout their antero-posterior extent. The distribution and numbers of newly born cells in the SGZ-GCL of intact young adult, middle-aged and aged rats have been described in our earlier report (Rao et al., 2005). The injured hippocampus of young adult rats exhibited a dramatic increase in the number of newly born cells (i.e. BrdU+ cells) in the SGZ-GCL (Fig. 2 [D1, D2]), in comparison to age-matched intact hippocampi (Fig. 2 [A1–A2]). The degenerated regions (DH and the CA3 pyramidal cell layer) also showed a large number of BrdU+ cells (Fig. 2 [D1]). The BrdU+ cells in the DH are likely newborn cells (rather than dead cells) because BrdU injections commenced after the conclusion of KA-induced cell death (i.e. 4 days after the KA-induced injury) in this study. However, it is possible that hilar BrdU+ cells comprise a mixture of cells that divide locally (such as microglial cells and astrocytes) and cells that have migrated from the SGZ.
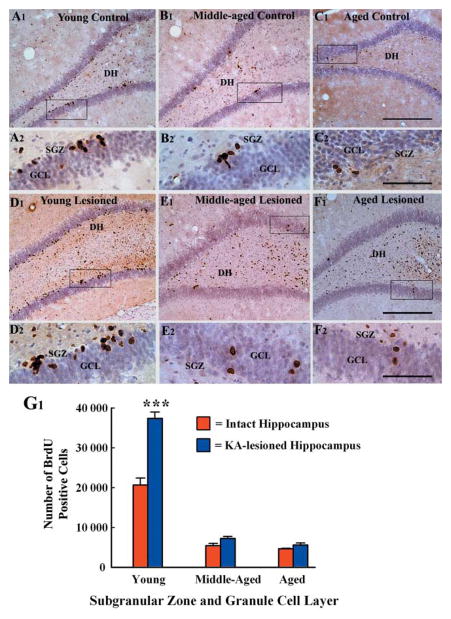
Fig 2.
Distribution of newly generated cells in the dentate gyrus (DG) at 24 h after 12 daily injections of 5′-bromodeoxyuridine (BrdU) in different groups, visualized with BrdU immunostaining and hematoxylin counterstaining. The groups include the dentate gyrus of naïve young adult (A1, A2), middle-aged (B1, B2) and aged (C1, C2) rats, and the dentate gyrus of hippocampi ipsilateral to kainic acid (KA) administration from young adult (D1, D2), middle-aged (E1, E2) and aged (F1, F2) rats. A2, B2, C2, D2, E2, and F2 are magnified views of regions from A1, B1, C1, D1, E1, and F1. Note that the density of newly born cells in the subgranular zone-granule cell layer (SGZ-GCL) increases in the young adult rat after KA administration (D1, D2), in comparison to age-matched naïve rat (A1, A2). In contrast, the density of newly born cells in these regions is unchanged in the middle-aged (E1, E2) and aged (F1, F2) rats after similar KA administration in comparison to respective naïve groups (B1, B2, C1, C2). The dentate hilus and CA3c subregion show increased density of BrdU immunopositive cells in KA-treated animals regardless of the age (D1, E1, F1). The bar chart in G1 compares the absolute numbers of newly born cells added over a period of 12 days to the SGZ-GCL in different groups of rats. Note that, KA administration considerably increases the numbers of newly born cells in the SGZ-GCL in young adult rats but not in middle-aged and aged rats. Scale bar: A1, B1, C1 D1, E1, F1 = 200 μm; A2, B2, C2, D2, E2, F2 = 50 μm.
In the injured hippocampus of middle-aged and aged rats, the density of BrdU+ cells in SGZ-GCL (Fig. 2 [E1–E2 and F1–F2]) remained similar to that observed in age-matched intact hippocampi (Fig. 2 [B1–B2 and C1–C2]). This suggests that no up-regulation in the number of newly born cells occurs in these regions following hippocampal injury. However, the degenerated regions such as the DH and CA3c subregion showed increased number of BrdU+ cells (Fig. 2 [E1 and F1]). Quantification of the number of new cells (i.e. BrdU+ cells) added to the SGZ-GCL over a period of 12 days (i.e. during the post-injury days 4–15) revealed a marked increase in the addition of new cells by NSCs in the injured young hippocampus (Fig. 2 [G1]). This is consistent with the earlier study in a similar model (Gray & Sundstrom, 1998). The overall increase in the addition of new cells to the SGZ-GCL of the injured young hippocampus was 81% (P < 0.001), in comparison to the number observed in the age-matched intact hippocampus (Fig. 2 [G1]). Interestingly, quantification of the total number of BrdU+ cells added to the SGZ-GCL during the post-injury days 4–15 (i.e. over a period of 12 days) demonstrated no significant increase in the addition of new cells in the injured middle-aged and aged hippocampi (Fig. 2 [G1]). In the injured middle-aged hippocampi, the average number of new cells added to the SGZ-GCL over a period of 12 days is 7201 (mean ± SEM = 7201 ± 512), in comparison to 5402 (5402 ± 596) in age-matched intact hippocampi (P > 0.05; Fig. 2 [G1]). Whereas, in the injured aged hippocampi, the average number of new cells added to the SGZ-GCL over a period of 12 days is 5569 (5569 ± 541), in comparison to 4644 (4644 ± 112) in age-matched intact hippocampi (P > 0.05; Fig. 2 [G1]). Thus, unlike the SGZ-GCL of the injured young hippocampus, the SGZ-GCL of the injured middle-aged and aged hippocampi display no significant changes in the addition of new cells, suggesting that the ability of the DG to increase the production of new cells in response to hippocampal injury is lost by middle age. Comparison across the injured groups reveals that the addition of new cells during the postinjury days 4–15 in the injured young hippocampus is ~5-folds greater than that of the injured middle-aged hippocampus and ~6-folds greater than that of the injured aged hippocampus.
Neuronal fate-choice decision of newly born cells after hippocampal injury in different age groups
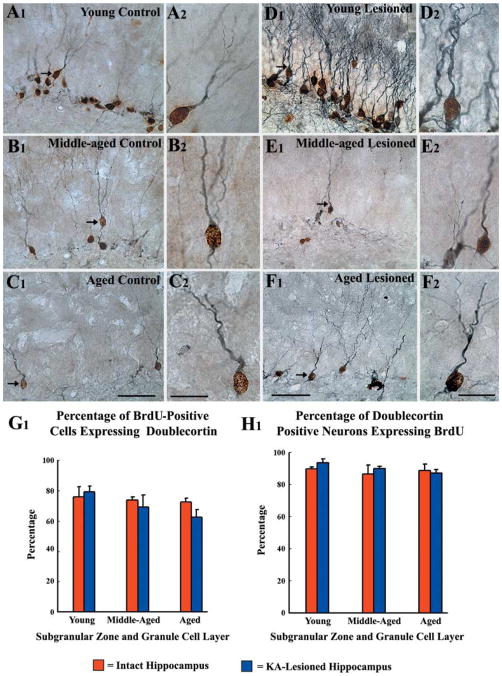
Neurons among newly born cells (BrdU+ cells) in the SGZ-GCL were characterized through dual immunostaining for BrdU and DCX in tissues harvested at 24 h after the last of 12 daily BrdU injections. This visualized BrdU expression in the nucleus and DCX expression within soma and dendrites of newly born neurons (Fig. 3 [A1–F2]). Doublecortin is an excellent marker of immature neurons in the DG, as DCX expression occurs within 3 h after birth in neuronally committed newly born cells (Kempermann et al., 2003) and persists for at least 2 weeks (Rao & Shetty, 2004). In intact animals, our earlier studies have shown that aging does not impair the neuronal fate-choice decision of newly born cells in the SGZ-GCL, as similar fractions of newly born cells differentiated into DCX+ neurons in intact young, middle-aged and aged animals (Rao et al., 2005, 2006a). In close similarity, a vast majority of newly born cells (BrdU+ cells) expressed DCX in the SGZ-GCL of injured hippocampi, regardless of the age of the hippocampus at the time of injury (Fig. 3 [A1–F2]). In all groups, DCX+ neurons exhibiting the phenotype of differentiated granule cells (i.e. cells with vertically oriented dendrites projecting into the molecular layer) and DCX+ neurons displaying a relatively immature morphology (i.e. cells with horizontally oriented dendrites in the SGZ) were clearly positive for BrdU (Fig. 3 [A1–F2]). The extent of neuronal differentiation of newly born cells in the SGZ-GCL was very similar across the three age groups after hippocampal injury and did not differ significantly from that observed in age-matched intact rats (Fig. 3 [G1]). The overall neuronal differentiation in the SGZ-GCL was 79 ± 3.6% in the injured young adult hippocampus, 69 ± 8% in the injured middle-aged hippocampus, and 62 ± 2% in the injured aged hippocampus (Fig. 3 [G1]). These percentages are closely comparable to percentages observed in intact age-matched hippocampi (Rao et al., 2005). Thus, majority of newly born cells in the SGZ-GCL of the DG differentiate into neurons in all age groups under both intact as well as lesioned conditions. This suggests that neither aging nor injury interferes with neuronal fate-choice decision of newly born cells in the SGZ-GCL. Quantification of the percentages of DCX+ neurons having BrdU immunopositive nucleus at 16 days postinjury demonstrated that over 87% of all DCX+ neurons in the SGZ-GCL were born during the 12 daily BrdU injections (i.e. at postinjury days 4–15) in all three age groups (Fig. 3 [H1]). This is similar to the earlier observation in age-matched intact rats (Rao et al., 2005). Thus, a vast majority of neurons visualized with DCX immunostaining in the SGZ-GCL of both intact and KA-treated young adult, middle-aged and aged rats are new granule cells that were generated during the preceding 12 days (i.e. during the BrdU injection period). Interestingly, hippocampal injury does not alter the DCX expression phase of newly born neurons in all groups examined in this study.
Fig 3.
Neuronal differentiation of newly born cells in the subgranular zone-granule cell layer (SGZ-GCL) of different groups at 24 h after the last of 12 daily injections of 5′-bromodeoxyuridine (BrdU) and visualized through BrdU and doublecortin (DCX) immunostaining. These include SGZ-GCL of naïve young adult (A1, A2), middle-aged (B1, B2) and aged (C1, C2) rats, and SGZ-GCL of hippocampi ipsilateral to kainic acid (KA) administration from young adult (D1, D2), middle-aged (E1, E2) and aged (F1, F2) rats at 16 days post-KA administration. A2, B2, C2, D2, E2, and F2 are magnified views of regions from A1, B1, C1, D1, E1, and F1. Note that a majority of newly born (BrdU +) cells (brown nuclei) are positive for DCX (blue-grey reaction product in the cytoplasm of the soma and dendrites) in all groups. Furthermore, majority of DCX + neurons are positive for BrdU in allthree age groups. Figure G1 shows that the percentages of BrdU + cells that are positive for DCX are comparable in different groups, suggesting that the rate of neuronal differentiation remains stable across the three age groups under both intact and lesioned conditions. The bar chart H1 illustrates that similar percentages (83–94%) of DCX + cells are immunoreactive for BrdU in different groups, implying that a vast majority of neurons visualized with DCX immunostaining in the SGZ-GCL of both intact and KA-treated young adult, middle-aged and aged rats are new granule cells that were generated during the preceding 12 days. Scale bar: A1, B1, C1, D1, E1, F1 = 50 μm; A2, B2, C2, D2, E2, F2 = 20 μm.
Hippocampal injury-related changes in the production of new neurons in different age groups, as assessed by measurement of DCX+ neurons in the dentate gyrus
As an additional measure of the status of dentate neurogenesis after hippocampal injury, we characterized DCX+ newly born neurons in the SGZ-GCL and the DH of all age groups of rats (Fig. 4 [A1–F2]). An increased density of DCX+ newly born neurons was observed in both SGZ-GCL and the DH of the injured young hippocampus (Fig. 4 [B1, B2]), in comparison to new neurons in respective regions of the age-matched intact hippocampus (Fig. 5 [A1, A2]). However, the densities of DCX+ newly born neurons in the SGZ-GCL of the injured middle-aged and aged hippocampi (Fig. 4 [D1–D2 and F1–F2]) were comparable to new neurons in respective regions of age-matched intact hippocampi (Fig. 4 [C1–C2 and E1–E2]). The DH showed a few DCX+ neurons following injury in both middle-aged and aged hippocampi. However, the ectopic migration of newly born cells into the DH following injury was very conspicuous in the young hippocampus (Fig. 4 [B1–B2]), which is consistent with the earlier reports from young rats after injury or seizures (Parent et al., 1997; Gray & Sundstrom, 1998; Hattiangady et al., 2004).
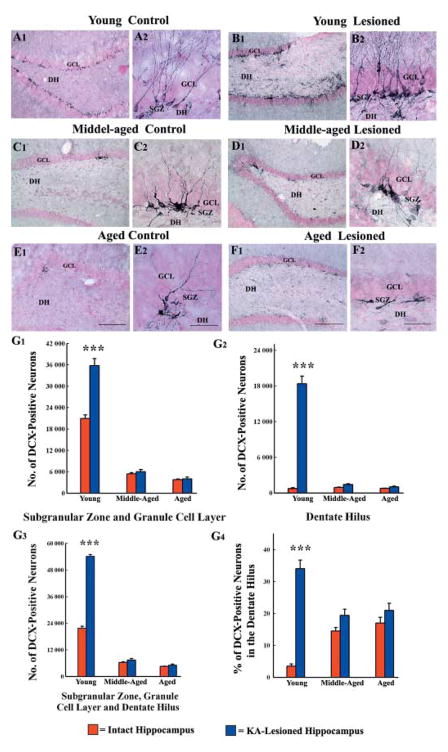
Fig 4.
Distribution of newly born neurons in the subgranular zone-granule cell layer (SGZ-GCL) of different groups, visualized through doublecortin (DCX) immunostaining. The groups include DG of naïve young adult (A1, A2), middle-aged (C1, C2) and aged (E1, E2) rats, and SGZ-GCL of hippocampi ipsilateral to kainic acid (KA) administration from young adult (B1, B2), middle-aged (D1, D2) and aged (F1, F2) rats at 16 days post-KA administration. A2, B2, C2, D2, E2, and F2 are magnified views of regions from A1, B1, C1, D1, E1, and F1. Note that the density of newly born neurons in the SGZ-GCL increases in the young adult rat after KA administration (B1, B2), in comparison to age-matched naïve rat (A1, A2). In contrast, the density of newly born cells in these regions is unchanged in the middle-aged (D1, D2) and aged (F1, F2) rats after similar KA administration in comparison to respective naïve groups (C1, C2 and E1, E2). The dentate hilus (DH) shows an increased density of ectopically migrated newly born neurons in the KA-treated young animal (B1, B2), whereas the KA-treated middle-aged and aged animals show only a few ectopically migrated neurons (D1, F1). Scale bar: A1, B1, C1, D1, E1, F1 = 200 μm; A2, B2, C2, D2, E2, F2 = 50 μm. The bar charts in G1–G3 compare the absolute numbers of newly born neurons added over 12 days to the SGZ-GCL (G1), the DH (G2), and the SGZ-GCL plus the DH (G3) in different groups of rats. Note that, KA administration considerably increases the numbers of newly born cells in all these regions in young adult rats but not in middle-aged and aged rats. The bar chart in G4 compares the percentages of newly born neurons that have migrated ectopically into the DH in different groups of rats. Note that KA administration considerably increases the ectopic migration of newly born cells in young adult rats but not in middle-aged and aged rats.
Fig 5.
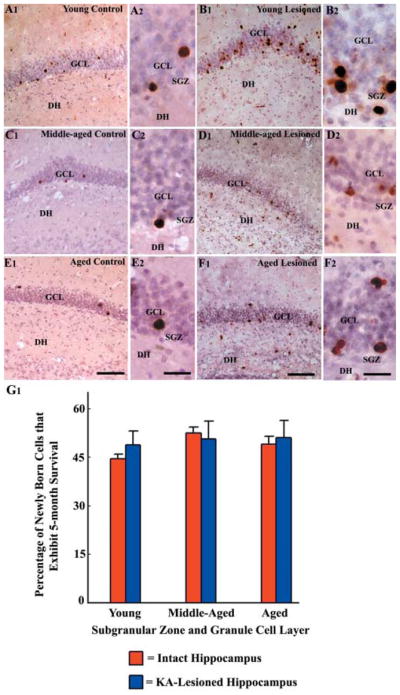
Distribution of newly born cells in the subgranular zone-granule cell layer (SGZ-GCL) of different groups at 5 months after 12 daily injections of 5′-bromodeoxyuridine (BrdU), visualized through BrdU immunostaining and hematoxylin counterstaining. The groups include the dentate gyrus of naïve young adult (A1, A2), middle-aged (C1, C2) and aged (E1, E2) rats, and the dentate gyrus of hippocampi ipsilateral to kainic acid (KA) administration from young adult (B1, B2), middle-aged (D1, D2) and aged (F1, F2) rats. A2, B2, C2, D2, E2, and F2 are magnified views of regions from A1, B1, C1, D1, E1, and F1. Note that newly added cells exhibit long-term survival and migrate up into the GCL in all groups. Scale bar: A1, B1, C1, D1, E1, F1 = 200 μm; A2, B2, C2, D2, E2, F2 = 50 μm. The bar chart G1 illustrates that similar percentages (44–53%) of newly added cells exhibit 5-month survival in different groups, suggesting that the long-term survival of newly added cells to the SGZ-GCL is independent of age as well as injury.
In every age group, the density and pattern of distribution of DCX+ neurons in the SGZ-GCL appeared comparable to that of BrdU+ cells under both intact and lesioned conditions. However, the overall density of DCX+ neurons in the DH was much lower than BrdU+ cells after hippocampal injury in all groups. This is likely because majority of BrdU+ cells in the DH after KA-induced injury are reactive glial cells such as activated microglia, as also observed with ED-1 staining described earlier. Furthermore, a vast majority of DCX+ neurons located in the SGZ-GCL of both intact and injured hippocampi of young rats exhibited the phenotype of differentiated granule cells with vertically oriented dendrites extending into the outer two-thirds of the dentate molecular layer (Rao et al., 2005). Contrastingly, in the injured middle-aged and aged hippocampi, the DCX+ neurons with vertically oriented dendrites reaching the outer two-thirds of the molecular layer were much fewer in number, as described earlier for naïve middle-aged and aged hippocampi (Rao et al., 2005). The morphology of DCX+ neurons that have migrated ectopically into the DH following hippocampal injury was similar in all three age groups. These neurons exhibited horizontally oriented dendrites or dendrites that projected mostly towards the DH (i.e. basal dendrites).
Measurement of the numbers of DCX+ neurons in the SGZ-GCL and the DH of different groups revealed the following. First, hippocampal injury considerably increases the number of new neurons in both SGZ-GCL and the DH of young adult rats. In comparison to numbers in age-matched intact rats, the overall increase was 71% in the SGZ-GCL, 25-fold in the DH and 150% in the entire DG (Fig. 4 [G1]). Second, hippocampal injury does not increase the numbers of new neurons in the SGZ-GCL of middle-aged and aged rats (Fig. 4 [G1]). The SGZ-GCL of intact middle-aged rats exhibit an average of 5380 ± 333 new neurons and this number remains stable after hippocampal injury (5980 ± 640; P > 0.05). The SGZ-GCL of intact aged rats display an average of 3763 ± 190 new neurons and this number does not change significantly after hippocampal injury (4009 ± 502; P > 0.05). Third, hippocampal injury leads to 1.4- to 1.5-fold increase in the numbers of new neurons in the DH of middle-aged and aged rats, though the extent of increase seen in these rats is insignificant in comparison to 25-fold increase observed in young adult rats after similar hippocampal injury (Fig. 4 [G2]). Fourth, even when taken as a whole entity (i.e. by adding new neurons in the SGZ-GCL and the DH), the DG of middle-aged and aged rats does not exhibit increased number of new neurons after hippocampal injury, unlike the DG of young adult rats exhibiting 150% increase in the number of new neurons (Fig. 4 [G3]).
Measurement of the fractions of newly born neurons that are located in the DH revealed that, under intact conditions, only 3% of newly born neurons migrate into the DH in young adult rats. In contrast, in middle-aged and aged rats, 15–17% of newly born neurons are located in the DH. Ectopic migration of newly born neurons into the DH increases to 34% of all newly born neurons (i.e. 11-fold increase) following hippocampal injury in young adult rats (Fig. 4 [G4]). On the other hand, hippocampal injury in middle-aged and aged rats does not increase the ectopic migration of newly born neurons (Fig. 4 [G4]). This is because, only 19 – 20% of all newly born neurons were located in the DH of these two groups after hippocampal injury, and this extent is equivalent to that observed during intact conditions (15–17% of all newly born cells). Thus, significant ectopic migration of newly born neurons into the DH following hippocampal injury appears to be a phenomenon restricted to the young DG. Furthermore, in the DH, the relative numbers of newly born neurons are dramatically less in the middle-aged and aged rats (middle age, 1404 ± 145; aged, 1026 ± 108), in comparison to young adult rats (18 357 ± 1263).
Long-term survival and migration of new cells that are born in the SGZ-GCL shortly after KA-induced hippocampal injury in different age groups
We examined whether the long-term survival and migration of new cells that are born in the SGZ-GCL shortly after the hippocampal injury depends on the age of the hippocampus at the time of their birth. For this, we examined and quantified the numbers of BrdU+ cells in the SGZ-GCL at 5 months after the last of 12 daily BrdU injections in all groups (Fig. 5). In the injured hippocampus of all age groups, the surviving BrdU+ cells at 5 months after birth were clearly isolated and majority of them were in the GCL (Fig. 5 [B1–B2, D1–D2, and F1–F2]), which is consistent with the pattern observed in age-matched control rats (Fig. 5 [A1–A2, C1–C2, and E1–E2]). Some of them were clearly in the outer third of the GCL in all age groups. These observations suggest that neither aging nor hippocampal injury alters the migration of newly born cells in the SGZ-GCL. Our earlier study in intact animals has demonstrated that the long-term survival of newly born cells in the SGZ-GCL is not impaired with aging, as comparable percentages (47 – 56%) of newly born cells exhibited 5-month survival in all three age groups (Rao et al., 2005). In line with these findings, the current study demonstrates that similar fractions of newly born cells in the SGZ-GCL exhibit 5-month survival in the injured hippocampus of young adult, middle-aged and aged rats (Fig. 5 [G1]). Comparison of the numbers of BrdU+ cells observed at 24 h and 5 months after the last of 12 daily BrdU injections revealed that the survival of newly born neurons in the SGZ-GCL of injured young adult, middle-aged and aged hippocampus was equivalent to 49 – 51% of new cells generated during post-injury days 4 and 15 (Fig. 5 [G1]). Thus, the long-term survival of newly born cells in the SGZ-GCL is independent of aging or hippocampal injury.
Neurons among newly born cells that exhibit 5-month survival in the SGZ-GCL of injured young adult, middle-aged and aged hippocampus
Characterization of neurons among the surviving BrdU+ cells using BrdU–NeuN dual immunofluorescence and confocal microscopy revealed that 79 – 84% of BrdU+ cells that exhibit 5-month survival are neurons in all groups (Fig. 6). Thus, vast majority of newly generated cells that exhibit 5-month survival in the SGZ-GCL become mature neurons and likely incorporate into the hippocampal circuitry, regardless of the age or injury at the time of their birth. Extrapolation of the percentages of cells exhibiting NeuN expression with the absolute numbers of BrdU+ cells at 5 months post-KA injury demonstrated the overall addition of mature neurons to the DG circuitry over a period of 12 days (i.e. during the post-injury days 4 and 15) in different groups. This was equivalent to ~14 770 neurons in injured young adult hippocampus, ~2887 neurons in injured middle-aged hippocampus and ~2268 neurons in the injured aged hippocampus. When compared to the numbers of mature neurons added over a period of 12 days to the DG circuitry in age-matched control rats (an average of 7703 neurons in the young adult, 2268 neuron in the middle-aged, and 1814 neurons in the aged) reported in our earlier study (Rao et al., 2005), it is clear that the addition of mature neurons to the GCL of young adult rats increases by ~92% after hippocampal injury. In contrast, the addition of mature neurons to the GCL does not increase significantly after hippocampal injury in middle-aged and aged rats.
Fig 6.
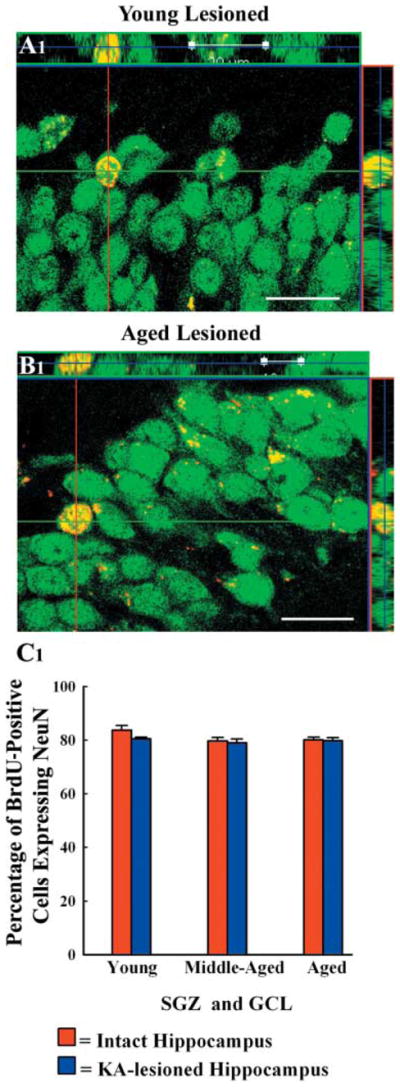
Orthogonal views of newly added cells that differentiated into neuron-specific nuclear antigen (NeuN) positive mature neurons in the granule cell layer of kainic acid-treated young adult (A1) and aged (B1) rats. The analysis was done at 5 months after 12 daily injections of 5′-bromodoxyuridine (BrdU) through BrdU and NeuN dual immunofluorescence and confocal microscopy. Scale bar: 20 μm. The bar chart in C1 demonstrates that 79–84% of new cells that persist in the granule cell layer differentiate into NeuN + positive neurons in different groups, implying that majority of new cells that exhibit long-term survival are neurons in all groups. SGZ, subgranular zone; GCL, granule cell layer.
Discussion
This quantitative study on injury-induced DG neurogenesis during aging offers three novel findings. First, aging considerably impairs the plasticity of hippocampal NSCs to enhance DG neurogenesis in response to KA-induced hippocampal injury. This was evidenced by unaltered neurogenesis in the middle-aged and aged DG following hippocampal injury, in contrast to dramatically increased neurogenesis in the young DG after similar injury. Second, both neuronal fate-choice decision of newly born cells and long-term survival of new neurons added to the SGZ-GCL are independent of aging and injury. This was demonstrated by similar rate of neuronal differentiation of newly born cells, and comparable percentages of newly added neurons in the SGZ-GCL exhibiting 5-month survival, across the three age groups under both intact and injured conditions. Third, injury to the middle-aged and aged hippocampi is not associated with increased ectopic migration of newly born neurons into the DH, in contrast to substantial increases in such abnormal migration after similar injury to the young hippocampus.
Age-related differences in DG neurogenesis after injury
An injury or status epilepticus, induced by chemoconvulsants, typically elicits an increased proliferative response from NSCs in the SGZ of the young hippocampus resulting in an enhanced DG neurogenesis (Parent et al., 1997; Gray & Sundstrom, 1998; Nakagawa et al., 2000; Hattiangady et al., 2004). Accordingly, we found that the DG neurogenesis increases considerably after an ICV KA-induced injury in the young hippocampus. The increase in the addition of new cells over a period of 12 days was ~81% based on BrdU labeling. Analyses of the status of DG neurogenesis at 16 days after injury through DCX immunostaining revealed ~150% increase in the addition of new neurons. Moreover, based on BrdU-NeuN analyses at 5 months after injury, there was 92% increase in the addition of new neurons to the DG circuitry between post-injury days 4 and 16 in the young hippocampus. In contrast, the middle-aged and aged hippocampi did not exhibit increase in neurogenesis after injury. Investigation of newly born neurons in the DH revealed that KA-induced injury in the young hippocampus induces 11-fold increases in the ectopic migration of newly born neurons. Contrastingly, similar injury in the middle-aged and aged hippocampi did not induce significant ectopic migration of newly born neurons. Thus, both increased DG neurogenesis and ectopic migration of newly born neurons following injury appear to be phenomena restricted to the young adult hippocampus.
Although earlier studies using stroke models have indicated that the NSCs in the aged (24 months old) hippocampus are no longer or only mildly capable of increasing neurogenesis in response to brain injury (Jin et al., 2004; Darsalia et al., 2005), a radical diminution of this injury-induced plasticity in the DG by middle age, as observed in this study, is intriguing. However, the finding is in line with the changes in NSC mitogenic factors and cognitive function observed at middle age in earlier studies. These include: (i) decreased concentrations of neurotrophic factors FGF-2, IGF-1, VEGF, and BDNF (Hattiangady et al., 2005; Shetty et al., 2005a); (ii) down-regulation of other NSC mitogenic factors such as neuropeptide Y (NPY) and the transcription factor phosphorylated cyclic AMP response element biding protein (Hattiangady et al., 2005; Howell et al., 2005, 2007); and (iii) impairments in memory and long-term potentiation (Park et al., 2002; Rex et al., 2005, 2006). Thus, the plasticity of the DG diminishes considerably by middle age. On the contrary, the response of the subventricular zone (SVZ, another neurogenic region in the brain) NSCs to injury such as stroke is retained even during the old age. This was evidenced by an increased production of new neurons by NSCs of the SVZ in the aged hippocampus following ischemia (Jin et al., 2004; Darsalia et al., 2005). These results suggest that NSCs in two distinct neurogenic regions (SGZ of the hippocampus and SVZ of the forebrain) of the brain respond differently to brain injury during aging.
Potential reasons for altered plasticity of NSCs in the middle-aged and aged hippocampi to injury
To begin with, the altered plasticity of NSCs following KA-induced injury in the middle-aged and aged hippocampi does not appear to be due to differences in the degree of hippocampal injury, as the lesion size after ICV KA administration was comparable across the three age groups, as also observed in our previous studies (Shetty & Turner, 1999a,b; Abdel-Rahman et al., 2004). Because the extent of inflammation can alter neurogenesis (Ekdahl et al., 2003; Monje et al., 2003), we examined whether the altered plasticity of NSCs in the injured middle-aged and aged hippocampi is linked to a different degree of inflammation in comparison to the injured young hippocampus. However, similar density of ED-1+ microglia in the injured hippocampus of all three age groups revealed that this is not the case. Furthermore, it is unlikely that intrinsic modifications in NSCs themselves such as decreased telomerase levels and telomerase shortening during aging (Shay & Wright, 2000; Brazel et al., 2005) have prevented an increased response from NSCs to changes such as injury. This is because NSCs in the middle-aged and aged hippocampi exhibit increased proliferation and neurogenesis in response to: (i) exogenous application of neurotrophic factors such as epidermal growth factor (EGF), FGF-2, and IGF-1 (Lichtenwalner et al., 2001; Jin et al., 2003; Rai et al., 2007); (ii) decreases in stress hormone levels induced through adrenalectomy (Cameron & McKay, 1999); and (iii) grafting of glial progenitors and neural stem cells (Hattiangady et al., 2007). Thus, the inability of the middle-aged and aged hippocampi to increase neurogenesis after injury does not appear to be due to age-related intrinsic changes in NSCs. In light of these, it is possible that injury to the middle-aged and aged hippocampi leads to a depletion in the number of NSCs, though normal aging does not alter the number or phenotype of NSCs in the DG (Hattiangady & Shetty, 2008).
Moreover, based on links observed between increased neurogenesis and multiple other changes in the young hippocampus after injury, the following two issues are of interest. The first issue, is, whether the unaltered DG neurogenesis after injury in the middle-aged and aged hippocampi is due to subdued up-regulation of factors that are known to be mitogenic to NSCs. A number of studies demonstrate that injury or seizures in the young hippocampus are associated with enhanced concentration of neurotrophic factors that are known to stimulate the production of new neurons from NSCs. These comprise BDNF, NGF, FGF-2, EGF, and VEGF (Lowenstein et al., 1993; Shetty et al., 2003, 2004; Hagihara et al., 2005). Interestingly, while injury increases the levels of BDNF in all age groups, the injured middle-aged and aged hippocampi contain 45–52% less BDNF than the lesioned young hippocampus (Shetty et al., 2004). Furthermore, unlike the injured young hippocampus, the IGF-1 does not seem to be up-regulated after injury in the aged hippocampus (Woods et al., 1998). Considering these, it is plausible that multiple other NSC mitogenic factors also display subdued response to injury in the middle-aged and aged hippocampi. This may include FGF-2, EGF, VEGF neurogenesin-1, wnt proteins, cysteine protease cystatin C expressed on reactive astrocytes and microglia, serotonin 1A receptors and neuropeptide Y (Radley & Jacobs, 2003; Ueki et al., 2003; Howell et al., 2005, 2007; Lie et al., 2005; Pirttila et al., 2005; Scharfman & Gray, 2006; Rai et al., 2007). Lower levels of neurotrophic factors in the injured middle-aged and aged hippocampi are also supported by the observation that pretreatment and grafting of fetal hippocampal cell grafts with neurotrophic factors FGF-2 and/or BDNF improves their survival in the injured middle-aged and aged hippocampi, in comparison to the poor survival observed for untreated fetal hippocampal cell grafts in these locations (Zaman & Shetty, 2002, 2003; Rao et al., 2006b). The reasons for subdued response of neurotrophic factors after injury in the middle-aged and aged hippocampi are unclear. However, it is interesting to note that an enhanced production of new neurons in the young hippocampus after injury closely parallels the phase of greatly increased number of nestin-positive reactive astrocytes (Abdel-Rahman et al., 2004). This is likely due to secretion of a variety of NSC mitogenic factors by nestin positive astrocytes. In contrast, the overall density of nestin-positive astrocytes was much less in the injured middle-aged and aged hippocampi (Abdel-Rahman et al., 2004). This alteration likely contributes to lower levels of neurotrophic factors and instructive signals for DG neurogenesis in the middle-aged and aged groups (Song et al., 2002). Thus, it appears that the injured middle-aged and aged hippocampi are incapable of increasing the concentration of critical neurotrophic factors to levels at which they can stimulate an increased proliferation of NSCs and produce an increased number of new neurons. However, the environment after injury in the middle-aged and aged hippocampi does not change either the neuronal fate-choice decision of newly born cells or the long-term survival of new neurons added to the SGZ-GCL, suggesting that these aspects of neurogenesis are independent of aging and injury in the hippocampus. Alternatively, it suggests that the concentration of the neurotrophic factors and signalling factors available is adequate for regulating these two aspects of neurogenesis.
The second issue is the role of increased neuronal activity on NSC proliferation and neurogenesis. A study by Deisseroth et al. (2004) demonstrates that increased excitatory stimuli acts directly on adult hippocampal NSCs and influences the production of new neurons and a mild reduction in net hippocampal excitatory activity through diazepam administration leads to reduced neurogenesis. Based on this, one may speculate that KA administration in middle-aged and aged rats is associated with reduced excitatory activity in comparison to the young hippocampus. Indeed, a study demonstrates that while both young and aged animals exhibit an increase in the EEG power during the KA treatment, visual inspection and spectral analysis reveal a reduction of the faster frequencies in the EEGs of aged animals despite a shorter latency to stage V seizures in comparison to young rats (Darbin et al., 2004). It is possible that this altered EEG activity after KA inadequately stimulates NSCs in the middle-aged and aged hippocampi and contributes to an unaltered DG neurogenesis following KA administration. Additional studies are needed in future to address the above possibilities.
Implications of loss of injury-induced NSC plasticity in the middle-aged and aged hippocampi
Studies show that the likelihood for chronic epilepsy in humans rises with aging, particularly after middle age (Eisenschenk & Gilmore, 1999; LaRoche & Helmers, 2003). The types of seizures mainly include partial complex and secondarily generalized. The primary reason for age-related rise in the incidence of chronic seizures appears to be the increase in structural lesions of the brain with aging such as stroke, head injury and tumors. Increased incidence of secondary seizures and cognitive impairments after brain injury in the elderly may be due to increased or progressive neuronal cell death and insufficient post-lesion plastic response after injury. These may include subdued up-regulation of neurotrophic factors and the endogenous anticonvulsants that support both residual neuron survival at the site of injury and also neurogenesis from NSCs, altered glial response and trophic activity, severe reductions in the numbers of inhibitory GABA-ergic interneurons and decreased synaptic reorganization (Woods et al., 1998; Shetty & Turner, 1999a,b, 2000a,b, 2001; Yurek & Fletcher-Turner, 2001; Abdel-Rahman et al., 2004; Shetty et al., 2004). In addition, unaltered DG neurogenesis in the middle-aged and aged groups after injury might contribute to an increased susceptibility of the hippocampus to secondary seizures. This is because an earlier study shows that ~14% of newly born neurons in the DG of adult rats differentiate into the inhibitory GABAergic basket cells (Liu et al., 2003). Since DG neurogenesis is already low during aging and is not up-regulated after injury, the addition of new GABAergic basket cells to the DG circuitry is likely to be minimal in the injured middle-aged and aged hippocampi. This may contribute to the persistence of reduced inhibitory neurotransmission in the DG of injured middle-aged and aged hippocampi.
On the other hand, studies suggest that increased neurogenesis and enhanced ectopic migration of newly born granule cells into the DH likely exacerbate the intensity of chronic epilepsy after injury or acute seizures (Scharfman & Gray, 2007). For example, studies suggest that ectopically migrated new granule cells: (i) retain granule cell intrinsic properties but get integrated abnormally into the CA3 network and exhibit activation during spontaneous seizures (Scharfman et al., 2000, 2002); (ii) exhibit robust responses following the perforant path stimulation but display a longer latency to onset of evoked responses suggesting polysynaptic activation of these cells following perforant path stimulation (Scharfman et al., 2003); and (iii) make synaptic contacts with mossy fibers through their dendrites (Pierce et al., 2005). Thus, ectopic granule cells develop a pattern of synaptic connections that predisposes them to discharge in epileptiform bursts. Additional studies suggest that preventing abnormal neurogenesis following acute seizures or injury may be useful for reducing the frequency and intensity of spontaneous seizures during chronic epilepsy (Jung et al., 2004). From the above perspectives, the observation that injury to middle-aged and aged hippocampi is not associated with increased ectopic migration of newly born neurons into the DH seems beneficial for reducing the incidence or intensity of chronic epilepsy after injury or seizures during middle age and old age. Additional studies are however, needed in the future to ascertain the impact of minimal ectopic granule cells observed in middle-aged and aged animals after injury on the development of chronic epilepsy. Furthermore, many studies show that the spatial memory performance in the aged rats predicts the level of dentate neurogenesis (van Praag et al., 2002; Drapeau et al., 2003, 2007; Bruel-Jungerman et al., 2005; Aimone et al., 2006; Siwak-Tapp et al., 2007), and newly formed neurons in the DG get involved into learning and memory circuits (Zhao et al., 2006; Ge et al., 2007; Toni et al., 2007). Since both middle-aged and aged injured hippocampi fail to increase the production of new neurons after injury, one might argue that cognitive impairments related to hippocampal injury are greater after middle age. However, it should be noted that increased production of new neurons in response to hippocampal injury is one of the features of DG plasticity. This phenomenon likely reflects an attempt of the endogenous stem/progenitor cells in the hippocampus to self-repair or to reduce the effects of injury on cognitive function. However, the newly formed cells differentiate mostly into dentate granule cells and do not give rise to CA3 pyramidal neurons, implying that replacement of lost CA3 pyramidal neurons does not occur in spite of increased production of new neurons. Nevertheless, the role of increased neurogenesis in reducing cognitive dysfunction after injury cannot be ruled out, as several studies have shown that an increased dentate neurogenesis is positively correlated with spatial learning and memory performance. Rigorous behavioral analyses of animals for hippocampal-dependent cognitive function with blocking of neurogenesis after injury are needed in the future to address this issue.
Additionally, studies on interventional strategies capable of increasing dentate neurogenesis in the injured middle-aged and aged hippocampi may be helpful to ascertain the links between injury-induced dentate neurogenesis, hyperexcitability and cognitive impairments during aging. Furthermore, if the interventional strategies after injury selectively increase the proportion of new granule cells in the granule cell layer, the overall effects may be advantageous. This is because an electrophysiological study in an electrical stimulation model of epilepsy reports that new granule cells that are added into the GCL after the status epilepticus exhibit reduced excitability (Jakubs et al., 2006). This phenomenon, if consistent and predominant, may be useful to mitigate dentate hyperexcitability after injury or the status epilepticus. However, it is currently not clear whether the phenomenon of reduced excitability of newly added granule cells is specific to the model investigated by Jakubs et al. (2006) or applicable to diverse models of epilepsy and brain injury.
Experimental procedures
Animals
Animals used in this study were obtained from the National Institutes of Aging colony of F344 rats maintained at Harlan Sprague–Dawley (Indianapolis, IN, USA). Six groups of rats were used for this study. These include intact young adult rats (4 months old, n = 12; group 1), intact middle-aged rats (12 months old, n = 12; group 2), intact aged rats (24 months old, n = 12; group 3), young adult rats receiving ICV KA (n = 12; Group 4), middle-aged rats receiving ICV KA (n = 12; group 5) and aged rats receiving ICV KA (n = 12; group 6). The animals were individually housed in an environmentally controlled room (~23 °C) with a 12: 12-h light-dark cycle, and were given food and water ad libitum. All experiments were performed as per the animal protocol approved by the institutional animal care and use committee of the Duke University Medical Center and the animal studies subcommittee of the Durham Veterans Affairs Medical Center.
Intracerebroventricular kainic acid administration
Kainic acid was administered to all rats belonging to the groups 4 – 6 (i.e. young adult, middle-aged and aged rats receiving ICV KA), using methods detailed elsewhere (Shetty & Turner, 1999a,b, 2000). Each rat was anesthetized through an intramuscular injection of an anesthetic mixture comprising ketamine (50 mg mL−1), xylazine (6 mg mL−1), and acepromazine (0.5 mg mL−1) at a dose of 1.25 mL kg−1 body weight, fixed into a stereotaxic apparatus with incisor bars set at 3.7 mm below the interaural line. Using aseptic techniques, the dorsal surface of the skull was exposed through a midline incision and a burr hole was drilled in the skull using the following stereotaxic coordinates: antero-posterior (AP) = 3.7 mm caudal to bregma; lateral (L) = 4.1 mm right lateral to the midline. A 10-μL Hamilton syringe (Hamilton; Reno, NV, USA) fitted with a 25-gauge needle and filled with KA solution in saline was placed over the burr hole using stereotaxis, and the needle was lowered through the brain into the lateral ventricle (equivalent to a depth of 4.5 mm from the surface of the brain). One microliter (containing 0.5 μg of KA) of the solution was injected slowly (0.2 μL min−1) into the lateral ventricle, the needle was left in place for 15 min and withdrawn slowly afterwards.
Administration of BrdU for labeling newly born cells in the dentate gyrus and harvesting of brain tissues
Both intact rats (groups 1–3) and rats receiving ICV KA (groups 4 – 6) received daily intraperitoneal injections of BrdU for 12 consecutive days at a dose of 100 mg kg−1 b.w. (Sigma, St Louis, MO, USA). In rats receiving ICV KA (groups 4 – 6), daily BrdU injections were commenced on post-KA day 4, and ended on post-KA day 15. In all groups, 50% of rats (n = 6 per group) were fatally anesthetized with halothane and perfused with 4% paraformaldehyde at 24 h after the last of 12 daily BrdU injections, and brains were collected for histological analyses of newly born cells and neurons that are added to the different regions of the DG over 12 days. The remaining 50% of rats in all groups (n = 6 per group) were fatally euthanized with halothane and perfused with 4% paraformaldehyde at 5 months after the last of 12 BrdU injections, and the brains were used for analyses of the long-term survival of newly born cells and neurons in the DG.
Analyses of hippocampal cytoarchitecture and inflammation after KA-induced injury
Every 15th section through the hippocampus in each of the rats belonging to different groups was stained for Nissl to ascertain the cytoarchitecture. In rats receiving KA, this analysis determined the extent of hippocampal injury following unilateral ICV KA administration in different age groups of rats. Furthermore, we quantified the extent of damage to CA3 and CA1 cell layers following ICV KA administration in the three age groups of rats. For this, we used Nissl-stained serial sections and measured the volume of injury in CA3 and CA1 cell layers using the StereoInvestigator (Microbrightfield, Inc., Williston, VT, USA). Moreover, as recent studies show that neurogenesis is sensitive to the extent of hippocampal inflammation (Ekdahl et al., 2003; Monje et al., 2003), we examined whether the extent of activated microglial cells following KA-induced injury varies in different groups. For this, we employed immunohistochemistry for ED-1, a specific marker of activated microglia. Serial sections (every 20th) through the entire hippocampus from all KA-treated rats were selected and processed for ED-1 immunohistochemistry using the ABC method (Hattiangady et al., 2004).
BrdU and DCX immunohistochemistry
The brains harvested from different groups following perfusions were post-fixed in 4% paraformaldehyde overnight at 4 °C and cryoprotected in 30% sucrose solution in phosphate buffer (PB). Thirty-micrometer-thick cryostat sections were cut coronally through the entire hippocampus and collected serially in PB. Serial sections (every 15th) through the entire hippocampus were selected in each animal belonging to different groups and processed for BrdU immunostaining using a monoclonal antibody to BrdU (Roche Diagnostics; Indianapolis, IN, USA). Another series (every 15th) of sections from animals killed at 24 h (groups 1–3) after the last of 12 daily BrdU injections were processed for DCX immunostaining using a polyclonal antibody to DCX (Santa Cruz Biotechnology; Santa Cruz, CA, USA) using avidin–biotin complex method described in our earlier studies (Rao & Shetty, 2004; Rao et al., 2005). The visualization of the peroxidase reaction was done using diaminobenzidine as the chromogen for BrdU and Vector Gray (Vector) as the chromogen for DCX (Vector Labs, Burlingame, CA, USA). The immunostained sections were mounted on gelatin-coated slides, air-dried, counter-stained with hematoxylin, dehydrated, cleared and cover-slipped.
Quantification of the total number of BrdU+ cells and DCX+ neurons in the dentate gyrus
In every animal belonging to each of the six groups (n = 5 per group) killed at two different time-points (24 h and 5 months after the last of 12 daily BrdU injections), BrdU+ cells in the dentate SGZ (two-cell-thick region from the inner margin of the dentate GCL) and the GCL were counted in every 15th section through the entire antero-posterior extent of the hippocampus. In addition, in every animal belonging to each of the six groups (n = 5 per group) killed at 24 h after the last of 12 daily BrdU injections, DCX+ neurons in the SGZ-GCL and the DH were counted separately. The counting of cells utilized the StereoInvestigator system (Microbrightfield Inc., Williston, VT, USA), consisting of a color digital video camera (Optronics Inc., Muskogee, OK, USA) interfaced with a Nikon E600 microscope (Nikon, Instruments, Melville, NY, USA).
In each animal, BrdU+/DCX+ cells were counted from 50 to 400 randomly and systematically selected frames (each measuring 40 × 40 μm, 0.0016 mm2 area) in every 15th section using the ×100 oil immersion lens. The detailed methodology employed for counting is detailed in our earlier reports (Rao & Shetty, 2004; Rao et al., 2005, 2006a). Briefly, in every section, the contour of the SGZ-GCL or DH regions was first delineated using the tracing function of the StereoInvestigator. Following this, the optical fractionator component was activated, and the number and location of counting frames and the counting depth for that section were determined by entering parameters such as the grid size, the thickness of the top guard zone (4 μm) and the optical dissector height (8 μm). A computer-driven motorized stage then allowed the section to be analyzed at each of the counting frame locations. In each location, all BrdU+/DCX+ cells that were present within the 8-μm section depths were counted. The StereoInvestigator program then calculated the total number of BrdU+/DCX+ cells per SGZ-GCL and the DH by utilizing the optical fractionator formula, N = 1/ssf.1/asf.1/hsf.EQ−. ssf stands for section sampling fraction, which was 15 in this study as every 15th section was sampled; asf stands for area sampling fraction, which is calculated by dividing the area sampled with the total area of the SGZ-GCL or DH (i.e. the sum of SGZ-GCL or DH areas sampled in every 15th section); hsf stands for the height sampling fraction, which is calculated by dividing the height sampled (i.e. 8 μm in this study) with the section thickness at the time of analysis (i.e. 19 – 20 μm in young adults and 15–16 μm in middle-aged and aged animals); and EQ− denotes the total count of particles sampled for the entire DG.
Measurement of neuronal differentiation of newly born cells via DCX and BrdU dual immunostaining
To measure the percentages of newly born cells (BrdU+ cells) that differentiate into neurons in the SGZ-GCL, sections from animals belonging to all six groups perfused at 24 h after the last of 12 daily BrdU injections were processed for DCX and BrdU dual immunostaining. The sections were first processed for DCX immunostaining using the ABC method (Rao et al., 2005, 2006a) and the immunoreaction was developed using Vector Gray (Vector), which gave bluish gray reaction product in the cytoplasm of both soma and dendrites of DCX+ neurons. Sections were then processed for BrdU immunostaining and the BrdU immunoreaction was visualized using diaminobenzidine, which gave brown color to BrdU-positive nuclei. Since DCX and BrdU immunostaining occurred in two different cell compartments, the reaction product resulting from DCX and BrdU could be clearly distinguished. The percentages of BrdU+ cells expressing DCX were calculated in all groups to ascertain the neuronal fate-choice of newly born cells. Furthermore, to confirm that vast majority of DCX+ neurons observed at 24 h after the last of 12 daily BrdU injections in different groups were born during the BrdU injection period as observed in intact animals earlier (Rao & Shetty, 2004; Rao et al., 2005), the percentages of DCX+ neurons expressing BrdU were also calculated in all six groups. These measurements were accomplished through exhaustive counting of total DCX+ cells, total BrdU+ cells, and the dual labeled cells (i.e. cells positive for both DCX and BrdU) present in the SGZ-GCL. Six to eight sections were used from each animal and the counting was performed at a magnification of ×400 using a ×40 lens. As described in our earlier reports, the above two-chromogen method is as efficient as dual immunofluorescence method for identifying BrdU+ cells expressing DCX or DCX+ cells expressing BrdU (Rao & Shetty, 2004; Rao et al., 2005). This was confirmed through sequential dual immunofluorescence analyses of cells positive for BrdU and DCX in representative tissue sections from animals belonging to different groups.
Analyses of NeuN+ neurons among newly added cells that exhibit 5-month survival
To visualize the fractions of BrdU+ cells that are neurons among BrdU+ cells that exhibit 5-month survival, representative sections from all groups were processed for BrdU and NeuN dual immunofluorescence staining. The sections were first processed for various BrdU preincubation treatments (Rao & Shetty, 2004; Rao et al., 2005, 2006a), washed in Tris-buffered saline (TBS), blocked in normal serum, incubated in a cocktail solution containing rat anti-BrdU (1: 50, Accurate Chemicals, Westbury, NY, USA) and mouse anti-NeuN (1: 1000), and washed in TBS. The sections were then treated with a mixture of goat antimouse IgG tagged with Alexa Fluor 488 (Invitrogen, Carlsbad, CA, USA) and biotinylated rabbit antirat IgG (Vector), washed in TBS, incubated in streptavidin Texas red, rinsed in TBS, and cover-slipped with slow fade/antifade mounting medium (Invitrogen, Carlsbad, CA, USA). Cells that exhibited BrdU and NeuN coexpression were identified using a Nikon E600 fluorescence microscope. The fractions of BrdU+ cells that express NeuN were then quantified by examination of individual BrdU+ cells at ×400 (n = 4 per age group, 50–100 cells per animal) in confocal laser scanning microscopes (LSM 410 and LSM 510). For this, 1-μm-thick optical Z-sections were sampled from different regions of the SGZ-GCL in all groups, and the images were analyzed using LSM image browser (Carl Zeiss, Thornwood, NY, USA).
Statistical analyses
For every parameter, the average value was first calculated separately for each animal before the means and standard errors were determined for the total number of animals included per group. The values from different age groups of animals were compared using two-way ANOVA.
Acknowledgments
This research was supported by grants from the National Institute for Aging (R01 AG20924 to A.K.S.), the National Institute of Neurological Disorders and Stroke (R01 NS054780 to A.K.S.) and Department of Veterans Affairs (VA Merit Review Award to A.K.S.).
References
- Abdel-Rahman A, Rao MS, Shetty AK. Nestin expression in hippocampal astrocytes after injury depends on the age of the hippocampus. Glia. 2004;47:299– 313. doi: 10.1002/glia.20047. [DOI] [PubMed] [Google Scholar]
- Aimone JB, Wiles J, Gage FH. Potential role for adult neurogenesis in the encoding of time in new memories. Nat Neurosci. 2006;9:723– 727. doi: 10.1038/nn1707. [DOI] [PubMed] [Google Scholar]
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319– 335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Bengzon J, Kokaia Z, Elmer E, Nanobashvili A, Kokaia M, Lindvall O. Apoptosis and proliferation of dentate gyrus neurons after single and intermittent limbic seizures. Proc Natl Acad Sci U S A. 1997;94:10432–10437. doi: 10.1073/pnas.94.19.10432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal GM, Peterson DA. Neural stem cells as therapeutic agents for age-related brain repair. Aging Cell. 2004;3:345– 351. doi: 10.1111/j.1474-9728.2004.00132.x. [DOI] [PubMed] [Google Scholar]
- Brazel CY, Limke TL, Osborne JK, Miura T, Cai J, Pevny L, Rao MS. Sox2 expression defines a heterogeneous population of neurosphere-forming cells in the adult murine brain. Aging Cell. 2005;4:197– 207. doi: 10.1111/j.1474-9726.2005.00158.x. [DOI] [PubMed] [Google Scholar]
- Bruel-Jungerman E, Laroche S, Rampon C. New neurons in the dentate gyrus are involved in the expression of enhanced long-term memory following environmental enrichment. Eur J Neurosci. 2005;21:513– 521. doi: 10.1111/j.1460-9568.2005.03875.x. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Restoring production of hippocampal neurons in old age. Nat Neurosci. 1999;2:894– 897. doi: 10.1038/13197. [DOI] [PubMed] [Google Scholar]
- Chadashvili T, Peterson DA. Cytoarchitecture of fibroblast growth factor receptor 2 (FGFR-2) immunoreactivity in astrocytes of neurogenic and non-neurogenic regions of the young adult and aged rat brain. J Comp Neurol. 2006;498:1–15. doi: 10.1002/cne.21009. [DOI] [PubMed] [Google Scholar]
- Darbin O, Naritoku D, Patrylo PR. Aging alters electroencephalographic and clinical manifestations of kainate-induced status epilepticus. Epilepsia. 2004;45:1219–1227. doi: 10.1111/j.0013-9580.2004.66103.x. [DOI] [PubMed] [Google Scholar]
- Darsalia V, Heldmann U, Lindvall O, Kokaia Z. Stroke-induced neurogenesis in aged brain. Stroke. 2005;36:1790–1795. doi: 10.1161/01.STR.0000173151.36031.be. [DOI] [PubMed] [Google Scholar]
- Deisseroth K, Singla S, Toda H, Monje M, Palmer TD, Malenka RC. Excitation-neurogenesis coupling in adult neural stem/progenitor cells. Neuron. 2004;42:535– 552. doi: 10.1016/s0896-6273(04)00266-1. [DOI] [PubMed] [Google Scholar]
- Drapeau E, Mayo W, Aurousseau C, Le Moal M, Piazza PV, Abrous DN. Spatial memory performances of aged rats in the water maze predict levels of hippocampal neurogenesis. Proc Natl Acad Sci U S A. 2003;100:14385–14390. doi: 10.1073/pnas.2334169100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapeau E, Montaron MF, Aguerre S, Abrous DN. Learning-induced survival of new neurons depends on the cognitive status of aged rats. J Neurosci. 2007;27:6037– 6044. doi: 10.1523/JNEUROSCI.1031-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenschenk S, Gilmore R. Strategies for successful management of older patients with seizures. Geriatrics. 1999;54:31,34,39–31,34,40. passim. [PubMed] [Google Scholar]
- Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci U S A. 2003;100:13632–13637. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Feng R, Rampon C, Tang YP, Shrom D, Jin J, Kyin M, Sopher B, Miller MW, Ware CB, Martin GM, Kim SH, Langdon RB, Sisodia SS, Tsien JZ. Deficient neurogenesis in forebrain-specific presenilin-1 knockout mice is associated with reduced clearance of hippocampal memory traces. Neuron. 2001;32:911–926. doi: 10.1016/s0896-6273(01)00523-2. [DOI] [PubMed] [Google Scholar]
- Ge S, Yang CH, Hsu KS, Ming GL, Song H. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron. 2007;54:559– 566. doi: 10.1016/j.neuron.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert ME, Kelly ME, Samsam TE, Goodman JH. (200%) Chronic developmental lead exposure reduces neurogenesis in adult rat hippocampus but does not impair spatial learning. Toxicol Sci. 2007;86:365– 374. doi: 10.1093/toxsci/kfi156. [DOI] [PubMed] [Google Scholar]
- Gong C, Wang TW, Huang HS, Parent JM. Reelin regulates neuronal progenitor migration in intact and epileptic hippocampus. J Neurosci. 2007;27:1803–1811. doi: 10.1523/JNEUROSCI.3111-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E. Serotonin and hippocampal neurogenesis. Neuropsychopharmacology. 1999;21:46S– 51S. doi: 10.1016/S0893-133X(99)00045-7. [DOI] [PubMed] [Google Scholar]
- Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci. 1997;17:2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Reeves AJ, Fallah M, Tanapat P, Gross CG, Fuchs E. Hippocampal neurogenesis in adult Old World primates. Proc Natl Acad Sci U S A. 1999a;96:5263– 5267. doi: 10.1073/pnas.96.9.5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Tanapat P, Hastings NB, Shors TJ. Neurogenesis in adulthood: a possible role in learning. Trends Cogn Sci. 1999b;3:186–192. doi: 10.1016/s1364-6613(99)01310-8. [DOI] [PubMed] [Google Scholar]
- Gray WP, Sundstrom LE. Kainic acid increases the proliferation of granule cell progenitors in the dentate gyrus of the adult rat. Brain Res. 1998;790:52– 59. doi: 10.1016/s0006-8993(98)00030-4. [DOI] [PubMed] [Google Scholar]
- Hagihara H, Hara M, Tsunekawa K, Nakagawa Y, Sawada M, Nakano K. Tonic-clonic seizures induce division of neuronal progenitor cells with concomitant changes in expression of neurotrophic factors in the brain of pilocarpine-treated mice. Brain Res. 2005;39:258–266. doi: 10.1016/j.molbrainres.2005.05.031. [DOI] [PubMed] [Google Scholar]
- Hattiangady B, Rao MS, Shetty AK. Chronic temporal lobe epilepsy is associated with severely declined dentate neurogenesis in the adult hippocampus. Neurobiol Dis. 2004;17:473– 490. doi: 10.1016/j.nbd.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Hattiangady B, Rao MS, Shetty GA, Shetty AK. Brain-derived neurotrophic factor, phosphorylated cyclic AMP response element binding protein and neuropeptide Y decline as early as middle age in the dentate gyrus and CA1 and CA3 subfields of the hippocampus. Exp Neurol. 2005;195:353– 371. doi: 10.1016/j.expneurol.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Hattiangady B, Shetty AK. Aging does not alter the number or phenotype of putative stem/progenitor cells in the neurogenic region of the hippocampus. Neurobiol Aging. 2008;29:129–147. doi: 10.1016/j.neurobiolaging.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattiangady B, Shuai B, Cai J, Coksaygan T, Rao MS, Shetty AK. Increased dentate neurogenesis after grafting of glial restricted progenitors or neural stem cells in the aging hippocampus. Stem Cells. 2007;25:2104– 2117. doi: 10.1634/stemcells.2006-0726. [DOI] [PubMed] [Google Scholar]
- He J, Nixon K, Shetty AK, Crews FT. Chronic alcohol exposure reduces hippocampal neurogenesis and dendritic growth of newborn neurons. Eur J Neurosci. 2005;21:2711–2720. doi: 10.1111/j.1460-9568.2005.04120.x. [DOI] [PubMed] [Google Scholar]
- Howell OW, Doyle K, Goodman JH, Scharfman HE, Herzog H, Pringle A, Beck-Sickinger AG, Gray WP. Neuropeptide Y stimulates neuronal precursor proliferation in the post-natal and adult dentate gyrus. J Neurochem. 2005;93:560– 570. doi: 10.1111/j.1471-4159.2005.03057.x. [DOI] [PubMed] [Google Scholar]
- Howell OW, Silva S, Scharfman HE, Sosunov AA, Zaben M, Shatya A, McKhann G, Herzog H, 2nd, Laskowski A, Gray WP. Neuropeptide Y is important for basal and seizure-induced precursor cell proliferation in the hippocampus. Neurobiol Dis. 2007;26:174–188. doi: 10.1016/j.nbd.2006.12.014. [DOI] [PubMed] [Google Scholar]
- Jakubs K, Nanobashvili A, Bonde S, Ekdahl CT, Kokaia Z, Kokaia M, Lindvall O. Environment matters: synaptic properties of neurons born in the epileptic adult brain develop to reduce excitability. Neuron. 2006;52:1047–1059. doi: 10.1016/j.neuron.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Jessberger S, Zhao C, Toni N, Clemenson GD, Jr, Li Y, Gage FH. Seizure-associated, aberrant neurogenesis in adult rats characterized with retrovirus-mediated cell labeling. J Neurosci. 2007;27:9400– 9407. doi: 10.1523/JNEUROSCI.2002-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Minami M, Lan JQ, Mao XO, Batteur S, Simon RP, Greenberg DA. Neurogenesis in dentate subgranular zone and rostral sub-ventricular zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci U S A. 2001;98:4710– 4715. doi: 10.1073/pnas.081011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Minami M, Xie L, Sun Y, Mao XO, Wang Y, Simon RP, Greenberg DA. Ischemia-induced neurogenesis is preserved but reduced in the aged rodent brain. Aging Cell. 2004;3:373– 377. doi: 10.1111/j.1474-9728.2004.00131.x. [DOI] [PubMed] [Google Scholar]
- Jin K, Sun Y, Xie L, Batteur S, Mao XO, Smelick C, Logvinova A, Greenberg DA. Neurogenesis and aging: FGF-2 and HB-EGF restore neurogenesis in hippocampus and subventricular zone of aged mice. Aging Cell. 2003;2:175–183. doi: 10.1046/j.1474-9728.2003.00046.x. [DOI] [PubMed] [Google Scholar]
- Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci U S A. 2002;99:11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung KH, Chu K, Kim M, Jeong SW, Song YM, Lee ST, Kim JY, Lee SK, Roh JK. Continuous cytosine-b-D-arabinofuranoside infusion reduces ectopic granule cells in adult rat hippocampus with attenuation of spontaneous recurrent seizures following pilocarpine-induced status epilepticus. Eur J Neurosci. 2004;19:3219– 3226. doi: 10.1111/j.0953-816X.2004.03412.x. [DOI] [PubMed] [Google Scholar]
- Kee N, Teixeira CM, Wang AH, Frankland PW. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci. 2007;10:355– 362. doi: 10.1038/nn1847. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Brandon EP, Gage FH. Environmental stimulation of 129/SvJ mice causes increased cell proliferation and neurogenesis in the adult dentate gyrus. Curr Biol. 1998;8:939– 942. doi: 10.1016/s0960-9822(07)00377-6. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Gage FH. Neuroplasticity in old age: sustained fivefold induction of hippocampal neurogenesis by long-term environmental enrichment. Ann Neurol. 2002;52:135–143. doi: 10.1002/ana.10262. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. 2003;130:391–399. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- Kleindienst A, McGinn MJ, Harvey HB, Colello RJ, Hamm RJ, Bullock MR. Enhanced hippocampal neurogenesis by intraventricular S100B infusion is associated with improved cognitive recovery after traumatic brain injury. J Neurotrauma. 2005;22:645– 655. doi: 10.1089/neu.2005.22.645. [DOI] [PubMed] [Google Scholar]
- Kornack DR, Rakic P. Continuation of neurogenesis in the hippocampus of the adult macaque monkey. Proc Natl Acad Sci U S A. 1999;96:5768– 5773. doi: 10.1073/pnas.96.10.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027– 2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRoche SM, Helmers SL. Epilepsy in the elderly. Neurologist. 2003;9:241–249. doi: 10.1097/01.nrl.0000087719.64343.be. [DOI] [PubMed] [Google Scholar]
- Lee J, Duan W, Mattson MP. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J Neurochem. 2002;82:1367–1375. doi: 10.1046/j.1471-4159.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- Leuner B, Gould E, Shors TJ. Is there a link between adult neurogenesis and learning? Hippocampus. 2006;16:216– 224. doi: 10.1002/hipo.20153. [DOI] [PubMed] [Google Scholar]
- Lichtenwalner RJ, Forbes ME, Bennett SA, Lynch CD, Sonntag WE, Riddle DR. Intracerebroventricular infusion of insulin-like growth factor-I ameliorates the age-related decline in hippocampal neurogenesis. Neuroscience. 2001;107:603– 613. doi: 10.1016/s0306-4522(01)00378-5. [DOI] [PubMed] [Google Scholar]
- Lie DC, Colamarino SA, Song HJ, Desire L, Mira H, Consiglio A, Lein ES, Jessberger S, Lansford H, Dearie AR, Gage FH. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437:1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- Liu J, Solway K, Messing RO, Sharp FR. Increased neurogenesis in the dentate gyrus after transient global ischemia in gerbils. J Neurosci. 1998;18:7768– 7778. doi: 10.1523/JNEUROSCI.18-19-07768.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Wang J, Zhu D, Fu Y, Lukowiak K, Lu YM. Generation of functional inhibitory neurons in the adult rat hippocampus. J Neurosci. 2003;23:732– 736. doi: 10.1523/JNEUROSCI.23-03-00732.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenstein DH, Seren MS, Longo FM. Prolonged increases in neurotrophic activity associated with kainate-induced hippocampal synaptic reorganization. Neuroscience. 1993;56:597– 604. doi: 10.1016/0306-4522(93)90359-n. [DOI] [PubMed] [Google Scholar]
- Mirescu C, Peters JD, Gould E. Early life experience alters response of adult neurogenesis to stress. Nat Neurosci. 2004;7:841–846. doi: 10.1038/nn1290. [DOI] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Montaron MF, Piazza PV, Aurousseau C, Urani A, Le Moal M, Abrous DN. Implication of corticosteroid receptors in the regulation of hippocampal structural plasticity. Eur J Neurosci. 2003;18:3105– 3111. doi: 10.1111/j.1460-9568.2003.03048.x. [DOI] [PubMed] [Google Scholar]
- Nacher J, Alonso-Llosa G, Rosell DR, McEwen BS. NMDA receptor antagonist treatment increases the production of new neurons in the aged rat hippocampus. Neurobiol Aging. 2003;24:273– 284. doi: 10.1016/s0197-4580(02)00096-9. [DOI] [PubMed] [Google Scholar]
- Nakagawa E, Aimi Y, Yasuhara O, Tooyama I, Shimada M, McGeer PL, Kimura H. Enhancement of progenitor cell division in the dentate gyrus triggered by initial limbic seizures in rat models of epilepsy. Epilepsia. 2000;41:10–18. doi: 10.1111/j.1528-1157.2000.tb01498.x. [DOI] [PubMed] [Google Scholar]
- Nilsson M, Perfilieva E, Johansson U, Orwar O, Eriksson PS. Enriched environment increases neurogenesis in the adult rat dentate gyrus and improves spatial memory. J Neurobiol. 1999;39:569– 578. doi: 10.1002/(sici)1097-4695(19990615)39:4<569::aid-neu10>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Parent JM. Injury-induced neurogenesis in the adult mammalian brain. Neuroscientist. 2003;9:261–272. doi: 10.1177/1073858403252680. [DOI] [PubMed] [Google Scholar]
- Parent JM, Elliott RC, Pleasure SJ, Barbaro NM, Lowenstein DH. Aberrant seizure-induced neurogenesis in experimental temporal lobe epilepsy. Ann Neurol. 2006;59:81–91. doi: 10.1002/ana.20699. [DOI] [PubMed] [Google Scholar]
- Parent JM, Yu TW, LeibowitZ RT, Geschwind DH, Sloviter RS, Lowenstein DH. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci. 1997;17:3727– 3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, Smith PK. Models of visuospatial and verbal memory across the adult life span. Psychol Aging. 2002;17:299– 320. [PubMed] [Google Scholar]
- Pierce JP, Melton J, Punsoni M, McCloskey DP, Scharfman HE. Mossy fibers are the primary source of afferent input to ectopic granule cells that are born after pilocarpine-induced seizures. Exp Neurol. 2005;196:316– 331. doi: 10.1016/j.expneurol.2005.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirttila TJ, Lukasiuk K, Hakansson K, Grubb A, Abrahamson M, Pitkanen A. Cystatin C modulates neurodegeneration and neurogenesis following status epilepticus in mouse. Neurobiol Dis. 2005;20:241–253. doi: 10.1016/j.nbd.2005.03.006. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266– 270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Jacobs BL. Pilocarpine-induced status epilepticus increases cell proliferation in the dentate gyrus of adult rats via a 5-HT1A receptor-dependent mechanism. Brain Res. 2003;966:1–12. doi: 10.1016/s0006-8993(02)03989-6. [DOI] [PubMed] [Google Scholar]
- Rai K, Hattiangady B, Shetty AK. Enhanced production and dendritic growth of new dentate granule cells in the middle-aged and aged hippocampus following intracerebroventricular FGF-2 infusions. Eur J Neurosci. 2007;26:1765– 1779. doi: 10.1111/j.1460-9568.2007.05820.x. [DOI] [PubMed] [Google Scholar]
- Rao MS, Hattiangady B, Abdel-Rahman A, Stanley DP, Shetty AK. Newly born cells in the ageing dentate gyrus display normal migration, survival and neuronal fate choice but endure retarded early maturation. Eur J Neurosci. 2005;21:464– 476. doi: 10.1111/j.1460-9568.2005.03853.x. [DOI] [PubMed] [Google Scholar]
- Rao MS, Hattiangady B, Reddy DS, Shetty AK. Hippocampal neurodegeneration, spontaneous seizures, and mossy fiber sprouting in the F344 rat model of temporal lobe epilepsy. J Neurosci Res. 2006c;83:1088–1105. doi: 10.1002/jnr.20802. [DOI] [PubMed] [Google Scholar]
- Rao MS, Hattiangady B, Shetty AK. The window and mechanisms of major age-related decline in the production of new neurons within the dentate gyrus of the hippocampus. Aging Cell. 2006a;5:545– 558. doi: 10.1111/j.1474-9726.2006.00243.x. [DOI] [PubMed] [Google Scholar]
- Rao MS, Hattiangady B, Shetty AK. Fetal hippocampal CA3 cell grafts enriched with FGF-2 and BDNF exhibit robust long-term survival and integration and suppress aberrant mossy fiber sprouting in the injured middle-aged hippocampus. Neurobiol Dis. 2006b;21:276– 290. doi: 10.1016/j.nbd.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Rao MS, Shetty AK. Efficacy of doublecortin as a marker to analyse the absolute number and dendritic growth of newly generated neurons in the adult dentate gyrus. Eur J Neurosci. 2004;19:234– 246. doi: 10.1111/j.0953-816x.2003.03123.x. [DOI] [PubMed] [Google Scholar]
- Rex CS, Kramar EA, Colgin LL, Lin B, Gall CM, Lynch G. Long-term potentiation is impaired in middle-aged rats: regional specificity and reversal by adenosine receptor antagonists. J Neurosci. 2005;25:5956–5966. doi: 10.1523/JNEUROSCI.0880-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rex CS, Lauterborn JC, Lin CY, Kramar EA, Rogers GA, Gall CM, Lynch G. Restoration of long-term potentiation in middle-aged hippocampus after induction of brain-derived neurotrophic factor. J Neurophysiol. 2006;96:677– 685. doi: 10.1152/jn.00336.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sairanen M, Lucas G, Ernfors P, Castren M, Castren E. Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. J Neurosci. 2005;25:1089–1094. doi: 10.1523/JNEUROSCI.3741-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805– 809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, Goodman JH, Sollas AL. Granule-like neurons at the hilar/CA3 border after status epilepticus and their synchrony with area CA3 pyramidal cells: functional implications of seizure-induced neurogenesis. J Neurosci. 2000;20:6144– 6158. doi: 10.1523/JNEUROSCI.20-16-06144.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Gray WP. Plasticity of neuropeptide Y in the dentate gyrus after seizures, and its relevance to seizure-induced neurogenesis. EXS. 2006:193–211. doi: 10.1007/3-7643-7417-9_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Gray WP. Relevance of seizure-induced neurogenesis in animal models of epilepsy to the etiology of temporal lobe epilepsy. Epilepsia. 2007;48 (Suppl 2):33– 41. doi: 10.1111/j.1528-1167.2007.01065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Sollas AE, Berger RE, Goodman JH, Pierce JP. Perforant path activation of ectopic granule cells that are born after pilocarpine-induced seizures. Neuroscience. 2003;121:1017–1029. doi: 10.1016/s0306-4522(03)00481-0. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, Sollas AL, Goodman JH. Spontaneous recurrent seizures after pilocarpine-induced status epilepticus activate calbindin-immunoreactive hilar cells of the rat dentate gyrus. Neuroscience. 2002;111:71–81. doi: 10.1016/s0306-4522(01)00599-1. [DOI] [PubMed] [Google Scholar]
- Shay JW, Wright WE. Hayflick, his limit, and cellular ageing. Nat Rev Mol Cell Biol. 2000;1:72– 76. doi: 10.1038/35036093. [DOI] [PubMed] [Google Scholar]
- Shetty AK, Hattiangady B. Prospects of stem cell therapy for temporal lobe epilepsy. Stem Cells. 2007;25:2396– 2407. doi: 10.1634/stemcells.2007-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty AK, Hattiangady B, Shetty GA. Stem/progenitor cell proliferation factors FGF-2, IGF-1, and VEGF exhibit early decline during the course of aging in the hippocampus: role of astrocytes. Glia. 2005a;51:173–186. doi: 10.1002/glia.20187. [DOI] [PubMed] [Google Scholar]
- Shetty AK, Rao MS, Hattiangady B, Zaman V, Shetty GA. Hippocampal neurotrophin levels after injury: relationship to the age of the hippocampus at the time of injury. J Neurosci Res. 2004;78:520– 532. doi: 10.1002/jnr.20302. [DOI] [PubMed] [Google Scholar]
- Shetty AK, Turner DA. Aging impairs axonal sprouting response of dentate granule cells following _target loss and partial deafferentation. J Comp Neurol. 1999a;414:238– 254. doi: 10.1002/(sici)1096-9861(19991115)414:2<238::aid-cne7>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Shetty AK, Turner DA. Vulnerability of the dentate gyrus to aging and intracerebroventricular administration of kainic acid. Exp Neurol. 1999b;158:491–503. doi: 10.1006/exnr.1999.7107. [DOI] [PubMed] [Google Scholar]
- Shetty AK, Turner DA. Fetal hippocampal grafts containing CA3 cells restore host hippocampal glutamate decarboxylase-positive interneuron numbers in a rat model of temporal lobe epilepsy. J Neurosci. 2000;20:8788– 8801. doi: 10.1523/JNEUROSCI.20-23-08788.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty AK, Turner DA. Glutamic acid decarboxylase-67-positive hippocampal interneurons undergo a permanent reduction in number following kainic acid-induced degeneration of ca3 pyramidal neurons. Exp Neurol. 2001;169:276– 297. doi: 10.1006/exnr.2001.7668. [DOI] [PubMed] [Google Scholar]
- Shetty AK, Zaman V, Hattiangady B. Repair of the injured adult hippocampus through graft-mediated modulation of the plasticity of the dentate gyrus in a rat model of temporal lobe epilepsy. J Neurosci. 2005b;25:8391–8401. doi: 10.1523/JNEUROSCI.1538-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty AK, Zaman V, Shetty GA. Hippocampal neurotrophin levels in a kainate model of temporal lobe epilepsy: a lack of correlation between brain-derived neurotrophic factor content and progression of aberrant dentate mossy fiber sprouting. J Neurochem. 2003;87:147–159. doi: 10.1046/j.1471-4159.2003.01979.x. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372– 376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002;12:578– 584. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siwak-Tapp CT, Head E, Muggenburg BA, Milgram NW, Cotman CW. Neurogenesis decreases with age in the canine hippocampus and correlates with cognitive function. Neurobiol Learn Mem. 2007;88:249– 259. doi: 10.1016/j.nlm.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417:39– 44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- Sun Y, Jin K, Childs JT, Xie L, Mao XO, Greenberg DA. Vascular endothelial growth factor-B (VEGFB) stimulates neurogenesis: evidence from knockout mice and growth factor administration. Dev Biol. 2006;289:329– 335. doi: 10.1016/j.ydbio.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Sun Y, Jin K, Xie L, Childs J, Mao XO, Logvinova A, Greenberg DA. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest. 2003;111:1843–1851. doi: 10.1172/JCI17977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, McGinn MJ, Zhou Z, Harvey HB, Bullock MR, Colello RJ. Anatomical integration of newly generated dentate granule neurons following traumatic brain injury in adult rats and its association to cognitive recovery. Exp Neurol. 2007;204:264– 272. doi: 10.1016/j.expneurol.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Tang H, Wang Y, Xie L, Mao X, Won SJ, Galvan V, Jin K. Effect of neural precursor proliferation level on neurogenesis in rat brain during aging and after focal ischemia. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.06.004. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toni N, Teng EM, Bushong EA, Aimone JB, Zhao C, Consiglio A, van Praag H, Martone ME, Ellisman MH, Gage FH. Synapse formation on neurons born in the adult hippocampus. Nat Neurosci. 2007;10:727– 734. doi: 10.1038/nn1908. [DOI] [PubMed] [Google Scholar]
- Ueki T, Tanaka M, Yamashita K, Mikawa S, Qiu Z, Maragakis NJ, Hevner RF, Miura N, Sugimura H, Sato K. A novel secretory factor, neuro-genesin-1, provides neurogenic environmental cues for neural stem cells in the adult hippocampus. J Neurosci. 2003;23:11732–11740. doi: 10.1523/JNEUROSCI.23-37-11732.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods AG, Guthrie KM, Kurlawalla MA, Gall CM. Deafferentation-induced increases in hippocampal insulin-like growth factor-1 messenger RNA expression are severely attenuated in middle aged and aged rats. Neuroscience. 1998;83:663– 668. doi: 10.1016/s0306-4522(97)00539-3. [DOI] [PubMed] [Google Scholar]
- Yoshimura S, Takagi Y, Harada J, Teramoto T, Thomas SS, Waeber C, Bakowska JC, Breakefield XO, MoskowitZ MA. FGF-2 regulation of neurogenesis in adult hippocampus after brain injury. Proc Natl Acad Sci U S A. 2001;98:5874– 5879. doi: 10.1073/pnas.101034998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurek DM, Fletcher-Turner A. Differential expression of GDNF, BDNF, and NT-3 in the aging nigrostriatal system following a neurotoxic lesion. Brain Res. 2001;891:228– 235. doi: 10.1016/s0006-8993(00)03217-0. [DOI] [PubMed] [Google Scholar]
- Zaman V, Shetty AK. Combined neurotrophic supplementation and caspase inhibition enhances survival of fetal hippocampal CA3 cell grafts in lesioned CA3 region of the aging hippocampus. Neuroscience. 2002;109:537– 553. doi: 10.1016/s0306-4522(01)00478-x. [DOI] [PubMed] [Google Scholar]
- Zaman V, Shetty AK. Fetal hippocampal CA3 cell grafts enriched with fibroblast growth factor-2 exhibit enhanced neuronal integration into the lesioned aging rat hippocampus in a kainate model of temporal lobe epilepsy. Hippocampus. 2003;13:618– 632. doi: 10.1002/hipo.10091. [DOI] [PubMed] [Google Scholar]
- Zhao C, Teng EM, Summers RG, Jr, Ming GL, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26:3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]