Abstract
BACKGROUND & AIMS
Germline PTEN mutations cause Cowden syndrome (CS), associated with breast and thyroid cancers. Case reports found 35–85% of CS patients had gastrointestinal (GI) hamartomas. The association of benign and malignant GI neoplasias with CS remains debatable. Our goal is to describe the GI phenotype in a prospective series of PTEN mutation carriers.
METHODS
Patients who met relaxed International Cowden Consortium criteria (N=2548) or with ≥5 GI polyps, ≥1 of which was hyperplastic or hamartomatous (N=397) were prospectively recruited. Germline PTEN mutation/deletion analysis was performed. Of the 2945, 127 patients having clear pathogenic PTEN mutations (123/2548+4/397) were eligible for this study. EGD and colonoscopy were performed and pathology reports reviewed. Fisher’s 2-tailed exact test, unpaired t-tests, and age- and gender-adjusted SIR were calculated.
RESULTS
Of 127 PTEN mutation carriers, 67 underwent ≥1 endoscopy with 62 (95%) having polyps, making GI polyps the second most common feature, after macrocephaly (74.8%). Of the 65, half had hyperplastic polyps and ¼ each with hamartomatous, ganglioneuromatous or adenomatous polyps. There were one to “innumerable” polyps in the colorectum, ileum, duodenum, stomach and/or esophagus, with 24 subjects having both upper and lower GI polyps. Nine (13%) subjects had colorectal cancer, all under the age of 50. The adjusted SIR was 224.1 (95%CI 109.3–411.3, p<0.0001). Cancers were commonly associated with adenomatous and/or hyperplastic polyps. One had gastric signet ring cell carcinoma.
CONCLUSIONS
PTEN-associated CS should be considered a mixed polyp syndrome, with hyperplastic polyps most prevalent, and a risk of early-onset colorectal cancer. Routine colonoscopy should be considered in PTEN-associated CS especially in the context of hyperplastic and/or adenomatous polyps.
Keywords: colorectal cancer, Cowden syndrome, hamartomatous polyposis, PTEN
Background
Cowden syndrome is often considered a rare hamartomatous polyposis syndrome caused by germline alterations in the tumor suppressor gene, PTEN.1 It is thought to occur in 1 in 200,000 individuals; however, experts believe this is likely an underestimate because of the variable expression and subtle physical manifestations.2 CS is one of the disorders that comprises the PTEN hamartoma tumor syndrome.3 It is an autosomal dominant disorder that is characterized by mucocutaneous lesions, macrocephaly, and an increased risk of benign and malignant diseases of the breast, thyroid, and endometrium.2, 3 Although the gastrointestinal (GI) tract is affected in individuals with CS, it has not been systematically assessed.
Published case reports and highly selected small series reveal that 35–85% of Cowden syndrome patients had GI hamartomatous polyps.4–9 Although the majority of patients have been described to have hamartomatous polyps, they have also been reported to have ganglioneuromatous polyps, colonic lipomas and lymphoid aggregates, and hyperplastic, adenomatous, and inflammatory polyps. These polyps have been reported to occur in the esophagus, stomach, duodenum, jejunum, ileum, colon and rectum, with the colon being the site most often affected.
Whether GI neoplasias, especially malignancies, are true component phenotypes of CS is not known because this association has not been systematically studied in large series.10 Various case reports of colorectal cancer in patients with CS have been published, mainly prior to the mid-2000’s and often without the advantage of PTEN mutation status.5, 11–13 In a series of 93 Japanese patients, nine (9.6%) were reported to have colon cancer.14 Gastric cancer has been highlighted in case reports of two individuals with CS.15, 16 The risk of benign and malignant GI neoplasias has yet to be characterized in a large series of individuals with PTEN mutation positive Cowden syndrome. We, therefore, sought to determine the prevalence and characteristics of the GI phenotype in our series of PTEN mutation positive subjects.
Materials and Methods
Study Design
Between October 2005 and June 2009, subjects were prospectively recruited into a DNA banking protocol approved by the Institutional Review of Board for Human Subjects’ Protection of the Cleveland Clinic. Subjects were selected from enrollees from two systematic prospective cohorts: (1) those who met relaxed International Cowden Consortium (ICC) operational criteria (a pathognomonic mucocutaneous lesion, at least one major criterion with or without minor criteria, or at least two minor criteria) or (2) those who had at least five gastrointestinal polyps, at least one of which must have been hyperplastic or hamartomatous (see schema in Figure 1). Enrollees into these two protocols originate from primary care clinics in the community setting to genetics or oncology clinics in academic medical centers throughout North America and Europe. Upon providing informed consent, subjects provided a DNA sample and self-reported personal and family medical history. When available, medical records documenting the subject’s history of neoplasias were obtained. All subjects underwent mutation analysis of the PTEN gene, and only those found to have a deleterious germline mutation were included in this present study. Subject medical records were reviewed for any endoscopy (EGD and/or colonscopy) records and surgical pathology reports documenting gastrointestinal neoplasms (polyps, carcinoma), and those findings are reported descriptively.
Figure 1.
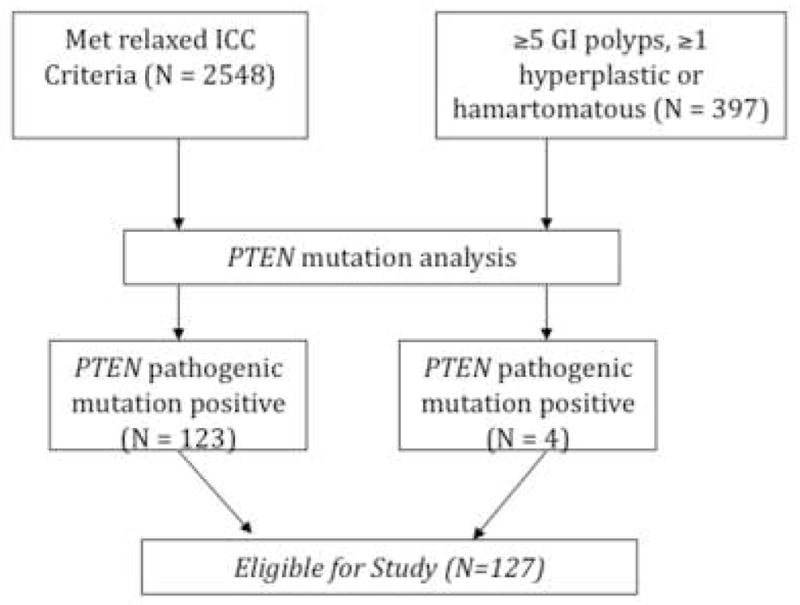
Schema for the prospective accrual of individuals meeting relaxed ICC criteria or the 5-polyp criteria and carrying clear pathogenic PTEN mutations. To be conservative, individuals with variants of unknown significance or promoter variants were excluded.
PTEN Mutation Analysis
Genomic DNA was extracted from peripheral blood leukocytes. Intragenic PTEN was analyzed with a combination of PCR-based DGGE and direct sequencing (ABI 3730xl) as previously reported.17 PTEN promoter mutations and large deletions/ rearrangements were assessed as previously described.18
Statistical Methods and Data Analysis
Fisher’s 2-tailed exact test and unpaired T-tests were utilized for comparison of PTEN mutation positive patients with and without polyps. An age- and gender-adjusted standardized incidence ratio (SIR) was calculated to compare incidence of colorectal and gastric cancers in our series to that of the Surveillance Epidemiology and End Results (SEER) database.
Results
Patients who met relaxed International Cowden Consortium (ICC) criteria (N=2548) or with ≥5 GI (any location) polyps, ≥1 of which was hyperplastic or hamartomatous (N=397) were prospectively recruited (see schema in Fig. 1). Of the 2945 total subjects from these two prospectively accruing protocols (see Methods for details), 127 patients having clear pathogenic PTEN mutations were eligible for this study (Fig. 1). Four of these subjects were enrolled from the cohort ascertained by presence of at least five GI polyps, at least one of which was hyperplastic or hamartomatous, and the remaining 123 were enrolled from those who met relaxed ICC criteria (Fig. 1). In our series of 127 PTEN mutation positive individuals, there were 8 individuals who might be clinically diagnosed with BRRS, comprising 5 male individuals with penile freckling and macrocephaly, 3 of whom also had lipomatosis. The remaining 3 were females, all of whom had macrocephaly, at least one lipoma (not lipomatosis) and one vascular anomaly. None of these 8 had GI malignancies. Average age at study enrollment was 34.6 years (range 1–73 years), and 63 (49.6%) were male. The majority of subjects were white (78), of which 4 were Hispanic/Latino, with the remaining black or African American (9), American Indian or Alaska Native (8), or Asian (6). Race was unknown for 26 subjects.
Of the 127 eligible subjects, GI polyps were reported in 65 (51.2%), with 24 having both upper and lower GI polyps, two only upper GI polyps and 41 only colorectal polyps. Subjects with polyps were significantly older (42.0 years) at the time of enrollment compared to those without polyps (26.6 years, p=0.0001). No gender differences were observed between those with and those without polyps. There was no clear genotype-phenotype correlations for those with or without polyps or those with or without malignancies.
Colorectal Polyps and Carcinomas
At least one colonoscopy was performed for 67 (52.8%) subjects. The average age at first colonoscopy was 36.4 years (1–73 years). Twenty patients underwent colonoscopy because they were symptomatic (abdominal pain, bleeding, constipation, protein-losing enteropathy); eight had the procedure because of the diagnosis of CS; seven underwent a general population screening colonoscopy; seven were due to a personal/family history of polyps; and two for other reasons (rectovaginal fistula and history of cervical cancer). For 23 subjects, the reason for the procedure was unknown. Only four patients had a normal GI examination. Colorectal polyps were identified in 62 subjects (representing 95% of those who underwent ≥1 colonoscopy, or 49% of all eligible subjects) and nine (representing 13% of all who underwent ≥1 colonoscopy, or 7.1% of all eligible subjects) had adenocarcinomas, with one, a rectal cancer, and the remaining 8, colon cancers (Table 1).
Table 1.
Colorectal polyps, their histology a nd colorectal adenocarcinomas in PTEN mutation carriers
| Subject | PTEN mutation | ||||||
|---|---|---|---|---|---|---|---|
| Hamartom | Hyperplastic | Ganglioneur | Adenoma | Inflamm | Other | ||
| 1306-3 | IVS6+2T>C | 1 | |||||
| 3159 | R335X | 1 | |||||
| 2834 | Q17X | 10 | |||||
| 1515 | P95L | >50 | |||||
| 68 | 302delT | Multiple | |||||
| 651-2 | 461delT | Multiple | |||||
| 3349 | 512insA | Multiple | |||||
| 86 | 470insG | Numerous | |||||
| 451 | R335X | Multiple | |||||
| 547 | PTEN/BMPR1A deletion | Multiple | |||||
| 2782 | 26delT | Multiple | |||||
| 521-2 | R130X | Multiple | |||||
| 178 | R130X | 1 | 2 | 1 | |||
| 3015 | 734del4 | 2 | 3 | ||||
| 2381 | C211X | Multiple | Multiple | ||||
| 1824-1 | Exon 2 deletion | Multiple | Multiple | Lipomas | |||
| 3028 | 10q23.2–10q23.31 deletion | Numerous | Numerous | Numerous | |||
| 385 | 592_601del10 | Numerous | Numerous | Numerous | Numerous | LA | |
| 3007 | R159T | 1 | |||||
| 393 | M35V | 4 | |||||
| 2447 | C136R | 9 | |||||
| 2736 | R173C | Multiple | |||||
| 1879 | R355X | 2 | Carpeting | ||||
| 2224 | R335X | 2 | 2 | ||||
| 958 | Exon 1 deletion | 5 | 2 | ||||
| 1140 | G132D | 3 | 2 | 1 | 2SSP | ||
| 1083 | 19insCT | 2 | 6 | Lipomas | |||
| 2438 | L345V | 3 | LA | ||||
| 1968 | 209+1G>T | Multiple | Multiple | Multiple | LA | ||
| 3605 | 210-2_211delAGTT | Multiple | Multiple | Multiple | Adenocarcinoma | ||
| 417 | 350insA | 3 | Pan- colonic | Adenocarcinoma | |||
| 907 | 895insTA | Multiple | 1 | ||||
| 2127 | Y240X | Carpet | 2 | Adenocarcinoma | |||
| 237-2 | C136Y | Numerous | Numerous | ||||
| 3577 | L181P | Multiple | Multiple | Multiple | |||
| 1824-2 | Exon 2 del | Multiple | Multiple | Multiple | |||
| 723 | 542delT | Multiple | Multiple | Multiple | |||
| 985 | 1019delA | Multiple | Multiple | ||||
| 111 | R130X | Innumerable | Lipomas | ||||
| 294 | 1027-2A>C | Multiple | Adenocarcinoma | ||||
| 37 | C211X | Multiple | Adenocarcinoma | ||||
| 1694 | 1026+1G>C | >3 | |||||
| 3935 | R130X | >40 | Adenocarcinoma | ||||
| 622 | C136R | Multiple | |||||
| 2370 | 491delA | Multiple | |||||
| 139 | 253+1G>A | Numerous | |||||
| 2544 | 407del17 | Numerous | |||||
| 2539 | 968delA | Numerous | 4 | ||||
| 1094 | 635-1G>C | Numerous | Numerous | ||||
| 47 | R130X | Multiple | Multiple | Adenocarcinoma | |||
| 559 | R130Q | 1 | |||||
| 2986 | R233X | 13 | Adenocarcinoma | ||||
| 1027 | A120E | Multiple | Multiple | ||||
| 1306-1 | IVS6+2T>C | 30-40 SSP; LA | |||||
| 77 | S229X | Polypoid mucosa | |||||
| 2466 | H61R | Adenocarcinoma | |||||
| 1334-2 | 210-1G>A | 1 polyp, unknown path | |||||
| 1495 | R335X | 2 polyps, unknown path | |||||
| 1334 | 210-1G>A | 50-100 polyps, unknown path | |||||
| 180 | P96R | Many polyps, unknown path | |||||
| 4-3 | G219X | Polyps, unknown path or number | |||||
| 367 | 870delA | Polyps, unknown path or number | |||||
| 2613 | 406insA | Polyps, unknown path or number |
Quantitation of polyps derives from endoscopy reports.
Hamartom, hamartomatous polyps; Hyperplastic, hyperplastic polyps; Ganglioneur, ganglioneuromatous polyps; Adenoma, adenomatous polyps; Inflamm, inflammatory polyps; LA, lymphoid aggregates; path, pathology; SSP, sessile serrated polyps.
Note that Subject 37 has previously been reported in the literature.13
The lower GI polyps were found throughout the colon and rectum (Table 1). Of the 62 subjects with colorectal polyps, 18 were found to have hamartomatous polyps, 27 hyperplastic, 16 ganglioneuromatous, 16 adenomatous, and 11 inflammatory (Table 1). At least nine subjects had polyps of three different histologic types. Of the 27 subjects with colorectal hyperplastic polyps, at least 16 meet the operational diagnosis of hyperplastic polyposis syndrome.
The nine colorectal cancers were diagnosed in five females and four males (Table 1). The average age at diagnosis was 44.4 years (range 35 to 49 years). Notably, the age- and gender-adjusted SIR for colorectal cancer in our series was 224.1 (95% CI 109.3–411.3, p<0.0001). One subject, 2466, presented with colorectal adenocarcinoma in the absence of polyps, and never went on to develop polyps. Three individuals with adenocarcinomas were found to have synchronous adenomatous polyps. In other words, of the 16 individuals with adenomatous polyps, three (18%) developed colorectal cancer. Two of these three individuals with adenomatous polyps and colorectal cancers also had hyperplastic polyps. Five individuals with colorectal carcinomas each had multiple (non-adenomatous) polyps, chief of which were hyperplastic (Table 1). Said another way, of the 27 subjects with hyperplastic polyps, four (15%) had colorectal carcinomas. Three of these four individuals meet the clinical diagnosis of hyperplastic polyposis as well.
Upper Gastrointestinal Findings
Thirty-nine (30.7%) subjects underwent at least one EGD. The average age at first EGD was 39.7 years (2–73 years). The most common indication for undergoing EGD were symptoms including weight loss (N=1), abdominal pain (3), esophagitis (1), gastroesophageal reflux disease (2), vomiting (1), dysphagia (4), diarrhea (2), hematochezia (1), anemia (1), and GI symptoms that were not otherwise specified (1). Five subjects had the examination because of the diagnosis of Cowden syndrome, six because of a history of colonic polyps, nine for unknown reasons, and two for other reasons (history of ulcerative colitis and an abnormal CT scan). Of the 39, only one subject had a normal examination. Gastritis or inflammation was present in 7 (17.9%) subjects and 8 (20.5%) had glycogenic acanthosis (Table 2). Upper GI polyps were found in 26 (66.7%) subjects within the esophagus, stomach, and duodenum (Table 2). Only two individuals had fundic gland polyps. Additionally, Subject 985, who was white Caucasian, was diagnosed in the late 60’s with invasive moderately to poorly differentiated adenocarcinoma with signet ring features arising in a large hyperplastic/hamartomatous gastric polyp. Although only a single individual, age- and sex-adjusted SIR for gastric cancer in this series is 148 (7.4–733, p<0.001). This individual also had diffuse hyperplastic polyposis of the upper (and lower) tracts.
Table 2.
The number of polyps and corresponding pathology of the upper GI polyps found in PTEN mutation positive carriers
| Subject | PTEN mutation | |||||
|---|---|---|---|---|---|---|
| Hamarto | Hyperplastic | Ganglioneur | Adenoma | Other | ||
| 68 | 302delT | 1-5 | ||||
| 1306-1 | IVS6+2T>C | Multiple | ||||
| 3349 | 512insA | Multiple | ||||
| 521-2 | R130X | Multiple | ||||
| 3028 | 10q23.2–10q23.31 deletion | Few | 1 | |||
| 912 | R130X | Several | 2 | Polypoid mucosa | ||
| 393 | M35V | 3 | Multiple | |||
| 417 | 350insA | Multiple | ||||
| 165 | 48insA | Innumerable | ||||
| 1027 | A120E | Myriads | ||||
| 1140 | G132D | Multiple | Few FGP | |||
| 2782 | 26delT | Multiple | Multiple inflammatory pol | |||
| 985 | 1019delA | Diffuse | Adenocarcinoma; FGP | |||
| 1083 | 19insCT | Multiple | Multiple inflammatory polyps | |||
| 2370 | 491delA | Multiple | ||||
| 178 | R130X | Polypoid mucosa | ||||
| 1879 | R355X | Polypoid mucosa | ||||
| 294 | 1027-2A>C | Polypoid mucosa | ||||
| 723 | 542delT | Polypoid mucosa | ||||
| 47 | R130X | Polypoid mucosa | ||||
| 86 | 470insG | Polypoid mucosa | ||||
| 180 | P96R | Many polyps, unknown path | ||||
| 2381 | C211X | Many polyps, unknown path | ||||
| 2986 | R233X | Multiple polyps, unknown path | ||||
| 2834 | Q17X | Multiple polyps, unknown path | ||||
| 37 | C211X | 100s polyps, unknown path |
Quantitation of polyps derives from endoscopy reports.
Hamartom, hamartomatous polyps; Hyperplastic, hyperplastic polyps; Ganglioneur, ganglioneuromatous polyps; Adenoma, adenomatous polyps; FGP, fundic gland polyps; path, pathology
Cowden Syndrome Clinical Features
Clinical features characteristic of or suspicious to be part of Cowden syndrome were also recorded for all 127 subjects (Table 3). As a control, we note that breast cancer occurred in ~37% (age- and sex-adjusted SIR~22, p<0.001) and thyroid cancer 16.5% (adjusted SIR~65, p<0.001) of these PTEN mutation carriers, within the ranges of previous estimates and the single population-based clinical epidemiologic study by Starink.3, 19 Macrocephaly was found in the great majority and was the most common clinical feature in our series of PTEN mutation carriers (95 [74.8%]). Somewhat surprisingly, GI polyps occurred in 51.2% of all eligible subjects or 95% of eligible subjects undergoing at least one colonoscopy, making them the second most common phenotypic feature only after macrocephaly.
Table 3.
Frequency of Cowden syndrome features observed in our PTEN mutation positive series
| Cowden Sydrome Feature | Frequency (percentage) |
|---|---|
| Macrocephaly | 95 (74.8) |
| GI Polyps | 65 (51.2) |
| Goiter/thyroid nodules | 56 (44.1) |
| Benign breast disease* | 24 (37.5) |
| Breast cancer* | 24 (37.5) |
| Lipomas | 44 (34.6) |
| Papillomatous papules | 43 (33.9) |
| Endometrial fibroids* | 17 (26.6) |
| Trichilemmomas | 26 (20.5) |
| Penile freckling^ | 12 (19.0) |
| Acral keratoses | 21 (16.5) |
| MR/DD | 21 (16.5) |
| Thyroid cancer | 21 (16.5) |
| Endometrial cancer* | 8 (12.5) |
| Colorectal cancer | 9 (7.1) |
| Lhermitte-Duclos disease | 8 (6.3) |
| Autism | 8 (6.3) |
Note that GI polyps are the second most common feature.
female subjects only;
male subjects only.
Discussion
Recognition of pertinent personal medical and family history features as well as physical manifestations is critical for accurate diagnosis, risk assessment, genetic testing, and medical management for individuals with hereditary cancer syndromes. Textbooks and single case reports have noted the association of CS and hamartomatous polyps for some time, but not necessarily based on systematic analysis. However, it remains an under-acknowledged manifestation of the disorder for several reasons, perhaps because the malignant potential of these polyps is not well characterized and because there has yet to be a systematic series addressing even the frequency and characteristics of the polyp histology. In our series of all comers with germline pathogenic PTEN mutations, GI polyps occurred in 51.2%, making it the second most common CS feature. Considering that colorectal polyps occurred in 95% of our eligible subjects with germline PTEN mutations who underwent at least one colonoscopy, and approximately half of the entire series, this 51.2% prevalence of any GI polyp is likely an underestimate. Notably, in addition to the textbook-acknowledged hamartomatous polyps, we show here that hyperplastic polyps, ganglioneuromatous polyps and adenomatous polyps are important components of the CS polyp histology. In fact, at least half of our mutation carriers who were shown to have colorectal polyps had two or more histologic types.
In an effort to create uniform criteria in 1995 for the purposes of identifying the predisposition gene, the ICC developed consensus operational diagnostic criteria,20, 21 which were revised in 2000, in an attempt to broaden the net for clinical usage.2 These criteria specify that GI hamartomas are a minor criterion because of lack of systematic study. If the ICC criteria were amended to include GI polyps as a major criterion, then an additional 21 (16.5%) subjects would have had a clinical diagnosis of Cowden syndrome at the time of study enrollment. The most recent version of the National Comprehensive Cancer Network Cowden syndrome testing criteria were updated to include multiple GI hamartomas or ganglioneuromas as a major criterion and a single GI hamartoma or ganglioneuroma as a minor criterion based on expert opinion in the context of single case reports and highly selected small series, often without PTEN mutation information.22 The observations from our prospective systematic study lend objective support to the changes to the NCCN testing criteria.
For patients who are suspicious for the PTEN hamartoma tumor syndrome, it is important to assess previous history of GI polyps. Efforts should be made to confirm the polyp burden in these patients with medical records. Additionally, given the inaccuracies with polyp histology,23 consideration should be given to having the pathology re-reviewed by a dedicated GI pathologist. For patients suspicious for Cowden syndrome or a PTEN hamartoma tumor syndrome who have not previously undergone an endoscopy, the risks and benefits of a baseline upper endoscopy and colonoscopy should be considered in the diagnostic workup.
Colorectal cancer occurred in 7.1% of our entire series and 13% of eligible subjects who underwent at least one colonoscopy (age- and gender-adjusted SIR=224). Currently, colorectal surveillance is not routinely recommended for individuals with Cowden syndrome beyond that for the general population. However, in our series, all nine subjects were diagnosed with colorectal cancer prior to age 50, with the youngest age at diagnosis being 35 years. Therefore, had our subjects initiated screening at age 50, their malignancies would likely not have been detected until an advanced stage. Individuals with PTEN mutations may benefit from earlier colonic surveillance. One group has advised a “vigorous” screening approach to patients with Cowden syndrome that includes colonoscopy beginning at age 15 with follow up every 1–2 years.24 This approach is rather aggressive. Based on our current observations, we recommend considering a baseline colonoscopy at age 35, or sooner if symptoms develop, with follow-up time based on polyp burden.
Based on our series, ~15% of PTEN mutation carriers with colorectal hyperplastic polyps developed colorectal cancer; and almost 20% of mutation carriers with colorectal adenomas had colorectal cancer. The patients who developed colorectal carcinomas also tended to have multiple polyps, based on small numbers. Therefore, if these observations can be reconfirmed in an independent series, then indications for colorectal surveillance should be offer to any PTEN mutation carrier with multiple lower GI polyps, and/or the presence of hyperplastic and/or adenomatous polyps.
There were 2 (13%) individuals with sessile serrated adenomas, both of whom were amongst the 16 individuals who met the criteria for hyperplastic polyposis syndrome. There are 2 interpretations to this observation: that this is a true representation, or there was uneven “calling” of this histology amongst the pathologists. We tried to gauge this by culling out our research subjects enrolled in another study (who may or may not have PTEN mutations) during a similar period, and we found that of those who met the diagnosis of HPS, ~50% also had sessile serrated adenomas (p=0.07).
Only one individual in our series developed gastric cancer. Although the formal adjusted SIR is elevated, we cannot draw genetic counseling or clinical conclusions given that this single subject was a white male who developed gastric cancer at 67 years old. It is nonetheless interesting to note the co-existing diffuse hyperplastic polyposis in the stomach and duodenum, almost mirroring the situation in the colon. In our series of PTEN mutations carriers, upper GI polyps do occur with some frequency, and, for a subset of patients, they do experience symptoms. Excluding the five individuals with unknown polyp histology in the upper GI tract, 15 of the 19 with polyps in the upper and lower tracts had concordant histologies. Interestingy, fundic gland polyps, often said to be indicative of CS, were only found in two mutation carriers. Notably, 20% of those with GI examinations had glycogenic acanthosis. Although no consensus guidelines exist regarding upper GI management in CS, Schreibmann et al. recommend upper endoscopy and an upper GI series with small bowel follow-through beginning at age 15 with follow up every two years.24 Again, based on our current observations, we believe that this approach is rather aggressive and recommend upper GI surveillance only for symptom management, at least in the white population. Based on anecdotal reports on Asian populations, we suspect that GI features, perhaps even upper GI malignancies, may be more prominent in the Asian population.
One of the major limitations of this study is that not all medical records were available to confirm all of the reported histories, although these remain in the minority. Therefore, for some patients, we do not have detailed information about their precise GI history. Further, since patients were recruited from multiple medical centers, there is wide variability in the medical reports and pathology expertise available. The most ideal study would be to offer at least baseline colonoscopy, by a limited number of endoscopists, and to have the pathology reviewed by a single pathologist with expertise in hereditary GI disease in order to accurately capture the frequency with which GI polyps occur in these patients. Nonetheless, despite these limitations, the conditions of the study do somewhat simulate the data available at a high-risk GI consultation.
Based on the observations from our prospectively accrued series of PTEN mutation carriers and the literature, we conclude that both upper and lower GI polyps are common component features of the PTEN hamartoma tumor syndrome. The presence of non-adenomatous polyps, and especially comprising mixed histologies, should signal a healthcare provider to refer such individuals to high-risk assessment. Furthermore, the presence of both macrocephaly and non-adenomatous GI polyps together should predict, to a high probability, for the presence of germline PTEN mutations. Colorectal adenomas are usually not considered part of the CS phenotype, but our series suggests that they most likely are, with almost 20% of PTEN mutation carriers with colorectal adenomatous polyps developing colorectal adenocarcinomas. Similarly, multiple colorectal polyps of any histology and hyperplastic polyps, especially a hyperplastic polyposis syndrome phenotype, may be red flags for PTEN mutation carriers developing colorectal cancers. Importantly, colorectal adenocarcinomas have an increased prevalence in PTEN mutation carriers, all occurring prior to the age of 50 years, and so, routine colonic surveillance should be considered.
Acknowledgments
Grant support: P01CA124570 from the National Cancer Institute, Doris Duke Distinguished Clinical Scientist Award, and ACS Clinical Research Professorship, generously funded, in part, by the F.M. Kirby Foundation (all to CE)
We are grateful to Jin-Lian Chen, Frank Mularo, Charissa Peterson and Yi-Ran Yang (all of the Eng lab) for technical assistance. CE is the Sondra J. and Stephen R. Hardis Endowed Chair of Cancer Genomic Medicine at the Cleveland Clinic.
Abbreviations
- CS
Cowden syndrome
- EGD
Esophagogastroduodenoscopy
- FGP
fundic gland polyps
- GI
gastrointestinal
- ICC
International Cowden Consortium
- SSP
sessile serrated polyps
- SIR
standardized incidence ratio
Footnotes
Disclosures: None
Transcript Profiling: None
Writing assistance: None
Author Roles:
Study Concept and Design: B. Heald and C. Eng
Acquisition of Data: B. Heald, J. Mester, M. Orloff
Analysis and Interpretation of Data: B. Heald, L. Rybicki, C. Eng
Statistical Analysis: L. Rybicki and C. Eng
Drafting of the Manuscript: B. Heald and C. Eng
Critical Revision of the Manuscript: B. Heald, J. Mester, L. Rybicki, M. Orloff, C. Burke, C. Eng
Obtained Funding: C. Eng
Study Supervision: C. Eng
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zbuk KM, Eng C. Hamartomatous polyposis syndromes. Nat Clin Pract Gastroenterol Hepatol. 2007;4:492–502. doi: 10.1038/ncpgasthep0902. [DOI] [PubMed] [Google Scholar]
- 2.Eng C. Will the real Cowden syndrome please stand up: revised diagnostic criteria. J Med Genet. 2000;37:828–30. doi: 10.1136/jmg.37.11.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zbuk K, Eng C. Cancer phenomics: RET and PTEN as illustrative models. Nature Rev Cancer. 2007;7:35–45. doi: 10.1038/nrc2037. [DOI] [PubMed] [Google Scholar]
- 4.Chen YM, Ott DJ, Wu WC, Gelfand DW. Cowden’s disease: a case report and literature review. Gastrointest Radiol. 1987;12:325–9. doi: 10.1007/BF01885173. [DOI] [PubMed] [Google Scholar]
- 5.Hanssen AMN, Fryns JP. Cowden syndrome. J Med Genet. 1995;32:117–9. doi: 10.1136/jmg.32.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marra G, Armelao F, Vecchio FM, Percesepe A, Anti M. Cowden’s disease with extensive gastrointestinal polyposis. J Clin Gastroenterol. 1994;18:42–7. doi: 10.1097/00004836-199401000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Salem OS, Steck WD. Cowden’s disease (multiple hamartoma and neoplasia syndrome). A case report and review of the English literature. J Am Acad Dermatol. 1983;8:686–96. doi: 10.1016/s0190-9622(83)70081-2. [DOI] [PubMed] [Google Scholar]
- 8.Starink TM, van der Veen JP, Goldschmeding R. Decreased natural killer cell activity in Cowden’s syndrome [letter] J Am Acad Dermatol. 1986;15:294–6. doi: 10.1016/s0190-9622(86)80258-4. [DOI] [PubMed] [Google Scholar]
- 9.Eng C. Cowden syndrome. J Genet Counsel. 1997;6:181–91. doi: 10.1023/A:1025664119494. [DOI] [PubMed] [Google Scholar]
- 10.Carlson GJ, Nivatvongs S, Snover DC. Colorectal polyps in Cowden’s disease (multiple hamartoma syndrome) Am J Surg Pathol. 1984;8:763–70. doi: 10.1097/00000478-198410000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Bosserhoff AK, Grussendorf-Conen EI, Rubben A, et al. Multiple colon carcinomas in a patient with Cowden syndrome. Int J Mol Med. 2006;18:643–7. [PubMed] [Google Scholar]
- 12.Burnett JW, Goldner R, Calton GJ. Cowden disease. Report of two additional cases. Br J Dermatol. 1975;93:329–36. doi: 10.1111/j.1365-2133.1975.tb06499.x. [DOI] [PubMed] [Google Scholar]
- 13.Hover AR, Cawthern T, McDanial W. A hereditary polyposis syndrome diagnosable by mucocutaneous inspection. J Clin Gastroenterol. 1986;8:576–9. doi: 10.1097/00004836-198610000-00019. [DOI] [PubMed] [Google Scholar]
- 14.Han S-Y, Kato H, Suzuki T, et al. Functional evaluation of PTEN missense mutations using in vitro phosphoinositide phosphatase assay. Cancer Res. 2000;60:3147–51. [PubMed] [Google Scholar]
- 15.Al-Thihli K, Palma L, Marcus V, et al. A case of Cowden’s syndrome presenting with gastric carcinomas and gastrointestinal polyposis. Nat Clin Pract Gastroenterol Hepatol. 2009;6:184–9. doi: 10.1038/ncpgasthep1359. [DOI] [PubMed] [Google Scholar]
- 16.Hamby LS, Lee EY, Schwartz RW. Parathyroid adenoma and gastric carcinoma as manifestations of Cowden’s disease. Surgery. 1995;118:115–7. doi: 10.1016/s0039-6060(05)80018-2. [DOI] [PubMed] [Google Scholar]
- 17.Mutter GL, Lin M-C, FitzGerald JT, Kum JB, Eng C. Changes in endometrial PTEN expression throughout the human menstrual cycle. J Clin Endocrinol Metab. 2000;85:2334–8. doi: 10.1210/jcem.85.6.6652. [DOI] [PubMed] [Google Scholar]
- 18.Zhou XP, Waite KA, Pilarski R, et al. Germline PTEN promoter mutations and deletions in Cowden/Bannayan-Riley-Ruvalcaba syndrome result in aberrant PTEN protein and dysregulation of the phosphoinositol-3-kinase/Akt pathway. Am J Hum Genet. 2003;73:404–11. doi: 10.1086/377109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Starink TM, van der Veen JPW, Arwert F, et al. The cowden syndrome: a clinical and genetic study in 21 patients. Clin Genet. 1986;29:222–33. doi: 10.1111/j.1399-0004.1986.tb00816.x. [DOI] [PubMed] [Google Scholar]
- 20.Eng C. Genetics of Cowden syndrome - through the looking glass of oncology. Intl J Oncol. 1998;12:701–10. doi: 10.3892/ijo.12.3.701. [DOI] [PubMed] [Google Scholar]
- 21.Nelen MR, Padberg GW, Peeters EAJ, et al. Localization of the gene for Cowden disease to 10q22–23. Nature Genet. 1996;13:114–6. doi: 10.1038/ng0596-114. [DOI] [PubMed] [Google Scholar]
- 22.NCCN. NCCN Practice Guidelines: Genetics/Familial High Risk Cancer. 2010. [Google Scholar]
- 23.Sweet K, Willis J, Zhou XP, et al. Molecular classification of patients with unexplained hamartomatous and hyperplastic polyposis. JAMA. 2005;294:2465–73. doi: 10.1001/jama.294.19.2465. [DOI] [PubMed] [Google Scholar]
- 24.Schreibman IR, Baker M, Amos C, McGarrity TJ. The hamartomatous polyposis syndromes: a clinical and molecular review. Am J Gastroenterol. 2005;100:476–90. doi: 10.1111/j.1572-0241.2005.40237.x. [DOI] [PubMed] [Google Scholar]


