Abstract
Propagation of the pressure wave along the arterial tree (pulse wave velocity [PWV]) is related to the intrinsic elasticity of the arterial wall. PWV is increased in stiffer arteries and, when measured over the aorta, is an independent predictor of cardiovascular morbidity and mortality. Given the predictive power of PWV, identifying strategies that prevent or reduce stiffening may be important in prevention of cardiovascular events. One view is that aortic stiffness occurs as a result of atherosclerosis along the aorta. However, there is little or no association between PWV and classical risk factors for atherosclerosis, other than age and blood pressure. Furthermore, PWV does not increase during early stages of atherosclerosis, as measured by intima-media thickness and non-calcified atheroma, but it does increase in the presence of aortic calcification that occurs within advanced atherosclerotic plaque. Age-related widening of pulse pressure is the major cause of age-related increase in prevalence of hypertension and has been attributed to arterial stiffening. This review summarizes the methods of measuring aortic stiffness in humans, the pathophysiological mechanisms leading to aortic stiffness, including its association with atherosclerosis, and the haemodynamic consequences of increased aortic stiffness.
Introduction
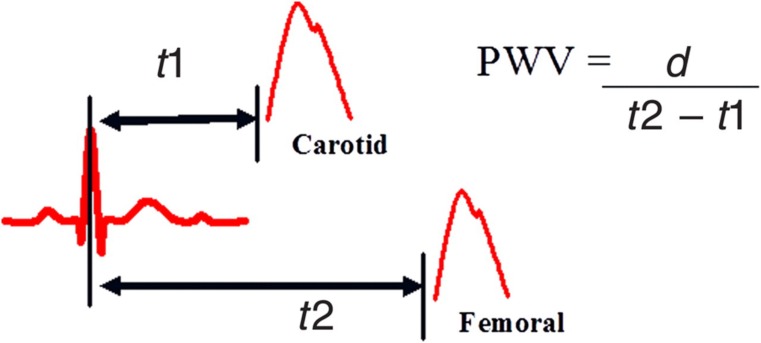
Arterial stiffness describes the reduced capability of an artery to expand and contract in response to pressure changes. Parameters that describe vessel stiffness include compliance and distensibility. Compliance (C) is a measure of volume change (ΔV) in response to a change in blood pressure (ΔP; C = ΔV/ΔP). In a stiff vessel the volume change, and therefore compliance, is reduced for any given pressure change. However, compliance also relates to initial arterial volume because a smaller volume reduces compliance for any given elasticity of the arterial wall. Distensibility (D) is compliance relative to initial volume (D = ΔV/ΔP × V) and therefore relates more closely to wall stiffness. The consequence of reduced compliance/distensibility is increased propagation velocity of the pressure pulse along the arterial tree, called pulse wave velocity (PWV), which relates to arterial distensibility by the Bramwell and Hill equation: PWV = √(V × ΔP/ρ × ΔV), where ρ is blood density. PWV is calculated by measuring time taken for a pressure pulse to travel between two set points (Figure 1). Commonly used points are the carotid and femoral artery because they are superficial and easy to access. Additionally, it covers the region over the aorta that exhibits greatest age-related stiffening.1
Figure 1.
PWV measurement. Carotid-to-femoral PWV is calculated by dividing the distance (d) between the two arterial sites by the difference in time of pressure wave arrival between the carotid (t1) and femoral artery (t2) referenced to the R wave of the electrocardiogram
Arterial stiffness, as measured by carotid-femoral PWV, is an independent predictor of cardiovascular morbidity and mortality in hypertensive patients, type 2 diabetes, end-stage renal disease and in elderly populations.2 Given the predictive power of PWV, identifying strategies that prevent or reduce stiffening may be important in the prevention of cardiovascular events.
Arterial stiffness and ageing
Arterial stiffness increases with age by approximately 0.1 m/s/y in East Asian populations with low prevalences of atherosclerosis.3 While some authors report a linear relationship between arterial stiffness and age,3 others have found accelerated stiffening between 50 and 60 years of age.4 In contrast, stiffness of peripheral arteries increases less3 or not at all with increasing age.1
The aorta is subject to age-related structural change in both the media and intima. In the media, these include fracture of elastin, increase in collagen and calcium deposits. In the intima, predominant changes include increase in intima-media thickness and prevalence of atherosclerosis in Western cultures. Age-related increase in PWV is found in populations with low prevalences of atherosclerosis, suggesting that medial degeneration is an important cause of arterial stiffening.3 One hypothesis is that repetitive cyclic stress over a life span is responsible for the fracture of elastin fibres.5 This suggests that stiffness is an inevitable consequence of ageing. However, there is considerable variation in the magnitude of age-related change between individuals3 and several indigenous populations do not show an age-related increase in pulse pressure (PP),6 which is determined in part by arterial stiffness.
An understanding of molecular pathways that influence arterial stiffness is now emerging. Ageing of the arterial media is associated with increased expression of matrix metalloproteinases (MMP), which are members of the zinc-dependent endopeptidase family and are involved in degradation of vascular elastin and collagen fibres. Several different types of MMP exist in the vascular wall, but in relation to arterial stiffness, much interest has focused on MMP-2 and MMP-9. Animal studies show that increased elastin thinning and fragmentation is associated with increased expression of intimal and medial MMP-2.7 Furthermore, MMP-2 co-localizes in regions of elastin lamellae fragmentation suggesting that it is involved in elastin degradation.8 In humans, serum MMP-2 and MMP-9 levels and MMP-9 gene polymorphisms have been associated with increased arterial stiffness.9 In addition, tissue expression of MMP-2 correlates with increased elastin fragmentation, PWV and calcium deposits in renal transplant patients.10 However, other investigators have reported a significant negative relationship between PWV and serum MMP-2 and MMP-9 in healthy subjects.11 Structural changes of collagen fibres, in particular collagen cross-linking by advanced glycation end-products (AGEs), may also affect arterial stiffness. In animals, suppression of AGEs by aminoguanidine prevents arterial stiffening without alterations in collagen or elastin content.12 In human studies, treatment of hypertensive patients with ALT-711, a non-enzymatic breaker of collagen cross-links, resulted in a significant reduction in PWV and systolic blood pressure in comparison to placebo without any difference in mean arterial pressure (MAP).13
Arterial stiffness and blood pressure
Structural proteins determine the material or intrinsic stiffness of the arterial wall and its ability to expand and recoil. However, measured or functional stiffness also depends on the pressure exerted by blood on the wall. Arterial stiffness increases at higher loading pressures without any structural change.14 Theoretically, this is because stress is transferred from elastin to collagen fibres.15 In human arteries, the association between pressure and diameter is non-linear and the slope of the curve per increment in pressure change is the Young's incremental modulus. Bergel14 showed that the incremental modulus of excised arterial segments increased non-linearly with increasing pressure, and that, at any given pressure, the modulus was higher in peripheral than central arterial segments. In vivo arterial stiffness is increased by an acute rise in blood pressure.16 Furthermore, stiffness is greater in hypertensive individuals when compared with age-matched controls.17 Sustained hypertension may also accelerate structural changes to the arterial wall, particularly in hypertensive individuals in whom blood pressure therapy does not normalize arterial stiffness.18 In the presence of structural change, blood pressure reduction might be expected to have less impact on arterial stiffness. In this regard, it is notable that failure to reduce PWV with antihypertensive therapy is a significant predictor of cardiovascular death in end-stage renal disease despite a similar reduction in MAP between survivors and non-survivors.19
Arterial stiffness and its relation to established cardiovascular risk factors, atherosclerosis and calcification
Arterial stiffness and established cardiovascular risk factors
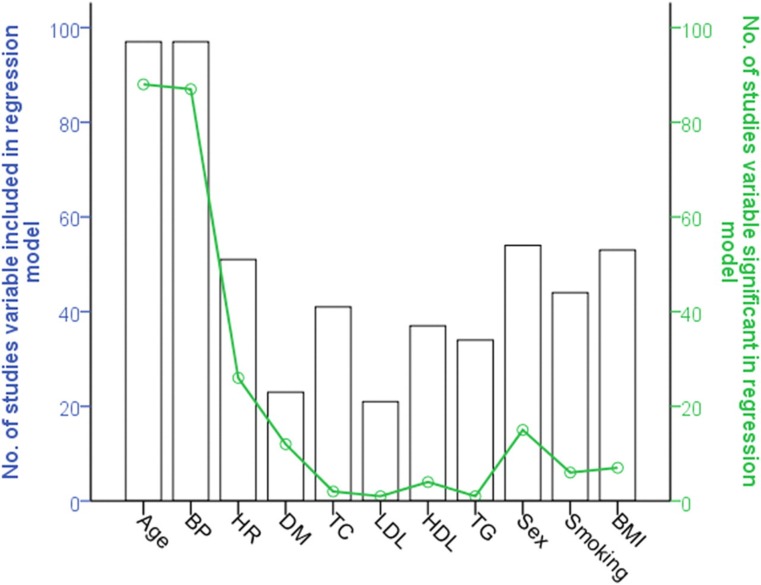
Classic cardiovascular risk factors, including hyperlipidaemia, diabetes mellitus, elevated body mass index and smoking, have been implicated in accelerated arterial stiffening. However, findings with respect to risk factors other than age and blood pressure have been inconsistent and negative findings not highlighted in many studies. Clustering of risk factors in the metabolic syndrome explains <1% of PWV variance.20 Furthermore, a systematic review of publications examining independent determinants of arterial stiffness showed a poor correlation between PWV and established cardiovascular risk factors other than age and blood pressure (Figure 2).21 This is in agreement with prospective studies where risk factors other than hypertension are not associated with progression of PWV.18
Figure 2.
Results of the systematic review. The number of studies in which classical risk factors and heart rate were included (bars) and the proportion of these (solid line) in which the risk factor was significantly independently associated with pulse wave velocity. Reprinted from Cecelja M, Chowienczyk P. Dissociation of aortic pulse wave velocity with risk factors for cardiovascular disease other than hypertension : a systematic review. Hypertension 2009; 54:1328–36
Arterial stiffness and atherosclerosis
Atherosclerosis is a pathological condition of the intima which is characterized by lipid accumulation, inflammatory cells, vascular smooth muscle cell (VSMC) migration, foam cell development, connective tissue fibres and calcium deposits. Early stages of plaque development begin with small lipid deposits and fatty streaks in the intima which may progress to complex plaque structures. The composition of advanced plaques is heterogeneous and includes a lipid core, fibrous connective tissue deposits and calcification.22 In the presence of atherosclerosis stiffness of large arteries is increased and it has been suggested that arterial stiffness may be a useful marker of the extent of atherosclerosis in the aorta.23
Increased intima media thickening (IMT) is thought to represent one of the earliest stages of atherosclerosis.24 In support of this, increased IMT co-localizes in areas susceptible to atherosclerosis;24 it predicts plaque development;23 and both IMT and presence of plaque are more prevalent in cardiovascular disease.25 IMT is characterized by an accumulation of VSMC, elastin and proteoglycans, but not lipid deposits.26 The association between IMT and arterial stiffness remains unclear. Although some studies have shown IMT and PWV to be correlated, such associations, where positive, have been relatively weak and several studies show no relation. In the Atherosclerosis Risk in Communities study consisting of over 10,000 subjects, reduced arterial elasticity was significant only in subjects in the 90th percentile of carotid IMT.27 Zureik et al. 28 found no association between PWV and IMT in 564 subjects after adjusting for age and blood pressure. Similary, in the Northern Manhattan Family Study of 693 subjects with mean age of 49 years, there was no significant association between IMT and measures of distensibility.29 An alternative hypothesis is that increased IMT normalizes circumferential wall stress in response to blood pressure increase.30 In support of this, middle-aged subjects with essential hypertension have increased radial IMT in comparison to age-matched controls, but circumferential stress is not significantly different.31 Additionally, in these subjects, radial artery Young's elastic modulus is comparable between the two groups when compared at similar pressure, suggesting that wall hypertrophy does not contribute to intrinsic wall stiffness of peripheral arteries.30,31 In line with these findings, brachial PWV changes little with age despite significantly increased blood pressure.32 Treatment with blood pressure lowering therapy can cause IMT to regress independently of a change in Young's elastic modulus.33 A uniting hypothesis suggests that IMT may be an adaptive response to maintain circumferential stress up to a certain level, after which it is more closely correlated with atherosclerosis.34
Studies examining the association between arterial stiffness and the presence of plaque have also reported conflicting findings. These inconsistencies may be due to heterogeneity of plaque composition. The relation of plaque structure to arterial stiffening is not fully understood. Farrar et al.35 showed an initial decrease in PWV at early stages of atherosclerosis in cynomolgus monkeys fed an atherogenic diet, followed by accelerated stiffening in the presence of advanced atherosclerosis. In humans, Paini et al. 36 showed both increased and decreased distensibility at the level of a plaque in comparison to adjacent plaque-free regions. The Rotterdam Study found no association between mild plaque score and PWV. However, in the presence of severe plaque score, as determined by ultrasound, or abdominal calcification determined by X-ray, PWV was significantly increased.37 This suggests that, in keeping with animal models of atherosclerosis,35 atherosclerotic change within the arterial wall is not necessarily associated with arterial stiffening.
Arterial stiffness and aortic calcification
Arterial calcification is the accumulation of calcium phosphate crystals in the media or intima of the arterial wall. Arterial calcium content increases with age and this accelerates after approximately 70 years of age.38 Intimal calcification is focal and occurs within atherosclerotic plaques. Medial calcification (also known as ‘Monckeberg's sclerosis’) is more diffuse, associates with elastic fibres and commonly affects the aorta and femoral arteries. Deposition of calcium in the arterial wall is increased in end-stage renal disease and diabetes mellitus.
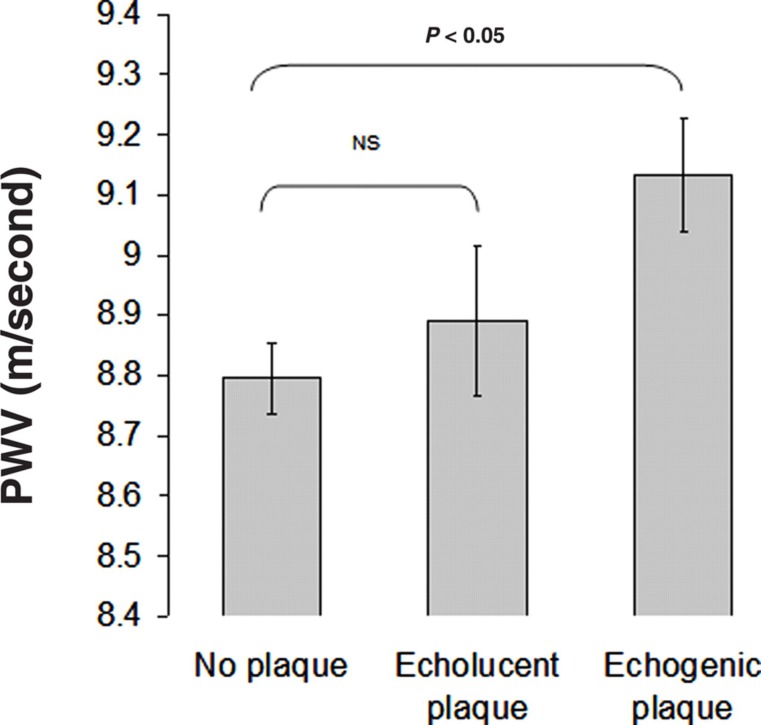
In animal models, medial calcification induced by warfarin plus vitamin K39 and vitamin D plus nicotine40 is associated with increased arterial stiffness, decreased elastin content and increased collagen content. These animals also develop isolated systolic hypertension.39,40 In humans, London et al. 41 reported higher PWV in individuals with medial and intimal calcification detected by soft-tissue X-rays in end-stage renal disease patients. In healthy individuals, Odink et al. 42 reported a correlation between aortic calcification detected using computerized tomography and PWV in 698 elderly subjects. However, calcification was measured in the ascending aorta and the arch whereas PWV is measured over the entire aorta. McEniery et al.,43 measured calcification over a more extensive region including the ascending, descending and a portion of the abdominal aorta. After adjustment for confounders, PWV was significantly associated with aortic calcification. What is not known is whether the association between arterial stiffness and calcification merely reflects an association between arterial stiffness and atherosclerosis. However, in a cohort of female twins we and others have found that calcified plaques, but not lipid-rich non-calcified plaques, correlate with increased stiffness (Figure 3).44,45 This suggests that the relation of PWV to vascular calcification may be independent of total plaque burden, and is consistent with a lack of association between PWV and early stages of atherosclerosis in animal models.
Figure 3.
Mean values of carotid-femoral PWV as a function of plaque type (adjusted for age, mean arterial pressure, heart rate and fasting glucose)
Genetic contribution to arterial stiffness
Several studies, using a variety of approaches, indicate that arterial stiffness is in part influenced by genetic factors. Firstly, monogenic traits, which are affected by single gene mutations, influence arterial stiffness. Monogenic traits follow Mendel's pattern of inheritance, where a trait is influenced by two variants of a gene, one of which is passed on from each parent. Marfan's syndrome is an autosomal dominant genetic disorder caused by a mutation of the FBN1 gene that encodes fibrillin-1. Fibrillin-1 regulates assembly of elastin fibres and is associated with increased arterial stiffness.46 William's syndrome results from microdeletion of chromosome 7q, which disrupts the elastin gene and correlates with reduced arterial stiffness.47 However, measures of arterial stiffness follow a normal distribution in population studies and are therefore unlikely to be influence by variation in one gene.
Family and twin studies support a role for genetic factors in arterial stiffness. The heritability from family studies has been estimated to be between 19% and 40% as summarized in Table 1. However, these estimates may be confounded by shared family environment. The classic twin design exploits known genetic relationships between monozygotic and dizygotic twins, and is also able to estimate the contribution of shared environment to phenotypic variation. Using the twin design, heritability of arterial stiffness has been reported at between 38% and 54%.44,48 Previous studies investigating heritability of atherosclerotic lesions as determined by ultrasound have reported non-significant49 or low heritability scores.50,51 In comparison, the contribution of genetic factors to vascular calcification was reported to be 49% by the Framingham Heart study.52 Twin studies can further be extended to allow analysis of the contribution of common genes and environment to correlated traits. Using structural equation modelling, the phenotypic correlation between arterial stiffness and arterial calcification was in the majority attributed to genetic factors that are common to both phenotypes.44
Table 1.
Results family and twin heritability studies of arterial stiffness phenotypes
| Author | Population | Number of participants | Measure of arterial stiffness | Adjusted for | Heritability estimate |
|---|---|---|---|---|---|
| North et al. 77 | The Strong Heart Family Study | 950 participants from three American Indian communities | β-stiffness Index | Age, sex, hypertension, centre | 0.23 ± 0.07 |
| Mitchell et al. 78 | Framingham Family Heart Study | 1480 representing 817 pedigrees in the Framingham study offsping cohort | PWV carotid-femoral | Age, age,2 height, weight | 0.40 ± 0.09 |
| Sayed-Tabatabaei et al. 51 | Erasmus Rucphen Family study | 930 individuals | PWV carotid-femoral | Age, gender, MAP, LDL-cholesterol, glucose, HR | 0.26 ± 0.08 |
| Ge et al. 48 | Georgia Cardiovascular Twin Study | 702 twins | PWV carotid-dorsalis pedis | – | 0.54 (CI: 0.43–0.63) |
| Seidlerová et al. 79 | Family Study | 494 participants | PWV carotid-femoral | Centre, sex, age, age,2 height, weight, MAP, HR, antihypertensive treatment, smoking, alcohol intake | 0.19 ± 0.11 |
HR, heart rate; LDL, low-density lipoprotein
Haemodynamic consequences of increased arterial stiffness: PWV and PP
Ageing is characterized by a widening of PP and in older subjects PP relates more closely to cardiovascular events than systolic blood pressure (SBP) or diastolic blood pressure (DBP) alone.53 A rise in PP is the major cause of the age-related increase in prevalence of hypertension and has generally been attributed to arterial stiffening. Bramwell and Hill54 were among the first to draw attention to the association of PP with arterial stiffness. They noted ‘the difference between SBP and DBP, that is the PP, other things being equal, will vary directly as the rigidity of the arterial walls’.54 Since then large epidemiological studies have confirmed a positive correlation between PP and PWV.55,56 However, PP when measured in the aorta can change independently of PWV, suggesting that this relationship is more complicated.57
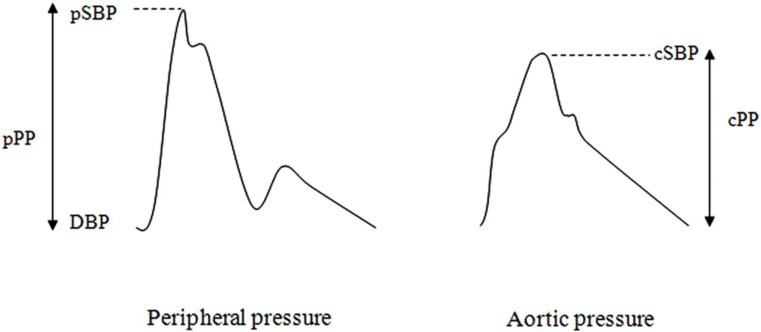
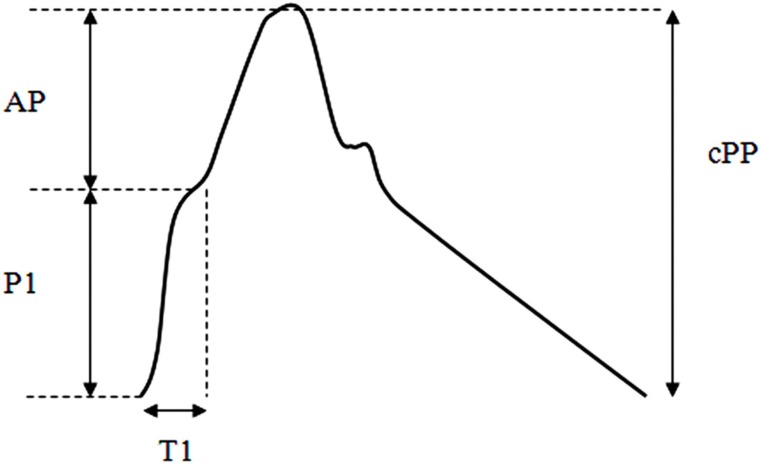
The pressure waveform changes in contour and amplitude as it propagates along the arterial tree. Whereas DBP and MAP remain relatively constant between the aorta and brachial artery, SBP is approximately 12 mmHg (range −6 to 35 mmHg) higher in the brachial artery; this is called pressure amplification (Figure 4).58 Typical peripheral pressure waveforms of healthy middle-aged adults are characterized by an uninterrupted systolic rise, followed by a second systolic shoulder in the downstroke of the wave (Figure 4). In contrast, in the central aorta the early pressure rise is augmented by a secondary pressure rise in late systole and creates a characteristic systolic shoulder on the upstroke of the aortic pressure waveform (Figure 5). Therefore, central PP (cPP) can be partitioned into two components: pressure of the first systolic shoulder (P1) resulting from left ventricular ejection and which coincides with peak flow velocity; and augmentation pressure (AP), which is peak systolic pressure minus P1 and is thought to result from wave reflection of the incident wave and arterial reservoir pressure (Figure 5). AP is often expressed as a percentage of total cPP (AP/cPP × 100) called augmentation index (AIx). AP increases linearly with increasing age.4,57 In contrast, AIx plateaus between the ages of 50 and 60 years.4,57
Figure 4.
Contours of peripheral and aortic pressure waveforms. Peripheral and aortic pressure waveforms showing peripheral PP (pPP), peripheral SBP (pSBP), cPP, central SBP (cSBP) and DBP. Reprinted from Cecelja M, Jiang B, Spector TD, Chowienczyk P. Progression of central pulse pressure over 1 decade of aging and its reversal by nitroglycerin: a twin study. J Am Coll Cardiol 2012; 59:475–83, with permission from the American College of Cardiology Foundation
Figure 5.
Aortic pressure waveform. The systolic pressure rise can be separated by a first systolic peak (P1) resulting from the ejection of blood from the left ventricle into the aorta; T1 is time to first peak and AP is the difference between central SBP (cSBP) and P1. P1 and AP sum to give cPP
Initial data suggest that cPP may be a better predictor of cardiovascular risk than PP measured in the brachial artery.59–64 In younger women the absolute increase in cPP is greater than peripheral PP.57 Furthermore, invasively measured AP is an independent predictor of cardiovascular events in male patients undergoing coronary angiography.65 Similarly, AP measured non-invasively predicts cardiovascular events in men, but not women.66 The predictive ability of AIx is less clear. Although AIx correlates with cardiovascular events in patients with end-stage renal disease67 and those undergoing percutaneous coronary intervention,68 other smaller studies have failed to find an association.59,69,70 However, a recent meta-analysis suggests AIx is an independent predictor of cardiovascular disease.63
Mechanisms relating central PP and arterial stiffness
A rise in local arterial stiffness will increase the ratio of pressure for a given volume flow, increasing the pressure of the forward wave (P1). Increased arterial stiffness is also proposed as the main mechanism of age-related increase in AP, either by affecting wave reflection or aortic reservoir. The rationale behind the association of PWV to wave reflection is that as the pressure wave travels along the arterial tree, it is partially reflected back to the aorta at sites of impedance and diameter mismatch. Arterial stiffening and hence an increase in PWV results in reflected waves arriving back to the aorta earlier, coinciding and augmenting pressure of the forward wave at P1.71 AP may also be influenced by reservoir pressure which is the product of stored stroke volume as the aorta expands during systole. A stiffer aorta will reduce aortic compliance, so the pressure generated for any given volume of stored blood will increase.72 Evidence of a link between AP and arterial stiffness comes from the observed correlation between AIx and PWV. However, because P1 is a denominator of AIx, the association between AIx and PWV may be driven by the correlation between P1 and PWV. Furthermore, in cross-sectional and longitudinal studies, AP does not correlate with PWV,55,57 suggesting that the primary mechanism linking cPP and arterial stiffness is through the association of PWV to the forward wave.
The pathophysiological consequences of increased cPP and AP can be explained by the adverse impact on left ventricular afterload. Elevated central SBP predisposes to left ventricular hypertrophy73 which is an independent predictor of cardiovascular death.74 Furthermore, a decrease in DBP reduces coronary perfusion, predisposing the heart to ischaemia.75 In addition, high pulsatile pressure may have adverse effects on microvessels, particularly those of the brain and kidneys.76 In this regard, it is notable that there is correlation between PP and glomerular filtration rate73 and between PP and cognitive impairment.32
Conclusion
Central arterial stiffness, measured using carotid-femoral PWV, is an independent predictor of cardiovascular morbidity and mortality. The prognostic value of PWV is unlikely to be explained by it being a simple marker of the effect of established risk factors, other than blood pressure, or extent of generalized atherosclerosis in the aorta. Accelerated arterial stiffening does correlate with vascular calcification, and this correlation is primarily due to shared genetic factors. Ageing is characterized by a widening of PP, which is the major cause of the age-related increase in prevalence of hypertension and has been attributed to arterial stiffening, influencing the outgoing pressure wave. The prognostic importance of carotid-femoral PWV is, therefore, likely to be attributed to adverse haemodynamic effects of aortic stiffening with increased systolic load and decreased myocardial perfusion pressure.
DECLARATIONS
Competing interests
PC has an interest in Centron Diagnostics Ltd., King's College London
Funding
Not applicable
Ethical approval
Not applicable
Guarantor
PC
Contributorship
MC drafted the manuscript. PC provided critical revision and final approval
Acknowledgements
None
References
- 1. Mitchell GF, Parise H, Benjamin EJ, et al. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension 2004;43:1239–45 [DOI] [PubMed] [Google Scholar]
- 2. Laurent S, Cockcroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 2006;27:2588–605 [DOI] [PubMed] [Google Scholar]
- 3. Avolio AP, Chen SG, Wang RP, Zhang CL, Li MF, O'Rourke MF Effects of aging on changing arterial compliance and left ventricular load in a northern Chinese urban community. Circulation 1983;68:50–8 [DOI] [PubMed] [Google Scholar]
- 4. McEniery CM, Yasmin, Hall IR, Qasem A, Wilkinson IB, Cockcroft JR Normal vascular aging: differential effects on wave reflection and aortic pulse wave velocity: the Anglo-Cardiff Collaborative Trial (ACCT). J Am Coll Cardiol 2005;46:1753–60 [DOI] [PubMed] [Google Scholar]
- 5. O'Rourke MF, Hashimoto J Mechanical factors in arterial aging: a clinical perspective. J Am Coll Cardiol 2007;50:1–13 [DOI] [PubMed] [Google Scholar]
- 6. Truswell AS, Kennelly BM, Hansen JD, Lee RB Blood pressures of Kung bushmen in Northern Botswana. Am Heart J 1972;84:5–12 [DOI] [PubMed] [Google Scholar]
- 7. Wang M, Lakatta EG Altered regulation of matrix metalloproteinase-2 in aortic remodeling during aging. Hypertension 2002;39:865–73 [DOI] [PubMed] [Google Scholar]
- 8. Li Z, Froehlich J, Galis ZS, Lakatta EG Increased expression of matrix metalloproteinase-2 in the thickened intima of aged rats. Hypertension 1999;33:116–23 [DOI] [PubMed] [Google Scholar]
- 9. Yasmin, McEniery C, O'Shaughnessy KM, et al. Variation in the human matrix metalloproteinase-9 gene is associated with arterial stiffness in healthy individuals. Arterioscler Thromb Vasc Biol 2006;26:1799–805 [DOI] [PubMed] [Google Scholar]
- 10. Chung AW, Yang HH, Kim JM, et al. Upregulation of matrix metalloproteinase-2 in the arterial vasculature contributes to stiffening and vasomotor dysfunction in patients with chronic kidney disease. Circulation 2009;120:792–801 [DOI] [PubMed] [Google Scholar]
- 11. Vlachopoulos C, Aznaouridis K, Dima I, et al. Negative association between serum levels of matrix metalloproteinases-2 and -9 and aortic stiffness in healthy adults. Int J Cardiol 2007;122:232–8 [DOI] [PubMed] [Google Scholar]
- 12. Corman B, Duriez M, Poitevin P, et al. Aminoguanidine prevents age-related arterial stiffening and cardiac hypertrophy. Proc Natl Acad Sci USA 1998;95:1301–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kass DA, Shapiro EP, Kawaguchi M, et al. Improved arterial compliance by a novel advanced glycation end-product crosslink breaker. Circulation 2001;104:1464–70 [DOI] [PubMed] [Google Scholar]
- 14. Bergel DH The static elastic properties of the arterial wall. J Physiol 1961;156:445–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Roach MR, Burton AC The reason for the shape of the distensibility curves of arteries. Can J Biochem Physiol 1957;35:681–90 [PubMed] [Google Scholar]
- 16. Stewart AD, Millasseau SC, Kearney MT, Ritter JM, Chowienczyk PJ Effects of inhibition of basal nitric oxide synthesis on carotid-femoral pulse wave velocity and augmentation index in humans. Hypertension 2003;42:915–8 [DOI] [PubMed] [Google Scholar]
- 17. Stewart AD, Jiang B, Millasseau SC, Ritter JM, Chowienczyk PJ Acute reduction of blood pressure by nitroglycerin does not normalize large artery stiffness in essential hypertension. Hypertension 2006;48:404–10 [DOI] [PubMed] [Google Scholar]
- 18. Benetos A, Adamopoulos C, Bureau JM, et al. Determinants of accelerated progression of arterial stiffness in normotensive subjects and in treated hypertensive subjects over a 6-year period. Circulation 2002;105:1202–7 [DOI] [PubMed] [Google Scholar]
- 19. Guerin AP, Blacher J, Pannier B, Marchais SJ, Safar ME, London GM Impact of aortic stiffness attenuation on survival of patients in end-stage renal failure. Circulation 2001;103:987–92 [DOI] [PubMed] [Google Scholar]
- 20. Scuteri A, Najjar SS, Muller DC, et al. Metabolic syndrome amplifies the age-associated increases in vascular thickness and stiffness. J Am Coll Cardiol 2004;43:1388–95 [DOI] [PubMed] [Google Scholar]
- 21. Cecelja M, Chowienczyk P Dissociation of aortic pulse wave velocity with risk factors for cardiovascular disease other than hypertension: a systematic review. Hypertension 2009;54:1328–36 [DOI] [PubMed] [Google Scholar]
- 22. Stary HC, Chandler AB, Dinsmore RE, et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler Thromb Vasc Biol 1995;15:1512–31 [DOI] [PubMed] [Google Scholar]
- 23. Hopkins KD, Lehmann ED, Gosling RG Aortic compliance measurements: a non-invasive indicator of atherosclerosis? Lancet 1994;343:1447 [DOI] [PubMed] [Google Scholar]
- 24. Stary HC, Blankenhorn DH, Chandler AB, et al. A definition of the intima of human arteries and of its atherosclerosis-prone regions. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation 1992;85:391–405 [DOI] [PubMed] [Google Scholar]
- 25. Ebrahim S, Papacosta O, Whincup P, et al. Carotid plaque, intima media thickness, cardiovascular risk factors, and prevalent cardiovascular disease in men and women: the British Regional Heart Study. Stroke 1999;30:841–50 [DOI] [PubMed] [Google Scholar]
- 26. Najjar SS, Scuteri A, Lakatta EG Arterial aging: is it an immutable cardiovascular risk factor? Hypertension 2005;46:454–62 [DOI] [PubMed] [Google Scholar]
- 27. Riley WA, Evans GW, Sharrett AR, Burke GL, Barnes RW Variation of common carotid artery elasticity with intimal-medial thickness: the ARIC Study. Atherosclerosis Risk in Communities. Ultrasound Med Biol 1997;23:157–64 [DOI] [PubMed] [Google Scholar]
- 28. Zureik M, Temmar M, Adamopoulos C, et al. Carotid plaques, but not common carotid intima-media thickness, are independently associated with aortic stiffness. J Hypertens 2002;20:85–93 [DOI] [PubMed] [Google Scholar]
- 29. Juo SH, Rundek T, Lin HF, et al. Heritability of carotid artery distensibility in Hispanics: the Northern Manhattan Family Study. Stroke 2005;36:2357–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Safar ME, Blacher J, Mourad JJ, London GM Stiffness of carotid artery wall material and blood pressure in humans: application to antihypertensive therapy and stroke prevention. Stroke 2000;31:782–90 [DOI] [PubMed] [Google Scholar]
- 31. Laurent S, Girerd X, Mourad J, et al. Elastic modulus of the radial artery wall material is not increased in patients with essential hypertension. Arterioscler Thromb 1994;14:1223–31 [DOI] [PubMed] [Google Scholar]
- 32. Mitchell GF Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end-organ damage. J Appl Physiol 2008;105:1652–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ariff B, Zambanini A, Vamadeva S, et al. Candesartan- and atenolol-based treatments induce different patterns of carotid artery and left ventricular remodeling in hypertension. Stroke 2006;37:2381–4 [DOI] [PubMed] [Google Scholar]
- 34. Bots ML, Hofman A, Grobbee DE Increased common carotid intima-media thickness. Adaptive response or a reflection of atherosclerosis? Findings from the Rotterdam Study. Stroke 1997;28:2442–7 [DOI] [PubMed] [Google Scholar]
- 35. Farrar DJ, Bond MG, Riley WA, Sawyer JK Anatomic correlates of aortic pulse wave velocity and carotid artery elasticity during atherosclerosis progression and regression in monkeys. Circulation 1991;83:1754–63 [DOI] [PubMed] [Google Scholar]
- 36. Paini A, Boutouyrie P, Calvet D, Zidi M, Agabiti-Rosei E, Laurent S Multiaxial mechanical characteristics of carotid plaque: analysis by multiarray echotracking system. Stroke 2007;38:117–23 [DOI] [PubMed] [Google Scholar]
- 37. van Popele NM, Grobbee DE, Bots ML, et al. Association between arterial stiffness and atherosclerosis: the Rotterdam Study. Stroke 2001;32:454–60 [DOI] [PubMed] [Google Scholar]
- 38. Bazan HA, Pradhan S, Mojibian H, Kyriakides T, Dardik A Increased aortic arch calcification in patients older than 75 years: implications for carotid artery stenting in elderly patients. J Vasc Surg 2007;46:841–5 [DOI] [PubMed] [Google Scholar]
- 39. Essalihi R, Dao HH, Yamaguchi N, Moreau P A new model of isolated systolic hypertension induced by chronic warfarin and vitamin K1 treatment. Am J Hypertens 2003;16:103–10 [DOI] [PubMed] [Google Scholar]
- 40. Niederhoffer N, Lartaud-Idjouadiene I, Giummelly P, Duvivier C, Peslin R, Atkinson J Calcification of medial elastic fibers and aortic elasticity. Hypertension 1997;29:999–1006 [DOI] [PubMed] [Google Scholar]
- 41. London GM, Guérin AP, Marchais SJ, Métivier F, Pannier B, Adda H Arterial media calcification in end-stage renal disease: impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant 2003;18:1731–40 [DOI] [PubMed] [Google Scholar]
- 42. Odink AE, van der Lugt A, Hofman A, et al. The association of arterial stiffness and arterial calcification: the Rotterdam Study. J Hum Hypertens 2008;22:205–7 [DOI] [PubMed] [Google Scholar]
- 43. McEniery CM, McDonnell BJ, So A, et al. Aortic calcification is associated with aortic stiffness and isolated systolic hypertension in healthy individuals. Hypertension 2009;53:524–31 [DOI] [PubMed] [Google Scholar]
- 44. Cecelja M, Jiang B, Bevan L, Frost ML, Spector TD, Chowienczyk PJ Arterial stiffening relates to arterial calcification but not to noncalcified atheroma in women a twin study. J Am Coll Cardiol 2011;57:1480–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zureik M, Bureau JM, Temmar M, et al. Echogenic carotid plaques are associated with aortic arterial stiffness in subjects with subclinical carotid atherosclerosis. Hypertension 2003;41:519–27 [DOI] [PubMed] [Google Scholar]
- 46. Jondeau G, Boutouyrie P, Lacolley P, et al. Central pulse pressure is a major determinant of ascending aorta dilation in Marfan syndrome. Circulation 1999;99:2677–81 [DOI] [PubMed] [Google Scholar]
- 47. Lacolley P, Boutouyrie P, Glukhova M, et al. Disruption of the elastin gene in adult Williams syndrome is accompanied by a paradoxical reduction in arterial stiffness. Clin Sci (Lond) 2002;103:21–9 [DOI] [PubMed] [Google Scholar]
- 48. Ge D, Young TW, Wang X, Kapuku GK, Treiber FA, Snieder H Heritability of arterial stiffness in black and white American youth and young adults. Am J Hypertens 2007;20:1065–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Moskau S, Golla A, Grothe C, Boes M, Pohl C, Klockgether T Heritability of carotid artery atherosclerotic lesions: an ultrasound study in 154 families. Stroke 2005;36:5–8 [DOI] [PubMed] [Google Scholar]
- 50. Hunt KJ, Duggirala R, Göring HHH, et al. Genetic basis of variation in carotid artery plaque in the San Antonio Family Heart Study. Stroke 2002;33:2775–80 [DOI] [PubMed] [Google Scholar]
- 51. Sayed-Tabatabaei FA, van Rijn MJ, Schut AF, et al. Heritability of the function and structure of the arterial wall: findings of the Erasmus Rucphen Family (ERF) study. Stroke 2005;36:2351–6 [DOI] [PubMed] [Google Scholar]
- 52. O'Donnell CJ, Chazaro I, Wilson PW, et al. Evidence for heritability of abdominal aortic calcific deposits in the Framingham Heart Study. Circulation 2002;106:337–41 [DOI] [PubMed] [Google Scholar]
- 53. Franklin SS, Larson MG, Khan SA, et al. Does the relation of blood pressure to coronary heart disease risk change with aging? The Framingham Heart Study. Circulation 2001;103:1245–9 [DOI] [PubMed] [Google Scholar]
- 54. Bramwell JC, Hill AV Velocity of transmission of the pulse wave and elasticity of arteries. Lancet 1922;1:891–2 [Google Scholar]
- 55. Cecelja M, Jiang B, McNeill K, et al. Increased wave reflection rather than central arterial stiffness is the main determinant of raised pulse pressure in women and relates to mismatch in arterial dimensions: a twin study. J Am Coll Cardiol 2009;54:695–703 [DOI] [PubMed] [Google Scholar]
- 56. Franklin SS, Jacobs MJ, Wong ND, L'Italien GJ, Lapuerta P Predominance of isolated systolic hypertension among middle-aged and elderly US hypertensives: analysis based on National Health and Nutrition Examination Survey (NHANES) III. Hypertension 2001;37:869–74 [DOI] [PubMed] [Google Scholar]
- 57. Cecelja M, Jiang B, Spector TD, Chowienczyk P Progression of central pulse pressure over 1 decade of aging and its reversal by nitroglycerin: a twin study. J Am Coll Cardiol 2012;59:475–83 [DOI] [PubMed] [Google Scholar]
- 58. Pauca AL, Wallenhaupt SL, Kon ND, Tucker WY Does radial artery pressure accurately reflect aortic pressure? Chest 1992;102:1193–8 [DOI] [PubMed] [Google Scholar]
- 59. Dart AM, Gatzka CD, Kingwell BA, et al. Brachial blood pressure but not carotid arterial waveforms predict cardiovascular events in elderly female hypertensives. Hypertension 2006;47:785–90 [DOI] [PubMed] [Google Scholar]
- 60. Jankowski P, Kawecka-Jaszcz K, Czarnecka D, et al. Pulsatile but not steady component of blood pressure predicts cardiovascular events in coronary patients. Hypertension 2008;51:848–55 [DOI] [PubMed] [Google Scholar]
- 61. Pini R, Cavallini MC, Palmieri V, et al. Central but not brachial blood pressure predicts cardiovascular events in an unselected geriatric population: the ICARe Dicomano Study. J Am Coll Cardiol 2008;51:2432–9 [DOI] [PubMed] [Google Scholar]
- 62. Roman MJ, Devereux RB, Kizer JR, et al. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: the Strong Heart Study. Hypertension 2007;50:197–203 [DOI] [PubMed] [Google Scholar]
- 63. Vlachopoulos C, Aznaouridis K, O'Rourke MF, Safar ME, Baou K, Stefanadis C Prediction of cardiovascular events and all-cause mortality with central haemodynamics: a systematic review and meta-analysis. Eur Heart J 2010;31:1865–71 [DOI] [PubMed] [Google Scholar]
- 64. Williams B, Lacy PS, Thom SM, et al. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation 2006;113:1213–25 [DOI] [PubMed] [Google Scholar]
- 65. Chirinos JA, Zambrano JP, Chakko S, et al. Aortic pressure augmentation predicts adverse cardiovascular events in patients with established coronary artery disease. Hypertension 2005;45:980–5 [DOI] [PubMed] [Google Scholar]
- 66. Wang KL, Cheng HM, Sung SH, et al. Wave reflection and arterial stiffness in the prediction of 15-year all-cause and cardiovascular mortalities: a community-based study. Hypertension 2010;55:799–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. London GM, Blacher J, Pannier B, Guérin AP, Marchais SJ, Safar ME Arterial wave reflections and survival in end-stage renal failure. Hypertension 2001;38:434–8 [DOI] [PubMed] [Google Scholar]
- 68. Weber T, Auer J, O'Rourke MF, et al. Increased arterial wave reflections predict severe cardiovascular events in patients undergoing percutaneous coronary interventions. Eur Heart J 2005;26:2657–63 [DOI] [PubMed] [Google Scholar]
- 69. Zoungas S, Cameron JD, Kerr PG, et al. Association of carotid intima-medial thickness and indices of arterial stiffness with cardiovascular disease outcomes in CKD. Am J Kidney Dis 2007;50:622–30 [DOI] [PubMed] [Google Scholar]
- 70. Covic A, Mardare N, Gusbeth-Tatomir P, Prisada O, Sascau R, Goldsmith DJ Arterial wave reflections and mortality in haemodialysis patients – only relevant in elderly, cardiovascularly compromised? Nephrol Dial Transplant 2006;21:2859–66 [DOI] [PubMed] [Google Scholar]
- 71. O'Rourke MF, Kelly RP Wave reflection in the systemic circulation and its implications in ventricular function. J Hypertens 1993;11:327–37 [DOI] [PubMed] [Google Scholar]
- 72. Ioannou CV, Stergiopulos N, Katsamouris AN, et al. Hemodynamics induced after acute reduction of proximal thoracic aorta compliance. Eur J Vasc Endovasc Surg 2003;26:195–204 [DOI] [PubMed] [Google Scholar]
- 73. Wang KL, Cheng HM, Chuang SY, et al. Central or peripheral systolic or pulse pressure: which best relates to _target organs and future mortality? J Hypertens 2009;27:461–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Brown DW, Giles WH, Croft JB Left ventricular hypertrophy as a predictor of coronary heart disease mortality and the effect of hypertension. Am Heart J 2000;140:848–56 [DOI] [PubMed] [Google Scholar]
- 75. Namasivayam M, Adji A, O'Rourke MF Influence of aortic pressure wave components determined noninvasively on myocardial oxygen demand in men and women. Hypertension 2011;57:193–200 [DOI] [PubMed] [Google Scholar]
- 76. O'Rourke MF, Safar ME Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension 2005;46:200–4 [DOI] [PubMed] [Google Scholar]
- 77. North KE, MacCluer JW, Devereux RB, et al. Heritability of carotid artery structure and function: the Strong Heart Family Study. Arterioscler Thromb Vasc Biol 2002;22:1698–703 [DOI] [PubMed] [Google Scholar]
- 78. Mitchell GF, DeStefano AL, Larson MG, et al. Heritability and a genome-wide linkage scan for arterial stiffness, wave reflection, and mean arterial pressure: the Framingham Heart Study. Circulation 2005;112:194–9 [DOI] [PubMed] [Google Scholar]
- 79. Seidlerová J, Bochud M, Staessen JA, et al. Heritability and intrafamilial aggregation of arterial characteristics. J Hypertens 2008;26:721–8 [DOI] [PMC free article] [PubMed] [Google Scholar]