Abstract
Aims
Exercise intolerance is a hallmark of heart failure with preserved ejection fraction (HFpEF), yet its mechanisms remain unclear. The current study sought to determine whether increases in cardiac output (CO) during exercise are appropriately matched to metabolic demands in HFpEF.
Methods and results
Patients with HFpEF (n = 109) and controls (n = 73) exercised to volitional fatigue with simultaneous invasive (n = 96) or non-invasive (n = 86) haemodynamic assessment and expired gas analysis to determine oxygen consumption (VO2) during upright or supine exercise. At rest, HFpEF patients had higher LV filling pressures but similar heart rate, stroke volume, EF, and CO. During supine and upright exercise, HFpEF patients displayed lower peak VO2 coupled with blunted increases in heart rate, stroke volume, EF, and CO compared with controls. LV filling pressures increased dramatically in HFpEF patients, with secondary elevation in pulmonary artery pressures. Reduced peak VO2 in HFpEF patients was predominantly attributable to CO limitation, as the slope of the increase in CO relative to VO2 was 20% lower in HFpEF patients (5.9 ± 2.5 vs. 7.4 ± 2.6 L blood/L O2, P = 0.0005). While absolute increases in arterial–venous O2 difference with exercise were similar in HFpEF patients and controls, augmentation in arterial–venous O2 difference relative to VO2 was greater in HFpEF patients (8.9 ± 3.4 vs. 5.5 ± 2.0 min/dL, P < 0.0001). These differences were observed in the total cohort and when upright and supine exercise modalities were examined individually.
Conclusion
While diastolic dysfunction promotes congestion and pulmonary hypertension with stress in HFpEF, reduction in exercise capacity is predominantly related to inadequate CO relative to metabolic needs.
Keywords: Diastolic heart failure, Exercise, Oxygen consumption, Cardiac output, Stroke volume, Heart rate
Introduction
Heart failure (HF) has been defined as an inability of the heart to provide cardiac output (CO) to the body at a rate commensurate with its needs, or to do so only at the cost of elevated filling pressures.1 Resting CO is generally preserved until the most advanced stages of disease, but CO reserve with exercise is impaired at earlier stages in HF with reduced ejection fraction (HFrEF).2,3 In practice, CO reserve is estimated indirectly by measuring the peak oxygen consumption (peak VO2) attained during exercise.4 However, because increases in CO are tightly coupled to changes in VO2,5,6 simultaneous measurement of CO and VO2 allows for more robust assessment of the adequacy of cardiac oxygen delivery relative to metabolic needs.2,4 This relationship (ΔCO/ΔVO2 slope) is characteristically depressed in HFrEF.2
Half of patients with HF have preserved EF (HFpEF).7,8 Peak VO2 is similarly depressed in HFpEF and HFrEF,9 yet the nature of VO2 impairment with exercise in HFpEF remains controversial.10–22 Potential mechanisms include CO limitation, subjective dyspnoea, impaired vasodilation, skeletal muscle dysfunction, deranged pulmonary gas exchange or mechanics, patient motivation, fitness level, body habitus, and medical co-morbidities. It has recently been reported that exertional capacity in HFpEF is constrained predominantly by abnormalities in cardiac filling13,22 or peripheral O2 extraction,18 rather than CO impairment. Distinguishing these possibilities is of fundamental importance when contemplating novel treatments for HFpEF, a disease with no proven therapy.7,8
The current study aimed to characterize the relationships between ventricular filling and ejection relative to metabolic demand, oxygen delivery, and extraction during exercise in patients with HFpEF. Because haemodynamics differ in the upright and supine positions, and because of potential for referral bias when exclusively studying a catheterization population, we include subjects studied using both invasive and non-invasive methods to measure CO in both the supine and upright positions. We hypothesized that CO reserve relative to VO2 would be impaired in HFpEF patients compared with controls.
Methods
Study population
The total study population is compiled from three cohorts of patients with HFpEF and controls. Cohort 1 (n = 112) includes consecutive patients and controls who underwent invasive supine exercise ergometry studies with simultaneous expired gas analysis at the Mayo Clinic from 2002 to 2011. No data from the cohort 1 patients have been previously published. Cohort 2 (n = 50)15 and cohort 3 (n = 36)12 are from previously published prospective studies examining upright exercise haemodynamics. Some clinical characteristics, exercise capacity, and ventricular–vascular function data from cohorts 2 and 3 have been published,12,15 but the cardiovascular responses as they relate to VO2, the primary aim of the current study, have not been reported. This study complies with the Declaration of Helsinki and has been approved by Institutional Review Boards of the Mayo Clinic and Johns Hopkins Hospital.
Heart failure with preserved EF was defined by LVEF ≥50% and cardiologist-adjudicated diagnosis of HF (Framingham criteria).12,15 Exclusion criteria were significant valvular disease (moderate or greater left-sided regurgitation, any stenosis), cor pulmonale, significant pulmonary disease, unstable coronary disease or coronary spasm, primary renal or hepatic disease, constrictive pericarditis, or infiltrative, restrictive, or hypertrophic cardiomyopathies. Controls in cohort 1 were referred to the cath lab for assessment of exertional dyspnoea and were found to display no cardiac pathology after thorough invasive and non-invasive evaluation. Controls in the non-invasive cohorts were recruited from the community.
Study measurements performed at rest and during exercise in cohorts 1–3 are shown in Supplementary material, Table S1. All patients were studied in the post-absorptive state. Baseline ventricular morphology and function, diastolic filling characteristics, and left atrial volume were measured by transthoracic echocardiography. Echocardiographic LV stroke volume (SV) was determined from LV outflow Doppler, EF was determined by Simpson's biplane method, and LV end-diastolic volume was determined by SV/EF.15,19 Heart rate (HR) was continuously recorded by electrocardiography. Breath-by-breath expired gas analysis was performed at rest and during exercise in all studies (MedGraphics, St Paul, MN, USA) to measure oxygen consumption (VO2).
Invasive haemodynamic exercise assessment (cohort 1)
Right heart catheterization was performed in the supine position through the internal jugular vein.17 Right atrial (RAP), pulmonary artery (PAP), and pulmonary capillary wedge (PCWP) pressures were assessed at end-expiration at rest and after ≥2 min had elapsed at peak workload during supine cycle ergometry. Systemic blood pressure (BP) was measured by intra-arterial (radial) catheter (n = 55) or by cuff sphygmomanometry (n = 41).
Arterial–venous oxygen content difference (AVO2diff) was measured directly as the difference between systemic and pulmonary arterial O2 contents (=saturation ×haemoglobin ×1.34). CO was determined by the direct Fick method (=VO2/AVO2diff). The SV was determined by CO/HR. Pulmonary vascular resistance [PVR = (mean PAP – PCWP)/CO] and effective arterial elastance (Ea = 0.9 ×systolic BP/SV)15,23 were determined as measures of pulmonary and systemic arterial afterload.
Non-invasive haemodynamic exercise assessment (cohorts 2 and 3)
Subjects in cohorts 2 and 3 underwent maximal-effort graded exercise testing on an upright cycle ergometer as previously described.12,15 Cardiac volumes, SV, and EF were assessed at rest and during the final 2 min of exercise by echocardiography in cohort 2 and nuclear gated blood pool scan in cohort 3. CO was determined by SV ×HR. AVO2diff was determined by the Fick method (=VO2/CO).19 Ea was determined as in cohort 1.
Statistical analysis
Data are reported as mean ± standard deviation or median (25th, 75th interquartile range). Between-group differences were compared by t-test, Wilcoxon rank-sum test, or χ2. Multivariable linear regression analysis was used to adjust for relevant baseline group differences and to examine differences with upright and supine exercise, in which the dependent variable was the normally distributed continuous outcome variable of interest, and factors entered into the model included age, gender, body mass, and history of hypertension, diabetes, creatinine, and medication use, or exercise posture. For non-normally distributed variables entered into regression models, the assumption of normally distributed residuals was verified by Quantile plots, and no violations were observed.
Results
Subject characteristics
Patients with HFpEF (n = 109) and controls (n = 73) were assembled from cohorts 1 (n = 71, 25), 2 (n = 21, 29), and 3 (n = 17, 19). The vast majority (n = 100) of patients met recently proposed diagnostic criteria for HFpEF.24 In the remaining nine HFpEF subjects, there was incomplete echocardiographic data limiting classification, though each of these patients had been previously hospitalized for pulmonary oedema that improved with diuresis. Compared with controls, HFpEF patients were older, heavier, and more likely to display co-morbidities and receive treatment with antihypertensives and diuretics (Table 1). Levels of BNP were higher in HFpEF patients compared with controls, whereas haemoglobin and estimated glomerular filtration rate were lower. LV chamber size was similar in HFpEF patients and controls, while LV mass, left atrial volume, and E/e' ratio were greater in HFpEF patients.
Table 1.
Baseline characteristics
| Control (n = 73) | HFpEF (n = 109) | P-value | |
|---|---|---|---|
| Clinical characteristics | |||
| Age (years) | 59 ± 14 | 67 ± 11 | <0.0001 |
| Female (%) | 75 | 72 | 0.6 |
| Body mass index (kg/m2) | 29.1 ± 5.5 | 33.2 ± 7.0 | <0.0001 |
| Body surface area (m2) | 1.95 ± 0.26 | 2.08 ± 0.29 | 0.002 |
| Hypertension (%) | 64 | 82 | 0.009 |
| Diabetes (%) | 16 | 33 | 0.01 |
| Beta-blockers (%) | 33 | 61 | 0.0003 |
| Diuretics (%) | 18 | 69 | <0.0001 |
| Haemoglobin (g/dL) | 13.1 ± 1.7 | 12.4 ± 1.5 | 0.006 |
| Estimated GFR (mL/min/1.73 m2) | 67 ± 20 | 55 ± 18 | 0.0001 |
| BNP (pg/mL) | 36 (15, 71) | 112 (49, 207) | <0.0001 |
| LV morphology and function | |||
| LV end-diastolic volume (mL) | 117 ± 25 | 115 ± 33 | 0.6 |
| LV end-systolic volume (mL) | 41 ± 14 | 38 ± 15 | 0.3 |
| LV mass (g/height2.7) | 45 ± 15 | 51 ± 18 | 0.015 |
| Left atrial volume (mL) | 59 ± 15 | 93 ± 29 | <0.0001 |
| Left atrial volume index (mL/m2) | 30 ± 7 | 45 ± 13 | <0.0001 |
| LVEF (%) | 63 ± 8 | 65 ± 7 | 0.09 |
| E/A ratio | 1.0 ± 0.3 | 1.3 ± 0.8 | 0.006 |
| E' (cm/s) | 7 ± 4 | 7 ± 3 | 0.9 |
| E/E' ratio | 11 ± 5 | 14 ± 8 | 0.004 |
| Resting haemodynamics | |||
| Heart rate (b.p.m.) | 71 ± 11 | 69 ± 10 | 0.4 |
| Systolic blood pressure (mmHg) | 138 ± 21 | 141 ± 24 | 0.5 |
| Mean blood pressure (mmHg) | 98 ± 15 | 95 ± 15 | 0.2 |
| Pulse pressure (mmHg) | 60 ± 14 | 70 ± 21 | 0.0009 |
| Systemic O2 content (mL/dL)a | 16.4 ± 1.6 | 15.6 ± 2.1 | 0.11 |
| PA O2 content (mL/dL)a | 12.6 ± 1.5 | 11.3 ± 1.7 | 0.001 |
| Right atrial pressure (mmHg)a | 4 ± 2 | 9 ± 4 | <0.0001 |
| PA systolic pressure (mmHg)a | 26 ± 6 | 39 ± 11 | <0.0001 |
| Mean PA pressure (mmHg)a | 16 ± 4 | 25 ± 7 | <0.0001 |
| PCWP (mmHg)a | 9 ± 3 | 16 ± 6 | <0.0001 |
| Cardiac output (L/min) | 5.4 ± 1.4 | 5.4 ± 1.7 | 0.9 |
| Cardiac index (L/min/m2) | 2.8 ± 0.7 | 2.6 ± 0.8 | 0.2 |
| PVR (mmHg/L/min)a | 1.2 ± 0.7 | 2.0 ± 1.3 | 0.008 |
| Ea (mmHg/mL) | 1.7 ± 0.4 | 1.8 ± 0.6 | 0.4 |
Data are reported as mean ± standard deviation, percentage of population, or median (25th, 75th interquartile range where appropriate.
E', mitral valve inflow tissue velocity; E/A, early to late mitral valve inflow velocity; GFR, glomerular filtration rate (Modified Diet in Renal Disease equation); HFpEF, heart failure with preserved ejection fraction; PA, pulmonary artery; PVR, pulmonary vascular resistance; SVR, systemic vascular resistance.
aData only available for Cohort 1 population (n = 71 HFpEF and 25 controls).
Baseline haemodynamics
Resting HR, BP, CO, and Ea were similar in HFpEF patients and controls (Table 1). Pulmonary artery O2 content was lower in those with HFpEF, suggesting relative inadequacy of O2 delivery compared with controls. Pulse pressure was higher in HFpEF patients, consistent with greater systemic arterial stiffening. Right and left heart filling pressures, PAPs, and PVR were higher in HFpEF patients than in controls.
Exercise performance
Resting VO2 was similar in HFpEF patients and controls, but when scaled to weight, resting VO2 was lower in those with HFpEF (Table 2). Compared with controls, HFpEF patients achieved lower peak workload, reduced peak VO2, and less increase in VO2 during exercise. Each of these differences persisted after adjusting for age, body mass index (BMI), hypertension, diabetes, glomerular filtration rate, haemoglobin, vasodilator use, and beta-blocker use. Exercise systemic O2 content was similar in HFpEF patients and controls, while pulmonary artery O2 content was lower in those with HFpEF. No difference between the groups was observed in resting, peak exercise, or absolute exercise change in AVO2diff.
Table 2.
Exercise responses
| Control (n = 73) | HFpEF (n = 109) | P-value | |
|---|---|---|---|
| Exercise performance | |||
| Peak workload (W) | 80 ± 30 | 40 ± 20 | <0.0001 |
| Resting VO2 (mL/min) | 261 ± 65 | 249 ± 78 | 0.3 |
| Resting VO2 (mL/kg/min) | 3.26 ± 0.63 | 2.72 ± 0.68 | <0.0001 |
| Exercise VO2 (mL/min) | 1269 ± 395 | 899 ± 312 | <0.0001 |
| Exercise VO2 (mL/kg/min) | 15.7 ± 4.2 | 9.8 ± 3.0 | <0.0001 |
| ΔVO2 (mL/min) | +1008 ± 354 | +651 ± 272 | <0.0001 |
| ΔVO2 (mL/kg/min) | +12.5 ± 3.9 | +7.1 ± 2.8 | <0.0001 |
| Exercise systemic O2 content (mL/dL)a | 17.0 ± 1.6 | 16.2 ± 2.1 | 0.11 |
| Exercise PA O2 content (mL/dL)a | 8.4 ± 1.9 | 6.9 ± 2.1 | 0.003 |
| Resting AVO2diff (mL/dL) | 5.1 ± 1.8 | 4.8 ± 1.3 | 0.2 |
| Exercise AVO2diff (mL/dL) | 10.1 ± 2.8 | 9.9 ± 3.2 | 0.7 |
| ΔAVO2diff (mL/dL) | +5.0 ± 1.8 | +5.2 ± 2.5 | 0.6 |
| Peak exercise haemodynamics | |||
| Heart rate (b.p.m.) | 128 ± 23 | 101 ± 20 | <0.0001 |
| Systolic BP (mmHg) | 183 ± 34 | 166 ± 34 | 0.001 |
| Mean BP (mmHg) | 122 ± 22 | 112 ± 22 | 0.005 |
| PA systolic pressure (mmHg)a | 41 ± 9 | 68 ± 13 | <0.0001 |
| Mean PA pressure (mmHg)a | 26 ± 6 | 46 ± 9 | <0.0001 |
| PCWP (mmHg)a | 14 ± 4 | 33 ± 8 | <0.0001 |
| Cardiac output (L/min) | 12.5 ± 2.8 | 9.2 ± 2.8 | <0.0001 |
| Cardiac index (L/min/m2) | 6.4 ± 1.3 | 4.4 ± 1.2 | <0.0001 |
| PVR (mmHg/L/min)a | 1.0 ± 0.4 | 1.5 ± 1.1 | <0.05 |
| Ea (mmHg/mL) | 1.7 ± 0.5 | 1.8 ± 0.7 | 0.8 |
| Integrated changes with exercise | |||
| ΔAVO2diff/ΔVO2 (min/dL) | +5.5 ± 2.0 | +8.9 ± 3.4 | <0.0001 |
| ΔCO/ΔVO2 (L blood/L O2) | +7.4 ± 2.6 | +5.9 ± 2.5 | 0.0005 |
Data are reported as mean ± standard deviation.
AVO2diff, arterial–venous oxygen difference; BP, blood pressure; CO, cardiac output; Ea, arterial elastance; HFpEF, heart failure with preserved ejection fracttion; PA, pulmonary artery; PVR, pulmonary vascular resistance; SVR, systemic vascular resistance, VO2, volume of oxygen consumed.
aData only available for Cohort 1 population (n = 71 HFpEF and 25 controls).
Exercise haemodynamics
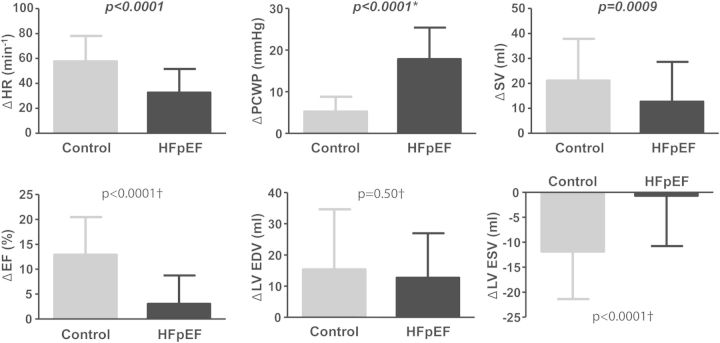
Compared with controls, HFpEF subjects demonstrated less increase in HR, BP, SV, EF, and CO with exercise (Table 2, Figure 1). Depressed EF reserve was due to lesser reduction in LV end-systolic volume in HFpEF patients, as changes in end-diastolic volume were similar in HFpEF patients and controls. Right and left heart filling pressures and PAPs with exercise were higher in those with HFpEF than in controls. Each of these differences persisted after adjusting for age, BMI, hypertension, diabetes, glomerular filtration rate, haemoglobin, vasodilator use, and beta-blocker use. Pulmonary hypertension in HFpEF patients was predominantly due to high PCWP, as PVR reductions with exercise were similar in HFpEF patients and controls (–0.5 ± 1.0 vs. –0.2 ± 0.7 mmHg/L/min, P = 0.3). Exercise Ea and changes in Ea were similar between HFpEF patients and controls.
Figure 1.
Haemodynamic changes with exercise in heart failure with preserved ejection fraction (HFpEF; black) compared with controls (grey). *Data available only for cohort 1 (n = 71 HFpEF and 25 controls); †Data available only for cohorts 2 and 3 (n = 38 HFpEF and 48 controls). EDV, end-diastolic volume; HR, heart rate; SV, stroke volume.
Integrated responses
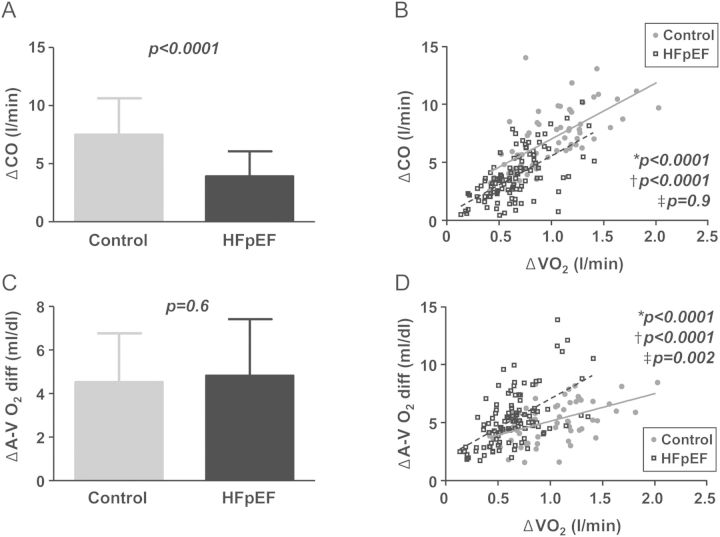
Depressed CO reserve in HFpEF patients could be caused by the lower absolute workload achieved or by cardiac limitation. To distinguish between these possibilities, the enhancement in CO relative to VO2 was then analysed, identifying CO limitation as the primary culprit in HFpEF on average (Figure 2A and B). This is reflected by a significantly lower ΔCO/ΔVO2 slope in HFpEF patients compared with controls (5.9 ± 2.5 vs. 7.4 ± 2.6, P = 0.0005; Table 2). Enhancement of CO with exercise was also reduced in HFpEF patients for any change in LV filling pressure (PCWP) or LV end-diastolic volume (Figure 3A and B). The ΔCO/ΔVO2 relationships were similar in HFpEF subjects treated or not treated with beta-blockers (5.6 ± 2.4 vs. 6.3 ± 2.6, P = 0.2). In contrast to VO2, scaling ΔCO to external work performed (in Watts) did not reveal a difference in HFpEF patients and controls overall or when substratified into lower and higher VO2 categories (Supplementary material, Table S2).
Figure 2.
(A and B) Absolute increases in cardiac output (ΔCO) and ΔCO as a function of metabolic requirements (ΔVO2) were impaired in heart failure with preserved ejection fraction (HFpEF; boxes–dashed line) compared with controls (circles–solid line). (C) Absolute increases in arterial–venous oxygen extraction (ΔAVO2diff) were similar at peak exercise, although O2 extraction relative to O2 consumption was greater in HFpEF (D). P-values refer to *bivariate comparisons, †HFpEF vs. control, and ‡interaction terms.
Figure 3.
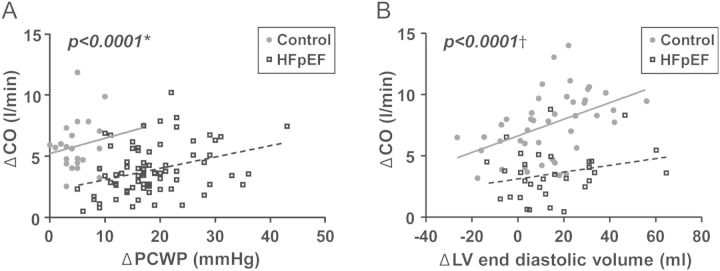
(A) Exercise increases in cardiac output (ΔCO) were impaired in heart failure with preserved ejection fraction (HFpEF; boxes–dashed line) compared with controls (circles–solid line) for any change in pulmonary capillary wedge pressure (ΔPCWP) or (B) LV end-diastolic volume. *Data available only for cohort 1 (n = 71 HFpEF and 25 controls); †Data available only for cohorts 2 and 3 (n = 38 HFpEF and 48 controls).
While absolute changes in oxygen extraction (AVO2diff) at peak exercise were similar in HFpEF patients and controls (Table 2, Figure 2C), HFpEF subjects relied on a greater increase in AVO2diff for any absolute change in VO2 (Figure 2D). The increase in O2 extraction relative to O2 consumption (ΔAVO2diff/ΔVO2 slope) was thus greater in HFpEF patients compared with controls (8.9 ± 3.4 vs, 5.5 ± 2.0 min/dL, P < 0.0001). Diabetics had higher resting AVO2diff (P = 0.04), but changes in AVO2diff with exercise were not different from those of non-diabetics. There were no differences in AVO2diff at rest or with exercise in the hypertensive and obese subgroups.
Importantly, these differences in ΔCO/ΔVO2 and ΔAVO2diff/ΔVO2 in HFpEF patients and controls were consistently observed when cohorts 1–3 were analysed separately rather than together (Table 3). Thus, regardless of body position during exercise, or method used to measure CO, the increase in CO was on average consistently impaired in those eith HFpEF, relative to metabolic demand. These differences persisted after adjusting for age, BMI, hypertension, diabetes, glomerular filtration rate, haemoglobin, vasodilator use, and beta-blocker use.
Table 3.
Uniformity of results across cohorts
| Cohort 1 (n = 96) |
Cohort 2 (n = 50) |
Cohort 3 (n = 36) |
||||
|---|---|---|---|---|---|---|
| Control (n = 25) | HFpEF (n = 71) | Control (n = 29) | HFpEF (n = 21) | Control (n = 19) | HFpEF (n = 17) | |
| Peak VO2 (mL/min) | 996 ± 280 | 823 ± 268* | 1492 ± 365 | 1168 ± 279* | 1215 ± 352 | 863 ± 343* |
| ΔVO2 (mL/min) | +781 ± 260 | +596 ± 233* | +1203 ± 335 | +867 ± 268* | +937 ± 304 | +598 ± 299* |
| Peak CO (L/min) | 11.5 ± 2.5 | 9.1 ± 2.8* | 12.5 ± 3.1 | 9.1 ± 2.5* | 13.4 ± 2.6 | 9.5 ± 2.9* |
| ΔCO (L/min) | +5.9 ± 2.2 | +3.8 ± 2.1* | +8.1 ± 2.4 | +4.3 ± 2.1* | +6.9 ± 2.9 | +3.0 ± 1.8* |
| Peak CI (L/min/m2) | 6.0 ± 1.0 | 4.4 ± 1.1* | 6.8 ± 1.5 | 4.5 ± 1.5* | 6.3 ± 1.3 | 4.5 ± 1.2* |
| ΔCI (L/min/m2) | +3.0 ± 1.0 | +1.8 ± 0.9* | +3.5 ± 1.7 | +1.4 ± 0.8* | +4.1 ± 1.0 | +2.1 ± 1.0* |
| Peak AVO2diff (mL/dL) | 8.4 ± 1.8 | 9.1 ± 2.3 | 12.4 ± 2.2 | 13.2 ± 3.6 | 9.2 ± 2.4 | 9.6 ± 3.9 |
| ΔAVO2diff (mL/dL) | +4.6 ± 1.5 | +4.7 ± 2.0 | +5.5 ± 1.8 | +6.9 ± 3.1 | +4.8 ± 2.0 | +5.4 ± 3.1 |
| ΔCO/ΔVO2 (L blood/L O2) | 7.6 ± 1.8 | 6.3 ± 2.4* | 6.6 ± 1.5 | 5.0 ± 1.9* | 8.3 ± 4.3 | 5.3 ± 3.0* |
| ΔAVO2/ΔVO2 (min/dL) | 6.5 ± 1.8 | 8.9 ± 3.4* | 4.7 ± 1.8 | 8.2 ± 2.7* | 5.3 ± 2.2 | 9.4 ± 4.1* |
AVO2diff, arterial–venous oxygen difference; CI, cardiac index; CO, cardiac output; HFpEF, heart failure with preserved ejection fraction; VO2, oxygen consumption.
*P < 0.05 vs control.
Cardiac output reserve impairment in HFpEF
Impact of body position
Compared with supine ergometry, upright exercise was associated with greater increases in VO2, CO, AVO2diff, HR, and BP (Supplementary material, Table S3). However, in multivariable regression analysis including exercise position in the model, HFpEF subjects continued to display highly significant impairments in the exercise augmentation in HR, SV, BP, CO, VO2, and ΔCO/ΔVO2, with greater ΔAVO2diff/ΔVO2 (Supplementary material, Table S3). Significant group ×position interactions were observed for exercise increases in CO and BP, where HFpEF patients displayed the smallest increases compared with controls during upright exercise.
Discussion
This study assessed haemodynamic responses to exercise in order to determine how cardiac filling and ejection capacity affect oxygen delivery and extraction to mediate exercise limitation (reduced VO2) in patients with HFpEF. We demonstrate that compared with controls, the increase in CO relative to metabolic requirements (VO2) is fundamentally impaired in HFpEF. CO reserve limitation in HFpEF was coupled to impairments in LV contractile and chronotropic reserve. Increases in LV preload (end-diastolic volume) during exercise were similar in HFpEF patients and controls, but similar preload recruitment in those with HFpEF required three-fold greater increases in LV filling pressures (PCWP), causing secondary elevation in PAP, which may impair right ventricular ejection and further contribute to blunted CO reserve. Increases in AVO2diff at peak exercise were similar in HFpEF patients and controls, but increases relative to absolute O2 consumption were enhanced in those with HFpEF, suggesting peripheral adaptation to the impairment in O2 delivery (CO reserve). These findings were consistently observed during both supine and upright exercise and employing both invasive and non-invasive modalities to assess CO, indicating that in addition to abnormalities in LV diastolic filling, impairments in CO reserve with stress contribute to the impairment in oxygen consumption in patients with HFpEF.
Cardiac output and peak VO2 in heart failure with preserved ejection fraction
Functional capacity (peak VO2) is similarly impaired in HFpEF and HFrEF.9 Peak VO2 is an integrated measure of cardiac reserve that is being used to diagnose HFpEF25 and as an endpoint in clinical trials.26–28 Thus, better characterization of the fundamental mechanisms underlying VO2 limitation in HFpEF is essential for improved understanding of pathophysiological mechanisms and to better inform future trial design and identify therapeutic _targets.
Most studies have reported that exercise VO2 and CO are individually depressed in HFpEF.10,12,15–20,23,29,30 However, VO2 and CO at any time during exercise are largely determined by the intensity of work being performed.4 Thus, group differences in CO might be caused by cardiac limitations or non-cardiovascular factors including patient motivation, peripheral limitations, fitness, or orthopaedic issues. Elevation in cardiac filling pressures (at rest or with stress) is the most conspicuous and consistently observed haemodynamic feature in HFpEF.17 Non-diastolic limitations have been reported,12,15,16,19,23,29,30 but their roles have been questioned.22 Impaired exercise reserve responses in non-diastolic parameters (e.g. HR or contractile response) may not be causal of exercise intolerance, but rather consequence of premature cessation of exercise in response to dyspnoea from high filling pressures,18 abnormal metabolic–neural signalling,31,32 or non-cardiac factors such as deconditioning or obesity.11,14 Indeed, textbooks of exercise physiology describe how patients with HFrEF are limited by inadequate CO reserve with exercise, whereas patients with HFpEF have normal CO responses but elevated filling pressures.33 It has even been questioned whether HFpEF truly represents a form of cardiac failure, since patients are frequently elderly with co-morbidities that might in themselves produce symptoms of effort intolerance that are not directly attributable to cardiac dysfunction.21
The current data provide compelling evidence that the reduction in exercise capacity in HFpEF is determined largely by inadequate CO reserve, which, when combined with stress-induced elevations in cardiac filling pressures,10,13,16,17,20 markedly limits exercise capacity. The strength of this experimental approach lies in the simultaneous assessment of both whole-body O2 delivery (CO) and O2 consumption (VO2). Because increases in CO during exercise are ultimately driven by increases in VO2,5,6 this analysis allows for direct comparisons of exercise responses between patients with HFpEF and controls, without the need to adjust for measures of effort adequacy (such as the respiratory exchange ratio) that are necessary to gauge metabolic status when CO is not directly measured. Intriguingly, scaling CO reserve to external work failed to reveal the cardiac limitation in HFpEF, suggesting that this index is less sensitive to haemodynamic impairments in HFpEF.
The relationship between CO and VO2 is typically depressed in patients with HFrEF,2 in keeping with the definition of HF as an inability to pump blood adequately to the body at normal filling pressures.1 However, only one previous study has examined the relationship between CO and VO2 in HFpEF.18 Bhella and colleagues found that peak VO2 and CO were reduced in HFpEF, similar to the current data, but, when plotting CO relative to VO2, the authors surprisingly found that the enhancement in CO was elevated in HFpEF. The authors speculated that abnormalities in skeletal muscle might generate metabolic signals that drive excessive increases in CO, leading to increased ventricular filling pressures during exercise in HFpEF.18
The current data argue against this hypothesis, showing that on average the increase in CO relative to VO2 was impaired in HFpEF. The reasons for the discordant findings are not obvious, although it is notable that the SV enhancement during exercise in HFpEF patients noted by Bhella et al. (+74% increase) was remarkably high, and well in excess of the +16% increase noted in the current study and the –7 to +10% changes with exercise reported by other groups.16,23,29,30 Secondly, Bhella and colleagues found that the resting VO2 was elevated in the HFpEF patients—suggesting a hypermetabolic state. In contrast, in the current study, the resting O2 consumption (scaled to body mass) was lower in HFpEF. These differences may relate to changes in metabolism reflecting variability in HF severity or chronicity between the two study populations.
Determinants of cardiac output limitation
Cardiac output reserve limitation in HFpEF was related to impaired SV and HR, similar to previous studies,10,12,15–17,19,23,29,30 Inadequate SV (and EF) reserve was due to an inability to reduce LV end-systolic volume with exercise, since end-diastolic volume increased similarly in HFpEF patients and controls. Impaired reduction in end-systolic volume could be caused by inadequate enhancement in contractility, blunted afterload reduction, or both. Previous studies have reported attenuated reductions in systemic vascular resistance (SVR) during exercise in HFpEF patients.12,29,30 In this study, effective arterial elastance (Ea), which characterizes total (resistive and pulsatile) arterial afterload, changed similarly in HFpEF patients and controls. This finding may appear at odds with previous studies showing increased arterial stiffness and impaired flow-mediated dilation in HFpEF.12,15,29 However, it is also known that Ea varies directly with HR, in addition to SVR.3 Because HR was ∼30% higher at peak exercise in controls, this would inflate the exercise Ea value in this group, even if other components of afterload were lower. The similar change in Ea observed during exercise in the current study suggests that the blunted SV and EF responses with exercise in HFpEF patients were caused primarily by limitations in contractile reserve.
Enhancement in LV end-diastolic volume was similar in HFpEF patients and controls, though diastolic reserve was clearly impaired, as evidenced by the three-fold greater elevation in PCWP in HFpEF patients. It is currently unknown if mitigation of PCWP elevation in HFpEF would directly improve aerobic capacity, though a recent trial found that exercise training was associated with a reduced resting E/e' ratio (a marker of PCWP), and the extent of resting E/e' improvement was correlated with the improvement in peak VO2.27 Elevation in LV diastolic pressure is often considered to limit exercise capacity by provoking dyspnoea, yet it is notable that PCWP increases during exercise in patients with HFpEF were associated with dramatic elevations in PAPs, increasing right ventricular afterload. Given the well-described impact of right ventricular dysfunction on exercise capacity in HFrEF,35 the enhanced load sensitivity of the right ventricle,36 and the deleterious impact of increased PCWP on pulsatile right ventricular load,37 it is likely that PCWP elevation from diastolic dysfunction in HFpEF has additional implications for right ventricular reserve that may also limit CO responses to exercise.
Arterial–venous oxygen extraction reserve
The Fick equation dictates that VO2 is equal to the product of CO and AVO2diff, and patients with HF may display reduced peak VO2 that is related to abnormalities in the latter, the former, or both.18,19,38 We found that AVO2diff at peak exercise was not different between HFpEF patients and controls, though the increase in AVO2diff as a function of VO2 (ΔAVO2 difference/ΔVO2 slope) was enhanced in those with HFpEF. We speculate that this functions to compensate partly for inadequate O2 delivery from CO reserve impairment at submaximal workloads in HFpEF.
The similar increase in peak exercise AVO2diff in cases and controls is similar to recent findings from Maeder and colleagues,16 but differs from two recent reports showing reduced AVO2diff at peak exercise in HFpEF patients.18,19 These discrepancies may relate to the populations studied and different analytical approaches. The latter studies enrolled disease-free controls, as opposed to the current study that included controls with co-morbidities that might influence O2 extraction. Haykowsky et al. performed comparisons of AVO2diff relative to workload (Watts) as opposed to VO2. They also found that the oxygen uptake/work slope was attenuated in HFpEF, meaning that VO2 was lower for any workload in HFpEF. Thus, the ΔAVO2diff/ΔVO2 slope in their HFpEF group might be expected to have been steeper if their data were examined according to VO2.
When CO is reduced, circulation time is increased, which may provide greater time for gas diffusion at the capillaries and greater O2 extraction.39 Thus, our findings should not be taken to indicate that patients with HFpEF are more ‘adept’ at peripheral O2 extraction, or that abnormalities in the periphery do not play key roles in limiting exercise capacity in HFpEF, as indicated in multiple recent studies.2,18,19,38 Indeed, even with limited CO reserve, improvements in peripheral function may be attainable with interventions such as exercise training, probably related to the greater plasticity in the vasculature and skeletal muscle.26,27,40 Future research may clarify whether clinical evaluation of both VO2 and CO reserve will allow for more refined insight as to whether limitations in a specific patient are due predominantly to cardiac or peripheral factors, possibly to better tailor therapy.4
Limitations
Patients with HFpEF and control subjects were drawn from three cohorts, two prospectively enrolled and one retrospective. Cohort 1 was a cath lab referral population, introducing potential bias. The protocols and methods to measure CO were different. However, all are well established, and the finding of impaired CO reserve relative to VO2 was uniformly and consistently observed in both upright and supine exercise when analysed separately and taking into account body position (Table 3; Supplementary material, Table S3). Cardiac pressure and volume were not measured simultaneously during exercise and in the same patient, but pressure and volume data in the three cohorts were included to provide insight into the mechanisms for CO limitation in HFpEF. Importantly, the primary endpoints (CO and VO2) were directly measured at rest and during exercise in all subjects. Baseline differences were present, including greater age, adiposity, beta-blocker use, and creatinine in those with HFpEF. However, all group differences remained highly significant after adjusting for each of these baseline differences. Subjective symptoms (dyspnoea, fatigue) were not quantified, and many subjects did not exercise to their ideal maximum capacity, particularly during supine ergometry, where peak HR and VO2 were lower. However, attainment of true maximal objective exercise workload is not necessary in this analysis, because adequacy of CO reserve was evaluated by scaling it to the physiological variable that drives it (VO2). This study did not assess for the development of mitral regurgitation during exercise, which may also contribute to impaired CO reserve and exertional pulmonary hypertension in HFpEF.29 Not all HFpEF subjects were taking chronic diuretics (69%), but this prevalence is similar to other studies of compensated HFpEF outpatients.19
Conclusions
The reduction in oxygen consumption during exercise in patients with HFpEF is determined predominantly by inadequate CO to the body relative to metabolic requirements. These data suggest that in addition to therapies _targeting elevation in cardiac filling pressures, treatments designed to enhance CO responses with stress may prove beneficial to improve exercise capacity and outcomes in HFpEF.
Supplementary material
Supplementary material is available at European Journal of Heart Failure online.
Funding
The Mayo Clinic Center for Translational Science Activities, the National Institutes of Health (UL RR024150); the Marie Ingalls Career Development Award in Cardiovascular Research (to B.A.B.).
Conflict of interest: none declared.
References
- 1.Denolin H, Kuhn H, Krayenbuehl HP, Loogen F, Reale A. The definition of heart failure. Eur Heart J. 1983;4:445–448. doi: 10.1093/oxfordjournals.eurheartj.a061500. [DOI] [PubMed] [Google Scholar]
- 2.Chomsky DB, Lang CC, Rayos GH, Shyr Y, Yeoh TK, Pierson RN, 3rd, Davis SF, Wilson JR. Hemodynamic exercise testing. A valuable tool in the selection of cardiac transplantation candidates. Circulation. 1996;94:3176–3183. doi: 10.1161/01.cir.94.12.3176. [DOI] [PubMed] [Google Scholar]
- 3.Sullivan MJ, Knight JD, Higginbotham MB, Cobb FR. Relation between central and peripheral hemodynamics during exercise in patients with chronic heart failure. Muscle blood flow is reduced with maintenance of arterial perfusion pressure. Circulation. 1989;80:769–781. doi: 10.1161/01.cir.80.4.769. [DOI] [PubMed] [Google Scholar]
- 4.Lang CC, Agostoni P, Mancini DM. Prognostic significance and measurement of exercise-derived hemodynamic variables in patients with heart failure. J Card Fail. 2007;13:672–679. doi: 10.1016/j.cardfail.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Dexter L, Whittenberger JL, Haynes FW, Goodale WT, Gorlin R, Sawyer CG. Effect of exercise on circulatory dynamics of normal individuals. J Appl Physiol. 1951;3:439–453. doi: 10.1152/jappl.1951.3.8.439. [DOI] [PubMed] [Google Scholar]
- 6.Astrand PO, Cuddy TE, Saltin B, Stenberg J. Cardiac output during submaximal and maximal work. J Appl Physiol. 1964;19:268–274. doi: 10.1152/jappl.1964.19.2.268. [DOI] [PubMed] [Google Scholar]
- 7.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, Bonet LA, Avraamides P, Ben Lamin HA, Brignole M, Coca A, Cowburn P, Dargie H, Elliott P, Flachskampf FA, Guida GF, Hardman S, Iung B, Merkely B, Mueller C, Nanas JN, Nielsen OW, Orn S, Parissis JT, Ponikowski P ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012;14:803–869. doi: 10.1093/eurjhf/hfs105. [DOI] [PubMed] [Google Scholar]
- 8.Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J. 2011;32:670–679. doi: 10.1093/eurheartj/ehq426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kitzman DW, Little WC, Brubaker PH, Anderson RT, Hundley WG, Marburger CT, Brosnihan B, Morgan TM, Stewart KP. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA. 2002;288:2144–2150. doi: 10.1001/jama.288.17.2144. [DOI] [PubMed] [Google Scholar]
- 10.Kitzman DW, Higginbotham MB, Cobb FR, Sheikh KH, Sullivan MJ. Exercise intolerance in patients with heart failure and preserved left ventricular systolic function: failure of the Frank–Starling mechanism. J Am Coll Cardiol. 1991;17:1065–1072. doi: 10.1016/0735-1097(91)90832-t. [DOI] [PubMed] [Google Scholar]
- 11.Caruana L, Petrie MC, Davie AP, McMurray JJ. Do patients with suspected heart failure and preserved left ventricular systolic function suffer from ‘diastolic heart failure’ or from misdiagnosis? A prospective descriptive study. BMJ. 2000;321:215–218. doi: 10.1136/bmj.321.7255.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borlaug BA, Melenovsky V, Russell SD, Kessler K, Pacak K, Becker LC, Kass DA. Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation. 2006;114:2138–2147. doi: 10.1161/CIRCULATIONAHA.106.632745. [DOI] [PubMed] [Google Scholar]
- 13.Westermann D, Kasner M, Steendijk P, Spillmann F, Riad A, Weitmann K, Hoffmann W, Poller W, Pauschinger M, Schultheiss HP, Tschope C. Role of left ventricular stiffness in heart failure with normal ejection fraction. Circulation. 2008;117:2051–2060. doi: 10.1161/CIRCULATIONAHA.107.716886. [DOI] [PubMed] [Google Scholar]
- 14.Ingle L, Cleland JG, Clark AL. Perception of symptoms is out of proportion to cardiac pathology in patients with ‘diastolic heart failure. Heart. 2008;94:748–753. doi: 10.1136/hrt.2007.131144. [DOI] [PubMed] [Google Scholar]
- 15.Borlaug BA, Olson TP, Lam CS, Flood KS, Lerman A, Johnson BD, Redfield MM. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2010;56:845–854. doi: 10.1016/j.jacc.2010.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maeder MT, Thompson BR, Brunner-La Rocca HP, Kaye DM. Hemodynamic basis of exercise limitation in patients with heart failure and normal ejection fraction. J Am Coll Cardiol. 2010;56:855–863. doi: 10.1016/j.jacc.2010.04.040. [DOI] [PubMed] [Google Scholar]
- 17.Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3:588–595. doi: 10.1161/CIRCHEARTFAILURE.109.930701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhella PS, Prasad A, Heinicke K, Hastings JL, Arbab-Zadeh A, Adams-Huet B, Pacini EL, Shibata S, Palmer MD, Newcomer BR, Levine BD. Abnormal haemodynamic response to exercise in heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13:1296–1304. doi: 10.1093/eurjhf/hfr133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haykowsky MJ, Brubaker PH, John JM, Stewart KP, Morgan TM, Kitzman DW. Determinants of exercise intolerance in elderly heart failure patients with preserved ejection fraction. J Am Coll Cardiol. 2011;58:265–274. doi: 10.1016/j.jacc.2011.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borlaug BA, Jaber WA, Ommen SR, Lam CS, Redfield MM, Nishimura RA. Diastolic relaxation and compliance reserve during dynamic exercise in heart failure with preserved ejection fraction. Heart. 2011;97:964–969. doi: 10.1136/hrt.2010.212787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Packer M. Can brain natriuretic peptide be used to guide the management of patients with heart failure and a preserved ejection fraction? The wrong way to identify new treatments for a nonexistent disease. Circ Heart Fail. 2011;4:538–540. doi: 10.1161/CIRCHEARTFAILURE.111.963710. [DOI] [PubMed] [Google Scholar]
- 22.Paulus WJ. Culprit mechanism(s) for exercise intolerance in heart failure with normal ejection fraction. J Am Coll Cardiol. 2010;56:864–866. doi: 10.1016/j.jacc.2010.04.041. [DOI] [PubMed] [Google Scholar]
- 23.Phan TT, Abozguia K, Nallur Shivu G, Mahadevan G, Ahmed I, Williams L, Dwivedi G, Patel K, Steendijk P, Ashrafian H, Henning A, Frenneaux M. Heart failure with preserved ejection fraction is characterized by dynamic impairment of active relaxation and contraction of the left ventricle on exercise and associated with myocardial energy deficiency. J Am Coll Cardiol. 2009;54:402–409. doi: 10.1016/j.jacc.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 24.Paulus WJ, Tschope C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite-Moreira AF, Borbely A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539–2550. doi: 10.1093/eurheartj/ehm037. [DOI] [PubMed] [Google Scholar]
- 25.Mahadevan G, Dwivedi G, Williams L, Steeds RP, Frenneaux M. Epidemiology and diagnosis of heart failure with preserved left ventricular ejection fraction: rationale and design of the study. Eur J Heart Fail. 2012;14:106–112. doi: 10.1093/eurjhf/hfr153. [DOI] [PubMed] [Google Scholar]
- 26.Kitzman DW, Brubaker PH, Morgan TM, Stewart KP, Little WC. Exercise training in older patients with heart failure and preserved ejection fraction: a randomized, controlled, single-blind trial. Circ Heart Fail. 2010;3:659–667. doi: 10.1161/CIRCHEARTFAILURE.110.958785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edelmann F, Gelbrich G, Dungen HD, Frohling S, Wachter R, Stahrenberg R, Binder L, Topper A, Lashki DJ, Schwarz S, Herrmann-Lingen C, Loffler M, Hasenfuss G, Halle M, Pieske B. Exercise training improves exercise capacity and diastolic function in patients with heart failure with preserved ejection fraction: results of the Ex-DHF (Exercise training in Diastolic Heart Failure) pilot study. J Am Coll Cardiol. 2011;58:1780–1791. doi: 10.1016/j.jacc.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 28.Redfield MM, Borlaug BA, Lewis GD, Mohammed SF, Semigran MJ, Lewinter MM, Deswal A, Hernandez AF, Lee KL, Braunwald E. PhosphodiesteRasE-5 inhibition to improve cLinical status and EXercise capacity in diastolic heart failure (RELAX) trial: rationale and design. Circ Heart Fail. 2012;5:653–659. doi: 10.1161/CIRCHEARTFAILURE.112.969071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ennezat PV, Lefetz Y, Marechaux S, Six-Carpentier M, Deklunder G, Montaigne D, Bauchart JJ, Mounier-Vehier C, Jude B, Neviere R, Bauters C, Asseman P, de Groote P, Lejemtel TH. Left ventricular abnormal response during dynamic exercise in patients with heart failure and preserved left ventricular ejection fraction at rest. J Card Fail. 2008;14:475–480. doi: 10.1016/j.cardfail.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 30.Tan YT, Wenzelburger F, Lee E, Heatlie G, Leyva F, Patel K, Frenneaux M, Sanderson JE. The pathophysiology of heart failure with normal ejection fraction: exercise echocardiography reveals complex abnormalities of both systolic and diastolic ventricular function involving torsion, untwist, and longitudinal motion. J Am Coll Cardiol. 2009;54:36–46. doi: 10.1016/j.jacc.2009.03.037. [DOI] [PubMed] [Google Scholar]
- 31.Clark AL, Poole-Wilson PA, Coats AJ. Exercise limitation in chronic heart failure: central role of the periphery. J Am Coll Cardiol. 1996;28:1092–1102. doi: 10.1016/S0735-1097(96)00323-3. [DOI] [PubMed] [Google Scholar]
- 32.Guazzi M, Samaja M, Arena R, Vicenzi M, Guazzi MD. Long-term use of sildenafil in the therapeutic management of heart failure. J Am Coll Cardiol. 2007;50:2136–2144. doi: 10.1016/j.jacc.2007.07.078. [DOI] [PubMed] [Google Scholar]
- 33.Froelicher VF, Myers J. Exercise and the Heart. 5th. Philadelphia, PA: WB Saunders; 2006. [Google Scholar]
- 34.Segers P, Stergiopulos N, Westerhof N. Relation of effective arterial elastance to arterial system properties. Am J Physiol Heart Circ Physiol. 2002;282:H1041–H1046. doi: 10.1152/ajpheart.00764.2001. [DOI] [PubMed] [Google Scholar]
- 35.Di Salvo TG, Mathier M, Semigran MJ, Dec GW. Preserved right ventricular ejection fraction predicts exercise capacity and survival in advanced heart failure. J Am Coll Cardiol. 1995;25:1143–1153. doi: 10.1016/0735-1097(94)00511-n. [DOI] [PubMed] [Google Scholar]
- 36.Abel FL, Waldhausen JA. Effects of alterations in pulmonary vascular resistance on right ventricular function. J Thorac Cardiovasc Surg. 1967;54:886–894. [PubMed] [Google Scholar]
- 37.Tedford RJ, Hassoun PM, Mathai SC, Girgis RE, Russell SD, Thiemann DR, Cingolani OH, Mudd JO, Borlaug BA, Redfield MM, Lederer DJ, Kass DA. Pulmonary capillary wedge pressure augments right ventricular pulsatile loading. Circulation. 2012;125:289–297. doi: 10.1161/CIRCULATIONAHA.111.051540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilson JR, Mancini DM, Dunkman WB. Exertional fatigue due to skeletal muscle dysfunction in patients with heart failure. Circulation. 1993;87:470–475. doi: 10.1161/01.cir.87.2.470. [DOI] [PubMed] [Google Scholar]
- 39.Morris NR, Snyder EM, Beck KC, Johnson BD. Lung-to-lung circulation times during exercise in heart failure. Eur J Appl Physiol. 2009;106:621–627. doi: 10.1007/s00421-009-1051-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kitzman DW. Exercise training in heart failure with preserved ejection fraction: beyond proof-of-concept. J Am Coll Cardiol. 2011;58:1792–1794. doi: 10.1016/j.jacc.2011.07.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.