Abstract
Acute and recurrent vulvovaginal candidiasis (VVC) remains a significant problem in women of childbearing age. While clinical studies of women with recurrent VVC (RVVC) and animal models have provided important data about a limited protective role of adaptive immunity, there remains a paucity of information on the protective mechanisms or factors associated with susceptibility to infection. In the present study, an intravaginal live Candida challenge in healthy adult women showed a differential susceptibility to symptomatic VVC, where 3 (15%) of 19 women with no history of VVC acquired a symptomatic infection compared to 6 (55%) of 11 women with an infrequent history of VVC. Furthermore, these studies revealed that protection against infection is noninflammatory while symptomatic infection correlates with a vaginal infiltration of polymorphonuclear neutrophils (PMNs) and a high vaginal fungal burden. Thus, the presence of symptomatic infection appears more dependent on host factors than on properties of the organism. Finally, vaginal lavage fluid from women with a symptomatic infection, but not those asymptomatically colonized, promoted the chemotaxis of PMNs. These results suggest that rather than RVVC/VVC being caused by an aberrant adaptive immune response, symptoms that define infection appear to be due to an aggressive innate response by PMNs.
Vulvovaginal candidiasis (VVC), caused primarily by Candida albicans, remains a significant problem in women of childbearing age (28). C. albicans is a commensal organism of the gastrointestinal and reproductive tracts (8). Several exogenous factors predispose menarchal women to acute VVC, including hormonal modulations associated with pregnancy, the luteal phase of the menstrual cycle, high-dose oral estrogen contraception, and hormone replacement therapy, and nonhormonal factors such as antibiotic usage and uncontrolled diabetes mellitus (29). There is also a population of women (5 to 10%) who suffer from recurrent VVC (RVVC), which is defined as three or more episodes per annum (28). While most women with RVVC have no known predisposing factors, hormonal or otherwise (idiopathic, and termed primary RVVC), another group of women have RVVC as a result of being unable to avoid certain predisposing factors (secondary RVVC) (28). Historically, primary RVVC has been attributed to a putative local immune deficiency (reviewed in reference 11).
Candida-specific cell-mediated immunity, acquired by exposure to Candida early in life, has been considered the predominant host defense mechanism against mucosal Candida infections. However, studies from a mouse model of vaginal candidiasis and many cross-sectional clinical studies evaluating women with primary RVVC over the past several decades have revealed a general lack of protection by local or systemic adaptive immunity (reviewed in reference 11). While data suggest that this may be the result of immunoregulatory mechanisms (31, 33, 34) and as such have provided important information, these studies provided few clues to the mechanisms that are protective against infection or the factors that are associated with susceptibility. Some level of protection against infection is suggested, however, based on the 15 to 25% rate of asymptomatic colonization in healthy adults or adolescents (29) and especially the high asymptomatic vaginal fungal burden in adolescents (2). Thus, the animal models and cross-sectional clinical studies have been limiting in providing insights into the immunopathogenesis of vaginal candidiasis.
As an alternative clinical approach to evaluation of the natural history of local immune responsiveness to Candida at the vaginal mucosa, we initiated a design that involved an intravaginal challenge with Candida antigen in healthy women without a history of VVC. Unfortunately, no appreciable responsiveness was detected on the basis of the presence of cytokines or immune mediators (12), despite some evidence to the contrary in an earlier pilot study (15). We therefore rationalized that a design that was more likely to provide appreciable information was a live intravaginal challenge protocol. This was deemed possible and potentially safe based on the fact that Candida is a commensal of the genital tract, VVC is superficial with no migration to extravaginal sites and is treatable, and there is no evidence that women with acute VVC are susceptible to RVVC. Live-challenge studies are not new, since they currently exist for Neisseria gonorrhoeae in males and for Haemophilus ducreyi (1, 10). A live Candida vaginal challenge is not new either, although it has not been reported in the literature since 1934, where Bland et al. (5) found a hormonal association with symptomatic infection.
The purpose of the present study was to renew this live challenge with new breadth and conduct a contemporary safe-conscious structured design with control women to study the natural history of infection and identify potential factors involved in resistance and/or susceptibility to infection.
(This work was presented in part at the 102nd General Meeting of the American Society for Microbiology 2002, Salt Lake City, Utah, 2002; the 6th ASM Conference on Candida and Candidiasis, Tampa, Fla., 2002; and the International Society for Human and Animal Mycology, San Antonio, Tex. 2003.)
MATERIALS AND METHODS
Subjects, enrollment, and screening.
Thirty healthy women between the ages of 18 and 55 years were enrolled. All eligible participants were enrolled through the Obstetrics and Gynecology Clinic at the Louisiana State University Health Sciences Center, New Orleans, La. Informed consent was obtained from all participants/patients, and all procedures in the conduct of clinical research were performed in accordance with the Institutional Review Board at the Louisiana State University Health Sciences Center. Prior to enrollment, a detailed questionnaire (information about past episodes of VVC and their causes) was administered and an extensive prescreening procedure (Candida-specific immune responsiveness, human immunodeficiency virus [HIV] test, pregnancy test, and vaginal yeast colonization status) was conducted. Candida-specific immune responsiveness was determined by a delayed- and immediate-hypersensitivity skin test for Candida. To determine immediate hypersensitivity, a surface scratch test was performed as previously described (19) using Candida antigen (Candin; Allermed Laboratories, San Diego, Calif.). To determine delayed hypersensitivity, an intradermal test was performed as previously described (19) with the same Candida antigen. A standard blood-based HIV test with appropriate pre- and post-test counseling and a standard human chorionic gonadotropin urine-based pregnancy test were conducted for each participant. Additionally, a vaginal swab was taken from each participant and cultured on Candida Chromagar (CHROMagar, Paris, France) at 37°C for 48 h to evaluate the vaginal yeast colonization status. Candida hyphae were evaluated by placing a drop of potassium hydroxide (KOH) on a vaginal smear made from the swab.
Specimen collection and laboratory analyses.
Specimens collected from enrolled subjects included a vaginal lavage specimen, vaginal swab, vaginal smears, and blood (10 ml) by venipuncture into a red-top tube (without EDTA) (Becton Dickinson, Franklin Lakes, N.J.). The vaginal lavage fluid was collected after a 30- to 40-s aspiration with 5 ml of nonpyrogenic sterile saline. The lavage fluid was processed as previously described (12), and the sterile filtered soluble fraction was stored at −70°C until use. Prior to filtration and storage, the lavage fluid was serially diluted and plated on Sabouraud dextrose (SAB) agar to quantify the vaginal fungal burden. The lavage cell pellet was stored at −70°C in cryopreservative medium (50% fetal bovine serum, 30% RPMI 1640 medium, 20% dimethyl sulfoxide [all from GIBCO, Langley, Okla.]) until use. The vaginal swab was plated on Candida Chromagar and incubated at 37°C for 48 h. Green CFU were further evaluated for germ tube formation (evidence for C. albicans) in fetal bovine serum for 2 to 3 h in a 37°C water bath. Non-germ-tube-forming colonies were identified to species level using API 20 AUX (bioMérieux, Hazelwood, Mo.). One vaginal smear was spray fixed and stained using the Papanicolaou technique. Leukocytes were identified by morphology. If present, the only leukocytes identified were polymorphonuclear neutrophils (PMNs). PMN scores were determined from the stained vaginal smear and defined as follows: 1, 1 to 10 PMNs/five fields at ×400 magnification; 2, 11 to 20 PMNs/five fields; 3, 21 to 30 PMNs/five fields; and continuing with a similar pattern up to 11, >100 PMNs/five fields. The second vaginal smear was Gram stained (as described below). Serum was collected from clotted blood following centrifugation and stored at −70°C until use.
Preparation of inocula.
The inocula were prepared using C. albicans clinical isolate DB597.94 from an RVVC patient; the isolate was kindly provided by J. D. Sobel, Wayne State University School of Medicine, Detroit, Mich. The isolate was grown on SAB agar at 34°C, and one colony was used to inoculate 10 ml of phytone-peptone (PP) broth (BBL, Syracuse, N.Y.) supplemented with 0.1% glucose and grown to stationary phase for 18 to 22 h at 25°C in a shaking water bath. The blastoconidia were collected and washed with phosphate-buffered saline (PBS), enumerated on a hemocytometer using trypan blue dye exclusion, and resuspended at specific concentrations in a variety of vehicles including PBS, PP, PP-0.1% glucose, and PP-0.1% glucose-1% estrogen (the general concentration employed in mice to stimulate or sustain vaginal infection). In one case, the known number of blastoconidia were incubated in tissue culture medium for 9 h to form hyphae and used as the inoculum.
Clinical protocol.
Eligible participants (immediate hypersensitive negative, delayed hypersensitive positive, HIV negative, nonpregnant, C. albicans culture negative, KOH negative for hyphae, not on any form of hormonal contraception, not having had more than two explained episodes of VVC in the past year, and with no known immunological disorders) were placed into one of two groups based on their history of VVC. Nineteen women were defined as having no history of VVC (no known episodes of VVC), and 11 women were defined as having an infrequent history of VVC (no more than two episodes in any year) due to antibiotic usage, oral contraceptive usage, hormone replacement therapy, and/or pregnancy (in most cases physician diagnosed). Women were asked to come to the clinic at a time coinciding with a stage of the menstrual cycle (within 5 days after menses [follicular phase] or days 17 to 19 [luteal phase]), at which time specimens were collected, vaginal pH was determined, and a KOH test was performed. Each participant was then given 103 to 107 blastoconidia intravaginally in a volume of 0.1 or 0.5 ml in one of several vehicles (PBS, PP, PP-0.1% glucose, PP-0.1% glucose-1% estrogen). Immediately after inoculation, a feminine pad was placed over the vagina and the subject remained in the stirrups for 15 min to reduce leakage. The pad remained in place for 2 h, was stored refrigerated, and was brought into the clinic on the next visit. The pad was swabbed on Candida Chromagar to determine a measure of leakage.
The participants were asked to return to the clinic an average of three times during a 7- to 14-day period for collection of specimens (vaginal lavage fluid, smear, and swab). The participants were also asked to collect self-obtained vaginal swabs daily for a total of five times on the days they did not attend the clinic and return them to the clinic at their earliest visit. Pilot studies where swabs known to be positive for Candida and were stored for up to 5 days refrigerated before plating, were positive for Candida in all cases. The swabs and lavage fluid were processed as described above. C. albicans that was recovered was confirmed by green colonies on Chromagar and germ tube formation. A symptom report card was given to each subject, and she was asked to record the severity of any symptoms or signs every day during the length of her participation. Signs included discharge and vulvar or vaginal erythema, and symptoms included vaginal burning, itching, and soreness. Each was quantified using a grading system of 0 (absent), 1 (mild), 2 (moderate), and 3 (severe). A self-obtained vaginal swab was collected and shipped overnight once every 2 weeks for 6 weeks after the end of the formal observation period and cultured as above. Topical antifungal treatment was offered to anyone who requested it at any time and was required for anyone who had a symptomatic infection at the end of the observation period.
Criteria for symptomatic infection.
The presence of hyphae on the KOH smear and in the vaginal lavage fluid, a positive swab culture, and the presence of visible signs and symptoms served as evidence of symptomatic infection. A cumulative grade of 1 to 3 was defined as mildly symptomatic, 4 to 8 was defined as moderately symptomatic, and >8 was defined as severely symptomatic. In addition to the criteria for infection, the organisms recovered were confirmed to be the same as the organism used for inoculation. For this procedure, several isolated colonies from the swabbed plates were grown overnight in yeast peptone dextrose medium at 30°C and genomic DNA was isolated by standard procedures. Genomic DNA (3 μg) was digested with EcoRI, and fragments were separated on a 0.8% agarose gel, transferred, and probed with the Ca3 probe (kindly provided by D. R. Soll, University of Iowa), which recognizes a repeat sequence in the Candida genome (27). The inoculating strain DB597.94 and the Ca3 reference strain were included as controls. In all cases, the recovered organism was identical to the inoculating organism.
Identification of the stage of the menstrual cycle.
The stage of the menstrual cycle was verified by measuring the estradiol and progesterone concentrations in sera by radioimmunoassay at the Clinical Endocrinology Laboratory, Division of Reproductive Endocrinology, Detroit Medical Center, Detroit, Mich.
Bacterial flora and determination of BV.
A portion of the vaginal lavage fluid was added to BBL Port-a-Cul transport vials (Becton Dickinson) and then aseptically aspirated from the vial. Three drops of the lavage fluid were placed on the appropriate media for the cultivation of both aerobes and anaerobes and then streaked for isolation to estimate semiquantitatively the concentration of each organism in the specimen. A subgroup of specimens were selected for performance of 10-fold dilutions to obtain quantitative CFU. Aerobic cultures were inoculated on BBL Columbia agar-5% sheep blood, BBL chocolate agar, or BBL human blood Tween medium and incubated at 37°C under a 7% CO2 atmosphere for 2 to 5 days. Anaerobic cultures were incubated in an anaerobic jar (O2 concentration of less than 5 ppm) at 37°C for 2 to 5 days. Isolated colonies were identified using the RapID ANA II system (Remel, Inc., Lenexa, Kans.) for anaerobes; API 20E (bioMérieux) for Enterobacteriacae; Gram stain and growth on inhibitory mold agar for yeast; Gram stain and beta-hemolysis of organism on human blood Tween medium for Gardnerella; Gram stain-3% hydrogen peroxide, BBL Staphyloslide latex test, BBL Streptocard acid latex test, BBL 6.5% sodium chloride (NaCl) broth, BBL bile esculin slants, and hemolysis on BBL Columbia agar-5% sheep blood for aerobic gram-positive cocci; and Gram stain for lactobacilli. All bacteria media were purchased from Becton Dickinson. The bacterial flora was scored by Nugent's criteria from a Gram stain (0 to 3, normal; 4 to 6, intermediate or disturbed flora; 7 to 10, bacterial vaginosis [BV]) (23).
Chemotaxis assay. (i) Neutrophil isolation.
Venous blood (20 ml) from healthy male and female adult volunteers was collected in preservative-free heparin tubes, mixed with equal volumes of a dextran-saline solution, and incubated for ∼20 min at room temperature. The leukocyte-rich plasma was then aspirated and centrifuged for 10 min at 250 × g. The cell pellet was resuspended in 0.9% NaCl, layered over 10 ml of Ficoll-Hypaque (Amersham Pharmacia Biotech, Uppsala, Sweden), and centrifuged for 40 min at 400 × g. The resulting pellet was then resuspended in 20 ml of cold 0.2% NaCl for 30 s to lyse any remaining red blood cells. Isotonic conditions were then restored by the addition of ice-cold 1.6% NaCl, and the mixture was centrifuged for 6 min at 250 × g. The cells were resuspended in chemotaxis buffer (RPMI 1640 containing 2 nM glutamine, 25 mM HEPES, and 1% bovine serum albumin BSA) (Sigma-Aldrich Co., St. Louis, Mo.), enumerated using a hemacytometer, and adjusted to the proper concentration in chemotaxis buffer.
(ii) Chemotaxis assay.
A transwell system (Corning Inc., New York, N.Y.) using the Boyden chamber principle (6) was used to determine neutrophil migration in vaginal lavage fluid from women who were symptomatically infected, asymptomatically colonized, or not colonized. Recombinant interleukin-8 (5 ng/ml) and RANTES (10 ng/ml) (R&D Systems, Minneapolis, Minn.) were used as positive and negative controls, respectively. C. albicans culture filtrate antigen (CaCF) and the supernatant of a 24-h C. albicans blastoconidia culture were used as controls for the presence of C. albicans antigen in the lavage fluid, and PBS was used as the true-negative control. All control experiments were done in triplicate, and sample experiments were done in duplicate. Chemotaxis buffer (150 μl) was added to the upper chambers of a transwell plate, and the chemoattractant (vaginal lavage or control fluids) was added to the bottom chambers at dilutions of 1:5 and 1:50. The chambers were equilibrated for 5 min at room temperature. Chemotaxis buffer (10 μl) containing 2 × 104 neutrophils was then added to the upper chambers, and the mixture was incubated for 30 min at 37°C under 5% CO2. After incubation, the upper chambers were removed and the neutrophils that migrated into the bottom chamber were harvested and quantified using a hemocytometer. Data were expressed as a chemotactic index (cell count in experimental chamber/cell count in buffer control chamber). An additional control included placing 2 × 104 neutrophils in the bottom chamber and confirming the cell number by using a hemocytometer and the procedure described above.
Statistics.
Student's t test was used for comparisons between vaginal fungal burden and PMN score and the chemotaxis assay. Fisher's exact test was conducted for any effects of BV, menstrual cycle, and inocula on the outcome of inoculation. Linear-regression analysis was performed to generate the correlation coefficient for vaginal fungal burden versus qualitative PMN score. Significance was defined as P < 0.05 using a two-tailed test.
RESULTS
Results from the intravaginal challenge with live C. albicans in women with no history of VVC.
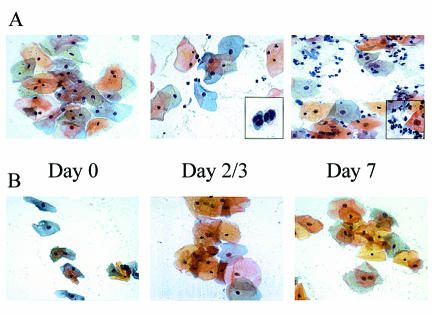
The first study initiated was a small trial with six women who had no history of VVC. Those eligible from the prescreening criteria were tested during either the follicular or luteal phase of the menstrual cycle. The inoculum of 103 to 105 stationary-phase C. albicans blastoconidia was given in a volume of 0.1 ml of sterile PBS. The women showed no evidence of immediate hypersensitivity in the vulva or vaginal area during the 15-min observation period. Results of the culture of the preinoculum swab and lavage fluid were confirmed negative for all women when obtained, and the inoculum was serially cultured to identify the actual amount given. The feminine pad worn for 2 h and presented at the first return visit, although positive for yeast in each case, showed no evidence of significant leakage (determined by the number of CFU on the SAB plate). At each return visit (day 2 or 3, 4 or 5, and 7), a vaginal swab and lavage fluid were collected, signs were recorded, and the women submitted the symptom report card. Of these six subjects, only one was categorized as acquiring a symptomatic infection (she showed symptoms, had a vaginal smear positive for hyphae by KOH, and had a vaginal swab that was culture positive for C. albicans). The mean symptom rating was mild (3.9), with signs that included a light discharge. The other five women either were colonized asymptomatically (n = 2) or had no detectable colonization in swabs and lavage fluid specimens (n = 3). There was no effect of inoculum concentration or stage of the menstrual cycle (follicular or luteal) on acquiring a symptomatic infection. Interestingly, the woman who became symptomatic had a vaginal cellular infiltrate consisting exclusively of PMNs (Fig. 1A), whereas those asymptomatically colonized (Fig. 1B) or not colonized showed no cellular infiltration. The symptomatic woman was instructed to use the sealed over-the-counter topical antifungal therapy and return to the clinic after 5 days. The KOH stain of the vaginal smear and the vaginal swab culture results showed complete resolution of the infection upon return to the clinic. Any minor signs and/or symptoms had been eliminated as well. Self-obtained swabs collected every 2 weeks over the next 6 weeks were negative for yeast. The women who were colonized remained so for a variable length of time (depending on the subject) but eventually became detectably culture negative over the 6-week self-swab follow-up. Follow-up phone conversations with all women for up to 1 year detected no adverse effects or problems.
FIG. 1.
Presence of neutrophils in vaginal lavage fluid of women who acquired a symptomatic infection. Vaginal smears collected from women on different days postinoculation were fixed and stained using the Papanicolaou technique. (A) Smears from a woman who acquired a symptomatic infection. Times are days postinoculation. (B) Smears from a woman who became asymptomatically colonized. The results are representative of the general pattern seen in all women tested (n = 29). Magnifications are ×400 for each frame and ×1,000 (inset, day 7) and 120% of ×1,000 (inset, day 2/3).
Following this low infection rate, the protocol for the next series of women tested included a higher inoculum (up to 108), longer period of observation (14 days), inoculum given in a volume of 0.5 ml, vaginal pH measurement, and modification of the vehicle in which the inoculum was given, including growth medium with or without glucose and with or without estrogen. Thirteen new women, again with no history of VVC, were enrolled. Of these, two women became symptomatic and were diagnosed with a C. albicans infection (mean symptom ratings were mild [2.0] to moderate [7.3] with signs that included discharge and/or erythema). Again, the women who became symptomatically infected showed a vaginal cellular response by PMNs. The women were treated as described above, with no adverse effects. Of the remaining women, six became asymptomatically colonized and five were not detectably colonized. Again, inoculum concentrations, stage of the menstrual cycle, and vaginal pH (which ranged from 4 to 6.5) had no effect on acquisition of the symptomatic infection, and there was no measurable enhanced susceptibility to symptomatic infection when the vehicle included a growth medium for fungi with or without glucose or estrogen to promote Candida growth. One subject was given the inoculum in the hyphal form. She also failed to become symptomatic or even detectably colonized. Women who became asymptomatically colonized became culture negative over time (1 to 2 months). Long-term follow-up via phone conversations showed no problems for a period up to 1 year. The overall incidence of symptomatic infection in these women with no history of vaginitis was 15%. Table 1 contains the details of each subject enrolled. Fisher's exact tests for the effects of low versus high inocula (<105 versus >105) or the menstrual cycle (follicular versus luteal) on susceptibility to infection were not significant (P = 0.40 and 1.0, respectively).
TABLE 1.
Demographics, inoculation conditions, and postinoculation results for enrolled women without a history of VVC
| Preinoculation
|
Inoculation
|
Postinoculation
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Racea | Age (yr) | BVb | Stage of MCc | Inoculum (theoretical/actual) | Vehicled | KOH | Yeast swab culture | Statuse | Symptomsf | Cell infiltrateg |
| AA | 38 | + | Follicular | 1 × 104/1 × 104 | PBS | + | + | I | 3.9 | + |
| C | 29 | − | Follicular | 1 × 103/5 × 102 | PBS | − | + | C | 0 | − |
| C | 47 | − | Luteal | 1 × 103/6 × 102 | PBS | − | + | C | 0 | − |
| AA | 43 | + | Follicular | 1 × 103/4 × 103 | PBS | − | − | NC | 0 | − |
| AA | 44 | + | Luteal | 1 × 104/4 × 103 | PBS | − | − | NC | 0 | − |
| C | 36 | − | Follicular | 1 × 104/1 × 104 | PBS | − | − | NC | 3.5 | − |
| C | 44 | − | Follicular | 1 × 103/5 × 103 | PBS | − | − | NC | 2.0 | − |
| AA | 34 | + | Follicular | 1 × 104/2 × 104 | PBS | − | − | NC | 0 | − |
| AA | 37 | + | Luteal | 1 × 103/1 × 103 | PPd | − | − | NC | 0 | − |
| AA | 21 | + | Follicular | 1 × 104/9 × 103 | PPw/glu | − | + | C | 0 | − |
| AA | 44 | + | Follicular | 1 × 106/1 × 106 | PBS | − | − | NC | 2.0 | − |
| C | 37 | − | Luteal | 1 × 107/1 × 107 | PBS | + | + | I | 6.6 | + |
| C | 47 | − | Luteal | 1 × 107/5 × 107 | PBS | − | + | C | 0 | − |
| C | 43 | − | Follicular | 1 × 103/2 × 103 | PBS hyphal | − | + | C | 0 | − |
| AA | 43 | + | Follicular | 1 × 107/5 × 107 | PP | − | + | C | 0 | − |
| C | 35 | ± | Follicular | 1 × 107/2 × 107 | PPw/glu | − | − | NC | 0 | − |
| AA | 33 | ± | Follicular | 1 × 107/1 × 107 | PPw/glu + est | + | + | C | 1 | − |
| C | 29 | − | Follicular | 1 × 107/9 × 106 | PPw/glu | − | + | C | 0 | − |
| AA | 46 | − | Follicular | 1 × 107/1 × 107 | PPw/glu | + | + | I | 2 | + |
AA, African American; C, Caucasian.
+, positive; −, negative; ±, intermediate.
MC, menstrual cycle.
PPw/glu, PP-0.1% glucose; PPw/glu + est, PP-0.1% glucose-1% estrogen.
I, infected; C, colonized; NC, not colonized.
Severity of symptoms out of a possible of 12; 1 to 3, mild; 4 to 8, moderate; 9 to 12, severe.
Presence of a cellular infiltrate postinoculation.
Results of the intravaginal challenge with live C. albicans in women with an infrequent history of VVC.
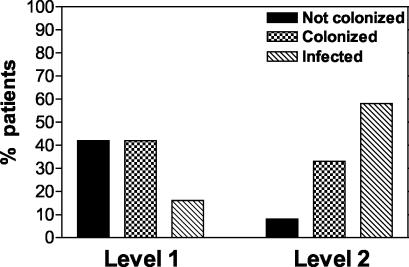
Based on the above results suggesting that the incidence of infection was based primarily on the host and not on the organism or inoculation conditions, we were granted approval to expand the inclusion criteria to include subjects with documented and explained infrequent episodes of VVC (i.e., VVC due to antibiotic usage, pregnancy, or oral contraceptive usage). We termed this group the “level 2” recruits. Inocula for the first of these prescreened eligible women included an intermediate inoculum (105 to 107) and escalating incremental inclusion of vehicle supplements (PP, glucose, and estrogen). No adverse safety issues associated with the vehicle supplements or inoculum concentration were observed in the first several subjects. Therefore, a more generous inoculum of 107 Candida cells in PP with glucose became the standard inoculum. The results from 11 women showed that 6 became symptomatically infected with C. albicans (mean symptom ratings were mild [2.0, 2.0, 2.0, 3.8, and 3.9] to moderate [7.3], with signs that included discharge and/or erythema), 4 became asymptomatically colonized, and 1 was not detectably colonized. One of the culture-positive women, although having mild symptoms, felt the need to request treatment after 1 day postinoculation, and so no additional specimens were collected. Like those tested previously, symptomatic culture-positive women had a vaginal cellular infiltrate consisting exclusively of PMNs (78% overall) and the stage of the menstrual cycle and the vaginal pH had no effect on acquisition of a symptomatic infection (data not shown). The infected women were treated as above with no adverse affects. All the level 2 recruits were treated with the antifungal agent as a precautionary measure at the end of the observation period. All the women cleared the organism, and long-term follow-up with self-obtained swabs showed no detectable fungal growth. Follow-up phone conversations detected no problems over the subsequent 6 months to 1 year. Figure 2 illustrates the difference in infection rates for level 1 and level 2 participants.
FIG. 2.
Comparison of the outcomes of intravaginal inoculation with C. albicans in women without a history of VVC (level 1) and those with an infrequent history of VVC (level 2). A total of 19 level 1 and 11 level 2 women were evaluated. An infrequent history of VVC was defined as no more than two acute VVC episodes in a year that were explained based on the usual predisposing factors (antibiotic usage, high-estrogen oral contraceptives, hormone replacement therapy, pregnancy). Results were based on the criteria of symptomatic infection over the entire observation period (up to 14 days). Women with symptomatic infection were defined as having symptoms consistent with vaginitis, vaginal swabs positive for C. albicans, and hyphae or yeasts visible in KOH preparations of vaginal smears. Women with asymptomatic colonization had vaginal swabs positive for C. albicans. Women not colonized had no detectable CFU on vaginal swabs.
Effect of intravaginal challenge with live C. albicans on the vaginal bacterial flora.
For the majority of the experimental protocol, vaginal lavage fluid and swab specimens were evaluated for changes in the bacterial flora and incidence of BV. The results showed that over the observation period, women who became symptomatically infected, asymptomatically colonized, or not colonized had no demonstrable changes in the type or concentration of bacterial flora. The floras included several anaerobic and aerobic bacteria such as Lactobacillus species, Staphylococcus species, diphtheroids, Streptococcus species, enterics, Gardnerella vaginalis, Prevotella species, Porphyromonas asaccharolyticus, Bacteroides fragilis group, and Peptostreptococcus species (data not shown). The incidence of BV in the subjects enrolled was ∼45%. There was no association between the presence of BV and the outcome of the inocula. This is illustrated in Table 1 for women without a history of VVC. The results for all women are as follows: of those with a symptomatic infection (n = 9), 3 were BV positive and 6 were BV negative; of those with asymptomatic colonization (n = 12), 5 were BV positive, 6 were BV negative, and 1 was intermediate; of those not colonized (n = 9), 5 were BV positive, 3 were BV negative, and 1 was intermediate. Fisher's exact test between infection (infected or uninfected) and BV (BV positive and BV negative) showed no significant differences (P > 0.22). There was also no appreciable change in BV status over the observation period.
Relationship between vaginal fungal burden and PMN score.
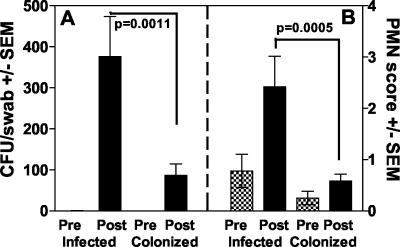
The vaginal fungal burden in all participants who became symptomatically infected (with or without a history of VVC) was significantly higher than that in those who became asymptomatically colonized (P = 0.0011) (Fig. 3A). Similarly, the PMN score was significantly higher in those who became symptomatic than in those who became asymptomatically colonized (P = 0.0005) (Fig. 3B). In fact, there was a positive correlation between PMN score and vaginal fungal burden in those with symptomatic infection (representing all visits where vaginal fungal burden was detected) (r = 0.500, P = 0.039). In some cases, PMNs were present in the vaginal lavage fluid at the time of inoculation and persisted unchanged throughout the observation period. However, only under conditions of symptomatic infection did the PMN score change significantly.
FIG. 3.
Vaginal fungal burden (A) and qualitative PMN score (B) before and after intravaginal inoculation. Results are from women who acquired a symptomatic infection (n = 9) compared to those who became asymptomatically colonized (n = 12). All women in the study irrespective of their history of VVC are represented. The PMN score listed for each woman was the mean for all visits. The mean PMN score for all women in a group was used to generate the graph. The criterion for PMN scoring is described in Materials and Methods. Pre and Post refer to pre- and postinoculation. SEM, standard error of the mean.
Chemotaxis assays with cervicovaginal lavage fluid.
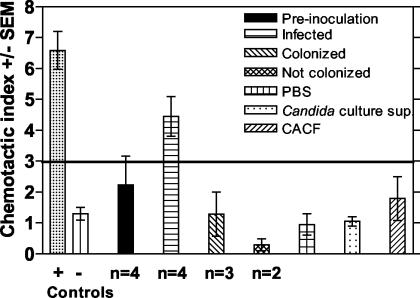
To determine whether the lavage fluids from those with symptomatic infection could cause the migration of PMNs from a healthy volunteer, we evaluated different concentrations of lavage fluid from women preinoculated and those symptomatically infected, asymptomatically colonized following inoculation, or not colonized in a chemotaxis assay. The results in Fig. 4 show that the lavage fluid (diluted 1:5) from women with a symptomatic infection promoted the migration of PMNs while lavage fluids from women preinoculated and women either colonized or not colonized postinoculation did not (P < 0.02). No chemotaxis was observed with any fluid at a 1:50 dilution (data not shown). Additionally, CaCF and a 24-h supernatant from a Candida blastospore culture that was similarly diluted also showed no chemotactic activity.
FIG. 4.
Ability of vaginal lavage fluid from women with symptomatic infection to induce PMN chemotaxis. Vaginal lavage fluid collected from women preinoculation (n = 4) or who acquired a symptomatic infection (n = 4) (infected), became asymptomatically colonized (n = 3) (colonized), or did not become colonized (n = 2) (not colonized) following inoculation with C. albicans was processed, sterile filtered, and tested in a PMN chemotaxis assay using a transwell chamber system. PMNs were acquired from normal volunteers. Lavage fluids were tested at 1:5 and 1:50 dilutions. The positive control was interleukin-8 (PMN chemokine), and the negative control was RANTES (T-cell chemokine). Other controls included CaCF, a 24-h supernatant of C. albicans blastoconidia cultured at 25°C (Candida sup), and PBS (vehicle for the lavage fluid). The chemotaxis index represents the ratio of cells in the experimental wells to those in the control well (chemotactic buffer). The horizontal line represents the line of positivity, above which active chemotaxis is considered positive. SEM, standard error of the mean.
DISCUSSION
A model for live Candida challenge to investigate vaginitis in women, while provocative, was a logical extension of data to date from animals and cross-sectional clinical studies that largely failed to reveal protective factors associated with vaginitis or the factors associated with susceptibility to infection. There was also good evidence, based on several properties of Candida in the reproductive tract, that a live Candida challenge protocol would be safe.
Surprisingly, fewer than 15% of women without a history of VVC became symptomatically infected, with the remaining women being equally split between those asymptomatically colonized and those not colonized. We assume that those not colonized did in fact have some vaginal fungal burden but that it was simply below the level of detection for swab cultures. Perhaps detection by PCR would have revealed a low-level colonization. Nevertheless, while these results did not aid significantly in identifying the events associated with infection or even the factors associated with susceptibility, some important observations were made. One was that protection against infection was noninflammatory, with no cellular infiltration. In fact, the vaginal environment appeared extremely quiescent through the entire observation period when no symptoms were observed. In contrast, symptomatic infection was associated with a cellular infiltrate consisting entirely of PMNs, with no influence by stage of the menstrual cycle, inoculum concentration, vaginal pH, vehicle, or race or age of the participant. There was also no influence of the vaginal bacterial flora on the outcome of the Candida inoculation since no changes in the flora were detected postinoculation. Finally, there was no evidence that the factors associated with infection were highly dependent on the Candida isolate used, since all the results observed (infected, colonized, or not colonized) were obtained with one Candida isolate derived from a woman with RVVC that is highly virulent in the mouse and macaque vaginitis models (14, 30). However, we do not exclude the fact that the organism may express virulence differently depending on the host vaginal environment. A structured microarray analysis would be needed to address this question. Interestingly, three of the women not colonized had symptoms that would be considered to be associated with vaginitis (itching, burning, and soreness). However, with the lack of any positive culture or hyphae on the KOH preparation, they could not be defined as infected. We do not rule out the possibility, however, that the symptoms were due to extremely low and undetectable levels of the organism. Alternatively, the symptoms could have been due to some other unknown condition during that period.
The results were very different for participants with an infrequent history of VVC: those who acquired infections easily as a result of known causes (not primary RVVC) including antibiotic usage, oral contraceptive usage, pregnancy, and hormone replacement therapy. In these women, the infection rates were increased to >55% and considerably more women were asymptomatically colonized than not colonized. Similar to those without a history of VVC, protection was noninflammatory and symptomatic infection was associated with a vaginal PMN infiltrate. The factors determining whether women with an infrequent history of VVC will acquire a symptomatic infection are not clear; there was no demonstrable effect of inoculum concentration, vehicle, stage of the menstrual cycle, or bacterial flora. There are conflicting reports of an inverse relationship between BV and VVC (20, 25, 35). However, in this study BV was equally common in those with and without a history of VVC and did not appear to influence the outcome.
In cases of symptomatic infection, both the vaginal fungal burden and PMN counts were significantly higher than that in those asymptomatically colonized. These results suggest that while the inoculum concentration was not critical in the infection process, the number of organisms that grew and became established and were then recovered from the vaginal cavity was important. This is consistent with observations obtained with women evaluated for natural infections (18, 24) and supports the interpretation that those in the present study with symptoms, but not detectably colonized, did not have a symptomatic Candida infection. Another important correlate was the positive relationship between the vaginal fungal burden and the qualitative score of PMNs. This suggests that PMNs are important for the symptomatology and that the signals to initiate infiltration are important for the role of these cells in this process. Indeed, vaginal lavage fluid from women with a symptomatic infection, but not those asymptomatically colonized or not colonized, had the ability to stimulate neutrophil migration in the chemotaxis assay. Candida antigen had no effect on chemotaxis, suggesting that Candida alone did not stimulate chemotaxis. Although we cannot exclude the possibility that Candida organisms interacting with the epithelium alone promote PMN chemotaxis, no PMN infiltration was observed in women showing detectable asymptomatic Candida colonization. As much as PMNs appear to play a formidable role in symptomatic VVC, we recognize that other factors (e.g., cytokines and immunomodulators) in addition to or instead of PMNs may play a role in the inflammatory process and concomitant symptoms. However, preliminary immunological analyses of pre- and postinoculation lavage fluids, while showing detectable levels of many immunomodulators and cytokines, have not revealed any patterns associated with protection and/or susceptibility to infection or with BV (P. L. Fidel, unpublished data).
Finally, the live Candida challenge protocol could not be complete without successful treatment of infection and significant follow-up to monitor any long-term effects. All symptomatic infections were cleared successfully, with no evidence of relapse over a period of 1 year. Similarly, the participants who were asymptomatically colonized or not colonized, whether treated or not, showed no detectable colonization over the postobservation period (6 to 8 weeks) and have shown no adverse effects of any kind for up to 1 year. It is interesting that even the women with symptomatic infection, irrespective of their history of VVC, did not have severe symptoms from a bolus inoculum. This increases our confidence that women with several levels of susceptibility to VVC can be safely tested to understand the pathogenesis of the infection. It also hints of regulatory mechanisms in the signals that initiate the symptoms.
Based on the consistent evidence that PMNs are playing a substantial role in the immunopathogenesis of induced VVC, albeit based on experiments with small numbers of women to date, several issues deserve comment. One question that arises is why PMNs have not been more evident in clinical cases of VVC or RVVC seen by physicians. One explanation involves the dynamics of the infection and the short half-life of PMNs. It is also possible that the KOH test used to identify hyphae in the vaginal smear lyses leukocytes. A second point is that this newfound PMN hyperresponse is quite distinct from the hyper-immunoglobulin E syndrome that appears to occur in a small population of women with primary RVVC (15, 26, 32). However, in either case, the cause of the symptoms is host mediated. This suggests than most, if not all, of the symptoms associated with VVC are host mediated instead of organism mediated, which has been the long-standing paradigm for VVC. This concept is supported by a limited number of reports suggesting a genetic predisposition to VVC in animals and humans (7, 9, 21), although there have been as many studies suggesting the contrary (4, 13). Additional support for the action of PMNs in symptomatic VVC comes from the fact that VVC is extremely uncommon in neutropenic patients (J. Sobel, personal communication). Finally, the presence of the PMNs in the vagina is also consistent with the presence of PMNs in the mouse model of vaginitis (4, 16, 17). While the PMNs in most animal studies were not found to have any demonstrable effects on vaginitis (4, 16) and PMN-dependent antifungal activity is inhibited by progesterone (22), it is possible that the presence of PMNs in the mouse vaginal cavity too is a sign of symptoms.
Another interesting result of our study is the potential dependence of the PMN infiltration on the number of Candida organisms present. As such, it is interesting to postulate that a lower threshold of Candida initiates the PMN migration in women with primary RVVC than in women with infrequent VVC and hence the higher frequency of episodes. Along similar lines, adolescents and women with no history of vaginitis have a high tolerance for Candida such that PMNs are rarely, if ever, stimulated to migrate. If this could be confirmed, it would suggest that women with primary RVVC and VVC are distinct populations by virtue of their toleration of Candida and the resulting frequency of infectious episodes rather than by virtue of distinct predisposing factors (i.e., immune deficiency, hormone imbalance).
Taken together, the live Candida challenge design appears to be safe and can be used to study the natural history of infection and to identify factors associated with infection. Furthermore, conditions have been identified where symptomatic infection can be predicted with some reliability. This represents a unique situation for humans, since the pathogenesis of an infectious disease can be studied systemically and in real time. In fact, the results collected during this short period have provided important new information about the natural history of vaginitis that had not been revealed in the animal models and studies of women with RVVC. Based on the present data, we now hypothesize that VVC is associated with signals following Candida-vaginal epithelial cell interactions that promote a nonprotective inflammatory leukocytic response and concomitant clinical symptoms while resistance to VVC is associated with a lack of signals and/or an elevated or enhanced appropriate local milieu of innate immune mediators. This hypothesis significantly alters the paradigm of immunopathogenesis of VVC and RVVC. Instead of RVVC being caused by a deficiency in adaptive immunity, we are now proposing that symptoms that define RVVC and VVC are due to an aggressive innate response by PMNs initiated by no more than signals from the interaction of Candida with vaginal epithelial cells.
Acknowledgments
This work was supported by National Institutes of Health Public Health Service grant AI 41693 from the National Institute of Allergy and Infectious Disease.
Editor: T. R. Kozel
REFERENCES
- 1.Al-Tawfig, J. A., K. L. Palmer, C. Chen, J. C. Haley, B. P. Katz, A. F. Hood, and S. M. Spinola. 1999. Experimental infection of human volunteers with Haemophilus decreyi does not confer protection against subsequent challenge. J. Infect. Dis. 179:1283-1287. [DOI] [PubMed] [Google Scholar]
- 2.Barousse, M., B. J. Van Der Pol, D. Fortenberry, D. Orr, and P. L. Fidel, Jr. 2003. Vaginal yeast colonization, prevalence of vaginitis, and associated local immunity in adolescents. Sex. Transm. Infect. 80:48-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Black, C. A., F. M. Eyers, M. L. Dunkley, R. L. Clancy, and K. W. Beagley. 1999. Major histocompatibility haplotype does not impact the course of experimentally induced murine vaginal candidiasis. Lab. Anim. Sci. 49:668-672. [PubMed] [Google Scholar]
- 4.Black, C. A., F. M. Eyers, A. Russell, M. L. Dunkley, R. L. Clancy, and K. W. Beagley. 1998. Acute neutropenia decreases inflammation associated with murine vaginal candidiasis but has no effect on the course of infection. Infect. Immun. 66:1273-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bland, P. B., A. E. Rakoff, and I. J. Pincus. 1934. Experimental vaginal and cutaneous moniliasis. Arch. Dermatol. Syphilol. 1934:760-780. [Google Scholar]
- 6.Bleul, C. C., R. C. Fulhbridge, J. M. Casasnovas, A. Aiouti, and T. A. Springer. 1996. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor-1 (SDF-1). J. Exp. Med. 184:1101-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calderon, L., R. William, M. Martinez, K. V. Clemons, and D. A. Stevens. 2003. Genetic susceptibility to vaginal candidiasis. Med. Mycol. 41:143-147. [DOI] [PubMed] [Google Scholar]
- 8.Calderone, R. A. (ed.). 2002. Candida and candidiasis. ASM Press, Washington, D.C.
- 9.Chaim, W., B. Foxman, and J. D. Sobel. 1997. Association of recurrent vaginal candidiasis and secretory ABO and Lewis phenotype. J. Infect. Dis. 176:828-830. [DOI] [PubMed] [Google Scholar]
- 10.Cohen, M. S., J. G. Cannon, A. E. Jerse, L. M. Charniga, S. F. Isbey, and L. G. Whicker. 1994. Human experimentation with Neissaria gonorrhoeae: rationale, methods, and implications for the biology of infection and vaccine development. J. Infect. Dis. 169:532-537. [DOI] [PubMed] [Google Scholar]
- 11.Fidel, P. L., Jr. 2002. Distinct protective host defenses against oral and vaginal candidiasis. Med. Mycol. 40:359-375. [PubMed] [Google Scholar]
- 12.Fidel, P. L., Jr., M. Barousse, T. Espinosa, R. R. Chesson, and K. Dunlap. 2003. Local immune responsiveness following intravaginal challenge with Candida antigen in adult women at different stages of the menstrual cycle. Med. Mycol. 41:97-109. [DOI] [PubMed] [Google Scholar]
- 13.Fidel, P. L., Jr., J. L. Cutright, and J. D. Sobel. 1995. Effects of systemic cell-mediated immunity on vaginal candidiasis in mice resistant and susceptible to Candida albicans infections. Infect. Immun. 63:4191-4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fidel, P. L., Jr., J. L. Cutright, and J. D. Sobel. 1997. Efficacy of D0870 treatment of experimental Candida vaginitis. Antimicrob. Agents Chemother. 41:1455-1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fidel, P. L., Jr., K. A. Ginsburg, J. L. Cutright, N. A. Wolf, D. Leaman, K. Dunlap, and J. D. Sobel. 1997. Vaginal-associated immunity in women with recurrent vulvovaginal candidiasis: evidence for vaginal Th1-type responses following intravaginal challenge with Candida antigen. J. Infect. Dis. 176:728-739. [DOI] [PubMed] [Google Scholar]
- 16.Fidel, P. L., Jr., W. Luo, C. Steele, J. Chabain, M. Baker, and F. L. Wormley. 1999. Analysis of vaginal cell populations during experimental vaginal candidiasis. Infect. Immun. 67:3135-3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fulurija, A., R. B. Ashman, and J. M. Papadimitriou. 1996. Neutrophil depletion increases susceptibility to systemic and vaginal candidiasis in mice, and reveals differences between brain and kidney in mechanisms of host resistance. Microbiology 142:3487-3496. [DOI] [PubMed] [Google Scholar]
- 18.Hopwood, V., T. Crowley, C. T. Horrocks, J. D. Milne, P. K. Taylor, and D. W. Warnock. 1988. Vaginal candidosis: relation between yeast counts and symptoms and clinical signs in non-pregnant women. Genitourin. Med. 64:331-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leigh, J. E., M. Barousse, R. K. Swoboda, T. Myers, S. Hager, N. A. Wolf, J. L. Cutright, J. Thompson, J. D. Sobel, and P. L. Fidel, Jr. 2001. Candida-specific systemic cell-mediated immune reactivities in HIV-infected persons with and without mucosal candidiaisis. J. Infect. Dis. 183:277-285. [DOI] [PubMed] [Google Scholar]
- 20.Mardh, P. A., A. G. Rodrigues, and C. Pina-Vas. 2001. Can in vitro observations explain interactions in vivo between a lactobacillus-dominated vaginal flora, bacterial vaginosis and vulvovaginal candidiasis. Ital. J. Obset. Gynecol. 183:89-93. [Google Scholar]
- 21.Mulero-Marchese, R. D., K. J. Blank, and T. G. Sieck. 1998. Genetic basis for protection against experimental vaginal candidiasis by peripheral immunization. J. Infect. Dis. 178:227-234. [DOI] [PubMed] [Google Scholar]
- 22.Nohmi, T., S. Abe, K. Dobashi, S. Tansho, and H. Yamaguchi. 1995. Suppression of anti-Candida activity of murine neutrophils by progesterone in vitro: a possible mechanism in pregnant women's vulnerability to vaginal candidiasis. Microbiol. Immunol. 39:405-409. [DOI] [PubMed] [Google Scholar]
- 23.Nugent, R. P., M. A. Krohn, and S. L. Hillier. 1991. Reliability of diagnosing bacterial vaginosis is improved by standardized method of Gram stain interpretation. J. Clin. Microbiol. 29:297-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Odds, F. C., C. E. Webster, P. Mayuranathan, and P. D. Simmons. 1988. Candida concentrations in the vagina and their association with signs and symptoms of vaginal candidosis. J. Med. Vet. Mycol. 26:277-283. [DOI] [PubMed] [Google Scholar]
- 25.Redondo-Lopez, V., C. Meriwether, C. Schmitt, M. Opitz, R. Cook, and J. D. Sobel. 1990. Vulvovaginal candidiasis complicating recurrent bacterial vaginosis. Sex. Transm. Dis. 17:51-53. [PubMed] [Google Scholar]
- 26.Rigg, D., M. M. Miller, and W. J. Metzger. 1990. Recurrent allergic vulvovaginitis: Treatment with Candida albicans allergen immunotherapy. Am. J. Obstet. Gynecol. 162:332-336. [DOI] [PubMed] [Google Scholar]
- 27.Schmid, J., E. Voss, and D. R. Soll. 1990. Computer-assisted methods for assessing strain relatedness in Candida albicans by fingerprinting with the moderately repetitive sequence Ca3. J. Clin. Microbiol. 28:1236-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sobel, J. D. 1992. Pathogenesis and treatment of recurrent vulvovaginal candidiasis. Clin. Infect. Dis. 14:S148-S153 [DOI] [PubMed] [Google Scholar]
- 29.Sobel, J. D., S. Faro, R. Force, B. Foxman, W. J. Ledger, P. R. Nyirjesy, B. D. Reed, and P. R. Summers. 1998. Vulvovaginal candidiasis: epidemiologic, diagnostic, and therapeutic considerations. Am. J. Obstet. Gynecol. 178:203-211. [DOI] [PubMed] [Google Scholar]
- 30.Steele, C., M. Ratterree, and P. L. Fidel, Jr. 1999. Differential susceptibility to experimental vaginal candidiasis in macaques. J. Infect. Dis. 180: 802-810. [DOI] [PubMed] [Google Scholar]
- 31.Taylor, B. N., M. Saavedra, and P. L. Fidel, Jr. 2000. Local Th1/Th2 cytokine production during experimental vaginal candidiasis. Med. Mycol. 38:419-431. [DOI] [PubMed] [Google Scholar]
- 32.Witkin, S. S. 1991. Immunologic factors influencing susceptibility to recurrent candidal vaginitis. Clin. Obstet. Gynecol. 34:662-668. [PubMed] [Google Scholar]
- 33.Wormley, F. L., Jr., C. Steele, K. Wozniak, K. Fujihashi, J. R. McGhee, and P. L. Fidel, Jr. 2001. Resistance of T-cell receptor δ-chain knock-out mice to experimental Candida vaginitis. Infect. Immun. 69:7162-7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wormley, F. L., Jr., J. Chaiban, and P. L. Fidel, Jr. 2001. Cell adhesion molecule and lymphocyte activation marker expression during experimental vaginal candidiasis. Infect. Immun. 69:5072-5079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zdolsek, B., D. Hellberg, G. Froman, S. Nilsson, and P. A. Mardh. 1995. Vaginal microbiological flora and sexually transmitted diseases in women with recurrent and current vulvovaginal candidosis. Infection 23:81-84. [DOI] [PubMed] [Google Scholar]