Abstract
BACKGROUND & AIMS
Liver stiffness measurement (LSM), using elastography, can independently predict outcomes of patients with chronic liver diseases (CLDs). However, there is much variation in reporting and consistency of findings. We performed a systematic review and meta-analysis to evaluate the association between LSM and outcomes of patients with CLDs.
METHODS
We performed a systematic review of the literature, through February 2013, for studies that followed up patients with CLDs prospectively for at least 6 months and reported the association between baseline LSM and subsequent development of decompensated cirrhosis or hepatocellular carcinoma (HCC), as well as mortality. Summary relative risk (RR) estimates per unit of LSM and 95% confidence intervals (CIs) were estimated using the random effects model.
RESULTS
Our final analysis included 17 studies, reporting on 7058 patients with CLDs. Baseline LSM was associated significantly with risk of hepatic decompensation (6 studies; RR, 1.07; 95% CI, 1.03–1.11), HCC (9 studies; RR, 1.11; 95% CI, 1.05–1.18), death (5 studies; RR, 1.22; 95% CI, 1.05–1.43), or a composite of these outcomes (7 studies; RR, 1.32; 95% CI, 1.16–1.51). We observed considerable heterogeneity among studies—primarily in the magnitude of effect, rather than the direction of effect. This heterogeneity could not be explained by variations in study locations, etiologies and stages of CLD, techniques to measure liver stiffness, adjustment for covariates, or method of imputing relationship in the meta-analysis.
CONCLUSIONS
Based on a meta-analysis of cohort studies, the degree of liver stiffness is associated with risk of decompensated cirrhosis, HCC, and death in patients with CLDs. LSM therefore might be used in risk stratification.
Keywords: Elastography, Prognosis, Outcomes, Cirrhosis, Cancer
Cirrhosis is the eighth leading cause of mortality worldwide and is associated with significant morbidity.1,2 Two distinct stages of cirrhosis with different prognostic implications have been defined: compensated cirrhosis (stage 4 fibrosis with or without esophageal varices) and decompensated cirrhosis (variceal bleeding, hepatic encephalopathy, ascites, spontaneous bacterial peritonitis, and/or hepatorenal syndrome).3 Most of the morbidity and mortality in patients with cirrhosis is attributable to transitioning to a decompensated state. In addition, the presence of cirrhosis increases the risk of hepatocellular carcinoma (HCC). Liver transplantation (LT) is the only effective treatment modality once these outcomes develop for highly selected candidates. Hence, early recognition of patients with chronic liver disease (CLD) at high risk for developing these outcomes in a noninvasive manner is warranted to allow implementation of optimal preventative management strategies that may modify the natural course of disease.4
Unfortunately, objective markers of hepatic synthetic function that accurately predict an individual’s risk of transitioning to a decompensated state are lacking.5 Hepatic venous pressure gradient (HVPG) is a potential prognostic measure, but it is an invasive test and is not widely available; in addition, serial testing is cumbersome and not without significant risks to patients. Liver stiffness measurement (LSM) using transient elastography (TE) or magnetic resonance elastography (MRE) is a noninvasive test that accurately predicts the presence of advanced fibrosis and cirrhosis in patients with CLD.6 Recent observational studies have shown that TE also detects the presence of portal hypertension, and correlates well with HVPG.7,8 Some prospective cohort studies have shown that LSM also may predict the future development of decompensated cirrhosis,9 HCC,10 and mortality in patients with chronic hepatitis C virus (HCV), chronic hepatitis B, and cholestatic liver diseases.11 However, the results from these studies are not consistent, potentially owing to a type II error related to the small sample size of individual studies and the small number of hepatic events.
Hence, we conducted a systematic review and meta-analysis of all cohort studies to better understand the relationship between liver stiffness at baseline and the subsequent development of clinically relevant outcomes—risk of decompensated cirrhosis, HCC, and overall mortality—in patients with CLD.
Methods
This systematic review was conducted following the guidance provided by the Cochrane Handbook12 and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.13 The process followed an a priori established protocol.
Search Strategy
First, a systematic literature search of Medline (January 1, 1966 through February 1, 2013), Embase (January 1, 1988 through February 1, 2013), and Web of Science (January 1, 1993 through February 1, 2013) databases was conducted with the help of an expert librarian to identify all relevant articles on the role of LSM in predicting clinically relevant outcomes in patients with CLD. Medical subject heading terms used in the search included a combination of “liver” AND “stiff*,” “elastogra*,” “Fibroscan,” combined with “outcome*,” “prognos*,” “predict*,” “course,” “cancer,” “death,” “mortal*,” “transplant*.” The details of the search strategy are included in Supplementary Table 1. The title and abstract of studies identified in the search were reviewed by 2 authors independently (S.S. and L.L.F.) to exclude studies that did not address the research question of interest, based on prespecified inclusion and exclusion criteria (see later). The full text of the remaining articles was examined to determine whether it contained relevant information. Next, the reference sections of the selected articles and review articles on the topic were searched manually for additional studies. Third, a manual search of conference proceedings of major gastroenterology and hepatology conferences (The Liver Meeting, organized by the American Association for the Study of the Liver; The International Liver Congress, organized by the European Association for the Study of the Liver; and Digestive Diseases Week, organized in conjunction with the American Gastroenterological Association) between 2008 and 2012 was conducted to identify additional studies published only in abstract form. This time period was chosen because elastographic techniques began to be used increasingly for the assessment of liver fibrosis in this time period.
Selection Criteria
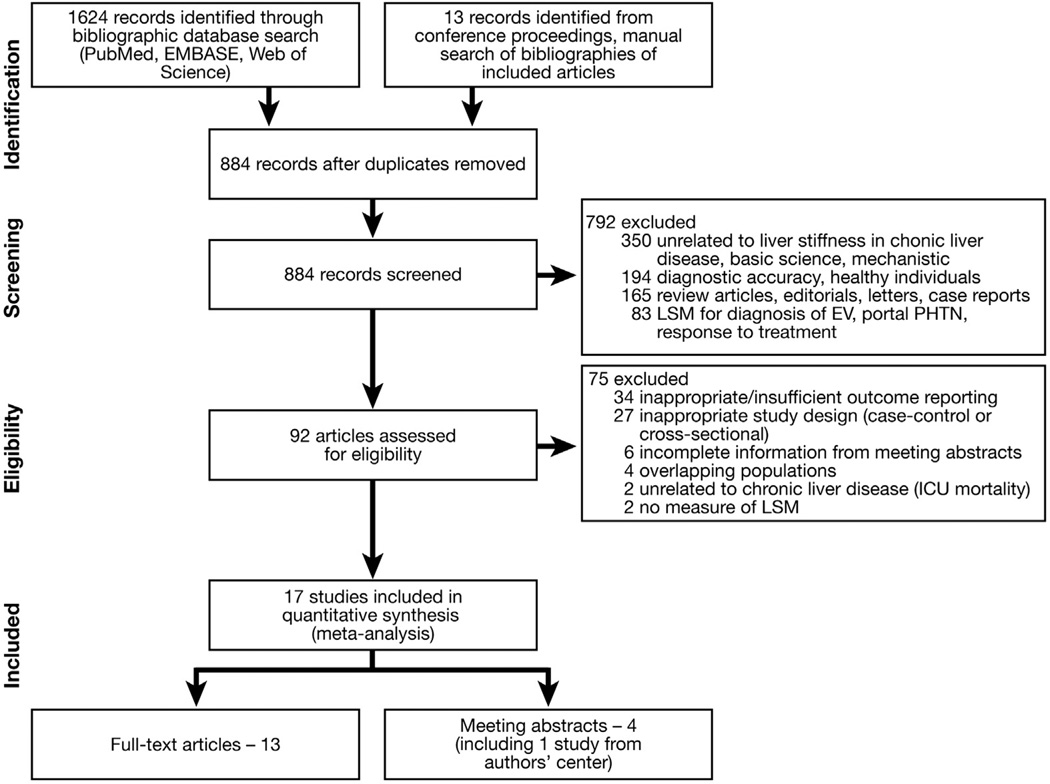
Considering the prognostic objective of this systematic review, we included prospective and historical cohort studies that met the following inclusion criteria: (1) LSM was performed (using TE or MRE) at time of cohort entry in patients with CLD (regardless of etiology and stage of disease) who were free of reported outcome at time of LSM; (2) systematically assessed the development of clinically relevant outcomes (decompensated cirrhosis, HCC in patients who previously were free of HCC, need for LT in patients who had a native liver and/ or all-cause or liver-related mortality); (3) had a minimum follow-up period of 6 months for all patients; and (4) reported a measure of association—hazard ratio (HR), relative risks (RR), sensitivity, and specificity of LSM for predicting outcomes or provided sufficient data for their calculation. Inclusion was not otherwise restricted by study size, language, or publication type. We excluded the following studies: (1) case-control studies, cross-sectional studies, and case series; (2) studies that assessed the use of LSM in patients with no evidence of CLD; or (3) provided insufficient data to allow estimation of RR with 95% confidence interval (CI) of outcomes. In case of multiple publications from the same cohort, data from the most recent comprehensive report were included. If, however, different outcomes were reported in different publications on the same cohort, then data from these studies were pooled together to interpret as one study with different outcome measures to minimize the risk of double-counting while at the same time maximizing available data. Figure 1 summarizes the process of study identification, inclusion, and exclusion.
Figure 1.
Flowchart showing the study identification and selection process. EV, esophageal varices; ICU, intensive care unit; PHTN, portal hypertension.
Data Abstraction
Data on the following were abstracted onto a standardized form: (1) study characteristics: primary author; time period of study/year of publication; country of the population studied; duration of follow-up evaluation (mean or median, total person-years of follow-up evaluation), as well as attrition; (2) patient characteristics: age, sex, body mass index; etiology of CLD (viral hepatitis, other causes); stage of CLD (early fibrosis defined as stages 0–2, advanced fibrosis, compensated cirrhosis, decompensated cirrhosis); treatment for underlying liver disease (and its distribution among those who developed outcomes and those who did not); (3) exposure assessment: technique of LSM, reported failure of LSM, whether LSM was reported as a continuous variable or categorical variable (and, if so, the categories reported); (4) outcomes reported: development of decompensated cirrhosis (variceal bleeding, ascites, hepatic encephalopathy, hepatorenal syndrome, spontaneous bacterial peritonitis), HCC, need for LT, overall- or liver-related mortality; (5) statistical analysis: HR or RR and 95% CIs with and without adjustment for confounding factors, or sensitivity/specificity of LSM for predicting outcomes; and (6) confounding variables, adjusted for in each study, especially for model for end-stage liver disease (MELD) score (or its components) and treatment of the underlying CLD. The authors of included studies were contacted for missing data. Conflicts in data abstraction were resolved by consensus, referring back to the original article and in consultation with the principal investigator (J.A.T.).
Quality Assessment
The quality assessment of prognostic studies was performed by 2 study investigators (S.S., L.L.F.) independently, using the Quality In Prognosis Studies tool,14 which evaluates validity and bias in studies of prognostic factors across 6 domains: participation, attrition, prognostic factor measurement, confounding measurement, outcome measurement, and analysis and reporting.
Outcomes
The primary analysis focused on assessing the prognostic use of LSM in predicting each clinically relevant outcome in patients with CLD: decompensated cirrhosis, HCC, and mortality. Because these outcomes are interdependent and not independent of each other, we report the association between LSM and each of these outcomes separately. In addition, some studies reported the risk of a composite outcome (hepatic decompensation, HCC, need for LT, and/or mortality), and these were pooled separately. Anticipating potential heterogeneity in the direction and magnitude of effect among the studies, we performed preplanned subgroup analyses on study-related variables to explore sources of heterogeneity. For each individual outcome, these included the following: (1) patient characteristics: location of study (Asian population vs Western population), etiology (viral hepatitis vs mixed), and stage (compensated cirrhosis vs all stages) of underlying liver disease, and (2) elastography-related variables: measure of LSM used (ultrasound-based vs magnetic resonance–based).
Statistical Analysis
We used the random-effects model using the DerSimonian and Laird15 method to calculate meta-analytic RR and the 95% CI for each outcome. We assumed HR to be equivalent to RR. When studies reported sensitivity and specificity of LSM for predicting outcomes, we calculated an unadjusted RR by formulating a 2 × 2 table.
When studies reported exposure grouped into categories to provide a dose-specific RR (using the lowest category as the referent category), we transformed this into a risk estimate per unit of LSM (called imputed RR), using linear-trend meta-analytic statistical methodology.16,17 Briefly, we assigned the midpoint of the cut-off points of the class as the dose value. For studies with open-ended categories, we used the lowest and highest reported LSM from the study to calculate the midpoint. We then calculated the RR for that range of LSM (subtracting the midpoints from the highest risk category with the lowest risk category) to estimate a per-unit RR, after log-transformation. This methodology assumes a linear relationship between LSM and logarithm of RR. If studies reported the relationship between LSM and outcomes as per unit, these were preferred for estimation (called reported RR).
We assessed heterogeneity between study-specific estimates using 2 methods: first, the Cochran Q test, which tests the null hypothesis that all studies in a meta-analysis have the same underlying magnitude of effect, was measured.18 Because this test is underpowered to detect moderate degrees of heterogeneity,19 a P value of less than .10 was considered suggestive of significant heterogeneity. Second, to estimate what proportion of total variation across studies was related to heterogeneity rather than chance, the I2 statistic was calculated. A value of greater than 50% was suggestive of considerable heterogeneity.18 We explored potential sources of heterogeneity using multivariable meta-regression according to predefined characteristics: study location (Asian vs Western population), etiology (viral vs mixed) and stage of CLD (compensated cirrhosis vs all stages), method of estimating RR (reported vs imputed), and whether analysis was adjusted for hepatic synthetic function, HVPG, or MELD (yes vs no). A P value less than .05 was suggestive of the grouping variable being a significant source of heterogeneity.
Because of the limited number of studies, a formal estimation of publication bias was not performed.20 Analyses and graphs were conducted using Comprehensive Meta-Analysis version 2 (Biostat, Englewood, NJ) and Stata version 12 (StataCorp, College Station, TX).
Results
From 884 unique studies identified using the search strategy, 17 studies met the inclusion criteria (13 full-text publications,11,21–32 4 abstracts33–36). Five studies were from overlapping populations,9,10,25,37,38 and hence only one of them reporting on hepatic decompensation and HCC was included25; the other studies were used for a sensitivity analysis as described later. Two studies reported the ability of LSM to predict short-term mortality in unselected hospitalized patients, and hence were excluded.39,40 The coefficient of agreement between the 2 reviewers for article selection (Cohen’s κ = 0.88; 95% CI, 0.77–0.99) was excellent.
Studies were variable with regard to the primary outcomes reported. Of the included studies, 6 studies reported the association between LSM and risk of hepatic decompensation.22,25,28,29,33,34 Nine studies reported the association between LSM and risk of HCC in patients with CLD21,22,24,25,27,28,30,34,35; of these, 2 studies reported a composite outcome of HCC or hepatic decompensation.22,28 Five studies reported the association between LSM and future risk of mortality.22,24,28,31,32 Because of the small number of individual events, 6 studies reported a composite outcome of hepatic decompensation, HCC, need for LT, or mortality.11,23,26,29,33,36 Through contact with authors, we were able to obtain additional information regarding 3 studies.11,22,25
Characteristics of Included Studies
All studies, except one,33 used ultrasound-based TE for measurement of liver stiffness; one study used MRE for assessing liver stiffness. The range of LSM in patients in individual studies varied from 1.5 to 75 kPa. The earliest study period started in 2004 and the latest follow-up period ended in 2012. The median follow-up period in individual studies ranged from 16 to 71 months, with the minimum patient follow-up period being at least 7 months. The majority of the studies were conducted in the European population (9 studies)11,22,23,28–32,34; 3 studies were performed in the North American population26,33,36 and 5 studies were conducted in the Asian population.21,24,25,27,35 In 9 of the studies, HCV, with or without human immunodeficiency virus, was the leading cause of CLD.22,27,28,30–32,34–36 Two Asian studies were performed exclusively in patients with chronic hepatitis B.24,25 One study was performed exclusively in patients with primary biliary cirrhosis.11 All of the remaining studies were performed on patients with mixed etiologies of CLD; nonalcoholic steatohepatitis was the leading cause of CLD (41%) in only one of the included studies performed using MRE.33 Five studies were conducted exclusively in patients with compensated cirrhosis,23,28,30,33,34 whereas others included a mix of patient populations at varying stages of fibrosis or cirrhosis (defined based on liver biopsy or LSM). Of note, in studies that included patients with decompensated cirrhosis at entry into the cohort, the subsequent risk of decompensated cirrhosis was not a valid outcome and was not reported; these patients were still at risk for HCC and mortality. Tables 1 and 2 show the baseline characteristics of the included studies.
Table 1.
Baseline Characteristics of the Included Studies
| Study | Location | Time period; follow-up period, mo (range or SD) |
Patients | Outcomes assessed |
Events (incidence rate per 1000 person-years) |
||
|---|---|---|---|---|---|---|---|
| Decompensation | HCC | Mortality | |||||
| Full-text publications | |||||||
| Akima et al21 | Saitama, Japan | 2005–2009; 41 (7–53) | 106a | HCC | - | 10 (27.8) | - |
| Corpechot et al11 | Paris, France | 2004–2011; 31 (14) | 150 | Composite | 5 (22.7) | 2 (5.1); +4 LT | |
| Fernandez-Montero et al22 | Madrid, Spain | 2004–2012; 71 (16) | 545 | Decompensation + HCC; mortality | 49 (15.2) | 4 (1.2) | 12 (3.7) |
| Forestier et al23 | Lyon, France | 2007–2008; 8–26 | 142b | Composite | 19 (unable to estimate IR) | 11 (unable to estimate IR) | |
| Fung et al24 | Hong Kong | 2006; 35 (6–42) | 528 | HCC; mortality | - | 7 (4.5) | 3 (1.9) |
| Chon et al25 | Seoul, South Korea | 2005–2009; 31 (24–51) | 1126 | Decompensation; HCC | 68 (23.4) | 63 (21.6) | - |
| Klibansky et al26 | Boston, MA | 2004–2009; 29 | 667 | Composite | 48 (29.8) | 16 (9.9) | 3 (1.9) |
| Masuzaki et al27 | Tokyo, Japan | 2004–2008; 36 | 866 | HCC | - | 77 (29.3) | - |
| Merchante et al28 | Andalusia, Spain | 2006–2011; 21 (10–35) | 239 | Decompensation + HCC; mortality | 28 (70.0) | 3 (7.2) | 15 (35.9) |
| Robic et al29 | Purpan, France | 2005–2006; 16 (9) | 100 | Decompensation; composite | 41 (307.5) | ||
| Salmon et al30 | Paris, France | 2012; 31 (22–42) | 244 | HCC | - | 21 (33.3) | - |
| Tuma et al31 | Madrid, Spain | 2004–2008; 28 (17–66) | 194 | Mortality | - | - | 25 (55.2) |
| Vergniol et al32 | Bordeaux, France | 2003–2009; 47 (41–53) | 1457 | Mortality | - | - | 89 (15.6) |
| Meeting abstracts | |||||||
| Asrani et al33 | Rochester, MN | 2007–2012; 27 | 167 | Decompensation; composite | 12 (31.9) | 8 (21.2) | |
| Calvaruso et al34 | Palermo, Italy | 2006–2009; 42 (18) | 233 | Decompensation; HCC | 20 (24.5) | 20 (24.5) | - |
| Narita et al35 | Izunokoni, Japan | 2012; 20 | 151 | HCC | - | 9 (37.8) | - |
| Vu et al36 | Baltimore, MD | 2005–2009; 23 (11–32) | 293 | Composite | NR | ||
SD, standard deviation.
Composite, composite of decompensated cirrhosis; decompensation, decompensated cirrhosis (portal hypertensive bleeding, ascites, spontaneous bacterial peritonitis, hepatic encephalopathy); HCC, need for LT, mortality; IR, incidence rate per 1000 person-years; NR, not reported.
Of 157 total patients, 116 patients were free of HCC at baseline, and, of these, 106 patients had a minimum of 6 months of follow-up evaluation.
Of 296 patients, 142 patients had cirrhosis at baseline, and these patients were followed up prospectively for a minimum of 8 months.
Table 2.
Demographics, Etiology, Stage, and Treatment of CLD in Individual Studies, and Measures of Association Between Liver Stiffness and Liver-Related Outcomes
| Study | Mean age, y; sex, % males |
Mean body mass index, kg/m2 |
Etiology of CLD | Stage of CLD | Treatment for CLD | RR/HR for association between LSM and outcome (95% CI) |
Adjustmentsa | ||
|---|---|---|---|---|---|---|---|---|---|
| Decompensation | HCC | Mortality | |||||||
| Full-text publications | |||||||||
| Akima et al21 | NR; NR | NR | HCV, 85%; HBV, 15% | NR | 5 of 16 on HBV Tx; 51 of 90 on HCV Tx | - | Adjusted HR, 14.9 (1.9–115.8) (≥12.5 kPa vs <12.5 kPa) | - | 1, 2, 4, 6, 8, 9, 10, 11 |
| Corpechot et al11 | 57; 11 | 23.7 | PBC, 100% | F0–2, 75%; F3–4, 25% (based on TE); no decomp cirrhosis | All on UDCA; 35% on steroids | Adjusted HR, 5.1 (1.5–15.9) (≥9.6 kPa vs <9.6 kPa) | 1, 2, 3, 5, 6, 7, 8, 9, 10 | ||
| Fernandez- Montero et al22 | 41; 71 | 23.3 | HIV/HCV, 100% | F0–2, 66%; F3–4, 34%; no decomp cirrhosis | 542 of 545 on HAART; 335 of 545 on HCV Tx | Adjusted HR (per unit kPa), 1.12 (1.08–1.16) | Adjusted HR (per unit kPa), 1.09 (1.01–1.19) | 1, 2, 6, 11 | |
| Forestier et al23 | 57; 74 | 26 | EtOH, 51%; HCV, 21%; HBV, 3%; NASH, 8%; others, 17% | F4, 100% (CPT A, 63%; CPT B, 30%; CPT C, 7%) | NR | Unadjusted RR, 5.6 (2.4–12.7) | Unadjusted | ||
| Fung et al24 | 42; 61 | NR | HBV, 100% | NR | 106 of 528 on HBV Tx | - | Unadjusted RR, 6.40 (4.86–8.41) | Unadjusted RR, 5.72 Unadjusted (3.75–8.71) | |
| Chon et al25 | 50; 68 | 30.8% overweight | HBV, 100% | Cirrhosis, 17.5% (all CPT A) | 443 of 1126 on HBV Tx | Adjusted HR (per unit kPa), 1.033 (1.007–1.060) | Adjusted HR (per unit kPa), 1.040 (1.012–1.070) | - | 1, 2, 4, 5, 7, 9, 10, 11 |
| Klibansky et al26 | 51; 62 | 26.8 | EtOH, 2%; HCV, 67%; HBV, 8%; NASH, 14%; others, 9% | F0–2, 60%; F3, 16%; F4, 13%; (+clinical evidence of cirrhosis, 11%) | NR | Univariate HR (using <10.5 kPa as reference); 10.5–12.5 kPa, 3.23 (0.29–35.73); >12.5 kPa, 2.87 (0.39–20.97) | Unadjusted | ||
| Masuzaki et al27 | 62; 46 | 22.5 | HCV, 100% | Clinical evidence of cirrhosis, 23% | 173 of 866 (20%) on IFN | - | Adjusted HR (using ≤10 kPa as reference) 10.1–15.0 kPa, 16.7 (3.71–75.2); 15.1–20.0 kPa, 20.9 (4.43–98.8); 20.1–25.0 kPa, 25.6 (5.21–126.1); >25.0 kPa, 45.5 (9.75–212.3) | - | 1, 2, 4, 5, 6, 9, 10, 11 |
| Merchante et al28 | 44; 90 | NR | HIV/HCV, 100% | Compen cirrhosis, 100% (CPT A, 90%; CPT B, 10%) | 93 of 239 on HCV Tx | Adjusted HR (per unit kPa), 1.03 (1.01–1.05) | Adjusted HR (per unit kPa), 0.9 (0.8–1.1) | 1, 2, 3, 4, 5, 6 | |
| Robic et al29 | 56; 59 | 25.0 | EtOH, 38%; HCV/HBV, 28%; NASH, 8%; others, 26% | F0–2, 25%; F3, 10%; F4, 65% | NR | Decompensation: unadjusted RR, 43.3 (3.99–469.9) Composite: unadjusted RR, 3.39 (2.09–5.49) | Unadjusted | ||
| Salmon et al30 | 47; 79 | 22.8 | HIV/HCV, 100% | Cirrhosis, 100% (CPT A, 87%; CPT B, 12%; CPT C, 1%) | HIV Tx, 92%; HCV Tx, 58% | - | Unadjusted HR (per unit kPa), 1.0 (0.9–1.0) | - | Unadjusted |
| Tuma et al31 | 44; 80 | NR | HIV, 100%; HCV, 89%; HBV, 10% | Compensated cirrhosis, 100% (based on LSM); CPT A, 77% CPT B, 17%; unknown 6% | HIV Tx, 93%; | - | - | Adjusted HR, 3.46 (1.24–9.69) (using cut-off >28.75 kPa) | 1, 2, 5, 11 |
| Vergniol et al32 | 51; 53 | 23.8 | HCV, 100% | F0–2, 67%; F3, 15%; F4, 18% | HCV Tx, 52% | - | - | Adjusted HR, 2.9 (2.0–4.3) (using <9.5 kPa as reference) | 1, 6, 7 |
| Meeting abstracts | |||||||||
| Asrani et al33 | 55; 55 | 28.0 | EtOH, 2%; HCV, 26%; NASH, 41%; other, 31% | F3–4, 100% | NR | Decompensation: adjusted HR (per unit kPa), 1.57 (1.24–1.99) Composite: adjusted HR (per unit kPa), 1.28 (1.10–1.49) | 3 | ||
| Calvaruso et al34 | NR | NR | HCV, 100% | Compensated cirrhosis, 100% | NR | Adjusted RR (per unit kPa), 1.06 (1.03–1.09) | Adjusted RR (per unit kPa), 1.10 (1.05–1.15) | - | NR |
| Narita et al35 | NR | NR | HCV, 100% | NR | HCV Tx, 100% | - | Adjusted HR, 5.58 (P = .002) (using <14 kPa as reference) | - | 1, 4, 6 |
| Vu et al36 | 50; 68 | NR | HIV/HCV, 100% | F0, 49%; F1–3, 27%; F4, 24% (based on LSM) | NR | Adjusted RR, 3.9 (1.1–13.3) | 1, 5 | ||
Composite, composite of decompensated cirrhosis; CPT, Child-Pugh-Turcotte; Decomp, decompensated cirrhosis (portal hypertensive bleeding, ascites, spontaneous bacterial peritonitis, hepatic encephalopathy); EtOH, alcoholic liver disease; HAART, highly active antiretroviral therapy; HBV, hepatitis B virus; HIV, human immunodeficiency virus; IFN, interferon; NASH, nonalcoholic steatohepatitis; NR, not reported; PBC, primary biliary cirrhosis; Tx, treatment; UDCA, ursodeoxycholic acid.
Adjustments were made for the following: 1, age; 2, sex; 3, MELD or its components; 4, platelet count; 5, severity of underlying liver disease (HCV, human immunodeficiency virus, hepatitis B virus); 6, treatment of underlying disease; 7, noninvasive fibrosis markers; 8, hyaluronic acid; 9, liver synthetic function; 10, liver enzymes; 11, other factors (body mass index, diabetes mellitus, α-fetoprotein concentration).
Quality of Included Studies
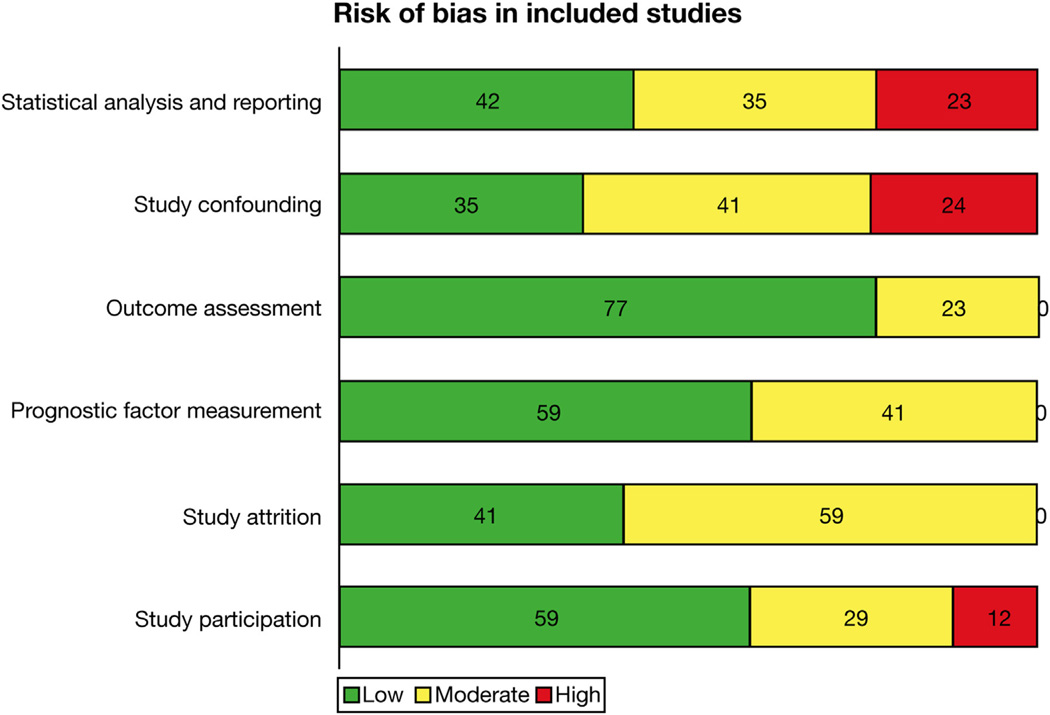
The included studies were usually at low to moderate risk of bias with regard to study participation, attrition from study, measurement of liver stiffness, and defining outcomes (except with regard to features of hepatic decompensation). Several studies did not adjust for important confounders, especially with regard to hepatic synthetic function, severity of liver disease, and whether patients were treated for their underlying CLD. In addition, some studies were at high risk of bias because they reported only univariate and/or unadjusted analysis. Figure 2 and Supplementary Table 2 show the overall quality assessment of the included studies using the Quality In Prognosis Studies tool. The agreement between the 2 reviewers for quality assessment (88.5%) was very good.
Figure 2.
Quality assessment of included studies using the Quality In Prognosis Studies tool. All studies were scored as low, moderate, or high risk of bias across 6 domains: study participation, study attrition, prognostic factor measurement (ie, elastographic technique), outcome assessment, confounding factors, and statistical analysis and reporting.
Risk of Hepatic Decompensation
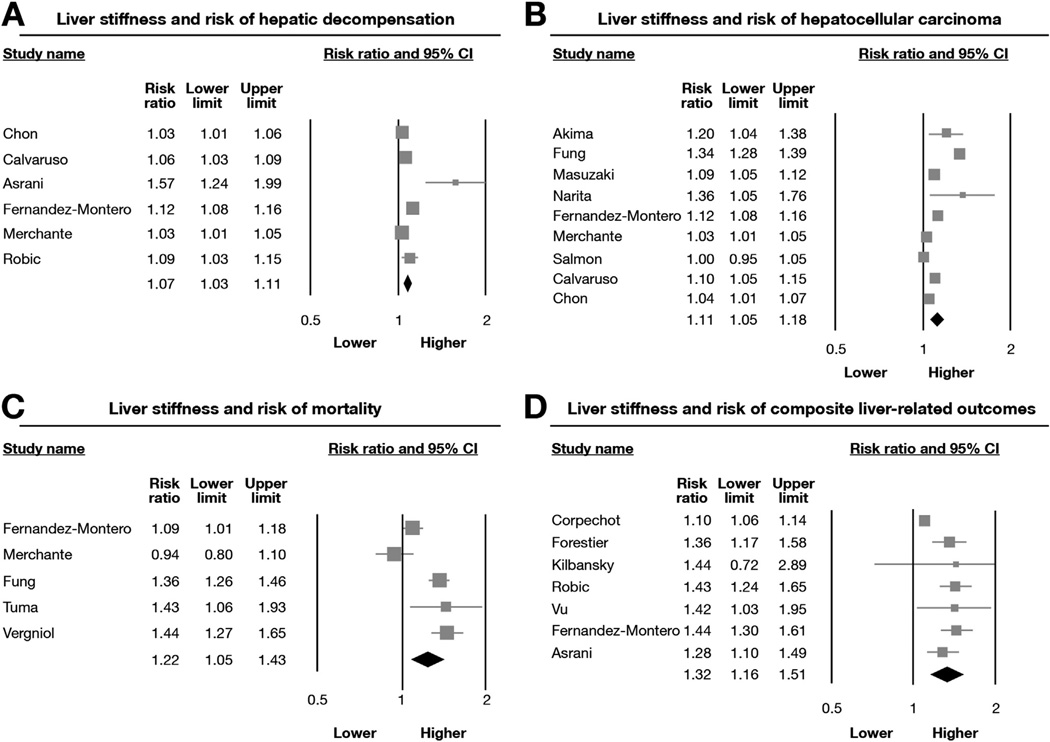
On meta-analysis of 6 studies (216 cases of hepatic decompensation),22,25,28,29,33,34 each unit increase in LSM was associated with a 7% higher risk of developing decompensated cirrhosis on follow-up evaluation (Figure 3A). The results were stable across etiology and stage of CLD, across populations, and in studies using TE or MRE for measurement of liver stiffness (Table 3). The results also were similar after restricting the analysis to studies that adjusted for conventional measures of hepatic function and prognostic markers. When we replaced the study by Chon et al25 with another study from the same cohort,38 the results were stable (RR, 1.10; 95% CI, 1.05–1.16).
Figure 3.
Association between baseline LSM and subsequent risk of (A) hepatic decompensation, (B) HCC, (C) mortality, and (D) composite outcome of liver-related events in patients with CLD. Pooled estimate represents increase in risk of liver-related event per unit of LSM.
Table 3.
Association Between Baseline LSM and Subsequent Risk of Hepatic Decompensation in Patients With CLD
| Groups | Categories | Studies, n | Adjusted RR | 95% CI |
|---|---|---|---|---|
| Location | West | 5 | 1.09 | 1.04–1.14 |
| Asia | 1 | 1.03 | 1.01–1.06 | |
| Etiology of CLD | Mixed | 2 | 1.28 | 0.90–1.83 |
| Viral | 4 | 1.06 | 1.02–1.09 | |
| Stage of CLD | All | 3 | 1.06 | 1.01–1.11 |
| Compensated cirrhosis | 3 | 1.12 | 1.02–1.23 | |
| Analysis | Adjusted | 5 | 1.07 | 1.03–1.11 |
| Unadjusted | 1 | 1.09 | 1.03–1.15 |
NOTE. The results represent change in risk of outcomes per unit (kPa) of LSM.
There was considerable heterogeneity observed in this analysis (P < .01, I2 = 84%); this was seen primarily in the magnitude of effect, but not in the direction of effect. In the multivariable meta-regression analysis, the heterogeneity was attributable to the technique of LSM used. In the one study using MRE, the risk estimates were significantly higher (RR, 1.57; 95% CI, 1.24–1.99) as compared with 5 studies performed using TE (RR, 1.06; 95% CI, 1.03–1.09). The results were stable when restricting analysis to studies in which per-unit analysis was reported, rather than imputed (5 studies; RR, 1.07; 95% CI, 1.03–1.11).
Risk of HCC
Of the 9 included studies (214 cases of HCC),21,22,24,25,27,28,30,34,35 8 reported a significant association between baseline LSM and subsequent risk of developing HCC. On meta-analysis, each unit increase in LSM was associated with an 11% higher risk of HCC (Figure 3B). The results were consistent across Asian and Western populations,aswell as across studies that included patients with compensated cirrhosis only or at all stages of CLD (Table 4). All studies were performed in patients with viral hepatitis as the etiology of CLD, and all studies used TE as a modality for LSM. The results were consistent when replacing the studybyChonetal25 withanother study from the same cohort(RR, 1.14; 95% CI, 1.07–1.22).10 Considerable heterogeneity was observed in the overall analysis (P < .01, I2 = 94%); meta-regression analysis was not able to explain heterogeneity based on stage of CLD, study location, level of adjustment in original studies, or method of imputation. The relationship between LSM and future risk of HCC continued to be significant on restricting analysis to studies in which per-unit analysis was reported, rather than imputed (5 studies; RR, 1.05; 95% CI, 1.00–1.09).
Table 4.
Association Between Baseline LSM and Subsequent Risk of HCC in Patients With CLD
| Groups | Categories | Studies, n | Adjusted RR | 95% CI |
|---|---|---|---|---|
| Location | West | 4 | 1.06 | 1.01–1.12 |
| Asia | 5 | 1.18 | 1.05–1.32 | |
| Stage of CLD | All | 6 | 1.16 | 1.07–1.26 |
| Compensated cirrhosis | 3 | 1.04 | 1.00–1.09 | |
| Analysis | Adjusted | 7 | 1.08 | 1.05–1.12 |
| Unadjusted | 2 | 1.16 | 0.87–1.54 |
NOTE. The results represent change in risk of outcomes per unit (kPa) of LSM. All studies were performed in patients with viral hepatitis as the etiology of CLD, and all studies used TE as the modality for LSM.
Risk of Mortality
Meta-analysis of 5 studies (144 deaths) showed that baseline LSM significantly predicted the risk of mortality in patients with CLD (Figure 3C).22,24,28,31,32 Four studies showed a significant positive association with effect sizes varying from 1.09 to 1.44. All studies were performed in patients with viral hepatitis with or without human immunodeficiency virus. The results were stable across location. Significant heterogeneity was observed in the analysis (P < .01; I2 = 88%). The heterogeneity might be explained on the basis of analysis—studies in which the imputed per-unit RR showed a significant difference from those that reported per-unit RR (Table 5). In a sensitivity analysis limited to 2 studies that reported per-unit analysis, one of the studies noted the presence of a dose-response relationship,22 and the other study did not observe such an association.28 Overall, a significant relationship was not observed (RR, 1.03; 95% CI, 0.89–1.19).
Table 5.
Association Between Baseline LSM and Subsequent Risk of Mortality in Patients With CLD
| Groups | Categories | Studies, n | Adjusted RR | 95% CI |
|---|---|---|---|---|
| Location | West | 4 | 1.19 | 0.98–1.44 |
| Asia | 1 | 1.36 | 1.26–1.46 | |
| Stage of CLD | All | 4 | 1.30 | 1.12–1.51 |
| Compensated cirrhosis | 4 | 0.94 | 0.80–1.10 | |
| Analysis | Adjusted | 4 | 1.19 | 0.98–1.44 |
| Unadjusted | 1 | 1.36 | 1.26–1.46 |
NOTE. The results represent change in risk of outcomes per unit (kPa) of LSM. All studies were performed in patients with viral hepatitis as the etiology of CLD, and all studies used TE as the modality for LSM.
Risk of Composite Outcome
In 7 studies (all performed in Western populations),11,23,26,29,33,36 the relationship between LSM and a composite outcome (hepatic decompensation, HCC, need for LT, or mortality) was reported. Meta-analysis of these studies showed a significant positive association in 6 of these 7 studies, with a summary estimate of 1.32 (Figure 3D). The results were stable across study location, etiology, and stage of CLD, as well as based on whether analysis was adjusted for other known predictors of outcomes (Table 6). Considerable heterogeneity (P < .01; I2 = 85%) was identified. However, meta-regression analysis could not identify any obvious sources of heterogeneity based on study, patient, or statistical characteristics.
Table 6.
Association Between Baseline LSM and Subsequent Risk of Composite Outcomes (Hepatic Decompensation, HCC, Need for LT or Mortality) in Patients With CLD
| Groups | Categories | Studies, n | Adjusted RR | 95% CI |
|---|---|---|---|---|
| Location | West | 6 | 1.33 | 1.14–1.56 |
| Asia | 1 | 1.44 | 1.19–1.75 | |
| Etiology of CLD | Mixed | 4 | 1.29 | 1.07–1.54 |
| Viral | 3 | 1.44 | 1.32–1.58 | |
| Stage of CLD | All stages | 5 | 1.33 | 1.11–1.60 |
| Compensated cirrhosis | 2 | 1.32 | 1.19–1.47 | |
| Analysis | Adjusted | 4 | 1.44 | 1.32–1.58 |
| Unadjusted | 3 | 1.28 | 1.05–1.55 |
NOTE. The results represent the change in risk of outcomes per unit (kPa) of LSM. All studies used TE as the modality for LSM.
Sensitivity Analyses
Because meeting abstracts have limited reporting of events and do not undergo rigorous peer-review, we performed sensitivity analyses restricted only to articles published in peer-reviewed journals. A meta-analysis of these studies showed a stable association between baseline LSM and risk of hepatic decompensation (N = 6 studies including our own study; RR, 1.08; 95% CI, 1.03–1.13), HCC (N = 7 studies; RR, 1.11; 95% CI, 1.04–1.18), and composite outcome (N = 6 studies; RR, 1.31; 95% CI, 1.14–1.51). All studies assessing the relationship between LSM and mortality were published as full-text articles.
On limiting analyses to studies that were at low risk for bias based on statistical analysis and reporting and appropriately controlled for confounding factors (hepatic synthetic function and treatment of CLD), the association between LSM and risk of hepatic decompensation (N = 3 studies; RR, 1.05; 95% CI, 1.00–1.11), HCC (N = 3 studies; RR, 1.05; 95% CI, 1.02–1.08), and composite outcome (N = 2 studies; RR, 1.16; 95% CI, 1.01–1.34) remained stable. Only one study was deemed to be at low risk of bias based on appropriate controlling for confounding and adequate statistical analysis and reporting, for assessing the association between LSM and future risk of mortality, and this study was negative (RR, 0.94; 95% CI, 0.80–1.10).
Discussion
Until recently, cirrhosis was considered a single and terminal disease stage, with an inevitably poor prognosis. However, it is now clear that cirrhosis encompasses a complex dynamic pathologic spectrum. Although patients with compensated cirrhosis have a median survival of 12 years, patients with decompensated cirrhosis have a median survival of 2 years.41 The average risk of progressing from a compensated to a decompensated state is 6% to 9% per year.41 Preventive strategies that help decrease the risk of progression from a compensated to a decompensated state have been proposed and include lifestyle changes (focusing on abstinence from alcohol, weight reduction, and smoking cessation), nonspecific therapies (such as nonselective β-blockers, statins, and antibiotics), as well as disease-specific therapies (such as antiviral treatment for viral hepatitis).4 However, objective tests that can identify patients at risk for progression to a decompensated state, for which these strategies may be most clinically relevant and cost effective, currently are lacking.
In this comprehensive meta-analysis of 17 prospective cohort studies on 7058 patients with CLD, we have shown that noninvasive measurement of liver stiffness may be a useful tool in identifying patients with CLD at risk for progression to clinical events. We observed that a high LSM, independent of other markers of severity of liver disease and hepatic synthetic function, is able to predict future risk of hepatic decompensation, HCC, and overall mortality in a dose-dependent manner. Each unit of LSM is associated with an incremental 7% and 11% increased risk of decompensation and HCC, respectively. The strong correlation between LSM and portal hypertension, as well as its diagnostic accuracy in detecting esophageal varices, has already been shown.7,8 Because elastography measures hepatic fibrosis, which only correlates with the fixed component of portal hypertension related to intrahepatic resistance, but is unable to account for the dynamic component related to hyperdynamic splanchnic circulation and portal venous blood flow, it is probable that LSM may not capture the risk of decompensation as completely as HVPG. Regardless, given that the development of decompensated cirrhosis and HCC represent an ominous milestone in the natural history of cirrhosis, the ability of this noninvasive technique to potentially capture this risk of transition is encouraging. Besides assessment of baseline LSM, some studies have shown that serial changes in LSM potentially may add to the prognostic utility of this technique. Corpechot et al,11 in a cohort of treated patients with primary biliary cirrhosis, observed that clinically relevant liver-related events occurred only in patients with advanced fibrosis who had an increase in liver stiffness of more than 2.1 kPa/y. Vergniol et al42 also observed that an evolution of LSM over 3 years predicted the risk of mortality over 4 years in a cohort of patients with HCV.
The strengths of this analysis included the following: (1) assessment of the longitudinal association between baseline LSM and multiple different relevant outcomes—hepatic decompensation, development of HCC, and mortality; (2) incorporating multiple etiologies and stages of CLD; (3) focusing only on cohort studies allowed us to assess the prognostic utility of LSM as opposed to its diagnostic utility, which may be inferred from case-control and cross-sectional studies; (4) inclusion of all available cohort studies and not restricting analysis based on publication type or language, and hence minimizing the risk of selection bias; and (5) performance of analyses of maximally adjusted risk estimates reported in the studies to account for the effect of potential confounders.
There were several limitations in our analysis that merit further discussion. First, meta-analyses of prognostic studies have inherent limitations given the clinical heterogeneity of patients included in studies, variability in treatment on follow- up evaluation, poor methodologic quality of prognostic studies, as well as potential reporting and publication bias.43 Ideally, pooled analysis of individual patient data would be best suited to assess the role of LSM in prognostication. Second, we imputed the relative risk per unit of LSM assuming a log-linear relationship across the ranges of 1.5 to 75 kPa. We assumed normality of LSM distribution, and a linear association between the log (RR) or log (HR) and LSM for all its range of values. It is possible that this relationship is not linear at low levels of liver stiffness, but potentially increases at a faster pace beyond a threshold of stiffness, which has not been identified consistently in studies. To support this linear relationship, we performed a sensitivity analysis including only studies in which per-unit RR was reported (not imputed), and the results were stable. Third, there was considerable heterogeneity observed in the analysis. However, this heterogeneity was seen primarily in the strength of the association between LSM and clinical outcomes, and not in the direction of association. The statistical heterogeneity could not be explained on meta-regression analysis based on study location, etiology and stages of CLD, technique of LSM, whether the study adjusted for important covariates, and method of imputing relationship. However, given the clinical variability in the included studies, other unmeasured confounders could have contributed to this heterogeneity. Fourth, follow-up evaluation was variable in the included studies and time-to-event analysis was variably reported in the included studies. Hence, we estimated a conservative meta-analytic RR of liver-related events, as opposed to time-to-event analysis, which may better reflect the prognostic value of LSM. Fifth, there was variable adjustment for confounding variables in these studies, especially for measures of severity of liver disease (MELD), hepatic synthetic function, and HVPG. We could not exclude confounding by unmeasured exposures or incomplete control of confounding from measured factors, such as treatment for underlying disease, although it did not appear that patients with high baseline LSM were treated differently from patients with low baseline LSM in the included studies. Sixth, because of the limited number of studies reporting each outcome, sufficient information was not available to perform all the preplanned subgroup analyses. Finally, although the number of published studies examining LSM was small given the recent emergence of this technology, it remains possible that investigations showing poor reproducibility or accuracy have not been published because of negative results. We tried to minimize the effect of publication bias by including reports from conference proceedings, including non-English language literature in our search, and contacting individual authors. Because of the limited number of studies as well as considerable heterogeneity, statistical testing for funnel plot asymmetry was not performed.20
In conclusion, assessment of liver stiffness using noninvasive elastographic techniques appears to be a useful modality to predict risk of clinically relevant outcomes in patients with CLD. These data suggest that LSM may evolve from its role as a diagnostic test to a surveillance procedure that actively helps in the management of patients at the highest need for vigilance. Future high-quality, prospective cohort studies in patients at similar (early) stages of CLD receiving similar treatment are warranted, to assess the prognostic utility of LSM more clearly in discriminating patients at high and low risk of hepatic decompensation, HCC, and liver-related mortality, to alter treatment or management decisions to finally have a chance to improve patients’ outcomes. Ideally, these studies should be registered to minimize risk of reporting bias. Moreover, individual patient data analysis of multiple studies would be ideal to overcome the limitation of a small number of events in individual studies as well as variability in exposure and outcome measurement in included studies. In addition, studies should focus on assessing whether a prognostic model including measures of liver severity such as MELD and hepatic synthetic function, in combination with LSM, may provide additional discriminative ability in predicting outcomes. Assessing the relationship between serial changes in LSM and the development of clinically relevant outcomes also would be important.
Supplementary Material
Acknowledgements
The authors sincerely thank the following authors for kindly sharing additional data from their cohort, which enabled this meta-analysis to be performed: Christopher Corpechot, MD (Service d’Hepatologie, Centre de reference des Maladies Inflammatoires des Voies Biliaires, Hospital Saint-Antoine, Assistance Publique–Hopitaux de Paris, Paris, France); José Vicente Fernandez-Montero, MD (Department of Infectious Diseases, Hospital Carlos III, C/Sinesio, Madrid, Spain); and Kwang-Hyub Han, MD, and Seung Up Kim, MD (Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea). The authors also wish to thank Ms Patricia Erwin, Medical Librarian at the Mayo Clinic Library, for helping with the literature search for this systematic review and meta-analysis, and Dr Colin West and Dr Victor Montori, Knowledge and Evaluation Research Unit, Mayo Clinic, for their critical input throughout the conduct of this systematic review.
Funding
This study was made possible by support from the Center for the Science of Healthcare Delivery, Mayo Clinic, and a Center for Trans-lational Science Activities (CTSA) grant (UL1 TR000135) from the National Center for Advancing Translational Sciences, a component of the National Institutes of Health, as well as a National Institutes of Health grant (EB001981). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Abbreviations used in this paper
- CI
confidence interval
- CLD
chronic liver disease
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HR
hazard ratio
- HVPG
hepatic venous pressure gradient
- LSM
liver stiffness measurement
- LT
liver transplantation
- MELD
model for end-stage liver disease
- MRE
magnetic resonance elastography
- RR
relative risk
- TE
transient elastography
Footnotes
Conflicts of interest
This author discloses the following: Richard Ehman has intellectual property rights and a financial conflict of interest related to the technology used in this study. The remaining authors disclose no conflicts.
The Mayo Clinic also has intellectual property rights and a financial conflict of interest related to the technology used in this study. This research has been conducted in compliance with oversight by the Mayo Clinic Conflict of Interest review board.
References
- 1.Asrani SK, Larson JJ, Yawn B, et al. Underestimation of liver-related mortality in the United States. Gastroenterology. 2013;145:375–382. doi: 10.1053/j.gastro.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manos MM, Leyden WA, Murphy RC, et al. Limitations of conventionally derived chronic liver disease mortality rates: results of a comprehensive assessment. Hepatology. 2008;47:1150–1157. doi: 10.1002/hep.22181. [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Tsao G, Friedman S, Iredale J, et al. Now there are many (stages) where before there was one: in search of a pathophysiological classification of cirrhosis. Hepatology. 2010;51:1445–1449. doi: 10.1002/hep.23478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsochatzis EA, Bosch J, Burroughs AK. New therapeutic paradigm for patients with cirrhosis. Hepatology. 2012;56:1983–1992. doi: 10.1002/hep.25915. [DOI] [PubMed] [Google Scholar]
- 5.Ripoll C, Groszmann R, Garcia-Tsao G, et al. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology. 2007;133:481–488. doi: 10.1053/j.gastro.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 6.Tsochatzis EA, Gurusamy KS, Ntaoula S, et al. Elastography for the diagnosis of severity of fibrosis in chronic liver disease: a meta-analysis of diagnostic accuracy. J Hepatol. 2011;54:650–659. doi: 10.1016/j.jhep.2010.07.033. [DOI] [PubMed] [Google Scholar]
- 7.Castera L, Pinzani M, Bosch J. Non invasive evaluation of portal hypertension using transient elastography. J Hepatol. 2012;56:696–703. doi: 10.1016/j.jhep.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Shi KQ, Fan YC, Pan ZZ, et al. Transient elastography: a meta-analysis of diagnostic accuracy in evaluation of portal hypertension in chronic liver disease. Liver Int. 2013;33:62–71. doi: 10.1111/liv.12003. [DOI] [PubMed] [Google Scholar]
- 9.Kim BK, Park YN, Kim do Y, et al. Risk assessment of development of hepatic decompensation in histologically proven hepatitis B viral cirrhosis using liver stiffness measurement. Digestion. 2012;85:219–227. doi: 10.1159/000335430. [DOI] [PubMed] [Google Scholar]
- 10.Jung KS, Kim SU, Ahn SH, et al. Risk assessment of hepatitis B virus-related hepatocellular carcinoma development using liver stiffness measurement (FibroScan) Hepatology. 2011;53:885–894. doi: 10.1002/hep.24121. [DOI] [PubMed] [Google Scholar]
- 11.Corpechot C, Carrat F, Poujol-Robert A, et al. Noninvasive elastography-based assessment of liver fibrosis progression and prognosis in primary biliary cirrhosis. Hepatology. 2012;56:198–208. doi: 10.1002/hep.25599. [DOI] [PubMed] [Google Scholar]
- 12.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] The Cochrane Collaboration. 2011 Available at: www.cochrane-handbook.org.
- 13.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. W64. [DOI] [PubMed] [Google Scholar]
- 14.Hayden JA, van der Windt DA, Cartwright JL, et al. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158:280–286. doi: 10.7326/0003-4819-158-4-201302190-00009. [DOI] [PubMed] [Google Scholar]
- 15.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 16.Chene G, Thompson SG. Methods for summarizing the risk associations of quantitative variables in epidemiologic studies in a consistent form. Am J Epidemiol. 1996;144:610–621. doi: 10.1093/oxfordjournals.aje.a008971. [DOI] [PubMed] [Google Scholar]
- 17.Cook MB, Greenwood DC, Hardie LJ, et al. A systematic review and meta-analysis of the risk of increasing adiposity on Barrett’s esophagus. Am J Gastroenterol. 2008;103:292–300. doi: 10.1111/j.1572-0241.2007.01621.x. [DOI] [PubMed] [Google Scholar]
- 18.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson SG, Pocock SJ. Can meta-analyses be trusted? Lancet. 1991;338:1127–1130. doi: 10.1016/0140-6736(91)91975-z. [DOI] [PubMed] [Google Scholar]
- 20.Sterne JA, Egger M, Smith GD. Systematic reviews in health care: investigating and dealing with publication and other biases in meta-analysis. BMJ. 2001;323:101–105. doi: 10.1136/bmj.323.7304.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akima T, Tamano M, Hiraishi H. Liver stiffness measured by transient elastography is a predictor of hepatocellular carcinoma development in viral hepatitis. Hepatol Res. 2011;41:965–970. doi: 10.1111/j.1872-034X.2011.00846.x. [DOI] [PubMed] [Google Scholar]
- 22.Fernandez-Montero JV, Barreiro P, Vispo E, et al. Liver stiffness predicts liver-related complications and mortality in HIV patients with chronic hepatitis C on antiretroviral therapy. AIDS. 2013;27:1129–1134. doi: 10.1097/QAD.0b013e32835e063f. [DOI] [PubMed] [Google Scholar]
- 23.Forestier J, Dumortier J, Guillaud O, et al. Noninvasive diagnosis and prognosis of liver cirrhosis: a comparison of biological scores, elastometry, and metabolic liver function tests. Eur J Gastroenterol Hepatol. 2010;22:532–540. doi: 10.1097/MEG.0b013e3283343f58. [DOI] [PubMed] [Google Scholar]
- 24.Fung J, Lai CL, Seto WK, et al. Prognostic significance of liver stiffness for hepatocellular carcinoma and mortality in HBeAg-negative chronic hepatitis B. J Viral Hepat. 2011;18:738–744. doi: 10.1111/j.1365-2893.2010.01355.x. [DOI] [PubMed] [Google Scholar]
- 25.Chon YE, Jung ES, Park JY, et al. The accuracy of noninvasive methods in predicting the development of hepatocellular carcinoma and hepatic decompensation in patients with chronic hepatitis B. J Clin Gastroenterol. 2012;46:518–525. doi: 10.1097/MCG.0b013e31825079f1. [DOI] [PubMed] [Google Scholar]
- 26.Klibansky DA, Mehta SH, Curry M, et al. Transient elastography for predicting clinical outcomes in patients with chronic liver disease. J Viral Hepat. 2012;19:e184–e193. doi: 10.1111/j.1365-2893.2011.01493.x. [DOI] [PubMed] [Google Scholar]
- 27.Masuzaki R, Tateishi R, Yoshida H, et al. Prospective risk assessment for hepatocellular carcinoma development in patients with chronic hepatitis C by transient elastography. Hepatology. 2009;49:1954–1961. doi: 10.1002/hep.22870. [DOI] [PubMed] [Google Scholar]
- 28.Merchante N, Rivero-Juarez A, Tellez F, et al. Liver stiffness predicts clinical outcome in human immunodeficiency virus/hepatitis C virus-coinfected patients with compensated liver cirrhosis. Hepatology. 2012;56:228–238. doi: 10.1002/hep.25616. [DOI] [PubMed] [Google Scholar]
- 29.Robic MA, Procopet B, Metivier S, et al. Liver stiffness accurately predicts portal hypertension related complications in patients with chronic liver disease: a prospective study. J Hepatol. 2011;55:1017–1024. doi: 10.1016/j.jhep.2011.01.051. [DOI] [PubMed] [Google Scholar]
- 30.Salmon D, Bani-Sadr F, Loko MA, et al. Insulin resistance is associated with a higher risk of hepatocellular carcinoma in cirrhotic HIV/HCV-co-infected patients: results from ANRS CO13 HEPAVIH. J Hepatol. 2012;56:862–868. doi: 10.1016/j.jhep.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 31.Tuma P, Jarrin I, Del Amo J, et al. Survival of HIV-infected patients with compensated liver cirrhosis. AIDS. 2010;24:745–753. doi: 10.1097/QAD.0b013e3283366602. [DOI] [PubMed] [Google Scholar]
- 32.Vergniol J, Foucher J, Terrebonne E, et al. Noninvasive tests for fibrosis and liver stiffness predict 5-year outcomes of patients with chronic hepatitis C. Gastroenterology. 2011;140:1970–1979. doi: 10.1053/j.gastro.2011.02.058. [DOI] [PubMed] [Google Scholar]
- 33.Asrani SK, Kamath PS, Shah VS, et al. Prediction of hepatic decompensation by magnetic resonance elastography among subjects with compensated cirrhosis. Hepatology. 2012;56:914A. [Google Scholar]
- 34.Calvaruso VBF, Simone F, Bavetta MG, et al. Liver stiffness at baseline predicts decompensation and hepatocellular carcinoma in patients with compensated HCV cirrhosis. Hepatology. 2012;56:931A–932A. [Google Scholar]
- 35.Narita YGT, Tsuzura H, Sato S, et al. Liver stiffness as a predictor of hepatocellular carcinoma development in chronic hepatitis C patients receiving interferon-based antiviral therapy. Hepatology. 2012;56:451A. [Google Scholar]
- 36.Vu TMSC, Mehta S, Higgins Y, et al. Baseline liver stiffness measured by transient elastography is independently associated with risk of end-stage liver disease and death among HIV/HCV co-infected adults. J Hepatol. 2011;54:S470. [Google Scholar]
- 37.Kim BK, Kim do Y, Han KH, et al. Risk assessment of esophageal variceal bleeding in B-viral liver cirrhosis by a liver stiffness measurement-based model. Am J Gastroenterol. 2011;106:1654–1662. doi: 10.1038/ajg.2011.160. 1730. [DOI] [PubMed] [Google Scholar]
- 38.Kim SU, Lee JH, Kim do Y, et al. Prediction of liver-related events using fibroscan in chronic hepatitis B patients showing advanced liver fibrosis. PLoS One. 2012;7:e36676. doi: 10.1371/journal.pone.0036676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koch A, Horn A, Duckers H, et al. Increased liver stiffness denotes hepatic dysfunction and mortality risk in critically ill non-cirrhotic patients at a medical ICU. Crit Care. 2011;15:R266. doi: 10.1186/cc10543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lindvig K, Mossner BK, Pedersen C, et al. Liver stiffness and 30-day mortality in a cohort of patients admitted to hospital. Eur J Clin Invest. 2012;42:146–152. doi: 10.1111/j.1365-2362.2011.02571.x. [DOI] [PubMed] [Google Scholar]
- 41.D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217–231. doi: 10.1016/j.jhep.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 42.Vergniol JBJ, Coutzac C, Bertrais S, et al. The evolution of liver stifff-ness predicts 4-year survival of patients with chronic hepatitis C. Hepatology. 2012;56:682A. [Google Scholar]
- 43.Altman DG. Systematic reviews of evaluations of prognostic variables. BMJ. 2001;323:224–228. doi: 10.1136/bmj.323.7306.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.