Abstract
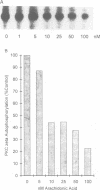
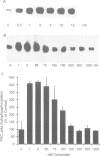
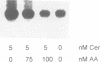
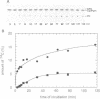
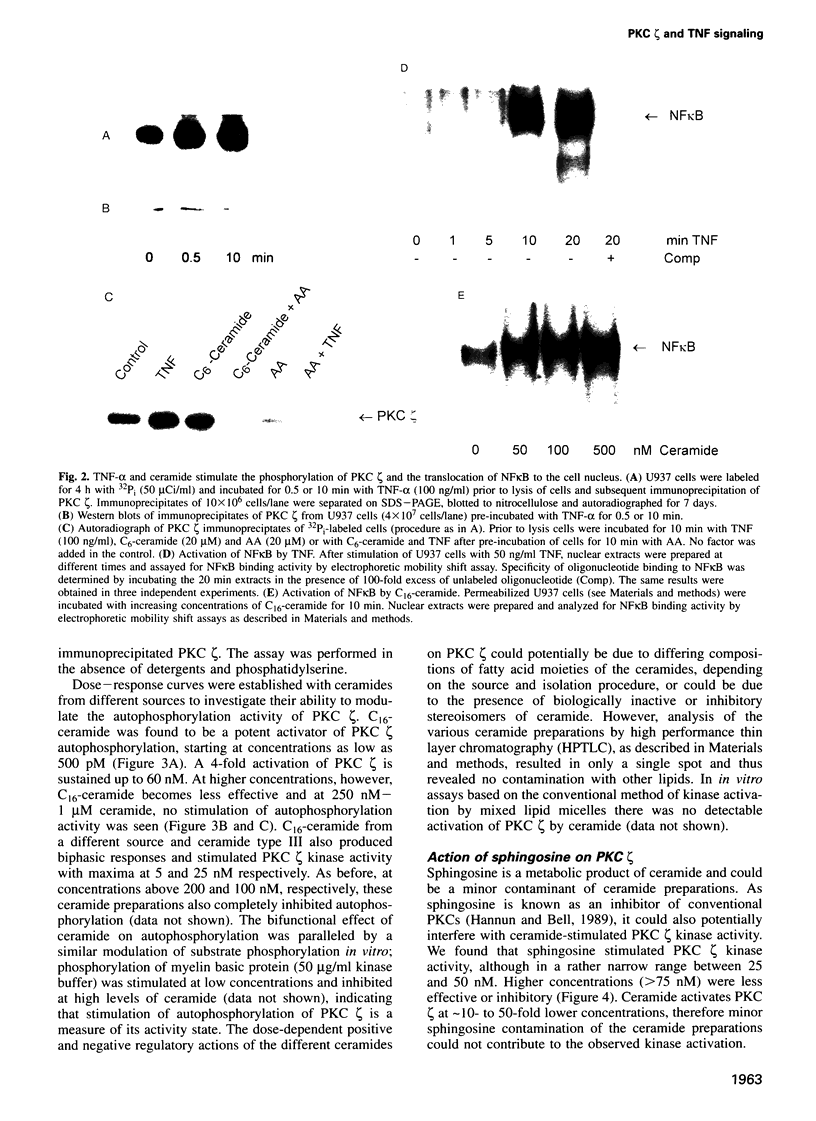
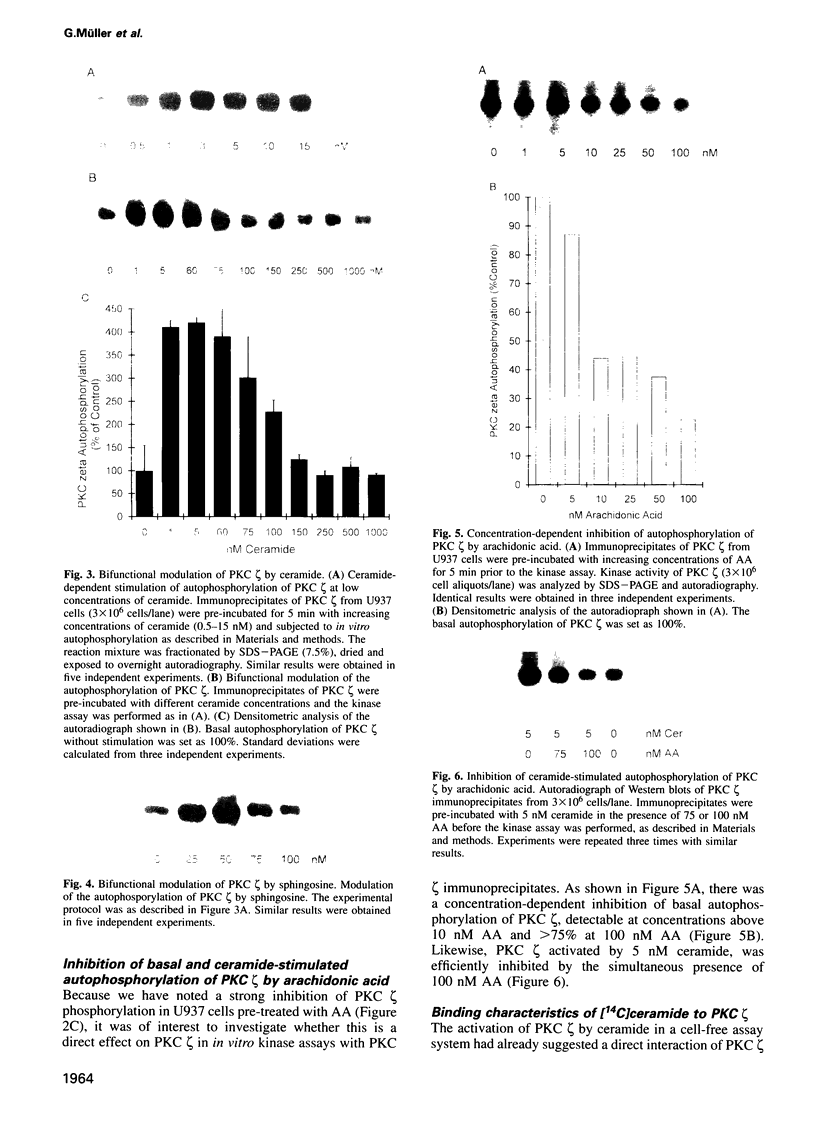
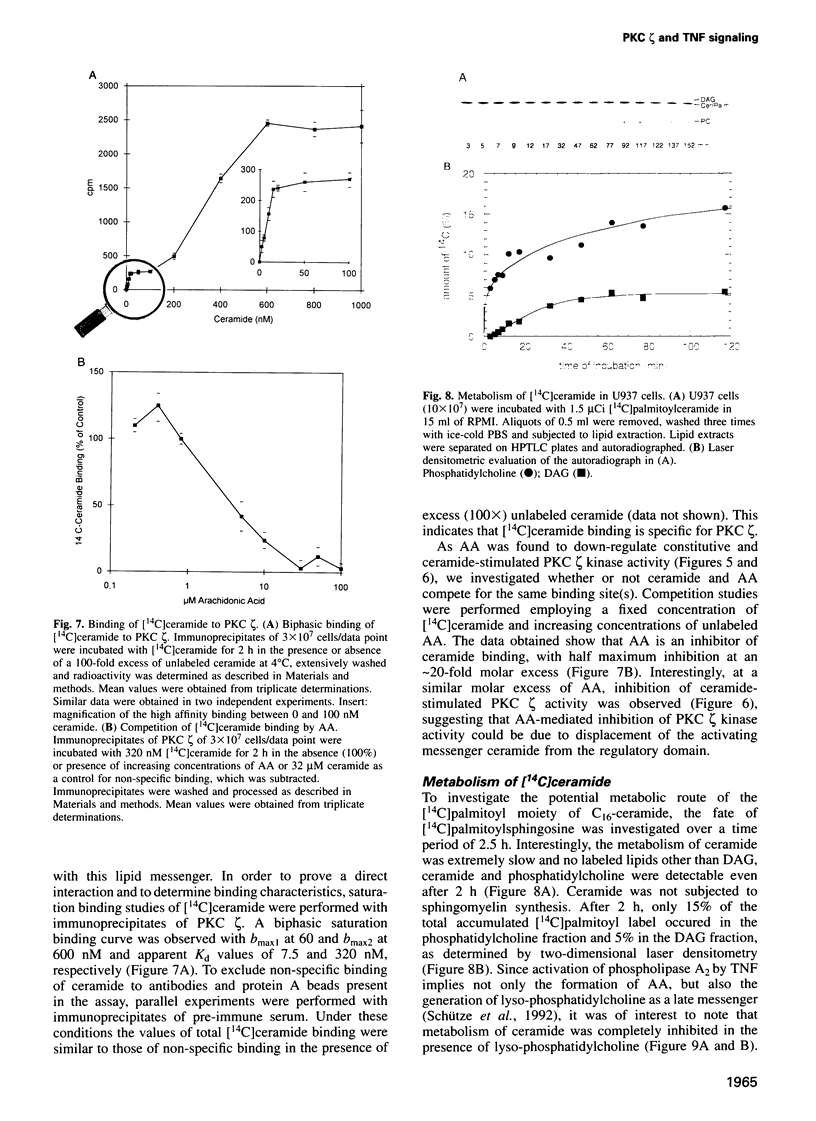
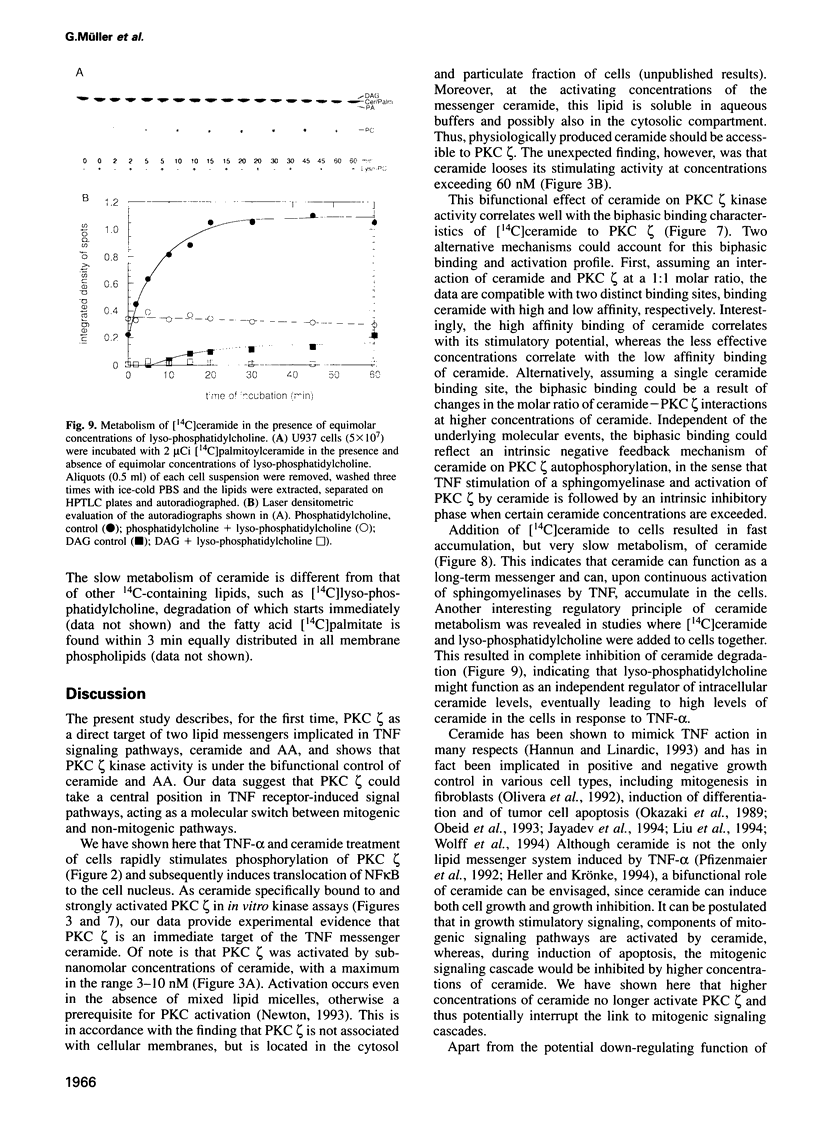
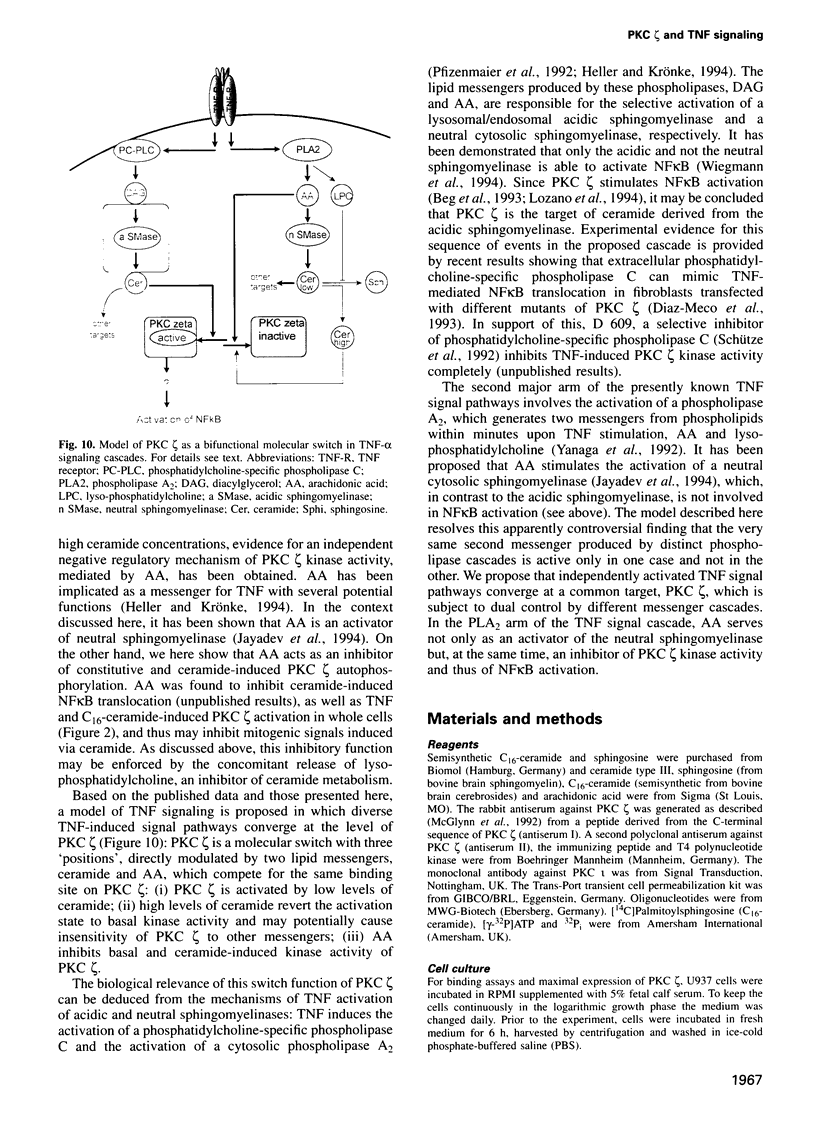
Tumor necrosis factor (TNF-alpha) stimulates a number of signal transduction pathways in which phospholipases produce lipid second messengers. However, the immediate molecular _targets of these messengers, in particular those of ceramide and arachidonic acid (AA) and their role in TNF signaling are not well defined. In this study we investigated the relationship of ceramide and AA in regulating an atypical PKC isozyme, PKC zeta. U937 cells responding to TNF-alpha treatment with NF kappa B activation displayed enhanced phosphorylation of PKC zeta, which is already detectable 30 s after stimulation. [14C]ceramide specifically binds to and regulates kinase activity of PKC zeta in a biphasic manner. Binding studies indicate high and low affinity binding with bmax values of 60 and 600 nM and Kd values of 7.5 and 320 nM respectively. At ceramide concentrations as low as 0.5 nM an up to 4-fold increase in autophosphorylation is obtained, which, at concentrations > 60 nM, again declines to basal levels. Interestingly, AA competes for ceramide binding and inhibits basal and ceramide-stimulated PKC zeta kinase activity at < 100 nM. Metabolism of [14C]ceramide in cells is slow and is inhibited in the presence of equimolar concentrations of lyso-phosphatidylcholine. Based on the bifunctional modulation of PKC zeta by the lipid messengers ceramide and AA, a model of TNF signal pathways is suggested in which PKC zeta takes a central position, acting as a molecular switch between mitogenic and growth inhibitory signals of TNF-alpha.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed S., Kozma R., Lee J., Monfries C., Harden N., Lim L. The cysteine-rich domain of human proteins, neuronal chimaerin, protein kinase C and diacylglycerol kinase binds zinc. Evidence for the involvement of a zinc-dependent structure in phorbol ester binding. Biochem J. 1991 Nov 15;280(Pt 1):233–241. doi: 10.1042/bj2800233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Beg A. A., Finco T. S., Nantermet P. V., Baldwin A. S., Jr Tumor necrosis factor and interleukin-1 lead to phosphorylation and loss of I kappa B alpha: a mechanism for NF-kappa B activation. Mol Cell Biol. 1993 Jun;13(6):3301–3310. doi: 10.1128/mcb.13.6.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell R. M., Burns D. J. Lipid activation of protein kinase C. J Biol Chem. 1991 Mar 15;266(8):4661–4664. [PubMed] [Google Scholar]
- Berra E., Diaz-Meco M. T., Dominguez I., Municio M. M., Sanz L., Lozano J., Chapkin R. S., Moscat J. Protein kinase C zeta isoform is critical for mitogenic signal transduction. Cell. 1993 Aug 13;74(3):555–563. doi: 10.1016/0092-8674(93)80056-k. [DOI] [PubMed] [Google Scholar]
- Diaz-Meco M. T., Berra E., Municio M. M., Sanz L., Lozano J., Dominguez I., Diaz-Golpe V., Lain de Lera M. T., Alcamí J., Payá C. V. A dominant negative protein kinase C zeta subspecies blocks NF-kappa B activation. Mol Cell Biol. 1993 Aug;13(8):4770–4775. doi: 10.1128/mcb.13.8.4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Meco M. T., Dominguez I., Sanz L., Dent P., Lozano J., Municio M. M., Berra E., Hay R. T., Sturgill T. W., Moscat J. zeta PKC induces phosphorylation and inactivation of I kappa B-alpha in vitro. EMBO J. 1994 Jun 15;13(12):2842–2848. doi: 10.1002/j.1460-2075.1994.tb06578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiers W. Tumor necrosis factor. Characterization at the molecular, cellular and in vivo level. FEBS Lett. 1991 Jul 22;285(2):199–212. doi: 10.1016/0014-5793(91)80803-b. [DOI] [PubMed] [Google Scholar]
- Hannun Y. A., Bell R. M. Functions of sphingolipids and sphingolipid breakdown products in cellular regulation. Science. 1989 Jan 27;243(4890):500–507. doi: 10.1126/science.2643164. [DOI] [PubMed] [Google Scholar]
- Hannun Y. A., Linardic C. M. Sphingolipid breakdown products: anti-proliferative and tumor-suppressor lipids. Biochim Biophys Acta. 1993 Dec 21;1154(3-4):223–236. doi: 10.1016/0304-4157(93)90001-5. [DOI] [PubMed] [Google Scholar]
- Hannun Y. A. The sphingomyelin cycle and the second messenger function of ceramide. J Biol Chem. 1994 Feb 4;269(5):3125–3128. [PubMed] [Google Scholar]
- Heller R. A., Krönke M. Tumor necrosis factor receptor-mediated signaling pathways. J Cell Biol. 1994 Jul;126(1):5–9. doi: 10.1083/jcb.126.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis W. D., Kolesnick R. N., Fornari F. A., Traylor R. S., Gewirtz D. A., Grant S. Induction of apoptotic DNA damage and cell death by activation of the sphingomyelin pathway. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):73–77. doi: 10.1073/pnas.91.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayadev S., Linardic C. M., Hannun Y. A. Identification of arachidonic acid as a mediator of sphingomyelin hydrolysis in response to tumor necrosis factor alpha. J Biol Chem. 1994 Feb 25;269(8):5757–5763. [PubMed] [Google Scholar]
- Kochs G., Hummel R., Meyer D., Hug H., Marmé D., Sarre T. F. Activation and substrate specificity of the human protein kinase C alpha and zeta isoenzymes. Eur J Biochem. 1993 Sep 1;216(2):597–606. doi: 10.1111/j.1432-1033.1993.tb18179.x. [DOI] [PubMed] [Google Scholar]
- Kolesnick R. N. Sphingomyelin and derivatives as cellular signals. Prog Lipid Res. 1991;30(1):1–38. doi: 10.1016/0163-7827(91)90005-p. [DOI] [PubMed] [Google Scholar]
- Liu J., Mathias S., Yang Z., Kolesnick R. N. Renaturation and tumor necrosis factor-alpha stimulation of a 97-kDa ceramide-activated protein kinase. J Biol Chem. 1994 Jan 28;269(4):3047–3052. [PubMed] [Google Scholar]
- Lozano J., Berra E., Municio M. M., Diaz-Meco M. T., Dominguez I., Sanz L., Moscat J. Protein kinase C zeta isoform is critical for kappa B-dependent promoter activation by sphingomyelinase. J Biol Chem. 1994 Jul 29;269(30):19200–19202. [PubMed] [Google Scholar]
- Mayer R. J., Marshall L. A. New insights on mammalian phospholipase A2(s); comparison of arachidonoyl-selective and -nonselective enzymes. FASEB J. 1993 Feb 1;7(2):339–348. doi: 10.1096/fasebj.7.2.8440410. [DOI] [PubMed] [Google Scholar]
- McGlynn E., Liebetanz J., Reutener S., Wood J., Lydon N. B., Hofstetter H., Vanek M., Meyer T., Fabbro D. Expression and partial characterization of rat protein kinase C-delta and protein kinase C-zeta in insect cells using recombinant baculovirus. J Cell Biochem. 1992 Jul;49(3):239–250. doi: 10.1002/jcb.240490306. [DOI] [PubMed] [Google Scholar]
- Nakanishi H., Brewer K. A., Exton J. H. Activation of the zeta isozyme of protein kinase C by phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1993 Jan 5;268(1):13–16. [PubMed] [Google Scholar]
- Newton A. C. Interaction of proteins with lipid headgroups: lessons from protein kinase C. Annu Rev Biophys Biomol Struct. 1993;22:1–25. doi: 10.1146/annurev.bb.22.060193.000245. [DOI] [PubMed] [Google Scholar]
- Newton A. C., Koshland D. E., Jr Protein kinase C autophosphorylates by an intrapeptide reaction. J Biol Chem. 1987 Jul 25;262(21):10185–10188. [PubMed] [Google Scholar]
- Obeid L. M., Linardic C. M., Karolak L. A., Hannun Y. A. Programmed cell death induced by ceramide. Science. 1993 Mar 19;259(5102):1769–1771. doi: 10.1126/science.8456305. [DOI] [PubMed] [Google Scholar]
- Okazaki T., Bell R. M., Hannun Y. A. Sphingomyelin turnover induced by vitamin D3 in HL-60 cells. Role in cell differentiation. J Biol Chem. 1989 Nov 15;264(32):19076–19080. [PubMed] [Google Scholar]
- Olivera A., Buckley N. E., Spiegel S. Sphingomyelinase and cell-permeable ceramide analogs stimulate cellular proliferation in quiescent Swiss 3T3 fibroblasts. J Biol Chem. 1992 Dec 25;267(36):26121–26127. [PubMed] [Google Scholar]
- Ono Y., Fujii T., Igarashi K., Kuno T., Tanaka C., Kikkawa U., Nishizuka Y. Phorbol ester binding to protein kinase C requires a cysteine-rich zinc-finger-like sequence. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4868–4871. doi: 10.1073/pnas.86.13.4868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono Y., Fujii T., Ogita K., Kikkawa U., Igarashi K., Nishizuka Y. Protein kinase C zeta subspecies from rat brain: its structure, expression, and properties. Proc Natl Acad Sci U S A. 1989 May;86(9):3099–3103. doi: 10.1073/pnas.86.9.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schütze S., Nottrott S., Pfizenmaier K., Krönke M. Tumor necrosis factor signal transduction. Cell-type-specific activation and translocation of protein kinase C. J Immunol. 1990 Apr 1;144(7):2604–2608. [PubMed] [Google Scholar]
- Schütze S., Potthoff K., Machleidt T., Berkovic D., Wiegmann K., Krönke M. TNF activates NF-kappa B by phosphatidylcholine-specific phospholipase C-induced "acidic" sphingomyelin breakdown. Cell. 1992 Nov 27;71(5):765–776. doi: 10.1016/0092-8674(92)90553-o. [DOI] [PubMed] [Google Scholar]
- Selbie L. A., Schmitz-Peiffer C., Sheng Y., Biden T. J. Molecular cloning and characterization of PKC iota, an atypical isoform of protein kinase C derived from insulin-secreting cells. J Biol Chem. 1993 Nov 15;268(32):24296–24302. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi A., Kubo M., Fujii H., Freire-Moar J., Turck C. W., Ransom J. T. Regulation of protein kinase C isoform proteins in phorbol ester-stimulated Jurkat T lymphoma cells. J Immunol. 1993 Mar 1;150(5):1746–1754. [PubMed] [Google Scholar]
- Ways D. K., Cook P. P., Webster C., Parker P. J. Effect of phorbol esters on protein kinase C-zeta. J Biol Chem. 1992 Mar 5;267(7):4799–4805. [PubMed] [Google Scholar]
- Wiegmann K., Schütze S., Machleidt T., Witte D., Krönke M. Functional dichotomy of neutral and acidic sphingomyelinases in tumor necrosis factor signaling. Cell. 1994 Sep 23;78(6):1005–1015. doi: 10.1016/0092-8674(94)90275-5. [DOI] [PubMed] [Google Scholar]
- Wolff R. A., Dobrowsky R. T., Bielawska A., Obeid L. M., Hannun Y. A. Role of ceramide-activated protein phosphatase in ceramide-mediated signal transduction. J Biol Chem. 1994 Jul 29;269(30):19605–19609. [PubMed] [Google Scholar]
- Yanaga F., Abe M., Koga T., Hirata M. Signal transduction by tumor necrosis factor alpha is mediated through a guanine nucleotide-binding protein in osteoblast-like cell line, MC3T3-E1. J Biol Chem. 1992 Mar 15;267(8):5114–5121. [PubMed] [Google Scholar]