Abstract
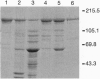
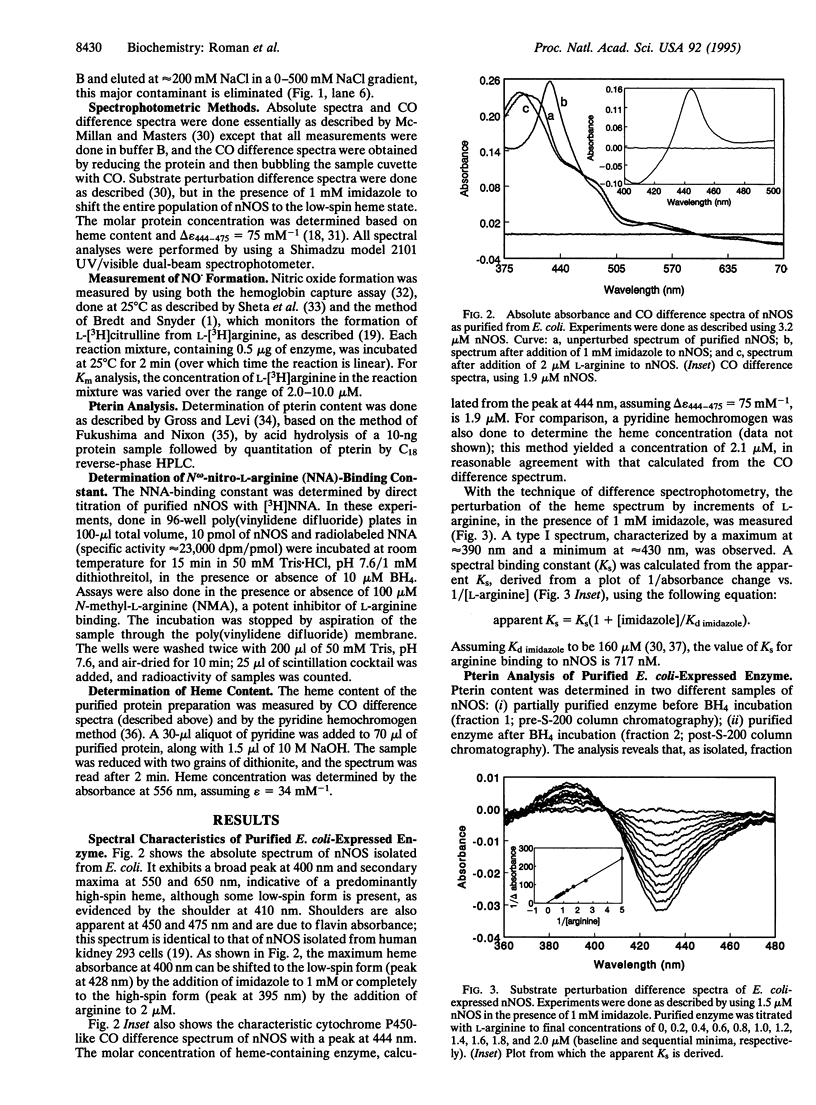
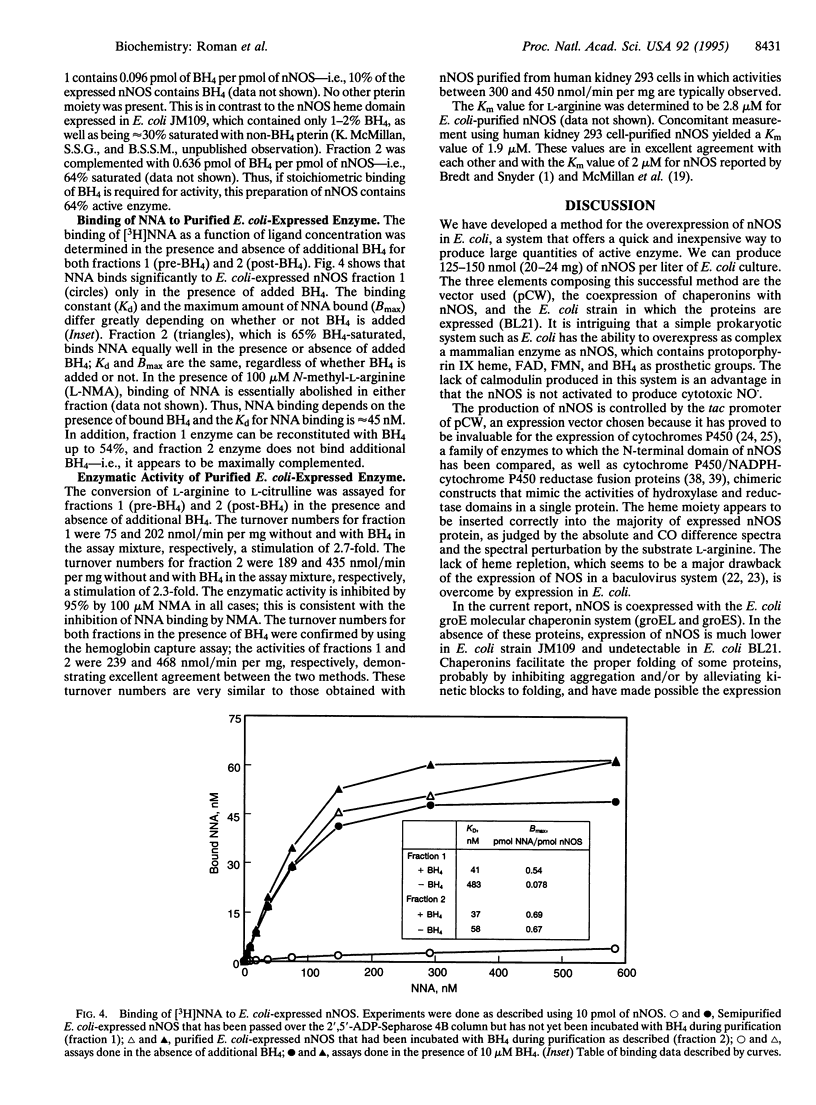
The neuronal nitric oxide synthase (nNOS) has been successfully overexpressed in Escherichia coli, with average yields of 125-150 nmol (20-24 mg) of enzyme per liter of cells. The cDNA for nNOS was subcloned into the pCW vector under the control of the tac promotor and was coexpressed with the chaperonins groEL and groES in the protease-deficient BL21 strain of E. coli. The enzyme produced is replete with heme and flavins and, after overnight incubation with tetrahydrobiopterin, contains 0.7 pmol of tetrahydrobiopterin per pmol of nNOS. nNOS is isolated as a predominantly high-spin heme protein and demonstrates spectral properties that are identical to those of nNOS isolated from stably transfected human kidney 293 cells. It binds N omega-nitroarginine dependent on the presence of bound tetrahydrobiopterin and exhibits a Kd of 45 nM. The enzyme is completely functional; the specific activity is 450 nmol/min per mg. This overexpression system will be extremely useful for rapid, inexpensive preparation of large amounts of active nNOS for use in mechanistic and structure/function studies, as well as for drug design and development.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes H. J., Arlotto M. P., Waterman M. R. Expression and enzymatic activity of recombinant cytochrome P450 17 alpha-hydroxylase in Escherichia coli. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5597–5601. doi: 10.1073/pnas.88.13.5597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredt D. S., Hwang P. M., Glatt C. E., Lowenstein C., Reed R. R., Snyder S. H. Cloned and expressed nitric oxide synthase structurally resembles cytochrome P-450 reductase. Nature. 1991 Jun 27;351(6329):714–718. doi: 10.1038/351714a0. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci U S A. 1990 Jan;87(2):682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Nitric oxide: a physiologic messenger molecule. Annu Rev Biochem. 1994;63:175–195. doi: 10.1146/annurev.bi.63.070194.001135. [DOI] [PubMed] [Google Scholar]
- Charles I. G., Chubb A., Gill R., Clare J., Lowe P. N., Holmes L. S., Page M., Keeling J. G., Moncada S., Riveros-Moreno V. Cloning and expression of a rat neuronal nitric oxide synthase coding sequence in a baculovirus/insect cell system. Biochem Biophys Res Commun. 1993 Nov 15;196(3):1481–1489. doi: 10.1006/bbrc.1993.2419. [DOI] [PubMed] [Google Scholar]
- Cho H. J., Xie Q. W., Calaycay J., Mumford R. A., Swiderek K. M., Lee T. D., Nathan C. Calmodulin is a subunit of nitric oxide synthase from macrophages. J Exp Med. 1992 Aug 1;176(2):599–604. doi: 10.1084/jem.176.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinerman J. L., Lowenstein C. J., Snyder S. H. Molecular mechanisms of nitric oxide regulation. Potential relevance to cardiovascular disease. Circ Res. 1993 Aug;73(2):217–222. doi: 10.1161/01.res.73.2.217. [DOI] [PubMed] [Google Scholar]
- Faeder E. J., Siegel L. M. A rapid micromethod for determination of FMN and FAD in mixtures. Anal Biochem. 1973 May;53(1):332–336. doi: 10.1016/0003-2697(73)90442-9. [DOI] [PubMed] [Google Scholar]
- Fisher C. W., Shet M. S., Caudle D. L., Martin-Wixtrom C. A., Estabrook R. W. High-level expression in Escherichia coli of enzymatically active fusion proteins containing the domains of mammalian cytochromes P450 and NADPH-P450 reductase flavoprotein. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10817–10821. doi: 10.1073/pnas.89.22.10817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima T., Nixon J. C. Analysis of reduced forms of biopterin in biological tissues and fluids. Anal Biochem. 1980 Feb;102(1):176–188. doi: 10.1016/0003-2697(80)90336-x. [DOI] [PubMed] [Google Scholar]
- Förstermann U., Pollock J. S., Schmidt H. H., Heller M., Murad F. Calmodulin-dependent endothelium-derived relaxing factor/nitric oxide synthase activity is present in the particulate and cytosolic fractions of bovine aortic endothelial cells. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1788–1792. doi: 10.1073/pnas.88.5.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegner J. A., Dahlquist F. W. Signal transduction in bacteria: CheW forms a reversible complex with the protein kinase CheA. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):750–754. doi: 10.1073/pnas.88.3.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goloubinoff P., Gatenby A. A., Lorimer G. H. GroE heat-shock proteins promote assembly of foreign prokaryotic ribulose bisphosphate carboxylase oligomers in Escherichia coli. Nature. 1989 Jan 5;337(6202):44–47. doi: 10.1038/337044a0. [DOI] [PubMed] [Google Scholar]
- Gross S. S., Levi R. Tetrahydrobiopterin synthesis. An absolute requirement for cytokine-induced nitric oxide generation by vascular smooth muscle. J Biol Chem. 1992 Dec 25;267(36):25722–25729. [PubMed] [Google Scholar]
- Hevel J. M., White K. A., Marletta M. A. Purification of the inducible murine macrophage nitric oxide synthase. Identification as a flavoprotein. J Biol Chem. 1991 Dec 5;266(34):22789–22791. [PubMed] [Google Scholar]
- Kelm M., Schrader J. Control of coronary vascular tone by nitric oxide. Circ Res. 1990 Jun;66(6):1561–1575. doi: 10.1161/01.res.66.6.1561. [DOI] [PubMed] [Google Scholar]
- Klatt P., Schmidt K., Brunner F., Mayer B. Inhibitors of brain nitric oxide synthase. Binding kinetics, metabolism, and enzyme inactivation. J Biol Chem. 1994 Jan 21;269(3):1674–1680. [PubMed] [Google Scholar]
- Klatt P., Schmidt K., Mayer B. Brain nitric oxide synthase is a haemoprotein. Biochem J. 1992 Nov 15;288(Pt 1):15–17. doi: 10.1042/bj2880015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamas S., Marsden P. A., Li G. K., Tempst P., Michel T. Endothelial nitric oxide synthase: molecular cloning and characterization of a distinct constitutive enzyme isoform. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6348–6352. doi: 10.1073/pnas.89.14.6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenstein C. J., Glatt C. S., Bredt D. S., Snyder S. H. Cloned and expressed macrophage nitric oxide synthase contrasts with the brain enzyme. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6711–6715. doi: 10.1073/pnas.89.15.6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J., Langer T., Boteva R., Schramel A., Horwich A. L., Hartl F. U. Chaperonin-mediated protein folding at the surface of groEL through a 'molten globule'-like intermediate. Nature. 1991 Jul 4;352(6330):36–42. doi: 10.1038/352036a0. [DOI] [PubMed] [Google Scholar]
- Masters B. S. Nitric oxide synthases: why so complex? Annu Rev Nutr. 1994;14:131–145. doi: 10.1146/annurev.nu.14.070194.001023. [DOI] [PubMed] [Google Scholar]
- Mayer B., John M., Heinzel B., Werner E. R., Wachter H., Schultz G., Böhme E. Brain nitric oxide synthase is a biopterin- and flavin-containing multi-functional oxido-reductase. FEBS Lett. 1991 Aug 19;288(1-2):187–191. doi: 10.1016/0014-5793(91)81031-3. [DOI] [PubMed] [Google Scholar]
- McMillan K., Bredt D. S., Hirsch D. J., Snyder S. H., Clark J. E., Masters B. S. Cloned, expressed rat cerebellar nitric oxide synthase contains stoichiometric amounts of heme, which binds carbon monoxide. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11141–11145. doi: 10.1073/pnas.89.23.11141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan K., Masters B. S. Optical difference spectrophotometry as a probe of rat brain nitric oxide synthase heme-substrate interaction. Biochemistry. 1993 Sep 28;32(38):9875–9880. doi: 10.1021/bi00089a001. [DOI] [PubMed] [Google Scholar]
- McMillan K., Masters B. S. Prokaryotic expression of the heme- and flavin-binding domains of rat neuronal nitric oxide synthase as distinct polypeptides: identification of the heme-binding proximal thiolate ligand as cysteine-415. Biochemistry. 1995 Mar 21;34(11):3686–3693. doi: 10.1021/bi00011a025. [DOI] [PubMed] [Google Scholar]
- Narhi L. O., Fulco A. J. Characterization of a catalytically self-sufficient 119,000-dalton cytochrome P-450 monooxygenase induced by barbiturates in Bacillus megaterium. J Biol Chem. 1986 Jun 5;261(16):7160–7169. [PubMed] [Google Scholar]
- Nishimoto M., Clark J. E., Masters B. S. Cytochrome P450 4A4: expression in Escherichia coli, purification, and characterization of catalytic properties. Biochemistry. 1993 Aug 31;32(34):8863–8870. doi: 10.1021/bi00085a018. [DOI] [PubMed] [Google Scholar]
- Pollock J. S., Förstermann U., Mitchell J. A., Warner T. D., Schmidt H. H., Nakane M., Murad F. Purification and characterization of particulate endothelium-derived relaxing factor synthase from cultured and native bovine aortic endothelial cells. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10480–10484. doi: 10.1073/pnas.88.23.10480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards M. K., Marletta M. A. Characterization of neuronal nitric oxide synthase and a C415H mutant, purified from a baculovirus overexpression system. Biochemistry. 1994 Dec 13;33(49):14723–14732. doi: 10.1021/bi00253a010. [DOI] [PubMed] [Google Scholar]
- Schmidt H. H., Pollock J. S., Nakane M., Gorsky L. D., Förstermann U., Murad F. Purification of a soluble isoform of guanylyl cyclase-activating-factor synthase. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):365–369. doi: 10.1073/pnas.88.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessa W. C., Harrison J. K., Barber C. M., Zeng D., Durieux M. E., D'Angelo D. D., Lynch K. R., Peach M. J. Molecular cloning and expression of a cDNA encoding endothelial cell nitric oxide synthase. J Biol Chem. 1992 Aug 5;267(22):15274–15276. [PubMed] [Google Scholar]
- Shet M. S., Fisher C. W., Arlotto M. P., Shackleton C. H., Holmans P. L., Martin-Wixtrom C. A., Saeki Y., Estabrook R. W. Purification and enzymatic properties of a recombinant fusion protein expressed in Escherichia coli containing the domains of bovine P450 17A and rat NADPH-P450 reductase. Arch Biochem Biophys. 1994 Jun;311(2):402–417. doi: 10.1006/abbi.1994.1255. [DOI] [PubMed] [Google Scholar]
- Sheta E. A., McMillan K., Masters B. S. Evidence for a bidomain structure of constitutive cerebellar nitric oxide synthase. J Biol Chem. 1994 May 27;269(21):15147–15153. [PubMed] [Google Scholar]
- Stuehr D. J., Cho H. J., Kwon N. S., Weise M. F., Nathan C. F. Purification and characterization of the cytokine-induced macrophage nitric oxide synthase: an FAD- and FMN-containing flavoprotein. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7773–7777. doi: 10.1073/pnas.88.17.7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuehr D. J., Ikeda-Saito M. Spectral characterization of brain and macrophage nitric oxide synthases. Cytochrome P-450-like hemeproteins that contain a flavin semiquinone radical. J Biol Chem. 1992 Oct 15;267(29):20547–20550. [PubMed] [Google Scholar]
- White K. A., Marletta M. A. Nitric oxide synthase is a cytochrome P-450 type hemoprotein. Biochemistry. 1992 Jul 28;31(29):6627–6631. doi: 10.1021/bi00144a001. [DOI] [PubMed] [Google Scholar]
- Wolff D. J., Datto G. A., Samatovicz R. A., Tempsick R. A. Calmodulin-dependent nitric-oxide synthase. Mechanism of inhibition by imidazole and phenylimidazoles. J Biol Chem. 1993 May 5;268(13):9425–9429. [PubMed] [Google Scholar]
- Wynn R. M., Davie J. R., Zhi W., Cox R. P., Chuang D. T. In vitro reconstitution of the 24-meric E2 inner core of bovine mitochondrial branched-chain alpha-keto acid dehydrogenase complex: requirement for chaperonins GroEL and GroES. Biochemistry. 1994 Aug 2;33(30):8962–8968. doi: 10.1021/bi00196a014. [DOI] [PubMed] [Google Scholar]
- Xie Q. W., Cho H. J., Calaycay J., Mumford R. A., Swiderek K. M., Lee T. D., Ding A., Troso T., Nathan C. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science. 1992 Apr 10;256(5054):225–228. doi: 10.1126/science.1373522. [DOI] [PubMed] [Google Scholar]