Abstract
Background
Bacterial infections are a major cause of morbidity and mortality in patients who are neutropenic following chemotherapy for malignancy. Trials have shown the efficacy of antibiotic prophylaxis in reducing the incidence of bacterial infections but not in reducing mortality rates. Our systematic review from 2006 also showed a reduction in mortality.
Objectives
This updated review aimed to evaluate whether there is still a benefit of reduction in mortality when compared to placebo or no intervention.
Search methods
We searched the Cochrane Cancer Network Register of Trials (2011), Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library Issue 2, 2011), MEDLINE (1966 to March 2011), EMBASE (1980 to March 2011), abstracts of conference proceedings and the references of identified studies.
Selection criteria
Randomised controlled trials (RCTs) or quasi‐RCTs comparing different types of antibiotic prophylaxis with placebo or no intervention, or another antibiotic, to prevent bacterial infections in afebrile neutropenic patients.
Data collection and analysis
Two authors independently appraised the quality of each trial and extracted data from the included trials. Analyses were performed using RevMan 5.1 software.
Main results
One‐hundred and nine trials (involving 13,579 patients) that were conducted between the years 1973 to 2010 met the inclusion criteria. When compared with placebo or no intervention, antibiotic prophylaxis significantly reduced the risk of death from all causes (46 trials, 5635 participants; risk ratio (RR) 0.66, 95% CI 0.55 to 0.79) and the risk of infection‐related death (43 trials, 5777 participants; RR 0.61, 95% CI 0.48 to 0.77). The estimated number needed to treat (NNT) to prevent one death was 34 (all‐cause mortality) and 48 (infection‐related mortality).
Prophylaxis also significantly reduced the occurrence of fever (54 trials, 6658 participants; RR 0.80, 95% CI 0.74 to 0.87), clinically documented infection (48 trials, 5758 participants; RR 0.65, 95% CI 0.56 to 0.76), microbiologically documented infection (53 trials, 6383 participants; RR 0.51, 95% CI 0.42 to 0.62) and other indicators of infection.
There were no significant differences between quinolone prophylaxis and TMP‐SMZ prophylaxis with regard to death from all causes or infection, however, quinolone prophylaxis was associated with fewer side effects leading to discontinuation (seven trials, 850 participants; RR 0.37, 95% CI 0.16 to 0.87) and less resistance to the drugs thereafter (six trials, 366 participants; RR 0.45, 95% CI 0.27 to 0.74).
Authors' conclusions
Antibiotic prophylaxis in afebrile neutropenic patients significantly reduced all‐cause mortality. In our review, the most significant reduction in mortality was observed in trials assessing prophylaxis with quinolones. The benefits of antibiotic prophylaxis outweighed the harm such as adverse effects and the development of resistance since all‐cause mortality was reduced. As most trials in our review were of patients with haematologic cancer, we strongly recommend antibiotic prophylaxis for these patients, preferably with a quinolone. Prophylaxis may also be considered for patients with solid tumours or lymphoma.
Plain language summary
Antibiotics to prevent bacterial infections due to chemotherapy in cancer patients with a low white blood cell count and no fever
For patients receiving chemotherapy, there is an increased risk of infection due to a low white blood cell count (neutropenia) caused by a toxic effect of chemotherapy on the bone marrow. The objective of this review was to establish whether preventive antibiotic therapy (prophylaxis) before the development of fever prevents illness and death in people with a low white blood cell count after chemotherapy and to assess whether certain types of antibiotics are better than others. We included 109 randomised controlled trials conducted between the years 1973 to 2010.
Antibiotic prophylaxis significantly decreased the risk of death when compared to no intervention. We estimated that the number of patients needed to be treated with antibiotics in order to prevent one death from all causes was 34. Antibiotic prophylaxis also decreased the risk of death from infection and the risk of development of fever. Although antibiotic prophylaxis may be associated with unfavourable effects and may encourage new and more resistant infection, this was not shown in existing trials. Recent studies used antibiotics of the quinolone class, which showed fewer adverse events and better outcomes than other classes of antibiotics.
Most studies were limited to haematological cancer patients (mostly leukaemia).
In conclusion, patients with a low white blood count following chemotherapy who received preventive antibiotic treatment in the absence of fever had a reduced risk of dying. This was shown mainly for haematological cancer patients. Antibiotic prophylaxis, preferably from the quinolone class of antibiotics, should be recommended for routine use in these patients.
Summary of findings
Background
Description of the condition
Patients with cancer are subject to infections as a result of several factors, notably breakdown of normal skin and mucosal barriers, obstruction related to the tumour, alteration of host defences secondary to infiltration of bone marrow, reduced or altered immunoglobulin or cytokine production, or neutropenia related to chemotherapy. Neutropenia, a deficiency in white blood cells, is the most frequently encountered host cell defect in patients with cancer and predicts the development of bacteraemia caused by Gram‐positive and Gram‐negative bacteria. In the absence of preventive measures, between 48% and 60% of neutropenic patients who become febrile have an established or occult infection, and around 16% to 20% or more of patients with profound neutropenia (neutrophil counts of less than 100/mm³) have bacteraemia (Bodey 1966; Lucas 1996; Schimpff 1986). During the past two decades there have been changes in the organisms that cause infection. In the 1990s the incidence of Gram‐negative infections declined and Gram‐positive organisms accounted for 60% to 70% of microbiologically documented infections (EORTC 1990; Hughes 2002). Currently, coagulase‐negative staphylococci are the most common blood isolates in most centres, however Gram‐negative pathogens are on the rise in some centres (Freifeld 2011).
Description of the intervention
A number of prophylactic strategies have been used in order to reduce the risk of infection during severe neutropenia. Different measures that have been investigated include isolation of the patient, granulocyte transfusions in patients with severe infections (Massey 2009; van de Wetering 2007), active or passive immunisation, and acceleration of granulocyte recovery by administration of granulocyte stimulating growth factors (GSCF) (Frank 2008; Kuderer 2007). However, these are still not enough to reduce infections. Numerous studies since the 1980s, evaluating prophylactic use of antibacterial agents, have shown that the frequency of febrile episodes can be reduced by administering antibiotics during the early afebrile period (Hughes 1990; Kerr 1999). Several prophylactic regimens have been studied in patients with malignancies. Selective intestinal decontamination has been suggested as a method of preventing bacterial infections in these patients. This consists of inhibition of the Gram‐negative flora of the gut with preservation of the remaining flora, especially anaerobic bacteria, which is important in maintaining resistance of the gut against intestinal colonisation and overgrowth and extra‐intestinal spread of pathogenic bacteria (Verhoef 1993).
Oral nonabsorbable antibiotics (such as polymyxin, neomycin, aminoglycoside, vancomycin) and absorbable antibiotics (quinolones, trimethoprim‐sulfamethoxazole (TMP‐SMZ)) as well as intravenous antibiotics (ceftriaxone, vancomycin) have been evaluated. The oral nonabsorbable drugs, which were studied in the early trials, have been abandoned due to poor tolerance and low patient compliance.
Studies of prophylaxis with TMP‐SMZ have shown a reduced infection rate for TMP‐SMZ treated patients when compared with placebo or a different agent (Hughes 1990; Walsh 1994). However, these studies failed to demonstrate a significant difference in mortality. Disadvantages of this regimen include side effects of the sulfamethoxazole component, myelosuppression and prolongation of neutropenia, the emergence of resistant bacteria, fungal overgrowth, Clostridium difficile colitis and inadequate coverage of Pseudomonas aeruginosa.
Quinolones were first introduced in the 1980s and since then they have become an attractive option for prophylaxis in neutropenic cancer patients. This is due to their broad antimicrobial spectrum (increased activity against Gram‐negative bacteria, including P. aeruginosa), preservation of the anaerobic flora of the alimentary tract (selective decontamination), high concentration in the faeces, systemic bactericidal activity, good tolerability and lack of myelosuppression (Del Favero 1993; Patrick 1997a). They were proved in published randomised trials to be more effective than placebo, oral nonabsorbable antibiotics or cotrimoxazole in the prevention of Gram‐negative infections. However, most of these studies were underpowered to detect an advantage in survival. In addition, some investigators did not show a reduction in the number of febrile episodes in patients receiving quinolones (Bow 1996; de Marie 1993). Moreover, not all studies demonstrate superiority of quinolones against comparable regimens (Donnelly 1992a). Whatever the perceived advantages, the problem of inadequate coverage for Gram‐positive bacteria cannot be ignored (Cruciani 1996; Kern 1991). Furthermore, the administration of quinolones has already been associated with the emergence and spread of resistant Staphylococcus‐coagulase negative bacteria (Oppenheim 1989). This has led to the addition of agents with increased anti‐Gram positive activity to the quinolone‐based regimens (penicillin or rifampin) (Kerr 1999). Another potential problem related to the prophylactic use of fluoroquinolones is the reported emergence of quinolone‐resistant Gram‐negative bacilli (Cometta 1994; Kern 1994).
Why it is important to do this review
When we originally started to work on the review, guidelines existed on antibiotic treatment for fever and neutropenia in cancer patients but the use of antibiotics for afebrile neutropenia was highly controversial and lacked consensus (Hughes 2002), with the exception of the use of TMP‐SMZ for all patients at risk of Pneumocystis pneumonia (those with childhood leukaemia, AIDS) regardless of whether they had neutropenia. It was only in cases of profound and prolonged neutropenia that a quinolone plus penicillin or TMP‐SMZ might have been recommended.
Although data supported the efficacy of TMP‐SMZ and quinolones in reducing the number of infectious episodes, such prophylaxis had not been shown to reduce mortality rates. In addition, there were concerns about adverse effects and the emergence of drug‐resistant bacteria. Several meta‐analyses have been conducted to assess the efficacy of quinolones for preventing bacterial infections in neutropenic patients (Cruciani 1996; Cruciani 2003; Engels 1998; Rotstein 1997; van de Wetering 2005). They all concluded that quinolone prophylaxis reduces the various infection‐related outcomes but not mortality.
Our original systematic review demonstrated a significant reduction in mortality with the use of prophylactic antibiotics (Gafter‐Gvili 2005a; Gafter‐Gvili 2005b; Leibovici Cancer 2006). This advantage in reducing mortality was not detected in individual studies due to small sample sizes. By updating the review to include new randomised controlled trials (RCTs), we aimed to assess whether the benefit of prophylaxis in terms of a reduction in mortality was robust and whether the rise in resistance to antibiotics nullifies or reduces the efficiency of prophylaxis.
Objectives
Our primary objective was to evaluate the effect of antibiotic prophylaxis on mortality and infection in neutropenic patients following chemotherapy.
Our secondary objectives were to assess:
whether the effectiveness of different antibiotic regimens are similar;
subgroups of patients and which may benefit most from prophylaxis;
emergence of quinolone‐resistant Gram‐negative bacteria;
adverse effects of the antibiotic regimens.
Methods
Criteria for considering studies for this review
Types of studies
For the 2005 review, RCTs and quasi‐RCTs comparing different types of antibiotic therapy with placebo, no intervention, or with another antibiotic for the prophylaxis of bacterial infections in afebrile neutropenic patients. For the 2011 update, only RCTs identified by the updated search were added. Trials were included irrespective of publication status, language and blinding.
Types of participants
Patients with cancer and neutropenia induced by chemotherapy or following bone marrow transplantation.
Types of interventions
The following medications, used alone or in combination, were considered regardless of the mode of administration (intravenous or oral):
quinolones (e.g. ciprofloxacin, ofloxacin, norfloxacin, pefloxacin) alone or in combination with gram‐positive prophylaxis (penicillin, rifampin, roxythromycin, vancomycin);
trimethoprim‐sulphamethoxazole (TMP‐SMZ);
nonabsorbable oral antibiotics: aminoglycoside (e.g. gentamicin, neomycin, tobramycin), colistin, polymyxin;
rifampin;
intravenous cephalosporins (e.g. ceftriaxone);
intravenous vancomycin;
other antibiotics.
The control groups received any of the above medications, placebo, or no intervention.
Types of outcome measures
Primary outcomes
All‐cause mortality (at 30 day follow‐up or at the end of the follow‐up in each study)
Secondary outcomes
Indicators of infection
Infection‐related mortality
Incidence of febrile patients or febrile episodes
Clinically documented infection, defined as the presence of symptoms or signs of inflammation at an anatomic site whether pathogens were recovered from the affected site or not
Microbiologically documented infection, defined as the presence of symptoms or signs of inflammation at an anatomic site where pathogens were recovered from the affected site
Microbiologically documented infections caused by Gram‐positive bacteria
Microbiologically documented infections caused by Gram‐negative bacteria
Bacteraemia, defined as the recovery of bacteria from one or more blood cultures
Incidence of superinfection or bacteria resistant to the given antibiotic in at least one of the follow‐up cultures
Incidence of hospital admissions and length of hospital stay
Duration of fever
Adverse events
Any serious adverse events that were fatal, life‐threatening, or requiring inpatient hospitalisation or prolongation of existing hospitalisation
Any adverse events that resulted in significant disability or incapacity
Any important medical events that might not have been immediately life‐threatening or result in death or hospitalisation, but might have jeopardised the patient or required intervention to prevent one of the above outcomes. Specifically we attempted to extract data on Clostridium difficile associated diarrhea (CDAD)
Any adverse events that required discontinuation of medication
Search methods for identification of studies
Electronic searches
For the original review, searches were conducted spanning from 1966 to 2005, see Appendix 1. The updated search was performed in March 2011 (from November 2005 to March 2011) and included the following databases: Cochrane Cancer Network Register of Trials, Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library Issue 2, 2011), MEDLINE, EMBASE, and the following conference proceedings (2005 to 2010): Abstracts of the Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC), Annual Meetings of the Infectious Diseases Society of America (IDSA) and European Congress of Clinical Microbiology and Infectious Diseases (ECCMID). For the present update, the following conference proceedings were also included: the American Society of Hematology (ASH), the European Society of Hematology (EHA) and the European Society for Bone Marrow and Transplantation (EBMT).
MEDLINE (Appendix 2) was searched and the search strategy adapted for searching the other databases (Appendix 3; Appendix 4).
Searching other resources
The references of all identified studies were inspected for more trials. Additionally, we attempted to contact the first or corresponding author of each included trial and researchers who are active in the field for information regarding unpublished trials or complementary information.
Data collection and analysis
Selection of studies
For the 2005 review (AGG, AF) and the update (AGG, LV), two authors independently assessed the titles and abstracts for inclusion of all the potential studies identified as a result of the search strategy . For potentially relevant articles, or in cases where there was disagreement between the two review authors, the full article was obtained and inspected independently by the two review authors. We resolved any further disagreement through discussion or, if required, we consulted MP.
Data extraction and management
For the 2005 review (AGG, AF) and the 2011 update (AGG, LV), two authors independently extracted the data of included trials to our specifically‐designed data extraction form. We resolved discrepancies through discussion or, if required, we consulted MP who then also extracted data. We documented our decisions and, where necessary, we contacted the authors of the trials for clarification. We identified trials by the name of the first author and year in which the trial was first published and ordered them chronologically. We entered data into Review Manager software (RevMan 2008) and checked them for accuracy. The following data were recorded:
(1) Characteristics of trials
Date, location and setting of trial
Publication status
Case definitions used (clinical, serological, bacteriological)
Sponsor of trial (specified, known or unknown)
Duration of follow‐up
(2) Characteristics of participants
Number of participants in each group
Age, gender, nationality
Underlying malignancy (haematological or solid)
Neutrophil count below 1000 or 500 or 100/mm³, in each group
Percentage of patients with acute leukaemia in each group
(3) Characteristics of interventions
Type of antibiotic, dose, mode of administration, schedule (started with chemotherapy or at onset of neutropenia), length of follow‐up (in months)
Number of days that the antibiotic prophylaxis was provided
(4) Characteristics of outcome measures
Whenever possible, the numbers of events previously listed under 'Types of outcome measures' were recorded in each arm of the randomised trials together with the numbers evaluated
When intention‐to‐treat (ITT) analysis was not performed by trial authors, we extracted data and performed an available case analysis
For trials which included three arms, the data collection was influenced by the different arms.
In trials in which there was a quinolone versus another antibiotic versus placebo arm, the patients and events in the control arm were divided so as to avoid counting them twice in two different comparisons, as suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2009), that is quinolone versus placebo and the other antibiotic versus placebo.
In the event that two arms contained a quinolone and the third arm was placebo, the patients and events in the quinolone arm were combined (for example an arm of quinolone only, an arm of quinolone plus another antibiotic and an arm of placebo). In the event that two of the arms were of different quinolones (for example quinolone versus quinolone versus placebo or quinolone versus quinolone versus another antibiotic) the patients and events in the quinolone arms were merged and counted in only one comparison (quinolone versus placebo or quinolone versus another antibiotic, respectively).
When information regarding any of the above was unclear, we attempted to contact authors of the original reports for them to provide further details.
Assessment of risk of bias in included studies
See Appendix 1 for the methodology of the original review. For the updated review, AGG and LV independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2009).
We assessed the following and resolved any disagreement by discussion:
selection bias (random sequence generation; allocation concealment);
performance bias (blinding of participants and personnel);
detection bias (blinding of outcome assessment);
attrition bias (incomplete outcome data); and
reporting bias (selective reporting of outcomes).
For further details see Appendix 5.
Measures of treatment effect
Dichotomous data were analysed by calculating the risk ratio (RR) for each trial with the uncertainty in each result being expressed using 95% confidence intervals (CIs). We had planned to analyse continuous data by using the mean and standard deviation (SD) of each trial and calculating the effect size (average mean difference) and the 95% CI, where comparisons in the two groups were normally distributed. However, data could not be combined for days of hospitalisation and fever days as these outcomes were summarised heterogeneously in the various included trials as means or medians without appropriate CIs.
Dealing with missing data
For included studies, we noted levels of attrition. For all outcomes we carried out analyses, as far as possible, on an intention‐to‐treat basis, that is we attempted to include all participants randomised to each group in the analysis, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the I² and Chi² statistics. We regarded heterogeneity as substantial if I² was greater than 30%, and if there was a low P value (< 0.10) in the Chi² test for heterogeneity. We anticipated inter‐trial variation in estimation of morbidity and mortality for trials comparing patients at different risk levels.
Assessment of reporting biases
If there were 10 or more studies in the meta‐analysis of the main outcomes, we investigated reporting biases (such as publication bias) using funnel plots. We assessed funnel plot asymmetry visually (Egger 1997). If asymmetry was suggested by a visual assessment we performed exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2008). We used fixed‐effect model meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect, that is where trials were examining the same intervention and the trials’ populations and methods were judged to be sufficiently similar. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we used random‐effects model meta‐analysis to produce an overall summary if an average treatment effect across trials was considered clinically meaningful. The random‐effects model summary was treated as the average range of possible treatment effects. If the average treatment effect was not clinically meaningful we did not combine trials. If we used random‐effects model analyses, the results were presented as the average treatment effect with 95% CI and the estimates of the Chi² and I² statistics.
Studies were sorted by publication year in the meta‐analyses to allow for a visual inspection of trends by year.
Subgroup analysis and investigation of heterogeneity
We subgrouped studies according to the type of antibiotic used, that is:
quinolone versus placebo or no intervention;
trimethoprim‐sulphamethoxazole (TMP‐SMZ) versus placebo or no intervention;
other systemic antibiotic versus placebo or no intervention;
nonabsorbable antibiotic versus placebo or no intervention.
In addition, we assessed the effects of underlying cancer (haematological or solid), timing of prophylaxis initiation (with start of chemotherapy or at onset of neutropenia), type of quinolone, and study year (published before 2000 or thereafter) on results for mortality through subgroup analyses. These analyses were performed only for the comparison of quinolone versus placebo or no treatment, which is the main intervention currently considered in clinical practice.
Sensitivity analysis
Sensitivity analyses were performed by the assessment of bias indicators, namely randomisation (low ‐ A, unclear ‐ B and high ‐ C risk), allocation concealment (low ‐ A, unclear ‐ B risk), and by whether the trials were double blind.
We included four outcomes for sensitivity analysis: mortality, incidence of fever, clinically documented infection and microbiologically documented infection.
Results
Description of studies
Results of the search
For the original review, 162 studies were identified from the search and 101 studies, conducted between the years 1973 to 2005, were included. For the 2011 update, we identified 18 potentially eligible studies. After independent assessment, we included eight of these studies. This makes a total of 109 studies included in the review.
Included studies
Studies were conducted between the years 1973 to 2010 and randomised 13,579 patients. One trial included 111 neutropenic episodes without specifying the number of patients (Gurwith 1979).
Sixty‐four studies compared a prophylactic antibiotic given orally or intravenously to placebo or no intervention (Characteristics of included studies).
Twenty‐seven studies compared quinolones to placebo or no intervention, the last published in 2010. Two studies included three arms: in one of the studies there was an additional arm in which patients were given vancomycin (Moreau 1995) and in the other there was an additional arm in which patients were given a quinolone plus vancomycin (Thomas 2000). Of the 27 studies that compared quinolones to placebo, in five of them the quinolone arm also included coverage against Gram‐positive bacteria (such as vancomycin, amoxicillin‐clavulonic acid or roxythromycin) (Lee 2002; Lalami 2004; Papaiakovou 2010; Thomas 2000; Tjan Heijnen 2001).
Nineteen studies compared TMP‐SMZ to placebo or no intervention. in two of them a macrolide (roxythromycin or erythromycin) was added to the antibiotic regimen (Kramer 1984; Pizzo 1983).
Eleven studies compared other systemic antibiotics with placebo or no intervention: intravenous vancomycin (five studies), intravenous cefipime (one study), intravenous imipenem (one study), intravenous ceftriaxone (two studies), intravenous teicoplanin (one study), oral amoxicillin‐clavulanate (one study).
Six studies compared oral nonabsorbable antibiotics with placebo or no intervention. The nonabsorbable antibiotics arm used combinations of oral gentamicin, vancomycin, neomycin, polymyxin, colistin, nalidixic acid, bacitracin or kanamycin.
Forty‐five studies compared different prophylactic regimens to each other, of which 35 studies compared quinolones to other antibiotics, including nonabsorbable antibiotics, or to each other.
Ten trials compared quinolones to quinolones plus antibiotics active against Gram‐positive pathogens. The antibiotics against Gram‐positive pathogens included: penicillin V in two trials, phenethicillin in one trial, amoxicillin‐clavulanate in one, vancomycin in two trials, rifampin in three trials and roxythromycin in one.
Thirteen studies compared quinolones to TMP‐SMZ.
Five studies compared different types of quinolones (ciprofloxacin, ofloxacin, norfloxacin, pefloxacin) in the two study arms. Results for these studies were not part of the meta‐analysis and are given separately (Table 3).
Three studies compared TMP‐SMZ to other antibiotics (trimethoprim, penicillin V, TMP‐SMZ plus ciprofloxacin) (Bow 1984; Guiot 1992; Murase 1995).
Twelve studies compared nonabsorbable antibiotics to the combination of nonabsorbable antibiotics and systemic antibiotics (Characteristics of included studies). In eight of the studies, quinolones were the systemic antibiotic. Three studies compared systemic antibiotics to the combination of nonabsorbable antibiotics and systemic antibiotics (Malarme 1981; Nemet 1989; Starke 1982). One study compared two different regimens of nonabsorbable antibiotics (Bender 1979).
1. Other studies.
| Study ID | Intervention 1 | Intervention 2 | Overall mortality | Inf‐related mortality | febrile patients | clin.doc.inf | micro.doc.inf | gram neg. inf | gram pos. inf |
| D'Antonio 1994 | ciprofloxacin | ofloxacin | 0.77(0.18‐3.33) | 0.77(0.18‐3.33) | 1.41(0.60‐3.32) | 0.9(0.47‐1.71) | 0.68(0.12‐3.98) | 0.95(0.46‐1.94) | |
| GIMEMA 1991 | ciprofloxacin | norfloxacin | 0.97(0.64‐1.47) | 1.11(0.72‐1.72) | 0.89(0.81‐0.99) | 0.92(0.62‐1.37) | 0.71(0.52‐0.98) | 0.46(0.24‐0.88) | 0.92(0.59‐1.44) |
| Maschmeyer 1988 | ciprofloxacin | norfloxacin | 1.43(0.22‐9.44) | 0.46(0.04‐4.74) | 0.92(0.64‐1.32) | 1.84(0.52‐6.52) | 0.51(0.20‐1.30) | 0.18(0.01‐3.65) | 0.66(0.24‐1.78) |
| D'Antonio 1991 | norfloxacin | ofloxacin | 3.08(0.13‐73.23) | 3.08(0.13‐73.23) | 9.25(0.52‐165.69) | 9.25(0.52‐165.69) | 11.31(0.65‐197.11) | 1.54(0.61‐3.88) | |
| D'Antonio 1992 | norfloxacin | pefloxacin | 1.03(0.22‐4.92) | 1.03(0.07‐16.13) | 1.3(1‐1.69) | 2.83(0.95‐8.46) | 2.06(1.06‐4.00) | 7.21(0.91‐57.02) | 1.69(0.86‐3.30) |
| Bender 1979 | gentamycin+vancomycin | gentamycin | 3.15(0.14‐72.88) | 0.95(0.62‐1.47) | 0.48(0.14‐1.57) | 2.06(1.06‐4.00) | 4.44(1.08‐18.25) | 0.44(0.1‐2.01) |
Six studies had three arms, thus the total number of comparisons listed above is larger than the number of trials (Arning 1990; Bow 1996; D'Antonio 1994; Malarme 1981; Moreau 1995; Thomas 2000).
Patients and settings
Seventy‐six studies included adult patients only. Twenty‐six studies included children less than 16 years, 10 exclusively. The other studies did not specify the patients' ages. Most patients had haematological malignancies, mostly acute leukaemia, acute myeloid leukaemia or acute lymphoblastic leukaemia but also lymphoma, chronic myelocytic leukaemia in blast crisis and multiple myeloma.
Seventy trials included only patients with haematological malignancies.
Bone marrow transplant patients were included in 33 studies. In 18 of these, more than half of the patients underwent bone marrow transplantation.
In 13 studies more than 80% of the patients had solid tumours (mostly breast, lung, ovary and germ cell tumours).
Patients were hospitalised for the duration of prophylaxis in 86 studies, both outpatients and inpatients were included in two studies, and 11 studies included only outpatients. The remaining studies did not report on the trial setting.
Prophylaxis was initiated either upon initiation of chemotherapy (87 studies) or when the patient became neutropenic (22 studies). Initiation time was not specified in one study. Prophylaxis was continued until: the peripheral granulocyte count reached greater than 500/mm3 or greater than 1000/mm3, the development of fever, remission, or a maximum of six weeks of treatment. Duration was different in several trials: in the Cullen 2005 study prophylaxis was administered during six cycles of chemotherapy, and in each cycle for seven consecutive days just before and during the anticipated period of neutropenia (thus, for a total of 42 days). In two other trials, treatment was prolonged to 40 weeks in one trial and three years in the other (Goorin 1985; van Eys 1987). Both of these studies included pediatric patients with acute lymphoblastic leukaemia (ALL). In the study in which prophylaxis was administered for 40 weeks the patients randomised to prophylaxis received it throughout the whole course of induction, consolidation and maintenance therapy. In the other study prophylaxis was administered throughout the whole course and even after.
In 15 studies the mean duration ranged between 10 to 151 days. In eight studies the median duration ranged between 8 to 37.5 days. Specific treatment duration was not reported in remaining studies.
In 57 studies anti‐fungal prophylaxis was administered to both study groups, unrelated to randomisation. The vast majority of studies did not report compliance.
Reporting of outcomes
Seventy studies, including 7502 participants, reported overall mortality (Characteristics of included studies).
Seventy‐one studies, including 9289 participants, reported infection‐related mortality, four of which did not report all‐cause mortality. Infection‐related mortality was not defined a priori in most of the original trials.
Eighty‐six studies, including 10,002 participants, reported the number of febrile patients or number of febrile episodes. Of these studies, 18 reported only the number of episodes.
Seventy‐nine studies, including 8811 participants, reported the number of clinically documented infections. Ninety‐three studies, including 10,922 participants, reported the number of microbiologically documented infections.
Eighty‐seven studies, including 9304 participants, reported the number of episodes of bacteraemia. Sixty‐six studies, including 8031 participants, reported the number of episodes of any side effects.
Sixty‐nine studies, including 5271 participants, reported the number of episodes of fungal infection.
Excluded studies
A total of 71 studies were excluded (Characteristics of excluded studies).
The design of 58 of these was incompatible with inclusion criteria: 25 non‐randomised trials, 26 review articles, six trials were trials of treatment of febrile neutropenia (Garcia 2000; Gilbert 1994; Karp 1986; Mantovani 1998; Schaison 1991; Takemoto 1990) and one trial assessed Pneumocystis pneumonia prophylaxis in AIDS patients (May 1994).
The randomised trials were excluded for the following reasons:
peri‐procedural (central line insertion) prophylactic antibiotic administration (Ljungman 1997),
vancomycin solution administration for prevention of catheter infection (Barriga 1997),
high attrition (EORTC 1982),
prophylactic antibiotic therapy combined with a protected environment (Lohner 1979),
prophylactic antibiotic therapy combined with lactobacilli (Ekert 1980),
the intervention evaluated was granulocyte colony stimulating factor and both arms received prophylactic antibiotics (Timmer‐Bonte 2005),
seven reports were identified as duplicate publications and were considered under their primary references (Bow 1984; Castagnola 2003; Donnelly 1992b; Harousseau 1987; Karp 1987; Sleijfer 1980; Winston 1986).
Risk of bias in included studies
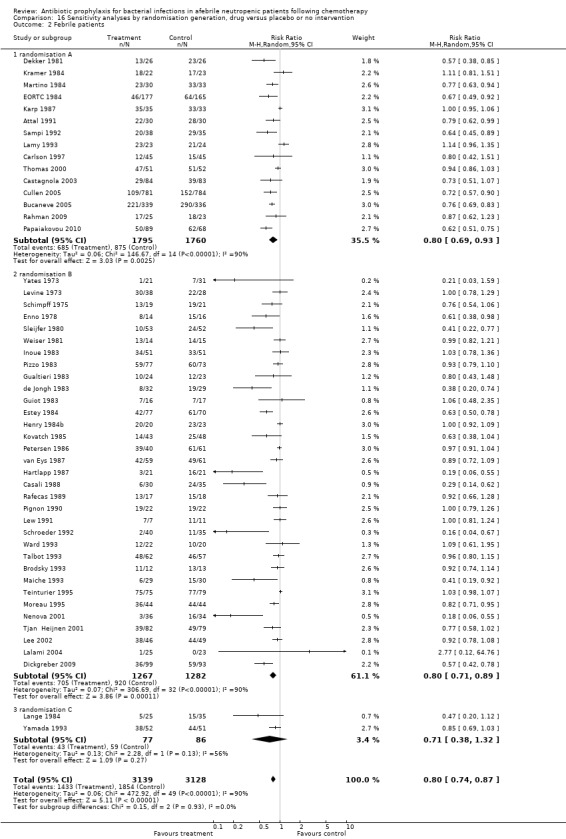
Results are summarised in Figure 1 showing that the large majority of risk of bias items were not described. The method of generating the randomisation sequence was adequate in 33 studies (classified as A, or low risk of bias) (Characteristics of included studies). In two studies generation of randomisation was inadequate (classified as C, or high risk of bias). In one, the randomisation generation was by birth dates (Lange 1984) and in the other by order of admission (Yamada 1993). In the remaining trials it was not clearly described (classified as B, or unclear risk of bias).
1.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation concealment was adequate (A, low risk) in 27 studies, and seven additional studies used sealed envelopes that were not described as opaque (classified as B). In the remaining studies allocation concealment was not described (also classified as B). Thirty studies were conducted in a double‐blinded fashion. All remaining trials were open.
Full intention‐to‐treat (ITT) analyses for mortality and infection were reported in 24 studies, and for mortality alone in six. In 14 studies the number evaluated was the same as the number randomised, with no mention of loss to follow‐up. In the remaining studies ITT analysis was not performed.
Fifty‐six studies reported that patients gave their consent to participate in the research. Approval of the ethics committee was reported in 27 of them.
Effects of interventions
Summary of findings for the main comparison. Summary of findings: antibiotics versus placebo or no intervention.
| Antibiotics compared with placebo or no intervention for afebrile neutropenia | ||||||
|
Patient or population: patients with afebrile neutropenia induced by chemotherapy Settings: hospital or outpatient Intervention: antibiotics Comparison: placebo or no intervention | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Antibiotic drug | |||||
| All cause mortality | 88 per 1000 | 57 per 1000 (47 to 68) | RR 0.66 (0.55‐0.79) | 5,635 participants (46 trials) |
⊕⊕⊕⊕ high |
I² = 20%. NNT to prevent one death from any cause is 34 (95% CI 26‐56). The greatest effect was seen in the quinolone prophylaxis subgroup (20 trials, 3,798 participants; RR 0.54 (95% CI 0.40 to 0.74). Test for subgroup differences: I²= 42%, P=0.16. Quality was not downgraded despite a high risk of bias: (allocation concealment was unclear in most of the trials) because when results of low risk allocation concealment were compared to unclear allocation concealment, they were similar. |
| Febrile patients and episodes | 607 per 1000 | 486 per 1000 (449 to 528) | RR 0.80 (0.74‐0.87) | 6,658 participants (54 trials) | ⊕⊕⊕ moderate | NNT to prevent one febrile patient or febrile episode was 7 (95%CI 5‐10). Quality was downgraded because of heterogeneity and unit of analysis issues, not because of the high risk of bias (allocation concealment was unclear in most of the trials) as, when results between low risk allocation concealment were compared to unclear allocation concealment, the results were similar. |
| Bacteraemia | 209 per 1000 | 105 per 1000 (88 to 125) | RR 0.50 (0.43‐0.60) | 6,390 participants (53 trials) |
⊕⊕⊕⊕ high |
This reduction occurred for all subgroups. NNT to prevent bacteraemia is 10 (95% CI 8‐12). Quality was first downgraded due to a high risk of bias (allocation concealment was unclear in most of the trials) and then upgraded due to large number of participants and large effect (RR 0.50). |
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; NS: not significantly different; NNT: number needed to treat | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Summary of findings 2. Summary of findings: quinolone prophylaxis compared with TMP‐SMZ prophylaxis.
| Quinolones compared with TMP‐SMZ for afebrile neutropenia | |||||
|
Patient or population: cancer patients with afebrile neutropenia following chemotherapy Settings: hospital or outpatient Intervention: quinolones Comparison: TMP‐SMZ |
|||||
| Outcomes | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE)* | Comments | |
| All cause‐mortality | RR 1.07 (0.66‐1.72) | 917 participants (10 trials) |
⊕⊕⊕ moderate | Quality was downgraded due to imprecision. | |
| Febrile patients and episodes | RR 0.92 (0.78‐1.09) | 931 participants (10 trials) |
⊕⊕⊕ moderate | Quality was downgraded due heterogeneity. | |
| Bacteraemia | RR 0.89 (0.56‐1.42) | 931 participants (10 trials) |
⊕⊕⊕ moderate | Quality was downgraded due heterogeneity and imprecision. | |
| CI: Confidence interval; RR: Risk Ratio | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
*Quality was downgraded to moderate for these outcomes due to a high risk of bias (allocation concealment was unclear in most of the trials).
Antibiotic versus placebo or no intervention
Primary outcome
1. All‐cause mortality
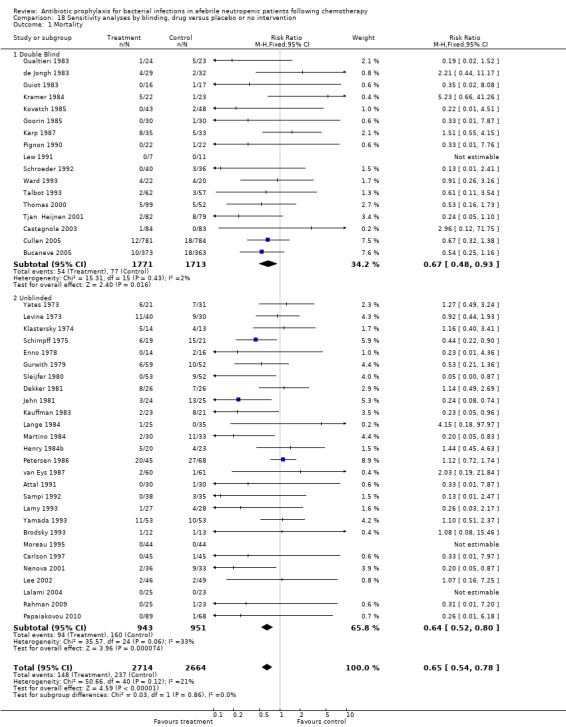
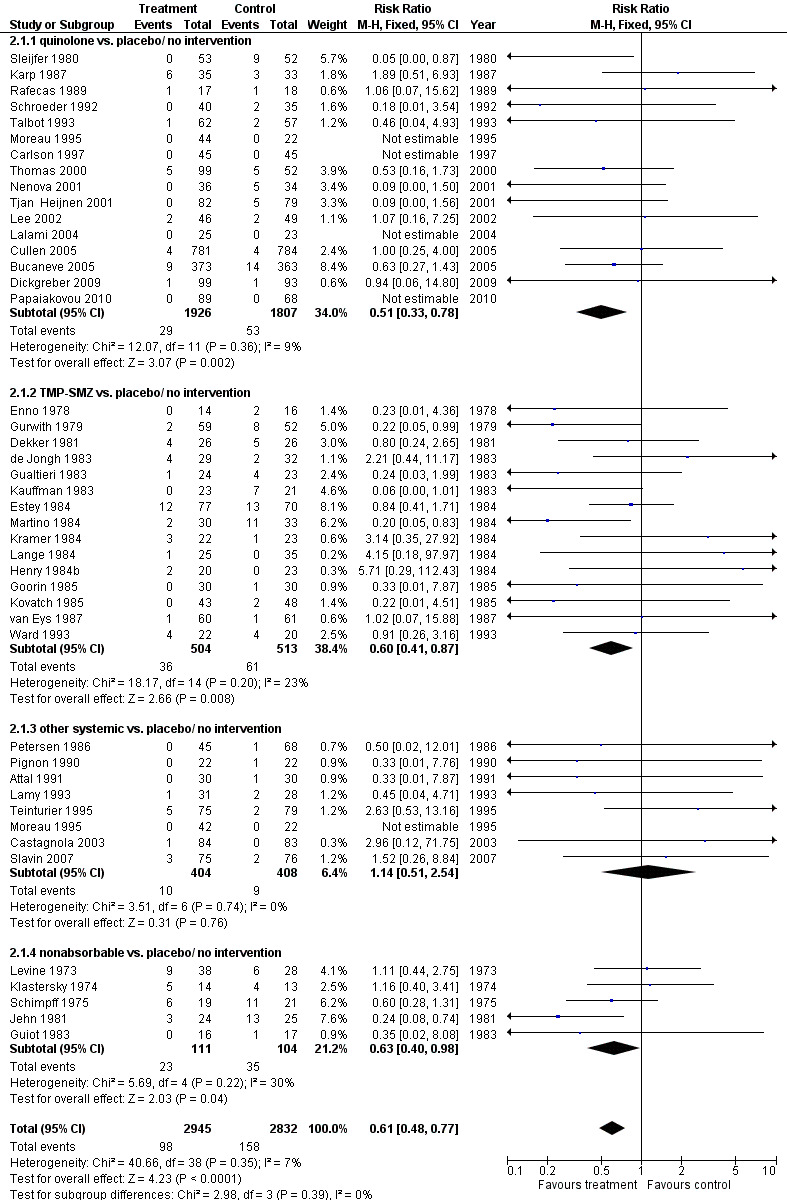
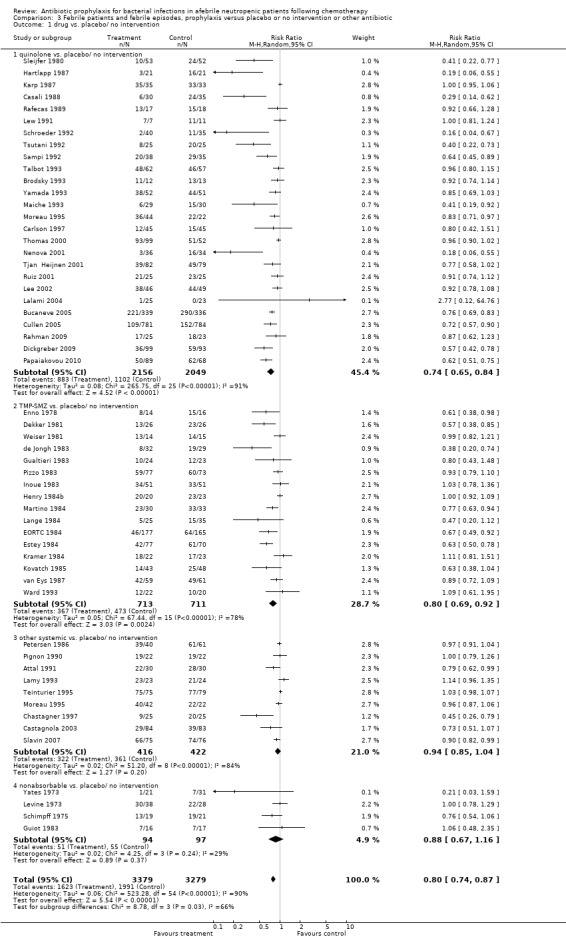
Antibiotic prophylaxis resulted in a significant reduction in the risk of mortality (46 trials, 5635 participants; RR 0.66, 95% CI 0.55 to 0.79) (Analysis 1.1; Figure 2). NNT to prevent one death from any cause was 34 (95% CI 26 to 56).
1.1. Analysis.
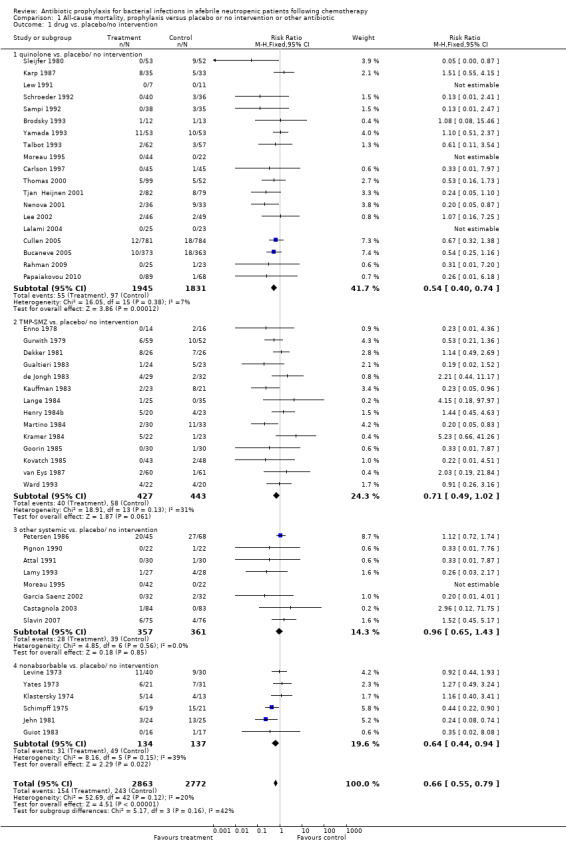
Comparison 1 All‐cause mortality, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 1 drug vs. placebo/no intervention.
2.
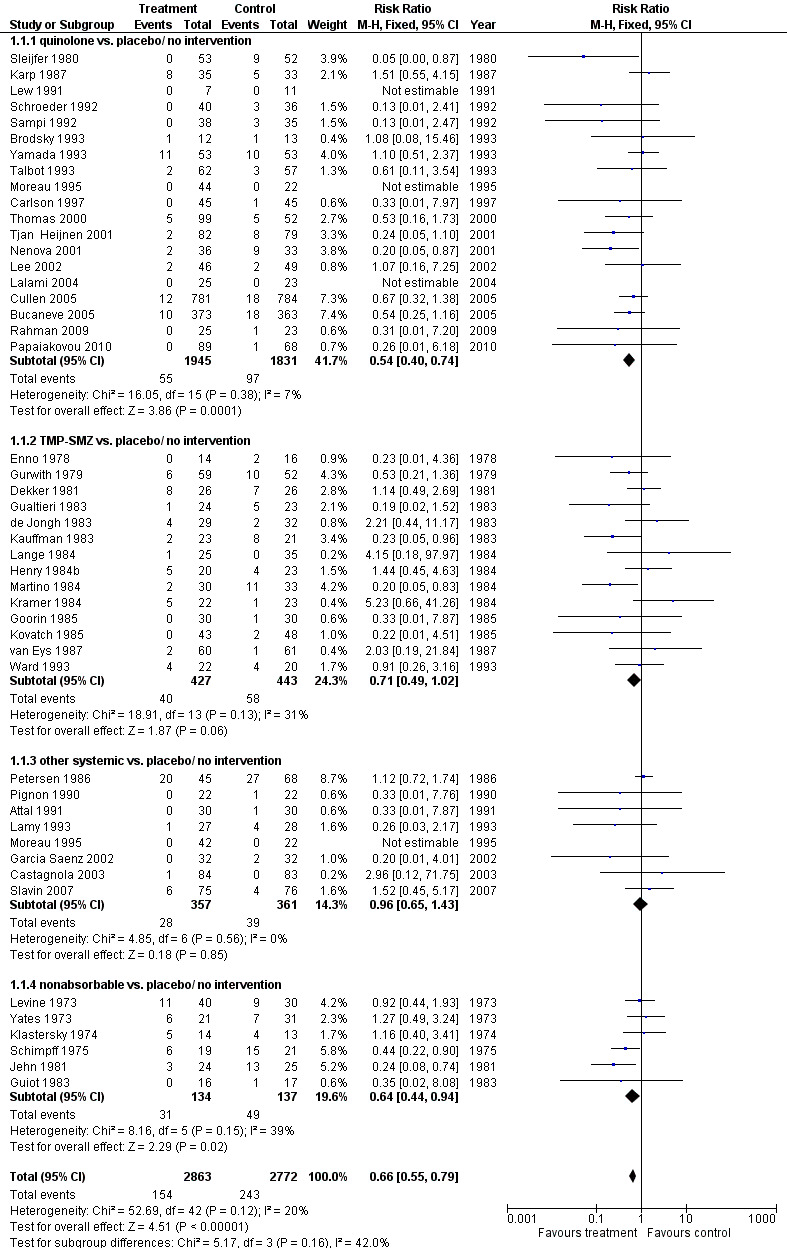
Forest plot of comparison: 1 All‐cause mortality, prophylaxis vs. placebo/no intervention or other antibiotic, outcome: 1.1 drug vs. placebo/no intervention.
The greatest effect was seen in the quinolone prophylaxis subgroup (19 trials, 3,776 participants; RR 0.54, 95% CI 0.40 to 0.74) although tests for subgroup differences were not significant (I² = 42%, P = 0.16).
Results for mortality for this comparison of antibiotic versus placebo or no treatment were not affected by the studies’ risk of bias; mortality was significantly lower with antibiotic prophylaxis in adequately randomised, concealed and double‐blind trials (Analysis 16.1; Analysis 17.1; Analysis 18.1).
16.1. Analysis.
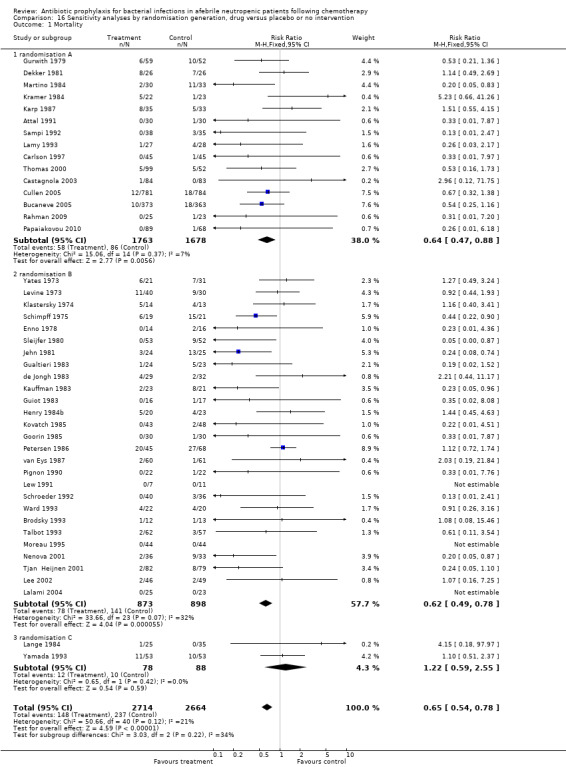
Comparison 16 Sensitivity analyses by randomisation generation, drug versus placebo or no intervention, Outcome 1 Mortality.
17.1. Analysis.
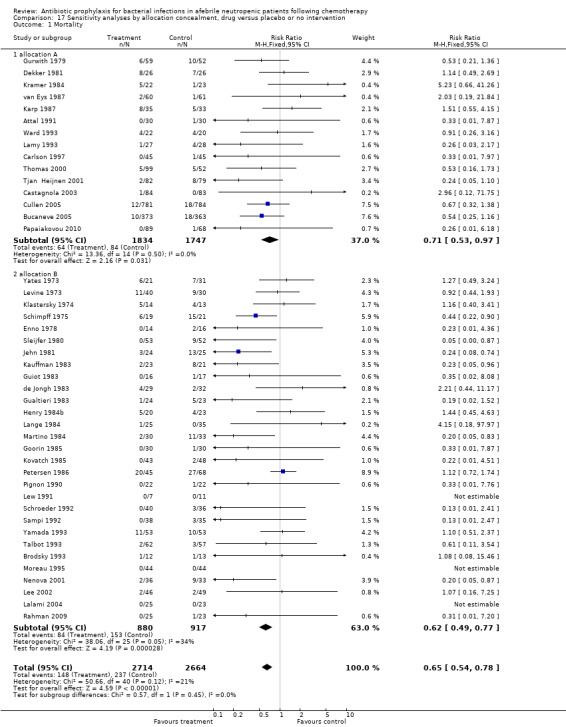
Comparison 17 Sensitivity analyses by allocation concealment, drug versus placebo or no intervention, Outcome 1 Mortality.
18.1. Analysis.
Comparison 18 Sensitivity analyses by blinding, drug versus placebo or no intervention, Outcome 1 Mortality.
Further to this outcome we performed some exploratory subgroup analyses on the quinolone prophylaxis subgroup, as follows.
Quinolones versus placebo or no treatment
A. All‐cause mortality by disease status
Most of the trials included haematological cancer patients, showing an advantage of prophylaxis. In patients with acute leukaemia or patients undergoing haematopoietic cell transplant (mainly allogeneic haematopoietic cell transplantation but also autologous stem cell transplant) quinolone prophylaxis resulted in a significant decrease in mortality (13 trials, 1818 participants; RR 0.57, 95% CI 0.40 to 0.82); NNT to prevent one death from any cause for haematological malignancies was 33 (95% CI 16 to 100). In trials assessing patients with solid cancer or lymphoma the effect was also statistically significant (5 trials, 1940 participants; RR 0.48, 95% CI 0.26 to 0.88) (Analysis 15.1); with a larger NNT of 50 (95% CI 33 to 1000).
15.1. Analysis.
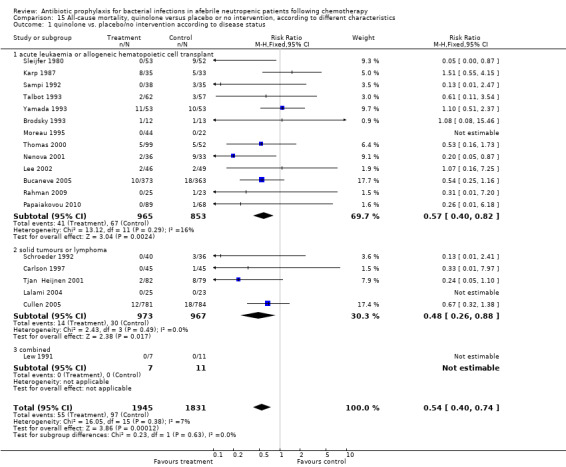
Comparison 15 All‐cause mortality, quinolone versus placebo or no intervention, according to different characteristics, Outcome 1 quinolone vs. placebo/no intervention according to disease status.
The group of patients with solid tumours or lymphoma included tumours of the lung, ovary, breast, testis and other. These were mostly outpatients. Tests for subgroup differences were nonsignificant and the funnel plot was symmetrical.
B. All‐cause mortality by type of quinolone
An advantage was seen with all quinolones except for norfloxacin. Levofloxacin reduced all‐cause mortality (4 trials, 2349 patients; RR 0.59, 95% CI 0.35 to 0.99) as did ciprofloxacin (8 trials, 726 patients; RR 0.30, 95% CI 0.13 to 0.69) and other quinolones (ofloxacin, pefloxacin or enoxacin) (4 trials, 451 patients; RR, 0.28, 95% CI 0.12 to 0.64). Norfloxacin had no significant effect on all‐cause mortality compared with placebo (4 trials, 271 patients; RR 1.03, 95% CI 0.58 to 1.81) (Analysis 15.2). Tests for subgroup differences were significant (I² = 67.8%, P = 0.03).
15.2. Analysis.
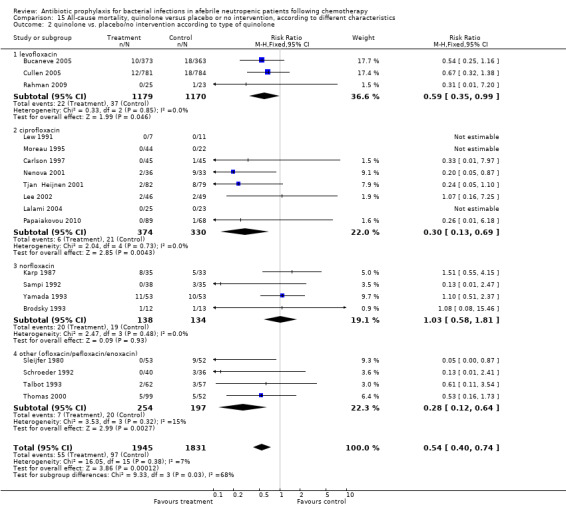
Comparison 15 All‐cause mortality, quinolone versus placebo or no intervention, according to different characteristics, Outcome 2 quinolone vs. placebo/no intervention according to type of quinolone.
C. All‐cause mortality by timing of prophylaxis initiation
Most trials initiated antibiotic prophylaxis with the start of chemotherapy. Results were similar for this set of trials (15 trials, 1947 patients; RR 0.63, 95% CI 0.44 to 0.92); or when prophylaxis was initiated at onset of neutropenia (4 trials, 1829 patients; RR 0.39, 95% CI 0.22 to 0.70) (Analysis 15.3). As shown, the effect was even larger for trials which initiated prophylaxis at the onset of neutropenia, without a statistically significant difference between these subgroups.
15.3. Analysis.
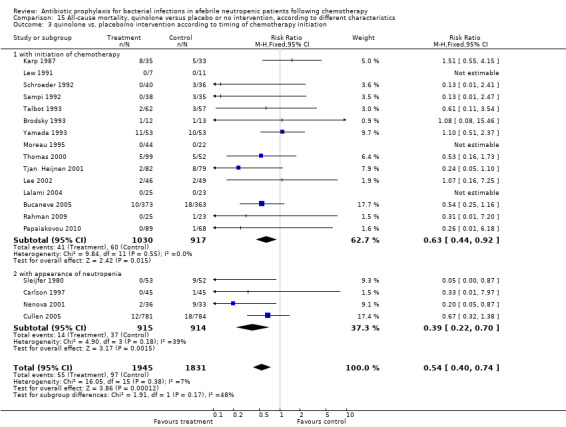
Comparison 15 All‐cause mortality, quinolone versus placebo or no intervention, according to different characteristics, Outcome 3 quinolone vs. placebo/no intervention according to timing of chemotherapy initiation.
D. All‐cause mortality by publication years
Finally, we analysed all‐cause mortality according to year of publication, that is before 2000 or thereafter. Studies conducted in the last decade (studies published after 2000) showed a larger effect of quinolone prophylaxis on mortality (8 trials, 2879 patients; RR 0.49, 95% CI 0.32 to 0.75) than older studies (conducted before and until 2000) (11 trials, 897 patients; RR 0.61, 95% CI 0.39 to 0.96), without a statistically significant difference between these subgroups (Analysis 15.4). There was no evidence to suggest publication bias in the funnel plot for mortality (Figure 3).
15.4. Analysis.
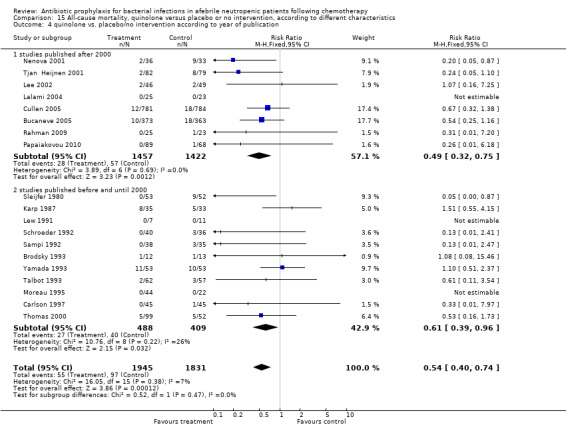
Comparison 15 All‐cause mortality, quinolone versus placebo or no intervention, according to different characteristics, Outcome 4 quinolone vs. placebo/no intervention according to year of publication.
3.
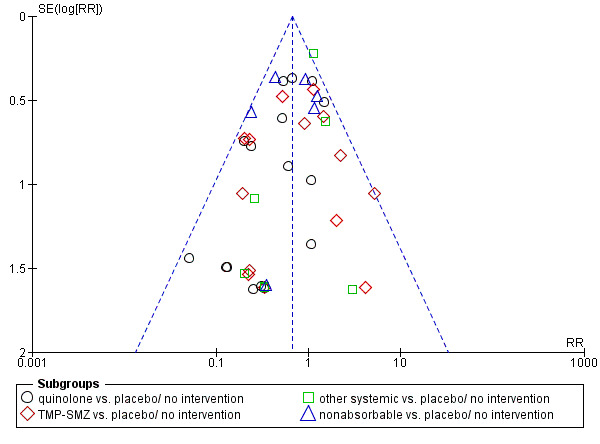
Funnel plot of comparison: 1.1 drug vs. placebo/no intervention for the outcome: All‐cause mortality.
Secondary outcomes
2. Infection‐related mortality
Antibiotic prophylaxis resulted in a significant reduction in the risk of infection‐related death (43 trials, 5777 participants; RR 0.61, 95% CI 0.48 to 0.77) (Analysis 2.1); NNT to prevent one death from infection was 48 (95% CI 34 to 77). This effect was consistent across subgroups with the greatest risk reduction seen in the quinolone prophylaxis subgroup (16 studies, 3733 participants; RR 0.51, 95% CI 0.33 to 0.78). TMP‐SMZ was associated with a RR of 0.60 (95% CI 0.41 to 0.87) (Figure 4; Figure 5).
2.1. Analysis.
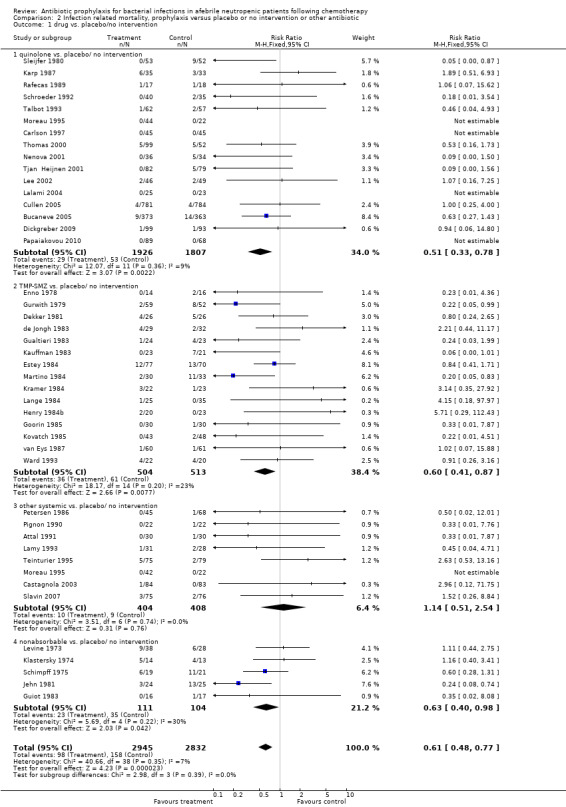
Comparison 2 Infection related mortality, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 1 drug vs. placebo/no intervention.
4.
Forest plot of comparison: 2 Infection related mortality, prophylaxis vs. placebo/no intervention or other antibiotic, outcome: 2.1 drug vs. placebo/no intervention.
5.
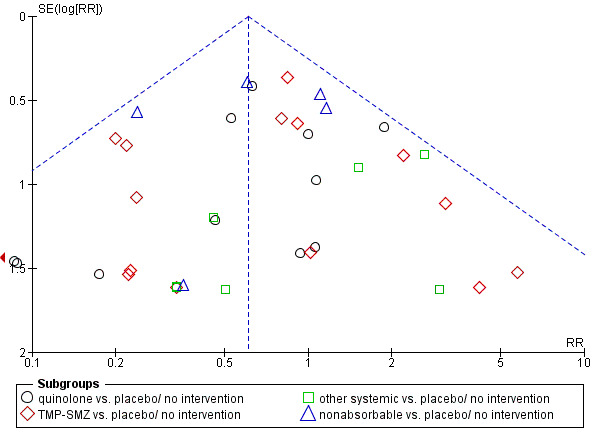
Funnel plot of comparison: 2 Infection related mortality, prophylaxis vs. placebo/no intervention or other antibiotic, outcome: 2.1 drug vs. placebo/no intervention.
3. Febrile episodes
Antibiotic prophylaxis resulted in a significant decrease in the occurrence of fever (54 trials, 6658 participants; RR 0.80, 95% CI 0.74 to 0.87) (Analysis 3.1; Figure 6) when both febrile patients and episodes were included in the analysis (when data on febrile patients were not available, data on febrile episodes were used for the numerator). The NNT to prevent one febrile patient or febrile episode was 7 (95% CI 5 to 10). Data were substantially heterogenous for this outcome, overall and across subgroups (I² = 89% and I² = 67%, respectively). Quinolones and TMP‐SMZ were the only subgroups associated with a reduction in febrile episodes (26 trials, 4205 participants; RR 0.74, 95% CI 0.65 to 0.84; and 16 trials, 1424 participants; RR 0.80, 95% CI 0.69 to 0.92, respectively).
3.1. Analysis.
Comparison 3 Febrile patients and febrile episodes, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 1 drug vs. placebo/ no intervention.
6.
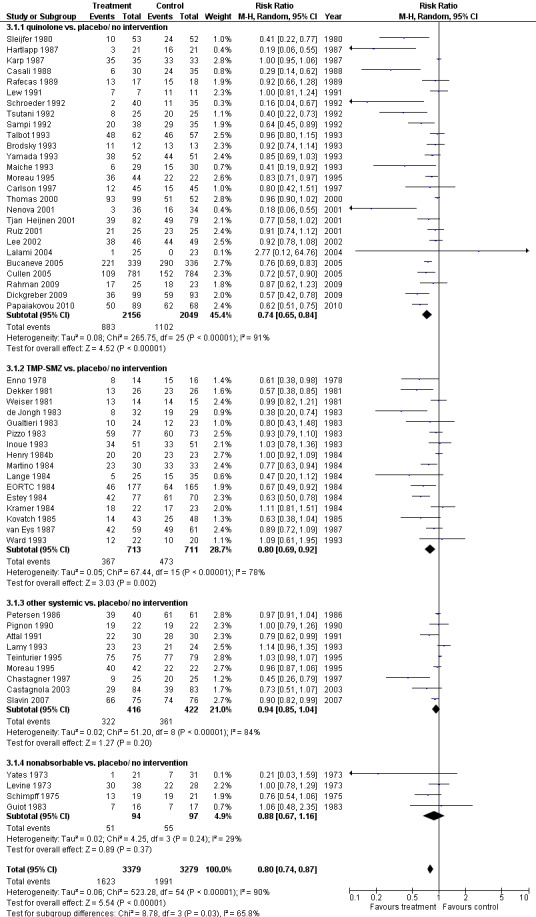
Forest plot of comparison: 3 Febrile patients and febrile episodes, prophylaxis vs. placebo/no intervention or other antibiotic, outcome: 3.1 drug vs. placebo/ no intervention.
Sensitivity analysis for this outcome showed that results did not differ significantly according to randomisation generation, concealment and blinding (for example Analysis 16.2; Analysis 17.2; Analysis 18.2).
16.2. Analysis.
Comparison 16 Sensitivity analyses by randomisation generation, drug versus placebo or no intervention, Outcome 2 Febrile patients.
17.2. Analysis.
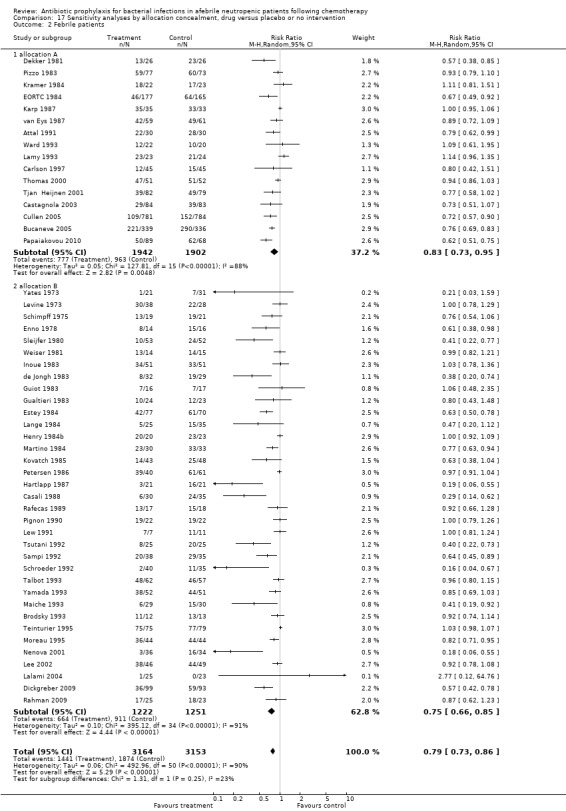
Comparison 17 Sensitivity analyses by allocation concealment, drug versus placebo or no intervention, Outcome 2 Febrile patients.
18.2. Analysis.
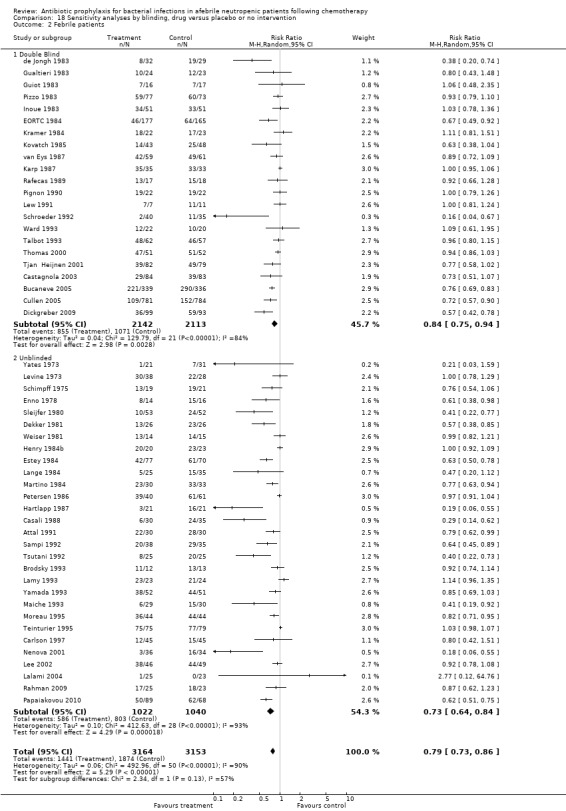
Comparison 18 Sensitivity analyses by blinding, drug versus placebo or no intervention, Outcome 2 Febrile patients.
4. Clinically documented infection
Antibiotic prophylaxis resulted in a significant decrease in the occurrence of clinically documented infection (48 trials, 5758 participants; RR 0.65, 95% CI 0.56 to 0.76) (Analysis 4.1).
4.1. Analysis.
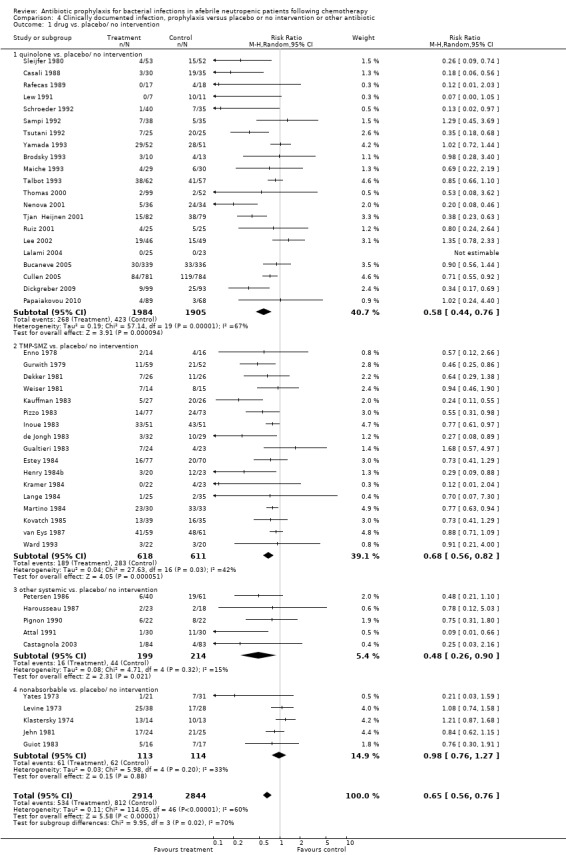
Comparison 4 Clinically documented infection, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 1 drug vs. placebo/ no intervention.
This reduction occurred for quinolones (21 trials, 3889 participants; RR 0.58, 95% CI 0.44 to 0.76), TMP‐SMZ (17 trials, 1229 participants; RR 0.68, 95% CI 0.56 to 0.82) and other systemic antibiotics (five trials, 413 participants; RR 0.48, 95% CI 0.26 to 0.90) but not nonabsorbables.
Sensitivity analysis for this outcome showed that results did not differ significantly according to randomisation generation, concealment and blinding (for example Analysis 16.3; Analysis 17.3; Analysis 18.3).
16.3. Analysis.
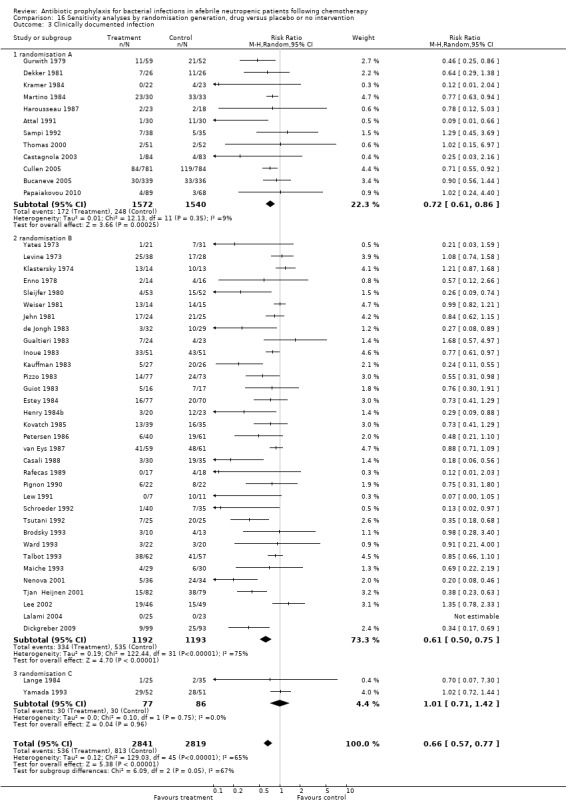
Comparison 16 Sensitivity analyses by randomisation generation, drug versus placebo or no intervention, Outcome 3 Clinically documented infection.
17.3. Analysis.
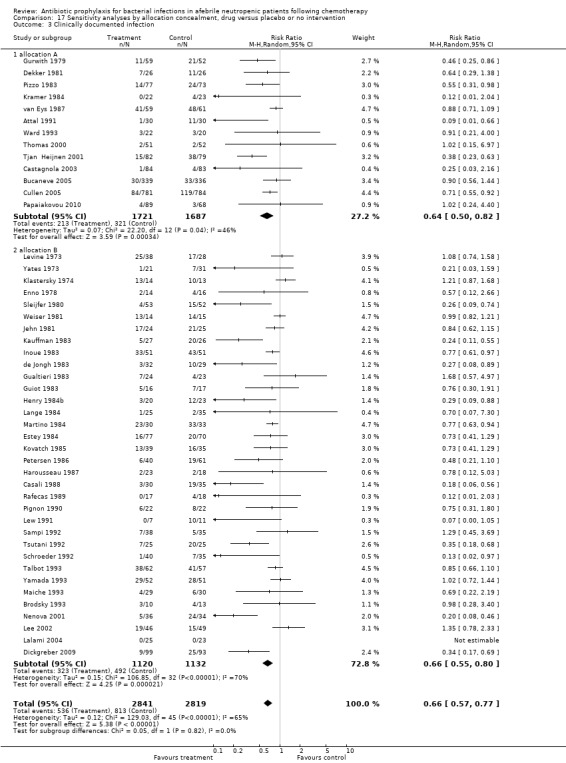
Comparison 17 Sensitivity analyses by allocation concealment, drug versus placebo or no intervention, Outcome 3 Clinically documented infection.
18.3. Analysis.
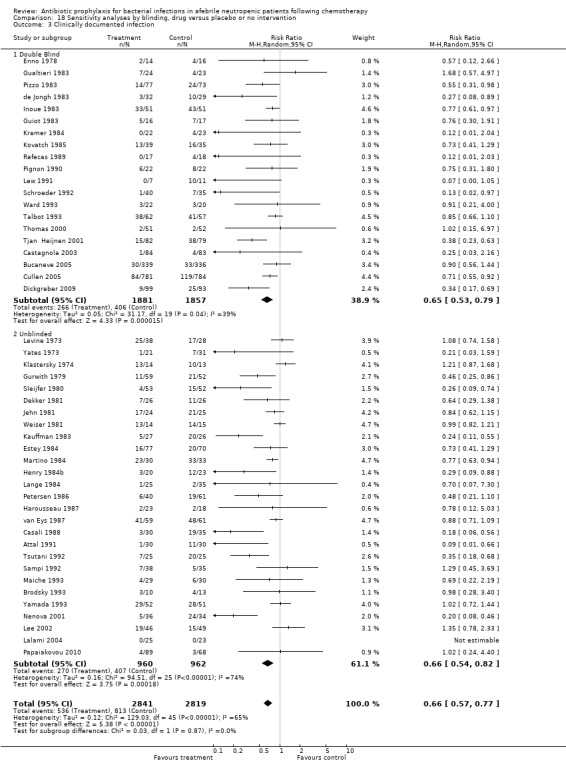
Comparison 18 Sensitivity analyses by blinding, drug versus placebo or no intervention, Outcome 3 Clinically documented infection.
5. Microbiologically documented infection
Antibiotic prophylaxis resulted in a significant decrease in the occurrence of microbiologically documented infection (53 trials, 6383 participants; RR 0.51, 95% CI 0.42 to 0.62) (Analysis 5.1); NNT to prevent one microbiologically documented infection was 7 (95% CI 6 to 9). This reduction occurred for quinolones (24 trials, 3953 participants; RR 0.46, 95% CI 0.32 to 0.66); TMP‐SMZ (17 trials, 1400 participants; RR 0.50, 95% CI 0.38 to 0.65); and other systemic antibiotics (10 trials, 882 participants; RR 0.63, 95% CI 0.45 to 0.87) but not nonabsorbables.
5.1. Analysis.
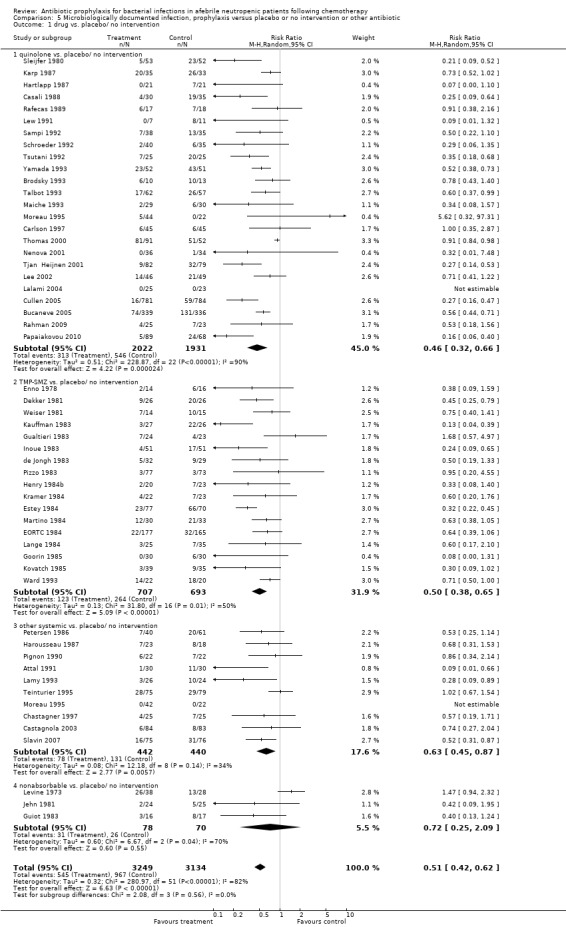
Comparison 5 Microbiologically documented infection, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 1 drug vs. placebo/ no intervention.
Sensitivity analysis for this microbiologically documented infection showed that results did not differ significantly according to randomisation generation, concealment and blinding (for example Analysis 16.4; Analysis 17.4; Analysis 18.4).
16.4. Analysis.
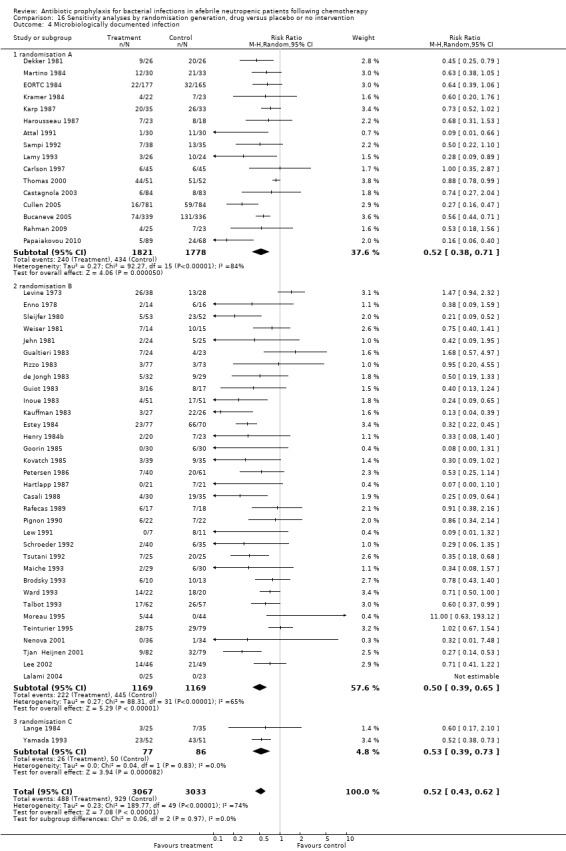
Comparison 16 Sensitivity analyses by randomisation generation, drug versus placebo or no intervention, Outcome 4 Microbiologically documented infection.
17.4. Analysis.
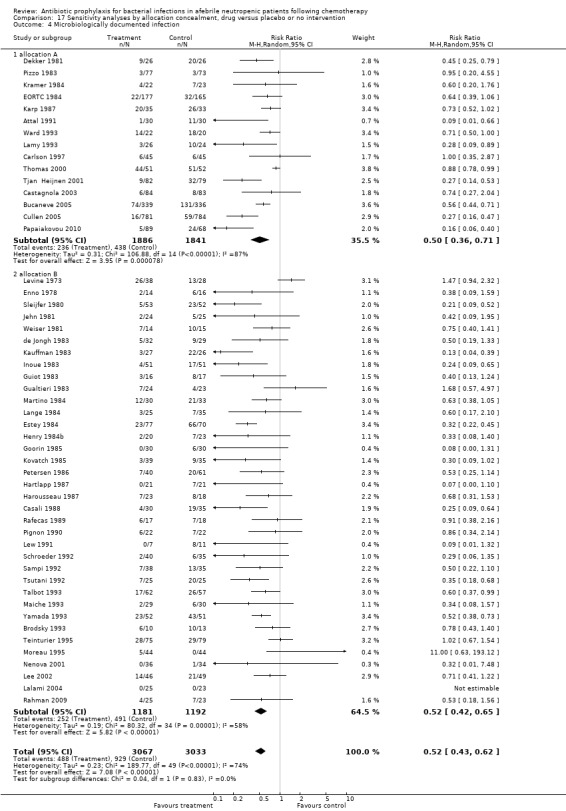
Comparison 17 Sensitivity analyses by allocation concealment, drug versus placebo or no intervention, Outcome 4 Microbiologically documented infection.
18.4. Analysis.
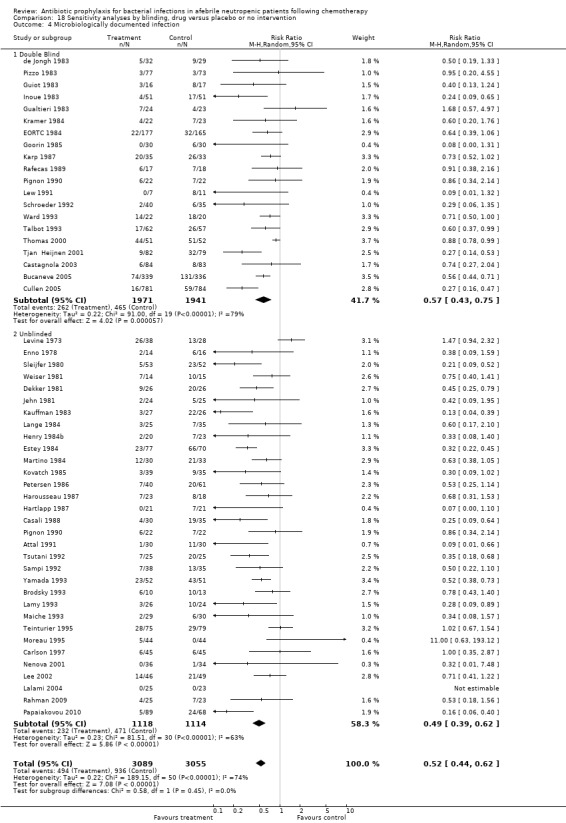
Comparison 18 Sensitivity analyses by blinding, drug versus placebo or no intervention, Outcome 4 Microbiologically documented infection.
6. Gram‐negative infection
Antibiotic prophylaxis resulted in a significant decrease in the occurrence of microbiologically documented Gram‐negative infection (44 trials, 5607 participants; RR 0.38, 95% CI 0.28 to 0.52) (Analysis 6.1). This reduction occurred with quinolones (21 trials, 3752 participants; RR 0.30, 95% CI 0.22 to 0.41) and TMP‐SMZ (13 trials, 1120 participants; RR 0.40, 95% CI 0.29 to 0.56) but not for other systemic or nonabsorbable antibiotics.
6.1. Analysis.
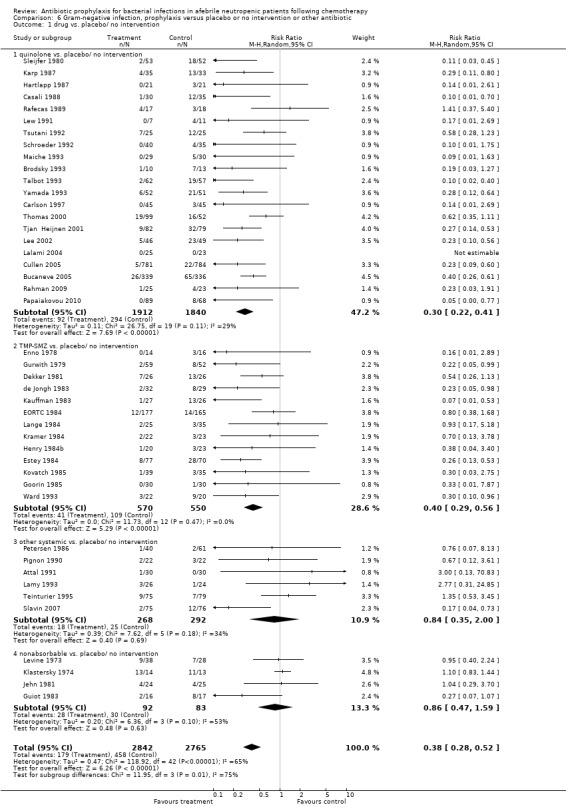
Comparison 6 Gram‐negative infection, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 1 drug vs. placebo/ no intervention.
7. Gram‐positive infection
Antibiotic prophylaxis resulted in a significant decrease in the occurrence of microbiologically documented Gram‐positive infection (45 trials, 5583 participants; RR 0.45, 95% CI 0.34 to 0.59) (Analysis 7.1). This reduction occurred with quinolones (21 trials, 3749 participants; RR 0.33, 95% CI 0.21 to 0.52) and TMP‐SMZ (12 trials, 1009 participants; RR 0.37, 95% CI 0.26 to 0.53) but not for nonabsorbable antibiotics. There was a trend towards reduction in the systemic antibiotic subgroup (7 trials, 610 participants; RR 0.59, 95% CI 0.34 to 1.02).
7.1. Analysis.
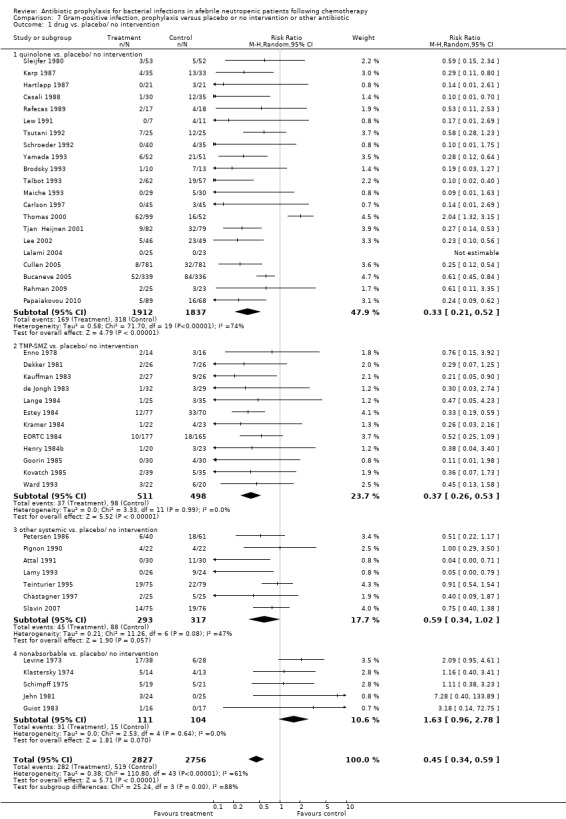
Comparison 7 Gram‐positive infection, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 1 drug vs. placebo/ no intervention.
8. Bacteraemia
Antibiotic prophylaxis resulted in a significant decrease in the occurrence of bacteraemia (53 trials, 6390 participants; RR 0.50, 95% CI 0.43 to 0.60) (Analysis 8.1); NNT to prevent bacteraemia was 10 (95% CI 8 to 12).
8.1. Analysis.
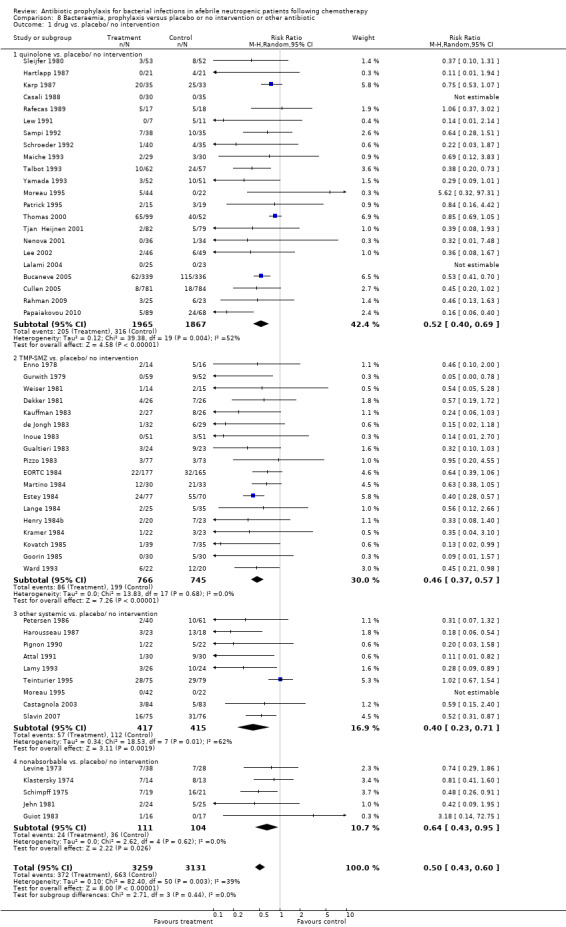
Comparison 8 Bacteraemia, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 1 drug vs. placebo/ no intervention.
This reduction occurred for quinolones (22 trials, 3806 participants; RR 0.52, 95% CI 0.40 to 0.69), TMP‐SMZ (18 trials, 1511 participants; RR 0.46, 95% CI 0.37 to 0.57), other systemic antibiotics (9 trials, 832 participants; RR 0.40, 95% CI 0.23 to 0.71) and nonabsorbable antibiotics (5 trials, 215 participants; RR 0.64, 95% CI 0.43 to 0.95).
9. Gram‐negative bacteraemia
Antibiotic prophylaxis significantly decreased the occurrence of Gram‐negative bacteraemia (40 trials, 5328 participants; RR 0.41, 95% CI 0.33 to 0.50) (Analysis 9.1). Overall, the NNT to prevent one episode of Gram‐negative bacteraemia was 17 (95% CI 14 to 22).
9.1. Analysis.
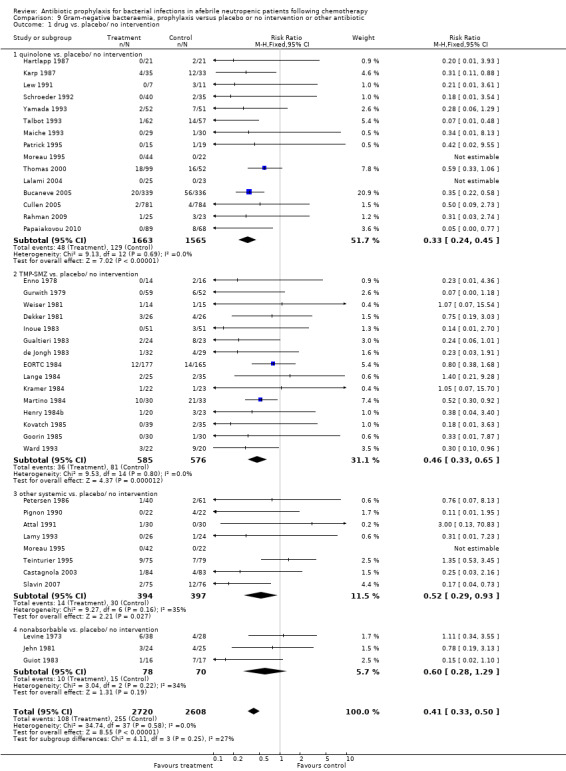
Comparison 9 Gram‐negative bacteraemia, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 1 drug vs. placebo/ no intervention.
The reduction occurred with quinolones (15 trials, 3228 participants; RR 0.33, 95% CI 0.24 to 0.45), TMP‐SMZ (RR 0.46, 95% CI 0.33 to 0.65) and other systemic antibiotics (8 trials, 791 participants; RR 0.52, 95% CI 0.29 to 0.93).
10. Gram‐positive bacteraemia
Antibiotic prophylaxis resulted in a significant decrease in the occurrence of Gram‐positive bacteraemia (39 trials, 5265 participants; RR 0.63, 95% CI 0.54 to 0.74) (Analysis 10.1); NNT to prevent one episode of Gram‐positive bacteraemia was 24 (95% CI 17 to 36). TMP‐SMZ resulted in a significant decrease in the occurrence of Gram‐positive bacteraemia (14 trials, 1098 participants; RR 0.38, 95% CI 0.24 to 0.60) as did quinolones (15 trials, 3228 participants; RR 0.70, 95% CI 0.57 to 0.86) and other systemic antibiotics (8 trials, 791 participants; RR 0.63, 95% CI 0.44 to 0.89).
10.1. Analysis.
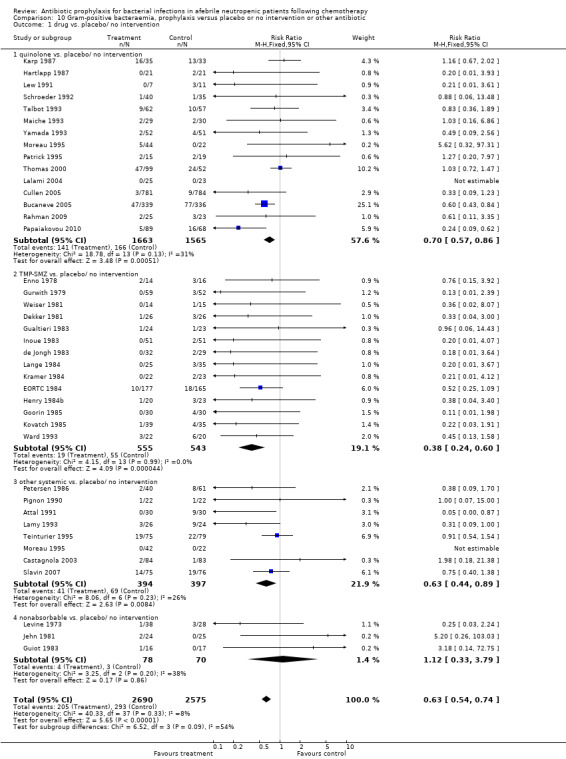
Comparison 10 Gram‐positive bacteraemia, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 1 drug vs. placebo/ no intervention.
11. Side effects
When compared to placebo or no intervention, prophylactic antibiotics caused more side effects (37 trials, 5103 participants; RR 1.58, 95% CI 1.19 to 2.12) (Analysis 11.1). This occurrence of side effects was significant in the quinolone (17 trials, 3324 participants; RR 1.51, 95% CI 1.12 to 2.04) and TMP‐SMZ (13 trials, 1240 participants; RR 1.70, 95% CI 1.12 to 2.59) subgroups only. These were mostly gastrointestinal side effects, including diarrhoea and nausea. C. difficile‐associated diarrhea specifically was reported on in only in two studies, with no events in one (Carlson 1997) and a similar event rate in the two arms in the other (Talbot 1993). Few other studies reported one to two cases in the antibiotic arm but did not report the number of events in the control arm.
11.1. Analysis.
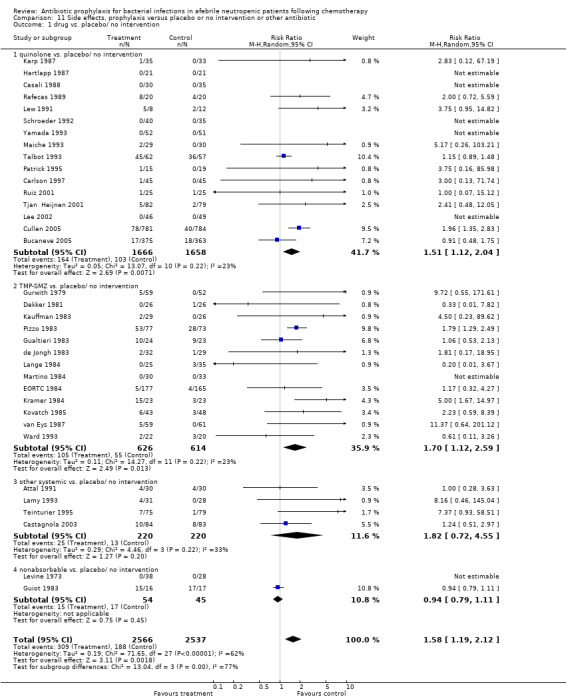
Comparison 11 Side effects, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 1 drug vs. placebo/ no intervention.
12. Side effects requiring discontinuation
When compared to placebo or no intervention, prophylactic antibiotics caused more side effects requiring discontinuation (18 trials, 2281 participants; RR 2.06, 95% CI 1.32 to 3.19) (Analysis 12.1). This was only significant for the quinolone (8 trials, 1513 participants; RR 2.04, 95% CI 1.10 to 3.81) and TMP‐SMZ subgroups (5 trials, 305 participants; RR 3.63, 95% CI 1.32 to 9.98).
12.1. Analysis.
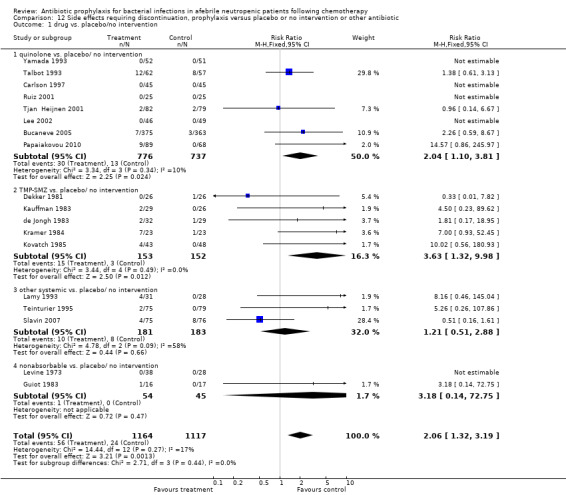
Comparison 12 Side effects requiring discontinuation, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 1 drug vs. placebo/no intervention.
13. Fungal infection
There was no statistically significant difference in the number of episodes of fungal infection when prophylactic antibiotics were compared to placebo (39 trials, 2887 participants; RR 1.04, 95% CI 0.82 to 1.33) (Analysis 13.1).
13.1. Analysis.
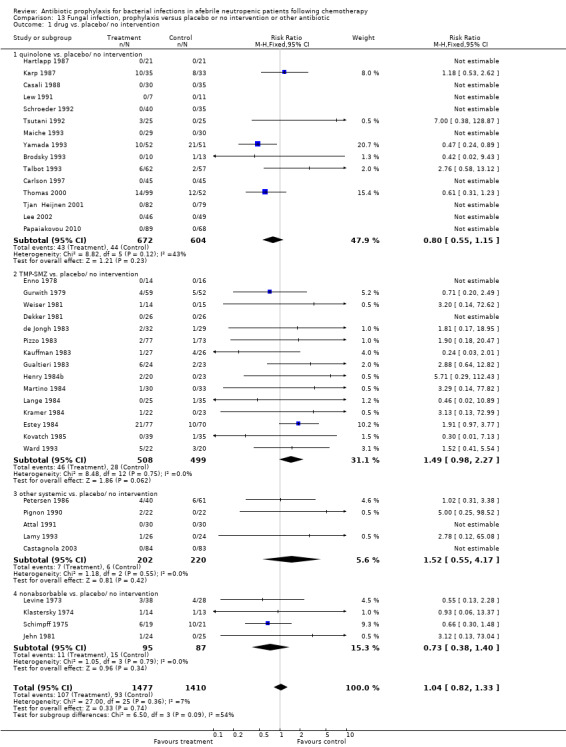
Comparison 13 Fungal infection, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 1 drug vs. placebo/ no intervention.
14. Resistance to antibiotics
For this comparison, the numerator was the number of episodes in which bacilli resistant to the specific drug (quinolones or TMP‐SMZ) were grown in cultures during follow‐up, and the denominator was the number of patients evaluated. When compared to placebo, participants receiving antibiotics were more likely to harbour resistant bacteria to the specific drug (19 trials, 3629 participants; RR 1.47, 95% CI 1.08 to 2.01) (Analysis 14.1; Figure 7). This applied specifically to the TMP‐SMZ subgroup (11 trials, 917 participants; RR 2.42, 95% CI 1.35 to 4.36). With quinolones there was no statistically significant difference between study groups.
14.1. Analysis.
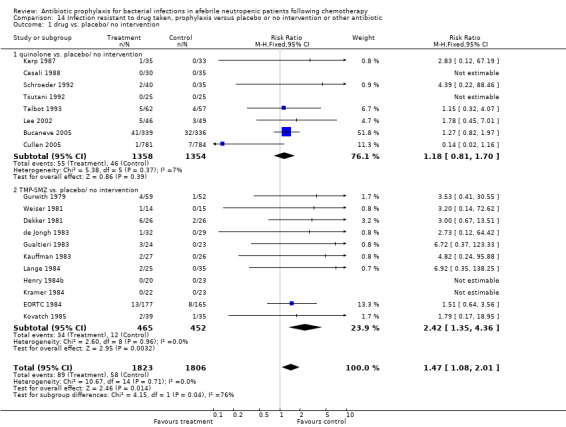
Comparison 14 Infection resistant to drug taken, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 1 drug vs. placebo/ no intervention.
7.
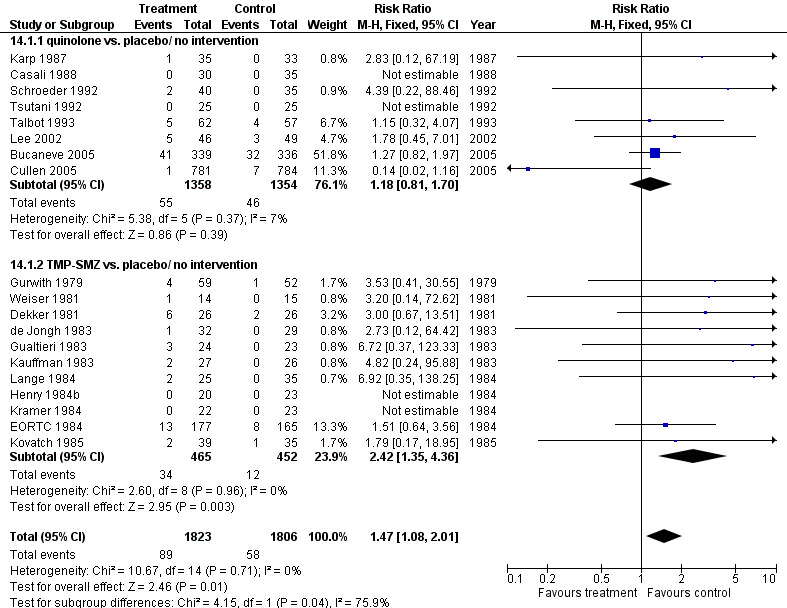
Forest plot of comparison: 14 Infection resistant to drug taken, prophylaxis vs. placebo/no intervention or other antibiotic, outcome: 14.1 drug vs. placebo/ no intervention.
15. Hospitalisations and fever days
Data on the number of hospitalisations, length of hospital stay and days of fever were too sparse for meta‐analyses.
Antibiotic versus antibiotic
Primary outcome
1. All‐cause mortality
There was no significant difference in all cause mortality between participants receiving quinolones compared with TMP‐SMZ (10 trials, 917 participants; RR 1.07, 95% CI 0.66 to 1.72) (Analysis 1.2). The last study was conducted in 1995. Ten studies (1474 participants) compared quinolones to quinolones plus prophylactic antibiotics active against Gram‐positive pathogens (Analysis 1.3). The addition of an antibiotic against Gram‐positive infection yielded no statistical significant difference (RR 1.28, 95% CI 0.69 to 2.38). When nonabsorbable antibiotics were compared to systemic antibiotics, again there was no difference in the risk for mortality in trials conducted between 1983 and 2001 (8 trials, 813 participants; RR 1.06, 95% CI 0.74 to 1.50) (Analysis 1.5). In two trials there was no advantage with the addition of nonabsorbable antibiotics to systemic antibiotic (Analysis 1.6). Six studies compared the different quinolones but no significant statistical differences were found (Table 3; Table 4). These studies were not summarised in a meta‐analysis.
1.2. Analysis.
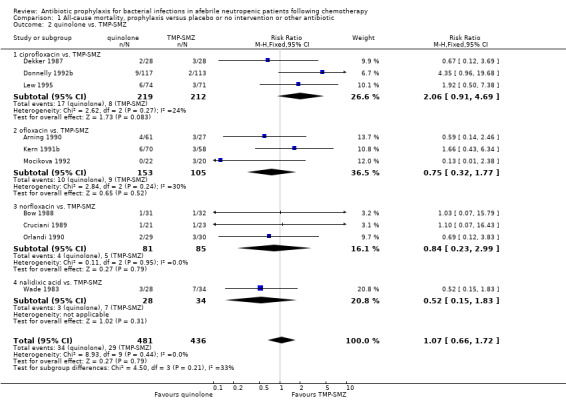
Comparison 1 All‐cause mortality, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 2 quinolone vs. TMP‐SMZ.
1.3. Analysis.
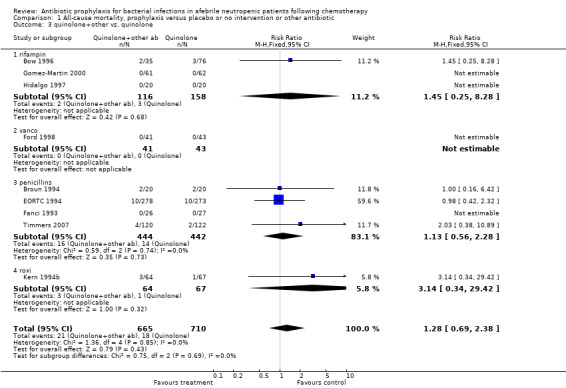
Comparison 1 All‐cause mortality, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 3 quinolone+other vs. quinolone.
1.5. Analysis.
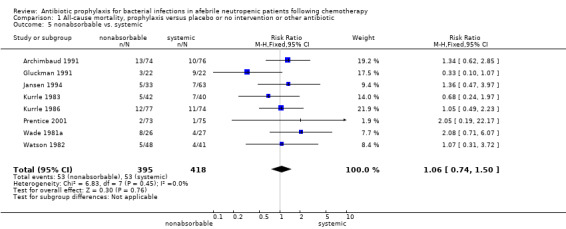
Comparison 1 All‐cause mortality, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 5 nonabsorbable vs. systemic.
1.6. Analysis.
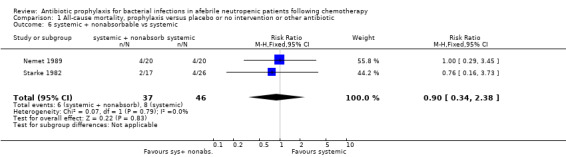
Comparison 1 All‐cause mortality, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 6 systemic + nonabsorbable vs systemic.
2. Other studies ‐ continued.
| Study ID | Intervention 1 | Intervention 2 | Bacteremia | Gram neg bacteraemia | Gram pos bacteraemia | Side effects | S/E requiring D/C | fungal infection | Inf.res. to quinolon |
| D'Antonio 1994 | ciprofloxacin | ofloxacin | 1.03(0.35‐3.04) | 0.15(0.01‐2.79) | 2.05(0.53‐7.92) | 1.32(0.52‐3.37) | 0.96(0.50‐1.85) | ||
| GIMEMA 1991 | ciprofloxacin | norfloxacin | 0.77(0.53‐1.13) | 0.57(0.23‐1.42) | 0.84(0.52‐1.36) | 1.58(0.75‐3.33) | 1.7(0.75‐3.84) | 1.06(0.22‐5.23) | 0.37(0.16‐0.87) |
| Maschmeyer 1988 | ciprofloxacin | norfloxacin | 0.92(0.21‐4.11) | 0.92(0.21‐4.11) | 0.46(0.04‐4.74) | 0.18(0.01‐3.65) | 0.31(0.01‐7.2) | ||
| D'Antonio 1991 | norfloxacin | ofloxacin | 6.17(0.78‐48.68) | 5.14(0.26‐103.37) | 4.11(0.48‐35.02) | 2.06(0.2‐21.68) | 3.08(0.13‐73.23) | 2.4(1.04‐5.53) | |
| D'Antonio 1992 | norfloxacin | pefloxacin | 2.47(0.92‐6.64) | 4.12(0.47‐35.91) | 2.27(0.83‐6.17) | 0.69(0.2‐2.32) | 2.06(0.19‐22.18) | 2.15(1.18‐3.91) | |
| Bender 1979 | gentamycin+vancomycin | gentamycin | 1.11(0.48‐2.55) | 1.48(0.38‐5.74) | 0.56(0.05‐5.62) |
Secondary outcomes
2. Infection‐related mortality
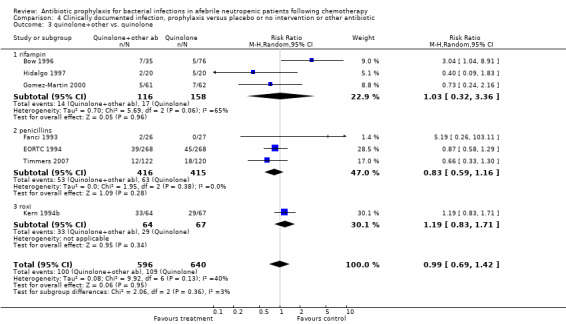
Eleven studies including 1019 participants compared quinolones with TMP‐SMZ. No statistically significant difference was found (RR 0.91, 95% CI 0.54 to 1.54) (Analysis 2.2). Ten studies including 1474 participants compared quinolones to quinolones plus prophylactic antibiotics active against Gram‐positive pathogens. The addition of antibiotic against Gram‐positive infection yielded no advantage in terms of infection‐related mortality (RR 1.01, 95% CI 0.56 to 1.81) (Analysis 2.3). Eleven studies which included 1005 patients compared nonabsorbable antibiotics to systemic antibiotics. For this comparison, there was a significant decrease in infection‐related mortality in favour of the systemic antibiotics arm (RR 2.48, 95% CI 1.65 to 3.73) (Analysis 2.5).
2.2. Analysis.
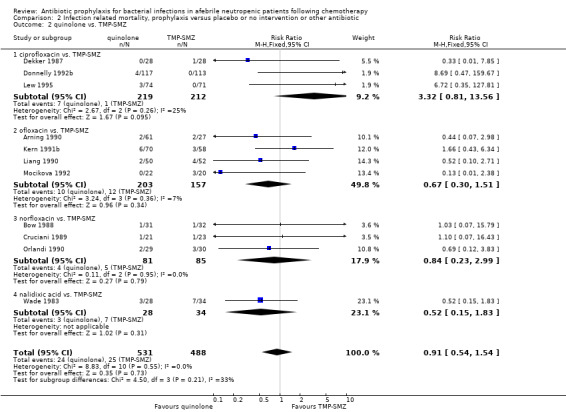
Comparison 2 Infection related mortality, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 2 quinolone vs. TMP‐SMZ.
2.3. Analysis.
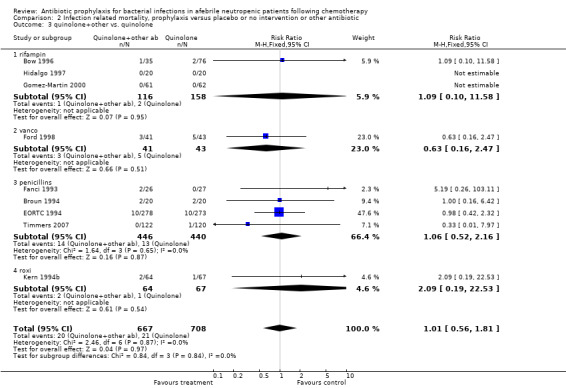
Comparison 2 Infection related mortality, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 3 quinolone+other vs. quinolone.
2.5. Analysis.
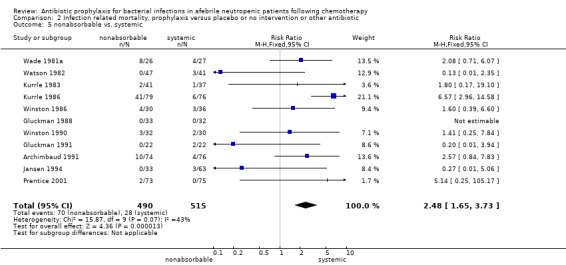
Comparison 2 Infection related mortality, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 5 nonabsorbable vs. systemic.
3. Febrile episodes
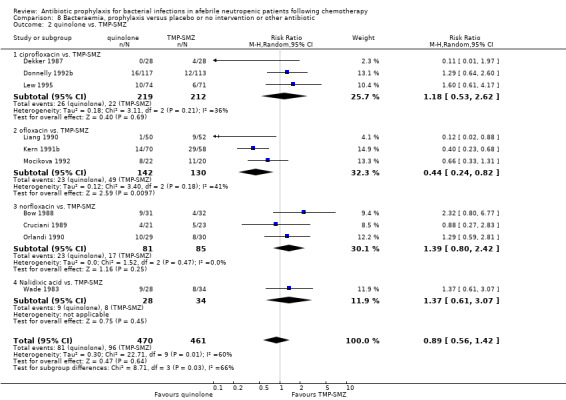
Ten studies including 931 participants compared quinolones with TMP‐SMZ, with no statistically significant difference (RR 0.92, 95% CI 0.78 to 1.09) (Analysis 3.2). The addition of an antibiotic against Gram‐positive infection yielded no statistically significant difference either (8 trials, 1375 participants; RR 1.03, 95% CI 0.97 to 1.11) (Analysis 3.3).
3.2. Analysis.
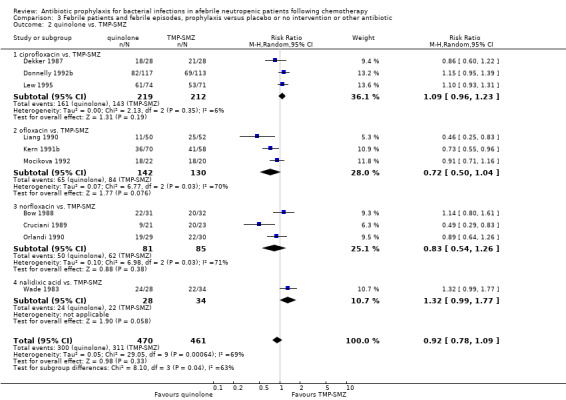
Comparison 3 Febrile patients and febrile episodes, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 2 quinolone vs. TMP‐SMZ.
3.3. Analysis.
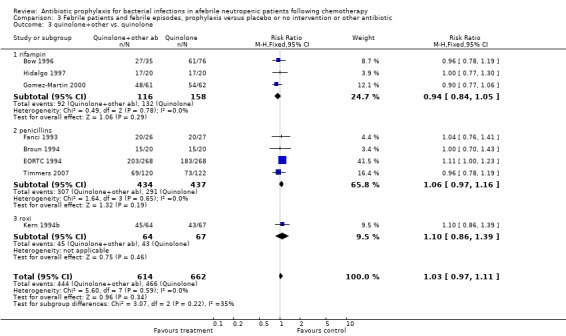
Comparison 3 Febrile patients and febrile episodes, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 3 quinolone+other vs. quinolone.
4. Clinically documented infection
Ten studies including 931 participants compared quinolones with TMP‐SMZ. A statistically significant difference in favour of TMP‐SMZ was shown (RR 1.33, 95% CI 1.06 to 1.66) (Analysis 4.2). The addition of an antibiotic against Gram‐positive infections yielded no statistically significant difference (7 studies, 1335 patients; RR 0.99, 95% CI 0.69 to 1.42) (Analysis 4.3).
4.2. Analysis.
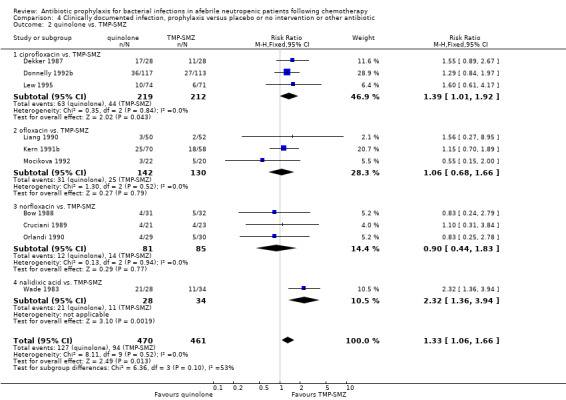
Comparison 4 Clinically documented infection, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 2 quinolone vs. TMP‐SMZ.
4.3. Analysis.
Comparison 4 Clinically documented infection, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 3 quinolone+other vs. quinolone.
5 to 7. Microbiologically documented infection
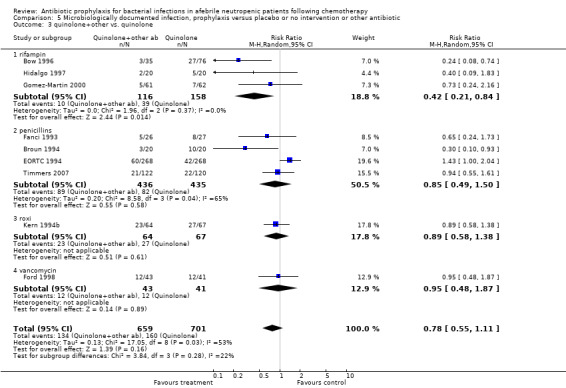
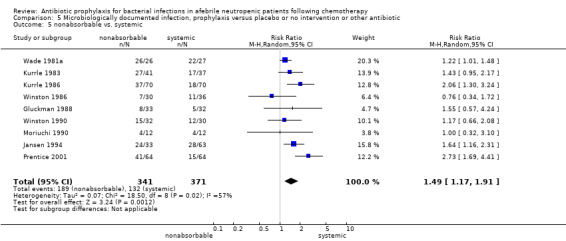
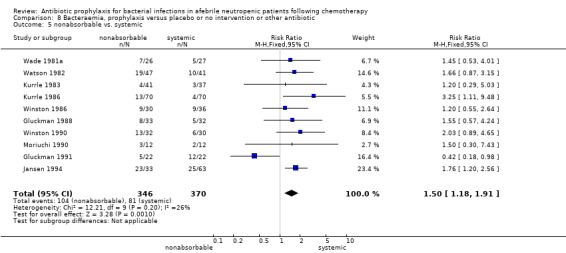
Eleven studies including 1019 participants compared quinolones with TMP‐SMZ. There was a trend to a reduction in microbiologically documented infection in the quinolone group (RR 0.75, 95% CI 0.56 to 1.01) (Analysis 5.2) and with the addition to quinolones of an antibiotic against Gram‐positive infection (RR 0.78, 95% CI 0.55 to 1.11) (Analysis 5.3). There was a clear benefit of systemic antibiotics when compared to nonabsorbable antibiotics (RR 1.49, 95% CI 1.17 to 1.91) (Analysis 5.5).
5.2. Analysis.
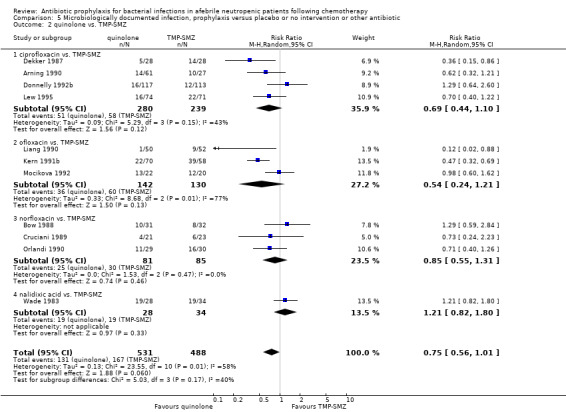
Comparison 5 Microbiologically documented infection, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 2 quinolone vs. TMP‐SMZ.
5.3. Analysis.
Comparison 5 Microbiologically documented infection, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 3 quinolone+other vs. quinolone.
5.5. Analysis.
Comparison 5 Microbiologically documented infection, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 5 nonabsorbable vs. systemic.
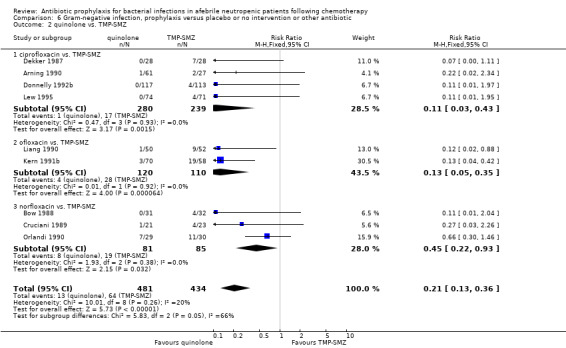
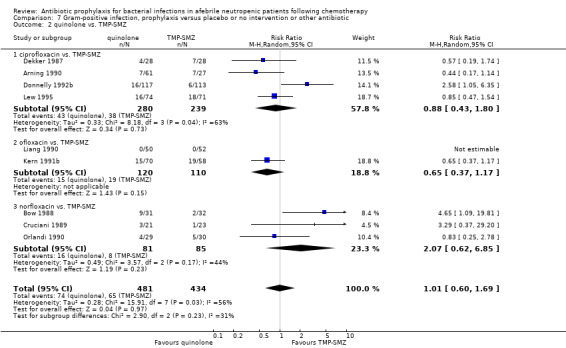
Quinolones resulted in a significant reduction in Gram‐negative infection compared with TMP‐SMZ prophylaxis (9 trials, 915 participants; RR 0.21, 95% CI 0.13 to 0.36; Analysis 6.2) but not Gram‐positive infections (9 trials, 915 participants; RR 1.01, 95% CI 0.60 to 1.69) (Analysis 7.2). The addition of an antibiotic against Gram‐positive infection to quinolones resulted in a significant decrease in documented Gram‐positive infection (7 trials, 740 participants; RR 0.40, 95% CI 0.22 to 0.72) (Analysis 7.3).
6.2. Analysis.
Comparison 6 Gram‐negative infection, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 2 quinolone vs. TMP‐SMZ.
7.2. Analysis.
Comparison 7 Gram‐positive infection, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 2 quinolone vs. TMP‐SMZ.
7.3. Analysis.
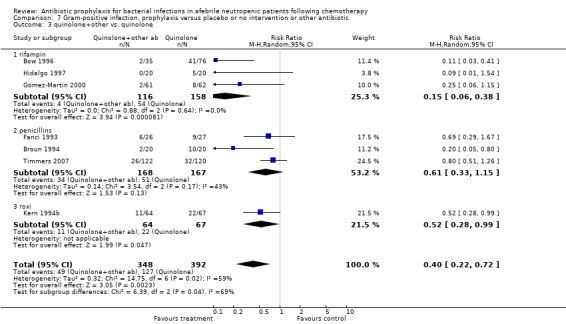
Comparison 7 Gram‐positive infection, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 3 quinolone+other vs. quinolone.
8 to 10. Bacteraemia
There was no significant difference in bacteraemia when quinolones were compared to TMP‐SMZ (10 trials, 931 participants; RR 0.89, 95% CI 0.56 to 1.42) (Analysis 8.2), however, the addition of an antibiotic against Gram‐positive infection to quinolones resulted in a significant decrease in bacteraemic episodes (8 trials, 824 participants; RR 0.74, 95% CI 0.56 to 0.97) (Analysis 8.6). There was also a clear benefit of other systemic antibiotics over nonabsorbable ones (10 trials, 716 participants; RR 1.50, 95% CI 1.18 to 1.91) (Analysis 8.5).
8.2. Analysis.
Comparison 8 Bacteraemia, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 2 quinolone vs. TMP‐SMZ.
8.6. Analysis.
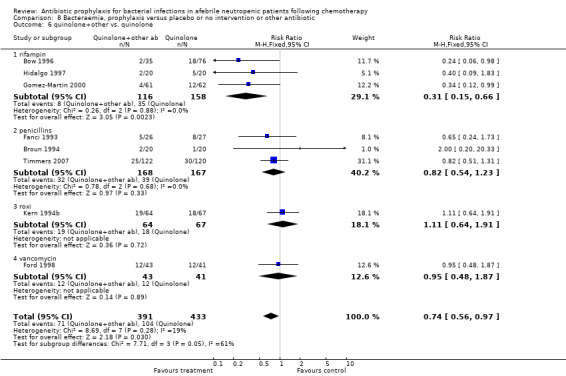
Comparison 8 Bacteraemia, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 6 quinolone+other vs. quinolone.
8.5. Analysis.
Comparison 8 Bacteraemia, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 5 nonabsorbable vs. systemic.
Quinolone prophylaxis resulted in a significant reduction in Gram‐negative bacteraemia compared to TMP‐SMZ prophylaxis (10 trials, 931 participants; RR 0.35, 95% CI 0.13 to 0.93) (Analysis 9.2) but there was no significant difference between them with regard to Gram‐positive bacteraemia (10 trials, 931 participants; RR 1.24, 95% CI 0.86 to 1.60) (Analysis 10.2). The addition of an antibiotic against Gram‐positive infections to quinolones resulted in a significant reduction in documented Gram‐positive bacteraemia (8 trials, 824 participants; RR 0.61, 95% CI 0.44 to 0.83) (Analysis 10.3).
9.2. Analysis.
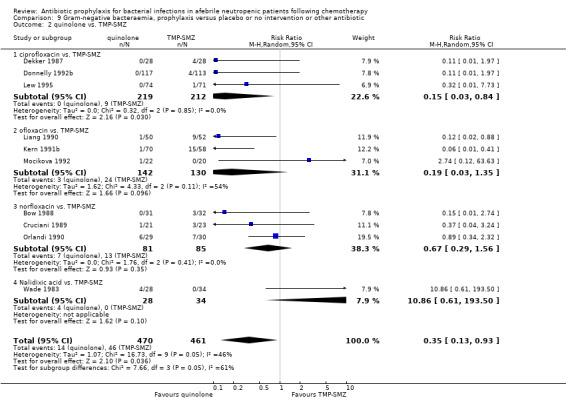
Comparison 9 Gram‐negative bacteraemia, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 2 quinolone vs. TMP‐SMZ.
10.2. Analysis.
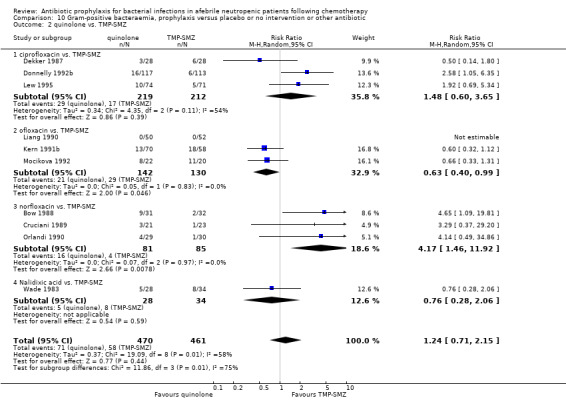
Comparison 10 Gram‐positive bacteraemia, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 2 quinolone vs. TMP‐SMZ.
10.3. Analysis.
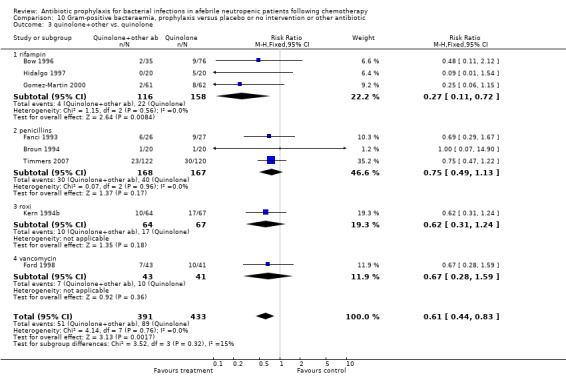
Comparison 10 Gram‐positive bacteraemia, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 3 quinolone+other vs. quinolone.
11 to 12. Side effects
When compared to TMP‐SMZ, quinolones caused fewer side effects (10 trials, 1027 participants; RR 0.62, 95% CI 0.43 to 0.90) (Analysis 11.2). There was a trend towards increased side effects with the addition to quinolones of an antibiotic against Gram‐positive infection (6 trials, 516 participants; RR 2.69, 95% CI 0.78 to 9.27) (Analysis 11.3).
11.2. Analysis.
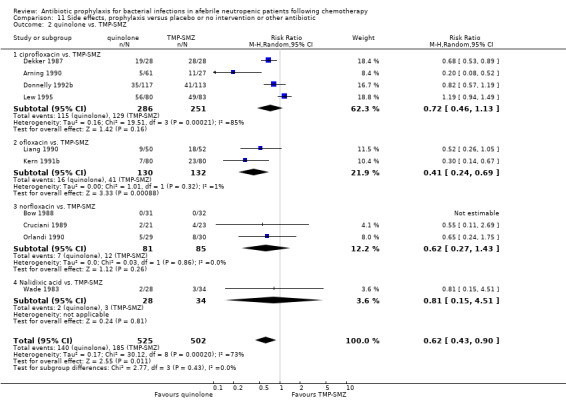
Comparison 11 Side effects, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 2 quinolone vs. TMP‐SMZ.
11.3. Analysis.
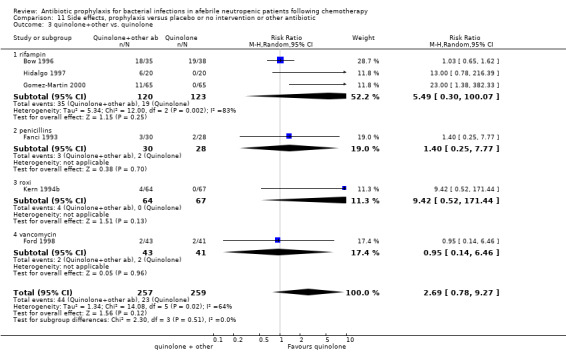
Comparison 11 Side effects, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 3 quinolone+other vs. quinolone.
Compared to TMP‐SMZ, quinolones caused fewer side effects requiring discontinuation (7 trials, 850 participants; RR 0.37, 95% CI 0.16 to 0.87) (Analysis 12.2). The addition to quinolones of antibiotic against Gram‐positive infection significantly increased side effects requiring discontinuation (RR 4.92, 95% CI 1.61 to 15.01) (Analysis 12.3).
12.2. Analysis.
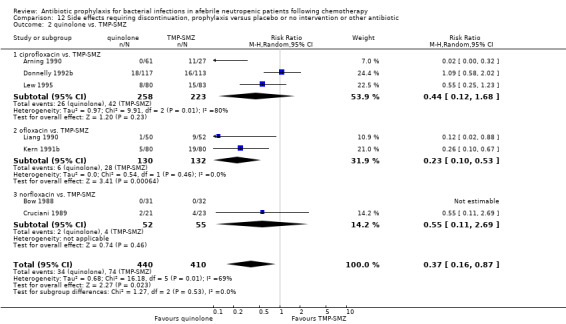
Comparison 12 Side effects requiring discontinuation, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 2 quinolone vs. TMP‐SMZ.
12.3. Analysis.
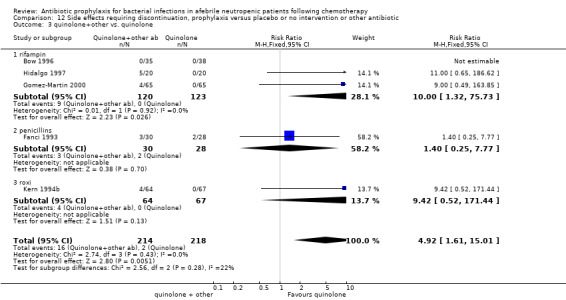
Comparison 12 Side effects requiring discontinuation, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 3 quinolone+other vs. quinolone.
13. Fungal infection
When quinolones were compared to TMP‐SMZ, no significant difference was found (10 trials, 789 participants; RR 0.65, 95% CI 0.36 to 1.16) (Analysis 13.2).
13.2. Analysis.
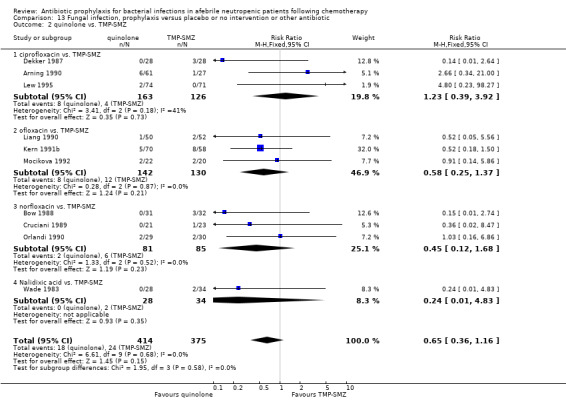
Comparison 13 Fungal infection, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 2 quinolone vs. TMP‐SMZ.
14. Resistance to antibiotics
For this comparison the numerator was the number of episodes in which bacilli resistant to the specific drug (quinolones or TMP‐SMZ) were grown in cultures, and the denominator was the number of evaluable patients. In studies comparing quinolones to TMP‐SMZ, less resistance to quinolones was observed following treatment with quinolones than resistance to TMP‐SMZ following treatment with TMP‐SMZ (6 trials, 366 participants; RR 0.45, 95% CI 0.27 to 0.74) (Analysis 14.2; Figure 8).
14.2. Analysis.
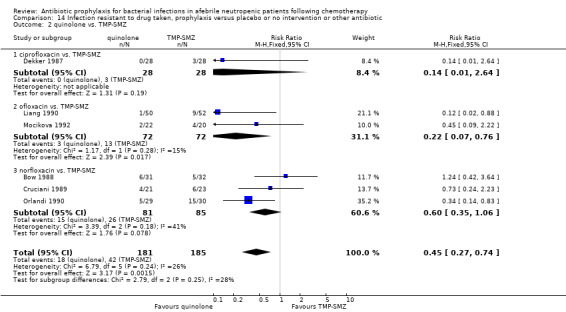
Comparison 14 Infection resistant to drug taken, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 2 quinolone vs. TMP‐SMZ.
8.
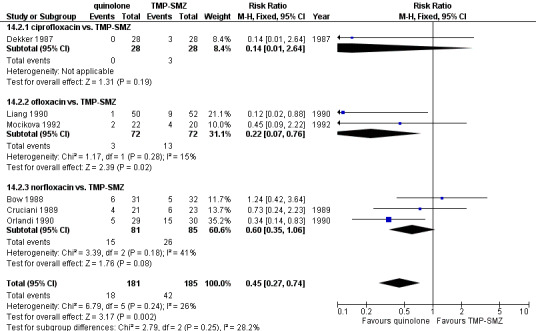
Forest plot of comparison: 14 Infection resistant to drug taken, prophylaxis vs. placebo/no intervention or other antibiotic, outcome: 14.2 quinolone vs. TMP‐SMZ.
Discussion
Summary of main results
In studies comparing antibiotic prophylaxis to placebo or no treatment in neutropenic patients, prophylaxis significantly reduced all‐cause mortality and infection‐related mortality. We estimated the NNT with antibiotic prophylaxis in order to prevent one death from all causes as 34 (95% CI 26 to 56). Prophylaxis significantly reduced febrile episodes. Patients receiving prophylaxis also experienced fewer clinically documented infections, fewer microbiologically documented infections, fewer Gram‐negative infections, fewer Gram‐positive infections, fewer episodes of bacteraemia, fewer episodes of Gram‐negative bacteraemia, and fewer episodes of Gram‐positive bacteraemia than patients who did not receive prophylaxis (Table 1). Side effects were increased by administration of prophylaxis, as was the development of resistance to the antibiotic regimen concerned.
When quinolones were compared to TMP‐SMZ, there was no significant difference in all‐cause mortality, febrile episodes or bacteraemia (Table 2), however Gram‐negative infections, Gram‐negative bacteraemia and side effects were significantly reduced.
The addition to quinolones of an antibiotic against Gram‐positive infection resulted in a significant decrease in the number of bacteraemic episodes, Gram‐positive infections, and Gram‐positive bacteraemia but an increase in side effects and no reduction in mortality.
Systemic antibiotics were more efficient than nonabsorbable ones in reducing the number of febrile patients, clinically documented infections, microbiologically documented infections, Gram‐negative infections, Gram‐positive infections, episodes of bacteraemia, episodes of Gram‐negative bacteraemia and episodes of Gram‐positive bacteraemia; however, side effects were increased.
Overall completeness and applicability of evidence
Overall, most trials included haematological patients and so our results apply mainly to this group. The haematological patients included mainly acute leukaemia and patients undergoing haematopoietic cell transplant. The group of patients with solid tumours or lymphoma was small and clinically heterogenous, including tumours of the lung, ovary, breast, testicular and other.
Our assessment of treatment effect supports quinolones as the prophylaxis of choice since they reduced the risk of death when compared to placebo or no intervention. This reduction occurred for patients with haematological malignancies (acute leukaemia and patients undergoing haematopoietic cell transplantation) and for patients with solid tumours and lymphoma. Quinolones are an attractive option for prophylaxis in neutropenic patients due to their broad antimicrobial spectrum, preservation of the anaerobic flora of the alimentary tract, high concentration in the faeces, systemic bactericidal activity, good tolerability and lack of myelosuppression (Engels 1998).
The majority of patients in the trials in our review were treated with either levofloxacin or ciprofloxacin. All types of quinolones reduced mortality when compared to placebo or no intervention except for norfloxacin. Furthermore, the efficacy of quinolone prophylaxis did not decrease in studies published in later years, with even a larger effect of quinolone prophylaxis on mortality reported than in older studies (although not statistically significant).
Our study demonstrates that quinolones also reduced the risk of infection‐related mortality, fever, clinically documented infections, microbiologically documented infections, Gram‐negative infections, Gram‐positive infections and bacteraemia when compared to placebo or no intervention. In addition, they reduced the risk for microbiologically documented infections, Gram‐negative infections and Gram‐negative bacteraemia and had fewer side effects when compared to TMP‐SMZ. A frequent misconception is that quinolone prophylaxis increases the incidence of Gram‐positive bacteraemia. Our meta‐analyses show that Gram‐positive bacteraemia is not significantly altered by quinolone prophylaxis.
One of the major concerns raised in regard to treatment with quinolones is the emergence of resistance and outbreaks of infections due to resistant organisms, such as coagulase‐negative Staphylococci (Oppenheim 1989) and E. coli (Kern 1994). When quinolones were compared to placebo or no intervention in our review there was no significant difference in the number of patients developing infections caused by organisms resistant to quinolones. Because the overall mortality was reduced by prophylaxis, the danger of infection caused by resistant pathogens to a particular patient evidently was much smaller than the gain. In studies in which quinolones were compared to TMP‐SMZ, resistance to the quinolone following quinolone treatment was less than resistance to TMP‐SMZ following treatment with TMP‐SMZ (RR 0.45, 95% CI 0.27 to 0.74). Furthermore, development of resistance to quinolones is not necessarily associated with development of resistance to other antibiotics which are administered for treatment of febrile neutropenia (Gentry 2002).
The addition to quinolones of antibiotics with coverage against Gram‐positive pathogens resulted in reduction of microbiologically documented infections, total episodes of bacteraemia and Gram‐positive bacteraemia. However, considering the lack of clear benefits in terms of mortality, it is probably not reasonable to recommend the addition of Gram‐positive coverage.
Hughes 1977 have shown that TMP‐SMZ is highly effective in the prevention of Pneumocystis pneumonia (PCP) among cancer patients at high risk of this infection. Currently identified risk factors for Pneumocystis jirovecii infections among cancer patients include prolonged corticosteroid therapy (equivalents of 20 mg prednisolone for over a month); intense chemotherapy, particularly with haematologic malignancies or mediastinal irradiation; and lymphopenia (Hughes 1977; Roblot 2004; Worth 2005). We could not assess the effect of TMP‐SMZ or other antibiotics on PCP since the studies included in our review do not report this particular infection among the outcomes assessed. PCP is not included within the fungal infections outcome since its classification within this class succeeded most of these studies. TMP‐SMZ prophylaxis should be administered to patients at high risk for PCP. The dosing schedule should follow that used in the studies included in our review (for example daily administration) to gain the survival benefit of TMP‐SMZ. The addition of quinolones to TMP‐SMZ has not been assessed in enough studies to draw conclusions. Thus, this decision should be based on local susceptibility patterns. We recommend that quinolones be added to TMP‐SMZ in locations where the prevalence of Gram‐negative bacteria resistant to TMP‐SMZ is high.
Combinations of oral nonabsorbable drugs which were studied in early studies have since been abandoned due to poor tolerance and low patient compliance (Hughes 2002). Our results support this, as oral nonabsorbable drugs were less efficient when compared to other regimens. TMP‐SMZ was also frequently used, although many centres have stopped its use due to possible prolongation of neutropenia, adverse reactions caused by sulfonamide drugs, development of drug‐resistant bacteria and oral candidiasis (Hughes 2002).
All‐cause mortality encompasses the personal harm associated with prophylactic antibiotic administration, side effects, and the emergence of resistant micro‐organisms. The number needed to treat to prevent one death (34) compares favourably with other interventions well accepted in medical practice. Thus, antibiotic prophylaxis for patients similar to those included in our review is clearly indicated. Even the reduction in the incidence of fever carries important implications, since the occurrence of fever in neutropenic patients prompts additional use of broad‐spectrum antibiotics, with the associated drawbacks.
Potential biases in the review process
Several limitations of our analyses should be noted.
We could obtain data on all‐cause mortality for only 47 studies out of 64 studies that compared antibiotic prophylaxis to placebo. Among studies that did not report mortality are some of the larger studies (EORTC 1984; EORTC 1994; GIMEMA 1991).
Data regarding the time period during which mortality was assessed were scarce and varied among the trials that reported it.
Many studies in our review are old. However, it seems that the RRs for efficacy and developing infections caused by quinolone‐resistant bacteria did not change over the years.
Length of follow‐up may have been too short to detect emergence of resistant bacteria and resistance data were not routinely collected in these studies. To actually assess the risk for resistance development studies must perform surveillance cultures prior to and following antibiotic treatment. None of these studies assessed resistance development.
Most studies assessed prophylaxis that was started at the onset of chemotherapy (rather than onset of neutropenia). Future studies should assess whether antibiotics started at onset of neutropenia are as effective, to limit unnecessary exposure to antibiotics.
Most studies were limited to haematological cancer patients. Seventy‐nine studies were conducted on inpatients. RRs for mortality obtained for patients with solid cancer or outpatients were not significantly different from those seen for haematological inpatients. However, the latter group of patients was smaller and should be studied further. Moreover, data regarding the specific chemotherapeutic protocols were scarce in the original trials and were not extracted.
Agreements and disagreements with other studies or reviews
Several previous meta‐analyses (Cruciani 1996; Cruciani 2003; Engels 1998; Rotstein 1997) have studied the efficacy of various prophylactic regimens compared to placebo or a to a different treatment regimen. All of these reviews demonstrated a reduced incidence of various infection‐related outcomes, but none demonstrated a significant effect of prophylaxis on mortality. Another recent meta‐analysis included only RCTs which were double blind and compared quinolones to placebo, only in adult patients (Imran 2008). It included eight trials. There was a statistically nonsignificant reduction in mortality with quinolone prophylaxis. These reviews included up to 20 trials while our review, which includes 64 trials comparing prophylaxis versus placebo or no treatment, has the power to detect a significant effect.
Before our original systematic review was published, there was no consensus to recommend antibiotic prophylaxis for afebrile neutropenic patients according to the IDSA guidelines (Hughes 2002). After our publication, several guidelines have changed their recommendation, taking into account the benefit of reduction in mortality. The recent Infectious Disease Society of America guidelines for antibiotic treatment in neutropenic patients with cancer, updated in 2010, now recommend antibiotic prophylaxis (Freifeld 2011). According to the guidelines, quinolone prophylaxis should be considered for high‐risk patients with expected durations of prolonged and profound neutropenia (ANC <100 cells/mm3 for more than 7 days). The First European Conference on Infections in Leukaemia (ECIL1) published their guidelines in July 2007 (Cordonnier 2007). They found quinolone prophylaxis to be effective in preventing bacterial infection and in reducing mortality in acute leukaemia and HSCT recipient afebrile neutropenic patients, and recommended ciprofloxacin or levofloxacin as the drug of choice.
Authors' conclusions
Implications for practice.
These updated findings support antibiotic prophylaxis, preferably with a quinolone where resistance permits, for routine use in neutropenic patients because it reduces mortality. We recommend levofloxacin or ciprofloxacin for this purpose, however the decision to use prophylaxis and the type of drugs, whether a quinolone or a combination of a quinolone and a drug effective against Gram‐positive bacteria, should be taken based on the local profile of pathogens in neutropenic patients and their susceptibility to antibiotics.
Prophylaxis is strongly recommended in patients with haematological malignancies, who are usually at higher risk for infection. However, a reduction in mortality was shown for patients with solid tumours or lymphoma as well and therefore these patients are likely to benefit from prophylactic antibiotics. Since studies of these patients were few and they were clinically heterogenous (different diseases), further research is needed to identify which patients with solid tumours or lymphoma may benefit the most.
Centres that implement prophylaxis should institute surveillance measures to monitor quinolone‐resistant Gram‐negative bacteria, as well as rates of other resistant organisms (vancomycin resistant enterococci (VRE), methicillin resistant S.aureus (MRSA) and Clostridium difficile.
Implications for research.
Current evidence points to an advantage in survival with antibiotic prophylaxis. Therefore, further RCTs comparing prophylaxis to placebo or no intervention are probably not warranted.
Although the evidence in favour of antibiotic prophylaxis is convincing, many of the studies included in the present analysis were of uncertain methodological quality and some of them were quite old. Thus an argument could be made in favour of a contemporary trial evaluating and comparing quinolones that has adequate randomisation, allocation concealment and blinding, and is powered to detect a difference in mortality. However, given that the mortality rates in recent trials of febrile neutropenic patients range between 1% to 8% (Cherif 2004; De Pauw 1994; Giamarellou 2000; Rolston 1992), a RCT powered to demonstrate a difference in mortality due to prophylaxis is probably not feasible since it would require an inordinately large sample size.
Further research should define which patients with solid tumours or lymphoma may benefit from prophylaxis. This could be assessed by conducting RCTs that include patients with a specific malignancy, or according to different chemotherapy protocols. Further studies should determine the advantage of antibiotic prophylaxis in the first chemotherapy cycle versus the next cycles. Data on all‐cause mortality should be reported, even if not as a primary outcome. Assessment of resistance development should be carefully planned and performed.
Future studies should assess whether antibiotics started at the onset of neutropenia are as effective as antibiotics started with chemotherapy, to limit unnecessary exposure to antibiotics.
What's new
| Date | Event | Description |
|---|---|---|
| 17 July 2018 | Amended | Next stage expected date amended |
| 28 June 2018 | Review declared as stable | Conclusions unlikely to change with the addition of new studies. |
History
Protocol first published: Issue 3, 2003 Review first published: Issue 4, 2005
| Date | Event | Description |
|---|---|---|
| 12 October 2011 | New citation required but conclusions have not changed | Eight new trials included:Garcia Saenz 2002, Lalami 2004, Rafecas 1989, Slavin 2007, Timmers 2007, Rahman 2009, Dickgreber 2009, Papaiakovou 2010. Ten newly identified trials excluded. New authors (LV and TL) added. |
| 1 March 2011 | New search has been performed | Search updated |
| 30 July 2009 | Amended | Tables linked to text |
| 19 August 2008 | Amended | Converted to new review format. |
| 29 July 2005 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We thank the Cochrane Gynaecological Cancer Review Group (CGCRG) for their support during the review process. In addition thanks to Jane Hayes for help with the search, and thanks to Clare Jess and Gail Quinn.
We thank the following authors who responded to our request for additional data:
A Casali, AW Dekker, E Estey, R Fanci, C Ford, JA Garcia‐Saenz, HFL Guiot, C Harman, J Harousseau, H Heimpel, J Jansen, J Karp, CA Kauffman, J Klastersky, B Kramer, T Lamy, G Maschmeyer, I Nenova, G Prentice, M M Rahman, FJ Rafecas, A Ronald, MA Slavin, X Thomas, J van Eys, T Ward, T Yamada, and SH Zinner.
We thank the CGCRG Co‐ordinating Editor Chris Williams and the peer reviewers M Clarke, M Cruciani, K Godfrey, and W Hughes for their helpful comments.
Appendices
Appendix 1. Methodology of the original 2005 review
Searches
Relevant RCTs were identified by searching The Cochrane Cancer Network Register of Trials (Oct 2005), Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library Issue 4, 2005), MEDLINE (January 1966 to Oct 2005), EMBASE (January 1980 to Oct 2005), and the following conference proceedings: Abstracts of the Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC) (1995 to 2004), Annual Meetings of the Infectious Diseases Society of America (IDSA) (2001 to 2004) and European Congress of Clinical Microbiology and Infectious Diseases (ECCMID) (2001 to 2004).
MEDLINE was searched using the following search phrase, which was adapted for searching the other databases: (((neutropenia*:ME or agranulocytosis*:ME or neutropenia* or neutropeni* or neutropaeni* or agranulocyt*) and ( quinolone:ME or trimethoprim‐sulfamethoxazole:ME or vancomycin:ME or quinolone* or trimethoprim‐sulfamethoxazole* or vancomycin* or antibiotic* or antimicrobial* or anti‐microbial* or antibacterial* or anti‐bacterial*) and (antibiotic prophylaxis*:ME or prophyla* or preventi*)))
The references of all identified studies were inspected for more trials. Additionally, the first or corresponding author of each included trial and the researchers active in the field were contacted for information regarding unpublished trials or complementary information on their own trial.
Risk of bias assessment
Trials fulfilling the review inclusion criteria were assessed for methodological quality by two authors (AGG and AF) independently. Trials which exceeded a threshold of 30% dropouts were excluded. We extracted information about randomisation and allocation concealment, blinding, sample size, exclusions after randomisation, and different lengths of follow‐up. This was done using the criteria described in the Cochrane Reviewer's handbook (Higgins 2005), which were based on the empirical evidence of a strong association between poor allocation concealment and overestimation of effect (Schulz 1995). This is defined as below:
A. Low risk of bias (adequate allocation concealment) B. Unclear risk of bias (unclear allocation concealment) C. High risk of bias (inadequate allocation concealment, i.e. quasi‐randomised studies)
In addition, sensitivity analyses was performed in order to assess the robustness of the findings to different aspects of the trials' methodology: randomisation generation, allocation concealment (adequate or unclear), blinding. Sensitivity analysis was conducted for the comparison of antibiotic versus placebo or no intervention. No data were found for conducting sensitivity analysis according to length of follow up (up to one month or one to 12 months).
Appendix 2. MEDLINE search strategy
1 Neutropenia/ 2 (neutropaeni* or neutropeni*).mp. 3 1 or 2 4 exp Antibiotic Prophylaxis/ 5 (antibiotic* or antimicrobial* or anti‐microbial* or antibacterial* or anti‐bacterial*).mp. 6 exp Quinolones/ 7 quinolone*.mp. 8 ciprofloxacin.mp. 9 ofloxacin.mp. 10 norfloxacin.mp. 11 pefloxacin.mp. 12 exp Trimethoprim‐Sulfamethoxazole Combination/ 13 trimethoprim‐sulfamethoxazole.mp. 14 TMP‐SMZ.mp. 15 exp Aminoglycosides/ 16 aminoglycoside*.mp. 17 gentamicin.mp. 18 neomycin.mp. 19 tobramycin.mp. 20 exp Colistin/ 21 colistin.mp. 22 exp Polymyxins/ 23 polymyxin*.mp. 24 exp Rifampin/ 25 rifampin.mp. 26 exp Cephalosporins/ 27 cephalosporin*.mp. 28 ceftriaxone.mp. 29 exp Vancomycin/ 30 vancomycin.mp. 31 or/5‐30 32 (prophyla* or prevent*).mp. 33 31 and 32 34 4 or 33 35 3 and 34 36 randomized controlled trial.pt. 37 controlled clinical trial.pt. 38 randomized.ab. 39 placebo.ab. 40 drug therapy.fs. 41 randomly.ab. 42 trial.ab. 43 groups.ab. 44 or/36‐43 45 35 and 44 46 (animals not (humans and animals)).sh. 47 45 not 46
key: mp=title, original title, abstract, name of substance word, subject heading word ab=abstract pt=publication type fs=floating subheading sh=subject heading
Appendix 3. CENTRAL search strategy
Issue 2 2009
#1 MeSH descriptor Neutropenia explode all trees #2 neutropaeni* or neutropeni* #3 (#1 OR #2) #4 MeSH descriptor Antibiotic Prophylaxis explode all trees #5 antibiotic* or antimicrobial* or anti‐microbial* or antibacterial* or anti‐bacterial* #6 MeSH descriptor Quinolones explode all trees #7 quinolone* #8 ciprofloxacin #9 ofloxacin #10 norfloxacin #11 pefloxacin #12 MeSH descriptor Trimethoprim‐Sulfamethoxazole Combination explode all trees #13 trimethoprim‐sulfamethoxazole #14 TMP‐SMZ #15 MeSH descriptor Aminoglycosides explode all trees #16 aminoglycoside* #17 gentamicin #18 neomycin #19 tobramycin #20 MeSH descriptor Colistin explode all trees #21 colistin #22 MeSH descriptor Polymyxins explode all trees #23 polymyxin* #24 MeSH descriptor Rifampin explode all trees #25 rifampin #26 MeSH descriptor Cephalosporins explode all trees #27 cephalosporin* #28 ceftriaxone #29 MeSH descriptor Vancomycin explode all trees #30 vancomycin #31 (#5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30) #32 prophyla* or prevent* #33 (#31 AND #32) #34 (#4 OR #33) #35 (#3 AND #34)
Appendix 4. EMBASE search strategy
1 exp neutropenia/ 2 (neutropaeni* or neutropeni*).mp. 3 1 or 2 4 exp antibiotic prophylaxis/ 5 (antibiotic* or antimicrobial* or anti‐microbial* or antibacterial* or anti‐bacterial*).mp. 6 exp quinolone derivative/ 7 quinolone*.mp. 8 ciprofloxacin.mp. 9 ofloxacin.mp. 10 norfloxacin.mp. 11 pefloxacin.mp. 12 trimethoprim‐sulfamethoxazole.mp. 13 TMP‐SMZ.mp. 14 exp aminoglycoside/ 15 aminoglycoside*.mp. 16 gentamicin.mp. 17 neomycin.mp. 18 tobramycin.mp. 19 exp colistin/ 20 colistin.mp. 21 exp polymyxin/ 22 polymyxin*.mp. 23 exp rifampicin/ 24 rifampin.mp. 25 cephalosporin derivative/ 26 cephalosporin*.mp. 27 ceftriaxone.mp. 28 exp vancomycin/ 29 vancomycin.mp. 30 or/5‐29 31 (prophyla* or prevent*).mp. 32 30 and 31 33 4 or 32 34 3 and 33 35 exp controlled clinical trial/ 36 randomized.ab. 37 placebo.ab. 38 dt.fs. 39 randomly.ab. 40 trial.ab. 41 groups.ab. 42 or/35‐41 43 34 and 42 44 exp animal/ 45 human/ 46 44 not (44 and 45) 47 43 not 46
key: mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name ab=abstract fs=floating subheading
Appendix 5. Assessment of risk of bias for the 2011 updated review
For each included study we assessed the following:
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups. We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator),
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number) or,
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment. We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judge that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes and assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel;
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes. We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information is reported, or can be supplied by the trial authors, we re‐included missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups; missing outcome data of < 20%);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found. We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not prespecified; outcomes of interest were reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias
Note: The risk of bias assessment performed for the original review only included points 1) to 3) above, therefore the updated 'risk of bias tables' for the original included trials in Characteristics of included studies were updated with details for points 4) to 5).
We explored the impact of the level of bias through undertaking sensitivity analyses ‐ see 'Sensitivity analysis'.
Appendix 6. Results and conclusions of the original 2005 review
2005 Results
One‐hundred and one trials (12,599 patients) conducted between the years 1973 to 2005 met the inclusion criteria. Antibiotic prophylaxis significantly decreased the risk for death when compared with placebo or no intervention (RR 0.66 [95% CI 0.55 to 0.79]). The authors estimated the number needed to treat (NNT) in order to prevent one death from all causes as 50 (95% CI 34 to 268).
Prophylaxis resulted in a significant decrease in the risk of infection‐related death, RR 0.59 (95% CI 0.47 to 0.75) and in the occurrence of fever, RR 0.77 (95% CI 0.74 to 0.81). A reduction in mortality was also evident when the more recently conducted quinolone trials were analysed separately. Quinolone prophylaxis reduced the risk for all‐cause mortality, RR 0.52 (95% CI, 0.37 to 0.74).
2005 Conclusions
Our review demonstrated that prophylaxis significantly reduced all‐cause mortality. The most significant reduction in mortality was observed in trials assessing prophylaxis with quinolones. The benefit demonstrated in our review outweighs harm, such as adverse effects and development of resistance, since all‐cause mortality is reduced. Since most trials in our review were of patients with haematologic cancer, prophylaxis, preferably with a quinolone, should be considered for these patients.
Data and analyses
Comparison 1. All‐cause mortality, prophylaxis versus placebo or no intervention or other antibiotic.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 drug vs. placebo/no intervention | 46 | 5635 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.55, 0.79] |
| 1.1 quinolone vs. placebo/ no intervention | 19 | 3776 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.40, 0.74] |
| 1.2 TMP‐SMZ vs. placebo/ no intervention | 14 | 870 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.49, 1.02] |
| 1.3 other systemic vs. placebo/ no intervention | 8 | 718 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.65, 1.43] |
| 1.4 nonabsorbable vs. placebo/ no intervention | 6 | 271 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.44, 0.94] |
| 2 quinolone vs. TMP‐SMZ | 10 | 917 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.66, 1.72] |
| 2.1 ciprofloxacin vs. TMP‐SMZ | 3 | 431 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.06 [0.91, 4.69] |
| 2.2 ofloxacin vs. TMP‐SMZ | 3 | 258 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.32, 1.77] |
| 2.3 norfloxacin vs. TMP‐SMZ | 3 | 166 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.23, 2.99] |
| 2.4 nalidixic acid vs. TMP‐SMZ | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.15, 1.83] |
| 3 quinolone+other vs. quinolone | 9 | 1375 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.69, 2.38] |
| 3.1 rifampin | 3 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.25, 8.28] |
| 3.2 vanco | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 penicillins | 4 | 886 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.56, 2.28] |
| 3.4 roxi | 1 | 131 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.14 [0.34, 29.42] |
| 4 TMP‐SMZ vs. other | 2 | 135 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.53, 2.89] |
| 5 nonabsorbable vs. systemic | 8 | 813 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.74, 1.50] |
| 6 systemic + nonabsorbable vs systemic | 2 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.34, 2.38] |
1.4. Analysis.
Comparison 1 All‐cause mortality, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 4 TMP‐SMZ vs. other.
Comparison 2. Infection related mortality, prophylaxis versus placebo or no intervention or other antibiotic.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 drug vs. placebo/no intervention | 43 | 5777 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.48, 0.77] |
| 1.1 quinolone vs. placebo/ no intervention | 16 | 3733 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.33, 0.78] |
| 1.2 TMP‐SMZ vs. placebo/ no intervention | 15 | 1017 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.41, 0.87] |
| 1.3 other systemic vs. placebo/ no intervention | 8 | 812 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.51, 2.54] |
| 1.4 nonabsorbable vs. placebo/ no intervention | 5 | 215 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.40, 0.98] |
| 2 quinolone vs. TMP‐SMZ | 11 | 1019 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.54, 1.54] |
| 2.1 ciprofloxacin vs. TMP‐SMZ | 3 | 431 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.32 [0.81, 13.56] |
| 2.2 ofloxacin vs. TMP‐SMZ | 4 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.30, 1.51] |
| 2.3 norfloxacin vs. TMP‐SMZ | 3 | 166 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.23, 2.99] |
| 2.4 nalidixic acid vs. TMP‐SMZ | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.15, 1.83] |
| 3 quinolone+other vs. quinolone | 9 | 1375 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.56, 1.81] |
| 3.1 rifampin | 3 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.10, 11.58] |
| 3.2 vanco | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.16, 2.47] |
| 3.3 penicillins | 4 | 886 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.52, 2.16] |
| 3.4 roxi | 1 | 131 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.09 [0.19, 22.53] |
| 4 TMP‐SMZ vs. other | 2 | 135 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.40, 2.82] |
| 5 nonabsorbable vs. systemic | 11 | 1005 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.48 [1.65, 3.73] |
| 6 systemic + nonabsorbable vs systemic | 2 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.23, 3.24] |
2.4. Analysis.
Comparison 2 Infection related mortality, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 4 TMP‐SMZ vs. other.
2.6. Analysis.
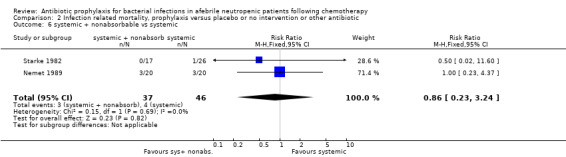
Comparison 2 Infection related mortality, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 6 systemic + nonabsorbable vs systemic.
Comparison 3. Febrile patients and febrile episodes, prophylaxis versus placebo or no intervention or other antibiotic.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 drug vs. placebo/ no intervention | 54 | 6658 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.74, 0.87] |
| 1.1 quinolone vs. placebo/ no intervention | 26 | 4205 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.65, 0.84] |
| 1.2 TMP‐SMZ vs. placebo/ no intervention | 16 | 1424 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.69, 0.92] |
| 1.3 other systemic vs. placebo/ no intervention | 9 | 838 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.85, 1.04] |
| 1.4 nonabsorbable vs. placebo/ no intervention | 4 | 191 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.67, 1.16] |
| 2 quinolone vs. TMP‐SMZ | 10 | 931 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.78, 1.09] |
| 2.1 ciprofloxacin vs. TMP‐SMZ | 3 | 431 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.96, 1.23] |
| 2.2 ofloxacin vs. TMP‐SMZ | 3 | 272 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.50, 1.04] |
| 2.3 norfloxacin vs. TMP‐SMZ | 3 | 166 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.54, 1.26] |
| 2.4 nalidixic acid vs. TMP‐SMZ | 1 | 62 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.99, 1.77] |
| 3 quinolone+other vs. quinolone | 8 | 1276 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.97, 1.11] |
| 3.1 rifampin | 3 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.84, 1.05] |
| 3.2 penicillins | 4 | 871 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.97, 1.16] |
| 3.3 roxi | 1 | 131 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.86, 1.39] |
| 4 TMP‐SMZ vs. other | 2 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.64, 1.31] |
| 5 nonabsorbable vs. systemic | 8 | 808 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.99, 1.13] |
| 6 systemic + nonabsorbable vs systemic | 2 | 103 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.72, 1.20] |
3.4. Analysis.
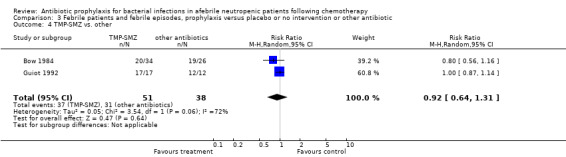
Comparison 3 Febrile patients and febrile episodes, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 4 TMP‐SMZ vs. other.
3.5. Analysis.
Comparison 3 Febrile patients and febrile episodes, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 5 nonabsorbable vs. systemic.
3.6. Analysis.
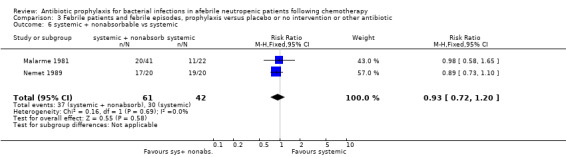
Comparison 3 Febrile patients and febrile episodes, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 6 systemic + nonabsorbable vs systemic.
Comparison 4. Clinically documented infection, prophylaxis versus placebo or no intervention or other antibiotic.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 drug vs. placebo/ no intervention | 48 | 5758 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.56, 0.76] |
| 1.1 quinolone vs. placebo/ no intervention | 21 | 3889 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.44, 0.76] |
| 1.2 TMP‐SMZ vs. placebo/ no intervention | 17 | 1229 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.56, 0.82] |
| 1.3 other systemic vs. placebo/ no intervention | 5 | 413 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.26, 0.90] |
| 1.4 nonabsorbable vs. placebo/ no intervention | 5 | 227 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.76, 1.27] |
| 2 quinolone vs. TMP‐SMZ | 10 | 931 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [1.06, 1.66] |
| 2.1 ciprofloxacin vs. TMP‐SMZ | 3 | 431 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [1.01, 1.92] |
| 2.2 ofloxacin vs. TMP‐SMZ | 3 | 272 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.68, 1.66] |
| 2.3 norfloxacin vs. TMP‐SMZ | 3 | 166 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.44, 1.83] |
| 2.4 nalidixic acid vs. TMP‐SMZ | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.32 [1.36, 3.94] |
| 3 quinolone+other vs. quinolone | 7 | 1236 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.69, 1.42] |
| 3.1 rifampin | 3 | 274 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.32, 3.36] |
| 3.2 penicillins | 3 | 831 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.59, 1.16] |
| 3.3 roxi | 1 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.83, 1.71] |
| 4 TMP‐SMZ vs. other | 2 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.56, 2.07] |
| 5 nonabsorbable vs. systemic | 10 | 862 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.89, 1.49] |
| 6 systemic + nonabsorbable vs systemic | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.25, 1.15] |
4.4. Analysis.
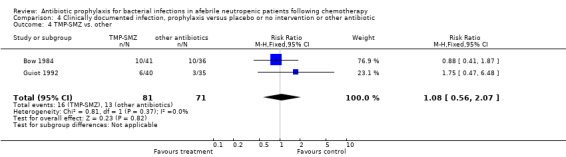
Comparison 4 Clinically documented infection, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 4 TMP‐SMZ vs. other.
4.5. Analysis.
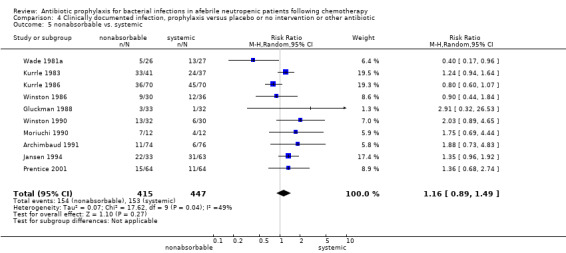
Comparison 4 Clinically documented infection, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 5 nonabsorbable vs. systemic.
4.6. Analysis.
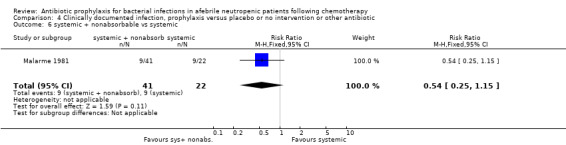
Comparison 4 Clinically documented infection, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 6 systemic + nonabsorbable vs systemic.
Comparison 5. Microbiologically documented infection, prophylaxis versus placebo or no intervention or other antibiotic.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 drug vs. placebo/ no intervention | 53 | 6383 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.42, 0.62] |
| 1.1 quinolone vs. placebo/ no intervention | 24 | 3953 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.32, 0.66] |
| 1.2 TMP‐SMZ vs. placebo/ no intervention | 17 | 1400 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.38, 0.65] |
| 1.3 other systemic vs. placebo/ no intervention | 10 | 882 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.45, 0.87] |
| 1.4 nonabsorbable vs. placebo/ no intervention | 3 | 148 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.25, 2.09] |
| 2 quinolone vs. TMP‐SMZ | 11 | 1019 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.56, 1.01] |
| 2.1 ciprofloxacin vs. TMP‐SMZ | 4 | 519 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.44, 1.10] |
| 2.2 ofloxacin vs. TMP‐SMZ | 3 | 272 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.24, 1.21] |
| 2.3 norfloxacin vs. TMP‐SMZ | 3 | 166 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.55, 1.31] |
| 2.4 nalidixic acid vs. TMP‐SMZ | 1 | 62 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.82, 1.80] |
| 3 quinolone+other vs. quinolone | 9 | 1360 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.55, 1.11] |
| 3.1 rifampin | 3 | 274 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.21, 0.84] |
| 3.2 penicillins | 4 | 871 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.49, 1.50] |
| 3.3 roxi | 1 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.58, 1.38] |
| 3.4 vancomycin | 1 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.48, 1.87] |
| 4 TMP‐SMZ vs. other | 3 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.71, 1.96] |
| 5 nonabsorbable vs. systemic | 9 | 712 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [1.17, 1.91] |
| 6 systemic + nonabsorbable vs systemic | 2 | 103 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [1.01, 2.65] |
5.4. Analysis.
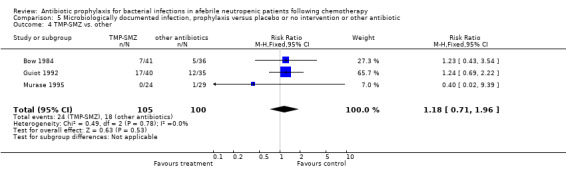
Comparison 5 Microbiologically documented infection, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 4 TMP‐SMZ vs. other.
5.6. Analysis.
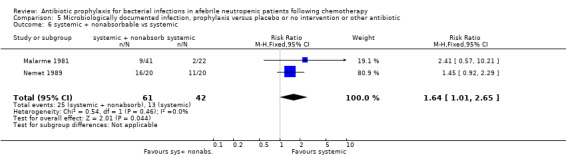
Comparison 5 Microbiologically documented infection, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 6 systemic + nonabsorbable vs systemic.
Comparison 6. Gram‐negative infection, prophylaxis versus placebo or no intervention or other antibiotic.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 drug vs. placebo/ no intervention | 44 | 5607 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.28, 0.52] |
| 1.1 quinolone vs. placebo/ no intervention | 21 | 3752 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.22, 0.41] |
| 1.2 TMP‐SMZ vs. placebo/ no intervention | 13 | 1120 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.29, 0.56] |
| 1.3 other systemic vs. placebo/ no intervention | 6 | 560 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.35, 2.00] |
| 1.4 nonabsorbable vs. placebo/ no intervention | 4 | 175 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.47, 1.59] |
| 2 quinolone vs. TMP‐SMZ | 9 | 915 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.13, 0.36] |
| 2.1 ciprofloxacin vs. TMP‐SMZ | 4 | 519 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.03, 0.43] |
| 2.2 ofloxacin vs. TMP‐SMZ | 2 | 230 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.05, 0.35] |
| 2.3 norfloxacin vs. TMP‐SMZ | 3 | 166 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.22, 0.93] |
| 3 quinolone+other vs. quinolone | 7 | 740 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.58 [1.21, 5.52] |
| 3.1 rifampin | 3 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.29, 3.14] |
| 3.2 penicillins | 3 | 335 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.37 [1.16, 35.01] |
| 3.3 roxi | 1 | 131 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.19 [0.92, 18.98] |
| 4 TMP‐SMZ vs. other | 3 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.41, 3.70] |
| 5 nonabsorbable vs. systemic | 11 | 950 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.15 [1.54, 3.00] |
| 6 systemic + nonabsorbable vs systemic | 2 | 103 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.90 [1.05, 8.02] |
6.3. Analysis.
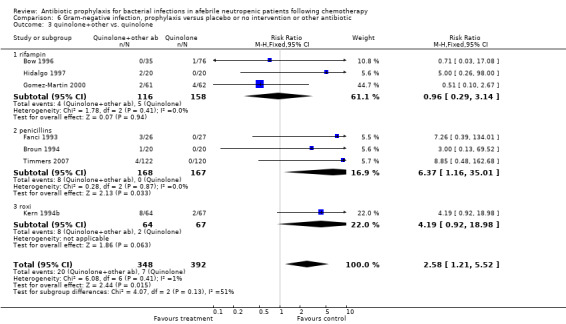
Comparison 6 Gram‐negative infection, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 3 quinolone+other vs. quinolone.
6.4. Analysis.
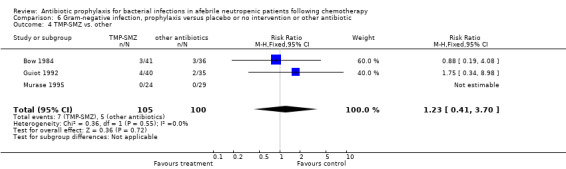
Comparison 6 Gram‐negative infection, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 4 TMP‐SMZ vs. other.
6.5. Analysis.
Comparison 6 Gram‐negative infection, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 5 nonabsorbable vs. systemic.
6.6. Analysis.
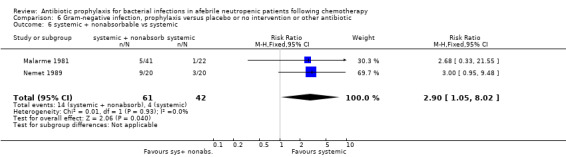
Comparison 6 Gram‐negative infection, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 6 systemic + nonabsorbable vs systemic.
Comparison 7. Gram‐positive infection, prophylaxis versus placebo or no intervention or other antibiotic.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 drug vs. placebo/ no intervention | 45 | 5583 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.34, 0.59] |
| 1.1 quinolone vs. placebo/ no intervention | 21 | 3749 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.21, 0.52] |
| 1.2 TMP‐SMZ vs. placebo/ no intervention | 12 | 1009 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.26, 0.53] |
| 1.3 other systemic vs. placebo/ no intervention | 7 | 610 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.34, 1.02] |
| 1.4 nonabsorbable vs. placebo/ no intervention | 5 | 215 | Risk Ratio (M‐H, Random, 95% CI) | 1.63 [0.96, 2.78] |
| 2 quinolone vs. TMP‐SMZ | 9 | 915 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.60, 1.69] |
| 2.1 ciprofloxacin vs. TMP‐SMZ | 4 | 519 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.43, 1.80] |
| 2.2 ofloxacin vs. TMP‐SMZ | 2 | 230 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.37, 1.17] |
| 2.3 norfloxacin vs. TMP‐SMZ | 3 | 166 | Risk Ratio (M‐H, Random, 95% CI) | 2.07 [0.62, 6.85] |
| 3 quinolone+other vs. quinolone | 7 | 740 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.22, 0.72] |
| 3.1 rifampin | 3 | 274 | Risk Ratio (M‐H, Random, 95% CI) | 0.15 [0.06, 0.38] |
| 3.2 penicillins | 3 | 335 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.33, 1.15] |
| 3.3 roxi | 1 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.28, 0.99] |
| 4 TMP‐SMZ vs. other | 3 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.53, 2.03] |
| 5 nonabsorbable vs. systemic | 10 | 800 | Risk Ratio (M‐H, Random, 95% CI) | 1.55 [1.06, 2.28] |
| 6 systemic + nonabsorbable vs systemic | 2 | 103 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.49, 2.25] |
7.4. Analysis.
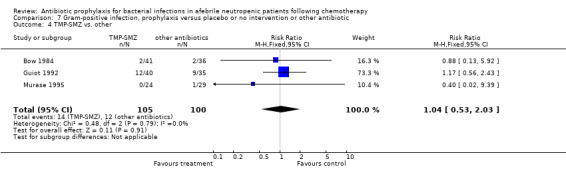
Comparison 7 Gram‐positive infection, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 4 TMP‐SMZ vs. other.
7.5. Analysis.
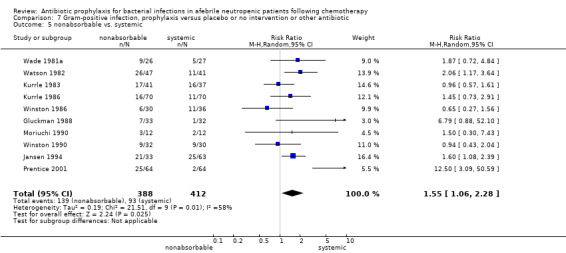
Comparison 7 Gram‐positive infection, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 5 nonabsorbable vs. systemic.
7.6. Analysis.
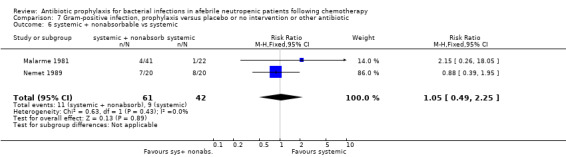
Comparison 7 Gram‐positive infection, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 6 systemic + nonabsorbable vs systemic.
Comparison 8. Bacteraemia, prophylaxis versus placebo or no intervention or other antibiotic.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 drug vs. placebo/ no intervention | 53 | 6390 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.43, 0.60] |
| 1.1 quinolone vs. placebo/ no intervention | 22 | 3832 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.40, 0.69] |
| 1.2 TMP‐SMZ vs. placebo/ no intervention | 18 | 1511 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.37, 0.57] |
| 1.3 other systemic vs. placebo/ no intervention | 9 | 832 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.23, 0.71] |
| 1.4 nonabsorbable vs. placebo/ no intervention | 5 | 215 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.43, 0.95] |
| 2 quinolone vs. TMP‐SMZ | 10 | 931 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.56, 1.42] |
| 2.1 ciprofloxacin vs. TMP‐SMZ | 3 | 431 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.53, 2.62] |
| 2.2 ofloxacin vs. TMP‐SMZ | 3 | 272 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.24, 0.82] |
| 2.3 norfloxacin vs. TMP‐SMZ | 3 | 166 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.80, 2.42] |
| 2.4 Nalidixic acid vs. TMP‐SMZ | 1 | 62 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.61, 3.07] |
| 3 systemic + nonabsorbable vs systemic | 2 | 106 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.38, 5.24] |
| 4 TMP‐SMZ vs. other | 3 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.80, 2.69] |
| 5 nonabsorbable vs. systemic | 10 | 716 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [1.18, 1.91] |
| 6 quinolone+other vs. quinolone | 8 | 824 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.56, 0.97] |
| 6.1 rifampin | 3 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.15, 0.66] |
| 6.2 penicillins | 3 | 335 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.54, 1.23] |
| 6.3 roxi | 1 | 131 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.64, 1.91] |
| 6.4 vancomycin | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.48, 1.87] |
8.3. Analysis.
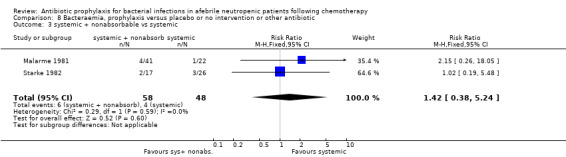
Comparison 8 Bacteraemia, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 3 systemic + nonabsorbable vs systemic.
8.4. Analysis.
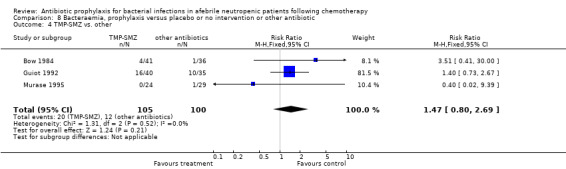
Comparison 8 Bacteraemia, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 4 TMP‐SMZ vs. other.
Comparison 9. Gram‐negative bacteraemia, prophylaxis versus placebo or no intervention or other antibiotic.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 drug vs. placebo/ no intervention | 40 | 5328 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.33, 0.50] |
| 1.1 quinolone vs. placebo/ no intervention | 15 | 3228 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.24, 0.45] |
| 1.2 TMP‐SMZ vs. placebo/ no intervention | 15 | 1161 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.33, 0.65] |
| 1.3 other systemic vs. placebo/ no intervention | 8 | 791 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.29, 0.93] |
| 1.4 nonabsorbable vs. placebo/ no intervention | 3 | 148 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.28, 1.29] |
| 2 quinolone vs. TMP‐SMZ | 10 | 931 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.13, 0.93] |
| 2.1 ciprofloxacin vs. TMP‐SMZ | 3 | 431 | Risk Ratio (M‐H, Random, 95% CI) | 0.15 [0.03, 0.84] |
| 2.2 ofloxacin vs. TMP‐SMZ | 3 | 272 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.03, 1.35] |
| 2.3 norfloxacin vs. TMP‐SMZ | 3 | 166 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.29, 1.56] |
| 2.4 Nalidixic acid vs. TMP‐SMZ | 1 | 62 | Risk Ratio (M‐H, Random, 95% CI) | 10.86 [0.61, 193.50] |
| 3 quinolone+other vs. quinolone | 8 | 824 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.30 [1.11, 4.78] |
| 3.1 rifampin | 3 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.29, 3.14] |
| 3.2 penicillins | 3 | 335 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.05 [0.90, 28.44] |
| 3.3 roxi | 1 | 131 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.38 [1.08, 65.08] |
| 3.4 vancomycin | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.14, 6.46] |
| 4 TMP‐SMZ vs. other | 3 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.80 [0.66, 21.74] |
| 5 nonabsorbable vs. systemic | 10 | 842 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.70 [1.63, 4.49] |
| 6 systemic + nonabsorbable vs systemic | 2 | 106 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.14, 5.83] |
9.3. Analysis.
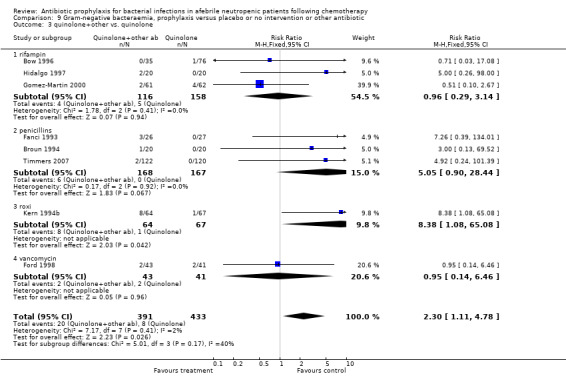
Comparison 9 Gram‐negative bacteraemia, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 3 quinolone+other vs. quinolone.
9.4. Analysis.
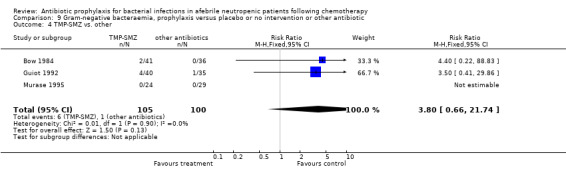
Comparison 9 Gram‐negative bacteraemia, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 4 TMP‐SMZ vs. other.
9.5. Analysis.
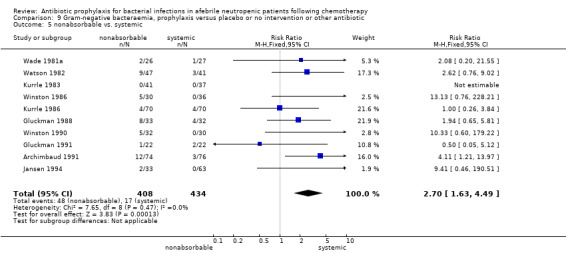
Comparison 9 Gram‐negative bacteraemia, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 5 nonabsorbable vs. systemic.
9.6. Analysis.
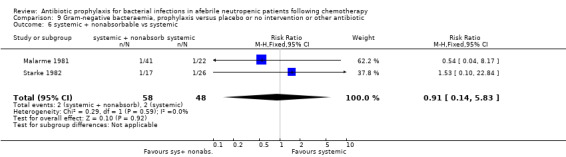
Comparison 9 Gram‐negative bacteraemia, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 6 systemic + nonabsorbable vs systemic.
Comparison 10. Gram‐positive bacteraemia, prophylaxis versus placebo or no intervention or other antibiotic.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 drug vs. placebo/ no intervention | 39 | 5265 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.54, 0.74] |
| 1.1 quinolone vs. placebo/ no intervention | 15 | 3228 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.57, 0.86] |
| 1.2 TMP‐SMZ vs. placebo/ no intervention | 14 | 1098 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.24, 0.60] |
| 1.3 other systemic vs. placebo/ no intervention | 8 | 791 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.44, 0.89] |
| 1.4 nonabsorbable vs. placebo/ no intervention | 3 | 148 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.33, 3.79] |
| 2 quinolone vs. TMP‐SMZ | 10 | 931 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.71, 2.15] |
| 2.1 ciprofloxacin vs. TMP‐SMZ | 3 | 431 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [0.60, 3.65] |
| 2.2 ofloxacin vs. TMP‐SMZ | 3 | 272 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.40, 0.99] |
| 2.3 norfloxacin vs. TMP‐SMZ | 3 | 166 | Risk Ratio (M‐H, Random, 95% CI) | 4.17 [1.46, 11.92] |
| 2.4 Nalidixic acid vs. TMP‐SMZ | 1 | 62 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.28, 2.06] |
| 3 quinolone+other vs. quinolone | 8 | 824 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.44, 0.83] |
| 3.1 rifampin | 3 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.11, 0.72] |
| 3.2 penicillins | 3 | 335 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.49, 1.13] |
| 3.3 roxi | 1 | 131 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.31, 1.24] |
| 3.4 vancomycin | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.28, 1.59] |
| 4 TMP‐SMZ vs. other | 3 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.57, 2.24] |
| 5 nonabsorbable vs. systemic | 9 | 692 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.63, 2.03] |
| 6 systemic + nonabsorbable vs systemic | 2 | 106 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.57 [0.36, 18.46] |
10.4. Analysis.
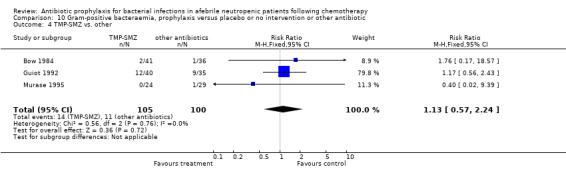
Comparison 10 Gram‐positive bacteraemia, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 4 TMP‐SMZ vs. other.
10.5. Analysis.
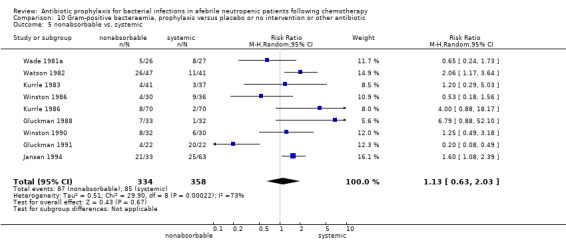
Comparison 10 Gram‐positive bacteraemia, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 5 nonabsorbable vs. systemic.
10.6. Analysis.
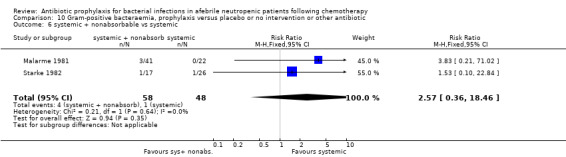
Comparison 10 Gram‐positive bacteraemia, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 6 systemic + nonabsorbable vs systemic.
Comparison 11. Side effects, prophylaxis versus placebo or no intervention or other antibiotic.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 drug vs. placebo/ no intervention | 35 | 5103 | Risk Ratio (M‐H, Random, 95% CI) | 1.58 [1.19, 2.12] |
| 1.1 quinolone vs. placebo/ no intervention | 16 | 3324 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [1.12, 2.04] |
| 1.2 TMP‐SMZ vs. placebo/ no intervention | 13 | 1240 | Risk Ratio (M‐H, Random, 95% CI) | 1.70 [1.12, 2.59] |
| 1.3 other systemic vs. placebo/ no intervention | 4 | 440 | Risk Ratio (M‐H, Random, 95% CI) | 1.82 [0.72, 4.55] |
| 1.4 nonabsorbable vs. placebo/ no intervention | 2 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.79, 1.11] |
| 2 quinolone vs. TMP‐SMZ | 10 | 1027 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.43, 0.90] |
| 2.1 ciprofloxacin vs. TMP‐SMZ | 4 | 537 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.46, 1.13] |
| 2.2 ofloxacin vs. TMP‐SMZ | 2 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.24, 0.69] |
| 2.3 norfloxacin vs. TMP‐SMZ | 3 | 166 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.27, 1.43] |
| 2.4 Nalidixic acid vs. TMP‐SMZ | 1 | 62 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.15, 4.51] |
| 3 quinolone+other vs. quinolone | 6 | 516 | Risk Ratio (M‐H, Random, 95% CI) | 2.69 [0.78, 9.27] |
| 3.1 rifampin | 3 | 243 | Risk Ratio (M‐H, Random, 95% CI) | 5.49 [0.30, 100.07] |
| 3.2 penicillins | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 1.4 [0.25, 7.77] |
| 3.3 roxi | 1 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 9.42 [0.52, 171.44] |
| 3.4 vancomycin | 1 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.14, 6.46] |
| 4 TMP‐SMZ vs. other | 2 | 128 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.06 [0.68, 13.79] |
| 5 nonabsorbable vs. systemic | 10 | 934 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.65, 1.96] |
| 6 systemic + nonabsorbable vs systemic | 3 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [1.02, 3.00] |
11.4. Analysis.
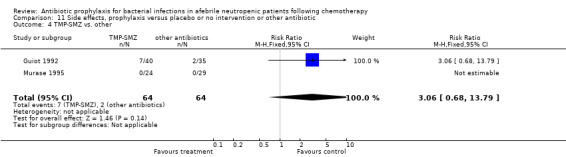
Comparison 11 Side effects, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 4 TMP‐SMZ vs. other.
11.5. Analysis.
Comparison 11 Side effects, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 5 nonabsorbable vs. systemic.
11.6. Analysis.
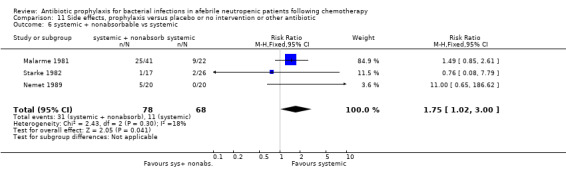
Comparison 11 Side effects, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 6 systemic + nonabsorbable vs systemic.
Comparison 12. Side effects requiring discontinuation, prophylaxis versus placebo or no intervention or other antibiotic.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 drug vs. placebo/no intervention | 18 | 2281 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.06 [1.32, 3.19] |
| 1.1 quinolone vs. placebo/ no intervention | 8 | 1513 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.04 [1.10, 3.81] |
| 1.2 TMP‐SMZ vs. placebo/ no intervention | 5 | 305 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.63 [1.32, 9.98] |
| 1.3 other systemic vs. placebo/ no intervention | 3 | 364 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.51, 2.88] |
| 1.4 nonabsorbable vs. placebo/ no intervention | 2 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.18 [0.14, 72.75] |
| 2 quinolone vs. TMP‐SMZ | 7 | 850 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.16, 0.87] |
| 2.1 ciprofloxacin vs. TMP‐SMZ | 3 | 481 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.12, 1.68] |
| 2.2 ofloxacin vs. TMP‐SMZ | 2 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.10, 0.53] |
| 2.3 norfloxacin vs. TMP‐SMZ | 2 | 107 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.11, 2.69] |
| 3 quinolone+other vs. quinolone | 5 | 432 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.92 [1.61, 15.01] |
| 3.1 rifampin | 3 | 243 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.0 [1.32, 75.73] |
| 3.2 penicillins | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.4 [0.25, 7.77] |
| 3.3 roxi | 1 | 131 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.42 [0.52, 171.44] |
| 4 TMP‐SMZ vs. other | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 nonabsorbable vs. systemic | 2 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.00, 0.69] |
| 6 systemic + nonabsorbable vs systemic | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.56, 2.80] |
12.4. Analysis.
Comparison 12 Side effects requiring discontinuation, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 4 TMP‐SMZ vs. other.
12.5. Analysis.
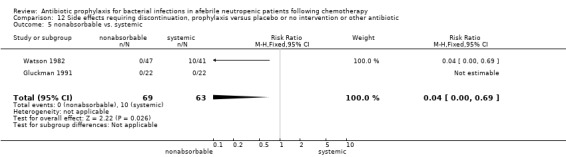
Comparison 12 Side effects requiring discontinuation, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 5 nonabsorbable vs. systemic.
12.6. Analysis.
Comparison 12 Side effects requiring discontinuation, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 6 systemic + nonabsorbable vs systemic.
Comparison 13. Fungal infection, prophylaxis versus placebo or no intervention or other antibiotic.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 drug vs. placebo/ no intervention | 39 | 2887 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.82, 1.33] |
| 1.1 quinolone vs. placebo/ no intervention | 15 | 1276 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.55, 1.15] |
| 1.2 TMP‐SMZ vs. placebo/ no intervention | 15 | 1007 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.98, 2.27] |
| 1.3 other systemic vs. placebo/ no intervention | 5 | 422 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.55, 4.17] |
| 1.4 nonabsorbable vs. placebo/ no intervention | 4 | 182 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.38, 1.40] |
| 2 quinolone vs. TMP‐SMZ | 10 | 789 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.36, 1.16] |
| 2.1 ciprofloxacin vs. TMP‐SMZ | 3 | 289 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.39, 3.92] |
| 2.2 ofloxacin vs. TMP‐SMZ | 3 | 272 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.25, 1.37] |
| 2.3 norfloxacin vs. TMP‐SMZ | 3 | 166 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.12, 1.68] |
| 2.4 Nalidixic acid vs. TMP‐SMZ | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.01, 4.83] |
| 3 quinolone+other vs. quinolone | 5 | 489 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.37, 3.47] |
| 3.1 rifampin | 3 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.42 [0.27, 153.69] |
| 3.2 roxi | 1 | 131 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.12, 4.04] |
| 3.3 vancomycin | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.14, 6.46] |
| 4 TMP‐SMZ vs. other | 2 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.20, 10.81] |
| 5 nonabsorbable vs. systemic | 10 | 800 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.64, 2.17] |
| 6 systemic + nonabsorbable vs systemic | 2 | 103 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.22, 3.02] |
13.3. Analysis.
Comparison 13 Fungal infection, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 3 quinolone+other vs. quinolone.
13.4. Analysis.
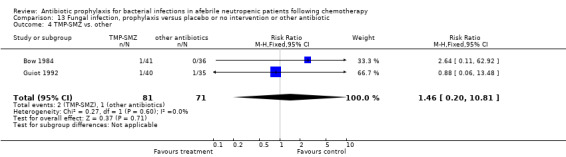
Comparison 13 Fungal infection, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 4 TMP‐SMZ vs. other.
13.5. Analysis.
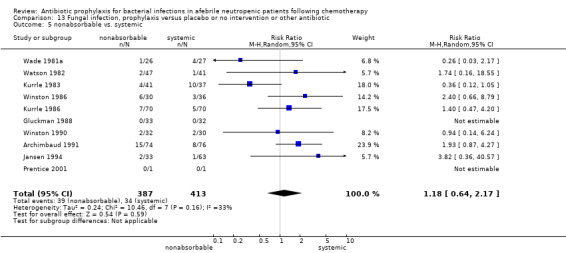
Comparison 13 Fungal infection, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 5 nonabsorbable vs. systemic.
13.6. Analysis.
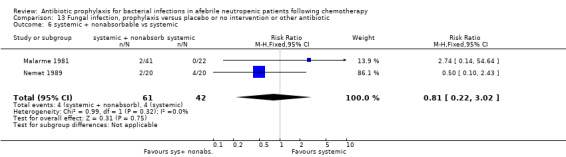
Comparison 13 Fungal infection, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 6 systemic + nonabsorbable vs systemic.
Comparison 14. Infection resistant to drug taken, prophylaxis versus placebo or no intervention or other antibiotic.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 drug vs. placebo/ no intervention | 19 | 3629 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [1.08, 2.01] |
| 1.1 quinolone vs. placebo/ no intervention | 8 | 2712 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.81, 1.70] |
| 1.2 TMP‐SMZ vs. placebo/ no intervention | 11 | 917 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.42 [1.35, 4.36] |
| 2 quinolone vs. TMP‐SMZ | 6 | 366 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.27, 0.74] |
| 2.1 ciprofloxacin vs. TMP‐SMZ | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.64] |
| 2.2 ofloxacin vs. TMP‐SMZ | 2 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.07, 0.76] |
| 2.3 norfloxacin vs. TMP‐SMZ | 3 | 166 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.35, 1.06] |
| 3 ciprofloxacin vs. norfloxacin | 1 | 619 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.16, 0.87] |
| 4 norfloxacin vs. pefloxacin | 1 | 136 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.15 [1.18, 3.91] |
| 5 quinolone+other vs. quinolone | 4 | 463 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.06, 5.69] |
| 5.1 rifampin | 2 | 163 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.01, 1.94] |
| 5.2 penicillin | 2 | 300 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.79, 2.04] |
| 6 nonabsorbable vs. systemic | 4 | 343 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.63, 1.12] |
| 7 TMP‐SMZ vs. other | 2 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.74 [1.27, 17.65] |
14.3. Analysis.
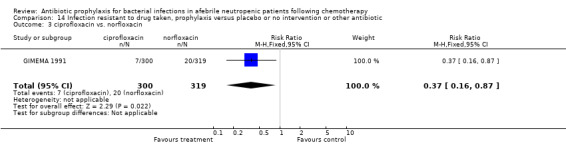
Comparison 14 Infection resistant to drug taken, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 3 ciprofloxacin vs. norfloxacin.
14.4. Analysis.
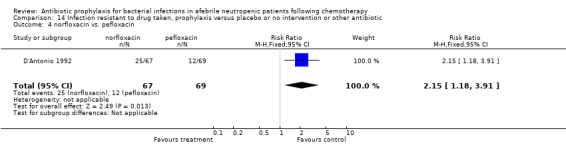
Comparison 14 Infection resistant to drug taken, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 4 norfloxacin vs. pefloxacin.
14.5. Analysis.
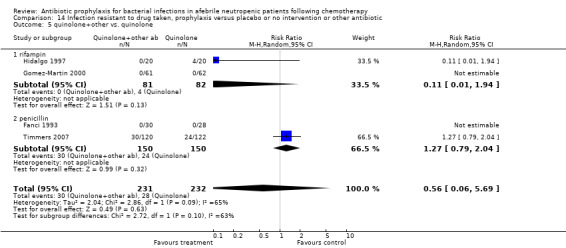
Comparison 14 Infection resistant to drug taken, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 5 quinolone+other vs. quinolone.
14.6. Analysis.
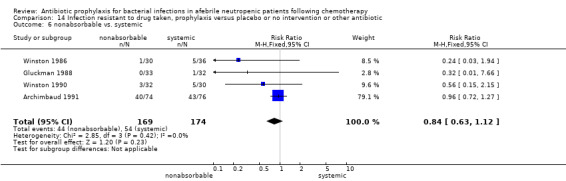
Comparison 14 Infection resistant to drug taken, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 6 nonabsorbable vs. systemic.
14.7. Analysis.
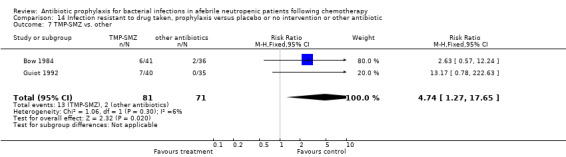
Comparison 14 Infection resistant to drug taken, prophylaxis versus placebo or no intervention or other antibiotic, Outcome 7 TMP‐SMZ vs. other.
Comparison 15. All‐cause mortality, quinolone versus placebo or no intervention, according to different characteristics.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 quinolone vs. placebo/no intervention according to disease status | 19 | 3776 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.40, 0.74] |
| 1.1 acute leukaemia or allogeneic hematopoietic cell transplant | 13 | 1818 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.40, 0.82] |
| 1.2 solid tumours or lymphoma | 5 | 1940 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.26, 0.88] |
| 1.3 combined | 1 | 18 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 quinolone vs. placebo/no intervention according to type of quinolone | 19 | 3776 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.40, 0.74] |
| 2.1 levofloxacin | 3 | 2349 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.35, 0.99] |
| 2.2 ciprofloxacin | 8 | 704 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.13, 0.69] |
| 2.3 norfloxacin | 4 | 272 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.58, 1.81] |
| 2.4 other (ofloxacin/pefloxacin/enoxacin) | 4 | 451 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.12, 0.64] |
| 3 quinolone vs. placebo/no intervention according to timing of chemotherapy initiation | 19 | 3776 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.40, 0.74] |
| 3.1 with initiation of chemotherapy | 15 | 1947 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.44, 0.92] |
| 3.2 with appearance of neutropenia | 4 | 1829 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.22, 0.70] |
| 4 quinolone vs. placebo/no intervention according to year of publication | 19 | 3776 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.40, 0.74] |
| 4.1 studies published after 2000 | 8 | 2879 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.32, 0.75] |
| 4.2 studies published before and until 2000 | 11 | 897 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.39, 0.96] |
Comparison 16. Sensitivity analyses by randomisation generation, drug versus placebo or no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 44 | 5378 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.54, 0.78] |
| 1.1 randomisation A | 15 | 3441 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.47, 0.88] |
| 1.2 randomisation B | 27 | 1771 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.49, 0.78] |
| 1.3 randomisation C | 2 | 166 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.59, 2.55] |
| 2 Febrile patients | 50 | 6267 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.74, 0.87] |
| 2.1 randomisation A | 15 | 3555 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.69, 0.93] |
| 2.2 randomisation B | 33 | 2549 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.71, 0.89] |
| 2.3 randomisation C | 2 | 163 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.38, 1.32] |
| 3 Clinically documented infection | 47 | 5660 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.57, 0.77] |
| 3.1 randomisation A | 12 | 3112 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.61, 0.86] |
| 3.2 randomisation B | 33 | 2385 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.50, 0.75] |
| 3.3 randomisation C | 2 | 163 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.71, 1.42] |
| 4 Microbiologically documented infection | 51 | 6100 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.43, 0.62] |
| 4.1 randomisation A | 16 | 3599 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.38, 0.71] |
| 4.2 randomisation B | 33 | 2338 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.39, 0.65] |
| 4.3 randomisation C | 2 | 163 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.39, 0.73] |
Comparison 17. Sensitivity analyses by allocation concealment, drug versus placebo or no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 44 | 5378 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.54, 0.78] |
| 1.1 allocation A | 15 | 3581 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.53, 0.97] |
| 1.2 allocation B | 29 | 1797 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.49, 0.77] |
| 2 Febrile patients | 51 | 6317 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.73, 0.86] |
| 2.1 allocation A | 16 | 3844 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.73, 0.95] |
| 2.2 allocation B | 35 | 2473 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.66, 0.85] |
| 3 Clinically documented infection | 47 | 5660 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.57, 0.77] |
| 3.1 allocation A | 13 | 3408 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.50, 0.82] |
| 3.2 allocation B | 34 | 2252 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.55, 0.80] |
| 4 Microbiologically documented infection | 51 | 6100 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.43, 0.62] |
| 4.1 allocation A | 15 | 3727 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.36, 0.71] |
| 4.2 allocation B | 36 | 2373 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.42, 0.65] |
Comparison 18. Sensitivity analyses by blinding, drug versus placebo or no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 44 | 5378 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.54, 0.78] |
| 1.1 Double Blind | 17 | 3484 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.48, 0.93] |
| 1.2 Unblinded | 27 | 1894 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.52, 0.80] |
| 2 Febrile patients | 51 | 6317 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.73, 0.86] |
| 2.1 Double Blind | 22 | 4255 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.75, 0.94] |
| 2.2 Unblinded | 29 | 2062 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.64, 0.84] |
| 3 Clinically documented infection | 47 | 5660 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.57, 0.77] |
| 3.1 Double Blind | 20 | 3738 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.53, 0.79] |
| 3.2 Unblinded | 27 | 1922 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.54, 0.82] |
| 4 Microbiologically documented infection | 51 | 6144 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.44, 0.62] |
| 4.1 Double Blind | 20 | 3912 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.43, 0.75] |
| 4.2 Unblinded | 32 | 2232 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.39, 0.62] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Archimbaud 1991.
| Methods | Randomisation: a table of random numbers, no data on allocation concealment; Blinding: double blind; Intention to treat: yes; Exclusion from analysis: none (0/150); Beginning of prophylaxis: chemotherapy; End of prophylaxis: PMN count 500 or death | |
| Participants | France, single centre; 150 afebrile adult patients presenting with neutropenia(<1000) expected to last more than two weeks after intensive chemotherapy for acute leukaemia or BMT for various haematological malignancies; Inpatients, reverse isolation | |
| Interventions | Nonabsorbable antibiotics: gentamicin, colistin sulphate, vancomycin (100mg, 3 million U, 800mg ‐ respectively) versus absorbable: pefloxacin, vancomycin (800mg, 800mg) | |
| Outcomes | All cause mortality; Infection related death; Number of febrile patients; Number of febrile patients or episodes; Fever days; Clinically documented febrile episodes; Microbiologically documented febrile episodes; Hospitalization days; Resistance to quinolones; Adverse events | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No post‐randomisation withdrawals |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
Arning 1990.
| Methods | Randomisation: no information (was done for neutropenic episodes,and not patients) Blinding: none; Intention to treat: no; Exclusion from analysis: 6/65 patients, 8/96 neutropenic episodes; Beginning of prophylaxis: chemotherapy; End of prophylaxis: PMN count 500 | |
| Participants | Germany, single centre; 65 afebrile adult patients presenting with neutropenia(<1000) after chemotherapy for acute leukaemia; Inpatients, reverse isolation | |
| Interventions | Trimethoprim‐sulfamethoxazole + colistin (160/800mg + 2 million units) versus ofloxacin 400mg versus ciprofloxacin 1g | |
| Outcomes | All cause mortality; Infection related death; Number of febrile patients or episodes; Microbiologically documented febrile episodes; Adverse events | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 6/65 patients, 8/96 neutropenic episodes |
Attal 1991.
| Methods | Randomisation: computer‐generated sequence unknown to physicians participating in trials, allocation by central randomisation calling a distant data coordinating system; Blinding: none; Intention to treat: yes; Exclusion from analysis: none (0/60); Beginning of prophylaxis: chemotherapy; End of prophylaxis: PMN count 500 or fever | |
| Participants | France, single centre; 60 patients (adult and children) after BMT for various haematological diseases; Inpatients | |
| Interventions | Intravenous vancomycin (15mg/kg X2/d day) versus no intervention | |
| Outcomes | All cause mortality; Infection related death; Number of febrile patients or episodes; Fever days; Clinically documented febrile episodes; Microbiologically documented febrile episodes; Adverse events | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated sequence |
| Allocation concealment (selection bias) | Low risk | Data coordinating system (A ‐ Adequate) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No exclusions |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Bartoloni 1989.
| Methods | Randomisation: no information; Blinding: double blind; Intention to treat: unknown; Exclusion from analysis: none; Beginning of prophylaxis: chemotherapy; End of prophylaxis: complete remission or a neutrophil count of 500 | |
| Participants | Italy, single centre; 19 adult patients with acute leukaemia or chronic leukaemia in blast crisis undergoing cytotoxic chemotherapy to induce remission; Inpatients | |
| Interventions | Ofloxaxin 300mgX2/d versus trimethoprim‐sulphamethoxazole 160/800mg twice daily | |
| Outcomes | No relevant outcomes: the trial evaluates effect of prophylactic antibiotics on the bacterial aerobic flora (nose, gingiva and perineal region) | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No exclusions |
| Selective reporting (reporting bias) | Unclear risk | No outcomes relevant to this review were reported. |
Bender 1979.
| Methods | Randomisation: no information Blinding: none; Intention to treat: no; Exclusion from analysis: 4/42 patients; Beginning of prophylaxis: chemotherapy; End of prophylaxis: remission, death or 5 weeks | |
| Participants | USA, single centre; 42 patients with acute leukaemia who were undergoing remission chemotherapy; Inpatients | |
| Interventions | Gentamicin 200mgX6, oral vancomycin 500mgX6 and nystatin 5 million units versus gentamicin 200mgX6 and nystatin 5 million units | |
| Outcomes | All cause mortality; Number of febrile patients or episodes; Clinically documented febrile episodes; Microbiologically documented febrile episodes | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 4/42 patients excluded from analysis |
| Selective reporting (reporting bias) | Unclear risk | Analysis not by ITT |
Bow 1984.
| Methods | Randomisation: central randomisation generated from a computer program, kept in sealed envelopes Blinding: none Intention to treat: no; Exclusion from analysis: 15/75; Beginning of prophylaxis: PMN count < 1000; End of prophylaxis: PMN count 1000 or death | |
| Participants | Canada, single centre; 75 patients with bone marrow failure due to haematological malignancies and neutropenia; Inpatients | |
| Interventions | Trimethoprim 150mgX2/d versus trimethoprim‐sulphamethoxazole 160mg/800mg X2/d | |
| Outcomes | All cause mortality; Infection related death; Number of febrile episodes; Clinically documented febrile episodes; Microbiologically documented febrile episodes; Resistance to quinolones | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | computer‐generated |
| Allocation concealment (selection bias) | Low risk | sealed envelopes (A ‐ Adequate) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 15/75 excluded from analysis |
| Selective reporting (reporting bias) | Unclear risk | Analysis not by ITT |
Bow 1988.
| Methods | Randomisation: central randomisation generated from a computer program, allocations assigned from pharmacies of the participating institutions; Blinding:none Intention to treat: no; Exclusion from analysis: 12/75; Beginning of prophylaxis: chemotherapy; End of prophylaxis: PMN count 1000 or fever | |
| Participants | Canada, multicentre; adult patients with haematological malignancies and cytotoxic induced neutropenia; Inpatients, university hospital | |
| Interventions | Norfloxacin 400mgX2/d versus trimethoprim‐sulphamethoxazole 160mg/800mgX2/d | |
| Outcomes | All cause mortality; Infection related death; Number of febrile patients or febrile episodes; Clinically documented febrile episodes; Microbiologically documented febrile episodes; Resistance to quinolones and trimethoprim‐sulphamethoxazole | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | computer‐generated |
| Allocation concealment (selection bias) | Low risk | pharmacy assigned allocation (A ‐ Adequate) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 12/75 excluded from analysis |
| Selective reporting (reporting bias) | Unclear risk | Analysis not by ITT |
Bow 1996.
| Methods | Randomisation: central randomisation, computerized random‐number generator, allocations sequentially assigned from a central office; Blinding: none; Intention to treat: no; Exclusion from analysis: 16/127; Beginning of prophylaxis: chemotherapy; End of prophylaxis: PMN count 500 or fever | |
| Participants | Canada, multicentre; adult patients with severe neutropenia receiving cytotoxic therapy for acute leukaemia or BMT; Inpatients | |
| Interventions | Norfloxacin 400mgX2/d, versus ofloxacin 400mg X2/d, versus ofloxacin 400mgX2/d + rifampin 300mgX2/d | |
| Outcomes | All cause mortality; Infection related death; Number of febrile episodes; Clinically documented febrile episodes; Microbiologically documented febrile episodes; Adverse events | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | sequential allocation from central office (A ‐ Adequate) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 16/127 excluded |
| Selective reporting (reporting bias) | Unclear risk | Analysis not by ITT |
Brodsky 1993.
| Methods | Randomisation: no information; Blinding: none; Intention to treat: no; Exclusion from analysis: unknown; Beginning of prophylaxis: chemotherapy; End of prophylaxis: PMN count 500 or fever | |
| Participants | Argentina, single centre; 14 adult patients with severe neutropenia receiving cytotoxic therapy for acute leukaemia; Inpatients | |
| Interventions | Norfloxacin 400mgX2/d or ciprofloxacin 500mgX2 versus no intervention | |
| Outcomes | All cause mortality; Number of febrile episodes; Clinically documented febrile episodes; Microbiologically documented febrile episodes | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Details not available for the 2011 review update |
| Selective reporting (reporting bias) | Unclear risk | Analysis not by ITT |
Broun 1994.
| Methods | Randomisation: no information; Blinding: none; Intention to treat: no; Exclusion from analysis: 3/40; Beginning of prophylaxis: chemotherapy; End of prophylaxis: PMN count 500 or fever | |
| Participants | USA, single centre; adult patients undergoing autologous BMT for haematological or solid tumours; Inpatients | |
| Interventions | Norfloxacin 400mgX3/d versus norfloxacin + penicillin (400mgX3/d, 10 million unitsX6/d) | |
| Outcomes | All cause mortality; Infection related death; Number of febrile patients; Clinically documented febrile episodes; Microbiologically documented febrile episodes | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 3/40 excluded |
| Selective reporting (reporting bias) | Unclear risk | Analysis not by ITT |
Bucaneve 2005.
| Methods | Randomisation: central, computer‐generated Blinding: triple blind; Intention to treat: none; Exclusion from analysis: 22/760; Beginning of prophylaxis: 1‐3 days before chemotherapy or reinfusion of stem cells; End of prophylaxis: neutropenia resolution (>1000) | |
| Participants | Italy, multicentre; 760 adult patients with acute leukaemia and solid tumour or lymphoma undergoing autologous blood stem cell transplantation; Inpatients | |
| Interventions | Levofloxacin 500mg X1/d versus placebo | |
| Outcomes | All cause mortality; Infection related death; Number of febrile patients; Clinically documented febrile episodes; Microbiologically documented febrile episodes; Infection resistant to quinolones; Adverse events | |
| Notes | Journal publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | computer‐generated |
| Allocation concealment (selection bias) | Low risk | central allocation (A ‐ Adequate) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | triple‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 22/760 excluded |
| Selective reporting (reporting bias) | Unclear risk | Analysis not by ITT |
Carlson 1997.
| Methods | Randomisation: central randomisation, computerized random‐number table; Blinding:none; Intention to treat: yes; Exclusion from analysis: 0/90; Beginning of prophylaxis: PMN count of 500; End of prophylaxis: PMN count 1000 | |
| Participants | USA, multicentre; Patients with ovarian cancer receiving chemotherapy (paclitaxel) and expected to be neutropenic; Outpatients | |
| Interventions | Ciprofloxacin 500mgX2/d versus no intervention | |
| Outcomes | All cause mortality; Infection related death; Number of febrile patients; Microbiologically documented febrile episodes; Hospitalization days; Adverse events | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Central allocation (A ‐ Adequate) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No exclusions |
| Selective reporting (reporting bias) | Low risk | Analyses by ITT; all expected outcomes reported |
Casali 1988.
| Methods | Randomisation: no information; Blinding: none; Intention to treat: unknown; Exclusion from analysis: unknown Beginning of prophylaxis: PMN count less than 1000 End of prophylaxis: PMN count 1000 | |
| Participants | Italy, single centre; 65 cancer patients with solid tumours presenting with neutropenia after chemotherapy; Setting not specified | |
| Interventions | Norfloxacin 400mgX3/d versus no intervention | |
| Outcomes | Number of febrile episodes; Clinically documented febrile episodes; Microbiologically documented febrile episodes; Resistance to quinolones; Adverse events | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Selective reporting (reporting bias) | Unclear risk | ITT not described |
Castagnola 2003.
| Methods | Randomisation: sequentially numbered batches according to a computer‐generated randomised list, allocation by central randomisation calling a data coordinating centre; Blinding: triple blind; Intention to treat: no; Exclusion from analysis: 6/173; Beginning of prophylaxis: PMN count less than 500; End of prophylaxis: PMN count 500, maximum 15 days | |
| Participants | Italy, multicentre (16 centres), 173 neutropenic children with cancer (solid or haematological) treated with chemotherapy; Inpatients | |
| Interventions | Amoxicillin/clavulanate 12mg/kgX2/d versus placebo | |
| Outcomes | All cause mortality; Infection related death; Number of febrile patients; Clinically documented febrile episodes; Microbiologically documented febrile episodes; Adverse events | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | computer‐generated list |
| Allocation concealment (selection bias) | Low risk | central allocation (A ‐ Adequate) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Triple blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 6/173 exclusions |
| Selective reporting (reporting bias) | Unclear risk | Analysis not by ITT |
Chastagner 1997.
| Methods | Randomisation: no information; Blinding: none; Intention to treat: none; Exclusion from analysis: 18 Beginning of prophylaxis: PMN count less than 500 End of prophylaxis: PMN count 500 | |
| Participants | France, single centre, 68 neutropenic children undergoing induction therapy for acute lymphoblastic leukaemia | |
| Interventions | IV Teicoplanin 10 mg/kg o.d. after initial dosing of 10 mg/kg every 12 hours for 3 doses versus no intervention | |
| Outcomes | Febrile episodes; Microbiologically documented infections | |
| Notes | Abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | no details |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 18/68 exclusions |
| Selective reporting (reporting bias) | Unclear risk | Analysis not by ITT |
Chung 1997.
| Methods | Randomisation: no information; Blinding: double blind; Intention to treat: none; Exclusion from analysis: 13 Beginning of prophylaxis: not specified; End of prophylaxis: not specified | |
| Participants | Korea, single centre, 65 neutropenic patients with acute leukaemia undergoing remission induction or consolidation chemotherapy | |
| Interventions | Tosufloxacin 150 mg X2/d versus placebo | |
| Outcomes | Number of febrile episodes; Microbiologically documented infections; Clinically documented infections. However, number of episodes for each outcome not specified, and therefore not included in the analysis. | |
| Notes | Abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 13/65 exclusions |
| Selective reporting (reporting bias) | Unclear risk | Analysis not by ITT |
Cruciani 1989.
| Methods | Randomisation: by an appropriate random‐number‐table, allocation unknown; Blinding: none; Intention to treat: no; Exclusion from analysis: 5/49; Beginning of prophylaxis: PMN count less than 1000 End of prophylaxis: PMN count 1000 | |
| Participants | Italy, single centre; 49 neutropenic children with haematological malignancies (and a few with neuroblastoma); Inpatients | |
| Interventions | Trimethoprim‐Sulphamethoxazole 15mg/kgX2/d versus norfloxacin 20mg/kgX2/d | |
| Outcomes | All cause mortality; Infection related death; Number of febrile patients or episodes; Clinically documented febrile episodes; Microbiologically documented febrile episodes; Resistance to quinolones and to trimethoprim‐sulphamethoxazole; Adverse events | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | random number table |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 5/49 exclusions |
| Selective reporting (reporting bias) | Unclear risk | Analysis not by ITT |
Cullen 2005.
| Methods | Randomisation: central, computer‐generated Blinding: double‐blind; Intention to treat: yes; Exclusion from analysis: none; Beginning of prophylaxis: day of anticipated neutropenia as determined by chemotherapy regimen; End of prophylaxis: 7 days per chemotherapy cycle | |
| Participants | UK, multicentre; 1565 adult patients with solid malignancies or lymphoma; Outpatients | |
| Interventions | Levofloxacin 500mgX1/d versus placebo | |
| Outcomes | Infection related death; Number of febrile patients and episodes; Clinically documented febrile episodes; Microbiologically documented febrile episodes; Resistance to quinolones; Adverse events | |
| Notes | Journal publication. Prophylaxis administered for multiple chemotherapy cycles. Results analysed per patient for all cycles | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | computer‐generated |
| Allocation concealment (selection bias) | Low risk | Central allocation (A ‐ Adequate) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No exclusions |
| Selective reporting (reporting bias) | Low risk | Analysis by ITT; all expected outcomes reported |
D'Antonio 1991.
| Methods | Randomisation: no information; Blinding: none; Intention to treat: yes; Exclusion from analysis: 0/71; Beginning of prophylaxis: PMN count less than 1000 End of prophylaxis: PMN count 1000 | |
| Participants | Italy, single centre; 71 neutropenic adult patients with haematological malignancies; Inpatients | |
| Interventions | Norfloxacin 400mgX2/d versus ofloxacin 400mgX2/d | |
| Outcomes | All cause mortality; Infection related death; Number of febrile patients or febrile episodes; Clinically documented febrile episodes; Microbiologically documented febrile episodes; Resistance to quinolones; Adverse events | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No exclusions |
| Selective reporting (reporting bias) | Low risk | Analysis by ITT; all expected outcomes reported. |
D'Antonio 1992.
| Methods | Randomisation: no information; Blinding: none; Intention to treat: none; Exclusion from analysis: 14/150; Beginning of prophylaxis: PMN count less than 1000 End of prophylaxis: PMN count 1000 | |
| Participants | Italy, single centre; neutropenic adult patients (PMN<1000, for more than 10 days); Inpatients | |
| Interventions | Norfloxacin 400mgX2/d versus pefloxacin 400mgX2/d | |
| Outcomes | All cause mortality; Infection related death; Number of febrile patients or febrile episodes; Clinically documented febrile episodes; Microbiologically documented febrile episodes; Resistance to quinolones; Adverse events | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 14/150 exclusions |
| Selective reporting (reporting bias) | Unclear risk | Analysis not by ITT; all expected outcomes reported. |
D'Antonio 1994.
| Methods | Randomisation: no information; Blinding: none; Intention to treat: none; Exclusion from analysis: unknown number of patients (data given in neutropenic episodes, 20/255 excluded); Beginning of prophylaxis: PMN count less than 1000 End of prophylaxis: PMN count 1000 | |
| Participants | Italy, single centre; neutropenic adult patients undergoing treatment for haematological malignancies; Inpatients | |
| Interventions | Ciprofloxacin 500mgX2/d versus ofloxacin 300mgX2/d versus pefloxacin 400mgX2/d | |
| Outcomes | All cause mortality; Infection related death; Number of febrile episodes; Clinically documented febrile episodes; Microbiologically documented febrile episodes; Resistance to quinolones; Adverse events | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 20/255 neutropenic episodes excluded |
| Selective reporting (reporting bias) | Unclear risk | Analysis not by ITT |
de Jongh 1983.
| Methods | Randomisation: no information; Blinding: double blind; Intention to treat: yes; Exclusion from analysis: none; Follow up period: three courses of chemotherapy or adverse effects or withdrawal or death | |
| Participants | USA, single centre; 61 adult patients with newly diagnosed small cell carcinoma of the lung during the initial courses of chemotherapy; Inpatients | |
| Interventions | Trimethoprim‐sulfamethoxazole 160mg/800mgX2/d versus placebo | |
| Outcomes | All cause mortality; Infection related death; Number of febrile episodes; Clinically documented febrile episodes; Microbiologically documented febrile episodes; Resistance to quinolones; Adverse events | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No exclusions |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported; analysis by ITT |
Dekker 1981.
| Methods | Randomisation: sealed opaque envelopes; Blinding: none; Intention to treat: no; Exclusion from analysis: 6/58; Beginning of prophylaxis: PMN count less than; End of prophylaxis: PMN count of 500 | |
| Participants | Netherlands, single centre; 58 adult patients with acute non‐lymphocytic leukaemia during remission induction treatment; Inpatients | |
| Interventions | Trimethoprim‐sulfamethoxazole 240mg/1200mgX2/d versus no intervention | |
| Outcomes | All cause mortality; Infection related death; Number of febrile episodes; Fever days; Clinically documented febrile episodes; Microbiologically documented febrile episodes; Resistance to trimethoprim‐sulphamethoxazole; Adverse events | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Details not available for the 2011 update |
| Allocation concealment (selection bias) | Low risk | sealed opaque envelopes (A ‐ Adequate) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 6/58 exclusions |
| Selective reporting (reporting bias) | Unclear risk | Analysis not by ITT. All expected outcomes reported. |
Dekker 1987.
| Methods | Randomisation: sealed opaque envelopes; Blinding: none; Intention to treat: no; Exclusion from analysis: 4/60; Beginning of prophylaxis: chemotherapy; End of prophylaxis: PMN count of 500 | |
| Participants | Netherlands, single centre; 60 adult patients with a first diagnosis or first relapse of acute leukaemia undergoing remission induction treatment; Inpatients | |
| Interventions | Ciprofloxacin 500mgX2/d versus trimethoprim‐sulphamethoxazole 160mg/800mgX2/d + colistin 200mgX3 | |
| Outcomes | All cause mortality; Infection related death; Number of febrile episodes; Fever days; Clinically documented febrile episodes; Microbiologically documented febrile episodes; Resistance to trimethoprim‐sulphamethoxazole; Adverse events | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Details not available for the 2011 review update |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes (A ‐ Adequate) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 4/60 exclusions |
| Selective reporting (reporting bias) | Unclear risk | Analysis not by ITT |
Dickgreber 2009.
| Methods | Randomisation: no information; Blinding: double blind; Intention to treat: not clear; Exclusion from analysis: not clear Beginning of prophylaxis: day 5 after chemotherapy End of prophylaxis: day 11 after chemotherapy | |
| Participants | all patients over the age of 65 with previously untreated advanced non small cell lung carcinoma | |
| Interventions | oral levofloxacin 500mg daily versus placebo | |
| Outcomes | Infection related mortality; Febrile patients; clinically documented infections, adverse events (only grade 3‐4 gastrointestinal events) | |
| Notes | Abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided ( B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | not clear how many excluded from study |
| Selective reporting (reporting bias) | Unclear risk | Analysis not by ITT |
Donnelly 1992b.
| Methods | Randomisation: no information; Blinding: none; Intention to treat: no; Exclusion from analysis: 48/278; Beginning of prophylaxis: chemotherapy; End of prophylaxis: PMN count of 1000 or 6 weeks | |
| Participants | Netherlands, multicentre (6 centres); 278 adult leukaemic patients expected to be neutropenic for at least one week following chemotherapy; Inpatients | |
| Interventions | Ciprofloxacin 500mgX2/d versus trimethoprim‐sulphamethoxazole 960mgX3/d + colistin 200mgX4/d | |
| Outcomes | All cause mortality; Infection related death; Number of febrile episodes; Fever days; Clinically documented febrile episodes; Microbiologically documented febrile episodes; Resistance to trimethoprim‐sulphamethoxazole; Adverse events | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 48/278 exclusions |
| Selective reporting (reporting bias) | Unclear risk | Analysis not by ITT |
Enno 1978.
| Methods | Randomisation: no information; Blinding: none; Intention to treat: no; Exclusion from analysis: 8/38; Beginning of prophylaxis: chemotherapy; End of prophylaxis: PMN count of 1000 or fever | |
| Participants | England, single centre; 30 patients over the age 15 with acute leukaemia being treated with chemotherapy; Inpatients | |
| Interventions | Trimethoprim‐sulfamethoxazole 160mg/800mgX2/d versus no intervention | |
| Outcomes | All cause mortality; Infection related death; Number of febrile patients; Clinically documented febrile episodes; Microbiologically documented febrile episodes; Adverse events | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 8/38 exclusions |
| Selective reporting (reporting bias) | Unclear risk | Analysis not by ITT. |
EORTC 1984.
| Methods | Randomisation generation by opening consecutively sealed envelopes, allocation by a central randomisation; Blinding: double blind; Intention to treat: no; Exclusion from analysis:203/545; Beginning of prophylaxis: PMN count less than 1000 for at least 6 days End of prophylaxis: resolution of neutropenia | |
| Participants | Europe, multicentre; 545 patients with haematological malignancies or solid tumours expected to be neutropenic for more than 6 days folllowing chemotherapy; Inpatients | |
| Interventions | Trimethoprim‐sulfamethoxazole 160mg/800mgX2/d versus placebo | |
| Outcomes | Number of febrile patients; Microbiologically documented febrile episodes; Resistance to trimethoprim‐sulphamethoxazole; Adverse events | |
| Notes | Journal publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centrally coordinated randomisation |
| Allocation concealment (selection bias) | Low risk | sequential sealed envelopes (A ‐ Adequate) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 203/545 exclusions |
| Selective reporting (reporting bias) | Unclear risk | Analysis not by ITT |
EORTC 1994.
| Methods | Randomisation: randomisation lists with different codes, allocation by consecutively opening sealed envelopes; Blinding: double blind; Intention to treat: no, yes‐ only for the mortality outcome; Exclusion from analysis: 15/551; Beginning of prophylaxis: chemotherapy; End of prophylaxis: resolution of neutropenia, fever, the use of IV antibiotics or death | |
| Participants | Europe, multicentre; 551 adult patients with leukaemia, lymphoma or solid tumours undergoing bone marrow transplantation; Inpatients | |
| Interventions | Pefloxacin 400mg2/d + oral penicillin v 500mgX2/d versus pefloxacin 400mg X2/d | |
| Outcomes | All cause mortality; Infection related death; Number of febrile episodes; Clinically documented febrile episodes; Microbiologically documented febrile episodes; Adverse events | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | randomisation lists |
| Allocation concealment (selection bias) | Low risk | Sequential sealed envelopes (A ‐ Adequate) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 15/551 exclusions |
| Selective reporting (reporting bias) | Unclear risk | ITT analysis done only for mortality outcome |
Estey 1984.
| Methods | Randomisation: no information; Blinding: none; Intention to treat: yes; Exclusion from analysis: unknown Beginning of prophylaxis: chemotherapy; End of prophylaxis: death or complete remission | |
| Participants | USA, single centre; 147 patients with acute leukaemia undergoing chemotherapy induction treatment; Outpatients or inpatients in standard rooms | |
| Interventions | Trimethoprim‐sulfamethoxazole 160mg/800mgX2/d versus no intervention | |
| Outcomes | All cause mortality; Infection related death; Number of febrile patients; Number of febrile episodes; Clinically documented febrile episodes; Microbiologically documented febrile episodes. | |
| Notes | ‐Journal publication ‐there were 4 arms:placebo/TMP+SMZ/ ketoconazole/ TMP‐SMZ+ketoconazole. We combined them into two groups: placebo and ketoconazole(=placebo) vs TMP‐SMZ+‐ketoconazole (=TMP‐SMZ) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Exclusions not stated |
| Selective reporting (reporting bias) | Low risk | Analysis by ITT; all expected outcomes reported. |
Fanci 1993.
| Methods | Randomisation: no information; Blinding: none; Intention to treat: no; Exclusion from analysis: 8/61; Beginning of prophylaxis: chemotherapy; End of prophylaxis: PMN count of 1000 or fever | |
| Participants | Italy, single centre; 61 adult patients with haematological malignancies designated to receive intensive chemotherapy expected to be neutropenic; Inpatients | |
| Interventions | Ciprofloxacin 500mgX2/d versus ciprofloxacin 500mgX2/d + amoxicillin 1gX1/d | |
| Outcomes | All cause mortality; Infection related death; Number of febrile episodes; Clinically documented febrile episodes; Microbiologically documented febrile episodes; Resistance to quinolones, Adverse events | |
| Notes | Journal publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 8/61 exclusions |
| Selective reporting (reporting bias) | Unclear risk | Analysis not by ITT |
Ford 1998.
| Methods | Randomisation: a table of random numbers, allocation concealment by sealed opaque envelopes; Blinding: none; Intention to treat: yes; Exclusion from analysis: none (0/84); Beginning of prophylaxis: chemotherapy; End of prophylaxis: PMN count 500 or the use of systemic antibiotics | |
| Participants | USA, single centre; 84 bone marrow recipients due to haematological malignancies or solid tumours; Inpatients | |
| Interventions | Ciprofloxacin 750mgX2/d and vancomycin versus ciprofloxacin 750mgX2/d | |
| Outcomes | All cause mortality; Infection related death; Fever days; Microbiologically documented febrile episodes; Hospitalization days; Adverse events. | |
| Notes | Journal publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | random number tables |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes (A ‐ Adequate) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No exclusions |
| Selective reporting (reporting bias) | Low risk | Analysis by ITT; all expected outcomes reported |
Garcia Saenz 2002.
| Methods | Randomisation: random number list, allocation concealment: not clear; Blinding: none;
Intention to treat: yes, according to chemotherapy cycles
Exclusion from analysis:not clear results are reported according to chemotherapy cycles
Beginning of prophylaxis: neutropenia
End of prophylaxis: neutrophil count >500 This was a randomized trial with a crossover design |
|
| Participants | 65 adult patients with solid tumors who received prior chemotherapy and were scheduled to receive intensive consolidation chemotherapy with or without autologous stem cell transplant. Malignancies included: ovarian cancer,breast cancer, sarcoma, peripheral neuro‐ectodermal tumor, other All inpatients, Hospitalized in single rooms |
|
| Interventions | intravenous Imipenem 1gr X2 daily and intravenous vancomycin 1gr X2 daily vs. no prophylaxis (administartion of imipenem when the patient became febrile Cross over: patients received either Imipenem or not after the first cycle of chemotherapy, and then crossed over to receive the opposite |
|
| Outcomes | All cause mortality; This was reported per patients after the first cycle of chemotherapy (before the crossover‐ thus, was usable for the meta‐analysis). All other outcomes reported per cycles, after the cross‐over ‐ not usable information |
|
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | random number list |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | none |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Details not available for the 2011 review update |
| Selective reporting (reporting bias) | Unclear risk | Details not available for the 2011 review update |
GIMEMA 1991.
| Methods | Randomisation: permuted blocks of ten, concealed by sealed envelopes (opaque not mentioned); Blinding:none; Intention to treat: no; Exclusion from analysis: 182/801; Beginning of prophylaxis: chemotherapy; End of prophylaxis: PMN count 1000 | |
| Participants | Italy, multicentre; 801 afebrile patients>14 y who had haematologic malignancies or BMT and chemotherapy induced neutropenia(<1000) expected to last >10 d; Inpatients, haematological units in tertiary care or university hospitals, conventional ward or single rooms | |
| Interventions | Norfloxacin 400mgX2 versus ciprofloxacin 500mgX2 + oral vancomycin 250mgX3/d | |
| Outcomes | All cause mortality; Infection related death; Number of febrile patients or episodes; Clinically documented febrile episodes; Microbiologically documented febrile episodes; Infection resistant to quinolones; Adverse events | |
| Notes | Journal publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | randomisation in blocks of ten |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes ‐ opaque not mentioned (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 182/801 exclusions |
| Selective reporting (reporting bias) | Unclear risk | Analysis not by ITT. |
Gluckman 1988.
| Methods | Randomisation: no information; Blinding: none; Intention to treat: yes; Exclusion from analysis: none (0/65); Beginning of prophylaxis: 8 days before bone marrow transplantation; End of prophylaxis: 15 days after bone marrow transplantation | |
| Participants | France, single centre; 65 patients treated by allogeneic bone marrow transplantation; Inpatients in laminar airflow rooms | |
| Interventions | Pefloxacin 400mg/d and IV penicillin 3 million units/d versus cephalotin, gentamicin and bacitracin 3g, 240mg and 1800IU respectively | |
| Outcomes | All cause mortality; Infection related death; Fever days; Clinically documented febrile episodes; Microbiologically documented febrile episodes; Infection resistant to quinolones | |
| Notes | Journal publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No exclusions |
| Selective reporting (reporting bias) | Low risk | Anaylsis by ITT. All expected outcomes reported. |
Gluckman 1991.
| Methods | Randomisation: no information; Blinding: none; Intention to treat: yes; Exclusion from analysis: none (0/44); Beginning of prophylaxis: 8 days before bone marrow transplantation; End of prophylaxis: 15 days after bone marrow transplantation | |
| Participants | France, single centre; 44 patients undergoing bone marrow transplantation for leukaemia or aplastic anaemia; Inpatients in laminar airflow rooms | |
| Interventions | Oral vancomycin 450mg/d, tobramycin 450mg/d and colistin 4.5 million units daily (divided in nine capsules) versus ofloxacin 200mgX2/d and amoxicillin 1gX2/d | |
| Outcomes | All cause mortality; Infection related death; Fever days; Microbiologically documented febrile episodes; Adverse events. | |
| Notes | Journal publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No exclusions |
| Selective reporting (reporting bias) | Low risk | Anaylsis by ITT. All expected outcomes reported. |
Gomez‐Martin 2000.
| Methods | Randomisation: consecutively drawn, sealed envelopes (opaque not mentioned); Blinding:none; Intention to treat: no; Exclusion from analysis: 7/130; Beginning of prophylaxis: 10 days before bone marrow transplantation; End of prophylaxis: PMN count 500 or fever | |
| Participants | Spain, multicentre; 130 patients undergoing high dose chemotherapy with peripheral stem cell transplantation; Inpatients in private rooms | |
| Interventions | Ciprofloxacin 500mgX3/d versus ciprofloxacin 500mgX3/d + rifampin 300mgX2/d | |
| Outcomes | All cause mortality; Infection related death; febrile patients; Fever days; Clinically documented febrile episodes; Microbiologically documented febrile episodes; Infection resistant to quinolone; Hospitalization days; Adverse events. | |
| Notes | Journal publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Details not available for the 2011 review update |
| Allocation concealment (selection bias) | Low risk | Sequential sealed envelopes (A ‐ Adequate) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 7/130 exclusions |
| Selective reporting (reporting bias) | Unclear risk | Analysis not by ITT; all expected outcomes reported. |
Goorin 1985.
| Methods | Randomisation: no information; Blinding:double blind; Intention to treat: no; Exclusion from analysis: 1/60; Beginning of prophylaxis: chemotherapy; End of prophylaxis: 40 weeks | |
| Participants | USA, single centre; 61 newly diagnosed children with acute lymphoblastic leukaemia expected to receive intensive chemotherapy; Inpatients | |
| Interventions | Trimethoprim‐sulfamethoxazole 80mg/400mg X2/d versus placebo | |
| Outcomes | All cause mortality; Infection related death; Number of febrile episodes; Clinically documented febrile episodes; Microbiologically documented febrile episodes; Hospitalization days; Number of hospitalisations | |
| Notes | Journal publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided. |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1/61 exclusions |
| Selective reporting (reporting bias) | Unclear risk | Analysis not by ITT |
Gualtieri 1983.
| Methods | Randomisation: no information; Blinding: double blind; Intention to treat: no; Exclusion from analysis: 19/66; Beginning of prophylaxis: PMN count less than 1000 End of prophylaxis: PMN count of 1000 | |
| Participants | USA, single centre; 66 adult patients with haematological malignancies and neutropenia; Inpatients | |
| Interventions | Trimethoprim‐sulfamethoxazole 160mg/800mg X2/d versus placebo | |
| Outcomes | All cause mortality; Infection related death; Number of febrile episodes; Fever days; Clinically documented febrile episodes; Microbiologically documented febrile episode; Infection resistant to trimethoprim‐sulphamethoxazole; Adverse events. | |
| Notes | Journal publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 19/66 exclusions |
| Selective reporting (reporting bias) | Unclear risk | high attrition |
Guiot 1983.
| Methods | Randomisation: no information; Blinding: double blind; intention to treat: no; Exclusion from analysis: 9/42; Beginning of prophylaxis: chemotherapy; End of prophylaxis: PMN count of 500 | |
| Participants | Netherlands, single centre; 42 adult patients with acute leukaemia undergoing induction chemotherapy; Inpatients | |
| Interventions | Neomycin, amphotericin, nalidixic acid, polymyxin in doses of 250mgX4/d, 250mgX4/d, 1gX2/d, 100mgX4/d respectively versus placebo | |
| Outcomes | All cause mortality; Infection related death; Number of febrile patients or febrile episodes; Fever days; Clinically documented febrile episodes; Microbiologically documented febrile episodes; Adverse events. | |
| Notes | Journal publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 9/42 exclusions |
| Selective reporting (reporting bias) | Unclear risk | Analysis not by ITT |
Guiot 1992.
| Methods | Randomisation: randomly drawn, envelopes containing lots coded for one of the two types of prophylaxis (opaque not mentioned); Blinding: none; Intention to treat: no; Exclusion from analysis: unknown (data given in episodes); Follow up period: 14 days, from beginning of chemotherapy | |
| Participants | Netherlands, single centre; 48 patients undergoing aggressive antileukaemic therapy; Inpatients in single rooms | |
| Interventions | IV Penicillin G 4 million units/d versus IV trimethoprim‐sulphamethoxazole 160mg/800mg X2/d | |
| Outcomes | All cause mortality; Infection related death; Number of febrile episodes; Clinically documented febrile episodes; Microbiologically documented febrile episodes; Infection resistant to trimethoprim‐sulphamethoxazole; Adverse events | |
| Notes | Journal publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | randomly drawn envelopes |
| Allocation concealment (selection bias) | Unclear risk | envelopes not stated as opaque (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Selective reporting (reporting bias) | Unclear risk | Analysis not by ITT |
Gurwith 1979.
| Methods | Randomisation: by a table of random numbers, allocation concealment by an independent pharmacy; Blinding: none; Intention to treat: unknown (randomisation and follow up according to neutropenic episodes, not patients); Exclusion from analysis: exclusion of patients unknown (no episodes excluded); Beginning of prophylaxis: PMN count less than 1000 End of prophylaxis: PMN count of 1000 | |
| Participants | Canada, single centre; granulocytopenic patients due to various malignancies (36% haematological malignancies), (a total of 111 neutropenic episodes); Inpatients | |
| Interventions | Trimethoprim‐sulfamethoxazole 160mg/800mg X2/d versus no intervention | |
| Outcomes | All cause mortality; Infection related death; Fever days; Clinically documented febrile episodes; Microbiologically documented febrile episodes; Resistance to trimethoprim‐sulphamethoxazole; Adverse events. | |
| Notes | Journal publication In the beginning ‐ there was the nonabsorbable group which received: nystatin 1mil X4, neomycin 500mg X4, polymyxin 50mg X4, chlorhexidine. After 4 months it was discontinued because of poor acceptance. Also, all patients were hospitalised | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | random number table |
| Allocation concealment (selection bias) | Low risk | pharmacy allocation (A ‐ Adequate) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Details not available for the 2011 review update |
| Selective reporting (reporting bias) | Unclear risk | The non‐absorbable group was discontinued due to poor acceptance |
Hargadon 1981.
| Methods | Randomisation: no information; Blinding: double blind; Intention to treat: no; Exclusion from analysis: none; Follow up period: 5‐6 weeks, from beginning of chemotherapy | |
| Participants | USA, single centre; 8 patients with small cell carcinoma of lung undergoing induction chemotherapy and 8 patients with leukaemia undergoing re induction; Setting not specified | |
| Interventions | for lung cancer patients: trimethoprim‐sulphamethoxazole 160mg/800mg X2/d versus placebo; for leukaemic patients: gentamicin liquid 200mg + nystatin tablets (4 million units) and nystatin suspension 1 million unit, all X6/d versus trimethoprim‐sulphamethoxazole 160mg/800mg X2/d + nystatin suspension 1 million units X6/d | |
| Outcomes | No relevant outcomes: the trial evaluates effect of prophylactic antibiotics on rectal flora | |
| Notes | Journal publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Details not available for the 2011 review update |
| Selective reporting (reporting bias) | Unclear risk | Analysis not by ITT. No relevant outcomes |
Harousseau 1987.
| Methods | Randomisation: random table of numbers, allocation concealed by envelopes; Blinding: none; Intention to treat: yes; Exclusion from analysis: none (0/41); Beginning of prophylaxis: PMN count less than 500 End of prophylaxis: PMN count 500 | |
| Participants | France, single centre; 64 neutropenic patients with haematological malignancies (and neuroblastoma) undergoing bone marrow transplantation; Inpatients | |
| Interventions | IV ceftriaxone 2gX1/d versus no intervention | |
| Outcomes | Number of febrile episodes; Fever days; Microbiologically documented febrile episodes | |
| Notes | Journal publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | random number tables |
| Allocation concealment (selection bias) | Unclear risk | allocation by envelopes not stated as sealed/opaque (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No exclusions/attrition |
| Selective reporting (reporting bias) | Low risk | Analysis by ITT. |
Hartlapp 1987.
| Methods | Randomisation: no information; Blinding: none; Intention to treat: unknown; Exclusion from analysis: unknown; Beginning of prophylaxis: 7 days after chemotherapy; End of prophylaxis: 10 days | |
| Participants | Germany, single centre; 42 patients with metastatic testicular germ cell tumours after cytostatic treatment; Outpatients | |
| Interventions | Ofloxacin 200mgX2/d versus no intervention | |
| Outcomes | Number of febrile patients; Microbiologically documented febrile episodes; Adverse events. | |
| Notes | Journal publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Details not available for the 2011 review update |
| Selective reporting (reporting bias) | Unclear risk | Details not available for the 2011 review update |
Henry 1984b.
| Methods | Randomisation: no information; Blinding: none; Intention to treat: yes; Exclusion from analysis: none (0/43); Beginning of prophylaxis: PMN count less than 1000; End of prophylaxis: PMN count of 1000 | |
| Participants | USA, single centre; 43 adult patients with newly diagnosed and relapsed acute leukaemia undergoing remission induction or reinduction chemotherapy; Outpatients | |
| Interventions | Trimethoprim‐sulfamethoxazole 160mg/800mgX2/d versus no intervention | |
| Outcomes | All cause mortality; Infection related death; Number of febrile patients; Fever days; Clinically documented febrile episodes; Microbiologically documented febrile episodes; Infection resistance to trimethoprim‐sulphamethoxazole; Adverse events. | |
| Notes | Journal publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No exclusions |
| Selective reporting (reporting bias) | Unclear risk | Details not available for the 2011 review update |
Hidalgo 1997.
| Methods | Randomisation: no information; Blinding: none; Intention to treat: unknown; Exclusion from analysis: unknown; Beginning of prophylaxis: 2 days before peripheral stem cell transplantation; End of prophylaxis: PMN count of 500 or fever | |
| Participants | Spain, single centre; 40 patients, most with solid malignancies, undergoing high dose chemotherapy with peripheral stem cell transplantation; Inpatients | |
| Interventions | Ciprofloxacin 500mgX3/d versus ciprofloxacin 500mgX3/d + rifampin 300mgX2/d | |
| Outcomes | All cause mortality; Infection related death; Febrile patients; Fever days; Clinically documented febrile episodes; Microbiologically documented febrile episodes; Infection resistant to quinolones; Hospitalization days; Adverse events. | |
| Notes | Journal publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Details not available for the 2011 review update |
| Selective reporting (reporting bias) | Unclear risk | Details not available for the 2011 review update |
Inoue 1983.
| Methods | Randomisation: no information; Blinding: double blind; Intention to treat: yes; Exclusion from analysis: 0/102; Beginning of prophylaxis: chemotherapy End of prophylaxis: end of chemotherapy (2‐8.5 months) | |
| Participants | Japan, multicentre; 102 children with acute leukaemia undergoing chemotherapy; Outpatients | |
| Interventions | Trimethoprim‐sulfamethoxazole 0.025g/kX2/d versus placebo | |
| Outcomes | Febrile patients; Fever days; Clinically documented febrile episodes; Microbiologically documented febrile episodes. | |
| Notes | Journal publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No exclusions |
| Selective reporting (reporting bias) | Low risk | Analysis by ITT. All outcomes reported. |
Jansen 1994.
| Methods | Randomisation: a table of random numbers, concealment in white envelopes stored in boxes Blinding: none; Intention to treat: no; Exclusion from analysis: 9/105; Beginning of prophylaxis: chemotherapy; End of prophylaxis: PMN count of 500 | |
| Participants | USA, single centre; 105 adult patients undergoing bone marrow transplantation or aggressive chemotherapy for acute leukaemia or blast crisis of chronic leukaemia; Inpatients | |
| Interventions | Ciprofloxacin 500mgX2/d versus neomycin 250mgX4/d + polymyxin 100mgX4/d + nalidixic acid 1gX2/d | |
| Outcomes | All cause mortality; Infection related death; Number of febrile patients; Clinically documented febrile episodes; Microbiologically documented febrile episodes; Adverse events. | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | random number table |
| Allocation concealment (selection bias) | Low risk | opaque envelopes (A ‐ Adequate) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 9/105 exclusions |
| Selective reporting (reporting bias) | Unclear risk | Details not available for the 2011 review update |
Jehn 1981.
| Methods | Randomisation: no information; Blinding: none; Intention to treat: unknown; Exclusion from analysis: unknown; Beginning of prophylaxis: chemotherapy; End of prophylaxis: PMN count of 1000 | |
| Participants | Germany, single centre; 49 adult patients with acute leukaemia undergoing chemotherapy; Inpatients in conventional wards | |
| Interventions | Neomycin, colistin (500mg, 1.5 million units respectively) versus no intervention | |
| Outcomes | All cause mortality; Infection related death; Number of febrile patients; Clinically documented febrile episodes; Microbiologically documented febrile episodes | |
| Notes | ‐Journal Publication ‐there were originally 3 treatment groups:nonabsorbable antibiotics,regular ward without antibiotics, strict isolation+nonabsorbable antibiotics. Only group1 and 2 were included | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Details not available for the 2011 review update |
| Selective reporting (reporting bias) | Unclear risk | The nonabsorbable group was not reported |
Karp 1987.
| Methods | Randomisation: by a random number table, allocation by a central pharmacy; Blinding: double blind; intention to treat: yes; Exclusion from analysis: none (0/68); Beginning of prophylaxis: chemotherapy; End of prophylaxis: PMN count of 500 | |
| Participants | USA, single centre; 68 adult patients with acute leukaemia and chemotherapy‐induced neutropenia; Inpatients | |
| Interventions | Norfloxacin 400mgX2/d versus placebo | |
| Outcomes | All cause mortality; Infection related death; Number of febrile patients; Microbiologically documented febrile episodes; Infection resistant to quinolone; Adverse events. | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | random number table |
| Allocation concealment (selection bias) | Low risk | allocation by pharmacy (A ‐ Adequate) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No exclusions |
| Selective reporting (reporting bias) | Low risk | ITT analysis; all outcomes reported. |
Kauffman 1983.
| Methods | Randomisation: no information; Blinding:none; Intention to treat: no; Exclusion from analysis: 11/55; Beginning of prophylaxis: chemotherapy; End of prophylaxis: duration of chemotherapy and discharge from hospital | |
| Participants | USA, multicentre; 55 patients over the age of 16 with haematological malignancies or solid tumours who were to be given high dose chemotherapy resulting in severe neutropenia; Inpatients | |
| Interventions | trimethoprim‐sulphamethoxazole 80mg/400mgX2/d versus no intervention | |
| Outcomes | All cause mortality; Infection related death; Fever days; Number of febrile episodes; Clinically documented febrile episodes; Microbiologically documented febrile episodes; Infection resistant to trimethoprim‐sulphamethoxazole; Adverse events | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 11/55 excluded |
| Selective reporting (reporting bias) | Unclear risk | Analysis not by ITT; 20% excluded from analysis |
Kern 1991b.
| Methods | Randomisation: no information; Blinding:none; Intention to treat: no; Exclusion from analysis: 32/160; Beginning of prophylaxis: chemotherapy; End of prophylaxis: PMN count of 1000 or termination of chemotherapy | |
| Participants | Germany, Single centre; 160 patients with acute leukaemia who received aggressive cytotoxic chemotherapy; Inpatients | |
| Interventions | Ofloxacin 200mgX3/d versus trimethoprim‐sulphamethoxazole 960mgX3/d | |
| Outcomes | All cause mortality; Infection related death; Number of febrile patients or episodes; Fever days; Clinically documented febrile episodes; Microbiologically documented febrile episodes; Adverse events | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 32/160 exclusions |
| Selective reporting (reporting bias) | Unclear risk | Analysis not by ITT; all expected outcomes reported |
Kern 1994b.
| Methods | Randomisation: no information; Blinding:none; Intention to treat: no; Exclusion from analysis: 10/141; Beginning of prophylaxis: chemotherapy; End of prophylaxis: development of fever | |
| Participants | Germany, Single centre; 141 afebrile patients>16 y who had acute leukaemia and received bone marrow transplantation and chemotherapy; Inpatients, most patients in standard rooms, bone marrow transplant patients in private rooms | |
| Interventions | Roxythromycin 150 mgX2 + ofloxacin 200 mgX3 versus ofloxacin 200 mg X3 | |
| Outcomes | All cause mortality; Infection related death; Number of febrile patients or febrile episodes; Clinically documented febrile episodes; Microbiologically documented febrile episodes; Infection resistant to quinolones; Adverse events. | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 10/141 exclusions |
| Selective reporting (reporting bias) | Unclear risk | Analysis not by ITT; all expected outcomes reported |
Klastersky 1974.
| Methods | Randomisation: no information; Blinding: none; Intention to treat: no; Exclusion from analysis: unknown; Beginning of prophylaxis: chemotherapy; End of prophylaxis: PMN count of 1000 | |
| Participants | France, single centre; 43 patients with haematological malignancies undergoing cytotoxic therapy | |
| Interventions | neomycin, bacitracin, kanamycin, polymyxin, nystatin (3.5g,10000 units, 3g, 850mg, 1 million units respectively) versus no prophylaxis | |
| Outcomes | All cause mortality; Infection related death; number of febrile episodes; clinically documented febrile episode; microbiologically documented febrile episode; | |
| Notes | ‐Journal Publication ‐there were originally 3 treatment groups: isolation+nonabsorbable antibiotics,nonabsorbable antibiotics alone, regular ward without prophylaxis. Only groups 2 and 3 were included | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Details not available for the 2011 review update |
| Selective reporting (reporting bias) | Unclear risk | Only two out of three allocation groups were reported |
Kovatch 1985.
| Methods | Randomisation: no information; Blinding: double blind; Intention to treat: no; Exclusion from analysis: 17/91; Beginning of prophylaxis: chemotherapy; End of prophylaxis: remission of leukaemia or infection or 60 days | |
| Participants | USA, single centre; 91 infants, children and adolescents with haematological malignancies or solid tumours, during intensive chemotherapy treatment; Inpatients | |
| Interventions | Trimethoprim‐sulfamethoxazole 3mg/kgX2/d versus placebo | |
| Outcomes | All cause mortality; Infection related death; Number of febrile patients or febrile episodes; Clinically documented febrile episodes; Microbiologically documented febrile episodes; Infection resistant to trimethoprim‐sulphamethoxazole; Adverse events. | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | double blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 17/91 exclusions |
| Selective reporting (reporting bias) | Unclear risk | Analysis not ITT |
Kramer 1984.
| Methods | Randomisation: by a randomisation list prepared by the pharmacy, allocation concealment by a pharmacy; Blinding:triple blind; Intention to treat: no; Exclusion from analysis: 21/66; Beginning of prophylaxis: chemotherapy; End of prophylaxis: PMN count of 500 or development of febrile neutropenia | |
| Participants | USA, single centre; 66 adult cancer patients (haematological and solid malignancies, mostly lung) receiving cytotoxic chemotherapy expected to result in significant neutropenia; Inpatients in private rooms with reverse isolation (patients in whom prolonged marrow suppression was anticipated) or outpatients | |
| Interventions | Trimethoprim‐sulfamethoxazole 320mg/1600mgX2/d + erythromycin 1gX2/d versus placebo | |
| Outcomes | All cause mortality; Infection related death; Number of febrile episodes; Clinically documented febrile episodes; Microbiologically documented febrile episodes; Adverse events | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | list prepared by pharmacy |
| Allocation concealment (selection bias) | Low risk | allocation concealed by pharmacy (A ‐ Adequate) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Triple blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 21/66 excluded |
| Selective reporting (reporting bias) | Unclear risk | High exclusion/attrition |
Kurrle 1983.
| Methods | Randomisation: computer generated, allocation by central randomisation from a remote centre; Blinding: none; Intention to treat: no (ITT only for mortality); Exclusion from analysis: 22/100; Beginning of prophylaxis: chemotherapy; End of prophylaxis: PMN count of 1000 | |
| Participants | Germany, single centre; 100 patients with acute leukaemia during remission induction chemotherapy; Inpatients | |
| Interventions | Trimethoprim‐sulfamethoxazole 160mg/800mgX3/d + polymyxin 100mgX4/d + nystatin (6 million units /d (divided by four) versus nalidixic acid 1gX4/d, neomycin 250mgX4/d, polymyxin 100mgX4/d and nystatin (6 million units /d (divided by four) | |
| Outcomes | All cause mortality; Infection related death; Number of febrile patients; Fever days; Clinically documented febrile episodes; Microbiologically documented febrile episodes; Adverse events | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | computer generated |
| Allocation concealment (selection bias) | Low risk | central coordinated allocation (A ‐ Adequate) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 22/100 exclusions |
| Selective reporting (reporting bias) | Unclear risk | ITT analysis done only for mortality |
Kurrle 1986.
| Methods | Randomisation: no information; Blinding: none; Intention to treat: no (ITT only for outcome of mortality); Exclusion from trial:15/155; Beginning of prophylaxis: chemotherapy; End of prophylaxis: PMN of 1000 | |
| Participants | Germany, single centre; 155 adult patients with a diagnosis of acute leukaemia undergoing remission induction chemotherapy; Inpatients in conventional ward | |
| Interventions | Oral nonabsorbable antibiotics: colistin 200mgX4/d + neomycin 250mgX4/d versus trimethoprim‐sulphamethoxazole 160mg/800mgX3/d and neomycin 250mgX4/d | |
| Outcomes | All cause mortality; Infection related death; Number of febrile patients; Fever days; Clinically documented febrile episodes; Microbiologically documented febrile episodes; Adverse events | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 15/155 exclusions |
| Selective reporting (reporting bias) | Unclear risk | ITT analysis only for outcome of mortality |
Lalami 2004.
| Methods | Randomisation: unclear, allocation unclear; Blinding: none; Intention to treat: yes Exclusion from analysis:no exclusions, 0/48; Beginning of prophylaxis: 48 hours after completion of chemotherapy; End of prophylaxis: PMN count of 2000 | |
| Participants | Belgium (Brussels) and Athens, multicenter centre; adult patients (age >18 years old) treated by chemotherapy for solid tumours who presented with a previous episode of febrile neutropenia after a previous chemotherapy cycle, patients were scheduled for another cycle of the same chemotherapy. All patients also received granulocyte‐colony stimulating factor |
|
| Interventions | ciprofloxacin 500mg X2 daily and amoxicillin+clavulonic acid 500mg X3 daily vs. no prophylaxis | |
| Outcomes | All cause mortality;
Infection related death;
Number of febrile patients; Fever days;
Clinically documented febrile episodes;
Microbiologically documented febrile episodes; Bacteremia Adverse events (reported only according to grades, not entered into the meta‐analysis) |
|
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | unclear |
| Allocation concealment (selection bias) | Unclear risk | unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no exclusions |
| Selective reporting (reporting bias) | Low risk | ITT analysis, all prespecified outcomes reported |
Lamy 1993.
| Methods | Randomisation: table of random numbers, allocation concealment by sealed and opaque envelopes; Blinding: none; Intention to treat: no, ITT only for mortality outcome; Exclusion from analysis: 9/59; Beginning of prophylaxis: chemotherapy; End of prophylaxis: PMN count of 500 | |
| Participants | France, single centre; 59 adult patients mostly with haematological malignancies undergoing induction chemotherapy, chemo‐consolidation or bone marrow transplantation and a few with solid tumours undergoing autologous bone marrow transplantation; Inpatients in laminar airflow rooms | |
| Interventions | IV vancomycin 15mg/kg twice daily versus no intervention | |
| Outcomes | All cause mortality; Infection related death; Number of febrile patients; Fever days; Microbiologically documented febrile episodes; Adverse events | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | random number tables |
| Allocation concealment (selection bias) | Low risk | sealed opaque envelopes (A ‐ Adequate) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 9/59 excluded |
| Selective reporting (reporting bias) | Unclear risk | ITT analysis only for mortality outcome |
Lange 1984.
| Methods | Randomisation: according to birth dates, allocation concealment unknown; Blinding:none; Intention to treat: no; Exclusion from analysis: 7/67; Beginning of prophylaxis: chemotherapy; End of prophylaxis: not specified | |
| Participants | USA, single centre; 67 children with acute lymphoblastic leukaemia during remission induction therapy; Outpatients | |
| Interventions | Trimethoprim‐sulfamethoxazole 5mg/kg, 25mg/kg respectively X2/d versus no intervention | |
| Outcomes | All cause mortality; Infection related death; Number of febrile patients; Clinically documented febrile episodes; Microbiologically documented febrile episodes; Adverse events | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | quasi‐randomisation according to birth dates |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 7/67 exclusions |
| Selective reporting (reporting bias) | Unclear risk | Analysis not by ITT; all expected outcomes reported |
Lee 2002.
| Methods | Randomisation: no information; Blinding: none; Intention to treat: yes; Exclusion from analysis: none, 0/95 Beginning of prophylaxis: chemotherapy; End of prophylaxis: PMN count of 500 or fever | |
| Participants | Korea, single centre; 95 patients with acute myeloid leukaemia receiving chemotherapy; Inpatients | |
| Interventions | Ciprofloxacin 250mgX2/d, roxythromycin 150mgX2/d, fluconazole 50mg/d versus no intervention | |
| Outcomes | All cause mortality; Infection related death; Number of febrile patients; Clinically documented febrile episodes; Microbiologically documented febrile episodes; Infection resistant to quinolones; Hospitalization days | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No exclusions |
| Selective reporting (reporting bias) | Low risk | Analysis by ITT. All expected outcomes reported. |
Levine 1973.
| Methods | Randomisation: no information; Blinding: none; Intention to treat: no; Exclusion from analysis: 4/70; Beginning of prophylaxis: chemotherapy; End of prophylaxis: PMN count of 750 or remission | |
| Participants | USA, single centre; 70 patients with acute leukaemia, between 15 and 65 years of age scheduled to receive remission induction chemotherapy; Inpatients in conventional ward | |
| Interventions | Nonabsorbable antibiotics: gentamicin, vancomycin and nystatin (dosage not specified in text) versus no intervention | |
| Outcomes | All cause mortality; Infection related death; Clinically documented febrile episodes; Microbiologically documented febrile episodes | |
| Notes | Journal Publication The trial compares three groups: gr 1 ‐ protected environment, gr 2 ‐ oral nonabsorbable, gr 3 ‐ placebo. gr 1 was excluded from our study, since it examines a different intervention | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 4/70 exclusions |
| Selective reporting (reporting bias) | Unclear risk | Analysis not by ITT |
Lew 1991.
| Methods | Randomisation: generation unclear, allocation not specified; Blinding: double blind; Intention to treat: no; Exclusion from analysis:8/26; Beginning of prophylaxis: chemotherapy; End of prophylaxis: Fever or PMN count of 500 | |
| Participants | USA, single centre; 26 oncology patients (mostly acute leukaemia and lymphoma but also solid malignancies) receiving chemotherapy, of whom 25 received bone marrow transplantation; Inpatients in private rooms | |
| Interventions | Ciprofloxacin 750mgX2/d versus placebo | |
| Outcomes | All cause mortality; Infection related death; Febrile patients; Fever days; Clinically documented febrile episodes; Microbiologically documented febrile episodes; Adverse events | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 8/26 exclusions |
| Selective reporting (reporting bias) | Unclear risk | High exclusion rate. No ITT analysis. |
Lew 1995.
| Methods | Randomisation: generation unclear, allocation by a remote pharmacy; Blinding: double blind; Intention to treat: no; Exclusion from analysis:22/167; Beginning of prophylaxis: chemotherapy; End of prophylaxis: Fever or PMN count of 400 | |
| Participants | USA, single centre; 67 adult patients about to undergo bone marrow transplantation for the treatment of haematological (64%) and solid malignancies; Inpatients in private rooms | |
| Interventions | Ciprofloxacin 750mgX2/d versus trimethoprim‐sulphamethoxazole 160mg/800mgX2/d | |
| Outcomes | All cause mortality; Infection related death; Febrile patients, Clinically documented febrile episodes; Microbiologically documented febrile episodes; Infection resistant to quinolones; Adverse events | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | randomisation and allocation by pharmacy |
| Allocation concealment (selection bias) | Low risk | done centrally by pharmacy (A ‐ Adequate) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 22/167 exclusions |
| Selective reporting (reporting bias) | Unclear risk | No ITT analysis |
Liang 1990.
| Methods | Randomisation: no information; Blinding:none; Intention to treat: no; Exclusion from analysis: 8/110; Beginning of prophylaxis: chemotherapy; End of prophylaxis: PMN count of 500 or development of side effects | |
| Participants | Hong Kong, single centre; 110 patients with haematological malignancies undergoing cytotoxic chemotherapy; Inpatients, university hospital | |
| Interventions | Ofloxacin 300mgX2/d versus trimethoprim‐sulphamethoxazole 80mg/400mgX2/d | |
| Outcomes | Infection related death; Febrile patients; Microbiologically documented febrile episodes; Infection resistant to quinolones; Adverse events | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 8/110 exclusions |
| Selective reporting (reporting bias) | Unclear risk | Analysis not by ITT |
Maiche 1993.
| Methods | Randomisation: no information; Blinding: none; Intention to treat: unknown; Exclusion from analysis: unknown; Beginning of prophylaxis: chemotherapy; End of prophylaxis: development of fever | |
| Participants | Finland, single centre; 59 patients with haematological and solid tumours, who have had a previous infection following chemotherapy; Inpatients in standard ward rooms | |
| Interventions | Ofloxacin 200mgX2/d or ciprofloxacin 750mgX2/d versus no intervention (both groups received GCSF) | |
| Outcomes | Febrile episodes; Clinically documented febrile episodes; Microbiologically documented febrile episodes; Adverse events | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Details not available for the 2011 review update |
| Selective reporting (reporting bias) | Unclear risk | ITT analysis and exclusions not described |
Malarme 1981.
| Methods | Randomisation: no information on generation, but concealment by a remote pharmacy providing the capsules; Blinding: triple blind; Intention to treat: unknown; Exclusion from analysis: unknown; Beginning of prophylaxis: chemotherapy; End of prophylaxis: PMN count of 1000, side effects or death | |
| Participants | Belgium, single centre; 63 adult patients with leukaemia, lymphoma or solid tumours receiving chemotherapy; Inpatients in single rooms | |
| Interventions | Oral vancomycin 500mg + gentamIcin 160mg + nystatin 2million units, all X3/d versus trimethoprim‐sulphamethoxazole 80mg/400mgX3/d + nystatin 2million unitsX3/d versus vancomycin + trimethoprim‐sulphamethoxazole + gentamIcin + nystatin (all in the same dosages) | |
| Outcomes | Number of febrile episodes; Clinically documented febrile episodes; Microbiologically documented febrile episodes; Adverse events | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Pharmacy coordinated |
| Allocation concealment (selection bias) | Low risk | pharmacy allocation (A ‐ Adequate) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Triple blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Details not available for the 2011 review update |
| Selective reporting (reporting bias) | Unclear risk | ITT analysis and exclusions not described. |
Martino 1984.
| Methods | Randomisation: randomisation by a casual choice of packages containing the indication for prophylaxis or not, allocation concealment not specified; Blinding: none; Intention to treat: unknown; Exclusion from analysis: unknown; Beginning of prophylaxis: chemotherapy; End of prophylaxis: PMN count of 500 | |
| Participants | Italy, single centre; 63 adult patients with acute leukaemia undergoing cytostatic treatment; Inpatients | |
| Interventions | Trimethoprim‐sulfamethoxazole 160mg/800mgX2/d versus no intervention | |
| Outcomes | All cause mortality; Infection related death; Number of febrile patients; Number of febrile episodes; Microbiologically documented febrile episodes; Adverse events | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | quasi randomisation |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Details not available for the 2011 review update |
| Selective reporting (reporting bias) | Unclear risk | ITT and exclusions not described |
Maschmeyer 1988.
| Methods | Randomisation: allocation concealment by central randomisation Blinding: none; Intention to treat: no; Exclusion from analysis: 3/51; Beginning of prophylaxis: chemotherapy; End of prophylaxis: PMN count of 1000 or adverse events | |
| Participants | Germany, single centre; 51 adult patients with acute leukaemia undergoing aggressive remission induction chemotherapy; Inpatients in standard ward rooms | |
| Interventions | Ciprofloxacin 500mgX2/d versus ciprofloxacin 250mgX2/d versus norfloxacin 200mgX2/d versus norfloxacin 400mg X2/d | |
| Outcomes | All cause mortality; Infection related death; Number of febrile patients; Clinically documented febrile episodes; Microbiologically documented febrile episodes; Adverse events | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centrally coordinated randomisation |
| Allocation concealment (selection bias) | Low risk | centrally coordinated (A ‐ Adequate) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 3/51 exclusions |
| Selective reporting (reporting bias) | Unclear risk | Analysis not by ITT |
Mocikova 1992.
| Methods | Randomisation: no information; Blinding: none; Intention to treat: unknown; Exclusion from analysis: unknown; Beginning of prophylaxis: chemotherapy; End of prophylaxis: PMN count of 500 | |
| Participants | Country not stated, single centre; 42 patients undergoing induction treatment for acute leukaemia; Setting not specified | |
| Interventions | Ofloxacin 200mgx2/d versus trimethoprim‐sulphamethoxazole 1440mgX2/d | |
| Outcomes | All cause mortality; Infection related death; Number of febrile patients; Clinically documented febrile episodes; Microbiologically documented febrile episodes; infection resistant to quinolones and trimethoprim‐sulphamethoxazole | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Details not available for the 2011 review update |
| Selective reporting (reporting bias) | Unclear risk | ITT and exclusions not described. |
Moreau 1995.
| Methods | Randomisation: no information; Blinding: none; Intention to treat: unknown; Exclusion from analysis: unknown; Beginning of prophylaxis: chemotherapy; End of prophylaxis: fever | |
| Participants | France, single centre; 130 patients treated for haematological malignancies with prolonged aplasia>14 days; Inpatients in laminar airflow rooms | |
| Interventions | IV ciprofloxacin 200mgX2/d plus amoxicillin‐clavulanic acid 1gX3/d versus IV vancomycin 1gX2/d versus no intervention | |
| Outcomes | All cause mortality; Infection related death; Number of febrile patients; Fever days; Microbiologically documented febrile episodes | |
| Notes | Abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Details not available for the 2011 review update |
| Selective reporting (reporting bias) | Unclear risk | ITT and exclusions not described |
Moriuchi 1990.
| Methods | Randomisation: no information; Blinding: none; Intention to treat: yes; Exclusion from analysis:none; Beginning of prophylaxis: chemotherapy; End of prophylaxis: PMN count of 1000 | |
| Participants | Japan, single centre; 24 patients receiving intensive chemotherapy for acute non‐lymphocytic leukaemia; Inpatients | |
| Interventions | Ciprofloxacin 600mgX3/d versus polymyxin B 300mgX3/d | |
| Outcomes | Number of febrile episodes; Fever days; Clinically documented febrile episodes; Microbiologically documented febrile episodes | |
| Notes | Journal Publication ‐ in Japanese | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Details not available for the 2011 review update |
| Selective reporting (reporting bias) | Low risk | Analysis by ITT; no exclusions |
Murase 1995.
| Methods | Randomisation: consecutively drawn, sealed envelopes, opaque not specified; Blinding: no; Intention to treat: yes; Exclusion from analysis:0/53; Beginning of prophylaxis: chemotherapy; End of prophylaxis: PMN count of 1000 | |
| Participants | Japan, single centre; 53 patients with haematological malignancies receiving chemotherapy; | |
| Interventions | Trimethoprim‐sulfamethoxazole 80mg/400mgX3/d versus trimethoprim‐sulphamethoxazole 80mg/400mgX3/d + ciprofloxacin 200mgX3/d | |
| Outcomes | Number of febrile episodes; Fever days; Microbiologically documented febrile episodes; Adverse events. | |
| Notes | Journal Publication ‐ in Japanese | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | sequential sealed envelopes, not stated as opaque (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No exclusions |
| Selective reporting (reporting bias) | Low risk | Analysis by ITT |
Nemet 1989.
| Methods | Randomisation: no information; Blinding:none; Intention to treat: unknown; Exclusion from analysis: unknown; Beginning of prophylaxis: chemotherapy; End of prophylaxis: PMN count of 1000 | |
| Participants | Yugoslavia, single centre; 40 adult patients with acute leukaemia receiving intensive chemotherapy expected to produce profound and prolonged neutropenia; Inpatients in reverse isolation rooms | |
| Interventions | Norfloxacin 400mgX2/d + trimethoprim‐sulphamethoxazole 80mg/800mg X2/d versus gentamicin 120mgX4 plus trimethoprim‐sulfamethoxazole 80mg/800mgX2/d | |
| Outcomes | All cause mortality; Infection related death; Number of febrile patients or episodes; Microbiologically documented febrile episodes; Adverse events | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Details not available for the 2011 review update |
| Selective reporting (reporting bias) | Unclear risk | Exclusions and ITT not described |
Nenova 2001.
| Methods | Randomisation: no information; Blinding: none; Intention to treat: no; Exclusion from analysis:1/70; Beginning of prophylaxis: PMN count less than 1000 End of prophylaxis: PMN count of 1000 or fever | |
| Participants | Bulgaria, single centre; 70 adult patients with haematologic malignancies undergoing chemotherapy; Inpatients | |
| Interventions | Ciprofloxacin 500mgX2/d (20 patients), pefloxacin or enoxacin or norfloxacin versus no intervention | |
| Outcomes | All cause mortality; Infection related death; Number of febrile episodes; Cinically documented febrile episodes; Microbiologically documented febrile episodes | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 1/70 exclusions |
| Selective reporting (reporting bias) | Unclear risk | Analysis not by ITT |
Orlandi 1990.
| Methods | Randomisation: no information; Blinding:none; Intention to treat: yes; Exclusion from analysis: unknown; Beginning of prophylaxis: chemotherapy; End of prophylaxis: PMN count of 1000 | |
| Participants | Italy, single centre; 60 adult patients with leukaemia undergoing chemotherapy treatment resulting in neutropenia; Inpatients | |
| Interventions | Norfloxacin 400mgX2/d versus trimethoprim‐sulphamethoxazole 160mg/800mgX2/d | |
| Outcomes | All cause mortality; Infection related death; Number of febrile patients; Fever days; Clinically documented febrile episodes; Microbiologically documented febrile episodes; Infection resistant to quinolones and trimethoprim‐sulphamethoxazole; Adverse events | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Details not available for the 2011 review update |
| Selective reporting (reporting bias) | Low risk | Analysis by ITT; all outcomes reported |
Papaiakovou 2010.
| Methods | Randomisation: computer generated, allocation concealment: central randomisation; Blinding: none; Intention to treat: yes Exclusion from analysis:no exclusions, 0/157; Beginning of prophylaxis: chemotherapy (day 0 of autologous stem cell transplant); End of prophylaxis: neutropenia resolution | |
| Participants | Single center, Greece (Athens); adult patients undergoing high dose chemotherapy (HDT) +autologous stem cell transplant. Malignancies included: Multiple Myeloma 79.6%, epithelial ovarian cancer,germ cell tumor, other All inpatients All received granulocyte‐colony stimulating factors |
|
| Interventions | oral ciprofloxacin 500mg X2 daily and intravenous vancomycin 1gr X2 daily vs. no prophylaxis | |
| Outcomes | All cause mortality;
Infection related death;
Number of febrile patients; Fever days;
Clinically documented febrile episodes;
Microbiologically documented febrile episodes; Gram negative and Gram positive Microbiologically documented febrile episodes; Bacteremia Gram negative and Gram positive Bacteremia Fungal infection Hospitalizations Adverse events |
|
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | computer generated |
| Allocation concealment (selection bias) | Low risk | central randomisation |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | no blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no exclusions |
| Selective reporting (reporting bias) | Low risk | ITT, all outcomes reported |
Patrick 1995.
| Methods | Randomisation: no information; Blinding:double blind; Intention to treat: unknown; Exclusion from analysis: unknown; Beginning of prophylaxis: one week prior to bone marrow transplantation (BMT); End of prophylaxis: PMN count of 500 | |
| Participants | USA, single centre; 48 children with leukaemia (23) or solid tumours (25) undergoing bone marrow transplantation | |
| Interventions | Ciprofloxacin 10 mg/kg /x2/d for 10 days (orally) and then 7.5 mg/kg X2/d (IV) versus placebo | |
| Outcomes | Microbiologically documented febrile episodes | |
| Notes | Abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided (D ‐ Not used) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Details not available for the 2011 review update |
| Selective reporting (reporting bias) | Unclear risk | ITT analysis and exclusions not described; abstract only, insufficient information |
Petersen 1986.
| Methods | Randomisation: no information; Blinding: none; Intention to treat: no; Exclusion from analysis:21/122; Beginning of prophylaxis: PMN count less than 500 End of prophylaxis: PMN count of 500 or bone marrow engraftment or death | |
| Participants | USA, single centre; 122 patients with haematological malignancies undergoing bone marrow transplantation; Inpatients in laminar airflow rooms | |
| Interventions | IV vancomycin 2g/d + ticarcillin 300mg/kg/d + tobramycin 3‐5mg/kg/d versus no intervention (all received oral nonabsorbable antibiotic as well) | |
| Outcomes | All cause mortality; Infection related death; Number of febrile episodes; Clinically documented febrile episodes; Microbiologically documented febrile episodes | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 21/122 exclusions |
| Selective reporting (reporting bias) | Unclear risk | Analysis not by ITT |
Pignon 1990.
| Methods | Randomisation: no information; Blinding: double blind; Intention to treat: unknown; Exclusion from analysis:unknown; Beginning of prophylaxis: chemotherapy; End of prophylaxis: PMN count of 500 or fever | |
| Participants | France, single centre; 44 patients with various haematological malignancies treated by intensive chemotherapy inducing a prolonged neutropenia; Inpatients in conventional rooms or laminar airflow rooms | |
| Interventions | IV ceftriaxone 2g X1/d versus placebo | |
| Outcomes | Number of febrile episodes; Fever days; Clinically documented febrile episodes; Microbiologically documented febrile episodes | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Details not available for the 2011 review update |
| Selective reporting (reporting bias) | Unclear risk | ITT and exclusions not described |
Pizzo 1983.
| Methods | Randomisation: a balanced randomised block design kept in the pharmacy, antibiotics dispensed by a central pharmacist; Blinding: triple blind; Intention to treat: no; Exclusion from analysis: 21/150 Beginning of prophylaxis: chemotherapy; End of prophylaxis: fever or PMN count of 500 | |
| Participants | USA, single centre; 150 patients (children and adults) with haematological or solid tumours receiving chemotherapy; Inpatients for induction‐ chemotherapy, outpatients for maintenance | |
| Interventions | Trimethoprim‐sulfamethoxazole 5mg/kg X2/d + erythromycin 15mg/kg X2/d versus placebo | |
| Outcomes | Number of first febrile episode. | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | blocked randomisation |
| Allocation concealment (selection bias) | Low risk | Pharmacy allocated (A ‐ Adequate) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Triple blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 21/150 exclusions |
| Selective reporting (reporting bias) | Unclear risk | Analysis not by ITT |
Prentice 2001.
| Methods | Randomisation: no information; Blinding: none; Intention to treat: no; Exclusion from analysis: 22/150 Beginning of prophylaxis: chemotherapy; End of prophylaxis:PMN count of 1000 or 30 days | |
| Participants | England, single centre; 150 patients receiving cytotoxic chemotherapy for treatment of haematological malignancies or undergoing intensive chemotherapy prior to bone marrow transplantation; Inpatients in private rooms | |
| Interventions | Ciprofloxacin 500mgX2/d + colistin 1.5 million unitsX2/d versus neomycin 500mgX2/d + colistin 1.5 million unitsX2/d | |
| Outcomes | All cause mortality; Infection related death; Number of febrile patients or febrile episodes; Clinically documented febrile episodes; Microbiologically documented febrile episodes; Adverse events | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 22/150 exclusions |
| Selective reporting (reporting bias) | Unclear risk | ITT analysis not described |
Rafecas 1989.
| Methods | Randomisation: not clear;allocation concealment not clear Blinding: double blind; Intention to treat: no; Exclusion from analysis: 5/40; Beginning of prophylaxis: chemotherapy (or once chemotherp‐indused emesis resolved); End of prophylaxis: resolution of neutropenia (neutrophil count >500) | |
| Participants | Spain; 40 adult patients with acute leukaemia undergoing aggressive chemotherapyt;70% of evaluable patients were hospitalized | |
| Interventions | ciprofloxacin 500mgX2daily versus placebo | |
| Outcomes | Infection‐related mortality;
Number of febrile episodes;
Clinically documented febrile episodes; Microbiologically documented febrile episodes; Gram negative and Gram positive Microbiologically documented febrile episodes fever days Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | unclear randomisation |
| Allocation concealment (selection bias) | Unclear risk | unclear allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | double blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 5/40 excluded |
| Selective reporting (reporting bias) | Unclear risk | not ITT |
Rahman 2009.
| Methods | Randomisation: table of random numbers;allocation concealment not clear Blinding: the trial was only single blinded; Intention to treat: no; Exclusion from analysis: 32/80;(the randomisation in this trial was conducted when patients with acute leukaemia at risk for chemotherapy‐induced neutropenia were included. 80 were randomized. Then, only those who became neutropenic were included for analysis. Thus, 32 were excluded after randomisation) Beginning of prophylaxis: chemotherapy; End of prophylaxis: resolution of neutropenia | |
| Participants | Bangladesh, single centre; 80 adult patients with acute leukaemia during remission induction treatment;All Inpatients | |
| Interventions | levofloxacin 500mg daily versus placebo | |
| Outcomes | All cause mortality;
Number of febrile patients;
Clinically documented febrile episodes; Microbiologically documented febrile episodes; Gram negative and Gram positive Microbiologically documented febrile episodes Bacteremia Gram negative and Gram positive bacteraemia |
|
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | not clear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Exclusion from analysis: 32/80 (more than 30%);(the randomisation in this trial was conducted when patients with acute leukaemia at risk for chemotherapy‐induced neutropenia were included. 80 were randomized. Then, only those who became neutropenic were included for analysis. Thus, 32 were excluded after randomisation) |
| Selective reporting (reporting bias) | Unclear risk | analysis not by ITT |
Ruiz 2001.
| Methods | Randomisation: no information; Blinding: double blind; Intention to treat: unknown; Exclusion from analysis: unknown; Beginning of prophylaxis: 5 days before autologous peripheral blood stem cell transplantation (A‐PBSC); End of prophylaxis: fever | |
| Participants | Spain, single centre, 50 patients undergoing autologous peripheral blood stem cell transplantation (A‐PBSC) for various malignancies | |
| Interventions | Ofloxacin 200mg X2/d versus placebo | |
| Outcomes | Number of febrile episodes; Clinically or microbiologically documented infections; Adverse events | |
| Notes | Abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | exclusions not described |
| Selective reporting (reporting bias) | Unclear risk | ITT not described; insufficient information |
Sampi 1992.
| Methods | Randomisation: by envelopes; blinding: none; Intention to treat: no; Exclusion from analysis: unknown; Beginning of prophylaxis: chemotherapy; End of prophylaxis: resolution of neutropenia | |
| Participants | Japan, single centre; 73 patients with acute leukaemia receiving consolidation therapy or autologous bone marrow transplantation; Inpatients, some in laminar airflow rooms | |
| Interventions | Norfloxacin 200mgX4/d versus no intervention | |
| Outcomes | All cause mortality; Number of febrile patients; fever days; Clinically documented febrile episodes; Microbiologically documented febrile episodes | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | Envelopes used but not stated as sealed/opaque (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Details not available for the 2011 review update |
| Selective reporting (reporting bias) | Unclear risk | Analysis not by ITT and exclusions not stated. |
Schimpff 1975.
| Methods | Randomisation: no information; Blinding: none; Intention to treat: yes; Exclusion from analysis: none Beginning of prophylaxis: chemotherapy; End of prophylaxis: remission or death | |
| Participants | USA, single centre; 40 patients with acute leukaemia undergoing induction chemotherapy; Inpatients in conventional rooms | |
| Interventions | Nonabsorbable antibiotics: gentamicin, vancomycin and nystatin versus no intervention | |
| Outcomes | All cause mortality; Infection related mortality; Number of febrile patients; Clinically documented febrile episodes; Microbiologically documented febrile episodes | |
| Notes | Journal Publication The trial compares three groups: group 1 ‐ protected environment with antibiotics, group 2 ‐ oral nonabsorbable antibiotics, grouo 3 ‐ no intervention. group 1 was excluded from our analysis, since it examines a different intervention | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no exclusions |
| Selective reporting (reporting bias) | Low risk | Analysis by ITT |
Schroeder 1992.
| Methods | Randomisation: no information; Blinding: double blind; Intention to treat: no; Exclusion from analysis:5/80; Beginning of prophylaxis: chemotherapy; End of prophylaxis: PMN count of 500 | |
| Participants | Germany, single centre; 80 patients with solid tumours, who had undergone aggressive chemotherapy and in whom granulocytes had fallen to below 500; Outpatients | |
| Interventions | Ofloxacin 200mgX2/d versus placebo | |
| Outcomes | All cause mortality; Infection related death; Number of febrile patients; Clinically documented febrile episodes; Microbiologically documented febrile episodes; Hospitalization days; Number of hospitalisations; Adverse events | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 5/80 exclusions |
| Selective reporting (reporting bias) | Unclear risk | Analysis not by ITT |
Slavin 2007.
| Methods | Randomisation: computer generated, allocation concealment: sealed and opaque envelopes; Blinding: none; Intention to treat: no Exclusion from analysis:2/153 excluded after randomisation, but this is explained: in one consent withdrawn before drug administered, in the second failure of transplant before drug Beginning of prophylaxis: chemotherapy (day 0 of autologous stem cell transplant); End of prophylaxis: neutropenia resolution | |
| Participants | Two adult bone marrow transplant centers in Australia adult patients undergoing high dose chemotherapy (HDT) + stem cell transplant: autologous 48%, allogeneic 52% Malignancies included: Almost 100% haematological malignancies‐NHL,AML,ALL,CML,MM,Hodgkins,other hemato; also 4% in antibiotic group and 7% in control ‐solid malignancies: breast,sarcoma,germ cell tumour All inpatients hospitalised in HEPA filter rooms |
|
| Interventions | intravenous cefipime 1g X2 daily vs. no prophylaxis (the same antibiotic administered as empiric therapy when the patient became febrile) | |
| Outcomes | All cause mortality;
Infection related death;
Number of febrile patients;
Microbiologically documented febrile episodes; Gram negative and Gram positive Microbiologically documented febrile episodes; Bacteremia Gram negative and Gram positive Bacteremia Hospitalizations Adverse events |
|
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | computer generated |
| Allocation concealment (selection bias) | Low risk | sealed and opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | no blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | two excluded |
| Selective reporting (reporting bias) | Unclear risk | not ITT (only 2 excluded), all outcomes reported |
Sleijfer 1980.
| Methods | Randomisation: no information; Blinding: none;Intention to treat: no; Exclusion from analysis: 8/113 Beginning of prophylaxis: PMN count less than 1000; End of prophylaxis: PMN count of 1000 or death | |
| Participants | Netherlands, single centre; 113 patients with haematological malignancies undergoing chemotherapy; Inpatients | |
| Interventions | Nalidixic acid 2gX4/d or Trimethoprim‐sulfamethoxazole 160/800mgX3/d or polymyxin 200mgX4/d versus no intervention | |
| Outcomes | All cause mortality; Infection related death; Number of febrile patients; Clinically documented febrile episodes; Microbiologically documented febrile episodes | |
| Notes | ‐Journal Publication ‐ prophylaxis was administered according to microbiological surveillance cultures. 35 patients received nalidixic acid, 18 patients received TMP‐SMZ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 8/113 exclusions |
| Selective reporting (reporting bias) | Unclear risk | Analysis not by ITT |
Starke 1982.
| Methods | Randomisation: no information; Blinding: none; Intention to treat: yes; Exclusion from analysis: 0/43 Beginning of prophylaxis: chemotherapy; End of prophylaxis: PMN count of 500 | |
| Participants | England, single centre; 43 patients undergoing treatment for acute leukaemia; Inpatients in single rooms with reverse isolation | |
| Interventions | Trimethoprim‐sulfamethoxazole 40mg/800mg2/d versus trimethoprim‐sulphamethoxazole 40mg/800mgX2/d plus framycetin 500mgX4/d + colistin 1.5 million unitsX4/d | |
| Outcomes | All cause mortality; Infection related death; Number of febrile episodes; Fever days; Microbiologically documented febrile episodes; Adverse events | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Details not available for the 2011 review update |
| Selective reporting (reporting bias) | Low risk | Analysis by ITT; No exclusions |
Talbot 1993.
| Methods | Randomisation: no information; Blinding: double blind; Intention to treat: yes; Exclusion from analysis: none, excluded from trial 23/119 Beginning of prophylaxis: chemotherapy; End of prophylaxis: PMN count of 500 for 7 days or PMN count of 1000 or 6 weeks | |
| Participants | USA, multicentre; 119 adult patients with acute leukaemia treated with chemotherapy | |
| Interventions | Enoxacin 200mgX2/d versus placebo | |
| Outcomes | All cause mortality; Infection related death; Number of febrile patients; Clinically documented febrile episode; Microbiologically documented febrile episodes; Adverse events | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 23/119 exclusions |
| Selective reporting (reporting bias) | Unclear risk | Analysis by ITT |
Teinturier 1995.
| Methods | Randomisation: no information; Blinding: none; Intention to treat: no; Exclusion from analysis: 1/155 Beginning of prophylaxis: 5 days before bone marrow transplantation; End of prophylaxis: one day after bone marrow transplantation | |
| Participants | France, single centre; 155 patients with various malignancies undergoing bone marrow transplantation; Inpatients | |
| Interventions | IV vancomycin 10mg/kg X4/d versus no intervention | |
| Outcomes | All cause mortality; Infection related death; Number of febrile patients; Microbiologically documented febrile episodes; Adverse events | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Details not available for the 2011 review update |
| Selective reporting (reporting bias) | Unclear risk | Analysis not by ITT; all expected outcomes reported. |
Thomas 2000.
| Methods | Randomisation: a table of random numbers, sealed and opaque envelopes for allocation concealment; Blinding: double blind; Intention to treat: no; Exclusion from analysis: 11/162 Beginning of prophylaxis: chemotherapy; End of prophylaxis: PMN count of 500 | |
| Participants | France, single centre; 152 patients with haematological malignancies undergoing induction chemotherapy or consolidation or bone marrow transplantation or solid malignancies undergoing autologous bone marrow transplantation; Inpatients | |
| Interventions | Pefloxacin 200mgX4/d versus pefloxacin 200mgX4/d + oral vancomycin 200mgX4/d versus placebo | |
| Outcomes | All cause mortality; Number of febrile patients; Fever days; Clinically documented infection; Microbiologically documented febrile episodes | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | random number table |
| Allocation concealment (selection bias) | Low risk | sealed opaque envelopes (A ‐ Adequate) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 11/162 exclusions |
| Selective reporting (reporting bias) | Unclear risk | Analysis not by ITT |
Timmers 2007.
| Methods | Randomisation and concealment: randomisation according to sealed envelopes, opaque not mentioned (A/B); Blinding: none; Intention to treat: no Exclusion from analysis:3 exclusions, 3/245; (3 excluded but this is explained: 1 withdrew consent,another erroneously enrolled twice, 1 died on randomisation day) Beginning of prophylaxis: chemotherapy End of prophylaxis: neutropenia resolution>500 |
|
| Participants | Single center, Netherlands 100% patients with haematological malignancies‐ALL,AML,MM,Lymphoma,MDS,and other The following underwent stem cell transplant: cipro plus phenethicillin arm: 83.3% autologous, 16.7%allogeneic levofloxacin arm:86%autologous, 14%allogeneic All inpatients |
|
| Interventions | oral ciprofloxacin 500mg X2 daily plus phenethicillin 250mgX4 daily vs. levofloxacin | |
| Outcomes | All cause mortality;
Infection related death;
Number of febrile patients; Fever days;
Clinically documented febrile episodes;
Microbiologically documented febrile episodes; Bacteremia Gram negative and Gram positive Bacteremia Infection resistance to quinolones Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Randomised' |
| Allocation concealment (selection bias) | Unclear risk | sealed envelopes, opaque not mentioned (A/B); |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | none |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 3 excluded from 245 |
| Selective reporting (reporting bias) | Unclear risk | not ITT, only 3 excluded, all outcomes reported |
Tjan Heijnen 2001.
| Methods | Randomisation: generation not specified, central randomisation; Blinding: double blind Intention to treat: no; Exclusion from analysis:2/163; Beginning of prophylaxis: chemotherapy; End of prophylaxis: fever, death or side effects | |
| Participants | Europe, multicentre; 163 adult patients with small cell lung cancer undergoing intensive chemotherapy; Outpatients | |
| Interventions | Ciprofloxacin 750mgX2/d + roxythromycin 150mgX2/d versus placebo | |
| Outcomes | All cause mortality; Infection related death; Number of febrile patients or febrile episodes; Fever days; Clinically documented infection; Microbiologically documented febrile episodes; number of Hospitalizations; Hospitalization days; Infection resistant to quinolones; Adverse events | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation centrally coordinated |
| Allocation concealment (selection bias) | Low risk | centrally coordinated (A ‐ Adequate) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2/163 exclusions |
| Selective reporting (reporting bias) | Unclear risk | Analysis not by ITT but all expected outcomes reported |
Tsutani 1992.
| Methods | Randomisation: no information; Blinding: none; Intention to treat: no; Exclusion from analysis: unknown; Beginning of prophylaxis: PMN count less than 500; End of prophylaxis: PMN count of 1500 or fever | |
| Participants | Japan, single centre; 22 patients with haematological malignancies during post remission chemotherapy; Inpatients | |
| Interventions | Ofloxacin 300mgX2 versus no intervention | |
| Outcomes | Number of febrile episodes; Clinically documented infection; Microbiologically documented febrile episodes | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Details not available for the 2011 review update |
| Selective reporting (reporting bias) | Unclear risk | Analysis not by ITT; exclusions not stated |
van Eys 1987.
| Methods | Randomisation: central randomisation with institutional balancing; Blinding: none; Intention to treat: no; Exclusion from analysis: 6/126 Beginning of prophylaxis: chemotherapy; End of prophylaxis: 3 years or relapse of leukaemia | |
| Participants | USA, multicentre; 126 children with lymphocytic leukaemia undergoing induction chemotherapy; Setting not specified | |
| Interventions | Trimethoprim‐sulfamethoxazole 4mg/kg/d versus no intervention | |
| Outcomes | All cause mortality; Infection related death; Number of febrile patients; Clinically documented infection; Adverse events | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisation |
| Allocation concealment (selection bias) | Low risk | Central allocation (A ‐ Adequate) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 6/126 exclusions |
| Selective reporting (reporting bias) | Unclear risk | Analysis not ITT |
Wade 1981a.
| Methods | Randomisation: no information; Blinding: none; Intention to treat: yes; Exclusion from analysis: none 0/53; Beginning of prophylaxis: chemotherapy; End of prophylaxis: PMN count of 1000, remission or death | |
| Participants | USA, single centre; 53 adult patients with acute leukaemia undergoing induction chemotherapy; Inpatients or outpatients | |
| Interventions | Trimethoprim‐sulfamethoxazole 160mg/800mg versus oral gentamIcin 200mgX4 | |
| Outcomes | All cause mortality; Infection related death; Number of febrile episodes; Clinically documented infection; Microbiologically documented febrile episodes; Adverse events | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Details not available for the 2011 review update |
| Selective reporting (reporting bias) | Low risk | ITT analysis; all expected outcomes reported. No exclusions |
Wade 1983.
| Methods | Randomisation: no information; Blinding: none; Intention to treat: yes; Exclusion from analysis: none 0/62; Beginning of prophylaxis: chemotherapy; End of prophylaxis: PMN count of 1000, remission or death | |
| Participants | USA, single centre; 62 adult patients with acute leukaemia undergoing induction chemotherapy; Outpatients | |
| Interventions | Trimethoprim‐sulfamethoxazole 160mg/800mg versus nalidixic acid 1gX4/d | |
| Outcomes | All cause mortality; Infection related death; Number of febrile patients or febrile episodes; Clinically documented infection; Microbiologically documented febrile episodes; Adverse events | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Details not available for the 2011 review update |
| Selective reporting (reporting bias) | Low risk | Analysis by ITT; all expected outcomes reported; no exclusions |
Ward 1993.
| Methods | Randomisation: no information on generation, central randomisation; Blinding: double blind; Intention to treat: no; Exclusion from analysis: none 9/51; Beginning of prophylaxis: chemotherapy; End of prophylaxis: PMN count of 1000 | |
| Participants | USA, multicentre (11 centres); 51 adult neutropenic patients undergoing chemotherapy for acute leukaemia; Inpatients in private rooms with reverse isolation | |
| Interventions | Trimethoprim‐sulfamethoxazole 160mg/800mg versus placebo | |
| Outcomes | All cause mortality; Infection related death; Number of febrile patients; Clinically documented infection; Microbiologically documented febrile episodes; Adverse events | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisation |
| Allocation concealment (selection bias) | Low risk | Centrally coordinated allocation (A ‐ Adequate) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 9/51 exclusions |
| Selective reporting (reporting bias) | Unclear risk | Analysis not by ITT |
Watson 1982.
| Methods | Randomisation: no information; Blinding: none; Intention to treat: no; Exclusion from analysis: 12/100; Beginning of prophylaxis: chemotherapy; End of prophylaxis: PMN count of 500,discharge from isolation, withdrawal or death | |
| Participants | England, single centre; 100 patients with acute leukaemia undergoing remission induction or allogeneic bone marrow transplantation; Inpatients in private rooms with reverse isolation | |
| Interventions | Trimethoprim‐sulfamethoxazole 160mg/800mgX2/d versus neomycin 500mgX2/d + colistin 1.5 million unitsX2/d 500mgX2 | |
| Outcomes | All cause mortality; Infection related death; Number of febrile patients; Clinically documented infection; Microbiologically documented febrile episodes; Adverse events | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 12/100 exclusions |
| Selective reporting (reporting bias) | Unclear risk | Analysis not by ITT but all expected outcomes reported |
Weiser 1981.
| Methods | Randomisation: no information; Blinding: none; Intention to treat: unknown; Exclusion from analysis: unknown; Beginning of prophylaxis: chemotherapy; End of prophylaxis: PMN count of 1000 | |
| Participants | USA, single centre; 29 adult patients with acute leukaemia in their first remission and undergoing consolidation chemotherapy; Outpatients | |
| Interventions | Trimethoprim‐sulfamethoxazole 160mg/800mgX2/d versus no intervention | |
| Outcomes | Number of febrile episodes; Fever days; Clinically documented infection; Microbiologically documented febrile episodes; Number of hospitalisations; Hospitalization days; Infection resistant to trimethoprim‐sulphamethoxazole | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Details not available for the 2011 review update |
| Selective reporting (reporting bias) | Unclear risk | ITT and exclusions not described |
Winston 1986.
| Methods | Randomisation: no information; Blinding: none; Intention to treat: unknown; Exclusion from analysis: unknown; Beginning of prophylaxis: chemotherapy; End of prophylaxis: PMN count of 1000 or death | |
| Participants | USA, single centre; 66 adult patients with haematologic malignancies undergoing chemotherapy; Inpatients | |
| Interventions | Norfloxacin 400mg X3/d versus oral vancomycin 400mgX3/d + polymyxin 100mg X3/d | |
| Outcomes | Infection related death; Number of febrile patients or episodes; Clinically documented infection; Microbiologically documented febrile episodes; Infection resistant to quinolones; Adverse events | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Details not available for the 2011 review update |
| Selective reporting (reporting bias) | Unclear risk | ITT and exclusions not described |
Winston 1990.
| Methods | Randomisation: no information; Blinding: none; Intention to treat: unknown; Exclusion from analysis: unknown; Beginning of prophylaxis: chemotherapy; End of prophylaxis: PMN count of 750 or death or withdrawal from trial | |
| Participants | USA, single centre; 62 adult patients with haematologic malignancies undergoing chemotherapy; Inpatients | |
| Interventions | Ofloxacin 400mgX3/d versus oral vancomycin 400mgX3/d + polymyxin 100mgX3/d | |
| Outcomes | Infection related death; Number of febrile patients or episodes; Fever days; Clinically documented infection; Microbiologically documented febrile episodes; Adverse events | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Details not available for the 2011 review update |
| Selective reporting (reporting bias) | Unclear risk | ITT and exclusions not described |
Yamada 1993.
| Methods | Randomisation: generation by order of admission, no information on allocation; Blinding: none; Intention to treat: no; Exclusion from analysis: 5/111; Beginning of prophylaxis: chemotherapy; End of prophylaxis: PMN count of 1000 or fever | |
| Participants | Japan, single centre; 111 adult patients with acute leukaemia receiving cytotoxic chemotherapy; Inpatients | |
| Interventions | Norfloxacin 200mgX2/d or X4/d versus no intervention | |
| Outcomes | All cause mortality; Number of febrile patients or episodes; Fever days; Clinically documented infection; Microbiologically documented febrile episodes; Adverse events | |
| Notes | Journal Publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | quasi randomisation |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 5/111 exclusions |
| Selective reporting (reporting bias) | Unclear risk | Analysis not by ITT |
Yates 1973.
| Methods | Randomisation: no information; Blinding: none; Intention to treat: no; Exclusion from analysis: 15/67; Beginning of prophylaxis: chemotherapy; End of prophylaxis: PMN count of 500, severe side effects or death | |
| Participants | USA, single centre; 67 adult patients with haematologic malignancies undergoing chemotherapy; Inpatients in conventional ward with reverse isolation | |
| Interventions | Nonabsorbable antibiotics: gentamicin,vancomycin and nystatin versus no intervention | |
| Outcomes | All cause mortality; Number of febrile patients | |
| Notes | ‐Journal Publication ‐there were originally 4 treatment groups: regular ward, regular ward + nonabsorbable, total isolation without nonabsorbable,total isolation with nonabsorbable . Only group 1 and 2 are included | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided (B ‐ Unclear) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 15/67 exclusions |
| Selective reporting (reporting bias) | Unclear risk | Analysis not by ITT; high exclusion rate |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Barriga 1997 | Use of vancomycin solution for prevention of catheter infection, not all patients neutropenic |
| Bunn 2001 | Not a randomised controlled trial |
| Capdevila 1997 | Not a randomised controlled trial |
| Corrado 1988 | A review article |
| Craig 2007 | A prospective cohort trial regarding prophylaxis with quinolones, not a randomised controlled trial |
| Cruciani 2000b | A review article |
| Davis 1998 | A review article |
| Del Favero 1989 | A review article |
| Del Favero 1993 | A review article |
| Delarive 2000 | A review article |
| Donnelly 1997 | A review article |
| Ekert 1980 | A trial comparing prophylactic antibiotic therapy with prophylactic antibiotic and another intervention‐ lactobacilli |
| Engerval 1996a | A review article |
| Engervall 1996b | Not a randomised controlled trial |
| EORTC 1982 | A high percentage of dropouts (53%, 40 patients out of 75 which were randomised were excluded) |
| Fernndez‐Aviles 2010 | A trial of ceftriaxone prophylaxis, but not randomised |
| Figueredo 1985 | In this trial the intervention was high dose or low dose chemotherapy. Only the arm which was randomised to high dose chemotherapy received prophylactic antibiotics |
| Frampton 1996 | A review article |
| Garcia 2000 | A randomised controlled trial of treatment of febrile neutropenia, and not of prophylaxis |
| Giamarellou 1995 | A review article |
| Gilbert 1994 | Not a randomised controlled trial, compared to a historical control |
| Guiot 1981 | A prospective clinical trial of antibiotic prophylaxis, not RCT |
| Haahr 1997 | A review article |
| Hallbk 2010 | A cohort study of autologous transplant patients who received prophylaxis, not a randomised trial |
| Henry 1984a | A review article |
| Horvathova 1998 | Not a randomised controlled trial, analysis of cases with bacteraemia only |
| Imrie 1995 | Not a randomised controlled trial, compared to a historical control |
| Karp 1986 | A randomised controlled trial of treatment of febrile neutropenia, and not of prophylaxis |
| Kerr 1999b | A review article |
| Klastersky 1996 | A review article |
| Krupova 1998 | A review article |
| Ljungman 1997 | A randomised controlled trial of antibiotic prophylaxis which is only periprocedural (central line insertion). The antibiotic coverage, the outcomes and the time frame are different |
| Lohner 1979 | A trial comparing prophylactic antibiotic therapy with prophylactic antibiotic therapy combined with a protected environment |
| Maltezou 1999 | Not a randomised controlled trial |
| Maltezou 1999 (a) | Not a randomised controlled trial |
| Mantovani 1998 | A trial of febrile neutropenia |
| Marchetti 2002 | A review article |
| Martino 1998 | A review article |
| Maschmeyer 1990 | A review article |
| May 1994 | A randomised trial for prophylaxis of PCP in HIV patients, not neutropenic cancer patients |
| Menichetti 1989 | A trial of consecutive patients, not RCT |
| Mihaylov 2007 | A review article |
| Minenko 2004 | Not a randomised controlled trial, in this trial moxifloxacin was compared to historical controls treated with ciprofloxacin. |
| Patrick 1997 | A review article |
| Persson 2000 | Not a randomised controlled trial |
| Pivkova 2005 | A comparative study of 35 patients receiving high dose VP16 and undergoing autologous transplant. The comparison is between three options:no antibiotic prophylactic regimens, vancomycin or ciprofloxacin and amoxicillin‐clavulonic acid. However, it is not randomised |
| Reuter 2005 | Not an RCT, a prospective observational study |
| Risi 1998 | A review article |
| Schaison 1991 | A study of febrile neutropenia |
| Solano 2005 | Not a randomised trial ‐ a prospective study of prophylaxis compared with historical controls |
| Spanik 1998 | A retrospective trial |
| Takemoto 1990 | A randomised controlled trial but for febrile neutropenia |
| Timmer‐Bonte 2005 | A randomised controlled trial, but the intervention was granulocyte colony‐stimulating factor and both arms received prophylactic antibiotics |
| Tjan Heijnen 2002 | A letter relating to another trial |
| Tunkel 2002 | A review article |
| Van De Leur 1995 | A study which evaluates antibiotic concentration in faeces, but not in neutropenics, not a RCT |
| Viscoli 2001 | A review article |
| Viscoli 1998 | A review article |
| Viscoli 2002 | A review article |
| von Baum 2006 | Controlled before and after observational study of prophylaxis but not randomised |
| von Minckwitz 2008 | This study compared four consecutive cohort studies of patients receiving chemotherapy for breast cancer, it was not randomised. The 4 methods of neutropenia prophylaxis administered were : ciprofloxacin alone vs figrastim vs. pegfilgratim vs pegfilgradtim+ciprofloxacin |
| Wade 1981 | A retrospective study |
| Wilson 1982 | A case report of two cases of failure of TMP‐SMZ prophylaxis |
| Zinner 1999 | A review article |
Differences between protocol and review
Since the original publication of this review, it has become clear that the main determinant of clinical practice in this area is all‐cause mortality. Therefore, for the 2011 update, we retained all‐cause mortality as the primary outcome, and considered infection‐related mortality and febrile neutropenia as secondary outcomes.
Contributions of authors
Anat Gafter‐Gvili ‐ coordinating the review, data collection and management, analysis, interpretation of results and writing of the review, securing funding for the review. Abigail Fraser ‐ data collection and management, analysis, interpretation of results and writing of the review. Mical Paul ‐ data collection, interpretation of results and writing the review. Liat Vidal ‐ extraction of data from trials for the update. Tess Lawrie ‐ updated methodological and statistical aspects of the review. Marianne van de Wetering ‐ article retrieval. Leontien Kremer ‐ article retrieval. Leonard Leibovici ‐ conceiving and designing the review, analysis of data, interpretation of data and writing of the review, securing funding for the review.
Sources of support
Internal sources
A grant from Rabin Medical Center, Israel.
External sources
European Commission (TREAT Project, Contract 1999‐11459), Other.
Declarations of interest
None
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Archimbaud 1991 {published and unpublished data}
- Archimbaud E, Guyotat D, Maupas J, Ploton C, Nageotte A, Devaux Y, et al. Pefloxacin and vancomycin vs. gentamicin, colistin sulphate and vancomycin for prevention of infections in granulocytopenic patients: a randomised double‐blind study. European Journal of Cancer 1991;27:174‐8. [DOI] [PubMed] [Google Scholar]
Arning 1990 {published data only}
- Arning M, Wolf H, Aul C, Heyll A, Scharf R, Scheider W. Infection prophylaxis in neutropenic patients with acute leukaemia‐‐a randomised, comparative study with ofloxacin, ciprofloxacin and co‐trimoxazole/colistin. Journal of Antimicrobial Chemotherapy 1990;26 Suppl D:137‐42. [DOI] [PubMed] [Google Scholar]
Attal 1991 {published data only}
Bartoloni 1989 {published data only}
- Bartoloni AF, Orsi A, Aquilini D, Bosi A, Rossi Ferrini P, Paradisi F. The influence of ofloxacin versus trimethoprim‐sulfamethoxazole on the aerobic flora in granulocytopenic subjects. Journal of Chemotherapy 1989;1:91‐4. [DOI] [PubMed] [Google Scholar]
Bender 1979 {published data only}
- Bender JF, Schimpff SC, Young VM, Fortner CL, Brouillet MD, Love LJ, et al. Role of vancomycin as a component of oral nonabsorbable antibiotics for microbial suppression in leukemic patients. Antimicrobial Agents and Chemotherapy 1979;15(3):455‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bow 1984 {published data only}
- Bow EJ, Louie TJ, Riben PD, McNaughton RD, Harding GK, Ronald AR. Randomized controlled trial comparing trimethoprim/sulfamethoxazole and trimethoprim for infection prophylaxis in hospitalized granulocytopenic patients. American Journal of Medicine 1984;76:223‐33. [DOI] [PubMed] [Google Scholar]
- Riben PD, Louie JT, Lank BA, Kornachuk E, Gurwith MJ, Harding GKM, et al. Reduction in mortality from gram‐negative sepsis in neutropenic patients receiving trimethoprim‐sulfamethoxazole therapy. Cancer 1983;51:1587‐92. [DOI] [PubMed] [Google Scholar]
Bow 1988 {published data only}
- Bow EJ, Raynor E, Thomas JL. Comparison of norfloxacin with cotrimoxazole for infection prophylaxis in acute leukemia. The trade‐off for reduced gram‐negative sepsis. American Journal of Medicine 1988;84:847‐54. [DOI] [PubMed] [Google Scholar]
Bow 1996 {published data only}
- Bow EJ, Mandell LA, Thomas JL, Feld R, Palmer M, Zee B, et al. Quinolone‐based antibacterial chemoprophylaxis in neutropenic patients: effect of augmented gram‐positive activity on infectious morbidity. National Cancer Institute of Canada Clinical Trials Group. Annals of Internal Medicine 1996;125(3):183‐90. [DOI] [PubMed] [Google Scholar]
Brodsky 1993 {published data only}
- Brodsky AL, Melero MJ, Sanchez Avalos JC. Prophylaxis with fluoroquinolones in patients with neutropenia. Medicina 1993;53:401‐7. [PubMed] [Google Scholar]
Broun 1994 {published data only}
- Broun ER, Wheat JL, Kneebone PH, Sundblad K, Hromas RA, Guido T. Randomized trial of the addition of gram‐positive prophylaxis to standard antimicrobial prophylaxis for patients undergoing autologous bone marrow transplantation. Antimicrobial Agents and Chemotherapy 1994;38(3):576‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bucaneve 2005 {published data only}
- Bucaneve G, Micozzi A, Menichetti F, Martino P, Stella Dionosi M, Martinelli G, et al. Levofloxacin to prevent bacterial infection in patients with cancer and neutropenia. New England Journal of Medicine 2005;353(10):977‐87. [DOI] [PubMed] [Google Scholar]
- Micozzi A, Bucaneve O, Martino P, Menichetti F, Favero A, GIMEMA Infection Program. Levofloxacin compared to placebo for preventing infection in neutropenic cancer patients in a multi‐centre, double‐blind, randomised, controlled trial. Abstracts of the 44th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2004 October 30 ‐ November 2 Washington DC, USA. 2004:Abstract L‐991.
Carlson 1997 {published data only}
- Carlson JW, Fowler JM, Mitchell SK, Carson LF, Mayer AR, Copelands LJ. Chemoprophylaxis with ciprofloxacin in ovarian cancer patients receiving paclitaxel: a randomised trial. Gynecologic Oncology 1997;65:325‐9. [DOI] [PubMed] [Google Scholar]
Casali 1988 {published data only}
- Casali A, Verri C, Paoletti G, Tropea F, Modugno G, Frasca AM, et al. Chemoprophylaxis of bacterial infections in granulocytopenic cancer patients using norfloxacin. Chemioterapia 1988;7:327‐9. [PubMed] [Google Scholar]
Castagnola 2003 {published data only}
- Boni L, Castagnola E, Giacchino M, Cesaro S, Sio L, Garaventa A, et al. Amoxicillin‐Clavulanate (AC) Vs Placebo (PB) in the Prevention of Febrile Neutropenia in Children with Cancer. Abstracts of the 40th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2000 September Toronto. 2000:Abstract 727:473.
- Castagnola E, Boni L, Giacchino M, Cesaro S, Sio L, Garaventa A, et al. A multicentre, randomised, double blind placebo‐controlled trial of amoxicillin/clavulanate for the prophylaxis of fever and infection in neutropenic children with cancer. Pediatric Infectious Disease Journal 2003;22(4):359‐65. [DOI] [PubMed] [Google Scholar]
Chastagner 1997 {published data only}
- Chastagner P, Leverger G, Perel Y, Lamagnere JP, Bergeron C, Vannier JP, et al. Prevention of gram positive infections with teicoplanin during the induction therapy of childhood acute lymphoblastic leukemia (ALL): results of a prospective randomised trial. Abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy; 1997 Sept 28 ‐ Oct 1; Toronto. 1997:Abstract LM‐58b.
Chung 1997 {published data only}
- Chung DR, Kim YS, Kim EO, JH Woo, Ryu JS. Prophylaxis with Tosufloxacin during Periods of Granulocytopenia in Patients with Acute Leukemia. Abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy; 1997 September 28 ‐ October 1; Toronto. 1997:Abstract LM‐58a.
Cruciani 1989 {published data only}
- Cruciani M, Concia E, Navarra A, Perversi L, Bonetti F, Arico M, et al. Prophylactic co‐trimoxazole versus norfloxacin in neutropenic children‐‐perspective randomised study. Infection 1989;17:65‐9. [DOI] [PubMed] [Google Scholar]
Cullen 2005 {published data only}
- Cullen M, Steven N, Billingham L, Gaunt C, Hastings M, Simmonds P, et al. Antibacterial prophylaxis after chemotherapy for solid tumors and lymphomas. New England Journal of Medicine 2005;353(10):988‐98. [DOI] [PubMed] [Google Scholar]
D'Antonio 1991 {published data only}
- D'Antonio D, Iacone A, Fioritoni G, Betti S, DiGirolamo A, Piccolomini R, et al. Antibacterial prophylaxis in granulocytopenic patients: A randomised study of ofloxacin versus norfloxacin. Currrent Therapeutic Research 1991;50:304‐11. [Google Scholar]
D'Antonio 1992 {published data only}
- D'Antonio D, Iacone A, Fioritoni G, Betti S, Dell'Isola M, Quaglietta AM, et al. Comparison of norfloxacin and pefloxacin in the prophylaxis of bacterial infection in neutropenic cancer patients. Drugs Under Experimental & Clinical Research 1992;18:141‐6. [PubMed] [Google Scholar]
D'Antonio 1994 {published data only}
- D'Antonio D, Piccolomini R, Iacone A, Fioritoni G, Parruti G, Betti S, et al. Comparison of ciprofloxacin, ofloxacin and pefloxacin for the prevention of the bacterial infection in neutropenic patients with haematological malignancies. Journal of Antimicrobial Chemotherapy 1994;33:837‐44. [DOI] [PubMed] [Google Scholar]
de Jongh 1983 {published data only}
- Jongh CA, Wade JC, Finley RS, Joshi JH, Aisner J, Wiernik PH, et al. Trimethoprim/sulfamethoxazole versus placebo: a double‐blind comparison of infection prophylaxis in patients with small cell carcinoma of the lung. Journal of Clinical Oncology 1983;1:302‐7. [DOI] [PubMed] [Google Scholar]
Dekker 1981 {published and unpublished data}
- Dekker AW, Rozenberg, Arska M, Sixma JJ, Verhoef J. Prevention of infection by trimethoprim‐sulfamethoxazole plus amphotericin B in patients with acute nonlymphocytic leukaemia. Annals of Internal Medicine 1981;95:555‐9. [DOI] [PubMed] [Google Scholar]
- Verhoef J, Rozenberg‐Arska M, Dekker A. Prevention of infection in the neutropenic patient. Reviews of Infectious Diseases 1989;11 Suppl 7:1545‐50. [DOI] [PubMed] [Google Scholar]
Dekker 1987 {published and unpublished data}
- Dekker AW, Rozenberg, Arska M, Verhoef J. Infection prophylaxis in acute leukemia: a comparison of ciprofloxacin with trimethoprim‐sulfamethoxazole and colistin. Annals of Internal Medicine 1987;106:7‐11. [DOI] [PubMed] [Google Scholar]
Dickgreber 2009 {published data only}
- Dickgreber N. Nagel S. Roscher K. Schuette W. Randomised, placebo‐controlled phase III trial of docetaxel plus carboplatin with or without levofloxacin prophylaxis in elderly patients with advanced non‐small cell lung cancer: The APRONTA Trial [19th European Congress of Clinical Microbiology and Infectious Diseases]. Clinical Microbiology and Infection. 2009; Vol. 15:S519.
Donnelly 1992b {published data only}
- Donnelly JP, Maschmeyer G, Daenen S. Selective oral antimicrobial prophylaxis for the selective oral antimicrobial prophylaxis for the pevention of infection in acute leukaemia‐ciprofloxacin versus co‐trimoxazole plus colistin. The EORTC‐Gnotobiotic Project Group. European Journal of Cancer 1992;28A:873‐8. [DOI] [PubMed] [Google Scholar]
- Verhoef J, Rozenberg‐Arska M, Dekker A. Prevention of infection in the neutropenic patient. Reviews of Infectious Diseases 1989;11(suppl 7):1545‐50. [DOI] [PubMed] [Google Scholar]
Enno 1978 {published data only}
- Enno A, Catovsky D, Darrell J, Goldman JM, Hows J, Galton DA. Co‐trimoxazole for prevention of infection in acute leukaemia. Lancet 1978;2:395‐7. [DOI] [PubMed] [Google Scholar]
EORTC 1984 {published and unpublished data}
- EORTC International Antimicrobial Therapy Project Group. Trimethoprim‐sulfamethoxazole in the prevention of infection in neutropenic patients. EORTC International Antimicrobial Therapy Project Group. Journal of Infectious Diseases 1984;150:372‐9. [DOI] [PubMed] [Google Scholar]
EORTC 1994 {published and unpublished data}
- nternational Antimicrobial Therapy Cooperative Group of the European Organization for Research and Treatment of Cancer. Reduction of fever and streptococcal bacteraemia in granulocytopenic patients with cancer: a trial of oral penicillin v or placebo combined with pefloxacin. International Antimicrobial Therapy Cooperative Group of the European Organization for Research and Treatment of Cancer. JAMA 1994;272(15):1183‐9. [PubMed] [Google Scholar]
Estey 1984 {published data only}
- Estey E, Maksymiuk A, Smith T, Fainstein V, Keating M, McCredie KB, et al. Infection prophylaxis in acute leukemia. Comparative effectiveness of sulfamethoxazole and trimethoprim, ketoconazole, and a combination of the two. Archives of Internal Medicine 1984;144(8):1562‐8. [DOI] [PubMed] [Google Scholar]
Fanci 1993 {published data only}
- Fanci R, Leoni F, Bosi A, Guidi S, Ciolli S, Longo G, et al. Chemoprophylaxis of bacterial infections in granulocytopenic patients with ciprofloxacin vs ciprofloxacin plus amoxicillin. Journal of Chemotherapy 1993;5:119‐23. [DOI] [PubMed] [Google Scholar]
Ford 1998 {published and unpublished data}
- Ford CD, Reilly W, Wood J, Classen DC, Burke JP. Oral antimicrobial prophylaxis in bone marrow transplant recipients: randomised trial of ciprofloxacin versus ciprofloxacin‐vancomycin. Antimicrobial Agents and Chemotherapy 1998;42:1402‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Garcia Saenz 2002 {published and unpublished data}
- García‐Sáenz JA, Martín M, Casado A, Pérez‐Segura P, Manrique I, Flores L, et al. Immediate vs. delayed imipenem treatment in cancer patients with profound neutropenia induced by high‐dose chemotherapy:Results of a randomised study [Tratamiento inmediato o tardío con imipenemen pacientes con cáncer y neutropenia por quimioterapia a dosis altas:Resultados de un estudio comparativo aleatorizado]. Rev Esp Quimioterap 2002;15(3):257‐63. [PubMed] [Google Scholar]
GIMEMA 1991 {published data only}
- Gruppo Italiano Malattie Ematologiche Maligne dell'Adulto. Prevention of bacterial infection in neutropenic patients with hematologic malignancies. A randomised, multicentre trial comparing norfloxacin with ciprofloxacin. The GIMEMA Infection Program. Gruppo Italiano Malattie Ematologiche Maligne dell'Adulto. Annals of Internal Medicine 1991;115:7‐12. [PubMed] [Google Scholar]
Gluckman 1988 {published data only}
- Gluckman E, Cavazzana M, Devergie A, Meletis J, Arlet G, Perol Y, et al. Prevention of bacterial infections after bone marrow graft by broad‐spectrum oral antibiotics, absorbable (pefloxacin, penicillin) and non absorbable (cephalosporin, gentamycin, bacitracin) [Prevention des infections bacteriennes apres greffe de moelle osseuse par antibiotique oraux a large spectre absorbables (pefloxacine, penicilline) ou non absorbables (cephalosporine, gentamicine, bacitracine)]. Pathologie Biologie 1988;36(7):902‐6. [PubMed] [Google Scholar]
Gluckman 1991 {published data only}
- Gluckman E, Roudet C, Hirsch I, Devergie A, Bourdeau H, Arlet C, et al. Prophylaxis of bacterial infections after bone marrow transplantation. A randomised prospective study comparing oral broad‐spectrum nonabsorbable antibiotics (vancomycin‐tobramycin‐colistin) to absorbable antibiotics (ofloxacin‐amoxicillin). Chemotherapy 1991;37 Suppl 1:33‐8. [DOI] [PubMed] [Google Scholar]
Gomez‐Martin 2000 {published data only}
- Gomez‐Martin C, Sola C, Hornedo J, Perea S, Lumbreras C, Valenti V, et al. Rifampin does not improve the efficacy of quinolone antibacterial prophylaxis in neutropenic cancer patients: results of a randomised clinical trial. Journal of Clinical Oncology 2000;18:2126‐34. [DOI] [PubMed] [Google Scholar]
Goorin 1985 {published data only}
- Goorin AM, Hershey BJ, Levin MJ, Siber GR, Gelber RD, Flynn K, et al. Use of trimethoprim‐sulfamethoxazole to prevent bacterial infections in children with acute lymphoblastic leukemia. Pediatric Infectious Disease 1985;4(3):265‐9. [DOI] [PubMed] [Google Scholar]
Gualtieri 1983 {published data only}
- Gualtieri RJ, Donowitz GR, Kaiser Dl, Hess CE, Sande MA. Double‐blind randomised study of prophylactic trimethoprim/sulfamethoxazole in granulocytopenic patients with hematologic malignancies. American Journal of Medicine 1983;74:934‐40. [DOI] [PubMed] [Google Scholar]
Guiot 1983 {published data only}
- Guiot HF, Broek PJ, Meer JW, Furth R. Selective antimicrobial modulation of the intestinal flora of patients with acute nonlymphocytic leukemia: a double‐blind, placebo‐controlled study. Journal of Infectious Diseases 1983;147:615‐23. [DOI] [PubMed] [Google Scholar]
Guiot 1992 {published data only}
- Guiot HF, Meer JWM, Broek PJ, Willemze R, Furth R. Prevention of viridans‐group streptococcal septicemia in oncohematologic patients: a controlled comparative study on the effect of penicillin G and cotrimoxazole. Annals of Hematology 1992;64:260‐5. [DOI] [PubMed] [Google Scholar]
Gurwith 1979 {published and unpublished data}
- Gurwith MJ, Brunton JL, Lank BA, Harding GK, Ronald AR. A prospective controlled investigation of prophylactic trimethoprim/sulfamethoxazole in hospitalized granulocytopenic patients. American Journal of Medicine 1979;66:248‐56. [DOI] [PubMed] [Google Scholar]
Hargadon 1981 {published data only}
- Hargadon MT, Young VM, Schimpff SC, Wade JC, Minah GE. Selective suppression of alimentary tract microbial flora as prophylaxis during granulocytopenia. Antimicrobial Agents & Chemotherapy 1981;20:620‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harousseau 1987 {published and unpublished data}
- Harousseau JL, Milpied N, Reynaud AE. Antibiotic prophylaxis with ceftriaxone for severe and durable neutropenia [Utilisation de la ceftriaxone en prophylaxie des infections bacteriennes au cours des]. Medecine et Maladies Infectieuses 1989;19:91‐5. [Google Scholar]
- Harousseau Jl, Milpied N, Reynaud AE, Derriennic M, Bourhis JH, Courtieu AL. Preventive systemic antibiotherapy with ceftriaxone alone in neutropenic patients treated in a protected environment. [French]. La Presse Medicale 1987;16:1737‐40. [PubMed] [Google Scholar]
Hartlapp 1987 {published data only}
- Hartlapp JH. Antimicrobial prophylaxis in immunocompromised patients. Drugs 1987;34 Suppl 1:131‐3. [DOI] [PubMed] [Google Scholar]
Henry 1984b {published data only}
- Henry SA, Armstrong D, Kempin S, Gee T, Arlin Z, Clarkson B. Oral trimethoprim/sulfamethoxazole in attempt to prevent infection after induction chemotherapy for acute leukemia. American Journal of Medicine 1984;77:663‐6. [DOI] [PubMed] [Google Scholar]
Hidalgo 1997 {published data only}
- Hidalgo M, Hornedo J, Lumbreras C, Trigo JM, Gomez C, Perea S, et al. Lack of ability of ciprofloxacin‐rifampin prophylaxis to decrease infection‐related morbidity in neutropenic patients given cytotoxic therapy and peripheral blood stem cell transplants. Antimicrobial Agents & Chemotherapy 1997;41:1175‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Inoue 1983 {published data only}
- Inoue M, Arai S, Kawamura Y, Kamiya H, Sakurai M, Izawa T, et al. The prophylactic effect of trimethoprim‐sulfamethoxazole on infections in children with acute leukaemia. Drugs Under Experimental and Clinical Research 1983;9:35‐43. [Google Scholar]
Jansen 1994 {published and unpublished data}
- Jansen J, Cromer M, Akard L, Black JR, Wheat J. Infection prevention in severely myelosuppressed patients: a comparison between ciprofloxacin and a regimen of selective antibiotic modulation of the intestinal flora. American Journal of Medicine 1994;96:335‐41. [DOI] [PubMed] [Google Scholar]
Jehn 1981 {published data only}
- Jehn U, Ruckdeschel G, Sauer H, Clemm C, Wilmanns W. Comparative study on the value of selective decontamination of the digestive tract in acute leukemia patients (author's transl)]. [German]. Klinische Wochenschrift 1981;59:1093‐9. [DOI] [PubMed] [Google Scholar]
Karp 1987 {published and unpublished data}
- Karp JE, Merz WG, Hendricksen C, Laughon B, Redden T, Bamberger BJ, et al. Infection Management during antileukemic treatment‐induced granulocytopenia: the role for oral norfloxacin prophylaxis against infections arising from gastrointestinal tract. Scandinavian Journal of Infectious Diseases 1986;Suppl 48:66‐78. [PubMed] [Google Scholar]
- Karp JE, Merz WG, Hendricksen C, Laughon B, Redden T, Bamberger BJ, et al. Oral norfloxacin for prevention of gram‐negative bacterial infections in patients with acute leukemia and granulocytopenia. A randomised, double‐blind, placebo‐controlled trial. Annals of Internal Medicine 1987;106:1‐7. [DOI] [PubMed] [Google Scholar]
Kauffman 1983 {published data only}
- Kauffman CA, Liepman MK, Bergman AG, Mioduszewski J. Trimethoprim/sulfamethoxazole prophylaxis in neutropenic patients. Reduction of infections and effect on bacterial and fungal flora. The American Journal of Medicine 1983;74:599‐607. [DOI] [PubMed] [Google Scholar]
Kern 1991b {published data only}
- Kern W, Kurrle E. Ofloxacin versus trimethoprim‐sulfamethoxazole for prevention of infection in patients with acute leukemia and granulocytopenia. Infection 1991;19:73‐80. [DOI] [PubMed] [Google Scholar]
Kern 1994b {published data only}
- Kern WV, Hay B, Kern P, Marre R, Arnold R. A randomised trial of roxithromycin in patients with acute leukemia and bone marrow transplant recipients receiving fluoroquinolone prophylaxis. Antimicrobial Agents and Chemotherapy 1994;38:465‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Klastersky 1974 {published and unpublished data}
- Klastersky J, Debusscher L, Weerts D, Daneau D. Use of oral antibiotics in protected units environment: clinical effectiveness and role in the emergence of antibiotic‐resistant strains. Pathologie Biologie 1974;22(1):5‐12. [PubMed] [Google Scholar]
Kovatch 1985 {published data only}
- Kovatch AL, Wald ER, Albo VC, Prin W, Orlando SJ, Wollman MR, et al. Oral trimethoprim‐sulfamethoxazole for prevention of bacterial infection during the induction phase of cancer chemotherapy in children. Pediatrics 1985;76(5):754‐60. [PubMed] [Google Scholar]
Kramer 1984 {published and unpublished data}
- Kramer BS, Carr DJ, Rand KH, Pizzo PA, Johnson A, Robichaud KJ, et al. Prophylaxis of fever and infection in adult cancer patients. A placebo‐controlled trial of oral trimethoprim‐sulfamethoxazole plus erythromycin. Cancer 1984;53(2):329‐35. [DOI] [PubMed] [Google Scholar]
Kurrle 1983 {published and unpublished data}
- Kurrle E, Bhaduri S, Pfleiger H, Heimpel H. Antimicrobial prophylaxis in acute leukemia: prospective randomised study comparing two methods of selective decontamination. Klinische Wochen‐schrift 1983;61:691‐8. [DOI] [PubMed] [Google Scholar]
Kurrle 1986 {published data only}
- Kurrle E, Dekker AW, Gaus W, Harlambie E, Krieger M, Rozenberg‐Arska M et al (EORTC writing committee). Prevention of infection in acute leukemia: a prospective randomised study on the efficacy of two different drug regimens for antimicrobial prophylaxis. Infection 1986;14(5):226‐32. [DOI] [PubMed] [Google Scholar]
Lalami 2004 {published data only}
- Lalami Y, Paesmans M, Aoun M, Munoz‐Bermeo R, Reuss K, Cherifi S, et al. A prospective randomised evaluation of G‐CSF or G‐CSF plus oral antibiotics in chemotherapy‐treated patients at high risk of developing febrile neutropenia. Support Care Cancer 2004;12:725‐30. [DOI] [PubMed] [Google Scholar]
Lamy 1993 {published and unpublished data}
- Lamy T, Michelet C, Dauriac C, Grulois I, Donio PY, Prise PY. Benefit of prophylaxis by intravenous systemic vancomycin in granulocytopenic patients: a prospective, randomised trial among 59 patients. Acta Haematologica 1993;90:109‐13. [DOI] [PubMed] [Google Scholar]
Lange 1984 {published data only}
- Lange B, Halpern S, Gale G, Kramer S. Trimethoprim‐sulfamethoxazole and nystatin prophylaxis in children with acute lymphoblastic leukemia. European Paediatric Haematology and Oncology 1984;1:231‐8. [Google Scholar]
Lee 2002 {published data only}
- Lee DG, Choi SM, Choi JH, Yoo JH, Park YH, Kim YJ, et al. Selective bowel decontamination for the prevention of infection in acute myelogenous leukemia: a prospective randomised trial. Korean Journal of Internal Medicine 2002;17(1):38‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Levine 1973 {published data only}
- Levine AS, Seigel SE, Schreiber AD, Hauser J, Preisler H, Goldstein IM, et al. Protected environments and prophylactic antibiotics. A prospective controlled study of their utility in the therapy of acute leukemia. New England Journal of Medicine 1973;288:477‐83. [DOI] [PubMed] [Google Scholar]
Lew 1991 {published data only}
- Lew MA, Kehoe K, Ritz J, Antman Kh, Nadler L, Takvorian T, et al. Prophylaxis of bacterial infections with ciprofloxacin in patients undergoing bone marrow transplantation. Transplantation 1991;51:630‐6. [DOI] [PubMed] [Google Scholar]
Lew 1995 {published data only}
- Lew MA, Kehoe K, Ritz J, Antman KH, Nadler L, Kalish LA, et al. Ciprofloxacin versus trimethoprim/sulfamethoxazole for prophylaxis of bacterial infections in bone marrow transplant recipients: a randomised, controlled trial. Journal of Clinical Oncology 1995;13:239‐50. [DOI] [PubMed] [Google Scholar]
Liang 1990 {published data only}
- Liang RH, Yung RWH, Chan TK, Chau PY, Lam WK, So SY, et al. Ofloxacin versus co‐trimoxazole for prevention of infection in neutropenic patients following cytotoxic chemotherapy. Antimicrobial Agents & Chemotherapy 1990;34:215‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maiche 1993 {published data only}
- Maiche AG, Muhonen T. Granulocyte colony‐stimulating factor (G‐CSF) with or without a quinolone in the prevention of infection in cancer patients. European Journal of Cancer 1993;29A(10):1403‐5. [DOI] [PubMed] [Google Scholar]
Malarme 1981 {published data only}
- Malarme M, Meunier‐Carpentier F, Klastersky J. Vancomycin plus gentamicin and cotrimoxazole for prevention of infections in neutropenic cancer patients (a comparative, placebo‐controlled pilot study). European Journal of Cancer and Clinical Oncology 1981;17:1315‐22. [DOI] [PubMed] [Google Scholar]
Martino 1984 {published data only}
- Martino P, Venditti M, Petti MC, Mandelli F, Serra P. Cotrimoxazole prophylaxis in patients with leukemia and prolonged granulocytopenia. American Journal of the Medical Sciences 1984;287:7‐9. [DOI] [PubMed] [Google Scholar]
Maschmeyer 1988 {published and unpublished data}
- Maschmeyer G, Haralambie E, Gaus W, Kern W, Dekker AW, Vries‐Hospers HG, et al. Ciprofloxacin and norfloxacin for selective decontamination in patients with severe granulocytopenia. Infection 1988;16:98‐104. [DOI] [PubMed] [Google Scholar]
Mocikova 1992 {published data only}
- Mocikova K, Svac J, Faber E, Wildova B, Banikova I. Comparison of Tarivid and Biseptol in the prevention of bacterial infections in patients with acute leukemia. Vnitrni Lekarstvi 1992;38(2):166‐72. [PubMed] [Google Scholar]
Moreau 1995 {published data only}
- Moreau P, Milplied N, Richard P, Bulbois CE, Richet H, Harousseau JL. Prospective randomised study comparing prophylactic ciprofloxacin (C) and amoxicillin + clavulanic acid (A‐CA) vs prophylactic vancomycin (V) vs non prophylactic conventional empiric antimicrobial therapy in neutropenic patients [abstract]. Bone Marrow Transplantation. 1995; Vol. 15 Suppl 2:129.
Moriuchi 1990 {published data only}
- Moriuchi Y, Kamihira S, Yamamura M, Mori H, Miyazaki Y, Tokunaga S, et al. Comparison of ciprofloxacin with polymyxin B for infection prophylaxis in neutropenic patients with acute non‐lymphocytic leukemia. Japanese Journal of Clinical Hematology 1990;31:1664‐9. [PubMed] [Google Scholar]
Murase 1995 {published data only}
- Murase T. Chemoprophylaxis of bacterial infections in granulocytopenic patients with new quinolone: a comparison of trimethoprim‐sulfamethoxazole (ST) alone with ST plus ciprofloxacin. Journal of the Japanese Association for Infectious Diseases 1995;69:28‐32. [DOI] [PubMed] [Google Scholar]
Nemet 1989 {published data only}
- Nemet D, Kalenic S, Badanjak A, Bogdanic V, Davila N, Francetic I, et al. Prevention of gram‐negative bacterial infection in granulocytopenic patients: a randomised study comparing oral norfloxacin with gentamycin. Bone Marrow Transplantation 1989;4(Suppl 3):105. [PubMed] [Google Scholar]
Nenova 2001 {published data only}
- Nenova IS, Ananostev NH, Goranov, SE, Mateva NG, Haidushka IA. Fluoroquinolone prophylaxis for bacterial infections in neutropenic patients with hematologic malignancies. Folia Medica Plovdiv 2001;43(4):40‐5. [PubMed] [Google Scholar]
Orlandi 1990 {published data only}
- Orlandi E, Navarra A, Cruciani M, Caldera D, Morra E, Castagnola C, et al. Norfloxacin versus cotrimoxazole for infection prophylaxis in granulocytopenic patients with acute leukemia. A prospective randomised study. Haematologica 1990;75:296‐8. [PubMed] [Google Scholar]
Papaiakovou 2010 {published data only}
- Eleutherakis‐Papaiakovou E, Kostis E, Migkou M, Christoulas D, Terpos E, Gavriatopoulou M, et al. Prophylactic antibiotics for the prevention of neutropenic fever in patients undergoing autologous stem‐cell transplantation: Results of a single institution, randomised phase 2 trial. American Journal of Hematology 2010;85(11):863‐7. [DOI] [PubMed] [Google Scholar]
Patrick 1995 {published data only}
- Patrick CC, Adair JR, Warner WC Jr, Church DA, Sanders RH, Krance R, et al. Safety of Ciprofloxacin as a Prophylactic Agent for Prevention of Bacterial Infections in Pediatric Bone Marrow Transplant (BMT) Patients. Abstracts of the 35th Interscience Conference on Antimicrobial Agents and Chemotherapy; 1995 October; San Francisco. 1995:Abstract LM32.
Petersen 1986 {published data only}
- Petersen FB, Buckner CD, Clift RA, Lee S, Nelson N, Counts GW, et al. Laminar air flow isolation and decontamination: a prospective randomised study of the effects of prophylactic systemic antibiotics in bone marrow transplant patients. Infection 1986;14(3):115‐21. [DOI] [PubMed] [Google Scholar]
Pignon 1990 {published data only}
- Pignon B, Thiriet L, Aubert D, Pennaforte JL, Vilque JP, Lartigue B, et al. Evaluation of the efficacy of prophylactic intravenous antibiotherapy with ceftriaxone in post‐chemotherapy agranulocytic patients. Nouvelle Revue Francaise d'Hematologie 1990;32(4):249‐52. [PubMed] [Google Scholar]
Pizzo 1983 {published data only}
- Pizzo PA, Robichaud K, Edwards BK, Schumaker C, Kramer BS, Johnson A. Oral antibiotic prophylaxis in patients with cancer: a double‐blind randomised placebo‐controlled trial. Journal of Pediatrics 1983;102:125‐33. [DOI] [PubMed] [Google Scholar]
Prentice 2001 {published data only}
- Prentice HG, Hann IM, Nazareth B, Paterson P, Bhamra A, Kibbler CC. Oral ciprofloxacin plus colistin: prophylaxis against bacterial infection in neutropenic patients. A strategy for the prevention of emergence of antimicrobial resistance. British Journal of Haematology 2001;115:46‐52. [DOI] [PubMed] [Google Scholar]
Rafecas 1989 {published data only}
- Rafecas FJ, Gil E, Martin G, Martinez J, Sanz G, Sempere A, et al. Oral ciprofloxacin in the prophylaxis of bacterial infection in neutropenic patients. A randomized, double‐blind, comparative clinical study [Ciprofloxacina oral en la profilaxis de la infeccion bacteriana en pacientes neutropenicos. Ensayo clinico aleatorio, doble ciego y comparativo vs. placebo]. Rev Esp Quimioter 1989;2(2):174‐7. [Google Scholar]
Rahman 2009 {published data only}
- Rahman MM, Khan MA. Levofloxacin prophylaxis to prevent bacterial infection in chemotherapy‐induced neutropenia in acute leukemia. Bangladesh Medical Research Council Bulletin 2009;35:91‐4. [DOI] [PubMed] [Google Scholar]
Ruiz 2001 {published data only}
- Ruiz I, Fuentes I, Capdevila JA, Juli A, Carreras J, Pahissa A, et al. Ofloxacin Prophylaxis in Preventing Bacterial Infection after Autologous Peripheral Blood Stem Cell Transplantation (A‐PBSC). Abstracts of the 41st Interscience Conference on Antimicrobial Agents and Chemotherapy; 2001 September and December; Chicago. 2001:Abstract L‐781:447.
Sampi 1992 {published data only}
- Sampi K, Maseki N, Hattori M. [A comparison of nystatin with norfloxacin for prevention of infection after consolidation therapy in patients with acute leukemia or autologous bone marrow transplantation: a randomised study]. Gan To Kagaku Ryoho 1992;19(6):823‐6. [PubMed] [Google Scholar]
Schimpff 1975 {published data only}
- Schimpff SC, Greene WH, Young VM, Fortner CL, Cusack N, Block JB, et al. Infection prevention in acute nonlymphocytic leukemia. Laminar air flow room reverse isolation with oral, nonabsorbable antibiotic prophylaxis. Annals of Internal Medicine 1975;82(3):351‐8. [DOI] [PubMed] [Google Scholar]
Schroeder 1992 {published data only}
- Schroeder M, Schadeck‐Gressel C, Selbach J, Westerhausen M. Antibiotic prophylaxis with gyrase inhibitors during cytostatically induced granulocytopenias in patients with solid tumors: A double‐blind prospective randomised study. Onkologie 1992;15:476‐9. [Google Scholar]
Slavin 2007 {published data only}
- Slavin MA, Grigg AP, Schwarer AP, Szer J, Spencer A, Sainani A, et al. A randomised comparison of empiric or pre‐emptive antibiotic therapy after hematopoietic stem cell transplantation. Bone Marrow Transplantation 2007;40:157‐163. [DOI] [PubMed] [Google Scholar]
Sleijfer 1980 {published data only}
- Sleijfer DT, Mulder NH, Vries‐Hospers HG, Fidler V, Nieweg HO, Waaij D, et al. Infection prevention in granulocytopenic patients by selective decontamination of the digestive tract. European Journal of Cancer 1980;16:859‐69. [DOI] [PubMed] [Google Scholar]
- Vries H, Sleijfer DT, Mulder NH, Waaj D, Nieweg OH, Saene HKF. Bacteriological aspects of selective decontamination of the digestive tract as a method of infection prevention in granulocytopenic patients. Antimicrobial Agents & Chemotherapy 1981;19(5):813‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Starke 1982 {published data only}
- Starke ID, Donnely P, Catovsky D, Darrell J, Johnson SA, Goldman JM, et al. Co‐trimoxazole alone for prevention of bacterial infection in patients with acute leukaemia. Lancet 1982;1(8262):5‐6. [DOI] [PubMed] [Google Scholar]
Talbot 1993 {published data only}
- Talbot GH, Cassileth GH, Paradiso L, Correa‐Coronas R, Bond L. Oral enoxacin for infection prevention in adults with acute nonlymphocytic leukemia. The Enoxacin Prophylaxis Study Group. Antimicrobial Agents and Chemotherapy 1993;3:474‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Teinturier 1995 {published data only}
- Teinturier C, Hartmann O, Lemerle J, Benhamou E, Maraninchi D. Prevention of gram‐positive infections in patients treated with high‐dose chemotherapy and bone marrow transplantation: a randomised controlled trial of vancomycin. Pediatric Hematology and Oncology 1995;12(1):73‐7. [DOI] [PubMed] [Google Scholar]
Thomas 2000 {published and unpublished data}
- Thomas X, Troncy J, Belhabri A, Thiebaut A, Bouheddou N, Michallet M, et al. Effectiveness of combined vancomycin and pefloxacine in gastrointestinal decontamination for preventing infections after chemotherapy‐induced bone marrow aplasia. A randomised double‐blind study. La Presse Medicale 2000;29(32):1745‐51. [PubMed] [Google Scholar]
Timmers 2007 {published data only}
- Timmers GJ, Simoons‐Smit AM, Leidekker ME, Janssen JJWM, Vandenbroucke‐Grauls CMJE, Huijgens PC. Levofloxacin vs. ciprofloxacin plus phenethicillin for the prevention of bacterial infections in patients with haematological malignancies. Clinical Microbiology and Infection 2007;13(5):497‐503. [DOI] [PubMed] [Google Scholar]
Tjan Heijnen 2001 {published data only}
- Tjan Heijnen VCG, Postmus PE, Ardizzoni A, Manegold Ch, Burghouts J, Meerbeeck J, et al. Reduction of chemotherapy‐induced febrile leucopenia by prophylactic use of ciprofloxacin and roxithromycin in small‐cell lung cancer patients: an EORTC double‐blind placebo‐controlled phase III study. Annals of Oncology 2001;12(10):1359‐68. [DOI] [PubMed] [Google Scholar]
Tsutani 1992 {published data only}
- Tsutani H, Imamura S, Ueda T, Yoshida H, Iwasaki H, Fukushima T. Prophylactic use of ofloxacin in granulocytopenic patients with hematological malignancies during post‐remission chemotherapy. Internal Medicine 1992;31:319‐24. [DOI] [PubMed] [Google Scholar]
van Eys 1987 {published and unpublished data}
- Eys J, Berry DM, Crist W, Doering EJ, Fernbach DJ, Pullen J, et al. Effect of trimethoprim/sulfamethoxazole prophylaxis on outcome of childhood lymphocytic leukemia. A Pediatric Oncology Group Study. Cancer 1987;1(59):19‐23. [DOI] [PubMed] [Google Scholar]
Wade 1981a {published data only}
- Wade JC, Schimpff SC, Hargadon MT, Fortner CL, Wiernik PH. A comparison of trimethoprim‐sulfamethoxazole plus nystatin with gentamicin plus nystatin in the prevention of infections in acute leukemia. New England Journal of Medicine 1981;304(18):1057‐62. [DOI] [PubMed] [Google Scholar]
Wade 1983 {published data only}
- Wade JC, Jongh CA, Newman KA, Crowley J, Wiernik PH, Schimpff SC. Selective antimicrobial modulation as prophylaxis against infection during granulocytopenia: trimethoprim‐sulfamethoxazole vs. nalidixic acid. Journal of Infectious Diseases 1983;147:624‐34. [DOI] [PubMed] [Google Scholar]
Ward 1993 {published data only}
- Ward TT, Thomas RG, Fye CL, Arbeit R, Coltman CA Jr, Craig W, et al. Trimethoprim‐sulfamethoxazole prophylaxis in granulocytopenic patients with acute leukemia: evaluation of serum antibiotic levels in a randomised, double‐blind, placebo‐controlled Department of Veterans Affairs Cooperative Study. Clinical Infectious Diseases 1993;17:323‐32. [DOI] [PubMed] [Google Scholar]
Watson 1982 {published data only}
- Watson JG, Jameson B, Powles RL, McElwain TJ, Lawson DN, Judson I, et al. Co‐trimoxazole versus nonabsorbable antibiotics in acute leukaemia. Lancet 1982;1(8262):6‐9. [DOI] [PubMed] [Google Scholar]
Weiser 1981 {published data only}
- Weiser B, Lange M, Fialk MA, Singer C, Szatrowski TH, Armstrong D. Prophylactic trimethoprim‐sulfamethoxazole during consolidation chemotherapy for acute leukemia: a controlled trial. Annals of Internal Medicine 1981;95:436‐8. [DOI] [PubMed] [Google Scholar]
Winston 1986 {published data only}
- Winston DJ, Ho WJ, Champlin RE, Karp J, Bartlett J, Finley RS, et al. Norfloxacin for prevention of bacterial infections in granulocytopenic patients. American Journal of Medicine 1987;82(suppl 6B):40‐6. [DOI] [PubMed] [Google Scholar]
- Winston DJ, Ho WJ, Nakao SL, Gale RP, Champlin RE. Norfloxacin versus vancomycin/polymyxin for prevention of infections in granulocytopenic patients. American Journal of Medicine 1986;80(5):884‐90. [DOI] [PubMed] [Google Scholar]
Winston 1990 {published data only}
- Winston DJ, Winston GH, Bruckner DA, Gale RP, Champlin RE. Ofloxacin versus vancomycin/polymyxin for prevention of infections in granulocytopenic patients. American Journal of Medicine 1990;88:36‐42. [DOI] [PubMed] [Google Scholar]
Yamada 1993 {published and unpublished data}
- Yamada T, Kazuo T, Nomura T. Prevention of bacterial and fungal infections in acute leukemia patients: a new potent combination of oral norfloxacin and amphotericin b. Internal Medicine 1993;32(9):710‐4. [DOI] [PubMed] [Google Scholar]
Yates 1973 {published data only}
- Yates JW, Holland JF Yates JW, Holland JF. A controlled study of isolation and endogenous microbial suppression in acute myelocytic leukemia patients. Cancer 1973;32(6):1490‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Barriga 1997 {published data only}
- Barriga F, Varas M, Potin M, Sapunar F, Rojo H, Martinez A, et al. Efficacy of Vancomycin solution to prevent bacteraemia associated with an indwelling central venous catheter in neutropenic and non‐neutropenic cancer patients. Medical and Pediatric Oncology 1997;28:196‐200. [DOI] [PubMed] [Google Scholar]
Bunn 2001 {published data only}
- Bunn, PA. Editorial comments on 'Reduction of chemotherapy‐induced febrile leucopenia by prophylactic use of ciprofloxacin and roxithromycin in small‐cell lung cancer patients: an EORTC double‐blind placebo‐controlled phase III study'. Annals of Oncology 2001;12(10):1339‐40. [DOI] [PubMed] [Google Scholar]
Capdevila 1997 {published data only}
- Capdevila J, Pahissa A. Infection prophylaxis in neutropenic patients [Profilaxis de las infecciones en los pacientes neutropenicos]. Enfermedades Infecciosas y Microbiologica Clininica 1997;15(10):544‐51. [PubMed] [Google Scholar]
Corrado 1988 {published data only}
- Corrado ML, Struble WE, Hesney M. The tolerability profile of prophylactic norfloxacin in neutropenic patients. European Journal of Cancer and Clinical Oncology 1988;24 Suppl 1:29‐33. [PubMed] [Google Scholar]
Craig 2007 {published data only}
- Craig M, Cumpston AD, Hobbs GR, Devetten MP, Sarwari AR, Ericson SG. The clinical impact of antibacterial prophylaxis and cycling antibiotics for febrile neutropenia in a hematological malignancy and transplantation unit. Bone Marrow Transplantation 2007;39(8):477‐82. [DOI] [PubMed] [Google Scholar]
Cruciani 2000b {published data only}
- Cruciani M. Antibacterial prophylaxis. International Journal of Antimicrobial Agents 2000;16(2):123‐5. [DOI] [PubMed] [Google Scholar]
Davis 1998 {published data only}
- Davis C. The antiviral prophylaxis of post‐transplant lymphoproliferative disorder. Springer Seminars in Immunopathology 1998;20(3‐4):437‐53. [DOI] [PubMed] [Google Scholar]
Delarive 2000 {published data only}
- Delarive P, Baumgartner JD. Evaluation of antibiotic prophylaxis in neutropenic patients with hematologic malignancies [Evaluation de la prophylaxie antibiotique chez les patients neutropeniques avec hemopathie maligne]. Schweizerisce Medizinische Wochenschrift 2000;130(48):1837‐44. [PubMed] [Google Scholar]
Del Favero 1989 {published data only}
- Favero A, Martino P, Bucaneve G, Micozzi A, D'Antonio D, Minetti B, et al. [Prophylaxis using quinolones in neutropenic patients. Preliminary results of a cooperative study by the Grupo Italiano Malattie Ematologiche maligne dell'adulto (GIMEMEA)]. Haematologica 1989;74 Suppl:278‐86. [PubMed] [Google Scholar]
Del Favero 1993 {published data only}
- Favero A, Menichetti F. The new fluorinated quinolones for antimicrobial prophylaxis in neutropenic cancer patients. European Journal of Cancer 1993;29A Suppl 1:2‐6. [DOI] [PubMed] [Google Scholar]
Donnelly 1997 {published data only}
- Donnelly JP. Is there a rationale for the use of antimicrobial prophylaxis in neutropenic patients?. Journal of Internal Medicine Suppl 1997;740:79‐88. [PubMed] [Google Scholar]
Ekert 1980 {published data only}
- Ekert H, Jurk IK, Waters KD, Tiedemann K. Prophylactic co‐trimoxazole and lactobacilli preparation in neutropenic patients. Medical & Pediatric Oncology 1980;8:47‐51. [DOI] [PubMed] [Google Scholar]
Engerval 1996a {published data only}
- Engervall P, Bjorkholm M. Infections in neutropenic patients II: Management. Medical Oncology 1996;13(1):63‐9. [DOI] [PubMed] [Google Scholar]
Engervall 1996b {published data only}
- Engervall P, Bjorkholm M. Ofloxacin versus co‐trimoxazole in prevention of infections in children with neutropenia after cytotoxic chemotherapy. (Short communication). Anti‐infective Drugs and Chemotherapy 1996;14(2):105‐7. [Google Scholar]
EORTC 1982 {published data only}
- EORTC ‐ Gnotobiotic Project Group. A prospective cooperative study of antimicrobial decontamination in granulocytopenic patients. Comparison of two different methods. Infection 1982;10(3):131‐8. [DOI] [PubMed] [Google Scholar]
Fernndez‐Aviles 2010 {published data only}
- Fernndez‐Aviles F, Rovira M, Martnez C, Rosiol L, Gallego C, Hernando A, et al. A comparison of the efficacy prophylactic ceftriaxone in patients managed at home after autologous stem cell transplantation using either BEAM or melphalan conditioning: A single‐centre experience. Bone Marrow Transplantation 2010;Conference Publication. 45:S394‐395. [Google Scholar]
Figueredo 1985 {published data only}
- Figueredo AT, Hryniuk WM, Strautmanis I, Frank G, Rendell S. Co‐trimoxazole prophylaxis during high‐dose chemotherapy of small‐cell lung cancer. Journal of Clinical Oncology 1985;3:54‐64. [DOI] [PubMed] [Google Scholar]
Frampton 1996 {published data only}
- Franpton JE, Faulds D. Filgrastim. A reappraisal of pharmacoeconomic considerations in the prophylaxis and treatment of chemotherapy‐induced neutropenia. Pharmacoeconomics 1996;9(1):76‐96. [DOI] [PubMed] [Google Scholar]
Garcia 2000 {published data only}
- Garcia G. Immediate vs. delayed imipenem treatment in cancer patients with profound neutropenia induced by high‐dose chemotherapy: Results of a randomised study. Revista Espanola de Quimioterapia 2000;15(3):257‐63. [PubMed] [Google Scholar]
Giamarellou 1995 {published data only}
- Giamarellou H. Current approach to the management of neutropenia. Journal of Intensive Care Medicine 1995;10(6):283‐93. [Google Scholar]
Gilbert 1994 {published data only}
- Gilbert C, Meisenberg B, Vredenburgh J, Ross M, Hussein A, Perfect J, Peters WP. Sequential prophylactic oral and empiric once‐daily parenteral antibiotics for neutropenia and fever after high‐dose chemotherapy and autologous bone marrow support. Journal of Clinical Oncology 1994;12(5):1005‐11. [DOI] [PubMed] [Google Scholar]
Guiot 1981 {published data only}
- Guiot HF, Meer JW, Furth R. Selective antimicrobial modulation of human microbial flora: infection prevention in patients with decreased host defense mechanisms by selective elimination of potentially pathogenic bacteria. The Journal of Infectious Diseases 1981;143(5):644‐54. [DOI] [PubMed] [Google Scholar]
Haahr 1997 {published data only}
- Haahr V, Peterslund NA, Moller JK. The influence of antimicrobial prophylaxis on the microbial and clinical findings in patients after autologous bone marrow transplantation. Scandinavian Journal of Infectious Diseases 1997;29(6):623‐6. [DOI] [PubMed] [Google Scholar]
Hallbk 2010 {published data only}
- Hallbk H, Lidstrm AK, Pauksens K. Antibacterial prophylaxis reduces the use of broad‐spectrum antibiotics in autologous stem cell transplantation. Bone Marrow Transplantation 2010;Conference Publication. 45:S230. [Google Scholar]
Henry 1984a {published data only}
- Henry SA. Symposium on infectious complications of neoplastic disease (Part II). Chemoprophylaxis of bacterial infections in granulocytopenic patients. [Review]. American Journal of Medicine 1984;76:645‐51. [DOI] [PubMed] [Google Scholar]
Horvathova 1998 {published data only}
- Horvathova Z, Spanik S, Sufliarsky J, Mardiak J, Pichna P, Pichnova E. Bacteremia due to methicillin‐resistant staphylococci occurs more frequently in neutropenic patients who received antimicrobial prophylaxis and is associated with higher mortality in comparison to methicillin‐sensitive bacteriemia. International Journal of Antimicrobial Agents 1998;10(1):55‐8. [DOI] [PubMed] [Google Scholar]
Imrie 1995 {published data only}
- Imrie KR, Prince HM, Couture F, Brandwein JM, Keating A. Effect of antimicrobial prophylaxis on hematopoietic recovery following autologous bone marrow transplantation: ciprofloxacin versus co‐trimoxazole. Bone Marrow Transplantation 1995;15:267‐70. [PubMed] [Google Scholar]
Karp 1986 {published data only}
- Karp JE, Dick JD, Angelopulos C, Carache P, Green L, et al. Empiric use of vancomycin during prolonged treatment‐induced granulocytopenia. The American Journal of Medicine 1986;81:237‐42. [DOI] [PubMed] [Google Scholar]
Kerr 1999b {published data only}
- Kerr KG. The prophylaxis of bacterial infections in neutropenic patients. Journal of Antimicrobial Chemotherapy 1999;44(5):587‐91. [DOI] [PubMed] [Google Scholar]
Klastersky 1996 {published data only}
- Klastersky J. Prevention of infection in neutropenic cancer patients. Current Opinion in Oncology 1996;8(4):270‐8. [DOI] [PubMed] [Google Scholar]
Krupova 1998 {published data only}
- Krupova Y, Novotny J, Sabo A, Mateicka F, Krcmry V Jr. Aetiology, cost of antimicrobial therapy and outcome in neutropenic patients who developed bacteraemia during antimicrobial prophylaxis: a case‐control study. International Journal of Antimicrobial Agents 1998;10(4):313‐6. [DOI] [PubMed] [Google Scholar]
Ljungman 1997 {published data only}
- Ljungman P, Hagglund H, Bjorkstrand B, Lonnqvist B, Ringden O. Perioperative teicoplanin for prevention of gram‐positive infections in neutropenic patients with indwelling central venous catheters: a randomised, controlled study. Supportive Care in Cancer 1997;5:485‐8. [DOI] [PubMed] [Google Scholar]
Lohner 1979 {published data only}
- Lohner D, Debusscher L, Prevost JM, Klastersky J. Comparative randomised study of protected environment plus oral antibiotics versus oral antibiotics alone in neutropenic patients. Cancer Treatment Reports 1979;63:363‐8. [PubMed] [Google Scholar]
Maltezou 1999 {published data only}
- Maltezou HC, Kafetzis DA, Rolston KVI. Comparison of Vancomycin (VA) Alone or with Norfloxacin (NO) for Antibacterial Prophylaxis in Adult Hematopoietic Stem Cell Transplant (HSCT) Recipients. Abstracts of the 39th Interscience Conference on Antimicrobial Agents and Chemotherapy; 1999 September; San Francisco. 1999:Abstract 734:599.
Maltezou 1999 (a) {published data only}
- Maltezou HC, Kafetzis DA, Rolston KVI. Bacterial Prophylaxis vs. Nonprophylaxis Policy in Patients (pts) with Hematopoietic Stem Cell Transplants (HSCT). Abstracts of the 39th Interscience Conference on Antimicrobial Agents and Chemotherapy; 1999 September; San Francisco. 1999:Abstract: 735:599.
Mantovani 1998 {published data only}
- Mantovani G, Ghiani M, Massidda S, Lai P, Mulas C, Massa E, et al. A clinical trial comparing the efficacy of a single b‐lactam antibiotic (Metropenem) with a combination of Metropenem plus Teicoplanin plus Netilmicin in the prophylactic and symptomatic treatment of febrile severe neutropenia following chemotherapy for cancer. Supportive Care in Cancer 1998;6 Suppl:315. [Google Scholar]
Marchetti 2002 {published data only}
- Marchetti O, Calandra T, Marchetti O. Infections in neutropenic cancer patients. Lancet 2002;359(9308):723‐5. [DOI] [PubMed] [Google Scholar]
Martino 1998 {published data only}
- Martino R, Subira M, Altes A, Lopez R, Sureda A, Domingo Albos A. Effect of discontinuing prophylaxis with norfloxacin in patients with hematologic malignancies and severe neutropenia. A matched case‐control study of the effect on infectious morbidity. Acta Haematologica 1998;99(4):206‐11. [DOI] [PubMed] [Google Scholar]
Maschmeyer 1990 {published data only}
- Maschmeyer G, Daenen S, Pauw Be, Vries Hospers HG, Dekker A W, Donnelly JP. Prevention of infection in acute leukemia. [Review]. Hamatologie und Bluttransfusion 1990;33:525‐30. [DOI] [PubMed] [Google Scholar]
May 1994 {published data only}
- May T, Beuscart C, Reynes J, Marchou B, Leclercq P, Lebas FB. Trimethoprim‐sulfamethoxazole versus aerosolized pentamidine for primary prophylaxis of Pneumocystis carinii pneumonia: A prospective, randomised, controlled clinical trial. Journal of Acquired Immune Deficiency Syndrome 1994;7:457‐62. [PubMed] [Google Scholar]
Menichetti 1989 {published data only}
- Menichetti F, Felicini R, Bucaneve G, Aversa F, Greco M, Pasqurella C, et al. Norfloxacin prophylaxis for neutropenic patients undergoing bone marrow transplantation. Bone Marrow Transplantation 1989;4:489‐92. [PubMed] [Google Scholar]
Mihaylov 2007 {published data only}
- Mihaylov G, Vasileva N, Ganeva P. Antibacterial prophylaxis and treatment of the patients with febrile neutropenia during hematopoietic stem cell transplantation. Clinical and Transfusion Haematology 2007;43(1‐2):3‐14. [Google Scholar]
Minenko 2004 {published data only}
- Minenko SV, Dmitrieva NV, Sokolova EN, Zhukov NV, Ptushkin VV. Efficacy of moxifloxacin (Avelox) in prophylaxis of infection in patients with profound neutropenia. Antibiotiki‐i‐khimioterapiia 2004;49(3):26‐31. [PubMed] [Google Scholar]
Patrick 1997 {published data only}
- Patrick CC. Use of fluoroquinolones as prophylactic agents in patients with neutropenia. Pediatric Infectious Disease Journal 1997;16(1):135‐9. [DOI] [PubMed] [Google Scholar]
Persson 2000 {published data only}
- Persson L, Vikerfors T, Sjoberg L, Engervall P, Tidefelt U. Increased incidence of bacteraemia due to viridans streptococci in an unselected population of patients with acute myeloid leukaemia. Scandinavian Journal of Infectious Diseases 2000;32(6):615‐21. [DOI] [PubMed] [Google Scholar]
Pivkova 2005 {published data only}
- Pivkova A, Stojanoski Z, Cevreska L, Siljanovski N, Karanfilski O, Genadieva‐Stravic S, et al. Prevention of neutropenic fever during administration of high dose VP‐16 plus G‐CSF for mobilization of PBSC ‐ the efficiency of prophylactic antibiotic treatment. Haematologica 2005;90 Suppl 2:448. [Google Scholar]
Reuter 2005 {published data only}
- Reuter S, Kern WV, Sigge A, Dhner H, Marre R, Kern P, et al. Impact of fluoroquinolone prophylaxis on reduced infection‐related mortality among patients with neutropenia and hematologic malignancies. Clinical Infectious Diseases 2005;40(8):1087‐93. [DOI] [PubMed] [Google Scholar]
Risi 1998 {published data only}
- Risi GF, Tomascak V. Prevention of infection in the immunocompromised host. American Journal of Infection Control 1998;26(6):594‐606. [DOI] [PubMed] [Google Scholar]
Schaison 1991 {published data only}
- Schaison GS, Decroly FC. Propylaxis, cost and effectiveness of therapy of infections caused by Gram‐positive organisms in neutropenic children. Journal of Antimicrobial Chemotherapy 1991;27:Suppl B:61‐7. [DOI] [PubMed] [Google Scholar]
Solano 2005 {published data only}
- Solano C, Gutierrez A, Martinez F, Gimeno C, Gomez C, Munoz I, Faus F. Prophylaxis of early bacterial infections after autologous peripheral blood stem cell transplantation (PBSCT): a matched‐pair study comparing oral fluoroquinolones and intravenous piperacillin‐tazobactam. Bone Marrow Transplantation 2005;36(1):59‐65. [DOI] [PubMed] [Google Scholar]
Spanik 1998 {published data only}
- Spanik S, Sufliarsky J, Mardiak J, Sorkovska D, Trupl J, Kunova A. Predictors of mortality in bacteremic cancer patients: retrospective analysis of 64 deaths occurring among 262 bacteremic episodes. Supportive Care in Cancer 1998;6(3):291‐4. [DOI] [PubMed] [Google Scholar]
Takemoto 1990 {published data only}
- Takemoto Y, Kanamaru A, Nagai K, Masaoka T, Kitani T, Horiuchi A. Randomized trial of combination antibiotic therapy in patients with hematological disorders. Japanese Journal of Antibiotics 1990;43:63‐9. [PubMed] [Google Scholar]
Timmer‐Bonte 2005 {published data only}
- Timmer‐Bonte JN, Boo TM, Smit HJ, Biesma B, Wilschut FA, Cheragwandi SA, et al. Prevention of chemotherapy‐induced febrile neutropenia by prophylactic antibiotics plus or minus granulocyte colony‐stimulating factor in small‐cell lung cancer: a Dutch Randomized Phase III Study. Journal of Clinical Oncology 2005;23(31):7974‐84. [DOI] [PubMed] [Google Scholar]
Tjan Heijnen 2002 {published data only}
- Tjan Heijnen VC, Postmus PE, Ardizzoni A. Reply to the article "Editorial comments on 'reduction of chemotherapy‐induced febrile leucopenia by prophylactic use of ciprofloxacin and roxithromycin in small‐cell lung cancer patients: an EORTC double‐blind placebo‐controlled phase III study'", by P. A. Bunn Jr (Ann Oncol 2001; 12: 1339‐1340). Annals of Oncology 2002;13(3):485‐6. [DOI] [PubMed] [Google Scholar]
Tunkel 2002 {published data only}
- Tunkel AR, Sepkowitz KA. Infections caused by viridans streptococci in patients with neutropenia. Clinical Infectious diseases 2002;34(11):1524‐9. [DOI] [PubMed] [Google Scholar]
Van De Leur 1995 {published data only}
- Leur JJ, Vollaard EJ, Janssen AJ, Dofferhoff AS. Concentration of pefloxacin in feces during infection prophylaxis in neutropenic patients. Antimicrobial Agents and Chemotherapy 1995;39:1182‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Viscoli 1998 {published data only}
- Viscoli C, Castagnola E. Planned progressive antimicrobial therapy in neutropenic patients. British Journal of Haematology 1998;102(4):879‐88. [DOI] [PubMed] [Google Scholar]
Viscoli 2001 {published data only}
- Viscoli C, Paesmans M, Sanz M, Castagnola E, Klastersky J, Martino P, International Antimicrobial Therapy Cooperative Group of the, European Organization for Research, and Treatment of Cancer. Association between antifungal prophylaxis and rate of documented bacteraemia in febrile neutropenic cancer patients. Clinical Infectious Diseases 2001;32:1532‐7. [DOI] [PubMed] [Google Scholar]
Viscoli 2002 {published data only}
- Viscoli C, Castagnola E. "Treatment of febrile neutropenia: what is new?". Current Opinion in Infectious Diseases 2002;15(4):377‐82. [DOI] [PubMed] [Google Scholar]
von Baum 2006 {published data only}
- Baum H, Sigge A, Bommer M, Kern WV, Marre R, Dohner H, et al. Moxifloxacin prophylaxis in neutropenic patients. Journal of Antimicrobial Chemotherapy 2006;58(4):891‐4. [DOI] [PubMed] [Google Scholar]
von Minckwitz 2008 {published data only}
- von Minckwitz G, Kummel S, du Bois A, Eiermann W, Eidtmann H, Gerber B, et al. Pegfilgrastim +/‐ ciprofloxacin for primary prophylaxis with TAC (docetaxel/doxorubicin/cyclophosphamide) chemotherapy for breast cancer. Results from the GEPARTRIO study. Annals of Oncology 2008;19(2):292‐8. [DOI] [PubMed] [Google Scholar]
Wade 1981 {published data only}
- Wade JC, Schimpff SC, Wiernik PH. Antibiotic combination‐associated nephrotoxicity in granulocytopenic patients with cancer. Archives of Internal Medicine 1981;141:1789‐93. [DOI] [PubMed] [Google Scholar]
Wilson 1982 {published data only}
- Wilson JM, Guiney DG. Failure of oral trimethoprim‐sulfamethoxazole prophylaxis in acute leukemia: isolation of resistant plasmids from strains of Enterobacteriaceae causing bacteraemia. New England Journal of Medicine 1982;306(1):16‐20. [DOI] [PubMed] [Google Scholar]
Zinner 1999 {published data only}
- Zinner SH. Changing epidemiology of infections in patients with neutropenia and cancer: emphasis on gram‐positive and resistant bacteria. Clinical Infectious Diseases 1999;29(3):490‐4. [DOI] [PubMed] [Google Scholar]
Additional references
Bodey 1966
- Bodey GP, Buckley M, Sathe YS, Friereich EJ. Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Annals of Internal Medicine 1966;64(2):328‐40. [DOI] [PubMed] [Google Scholar]
Cherif 2004
- Cherif H, Bjorkholm M, Engervall P, Johansson P, Ljungman P, Hast R, et al. A prospective, randomised study comparing cefepime and imipenem‐cilastatin in the empirical treatment of febrile neutropenia in patients treated for haematological malignancies. Scandinavian Journal of Infectious Diseases 2004;36(8):593‐600. [DOI] [PubMed] [Google Scholar]
Cometta 1994
- Cometta A, Calandra T, Bille J, Glauser MP. Escherichia coli resistant to fluoroquinolones in patients with cancer and neutropenia (letter). New England Journal of Medicine 1994;330:1240‐1. [DOI] [PubMed] [Google Scholar]
Cordonnier 2007
- Cordonnier C, Calandra T, Meunier F. Guidelines from the first European conference on infections in leukaemia: ECIL1. European Journal of Cancer 2007;Suppl 5(2):1‐60. [Google Scholar]
Cruciani 1996
- Cruciani M, Rampazzo R, Malena M, Lazzarini L, Todeschini G, Messori A, et al. Prophylaxis with fluoroquinolones for bacterial infections in neutropenic patients: a meta‐analysis. Clinical Infectious Diseases 1996;23:795‐805. [DOI] [PubMed] [Google Scholar]
Cruciani 2003
- Cruciani M, Malena M, Bosco O, Nardi S, Serpelloni G, Mengoli C. Reappraisal with meta‐analysis of the addition of gram‐positive prophylaxis to fluoroquinolone in neutropenic patients. Journal of Clinical Oncology 2003;21(22):4127‐37. [DOI] [PubMed] [Google Scholar]
de Marie 1993
- Marie S, Broek PJ, Willimze R, Furth R. Strategy for antibiotic therapy in febrile neutropenic patients on selective antimicrobial decontamination. European Journal of Clinical Microbiology and Infectious diseases 1993;12:897‐906. [DOI] [PMC free article] [PubMed] [Google Scholar]
De Pauw 1994
- Pauw BE, Deresinski SC, Feld R, Lane‐Allman EF, Donnelly JP. Ceftazidime compared with piperacillin and tobramycin for the empiric treatment of fever in neutropenic patients with cancer. A multicentre randomised trial. The Intercontinental Antimicrobial Study Group. Annals of Internal Medicine 1994;120(10):834‐44. [DOI] [PubMed] [Google Scholar]
Donnelly 1992a
- Donnelly JP, Maschmeyer G, Daenen S. Selective oral antimicrobial prophylaxis for the prevention of infection in acute leukaemia‐ciprofloxacin versus co‐trimoxazole plus colistin. European Journal of Cancer 1992;28A:873‐8. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Engels 1998
- Engels E, Lau J, Barza M. Efficacy of quinolone prophylaxis in neutropenic cancer patients: a meta‐analysis. Journal of Clinical Oncology 1998;16(3):1179‐87. [DOI] [PubMed] [Google Scholar]
EORTC 1990
- EORTC International Antimicrobial Therapy Cooperative Group. Gram‐positive bacteraemia in granulocytopenic cancer patients. European Journal of Cancer 1990;26(5):569‐74. [DOI] [PubMed] [Google Scholar]
Frank 2008
- Frank DA. Review: Prophylactic use of granulocyte colony‐stimulating factor reduces febrile neutropenia and short‐term mortality in cancer. ACP J Club 2008;148(1):7. [PubMed] [Google Scholar]
Freifeld 2011
- Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clinical Infectious Diseases 2011;52(4):e56‐e93. [DOI] [PubMed] [Google Scholar]
Gentry 2002
- Gentry C, Flournoy DJ, Reinert R. Analysis of antimicrobial resistance among gram‐negative bacilli and antimicrobial use in intensive care unit patients for 5 years in a Veterans Affairs medical centre. American Journal of Infection Control 2002;30(7):411‐6. [DOI] [PubMed] [Google Scholar]
Giamarellou 2000
- Giamarellou H, Bassaris HP, Petrikkos G, Busch W, Voulgarelis M, Antoniadou A, et al. Monotherapy with intravenous followed by oral high‐dose ciprofloxacin versus combination therapy with ceftazidime plus amikacin as initial empiric therapy for granulocytopenic patients with fever. Antimicrobial Agents and Chemotherapy 2000;44(12):3264‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2005
- Higgins JPT, Green S, editors. Handbook for Systematic Reviews of Interventions 4.2.5 [Updated May 2005]. The Cochrane Library, Issue 3, 2005. Chichester, UK: John Wiley & Sons, Ltd, 2005. [Google Scholar]
Higgins 2009
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009]. The Cochrane Collaboration, 2009. Available from www.cochrane‐handbook.org.
Hughes 1977
- Hughes WT, Kuhn S, Chaudhary S, Feldman S, Verzosa M, Aur RJ, et al. Successful chemoprophylaxis for Pneumocystis carinii pneumonitis. New England Journal of Medicine 1977;297:1419‐26. [DOI] [PubMed] [Google Scholar]
Hughes 1990
- Hughes WT, Armstrong D, Bodey GP, Feld R, Mandell GL, Meyers JD, et al. From the Infectious Diseases Society of America: guidelines for the use of antimicrobial agents in neutropenic patients with unexplained fever. Journal of Infectious Diseases 1990;161:381‐96. [DOI] [PubMed] [Google Scholar]
Hughes 2002
- Hughes WT, Armstrong D, Bodey GP, Bow EJ, Brown AE, Calandray T, et al. Guidelines for the use of antimicrobial agents in neutropenic patients with cancer. Clinical Infectious Diseases 2002;34(6):730‐51. [DOI] [PubMed] [Google Scholar]
Imran 2008
- Imran H, Tleyjeh IM, Arndt CAS, Baddour LM, Erwin PJ, Tsigrelis C, et al. Fluoroquinolone prophylaxis in patients wth neutropenia: a meta‐analysis of randomized placebo‐controlled trials. European Journal of Clinical Microbiology and Infectious Diseases 2008;27:53‐63. [DOI] [PubMed] [Google Scholar]
Kern 1991
- Kern Wv, Kurrle E. Ofloxacin versus trimethoprim‐sulfamethoxazole for prevention of infection in patients with acute leukemia and granulocytopenia. Infection 1991;19:73‐80. [DOI] [PubMed] [Google Scholar]
Kern 1994
- Kern WV, Androf E, Oethinger M, Kern P, Hacker J, Marre R. Emergence of fluoroquinolone‐resistant Escherichia coli at a cancer centre. Antimicrobial Agents and Chemotherapy 1994;38:681‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kerr 1999
- Kerr KG. The prophylaxis of bacterial infections in neutropenic patients. Journal of Antimicrobial Chemotherapy 1999;44(5):587‐91. [DOI] [PubMed] [Google Scholar]
Kuderer 2007
- Kuderer NM, Dale DC, Crawford J, Lyman GH. Impact of primary prophylaxis with granulocyte colony‐stimulating factor on febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: a systematic review. Journal of Clinical Oncology 2007;25(21):3158‐67. [DOI] [PubMed] [Google Scholar]
Lucas 1996
- Lucas KG, Brown AE, Armstrong D. The identification of febrile, neutropenic children with neoplastic disease at low risk for bacteraemia and complications of sepsis. Cancer 1996;77(4):791‐8. [PubMed] [Google Scholar]
Massey 2009
- Massey E, Paulus U, Doree C, Stanworth S. Granulocyte transfusions for preventing infections in patients with neutropenia or neutrophil dysfunction. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD005341] [DOI] [PubMed] [Google Scholar]
Oppenheim 1989
- Oppenheim BA, Hartley JW, Lee W, Burnie JP. Outbreak of coagulase negative staphylococci highly resistant to ciprofloxacin in a leukemia unit. British Medical Journal 1989;299:294‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Patrick 1997a
- Patrick CC. Use of fluoroquinolones as prophylactic agents in patients with neutropenia. Pediatric Infectious Disease 1997;16:135‐9. [DOI] [PubMed] [Google Scholar]
RevMan 2008 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Roblot 2004
- Roblot F, Imbert S, Godet C, Kauffmann C, Ragot S, Moal G, et al. Risk factors analysis for Pneumocystis jiroveci pneumonia (PCP) in patients with haematological malignancies and pneumonia. Scandinavian Journal of Infectious Diseases 2004;36:848‐54. [DOI] [PubMed] [Google Scholar]
Rolston 1992
- Rolston KV, Berkey P, Bodey GP, Anaissie EJ, Khardori NM, Joshi JH, et al. A comparison of imipenem to ceftazidime with or without amikacin as empiric therapy in febrile neutropenic patients. Archives of Internal Medicine 1992;152:283‐91. [PubMed] [Google Scholar]
Rotstein 1997
- Rotstein C, Mandell LA, Goldberg N. Fluoroquinolone prophylaxis for profoundly neutropenic cancer patients: a meta‐analysis. Current Oncology 1997;4 suppl 2:2‐7. [Google Scholar]
Schimpff 1986
- Schimpff SC. Empiric Antibiotic therapy for granulocytopenic cancer patients. American Journal of Medicine 1986;80(Suppl 5c):13‐20. [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment in controlled trials. JAMA 1995;273:408‐12. [DOI] [PubMed] [Google Scholar]
van de Wetering 2007
- Wetering MD, Weggelaar N, Offringa M, Caron HN, Kuijpers TW. Granulocyte transfusions in neutropaenic children: a systematic review of the literature. European Journal of Cancer 2007;43(14):2082‐92. [DOI] [PubMed] [Google Scholar]
Verhoef 1993
- Verhoef J. Prevention of infections in neutropenic patients. Clinical Infectious Diseases 1993;17 Suppl 2:359‐67. [DOI] [PubMed] [Google Scholar]
Walsh 1994
- Walsh T, Karp J, Hathorn JW, Pizzo PA. Prevention of bacterial infections in neutropenic patients. Bailliere's Clinical Infectious Diseases 1994;1:469‐98. [Google Scholar]
Worth 2005
- Worth LJ, Dooley MJ, Seymour JF, Mileshkin L, Slavin MA, Thursky KA. An analysis of the utilisation of chemoprophylaxis against Pneumocystis jirovecii pneumonia in patients with malignancy receiving corticosteroid therapy at a cancer hospital. British Journal of Cancer 2005;92:867‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Gafter‐Gvili 2005a
- Gafter‐Gvili A, Fraser A, Paul M, Leibovici L. Meta‐Analysis: Antibiotic prophylaxis reduces mortality in neutropenic patients. Annals of Internal Medicine 2005;142(12 pt 1):979‐95. [DOI] [PubMed] [Google Scholar]
Gafter‐Gvili 2005b
- Gafter‐Gvili A, Fraser A, Paul M, Wetering MD, Kremer LMC, Leibovici L. Antibiotic prophylaxis for bacterial infections in afebrile neutropenic patients following chemotherapy. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD004386] [DOI] [PubMed] [Google Scholar]
van de Wetering 2005
- Wetering MD, Witte MA, Kremer LCM, Offringa M, Scholten RJPM, Caron HN. Efficacy of oral prophylactic antibiotics in neutropenic afebrile oncology patients: A systematic review of randomised controlled trials. European Journal of Cancer 2005;41:1372‐82. [DOI] [PubMed] [Google Scholar]


