Abstract
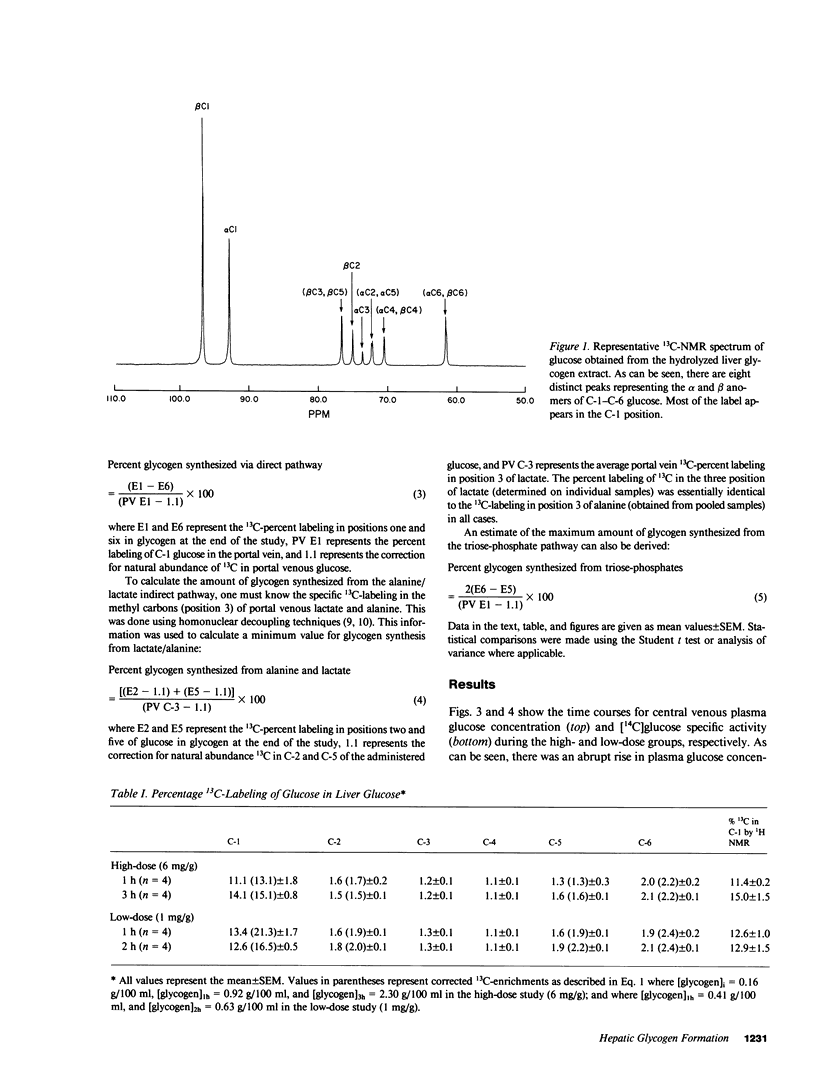
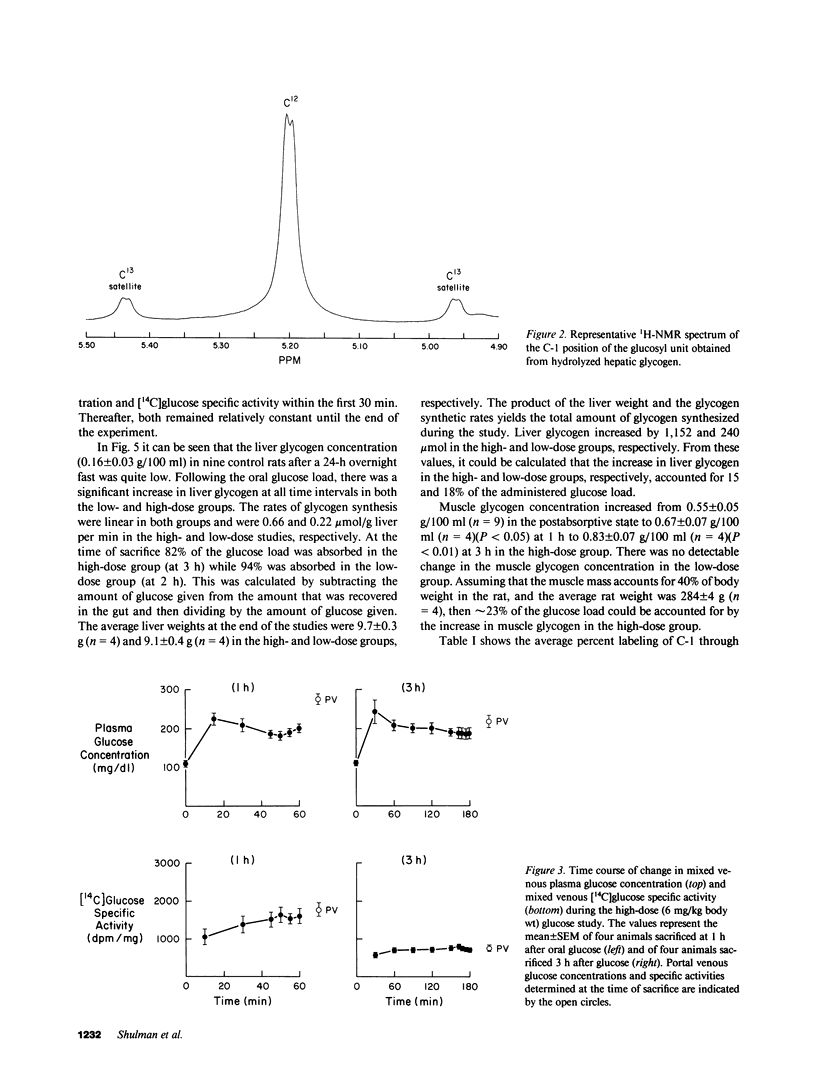
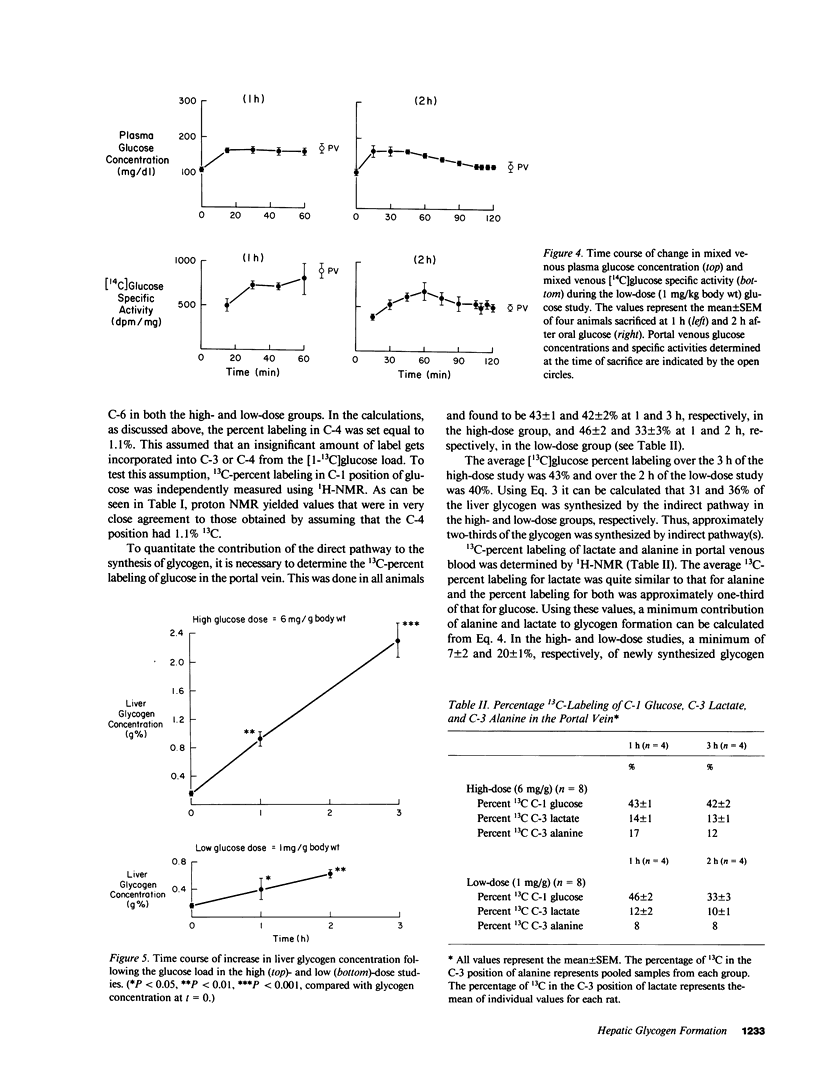
In order to quantitate the pathways by which liver glycogen is repleted, we administered [1-13C]glucose by gavage into awake 24-h fasted rats and examined the labeling pattern of 13C in hepatic glycogen. Two doses of [1-13C]glucose, 1 and 6 mg/g body wt, were given to examine whether differences in the plasma glucose concentration altered the metabolic pathways via which liver glycogen was replenished. After 1 and 3 h (high-dose group) and after 1 and 2 h (low-dose group), the animals were anesthetized and the liver was quickly freeze-clamped. Liver glycogen was extracted and the purified glycogen hydrolyzed to glucose with amyloglucosidase. The distribution of the 13C-label was subsequently determined by 13C-nuclear magnetic resonance spectroscopy. The percent 13C enrichment of the glucosyl units in glycogen was: 15.1 +/- 0.8%(C-1), 1.5 +/- 0.1%(C-2), 1.2 +/- 0.1%(C-3), 1.1 +/- 0.1%(C-4), 1.6 +/- 0.1%(C-5), and 2.2 +/- 0.1%(C-6) for the high-dose study (n = 4, at 3 h); 16.5 +/- 0.5%(C-1), 2.0 +/- 0.1%(C-2), 1.3 +/- 0.1%(C-3), 1.1 +/- 0.1%(C-4), 2.2 +/- 0.1%(C-5), and 2.4 +/- 0.1%(C-6) in the low-dose study (n = 4, at 2 h). The average 13C-enrichment of C-1 glucose in the portal vein was found to be 43 +/- 1 and 40 +/- 2% in the high- and low-dose groups, respectively. Therefore, the amount of glycogen that was synthesized from the direct pathway (i.e., glucose----glucose-6-phosphate----glucose-1-phosphate----UDP-glucose---- glycogen) was calculated to be 31 and 36% in the high- and low-dose groups, respectively. The 13C-enrichments of portal vein lactate and alanine were 14 and 14%, respectively, in the high-dose group and 11 and 8%, respectively, in the low-dose group. From these enrichments, the minimum contribution of these gluconeogenic precursors to glycogen repletion can be calculated to be 7 and 20% in the high- and low-dose groups, respectively. The maximum contribution of glucose recycling at the triose isomerase step to glycogen synthesis (i.e., glucose----triose-phosphates----glycogen) was estimated to be 3 and 1% in the high- and low-dose groups, respectively. In conclusion, our results demonstrate that (a) only one-third of liver glycogen repletion occurs via the direct conversion of glucose to glycogen, and that (b) only a very small amount of glycogen synthesis can be accounted for by the conversion of glucose to triose phosphates and back to glycogen; this suggests that futile cycling between fructose-6-phosphate and fructose-1,6-diphosphate under these conditions is minimal. Our results also show that (c) alanine and lactate account for a minimum of between 7 and 20% of the glycogen synthesized, and that (d) the three pathways through which the labeled flux is measured account for a total of only 50% of the total glycogen synthesized. These results suggest that either there is a sizeable amount of glycogen synthesis via pathway(s) that were not examined in the present experiment or that there is a much greater dilution of labeled alanine/lactate in the oxaloacetate pool than previously appreciated, or some combination of these two explanations.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BISHOP J. S., STEELE R., ALTSZULER N., DUNN A., BJERKNES C., DEBODO R. C. EFFECTS OF INSULIN ON LIVER GLYCOGEN SYNTHESIS AND BREAKDOWN IN THE DOG. Am J Physiol. 1965 Feb;208:307–316. doi: 10.1152/ajplegacy.1965.208.2.307. [DOI] [PubMed] [Google Scholar]
- COOK M., LORBER V. Conversion of 1-C14-Mannose and 1-C14-glucose to liver and muscle glycogen in the intact rat. J Biol Chem. 1952 Nov;199(1):1–8. [PubMed] [Google Scholar]
- Campbell I. D., Dobson C. M., Jeminet G., Williams R. J. Pulsed NMR methods for the observation and assignment of exchangeable hydrogens: application to bacitracin. FEBS Lett. 1974 Dec 1;49(1):115–119. doi: 10.1016/0014-5793(74)80645-9. [DOI] [PubMed] [Google Scholar]
- Cherrington A. D., Lacy W. W., Chiasson J. L. Effect of glucagon on glucose production during insulin deficiency in the dog. J Clin Invest. 1978 Sep;62(3):664–677. doi: 10.1172/JCI109174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. M., Glynn P., Shulman R. G. 13C NMR study of gluconeogenesis from labeled alanine in hepatocytes from euthyroid and hyperthyroid rats. Proc Natl Acad Sci U S A. 1981 Jan;78(1):60–64. doi: 10.1073/pnas.78.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. M., Ogawa S., Shulman R. G. 13C NMR studies of gluconeogenesis in rat liver cells: utilization of labeled glycerol by cells from euthyroid and hyperthyroid rats. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1603–1609. doi: 10.1073/pnas.76.4.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERS H. G. The conversion of fructose-1-C14 and sorbitol-1-C14 to liver and muscle glycogen in the rat. J Biol Chem. 1955 May;214(1):373–381. [PubMed] [Google Scholar]
- Hems D. A., Whitton P. D., Taylor E. A. Glycogen synthesis in the perfused liver of the starved rat. Biochem J. 1972 Sep;129(3):529–538. doi: 10.1042/bj1290529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hers H. G. The control of glycogen metabolism in the liver. Annu Rev Biochem. 1976;45:167–189. doi: 10.1146/annurev.bi.45.070176.001123. [DOI] [PubMed] [Google Scholar]
- Hetenyi G., Jr, Ferrarotto C. Correction for metabolic exchange in the calculation of the rate of gluconeogenesis in rats. Biochem Med. 1983 Jun;29(3):372–378. doi: 10.1016/0006-2944(83)90073-x. [DOI] [PubMed] [Google Scholar]
- Hostetler K. Y., Landau B. R. Estimation of the pentose cycle contribution to glucose metabolism in tissue in vivo. Biochemistry. 1967 Oct;6(10):2961–2964. doi: 10.1021/bi00862a001. [DOI] [PubMed] [Google Scholar]
- Katz J. Determination of gluconeogenesis in vivo with 14C-labeled substrates. Am J Physiol. 1985 Apr;248(4 Pt 2):R391–R399. doi: 10.1152/ajpregu.1985.248.4.R391. [DOI] [PubMed] [Google Scholar]
- Katz J., McGarry J. D. The glucose paradox. Is glucose a substrate for liver metabolism? J Clin Invest. 1984 Dec;74(6):1901–1909. doi: 10.1172/JCI111610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz L. D., Glickman M. G., Rapoport S., Ferrannini E., DeFronzo R. A. Splanchnic and peripheral disposal of oral glucose in man. Diabetes. 1983 Jul;32(7):675–679. doi: 10.2337/diab.32.7.675. [DOI] [PubMed] [Google Scholar]
- Krebs H. A., Hems R., Weidemann M. J., Speake R. N. The fate of isotopic carbon in kidney cortex synthesizing glucose from lactate. Biochem J. 1966 Oct;101(1):242–249. doi: 10.1042/bj1010242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANGDON R. G., TAYLOR W. R. Intestinal absorption of glucose in the rat. Biochim Biophys Acta. 1956 Aug;21(2):384–385. doi: 10.1016/0006-3002(56)90027-0. [DOI] [PubMed] [Google Scholar]
- Newgard C. B., Hirsch L. J., Foster D. W., McGarry J. D. Studies on the mechanism by which exogenous glucose is converted into liver glycogen in the rat. A direct or an indirect pathway? J Biol Chem. 1983 Jul 10;258(13):8046–8052. [PubMed] [Google Scholar]
- Newgard C. B., Moore S. V., Foster D. W., McGarry J. D. Efficient hepatic glycogen synthesis in refeeding rats requires continued carbon flow through the gluconeogenic pathway. J Biol Chem. 1984 Jun 10;259(11):6958–6963. [PubMed] [Google Scholar]
- Nilsson L. H., Hultman E. Liver and muscle glycogen in man after glucose and fructose infusion. Scand J Clin Lab Invest. 1974 Feb;33(1):5–10. doi: 10.3109/00365517409114190. [DOI] [PubMed] [Google Scholar]
- Radziuk J. Sources of carbon in hepatic glycogen synthesis during absorption of an oral glucose load in humans. Fed Proc. 1982 Jan;41(1):110–116. [PubMed] [Google Scholar]
- Reo N. V., Siegfried B. A., Ackerman J. J. Direct observation of glycogenesis and glucagon-stimulated glycogenolysis in the rat liver in vivo by high-field carbon-13 surface coil NMR. J Biol Chem. 1984 Nov 25;259(22):13664–13667. [PubMed] [Google Scholar]
- Rognstad R., Katz J. Effects of hormones and of ethanol on the fructose 6-P-fructose 1,6-P2 futile cycle during gluconeogenesis in the liver. Arch Biochem Biophys. 1976 Dec;177(2):337–345. doi: 10.1016/0003-9861(76)90447-1. [DOI] [PubMed] [Google Scholar]
- Rognstad R., Katz J. Gluconeogenesis in the kidney cortex. Quantitative estimation of carbon flow. J Biol Chem. 1972 Oct 10;247(19):6047–6054. [PubMed] [Google Scholar]
- Rothman D. L., Behar K. L., Hetherington H. P., Shulman R. G. Homonuclear 1H double-resonance difference spectroscopy of the rat brain in vivo. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6330–6334. doi: 10.1073/pnas.81.20.6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikama H., Ui M. Glucose load diverts hepatic gluconeogenic product from glucose to glycogen in vivo. Am J Physiol. 1978 Oct;235(4):E354–E360. doi: 10.1152/ajpendo.1978.235.4.E354. [DOI] [PubMed] [Google Scholar]
- Spence J. T., Koudelka A. P. Pathway of glycogen synthesis from glucose in hepatocytes maintained in primary culture. J Biol Chem. 1985 Feb 10;260(3):1521–1526. [PubMed] [Google Scholar]
- Van Schaftingen E., Hue L., Hers H. G. Study of the fructose 6-phosphate/fructose 1,6-bi-phosphate cycle in the liver in vivo. Biochem J. 1980 Oct 15;192(1):263–271. doi: 10.1042/bj1920263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Paetkau V., Lardy H. A. Paths of carbon in gluconeogenesis and lipogenesis. 3. The role and regulation of mitochondrial processes involved in supplying precursors of phosphoenolpyruvate. J Biol Chem. 1966 Jun 10;241(11):2523–2532. [PubMed] [Google Scholar]


