Abstract
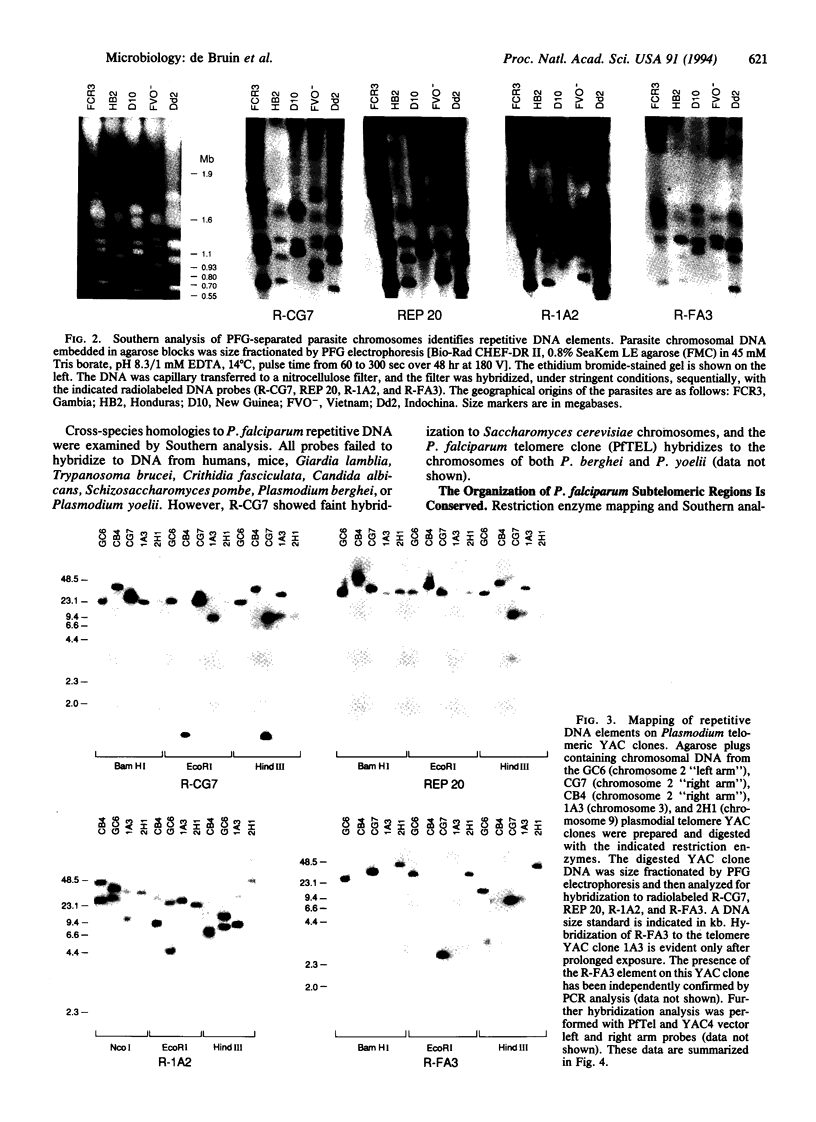
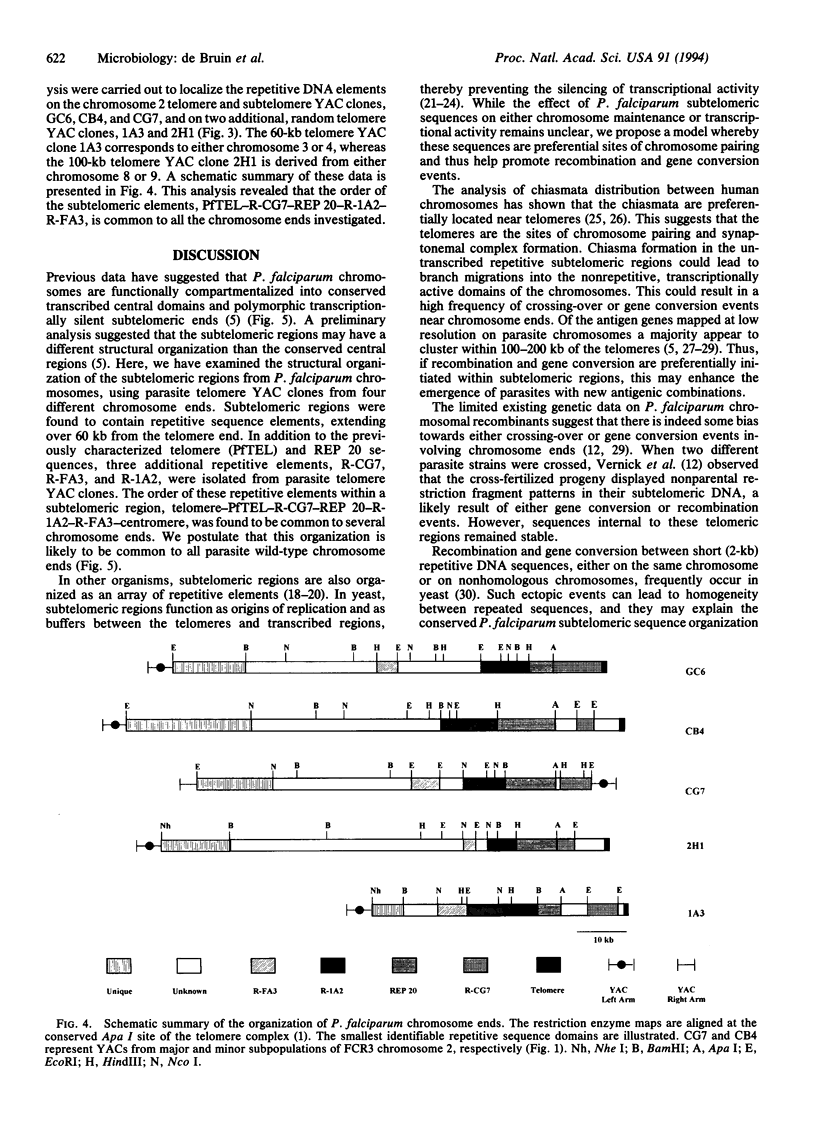
The human malaria parasite Plasmodium falciparum exhibits a high degree of chromosomal polymorphism, which may contribute to its ability to evade host defenses. The analysis of parasite chromosomes has revealed that these polymorphisms are confined to the subtelomeric regions, which are transcriptionally silent and contain repetitive sequence elements. Several subtelomeric repetitive elements have been isolated and mapped by using P. falciparum yeast artificial chromosome (YAC) clones. Structural analysis of parasite telomeric and subtelomeric YAC clones demonstrated that these repetitive elements are conserved between P. falciparum chromosome ends. We suggest that these subtelomeric elements promote chromosome pairing in P. falciparum and facilitate meiotic recombination and gene conversion between telomere-proximal genes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biessmann H., Mason J. M. Genetics and molecular biology of telomeres. Adv Genet. 1992;30:185–249. doi: 10.1016/s0065-2660(08)60321-1. [DOI] [PubMed] [Google Scholar]
- Biggs B. A., Kemp D. J., Brown G. V. Subtelomeric chromosome deletions in field isolates of Plasmodium falciparum and their relationship to loss of cytoadherence in vitro. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2428–2432. doi: 10.1073/pnas.86.7.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. R., MacKinnon P. J., Villasanté A., Spurr N., Buckle V. J., Dobson M. J. Structure and polymorphism of human telomere-associated DNA. Cell. 1990 Oct 5;63(1):119–132. doi: 10.1016/0092-8674(90)90293-n. [DOI] [PubMed] [Google Scholar]
- Cappai R., Kaslow D. C., Peterson M. G., Cowman A. F., Anders R. F., Kemp D. J. Cloning and analysis of the RESA-2 gene: a DNA homologue of the ring-infected erythrocyte surface antigen gene of Plasmodium falciparum. Mol Biochem Parasitol. 1992 Sep;54(2):213–221. doi: 10.1016/0166-6851(92)90113-x. [DOI] [PubMed] [Google Scholar]
- Corcoran L. M., Forsyth K. P., Bianco A. E., Brown G. V., Kemp D. J. Chromosome size polymorphisms in Plasmodium falciparum can involve deletions and are frequent in natural parasite populations. Cell. 1986 Jan 17;44(1):87–95. doi: 10.1016/0092-8674(86)90487-3. [DOI] [PubMed] [Google Scholar]
- Corcoran L. M., Thompson J. K., Walliker D., Kemp D. J. Homologous recombination within subtelomeric repeat sequences generates chromosome size polymorphisms in P. falciparum. Cell. 1988 Jun 3;53(5):807–813. doi: 10.1016/0092-8674(88)90097-9. [DOI] [PubMed] [Google Scholar]
- Foote S. J., Kemp D. J. Chromosomes of malaria parasites. Trends Genet. 1989 Oct;5(10):337–342. doi: 10.1016/0168-9525(89)90139-x. [DOI] [PubMed] [Google Scholar]
- Gottschling D. E., Aparicio O. M., Billington B. L., Zakian V. A. Position effect at S. cerevisiae telomeres: reversible repression of Pol II transcription. Cell. 1990 Nov 16;63(4):751–762. doi: 10.1016/0092-8674(90)90141-z. [DOI] [PubMed] [Google Scholar]
- Horowitz H., Haber J. E. Identification of autonomously replicating circular subtelomeric Y' elements in Saccharomyces cerevisiae. Mol Cell Biol. 1985 Sep;5(9):2369–2380. doi: 10.1128/mcb.5.9.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janse C. J. Chromosome size polymorphism and DNA rearrangements in plasmodium. Parasitol Today. 1993 Jan;9(1):19–22. doi: 10.1016/0169-4758(93)90158-c. [DOI] [PubMed] [Google Scholar]
- Lanzer M., de Bruin D., Ravetch J. V. Transcriptional differences in polymorphic and conserved domains of a complete cloned P. falciparum chromosome. Nature. 1993 Feb 18;361(6413):654–657. doi: 10.1038/361654a0. [DOI] [PubMed] [Google Scholar]
- Laurie D. A., Hultén M. A. Further studies on bivalent chiasma frequency in human males with normal karyotypes. Ann Hum Genet. 1985 Jul;49(Pt 3):189–201. doi: 10.1111/j.1469-1809.1985.tb01693.x. [DOI] [PubMed] [Google Scholar]
- Louis E. J., Haber J. E. The structure and evolution of subtelomeric Y' repeats in Saccharomyces cerevisiae. Genetics. 1992 Jul;131(3):559–574. doi: 10.1093/genetics/131.3.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolte D., Hundt E., Langsley G., Knapp B. A Plasmodium falciparum blood stage antigen highly homologous to the glycophorin binding protein GBP. Mol Biochem Parasitol. 1991 Dec;49(2):253–264. doi: 10.1016/0166-6851(91)90069-i. [DOI] [PubMed] [Google Scholar]
- Patarapotikul J., Langsley G. Chromosome size polymorphism in Plasmodium falciparum can involve deletions of the subtelomeric pPFrep20 sequence. Nucleic Acids Res. 1988 May 25;16(10):4331–4340. doi: 10.1093/nar/16.10.4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petes T. D., Hill C. W. Recombination between repeated genes in microorganisms. Annu Rev Genet. 1988;22:147–168. doi: 10.1146/annurev.ge.22.120188.001051. [DOI] [PubMed] [Google Scholar]
- Pologe L. G., Ravetch J. V. A chromosomal rearrangement in a P. falciparum histidine-rich protein gene is associated with the knobless phenotype. 1986 Jul 31-Aug 6Nature. 322(6078):474–477. doi: 10.1038/322474a0. [DOI] [PubMed] [Google Scholar]
- Pologe L. G., Ravetch J. V. Large deletions result from breakage and healing of P. falciparum chromosomes. Cell. 1988 Dec 2;55(5):869–874. doi: 10.1016/0092-8674(88)90142-0. [DOI] [PubMed] [Google Scholar]
- Pologe L. G., de Bruin D., Ravetch J. V. A and T homopolymeric stretches mediate a DNA inversion in Plasmodium falciparum which results in loss of gene expression. Mol Cell Biol. 1990 Jun;10(6):3243–3246. doi: 10.1128/mcb.10.6.3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherf A., Carter R., Petersen C., Alano P., Nelson R., Aikawa M., Mattei D., Pereira da Silva L., Leech J. Gene inactivation of Pf11-1 of Plasmodium falciparum by chromosome breakage and healing: identification of a gametocyte-specific protein with a potential role in gametogenesis. EMBO J. 1992 Jun;11(6):2293–2301. doi: 10.1002/j.1460-2075.1992.tb05288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotti R., Pace T., Ponzi M. A 40-kilobase subtelomeric region is common to most Plasmodium falciparum 3D7 chromosomes. Mol Biochem Parasitol. 1993 Mar;58(1):1–6. doi: 10.1016/0166-6851(93)90084-b. [DOI] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Vernick K. D., McCutchan T. F. Sequence and structure of a Plasmodium falciparum telomere. Mol Biochem Parasitol. 1988 Mar;28(2):85–94. doi: 10.1016/0166-6851(88)90055-2. [DOI] [PubMed] [Google Scholar]
- Vernick K. D., Walliker D., McCutchan T. F. Genetic hypervariability of telomere-related sequences is associated with meiosis in Plasmodium falciparum. Nucleic Acids Res. 1988 Jul 25;16(14B):6973–6985. doi: 10.1093/nar/16.14.6973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker-Jonah A., Dolan S. A., Gwadz R. W., Panton L. J., Wellems T. E. An RFLP map of the Plasmodium falciparum genome, recombination rates and favored linkage groups in a genetic cross. Mol Biochem Parasitol. 1992 Apr;51(2):313–320. doi: 10.1016/0166-6851(92)90081-t. [DOI] [PubMed] [Google Scholar]
- Weber J. L. Interspersed repetitive DNA from Plasmodium falciparum. Mol Biochem Parasitol. 1988 Jun;29(2-3):117–124. doi: 10.1016/0166-6851(88)90066-7. [DOI] [PubMed] [Google Scholar]
- Weiner A. M., Deininger P. L., Efstratiadis A. Nonviral retroposons: genes, pseudogenes, and transposable elements generated by the reverse flow of genetic information. Annu Rev Biochem. 1986;55:631–661. doi: 10.1146/annurev.bi.55.070186.003215. [DOI] [PubMed] [Google Scholar]
- Wellems T. E., Howard R. J. Homologous genes encode two distinct histidine-rich proteins in a cloned isolate of Plasmodium falciparum. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6065–6069. doi: 10.1073/pnas.83.16.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakian V. A., Blanton H. M. Distribution of telomere-associated sequences on natural chromosomes in Saccharomyces cerevisiae. Mol Cell Biol. 1988 May;8(5):2257–2260. doi: 10.1128/mcb.8.5.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zickler D., Moreau P. J., Huynh A. D., Slezec A. M. Correlation between pairing initiation sites, recombination nodules and meiotic recombination in Sordaria macrospora. Genetics. 1992 Sep;132(1):135–148. doi: 10.1093/genetics/132.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruin D., Lanzer M., Ravetch J. V. Characterization of yeast artificial chromosomes from Plasmodium falciparum: construction of a stable, representative library and cloning of telomeric DNA fragments. Genomics. 1992 Oct;14(2):332–339. doi: 10.1016/s0888-7543(05)80223-x. [DOI] [PubMed] [Google Scholar]




