Abstract
Background
Interleukin-1 plays a pivotal role in in the pathogenesis of systemic juvenile idiopathic arthritis (sJIA). We assessed the efficacy and safety of rilonacept (IL-1 trap), an IL-1 inhibitor, in a randomized, double-blind, placebo-controlled trial.
Methods
An initial 4-week double-blind placebo phase was incorporated into a 24-week randomized multi-center design, followed by an open label phase. We randomized 71 children with at least 2 active joints 1:1 to 2 arms of the study. Patients in the rilonacept arm received rilonacept (4.4mg/kg loading dose followed by 2.2mg/kg weekly, subcutaneously) from day 0; patients in the placebo arm received placebo for 4 weeks followed by a loading dose of rilonacept at week 4 followed by weekly maintenance doses. The primary endpoint was time to response, using adapted JIA ACR30 response criteria coupled with absence of fever and taper of systemic corticosteroids using pre-specified criteria.
Results
Time to response was shorter in the rilonacept arm than in the placebo arm (Chi-square 7.235, P=.007). Secondary analysis showed 20/35 (57%) of patients in the rilonacept arm responded at week 4 compared to 9/33 (27%) in the placebo arm (P=.016) using the same response criteria. Exacerbation of sJIA (4) was the most common SAE. More patients in the rilonacept arm had elevated liver transaminases, including more than three times the upper limits of normal, as compared to those in the placebo arm. Adverse events were similar in the two arms of the study.
Conclusions
Rilonacept was generally well tolerated and demonstrated efficacy in active sJIA.
Introduction
Systemic juvenile idiopathic arthritis (sJIA) is distinguished from other forms of JIA by its distinctive systemic features at onset, including high spiking fever, characteristic rash, hepatosplenomegaly, polyserositis, lymphadenopathy, anemia, leukocytosis and thrombocytosis and rarely macrophage activation syndrome1. More than 50% of children with sJIA have a polyphasic or chronic persistent disease course 2 and more than half suffer poor outcomes 3 and seldom death4 in the absence of highly active biologic treatment. Predictors of joint damage and poor functional outcome include young age at diagnosis, longer disease duration, persistent systemic long-term corticosteroid therapy thrombocytosis and high inflammatory markers 5,6.
This randomized controlled trial was designed to determine the safety and effectiveness of rilonacept in sJIA, and to confirm and extend findings from a number of anecdotal studies and trials showing effectiveness of IL-1 inhibition7-15. Rilonacept is a fusion protein consisting of human cytokine receptor extracellular domains of both receptor components required for IL-1 signaling ( IL-1 Type I receptor and the IL-1 receptor accessory protein) with the Fc portion of human IgG1. It binds IL-1α and IL-1β with picomolar affinity but potentially can bind to IL-1 receptor antagonist16. Although the primary efficacy endpoint was not met in a pilot, double-blind placebo-controlled study, rilonacept appeared to be well tolerated and efficacious enabling corticosteroid dose reduction in the open label long-term extension phase17.
Methods
Patients
The study was conducted in compliance with principles of the International Conference on Harmonization and Good Clinical Practice. The study was approved by the Institutional Review Boards of each study site. All patients or parents/guardians provided written informed consent. An independent Data Safety Monitoring Board, appointed by NIH/NIAMS, met every 6 months to evaluate study conduct and safety. Twenty Childhood Arthritis and Rheumatology Research Alliance (CARRA) centers in the US enrolled patients from 11/2008 to 5/2012. Key Inclusion criteria included: International League against Rheumatism criteria for sJIA 18; age ≥18 months to ≤19 years; ≥2 active joints; stable methotrexate dose for ≥4 weeks; stable corticosteroids ≥2 weeks; ≤ 2mg/kg or 60mg prednisone or equivalent. If previously treated with biologics, the following lengths of discontinuation were required: anakinra ≥4 days, etanercept ≥4 weeks, adalimumab ≥6 weeks, tocilizumab ≥6 weeks, abatacept ≥6 weeks, and infliximab ≥8 weeks. Key exclusion criteria included current treatment with disease modifying anti-rheumatic drug other than methotrexate; intra-articular corticosteroids or pulse steroids within 4 weeks; leflunomide without cholestyramine wash out; cyclophosphamide within 3 months; IVIG within 4 weeks; treatment in the past with an IL-1 inhibitor other than anakinra; renal insufficiency as defined as an elevated serum creatinine; aspartate aminotransferase (AST) or alanine aminotransferase (ALT) > 2 times the upper limit of normal; thrombo-, leuko-, or neutro-penia; prolonged PT or PTT; positive PPD without treatment documentation; live virus vaccine within 1 month; pregnancy or sexual activity without contraception.
Study Design
RAPPORT incorporated an initial 4-week double-blind placebo phase within a 24-week randomized multi-center design. As such, patients were randomized 1:1 to treatment with (a) 4 weeks of placebo followed by 20 weeks of rilonacept or (b) 24 weeks of rilonacept, resulting in a double-blind phase (weeks 0-4) and an all active treatment phase (weeks 4-24). This was followed by an open label Long Term Extension (LTE) phase (24 weeks-21 months). The randomized placebo phase study design is based on the assumption that if treatment is effective, patients who receive active drug earlier will respond sooner, on average, than patients who receive active drug later 19. This design is especially useful when testing highly effective therapies with rapid onset of action of several weeks, and when minimizing time on placebo or safety issues are important 19. IL-1 inhibition appears to have an onset of action of 2 weeks, based on data in sJIA subjects in the Amgen-sponsored study of anakinra in polyarticular-JIA that included subjects with sJIA 12. Randomization was performed using a Web-based randomization and drug supply management system (WebEZ, Almac, Durham, North Carolina). The central randomization scheme used a fixed-block size, stratified according to baseline corticosteroid use. Rilonacept and matching placebo were provided by Regeneron Pharmaceuticals, Inc. (New York).
In the rilonacept arm, a loading dose (4.4mg/kg, maximum dose 320mg) of rilonacept was given on day 0 followed by weekly maintenance doses (2.2mg/kg, to a maximum dose 160mg). In the placebo arm, a loading dose of placebo was given on day 0 followed by 3 maintenance doses of placebo; then a loading dose of rilonacept was given on day 28 followed by weekly maintenance doses of rilonacept. Evaluation of efficacy occurred during the first 12 weeks of the study; safety was assessed in the first 24 weeks and in the LTE. All patients who benefited from rilonacept treatment as judged by the treating physician were eligible for enrollment in the LTE phase. Patients in the LTE were initially offered open label rilonacept for 2 years or until rilonacept was commercially available. These criteria were shortened for some patients because of budgetary issues.
Clinical Assessments
Screening visits could occur up to 4 weeks before randomization. A randomization/baseline visit occurred at week 0 and follow up visits occurred at weeks 2, 4, 6, 8, 10, 12, 14, 18 (by telephone), and 24. Patients were seen every 3 months in the LTE. The 2 week interval between visits in the first 14 weeks was used to maximize the precision of estimation of time to response. Medical history, physical examination, concomitant medications, adverse events, fever (patients’ daily diary), blinded joint assessment, physician global assessment, parent/guardian/patient global assessment, clinical laboratory tests, and Childhood Health Assessment Questionnaire (CHAQ) were assessed at every (non-telephone) visit. Fasting lipoprotein profiles and PedsQL questionnaires were assessed at week 0, 12 and 24. Biospecimens (DNA, RNA, whole blood, serum, plasma) were obtained during the trial.
Endpoints
The primary endpoint was time to response during the 12 week efficacy period. Response was defined as a composite of 1)improvement in the JIA ACR30 20, 2) absence of fever ≥38.5°C in the previous 2 weeks, and 3)at least 10% taper in systemic corticosteroids from baseline if the patient were taking corticosteroids. The JIA ACR30 algorithm required at least 30% improvement from baseline in at least 3 of 6 core variables, with no more than 1 variable worsening by 30% or more. Corticosteroids were required to be tapered if all of the following criteria developed by consensus among the RAPPORT investigators 21 were met: fever ≤2 days in previous 7 days, absence of poor physical function, and absence of laboratory values associated with impending MAS. The time to response was defined as the visit designation at which the patient first achieved the response criteria and maintained response until the next scheduled visit. Absence of rash was not included in the response criteria.
Corticosteroids were increased or started if one or more of the following criteria developed by consensus of the RAPPORT investigators were met21: MAS 22, incomplete MAS, symptomatic anemia with a hemoglobin ≤6.5g/dL, myocarditis, or symptomatic pneumonitis, or serositis unresponsive to NSAIDs. Patients who met criteria for corticosteroid increase/start were deemed non-responders. Patients who met the criteria for non-response and started/increased corticosteroids were no longer eligible for response.
Secondary endpoints included the JIA ACR30, 50, 70, inactive disease 23, presence of fever, serositis, symptomatic anemia, abnormal liver function, rash, MAS, incomplete MAS, corticosteroid dose, CHAQ, and PedsQL Generic Core Modules. Adverse events (AEs) and Serious Adverse Events (SAEs) were collected throughout the study.
Data Analysis
Planned enrollment was 100 patients (50 per arm) to achieve ≥80% power to detect a difference at a 2-sided α level of 0.05. This sample size was determined based on the results from multiple Monte-Carlo simulations conducted under various assumptions for distribution of time to response in the two study arms.The trial was ended before reaching the enrollment goal due to slow enrollment and financial considerations. One pre-specified interim analysis of the primary endpoint to examine early stopping for overwhelming efficacy was performed when data were available for 50 patients. The Lan-DeMets flexible spending function corresponding to the O'Brian-Fleming stopping boundary was used to preserve the overall type I error of 0.0524. The stopping boundary P value at the interim analysis was 0.007. After reviewing the efficacy data at the interim analysis, the DSMB recommended to continue the study. The 2-sided P value for rejecting the null hypothesis of no difference between the treatment arms for the primary endpoint at the final analysis was 0.048. A 2-sided α level of 0.05 was considered statistically significant for all other endpoints. P values for the secondary analyses were not adjusted for multiple comparisons. All analyses were performed in SAS version 9.2 (SAS Institute Inc., Cary, NC).
Data were analyzed on an intent-to-treat basis. Descriptive statistics were summarized by treatment arm. The primary endpoint analysis compared time to response between treatment arms using Gehan-Wilcoxon test, which emphasizes early differences. Missing temperatures were imputed as fever free when calculating the response endpoint. A sensitivity analysis was performed treating the response endpoint as missing if the fever criterion could not be determined due to missing temperatures. In both the primary and sensitivity analyses, patients were censored if the response endpoint was missing at two consecutive visits, or if the patient met criteria for non-response and started/ increased corticosteroids prior to meeting the response criteria. Response rates were calculated using cumulative incidence estimation, treating non-response as a competing event. Comparison of the response to rilonacept vs. placebo at the end of the placebo phase (week 4) was evaluated using Fisher's exact test. Logistic regression was used to adjust for sJIA duration and presence of articular without systemic symptoms and to explore the association between those baseline characteristics and response at week 4.
JIA ACR30, 50, and corticosteroid dose were analyzed using a generalized estimating equation (GEE) repeated measures model. Improvement in JIA ACR70 was analyzed using Fisher's exact test. Adverse events were compared using chi-square test. Infection rates were compared using Poisson regression.
Results
Study Population
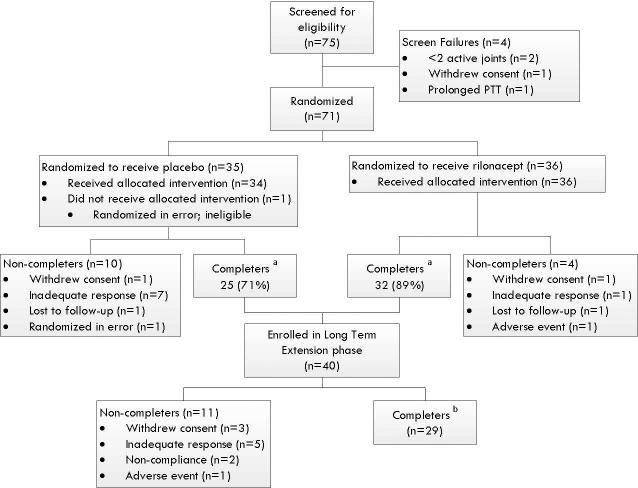
Seventy-one patients were randomized (Figure 1). Baseline demographic and disease characteristics were similar between the two arms of the study, except recent fever was more common in the rilonacept group (Table 1). Fourteen patients withdrew from the study prior to week 24: 8 for inadequate response (7 placebo arm, 1 rilonacept arm), 2 withdrew consent (1 in each arm), 1 for a serious adverse event (elevated liver transaminases) in the rilonacept arm, 1 excluded before drug exposure for not meeting inclusion/exclusion criteria, 2 lost to follow up (1 in each arm). Of the 57 completers, 40 enrolled in the LTE; 29 completed the LTE and 11 did not.
Figure 1. Flow of Patients in RAPPORT Trial.
RAPPORT, The RAndomized Placebo Phase Study Of Rilonacept in the Treatment of Systemic Juvenile Idiopathic Arthritis.
a Indicates patients who attended the week 24 study visit.
b Indicates patients who remained in long term extension until the LTE period ended.
Table 1.
Baseline Demographics and Disease Characteristics of Study Patients
| Characteristic | Rilonacept (N=36) | Placebo (N=35) |
|---|---|---|
| Sex–no. (%) | ||
| Male | 13 (36) | 12 (34) |
| Female | 23 (64) | 23 (66) |
| Race–no. (%)a | ||
| Black | 5 (14) | 7 (20) |
| White | 25 (69) | 23 (66) |
| Otherb | 6 (17) | 5 (14) |
| Ethnicity–no. (%)c | ||
| Hispanic | 7 (19) | 5 (14) |
| Non–Hispanic | 29 (81) | 30 (86) |
| Age–yr | ||
| Mean (SD) | 9.5 (4.6) | 10.5 (4.4) |
| Median (25th,75th) | 9.5 (6.0,13.0) | 11.0 (6.0,14.0) |
| Disease duration–yr | ||
| Mean(SD) | 2.6 (3.6) | 2.6 (3.1) |
| Median (25th,75th) | 0.7 (0.2, 4.0) | 1.4 (0.4, 3.6) |
| Number of active joints | ||
| Mean(SD) | 11.7 (9.6) | 10.5 (7.6) |
| Median (25th,75th) | 7.5 (4.0, 16.0) | 9.0 (5.0, 15.0) |
| Fever past 7 days–no. (%) | 10 (28) | 6 (17.1) |
| Articular without systemic symptoms–no. (%) | 16 (44) | 16 (46) |
| Prior medication use –no. (%) | ||
| Corticosteroids | 30 (83) | 33 (94) |
| Methotrexate | 21 (58) | 26 (74) |
| Leflunomide | 1 (3) | 2 (6) |
| Infliximab | 5 (14) | 6 (17) |
| Etanercept | 12 (33) | 16 (46) |
| Abatacept | 5 (14) | 4 (11) |
| Anakinrad | 13 (36) | 13 (37) |
| Baseline medication use–no. (%) | 30 (83) | 33 (94) |
| Corticosteroide | 25 (69) | 22 (63) |
| Corticosteroid dosef | .38(.23,.55) | .37(.19,.66) |
| Methotrexate | 16 (44) | 19 (54) |
| Characteristics in the past–no. (%) | ||
| Incomplete MAS | 1 (3) | 3 (9) |
| Complete MAS | 1 (3) | 1 (3) |
| Serositis | 9 (25) | 8 (23) |
| sJIA rash | 32 (89) | 33 (94) |
Race was collected via self-report . Categories consisted of American Indian/Alaska Native, Asian, black/African American, Native Hawaiian/other Pacific Islander, and white.
Other includes patients who self-identified as Asian, Native Hawaiian/other Pacific Islander, and multiracial. It also includes 3 patients where race was not reported.
Ethnicity was collected via self-report. Categories consisted of Hispanic/Latino and non-Hispanic/Latino.
Anakinra exposure data were missing in 8 subjects; 4 in each arm.
Corticosteroid includes oral, intravenous, and intramuscular steroids.
Median and inter quartile ranges prednisone equivalents mg/kg/d
Efficacy
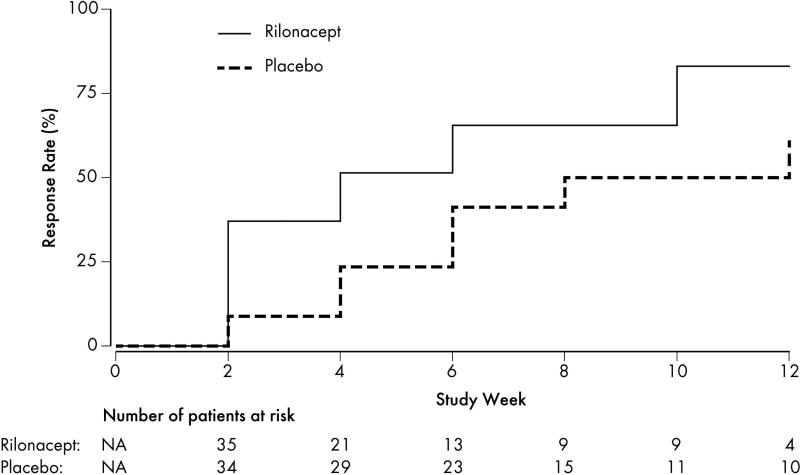
The primary endpoint, time to response as defined by time to achieve the composite endpoint of JIA ACR30, absence of fever, and corticosteroid tapering (for patients taking corticosteroids), was shorter in the rilonacept arm (median 4 weeks; 25th, 75th percentiles 2,10 weeks) than in the placebo arm (median 8 weeks; 25th percentile 6 weeks, 75th percentile not estimable from the data) (Figure 2; chi-square 7.235; P=0.007). By week 12, 27 of 35 patients (77%) receiving rilonacept continuously from onset of the trial and 20 of 34 patients (59%) receiving placebo for the initial 4 weeks, met the primary endpoint of response. The sensitivity analysis of time to response without fever imputation also demonstrated shorter time to response in the rilonacept arm as compared to the placebo arm (chi-square = 5.270; P=0.022). Secondary endpoint analysis of the response rate at 4 weeks showed 20 of 35 patients in the rilonacept arm compared to 9 of 33 patients in the placebo arm (57% vs. 27%; P=0.016). The JIA ACR30, 50, and 70 response rates were significantly better in the rilonacept arm at week 4 compared to the placebo arm (Table 2; all P<0.05). Twenty-six of 35 (74%) patients in the rilonacept arm compared to 13 of 33 (39%) in the placebo arm met JIA ACR30 response criteria at week 4 (OR=4.54; 95% CI 1.62, 12.72; P=0.004); 21 of 35 (60%) vs. 10 of 33 (30%) in the placebo arm met JIA ACR50 (OR=3.50; 95% CI 1.28, 9.56; P=0.015); 14 of 35 (40%) vs. 4 of 33 (12%) met JIA ACR70 (P=0.013).
Figure 2. Cumulative Incidence Estimates of Response by Treatment Arm.
The cumulative incidence curve shows a higher rate of response in the rilonacept arm compared to the placebo arm. The primary endpoint of response was assessed at bi-weekly study visits during the efficacy period. Response was defined as improvement in the American College of Rheumatology (ACR) Pediatric 30, absence of fevers ≥38.5°C in the previous 2 weeks, and at least 10% taper in systemic corticosteroids from baseline. Missing temperatures were imputed as fever free. Patients were no longer eligible for the primary endpoint if corticosteroids were increased or started based on the non-response algorithm prior to meeting the criteria for response. The cumulative incidence estimation treated non-response as a competing event. The number of patients at risk displayed below the figure shows the number of patients eligible to meet the response designation for the first time at each study week.
Table 2.
Change over time in Systemic Features, ACR Variables, and Laboratory Values
| Baseline |
Week 4 |
Week 12 |
Week 24 |
||||
|---|---|---|---|---|---|---|---|
| Variable | Rilonacept | Placebo | Rilonacept | Placebo | Rilonacept | Placebo | Rilonacept |
| Systemic features | |||||||
| Fevera | 10/36 (28%) | 6/35 (17%) | 3/36 (8%) | 5/34 (15%) | 4/33 (12%) | 1/29 (3%) | -- |
| Rash | 15/36 (42%) | 15/35 (43%) | 3/36 (8%) | 8/34 (24%) | 3/33 (9%) | 1/29 (3%) | 4/57 (7%) |
| JIA ACR 30 responseb,c | -- | -- | 26/35 (74%) | 13/33 (39%) | 29/33 (88%) | 22/29 (76%) | 45/55 (82%) |
| JIA ACR 50 responseb,d | -- | -- | 21/35 (60%) | 10/33 (30%) | 28/33 (85%) | 19/29 (66%) | 43/55 (78%) |
| JIA ACR 70 responseb,e | -- | -- | 14/35 (40%) | 4/33 (12%) | 23/33 (70%) | 17/29 (59%) | 35/55 (64%) |
| Inactive diseasef | -- | -- | 2/36 (6%) | 0/34 | 4/33 (12%) | 3/29 (10%) | 11/55 (20%) |
| JIA ACR core set of variables | |||||||
| No. of joints with active arthritis | |||||||
| Median | 7.5 | 9.0 | 3.0 | 7.0 | 2.0 | 4.0 | 1.0 |
| Interquartile range | 4.0-16.0 | 5.0-15.0 | 1.0-7.5 | 4.0-17.0 | 0.0-5.0 | 0.0-10.0 | 0.0-7.0 |
| Median % change from baseline | 70.0 | 12.5 | 81.3 | 63.6 | 90.0 | ||
| No. of joints with limited range of motion | |||||||
| Median | 4.0 | 8.0 | 2.0 | 8.0 | 1.0 | 2.0 | 1.0 |
| Interquartile range | 2.0-10.5 | 2.0-11.0 | 0.0-6.5 | 2.0-12.0 | 0.0-8.0 | 0.0-9.0 | 0.0-7.0 |
| Median % change from baseline | -- | -- | 50.0 | 0.0 | 75.0 | 47.7 | 66.7 |
| Score for physician's global assessment of disease activityg | |||||||
| Median | 43.0 | 59.5 | 12.5 | 40.5 | 10.0 | 18.0 | 6.0 |
| Interquartile range | 26.0-65.0 | 49.0-68.0 | 3.0-34.0 | 25.0-65.0 | 2.0-19.0 | 5.0-30.0 | 1.0-33.0 |
| Median % change from baseline | 73.7 | 27.9 | 81.7 | 73.1 | 85.8 | ||
| Score for parent's global assessment of overall well-beingh | |||||||
| Median | 49.5 | 53.0 | 12.0 | 34.0 | 3.5 | 8.0 | 7.0 |
| Interquartile range | 33.0-65.0 | 28.0-68.0 | 3.0-23.0 | 15.0-67.0 | 0.0-17.0 | 2.0-38.0 | 1.0-29.0 |
| Median % change from baseline | -- | -- | 60.8 | 6.2 | 81.5 | 66.7 | 79.4 |
| CHAQ-DI score | |||||||
| Median | 1.00 | 1.25 | 0.43 | 0.88 | 0.25 | 0.25 | 0.13 |
| Interquartile range | 0.75-1.63 | 0.50-1.63 | 0.00-1.13 | 0.38-1.63 | 0.00-0.88 | 0.00-1.25 | 0.00-1.00 |
| Median % change from baseline | -- | -- | 34.5 | 16.7 | 50.0 | 59.4 | 80.0 |
| C-reactive protein (mg/dL)i | |||||||
| Median | 4.40 | 4.48 | 0.40 | 4.12 | 0.40 | 0.40 | 0.40 |
| Interquartile range | 0.40-9.15 | 0.80-7.23 | 0.40-1.85 | 0.87-8.14 | 0.40-0.92 | 0.40-2.29 | 0.40-1.95 |
| Median % change from baseline | -- | -- | 52.4 | 0.0 | 72.6 | 50.0 | 50.0 |
| Laboratory variables | |||||||
| Hemoglobin (g/dL)k | |||||||
| Median | 11.0 | 11.8 | 11.8 | 11.2 | 12.0 | 11.6 | 12.1 |
| Interquartile range | 10.3-11.9 | 9.8-12.5 | 10.9-12.6 | 9.6-12.4 | 11.4-13.2 | 11.0-12.4 | 11.2-13.0 |
| Total neutrophils (×109/L)l | |||||||
| Median | 9.76 | 7.65 | 5.46 | 8.71 | 4.20 | 4.83 | 3.94 |
| Interquartile range | 5.43-19.07 | 5.69-12.06 | 3.07-8.48 | 6.69-13.14 | 2.36-6.16 | 3.51-6.24 | 2.27-5.74 |
| Platelet count (×109/L)m | |||||||
| Median | 449.0 | 357.0 | 341.0 | 376.0 | 315.0 | 338.0 | 312.0 |
| Interquartile range | 352.0-534.0 | 274.0-457.0 | 279.0-400.0 | 306.5-546.0 | 276.5-376.5 | 275.0-389.0 | 277.0-409.0 |
| D-dimer (ug/mL)j,n | |||||||
| Median | 1.38 | 1.63 | 0.52 | 1.31 | 0.37 | 0.59 | -- |
| Interquartile range | 0.36-3.66 | 0.30-3.05 | 0.34-1.58 | 0.32-3.59 | 0.25-0.98 | 0.23-1.86 | |
| Fibrinogen (mg/dL)o | |||||||
| Median | 361.0 | 361.0 | 243.5 | 365.0 | 231.0 | 258.0 | 257.0 |
| Interquartile range | 283.0-532.0 | 308.0-487.0 | 228.0-329.0 | 272.0-487.0 | 211.5-292.0 | 217.0-334.0 | 213.0-360.5 |
| Ferritin (ng/mL)j,p | |||||||
| Median | 112.0 | 80.0 | 29.0 | 52.5 | 24.0 | 20.0 | -- |
| Interquartile range | 52.5-291.0 | 37.0-207.0 | 13.0-68.0 | 25.5-181.5 | 11.0-44.0 | 8.0-36.0 | |
| ESR (mm/hr)q | |||||||
| Median | 40.0 | 47.0 | 12.0 | 37.0 | 11.0 | 15.5 | 10.5 |
| Interquartile range | 14.0-69.0 | 21.0-67.0 | 7.0-28.0 | 15.0-70.0 | 7.0-15.0 | 8.0-29.0 | 6.0-22.0 |
| Albumin (g/dL)r | |||||||
| Median | 4.1 | 4.1 | 4.4 | 4.1 | 4.4 | 4.3 | 4.4 |
| Interquartile range | 3.7-4.4 | 3.8-4.4 | 4.2-4.4 | 3.8-4.2 | 4.3-4.6 | 4.2-4.6 | 4.1-4.6 |
Abbreviations: ACR, American College of Rheumatology; CHAQ-DI, Childhood Health Assessment Questionnaire–Disability Index
Fever was assessed 7 days prior to baseline based on self-report. Fever (>=38.5° C) was assessed 14 days prior to Week 4 and Week 12 based on patient diaries. Days with missing temperature data were imputed as “fever free.” Fever data was not collected at Week 24.
The core variables for ACR Pediatric response included number of joints with active arthritis, number of joints with limited range of motion, score for physician's global assessment of disease activity, score for parent's global assessment of overall well-being, CHAQ-DI score, and C-reactive protein value. Absence of fever was not included.
The JIA ACR30 algorithm required at least 30% improvement from baseline in at least 3 of 6 core variables, with no more than 1 variable worsening by 30% or more.
The JIA ACR50 algorithm required at least 50% improvement from baseline in at least 3 of 6 core variables, with no more than 1 variable worsening by 50% or more.
The JIA ACR70 algorithm required at least 70% improvement from baseline in at least 3 of 6 core variables, with no more than 1 variable worsening by 70% or more.
Inactive disease was defined as the following: absence of joints with active arthritis, fever, rash, serositis, splenomegaly, or generalized lymphadenopathy; normal erythrocyte sedimentation rate or C-reactive protein; and score ≤1 for physician's global assessment of disease activity.
Physician's global assessment was based on a 100-mm visual-analogue scale. Score ranged from 0 (very well) to 100 (very poor).
Parent's global assessment was based on a 100-mm visual-analogue scale. Score ranged from 0 (not active) to 100 (very active).
The normal range for C-reactive protein was 0 to 0.90 mg/dL.
D-dimer and ferritin were not tested at week 24.
The normal range for Hgb was 11.5-13.5g/dL
The normal range for total neutrophils was 1.8-8.0 X 109/L
The normal range for platelets was 130-400 X 109/L
The normal range for D-dimer was <0.499ug/ml
The normal range for fibrinogen was 200-400mg/dl
The normal range for ferritin was 10-143ng/ml
The normal range for ESR varied by site
The normal range for albumin was 3.2-5.0g/dl
A pre-specified logistic regression analysis adjusted for sJIA duration and presence of articular involvement without systemic symptoms showed that the significant rilonacept effect on response at week 4 persisted after adjustment (OR=3.42; 95% CI 1.21, 9.70; P=0.020). We did not observe a statistically significant difference in odds of response at week 4 for patients without systemic manifestations at baseline compared to patients with systemic manifestations (OR=0.87; 95% CI 0.32, 2.40; P=0.794). There was no statistically significant difference in odds of responding at week 4 as a function of longer sJIA duration (OR=0.91; 95% CI 0.75, 1.11; P=0.359). No statistically significant difference in response rates at week 4 was observed for 26 patients unexposed to anakinra (44%) compared to 24 patients previously exposed to anakinra (40%); 8 subjects had missing data regarding anakinra exposure; 2 had missing response data at week 4.
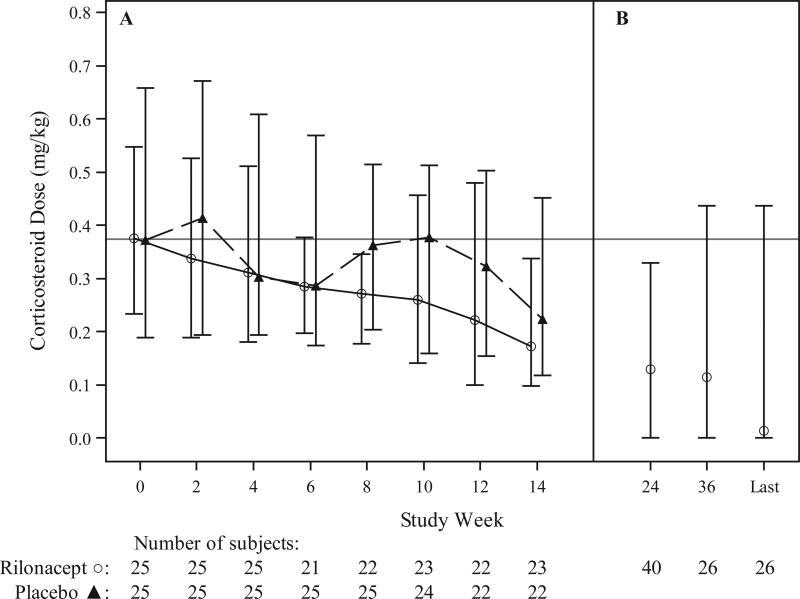
Seventeen of the 50 patients taking corticosteroids at baseline discontinued corticosteroids during the study. Overall, corticosteroid dose decreased more in the rilonacept arm than in the placebo arm during the efficacy period (P=0.036; Figure 3). Laboratory tests reflecting disease activity are reported by treatment arm in Table 2.
Figure 3. Change Over Time in Corticosteroid Dose.
Panel A shows the median corticosteroid dose by treatment group during the efficacy period, with I bars representing interquartile range. Panel B shows the median corticosteroid dose aggregated for all patients during the follow-up safety period, with I bars representing interquartile range. Patients’ oral dose at their last visit during the long term extension phase is displayed. The median time on study at the last visit was 16 months, with a range of 8 to 26 months.
Safety
In the double-blind and treatment phases, the rilonacept arm performed at least as well as the placebo arm in terms of safety (Table 3). There was not a higher incidence of infection in the rilonacept arm in either phase. Four patients, all in the rilonacept arm, developed elevations in liver transaminases of ≥2 times the upper limit of normal; 2 patients developed elevations ≥5 times the upper limit of normal (one of these was considered an SAE). There were 14 SAEs: 9 among patients in the rilonacept arm and 5 in the placebo arm with the most common being sJIA flare (4). The AST liver function test was consistently higher in the rilonacept arm.
Table 3.
Adverse Events
| Double-Blind Phase (0-4 weeks) |
Treatment Phase (4-24 weeks) |
LTE Open-Label Phase (24 weeks -21 months) |
|||
|---|---|---|---|---|---|
| Variable | Rilonacept (N=36) | Placebo (N=35) | Rilonacept (N=35) | Placebo (N=33) | Rilonacept (N=40) |
| Adverse event | |||||
| No. of events | 17 | 63 | 81 | 123 | 110 |
| No. of events per patient-year | 6.1 | 23.9 | 6.2 | 11.3 | 3.0 |
| Patients with an event - no. (%) | 10 (28%) | 19 (54%) | 27 (77%) | 28 (85%) | 28 (70%) |
| Most frequently reported events - no. of patients (%)a | |||||
| Abdominal pain upper | 1 (3%) | 2 (6%) | 3 (9%) | 1 (3%) | 0 |
| Arthralgia | 0 | 1 (3%) | 2 (6%) | 6 (18%) | 1 (3%) |
| Cough | 0 | 1 (3%) | 2 (6%) | 3 (9%) | 2 (5%) |
| Headache | 1 (3%) | 6 (17%) | 1 (3%) | 4 (12%) | 3 (8%) |
| Nausea | 0 | 1 (3%) | 1 (3%) | 2 (6%) | 3 (8%) |
| Pharyngitis streptococcal | 0 | 0 | 2 (6%) | 2 (6%) | 4 (10%) |
| Pyrexia | 0 | 1 (3%) | 5 (14%) | 1 (3%) | 1 (3%) |
| Rash | 2 (6%) | 1 (3%) | 1 (3%) | 3 (9%) | 1 (3%) |
| Upper respiratory tract infection | 0 | 1 (3%) | 5 (14%) | 9 (27%) | 2 (5%) |
| Vomiting | 1 (3%) | 2 (6%) | 1 (3%) | 2 (6%) | 4 (10%) |
| Serious adverse events (SAE) | |||||
| No. of events | 1 | 1 | 3 | 1 | 8 |
| No. of events per patient-year | 0.4 | 0.4 | 0.2 | 0.1 | 0.2 |
| Patients with an event - no. (%) | 1 (3%) | 1 (3%) | 3 (9%) | 1 (3%) | 6 (15%) |
| All reported events - no. of patients (%) | |||||
| Gastroenteritis salmonella | 0 | 0 | 0 | 0 | 1 (3%) |
| Histiocytosis haematophagicb | 0 | 0 | 0 | 0 | 1 (3%) |
| Juvenile arthritisc | 1 (3%) | 1 (3%) | 0 | 1 (3%) | 1 (3%) |
| Liver function test abnormald | 0 | 0 | 1 (3%) | 0 | 0 |
| Mental status changes | 0 | 0 | 0 | 0 | 1 (3%) |
| Pericarditis | 0 | 0 | 0 | 0 | 1 (3%) |
| Pharyngitis streptococcale | 0 | 0 | 0 | 0 | 1 (3%) |
| Pyrexia | 0 | 0 | 1 (3%) | 0 | 0 |
| Varicella | 0 | 0 | 1 (3%) | 0 | 0 |
| Viral upper respiratory tract infection | 0 | 0 | 0 | 0 | 1 (3%) |
| Infection | |||||
| No. of events | 2 | 2 | 25 | 29 | 37 |
| No. of events per patient-year | 0.7 | 0.8 | 1.9 | 2.7 | 1.0 |
| Patients with an event - no. (%) | 2 (6%) | 2 (6%) | 16 (46%) | 20 (61%) | 14 (35%) |
The most frequently reported events were defined as events that occurred in at least 10% of all patients during the entire study.
One patient developed EBV triggered macrophage activation syndrome (proven by polymerase chain reaction testing) deemed an SAE during the LTE phase.
The juvenile arthritis SAE summarized in the rilonacept arm during the double-blind phase was a pre-treatment event. The onset date occurred after consent but prior to randomization.
One patient had elevations in the liver function tests deemed an SAE during the treatment phase. The elevations subsided when drug was temporarily discontinued but recurred on re-challenge.
One patient had two separate episodes of streptococcal pharyngitis deemed SAEs during the LTE phase.
Discussion
sJIA has proven to be more difficult to treat than other categories of JIA with poor response to methotrexate and TNF inhibitors. This study demonstrates efficacy of rilonacept in active sJIA and confirms the remarkable effectiveness of IL-1 inhibition in sJIA, which is now demonstrated in 3 different IL-1 inhibitors, anakinra14, canakinumab10, rilonacept and in one IL-6 inhibitor, tocilizumab25 . The long-term dependence on corticosteroid therapy for many children with severe systemic manifestations has resulted in significant treatment-related comorbidities, and provided an important impetus to finding an effective no-corticosteroid treatment for sJIA. The remarkable clinical responsiveness to IL-1 inhibition 7-15 suggests that IL-1 plays a pivotal role in the pathogenesis of sJIA 7,26-28 and the lack of HLA association, autoantibodies, and other classical features of autoimmune diseases, suggest the sJIA should be reclassified as an autoinflammatory disease29.
This study utilized a novel design, the randomized placebo phase trial19 to meet several objectives. First and foremost, we were concerned that the randomized withdrawal study design which was so successful in other JIA trials30,31 might trigger a life threatening event when rilonacept was abruptly withdrawn in responders. Secondly, our study design directly tested corticosteroid tapering, by allowing inclusion of patients requiring high doses of corticosteroids, and incorporating a forced corticosteroid taper into the primary endpoint. Lastly, the placebo phase occurring at the beginning of the trial allowed collection of biospecimens for studying the effects of an IL-1 inhibitor on the immunobiology of sJIA in the context of highly curated clinical data. Currently, 4 translational studies are underway.
Previous observational studies suggest that sJIA patients without systemic manifestations, and/or longer disease duration have poorer responses to IL-1 inhibition11,32. We did not observe a statistically significant association between response at week 4 and those patient characteristics in our study population. However, a trend was noted with regard to duration of disease: the median disease duration was shorter among patients who responded at week 4 compared to those who didn't (9.6 vs. 15.3 months).
Rilonacept had an acceptable safety profile in this study. There were no opportunistic infections, however elevation in liver transaminases in at least one patient was clearly caused by rilonacept as the adverse event recurred on re-challenge. There was only one episode of MAS which was triggered by EBV infection during the LTE.
Recently, another IL-1 inhibitor – canakinumab – was shown to be effective in treating sJIA patients with systemic symptoms in two separate double-blind, placebo-controlled studies and has been FDA approved for sJIA10. Rilonacept could offer an alternative with its circulating half-life of 8.6 days33 in contrast to the long biologic activity of canakinumab (236 days) which could be a disadvantage in the setting of an SAE. The weekly administration of rilonacept may be preferred by families over anakinra which must be given by painful daily subcutaneous injections14. In contrast to all the IL-1 inhibitors tocilizumab, an IL-6 inhibitor which has also been shown to be effective in a double-blind, placebo controlled trial, and is approved by the FDA for sJIA, is given by infusion25.
Limitations of the study were missing data regarding prior anakinra exposure, difficulty in comparing adverse events between the two arms of the study because of the short placebo phase and not meeting our enrollment goals by the time the study was discontinued by the NIH/NIAMS.
In summary, rilonacept was generally well tolerated and demonstrated efficacy in active sJIA in our study. Rilonacept treatment facilitated corticosteroid tapering similarly to tocilizumab and canakinumab. In addition, the ability to integrate clinical data and biospecimens associated with this study will likely lead to advances in our understanding of this unique and challenging disease.
ACKNOWLEDGEMENTS
Marielis Rivera, Saima Siddiqui, Arisa Kapedani, Kathy Moore, Jane Winsor, Kristy Hwang, Barbara Kuzil, Thomas Phillips, Marsha Malloy, Kathleen Egla Rabinovich, Heather Van Mater, Jeffrey Dvergsten, Janet Wootton, Heather Bell-Brunson, Pamela Russell, Christel Gross, Sue Bowyer, Margaret Carson, Kabita Nanda, Gina Montealegre Sanchez, Dianne Morus, Dawn Debois, Aaron Eggebeen, Hendriana Gutierrez, Marilyn Orlando, B. Anne Eberhard, Cagri Yidirim-Toruner, Shirley Henry, Heather Benham, Alisa Gotte, Kathleen Haines, Suzanne Li, Jennifer Weiss, Mary Ellen Riordan, Jay Mehta, Dawn Wahezi, Kathleen Kenney-Riley, Kristen Hayward, Sarah Ringold, Audrey Hendrickson, Heather Bell-Brunson, Pamela Russell, Christel Gross, Patricia Irigoyen, Rayfel Schneider, Brian Feldman, Edward Giannini
This project was supported in part, by the:
Albert Einstein College of Medicine Clinical and Translational Science Awards 3UL1 TR00008605S1, 1UL1RR025750,
Indiana University Clinical and Translational Science Award TR000006
University of Washington Clinical and Translational Science Award 1 UL1 RR025014-01, UL1TR000423 Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 and the Dahms CRU at Case Medical Center
Footnotes
Financial Disclosures:
Dr. Ilowite received consulting fees from Novartis (Adjudication Committee), Janssen (Chair, DSMC). Dr. Schanberg received consulting fees from Novartis, Lilly, UCB, Glaxo Smith Kline, SOBI, Pfizer. Dr. Singer received consulting fees from Abbott, contracted with Merck, and conducts clinical trials for Genentech, Glaxo Smith Kline, Lilly, Pfizer, UCB and is on the DSMB for Pfizer. Dr. Wallace received grant funds from Amgen, Pfizer, Novartis.
Contributor Information
Norman T. Ilowite, The Children's Hospital at Montefiore, Albert Einstein College of Medicine
Kristi Prather, Duke Clinical Research Institute.
Yuliya Lokhnygina, Duke Clinical Research Institute.
Laura E. Schanberg, Duke University Medical Center
Melissa Elder, University of Florida.
Diana Milojevic, University of California, San Francisco.
James W. Verbsky, Medical College of Wisconsin
Steven J. Spalding, The Cleveland Clinic
Yukiko Kimura, Joseph M Sanzari Children's Hospital, Hackensack University Medical Center.
Lisa F. Imundo, Morgan Stanley Children's Hospital of New York-Presbyterian, Columbia University Medical Center
Marilynn G. Punaro, Texas Scottish Rite Hospital
David D. Sherry, Children's Hospital of Philadelphia
Stacey E. Tarvin, Riley Hospital for Children at Indiana University Health
Lawrence S. Zemel, Connecticut Children's Medical Center
James D. Birmingham, Michigan State University College of Human Medicine
Beth S. Gottlieb, Steven and Alexandra Cohen Children's Hospital
Michael L. Miller, Ann and Robert H. Lurie Children's Hospital
Kathleen O'Neil, Riley Hospital for Children at Indiana University Health.
Natasha M. Ruth, Medical University of South Carolina
Carol A. Wallace, Seattle Children's Hospital & Research Institute
Nora G. Singer, Rainbow Babies and Children's Hospital and Case Medical Center
Christy I. Sandborg, Lucille Salter Packard Children's Hospital, Stanford School of Medicine
References
- 1.Grom AA, Passo M. Macrophage activation syndrome in systemic juvenile rheumatoid arthritis. J Pediatr. 1996;129:630–2. doi: 10.1016/s0022-3476(96)70140-3. [DOI] [PubMed] [Google Scholar]
- 2.Singh-Grewal D, Schneider R, Bayer N, Feldman BM. Predictors of disease course and remission in systemic juvenile idiopathic arthritis: significance of early clinical and laboratory features. Arthritis Rheum. 2006;54:1595–601. doi: 10.1002/art.21774. [DOI] [PubMed] [Google Scholar]
- 3.Lomater C, Gerloni V, Gattinara M, Mazzotti J, Cimaz R, Fantini F. Systemic onset juvenile idiopathic arthritis: a retrospective study of 80 consecutive patients followed for 10 years. J Rheumatol. 2000;27:491–6. [PubMed] [Google Scholar]
- 4.Woo P. Systemic juvenile idiopathic arthritis: diagnosis, management, and outcome. Nat Clin Pract Rheumatol. 2006;2:28–34. doi: 10.1038/ncprheum0084. [DOI] [PubMed] [Google Scholar]
- 5.Spiegel LR, Schneider R, Lang BA, et al. Early predictors of poor functional outcome in systemic-onset juvenile rheumatoid arthritis: a multicenter cohort study. Arthritis Rheum. 2000;43:2402–9. doi: 10.1002/1529-0131(200011)43:11<2402::AID-ANR5>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 6.Sandborg C, Holmes TH, Lee T, et al. Candidate early predictors for progression to joint damage in systemic juvenile idiopathic arthritis. J Rheumatol. 2006;33:2322–9. [PubMed] [Google Scholar]
- 7.Pascual V, Allantaz F, Arce E, Punaro M, Banchereau J. Role of interleukin-1 (IL-1) in the pathogenesis of systemic onset juvenile idiopathic arthritis and clinical response to IL-1 blockade. The Journal of experimental medicine. 2005;201:1479–86. doi: 10.1084/jem.20050473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verbsky JW, White AJ. Effective use of the recombinant interleukin 1 receptor antagonist anakinra in therapy resistant systemic onset juvenile rheumatoid arthritis. J Rheumatol. 2004;31:2071–5. [PubMed] [Google Scholar]
- 9.Ruperto N, Quartier P, Wulffraat N, et al. A phase II, multicenter, open-label study evaluating dosing and preliminary safety and efficacy of canakinumab in systemic juvenile idiopathic arthritis with active systemic features. Arthritis Rheum. 2012;64:557–67. doi: 10.1002/art.33342. [DOI] [PubMed] [Google Scholar]
- 10.Ruperto N, Brunner HI, Quartier P, et al. Two randomized trials of canakinumab in systemic juvenile idiopathic arthritis. N Engl J Med. 2012;367:2396–406. doi: 10.1056/NEJMoa1205099. [DOI] [PubMed] [Google Scholar]
- 11.Nigrovic PA, Mannion M, Prince FH, et al. Anakinra as first-line disease-modifying therapy in systemic juvenile idiopathic arthritis: report of forty-six patients from an international multicenter series. Arthritis Rheum. 2011;63:545–55. doi: 10.1002/art.30128. [DOI] [PubMed] [Google Scholar]
- 12.Ilowite N, Porras O, Reiff A, et al. Anakinra in the treatment of polyarticular-course juvenile rheumatoid arthritis: safety and preliminary efficacy results of a randomized multicenter study. Clin Rheumatol. 2009;28:129–37. doi: 10.1007/s10067-008-0995-9. [DOI] [PubMed] [Google Scholar]
- 13.Zeft A, Hollister R, LaFleur B, et al. Anakinra for systemic juvenile arthritis: the Rocky Mountain experience. J Clin Rheumatol. 2009;15:161–4. doi: 10.1097/RHU.0b013e3181a4f459. [DOI] [PubMed] [Google Scholar]
- 14.Quartier P, Allantaz F, Cimaz R, et al. A multicentre, randomised, double-blind, placebo-controlled trial with the interleukin-1 receptor antagonist anakinra in patients with systemic-onset juvenile idiopathic arthritis (ANAJIS trial) Ann Rheum Dis. 2011;70:747–54. doi: 10.1136/ard.2010.134254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lequerre T, Quartier P, Rosellini D, et al. Interleukin-1 receptor antagonist (anakinra) treatment in patients with systemic-onset juvenile idiopathic arthritis or adult onset Still disease: preliminary experience in France. Ann Rheum Dis. 2008;67:302–8. doi: 10.1136/ard.2007.076034. [DOI] [PubMed] [Google Scholar]
- 16.Economides AN, Carpenter LR, Rudge JS, et al. Cytokine traps: multi-component, high-affinity blockers of cytokine action. Nat Med. 2003;9:47–52. doi: 10.1038/nm811. [DOI] [PubMed] [Google Scholar]
- 17.Lovell DJ, Giannini EH, Reiff AO, et al. Long-term safety and efficacy of rilonacept in patients with systemic juvenile idiopathic arthritis. Arthritis Rheum. 2013;65:2486–96. doi: 10.1002/art.38042. [DOI] [PubMed] [Google Scholar]
- 18.Petty RE, Southwood TR, Manners P, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31:390–2. [PubMed] [Google Scholar]
- 19.Feldman B, Wang E, Willan A, Szalai JP. The randomized placebo-phase design for clinical trials. J Clin Epidemiol. 2001;54:550–7. doi: 10.1016/s0895-4356(00)00357-7. [DOI] [PubMed] [Google Scholar]
- 20.Giannini EH, Ruperto N, Ravelli A, Lovell DJ, Felson DT, Martini A. Preliminary definition of improvement in juvenile arthritis. Arthritis Rheum. 1997;40:1202–9. doi: 10.1002/1529-0131(199707)40:7<1202::AID-ART3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 21.Ilowite NT, Sandborg CI, Feldman BM, et al. Algorithm development for corticosteroid management in systemic juvenile idiopathic arthritis trial using consensus methodology. Pediatr Rheumatol Online J. 2012;10:31. doi: 10.1186/1546-0096-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henter JI, Horne A, Arico M, et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48:124–31. doi: 10.1002/pbc.21039. [DOI] [PubMed] [Google Scholar]
- 23.Wallace CA, Ruperto N, Giannini E, et al. Preliminary criteria for clinical remission for select categories of juvenile idiopathic arthritis. J Rheumatol. 2004;31:2290–4. [PubMed] [Google Scholar]
- 24.O'Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35:549–56. [PubMed] [Google Scholar]
- 25.De Benedetti F, Brunner HI, Ruperto N, et al. Randomized trial of tocilizumab in systemic juvenile idiopathic arthritis. N Engl J Med. 2012;367:2385–95. doi: 10.1056/NEJMoa1112802. [DOI] [PubMed] [Google Scholar]
- 26.Stock CJ, Ogilvie EM, Samuel JM, Fife M, Lewis CM, Woo P. Comprehensive association study of genetic variants in the IL-1 gene family in systemic juvenile idiopathic arthritis. Genes Immun. 2008;9:349–57. doi: 10.1038/gene.2008.24. [DOI] [PubMed] [Google Scholar]
- 27.Allantaz F, Chaussabel D, Stichweh D, et al. Blood leukocyte microarrays to diagnose systemic onset juvenile idiopathic arthritis and follow the response to IL-1 blockade. The Journal of experimental medicine. 2007;204:2131–44. doi: 10.1084/jem.20070070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Macaubas C, Nguyen KD, Peck A, et al. Alternative activation in systemic juvenile idiopathic arthritis monocytes. Clin Immunol. 2012;142:362–72. doi: 10.1016/j.clim.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mellins ED, Macaubas C, Grom AA. Pathogenesis of systemic juvenile idiopathic arthritis: some answers, more questions. Nat Rev Rheumatol. 2011;7:416–26. doi: 10.1038/nrrheum.2011.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lovell DJ, Giannini EH, Reiff A, et al. Etanercept in children with polyarticular juvenile rheumatoid arthritis. Pediatric Rheumatology Collaborative Study Group. N Engl J Med. 2000;342:763–9. doi: 10.1056/NEJM200003163421103. [DOI] [PubMed] [Google Scholar]
- 31.Ruperto N, Lovell DJ, Quartier P, et al. Abatacept in children with juvenile idiopathic arthritis: a randomised, double-blind, placebo-controlled withdrawal trial. Lancet. 2008;372:383–91. doi: 10.1016/S0140-6736(08)60998-8. [DOI] [PubMed] [Google Scholar]
- 32.Gattorno M, Piccini A, Lasiglie D, et al. The pattern of response to anti-interleukin-1 treatment distinguishes two subsets of patients with systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 2008;58:1505–15. doi: 10.1002/art.23437. [DOI] [PubMed] [Google Scholar]
- 33.Kapur S, Bonk ME. Rilonacept (arcalyst), an interleukin-1 trap for the treatment of cryopyrin-associated periodic syndromes. P T. 2009;34:138–41. [PMC free article] [PubMed] [Google Scholar]