Figure 5.
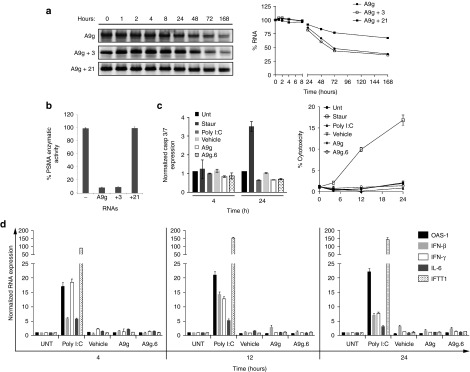
Stability and safety assessment of A9g aptamer in human serum and cells. (a) Effect of chemical modifications on stability of A9g aptamer in human serum. (Left panel) Denaturing PAGE gel of RNA aptamers following incubation with 100% human serum over a 1-week period. (Right panel) Band intensity was quantified using Image J version 1.47 and plotted relative to input/time 0 RNA. A9g = All pyrimidines modified with 2′-fluoro chemistry, A9g + 3 = All pyrimidines modified with 2′-fluoro chemistry and three purines modified with 2′-O methyl chemistry; A9g + 21 = All pyrimidines modified with 2′-fluoro chemistry and all 21 purines modified with 2′-O methyl chemistry. (b) Functional characterization of chemically modified A9g aptamers using NAALADAse Assay. Percentage of PSMA enzymatic activity is reported. (c) Assessment of potential toxicity of A9g in human peripheral blood mononuclear cells (hPBMC) from healthy adult volunteers. Staurosporine: positive control for apoptosis; Poly I:C: positive control for immune stimulation. Vehicle: Binding Buffer or a non-binding, control aptamer (A9g.6) for the indicated time points. (Left panel) Apoptosis assessed by caspase 3/7 activation. (Right panel) Cytotoxicity assessed by lactate dehydrogenase activity. (d) Assessment of potential immune stimulatory effect of A9g in hPBMCs as in Figure 3f for above.
