Abstract
Bloodstream infections caused by Candida species remain a significant cause of morbidity and mortality in hospitalized patients. Biofilm formation by Candida species is an important virulence factor for disease pathogenesis. A prospective analysis of patients with Candida bloodstream infection (n = 217) in Scotland (2012–2013) was performed to assess the risk factors associated with patient mortality, in particular the impact of biofilm formation. Candida bloodstream isolates (n = 280) and clinical records for 157 patients were collected through 11 different health boards across Scotland. Biofilm formation by clinical isolates was assessed in vitro with standard biomass assays. The role of biofilm phenotype on treatment efficacy was also evaluated in vitro by treating preformed biofilms with fixed concentrations of different classes of antifungal. Available mortality data for 134 patients showed that the 30-day candidaemia case mortality rate was 41%, with predisposing factors including patient age and catheter removal. Multivariate Cox regression survival analysis for 42 patients showed a significantly higher mortality rate for Candida albicans infection than for Candida glabrata infection. Biofilm-forming ability was significantly associated with C. albicans mortality (34 patients). Finally, in vitro antifungal sensitivity testing showed that low biofilm formers and high biofilm formers were differentially affected by azoles and echinocandins, but not by polyenes. This study provides further evidence that the biofilm phenotype represents a significant clinical entity, and that isolates with this phenotype differentially respond to antifungal therapy in vitro. Collectively, these findings show that greater clinical understanding is required with respect to Candida biofilm infections, and the implications of isolate heterogeneity.
Keywords: Antifungal, biofilm, Candida albicans, Candida glabrata, candidaemia, catheters, drug resistance
Introduction
Candida species bloodstream infection (BSI) remains a significant cause of morbidity and mortality [1], [2]. In the USA, Candida species are ranked as the fourth most common organisms responsible for all BSIs, and the third most common within the intensive-care unit [2], a clinical environment that is highly dependent on intravascular lines. Candida BSI is often associated with the ability of Candida to form biofilms on indwelling medical devices, such as central venous catheters (CVCs) and prostheses [3], [4]. Candida albicans remains one of the most important candidal pathogens in this context, owing in part to its greater capacity to form biofilms [5], and this has profound consequences for the clinical outcome of BSI. Therefore, removal of catheters is advocated to improve survival rates, on the basis of meta-analysis evidence from current guidelines [6], [7].
Retrospective studies have used multivariate approaches to attempt to analyse the risk factors associated with patients with Candida BSI. Biofilm formation has been reported as an independent predictor of mortality, in addition to inadequate antifungal therapy and APACHE III scores [8]. Analysis of the association of mortality with biofilm-forming ability demonstrated that both C. albicans and Candida parapsilosis were associated with increased mortality. A subsequent prospective case–control study showed that Candida BSI biofilm-forming isolates could be independently predicted by the presence of CVCs, urinary catheters, total parenteral nutrition, and diabetes mellitus [9]. Moreover, the hospital length of stay and cost of antifungal therapy were also greater in those with biofilm-forming isolates, and these patients had a greater risk of hospital mortality (OR 1.77). However, these studies used binary categorization of biofilm formation, i.e. biofilm formers or non-formers, on the basis of in vitro bioassays. Our group has recently reported that biofilm formation by C. albicans is heterogeneous, and that, rather than biofilm formation being a binary function, it can be considered on a spectrum or within defined categories [10]. Therefore, there remains a gap in our knowledge as to whether patients with isolates defined as low biofilm formers (LBFs) or high biofilm formers (HBFs) within the spectrum have differential clinical outcomes. The aim of this study was therefore to investigate the impact of biofilm formation by Candida species on the clinical outcomes of BSI in a defined Scottish cohort.
Patients and methods
Patients and variables
A retrospective study of all cases of Candida BSI was carried out within Scotland under NHS Caldicott Guardian approval from March 2012 to February 2013. Candida BSI was reported in 217 patients from 11 different health boards; clinical data were obtained from 157 patients. The complete datasets of patient demographics, underlying medical conditions and details of antimicrobial therapy were collected through a review of the medical case notes in each health board. Where available (134 patients), patient outcomes were followed from the first positive blood culture until 30 days or death, and clinical details, including the presence of indwelling medical devices in the 30 days prior to the detection of Candida BSI were also collected. All data were collected and stored electronically within a database (Excel, Microsoft).
Isolate collection
Blood cultures from 217 patients were processed according to routine standard operating procedures in each of the referring laboratories. When available, multiple isolates were collected from some of these patients within the observation period of 30 days. All clinical isolates obtained during this period were independently identified by the use of Colorex Candida chromogenic plates (E&O Laboratories, Bonnybridge, UK), as confirmed by matrix-assisted laser desorption ionization time-of-flight mass spectrometry at the Public Health England Southwest Laboratory (Bristol), and were stored in Microbank vials (Pro-Lab Diagnostics, Bromborough, UK) at −80°C until further use. These isolates were subcultured on Sabouraud's dextrose agar (Sigma-Aldrich, Poole, UK). Plates were incubated at 30°C for 48 h, and maintained at 4°C.
Biofilm formation
Candida species biofilms were grown according to our established protocols for 24 h [11], and the biomass of each isolate was assessed with the crystal violet (CV), XTT and SYTO9 assays, as previously reported [10], [12], [13]; isolates were grouped on the basis of their level of biomass distribution (optical density (OD)570 nm values). Isolates within the first quartile (Q1) were classed as LBFs, isolates with a biomass greater than the third quartile (Q3) were classed as HBFs, and those in between were classed as intermediate biofilm formers (IBFs) (second quartile (Q2)) [10]. The susceptibilities of C. albicans biofilm formation to different classes of antifungal were also assessed, with 24-h biofilms being treated with either 2 mg/L or 200 mg/L voriconazole, caspofungin or amphotericin B for 24 h. Following treatment, the proportional viability was compared with that untreated control by use of an XTT metabolic assay [13].
Statistical analysis
Initially, all data were numerically coded and labelled for each variable, and analysed with SPSS software (SPSS, Chicago, IL, USA). Categorical variables were compared between groups by use of the two-tailed χ2 test or Fisher’s exact test, as appropriate. Two groups of any continuous variables were compared by the use of Student’s t-test or the Mann–Whitney U-test, as appropriate. Pairwise correlations between biofilm assays were determined by calculating two-tailed Pearson correlation coefficients. The survival distribution function was estimated with the Kaplan–Meier method, and a non-parametric log-rank test was used to compare the survival curves among the different groups. Variables showing a significant association with survival according to Student’s t-test or the χ2 test were included in subsequent univariate and multivariate Cox regression analyses, to generate the survival curves, hazard ratios (HRs), and 95% CIs.
Results
Incidence and mortality associated with Candida species
Data from the most recent (2011) census (http://www.scrol.gov.uk/scrol/common/home.jsp) list the population of Scotland as 5 295 403. The population-based incidence of BSI in Scotland can therefore be calculated as 4.1 per 100 000 population. Of the 280 isolates collected in this study from 217 patients, 115 were found to be C. albicans and 98 were Candida glabrata. Of the 134 cases for which patient mortality data were available, the overall crude mortality rate was 41%, which was primarily associated with C. albicans (47.3%), followed by C. glabrata (34.5%) and other species (18.2%).
Clinical parameters influencing patient mortality
Initially, we assessed the influence of different clinical variables and underlying conditions on patient mortality (Table 1). The results showed that patient age was significantly associated with mortality (p 0.023). Other variables, including underlying clinical conditions, i.e. diabetes, liver disease, autoimmune disorders, and others, were also assessed, and found not to be statistically associated with patient mortality. Moreover, 96.4% of patients had lines in situ, including 93% of patients with CVCs. Further analysis showed an association between line removal after diagnosis of Candida BSI and mortality (p 0.032).
Table 1.
Variables stratified according to the survival or death status at the 30-day endpoint for the 134 patients studied
| Variables | % Survived | % Died | p |
|---|---|---|---|
| Age (years), mean ± SD | 58.6 ± 20.7 (79) | 66.9 ± 20.0 (55) | 0.023 |
| Male sex | 48.1 (79) | 83.6 (55) | 0.096 |
| Diabetes | 25.6 (78) | 32.7 (55) | 0.437 |
| Surgery | 63.6 (22) | 50.0 (18) | 0.523 |
| Radiotherapy | 37.5 (16) | 30.8 (13) | 1 |
| Chemotherapy | 64.3 (14) | 56.3 (16) | 0.722 |
| Solid organ transplant | 5.1 (79) | 0.0 (55) | 0.144 |
| Metastatic | 61.1 (18) | 85.7 (14) | 0.235 |
| Solid tumour | 32.1 (78) | 38.9 (54) | 0.460 |
| Autoimmune or genetic disorder | 11.4 (79) | 7.4 (54) | 0.559 |
| Renal failure | 32.9 (73) | 42.2 (45) | 0.330 |
| Liver disease | 8.3 (72) | 14.3 (49) | 0.374 |
| Alcohol abuse | 10.5 (76) | 15.4 (52) | 0.428 |
| ICU admission | 19.7 (76) | 28.8 (52) | 0.289 |
| Parenteral nutrition | 43.1 (72) | 37.3 (51) | 0.579 |
| Line removed | 82.2 (45) | 55.0 (20) | 0.032 |
| Antifungals in previous 3 months | 27.5 (69) | 14.0 (50) | 0.115 |
Values in parentheses indicate the total no. of patients assessed for each variable. Bold type indicates a significant result.
ICU, intensive care unit; SD, standard deviation.
Relationship between biofilm formation and mortality
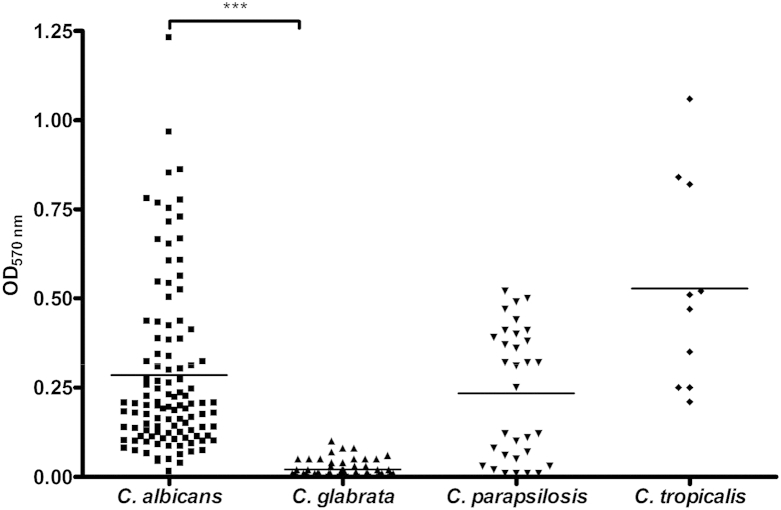
Biofilm formation (biomass) by different Candida species was assessed with CV, XTT and SYTO9 assays of C. albicans (n = 107), C. glabrata (n = 96), C. parapsilosis (n = 32), and Candida tropicalis (n = 10). When the different biomass assays were compared, significant positive correlations were found for C. albicans between CV and XTT (r = 0.8), between CV and SYTO9 (r = 0.6), and between XTT and SYTO9 (r = 0.4) (Fig. S1). On the basis of this, CV was used for further categorization of the isolates. Fig. 1 shows that biofilm formation by different Candida species was heterogeneous, irrespective of the species tested. Isolates were categorized as LBFs or HBFs if their CV absorbance was less than Q1 (OD570 nm = 0.15) or greater than Q3 (OD570 nm = 0.3), respectively. Those isolates between Q1 and Q3 were defined as IBFs. C. albicans, C. parapsilosis and C. tropicalis were found to be HBFs (n = 35, n = 16, and n = 5, respectively), IBFs (n = 36, n = 3, and n = 5, respectively), or LBFs (n = 35 n = 13, and n = 0, respectively), whereas C. glabrata organisms were found to be only LBFs (n = 98).
Fig. 1.
Biofilm formation by Candida species. Candida bloodstream isolates were evaluated for biofilm formation with standardized methods. Candida isolates standardized (1 × 106 cells/mL) in RPMI-1640 were grown in flat-bottomed 96-well microtitre plates for 24 h at 37°C. Mature biofilms were carefully washed with phosphate-buffered saline and allowed to air dry, and biomass quantified by staining with 0.05% w/v crystal violet solution. The biofilms were washed and destained with 100% ethanol. Biomass was quantified spectrophotometrically by reading the optical density (OD) at 570 nm in a microtitre plate reader (FluoStar Omega BMG Labtech, Aylesbury, UK). Six replicates were used for each isolate, and the mean of each is represented. ***p <0.0001.
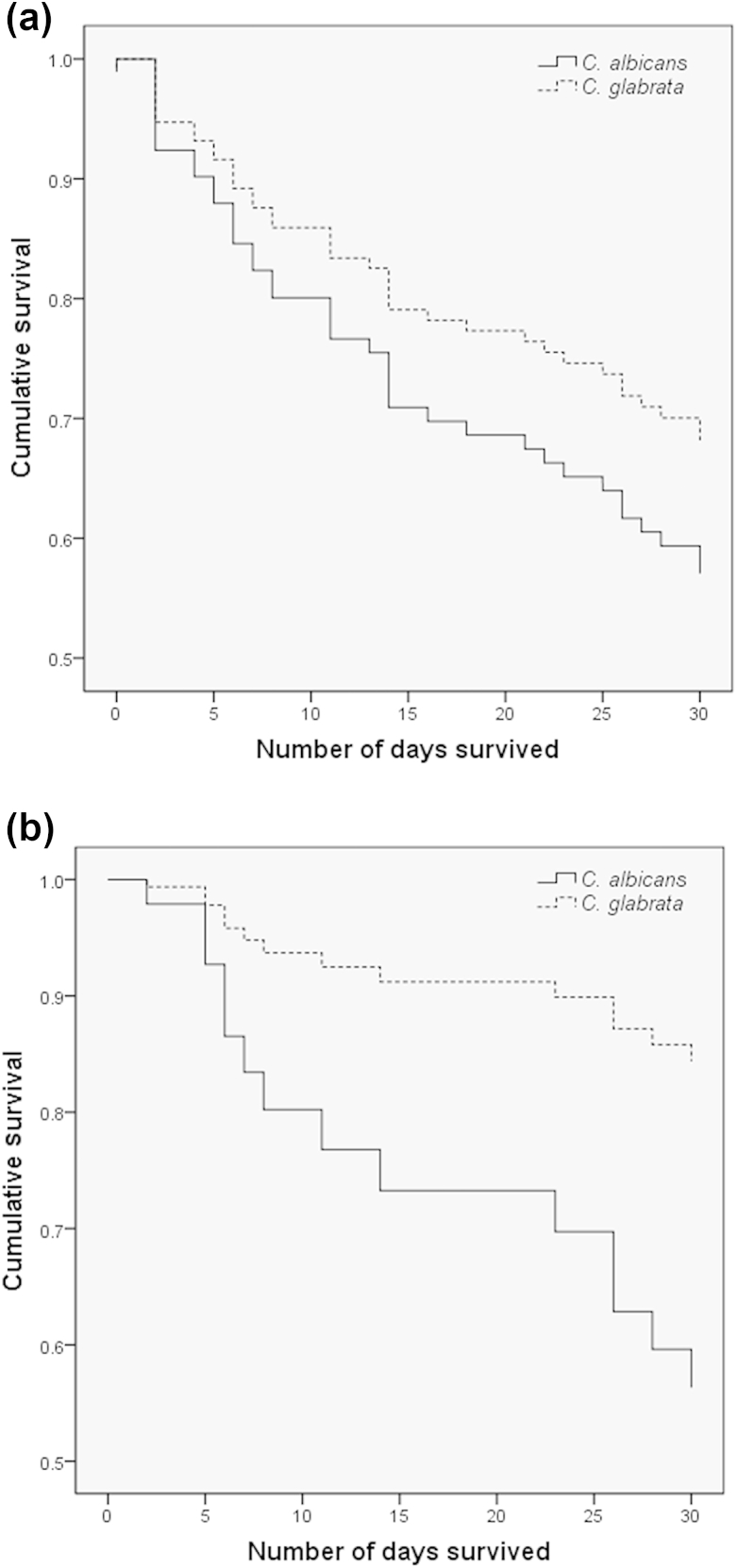
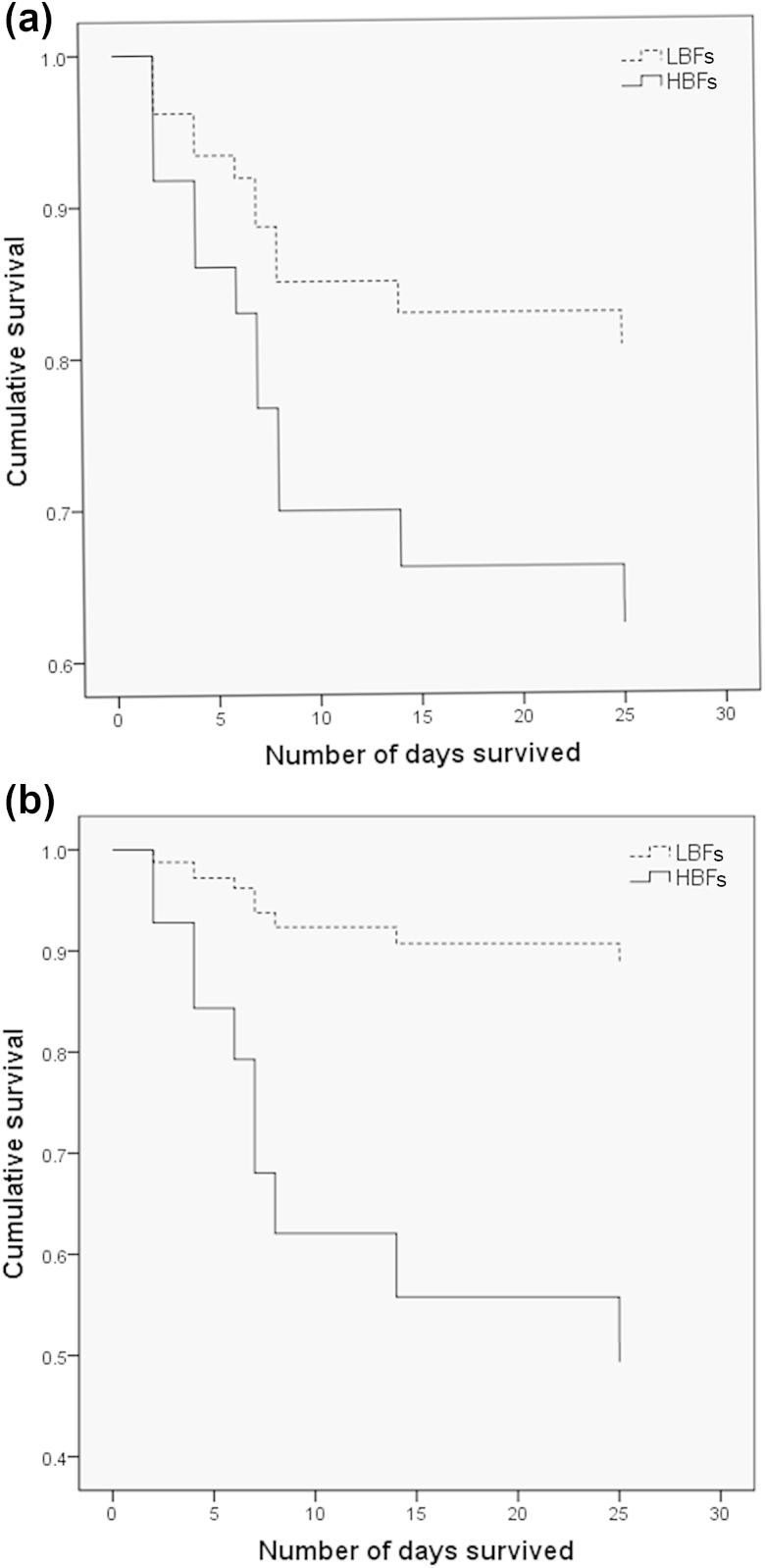
Given the significant disparity in biofilm formation between C. albicans and C. glabrata (p <0.0001), we analysed patient mortality (n = 95) with respect to these two groups on the basis of 30-day survival from the first positive blood culture (Fig. 2). Comparison of these by the use of Cox regression analysis adjusted for age showed a trend for higher mortality with C. albicans than with C. glabrata (p 0.260) (Fig. 2a), and, after adjustment for catheter line removal from patients (n = 42), showed a significant difference between these curves (Fig. 2b; p 0.048, HR 3.4, 95% CI 0.99–11.47). Next, we investigated whether the levels of biofilm formation by C. albicans showed an association with mortality, by specifically evaluating isolates defined as LBFs (n = 17) and HBFs (n = 17). The Cox regression plots adjusted for age showed a trend for a higher mortality rate with HBFs than with LBFs (p 0.192) (Fig. 3a). A previous study has shown that administration of parenteral nutrition induces C. albicans germination and biofilm formation [14]. Therefore, we performed analysis with adjustments for administration of parenteral nutrition, and these revealed a significant difference in survival between the LBF and HBF groups (Fig. 3b; p 0.024, HR 5.99, 95% CI 1.3–28.3).
Fig. 2.
Survival of patients with Candida albicans and Candida glabrata infection. Survival of patients infected with C. albicans (solid line) and C. glabrata (dotted line) was monitored over a period of 30 days from the first Candida-positive blood culture. Cox regression plots, adjusted only for patient age (n = 95) (a) or for age and catheter removal (n = 42) (b), in patients with C. albicans and C. glabrata infection are shown. Comparison between these curves showed a statistically significant difference in the mortality rate in (b) (p <0.05).
Fig. 3.
Survival of patients with Candida albicans high biofilm formers (HBFs) and low biofilm formers (LBFs). Survival of patients infected with C. albicans HBFs (n = 17) and LBFs (n = 17) was monitored over a period of 30 days from the first Candida-positive blood culture. Cox regression plots adjusted for (a) age only (n = 34) or (b) age and parenteral nutrition (n = 28) in patients with C. albicans HBFs (solid line) and LBFs (dotted line) are shown. Comparison between these curves showed a statistically significant difference in the mortality rate in (b) (p <0.05).
Biofilm sensitivity to antifungals
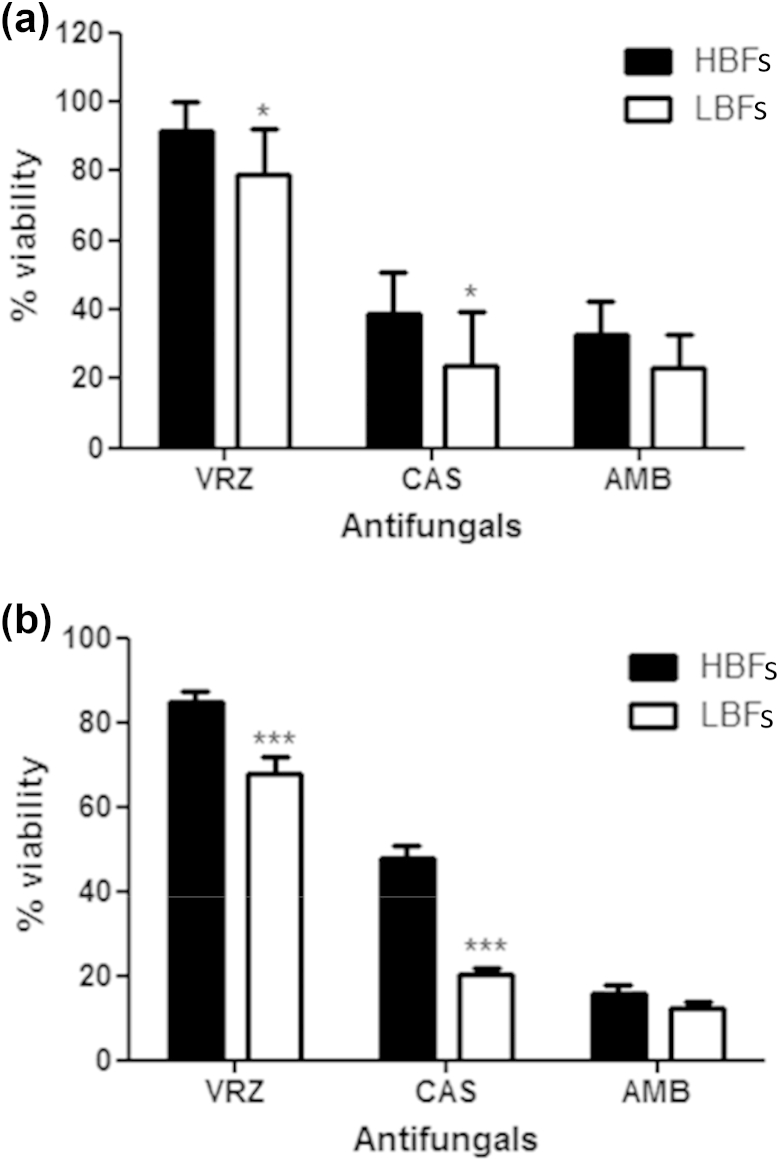
On the basis of these data, and given the positive correlations between biofilm formation and mortality, we tested C. albicans LBFs (n = 10) and HBFs (n = 10) for their response to azoles (voriconazole), polyenes (amphotericin B) and echinocandins (caspofungin) at low (2 mg/L) and high (200 mg/L) concentrations. Although both 2 mg/L and 200 mg/L voriconazole were equally ineffective against mature HBF and LBF biofilms (Fig. 4a,b), a significant difference in overall activity was observed between HBFs and LBFs at both 2 mg/L (p <0.05) and 200 mg/L (p <0.001). Conversely, caspofungin and amphotericin B were effective against both HBFs and LBFs, although the levels of biofilm formation significantly impacted on caspofungin sensitivity (2 mg/L, p <0.05; 200 mg/L, p <0.001). Furthermore, a paradoxical effect was found for caspofungin with HBFs, whereby 200 mg/L was significantly less effective than 2 mg/L (p <0.05). Conversely, no such effect was found in LBFs. Amphotericin B was shown to be equally effective against LBFs and HBFs, although a significant difference was found between 2 mg/L and 200 mg/L (HBFs, p <0.0005; LBFs, p <0.005).
Fig. 4.
Impact of Candida albicans biofilm formation on antifungal susceptibility. Ten low biofilm formers (LBFs) and high biofilm formers (HBFs) were standardized to 1 × 106 cells/mL in RPMI-1640, and grown as biofilms in flat-bottomed 96-well microtitre plates for 24 h. Biofilms were washed with phosphate-buffered saline before being treated with 2 mg/L (a) or 200 mg/L (b) voriconazole (VRZ), caspofungin (CAS), and amphotericin B (AMB). After incubation for 24 h, metabolic activity was measured with the XTT assay, with optical density being read at 492 nm. Percentage viability was calculated relative to untreated controls, and data are presented as mean ± standard deviation. Eight replicates were used for each isolate, and repeated on two separate occasions. *p <0.05, ***p <0.001.
Discussion
A retrospective analysis of patients with Candida BSI in Scotland was performed to determine the risk factors associated with mortality in the defined patient cohort. We report an adverse influence of biofilm formation by Candida species on the clinical outcomes of patients with C. albicans BSI.
The incidence of Candida BSI from this study in 2012–2013 (4.1 cases per 100 000 population per year) is comparable to that in 2005–2006 (4.8 cases per 100 000 population per year) [15]. The difference could be explained by an increasing population and by inaccurate reporting of the cases, and is therefore likely to represent a minimum estimate of the incidence of Candida BSI in Scotland. Our previous study reported that C. albicans was most prevalent within the population (50%), followed by C. glabrata (21%) [15]. The data from this study demonstrate a changing epidemiology, with a notable decrease in the incidence of C. albicans (41%) and a concurrent increase in the incidence of C. glabrata (35%). The reason for this is uncertain, but the overuse of azoles may have inadvertently selected C. glabrata, which has reduced sensitivity to fluconazole. The use of matrix-assisted laser desorption ionization time-of-flight mass spectrometry in addition to standard laboratory identification may have improved the isolation rate of this organism [16], [17], [18].
Among the patients with a BSI, we found a mortality rate of 41%, for which a number of risk factors were identified. Risk factors such as neutropenia, glucocorticosteroids, parenteral nutrition and CVC use were reported to be associated with Candida BSI [19]. In addition to Candida infection, the patient age covariate had a significant influence on patient mortality. It is of significance, however, that our data show that removal of catheter lines after diagnosis of candidaemia significantly improves the clinical outcome (p 0.032). These data support the current guidelines for the management of catheter-associated infection and their clinical management; that is, where possible, the catheter should be removed in non-neutropenic patients [7], [20], [21]. In a prospective randomized trial, it was shown that the removal of a catheter within the first 24 h of candidaemia resulted in a shorter duration of candidaemia [22]. Furthermore, a recent meta-analysis reported that removal of the CVC is associated with decreased mortality [6]. Biofilms are relatively refractory to antifungal therapy [3], [11]; therefore, unsurprisingly, inadequate antifungal therapy (OR 2.35, p 0.03) and biofilm formation (OR 2.33, p 0.007) were reported to be independent predictors of mortality in candidaemia patients [8]. Biofilm formation by clinical isolates of different Candida species was found to be highly variable, particularly between C. albicans and C. glabrata. Survival analysis was carried out to investigate the impact of their biofilm formation on clinical outcome, with Cox regression showing a trend for C. albicans to be more associated with mortality (p 0.026). After adjustment for the removal of lines, a significant difference in mortality was observed (p <0.05). These data are different from those previously reported, where no significant differences in survival between patients with C. glabrata and with C. albicans BSI was observed [23]. In addition, Cox regression analysis with the C. albicans HBF (41.2% mortality) and LBF (35.2% mortality) groups adjusted for patient age showed a trend for a lower survival rate with HBFs than with LBFs, which is in accordance with a previous study showing 51.2% mortality in the biofilm-forming group, as compared with 31.7% in the non-biofilm-forming group (p 0.004) [9]. Parenteral nutrition, including lipid emulsion, has been shown to induce C. albicans germination and increase biofilm formation in indwelling catheters [14]. Therefore, Cox regression analysis with adjustment for parenteral nutrition was performed, and revealed a significant impact on biofilm-related mortality. However, a caveat to the interpretation of these data is the low sample numbers, owing to the availability of complete datasets.
Given that biofilm formation by clinical isolates is heterogeneous and has an impact on patient mortality, we tested their antifungal sensitivity to determine whether it was affected. Biofilms were treated with antifungals at a clinically relevant concentration of 2 mg/L and another concentration that is potentially useful in antifungal lock therapy (200 mg/L) [24]. Our data illustrate a significant difference between HBFs and LBFs in sensitivity to voriconazole and caspofungin. However, amphotericin B is equally effective against both groups. Whereas the clinical data show that azoles were used extensively to treat 82.2% of patients in this study, our data show that this class of antifungal is less effective against matured biofilms. When echinocandins were compared with polyenes, the removal of the catheter showed no improved time to mycological eradication, possibly because of the effectiveness of both antifungal agents against biofilms [25]. Furthermore, with HBFs, we found a paradoxical effect of lower percentage kill with a higher concentration of caspofungin (200 mg/mL) than with 2 mg/L, in vitro (Fig. 4b). The exact mechanism causing this paradoxical effect with HBFs and its clinical relevance are unknown.
In summary, C. albicans remains a predominant Candida species associated with high mortality in candidaemia patients, and, within this species, biofilm heterogeneity has a direct impact on patient survival, and potentially on antifungal sensitivities, based on in vitro studies. These findings highlight the importance of biofilm stratification in the clinical management of candidaemia cases, to determine whether they should be managed with azoles or other fungicidal classes of antifungal agent. Moreover, understanding the genetic basis of these isolates will enable us to devise and develop a biomarker for biofilm-related Candida BSI, and to create more appropriate antifungal therapies, which, collectively, will facilitate improved clinical outcomes.
Transparency declaration
G. Ramage, B.J. Jones and C. Williams has received research grants and acted as a speaker for Gilead, MSD and Astellas.
Acknowledgements
This work was supported by the Wellcome Trust Strategic Award for Medical Mycology and Fungal Immunology 097377/Z/11/Z. Data collection was supported by a grant from Pfizer. G. Ramage was also supported by a research fellowship grant from Gilead Sciences. We are grateful to microbiology colleagues throughout Scotland for submitting isolates.
Editor: E. Roilides
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.cmi.2015.09.018.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Mensa J., Pitart C., Marco F. Treatment of critically ill patients with candidemia. Int J Antimicrob Agents. 2008;32(Suppl. 2):S93–S97. doi: 10.1016/S0924-8579(08)70007-4. [DOI] [PubMed] [Google Scholar]
- 2.Wisplinghoff H., Bischoff T., Tallent S.M., Seifert H., Wenzel R.P., Edmond M.B. Nosocomial bloodstream infections in US hospitals: Analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 3.Kojic E.M., Darouiche R.O. Candida infections of medical devices. Clin Microbiol Rev. 2004;17:255–267. doi: 10.1128/CMR.17.2.255-267.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lynch A.S., Robertson G.T. Bacterial and fungal biofilm infections. Annu Rev Med. 2008;59:415–428. doi: 10.1146/annurev.med.59.110106.132000. [DOI] [PubMed] [Google Scholar]
- 5.Sardi J.C., Scorzoni L., Bernardi T., Fusco-Almeida A.M., Mendes Giannini M.J. Candida species: Current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J Med Microbiol. 2013;62(Pt 1):10–24. doi: 10.1099/jmm.0.045054-0. [DOI] [PubMed] [Google Scholar]
- 6.Andes D.R., Safdar N., Baddley J.W., Playford G., Reboli A.C., Rex J.H. Impact of treatment strategy on outcomes in patients with candidemia and other forms of invasive candidiasis: A patient-level quantitative review of randomized trials. Clin Infect Dis. 2012;54:1110–1122. doi: 10.1093/cid/cis021. [DOI] [PubMed] [Google Scholar]
- 7.Cornely O.A., Bassetti M., Calandra T., Garbino J., Kullberg B.J., Lortholary O. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: Non-neutropenic adult patients. Clin Microbiol Infect. 2012;18(Suppl. 7):19–37. doi: 10.1111/1469-0691.12039. [DOI] [PubMed] [Google Scholar]
- 8.Tumbarello M., Posteraro B., Trecarichi E.M., Fiori B., Rossi M., Porta R. Biofilm production by Candida species and inadequate antifungal therapy as predictors of mortality for patients with candidemia. J Clin Microbiol. 2007;45:1843–1850. doi: 10.1128/JCM.00131-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tumbarello M., Fiori B., Trecarichi E.M., Posteraro P., Losito A.R., De Luca A. Risk factors and outcomes of candidemia caused by biofilm-forming isolates in a tertiary care hospital. PLoS One. 2012;7:e33705. doi: 10.1371/journal.pone.0033705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sherry L., Rajendran R., Lappin D.F., Borghi E., Perdoni F., Falleni M. Biofilms formed by Candida albicans bloodstream isolates display phenotypic and transcriptional heterogeneity that are associated with resistance and pathogenicity. BMC Microbiol. 2014;14:182. doi: 10.1186/1471-2180-14-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramage G., Vande Walle K., Wickes B.L., Lopez-Ribot J.L. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob Agents Chemother. 2001;45:2475–2479. doi: 10.1128/AAC.45.9.2475-2479.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Honraet K., Goetghebeur E., Nelis H.J. Comparison of three assays for the quantification of Candida biomass in suspension and CDC reactor grown biofilms. J Microbiol Methods. 2005;63:287–295. doi: 10.1016/j.mimet.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 13.Pierce C.G., Uppuluri P., Tristan A.R., Wormley F.L., Jr., Mowat E., Ramage G. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat Protoc. 2008;3:1494–1500. doi: 10.1038/nport.2008.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swindell K., Lattif A.A., Chandra J., Mukherjee P.K., Ghannoum M.A. Parenteral lipid emulsion induces germination of Candida albicans and increases biofilm formation on medical catheter surfaces. J Infect Dis. 2009;200:473–480. doi: 10.1086/600106. [DOI] [PubMed] [Google Scholar]
- 15.Odds F.C., Hanson M.F., Davidson A.D. One year prospective survey of Candida bloodstream infections in Scotland. J Med Microbiol. 2007;56(Pt 8):1066–1075. doi: 10.1099/jmm.0.47239-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Das I., Nightingale P., Patel M., Jumaa P. Epidemiology, clinical characteristics, and outcome of candidemia: Experience in a tertiary referral center in the UK. Int J Infect Dis. 2011;15:e759–e763. doi: 10.1016/j.ijid.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 17.Parmeland L., Gazon M., Guerin C., Argaud L., Lehot J.J., Bastien O. Candida albicans and non-Candida albicans fungemia in an institutional hospital during a decade. Med Mycol. 2013;51:33–37. doi: 10.3109/13693786.2012.686673. [DOI] [PubMed] [Google Scholar]
- 18.Arendrup M.C., Dzajic E., Jensen R.H., Johansen H.K., Kjaeldgaard P., Knudsen J.D. Epidemiological changes with potential implication for antifungal prescription recommendations for fungaemia: Data from a nationwide fungaemia surveillance programme. Clin Microbiol Infect. 2013;19:E343–E353. doi: 10.1111/1469-0691.12212. [DOI] [PubMed] [Google Scholar]
- 19.Dimopoulos G., Ntziora F., Rachiotis G., Armaganidis A., Falagas M.E. Candida albicans versus non-albicans intensive care unit-acquired bloodstream infections: Differences in risk factors and outcome. Anesth Analg. 2008;106:523–529. doi: 10.1213/ane.0b013e3181607262. table of contents. [DOI] [PubMed] [Google Scholar]
- 20.Mermel L.A., Allon M., Bouza E., Craven D.E., Flynn P., O'Grady N.P. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49:1–45. doi: 10.1086/599376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koehler P., Tacke D., Cornely O.A. Our 2014 approach to candidaemia. Mycoses. 2014;57:581–583. doi: 10.1111/myc.12207. [DOI] [PubMed] [Google Scholar]
- 22.Rex J.H., Bennett J.E., Sugar A.M., Pappas P.G., Serody J., Edwards J.E. Intravascular catheter exchange and duration of candidemia. NIAID Mycoses Study Group and the Candidemia Study Group. Clin Infect Dis. 1995;21:994–996. doi: 10.1093/clinids/21.4.994. [DOI] [PubMed] [Google Scholar]
- 23.Ruan S.Y., Huang Y.T., Chu C.C., Yu C.J., Hsueh P.R. Candida glabrata fungaemia in a tertiary centre in Taiwan: Antifungal susceptibility and outcomes. Int J Antimicrob Agents. 2009;34:236–239. doi: 10.1016/j.ijantimicag.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 24.Walraven C.J., Lee S.A. Antifungal lock therapy. Antimicrob Agents Chemother. 2013;57:1–8. doi: 10.1128/AAC.01351-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nucci M., Anaissie E., Betts R.F., Dupont B.F., Wu C., Buell D.N. Early removal of central venous catheter in patients with candidemia does not improve outcome: Analysis of 842 patients from 2 randomized clinical trials. Clin Infect Dis. 2010;51:295–303. doi: 10.1086/653935. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.