Abstract
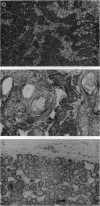
ApcMin (Min, multiple intestinal neoplasia) is a point mutation in the murine homolog of the APC gene. Min/+ mice develop multiple intestinal adenomas, as do humans carrying germ-line mutations in APC. Female mice carrying Min are also prone to develop mammary tumors. Min/+ mammary glands are more sensitive to chemical carcinogenesis than are +/+ mammary glands. Transplantation of mammary cells from Min/+ or +/+ donors into +/+ hosts demonstrates that the propensity to develop mammary tumors is intrinsic to the Min/+ mammary cells. Long-term grafts of Min/+ mammary glands also gave rise to focal alveolar hyperplasias, indicating that the presence of the Min mutation also has a role in the development of these lesions.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boman B. M., Levin B. Familial polyposis. Hosp Pract (Off Ed) 1986 May 15;21(5):155-65, 168, 170. [PubMed] [Google Scholar]
- Clarke A. R., Maandag E. R., van Roon M., van der Lugt N. M., van der Valk M., Hooper M. L., Berns A., te Riele H. Requirement for a functional Rb-1 gene in murine development. Nature. 1992 Sep 24;359(6393):328–330. doi: 10.1038/359328a0. [DOI] [PubMed] [Google Scholar]
- Donehower L. A., Harvey M., Slagle B. L., McArthur M. J., Montgomery C. A., Jr, Butel J. S., Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992 Mar 19;356(6366):215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- Ethier S. P., Ullrich R. L. Detection of ductal dysplasia in mammary outgrowths derived from carcinogen-treated virgin female BALB/c mice. Cancer Res. 1982 May;42(5):1753–1760. [PubMed] [Google Scholar]
- Groden J., Thliveris A., Samowitz W., Carlson M., Gelbert L., Albertsen H., Joslyn G., Stevens J., Spirio L., Robertson M. Identification and characterization of the familial adenomatous polyposis coli gene. Cell. 1991 Aug 9;66(3):589–600. doi: 10.1016/0092-8674(81)90021-0. [DOI] [PubMed] [Google Scholar]
- Hansen M. F., Cavenee W. K. Retinoblastoma and the progression of tumor genetics. Trends Genet. 1988 May;4(5):125–128. doi: 10.1016/0168-9525(88)90134-5. [DOI] [PubMed] [Google Scholar]
- Horii A., Nakatsuru S., Miyoshi Y., Ichii S., Nagase H., Ando H., Yanagisawa A., Tsuchiya E., Kato Y., Nakamura Y. Frequent somatic mutations of the APC gene in human pancreatic cancer. Cancer Res. 1992 Dec 1;52(23):6696–6698. [PubMed] [Google Scholar]
- Jacks T., Fazeli A., Schmitt E. M., Bronson R. T., Goodell M. A., Weinberg R. A. Effects of an Rb mutation in the mouse. Nature. 1992 Sep 24;359(6393):295–300. doi: 10.1038/359295a0. [DOI] [PubMed] [Google Scholar]
- Joslyn G., Carlson M., Thliveris A., Albertsen H., Gelbert L., Samowitz W., Groden J., Stevens J., Spirio L., Robertson M. Identification of deletion mutations and three new genes at the familial polyposis locus. Cell. 1991 Aug 9;66(3):601–613. doi: 10.1016/0092-8674(81)90022-2. [DOI] [PubMed] [Google Scholar]
- Kinzler K. W., Nilbert M. C., Su L. K., Vogelstein B., Bryan T. M., Levy D. B., Smith K. J., Preisinger A. C., Hedge P., McKechnie D. Identification of FAP locus genes from chromosome 5q21. Science. 1991 Aug 9;253(5020):661–665. doi: 10.1126/science.1651562. [DOI] [PubMed] [Google Scholar]
- Lee E. Y., Chang C. Y., Hu N., Wang Y. C., Lai C. C., Herrup K., Lee W. H., Bradley A. Mice deficient for Rb are nonviable and show defects in neurogenesis and haematopoiesis. Nature. 1992 Sep 24;359(6393):288–294. doi: 10.1038/359288a0. [DOI] [PubMed] [Google Scholar]
- Malkin D., Li F. P., Strong L. C., Fraumeni J. F., Jr, Nelson C. E., Kim D. H., Kassel J., Gryka M. A., Bischoff F. Z., Tainsky M. A. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science. 1990 Nov 30;250(4985):1233–1238. doi: 10.1126/science.1978757. [DOI] [PubMed] [Google Scholar]
- Medina D., Butel J. S., Socher S. H., Miller F. L. Mammary tumorigenesis in 7,12-dimethybenzanthracene-treated C57BL x DBA/2f F1 mice. Cancer Res. 1980 Feb;40(2):368–373. [PubMed] [Google Scholar]
- Medina D. Mammary tumorigenesis in chemical carcinogen-treated mice. I. Incidence in BALB-c and C57BL mice. J Natl Cancer Inst. 1974 Jul;53(1):213–221. doi: 10.1093/jnci/53.1.213. [DOI] [PubMed] [Google Scholar]
- Moser A. R., Dove W. F., Roth K. A., Gordon J. I. The Min (multiple intestinal neoplasia) mutation: its effect on gut epithelial cell differentiation and interaction with a modifier system. J Cell Biol. 1992 Mar;116(6):1517–1526. doi: 10.1083/jcb.116.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser A. R., Pitot H. C., Dove W. F. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science. 1990 Jan 19;247(4940):322–324. doi: 10.1126/science.2296722. [DOI] [PubMed] [Google Scholar]
- Nishisho I., Nakamura Y., Miyoshi Y., Miki Y., Ando H., Horii A., Koyama K., Utsunomiya J., Baba S., Hedge P. Mutations of chromosome 5q21 genes in FAP and colorectal cancer patients. Science. 1991 Aug 9;253(5020):665–669. doi: 10.1126/science.1651563. [DOI] [PubMed] [Google Scholar]
- Oomen L. C., van der Valk M. A., Hart A. A., Demant P., Emmelot P. Influence of mouse major histocompatibility complex (H-2) on N-ethyl-N-nitrosourea-induced tumor formation in various organs. Cancer Res. 1988 Dec 1;48(23):6634–6641. [PubMed] [Google Scholar]
- Russell L. B., Montgomery C. S. Supermutagenicity of ethylnitrosourea in the mouse spot test: comparisons with methylnitrosourea and ethylnitrosourethane. Mutat Res. 1982 Feb 22;92(1-2):193–204. doi: 10.1016/0027-5107(82)90223-8. [DOI] [PubMed] [Google Scholar]
- Shedlovsky A., Guenet J. L., Johnson L. L., Dove W. F. Induction of recessive lethal mutations in the T/t-H-2 region of the mouse genome by a point mutagen. Genet Res. 1986 Apr;47(2):135–142. doi: 10.1017/s0016672300022977. [DOI] [PubMed] [Google Scholar]
- Staats J. Standardized nomenclature for inbred strains of mice: sixth listing. Cancer Res. 1976 Dec;36(12):4333–4377. [PubMed] [Google Scholar]
- Su L. K., Kinzler K. W., Vogelstein B., Preisinger A. C., Moser A. R., Luongo C., Gould K. A., Dove W. F. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science. 1992 May 1;256(5057):668–670. doi: 10.1126/science.1350108. [DOI] [PubMed] [Google Scholar]
- Wallace M. R., Marchuk D. A., Andersen L. B., Letcher R., Odeh H. M., Saulino A. M., Fountain J. W., Brereton A., Nicholson J., Mitchell A. L. Type 1 neurofibromatosis gene: identification of a large transcript disrupted in three NF1 patients. Science. 1990 Jul 13;249(4965):181–186. doi: 10.1126/science.2134734. [DOI] [PubMed] [Google Scholar]
- Zhang R., Haag J. D., Gould M. N. Quantitating the frequency of initiation and cH-ras mutation in in situ N-methyl-N-nitrosourea-exposed rat mammary gland. Cell Growth Differ. 1991 Jan;2(1):1–6. [PubMed] [Google Scholar]