Abstract
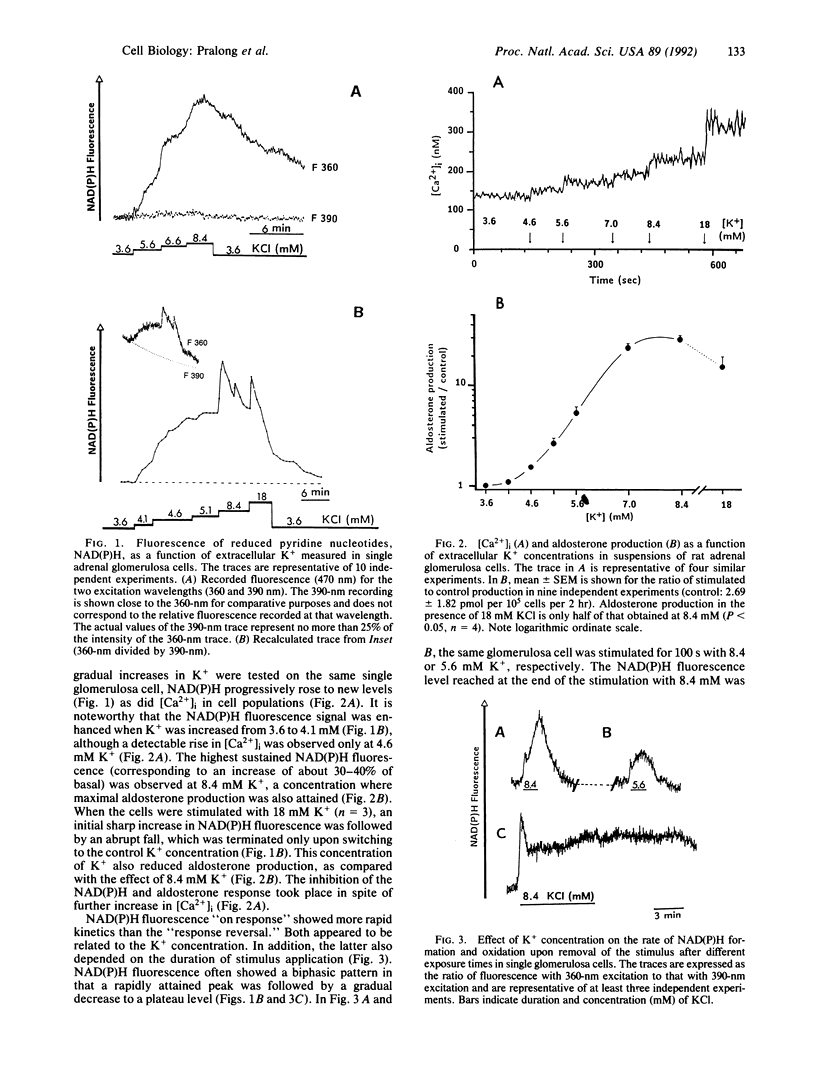
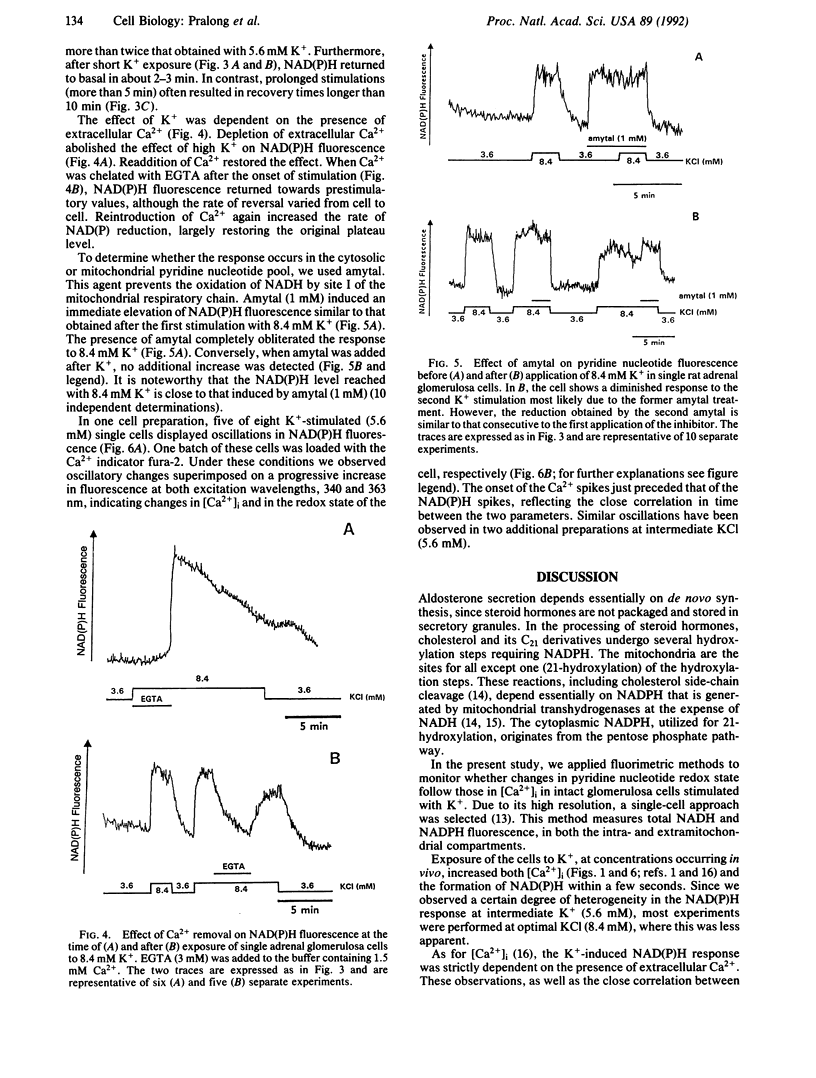
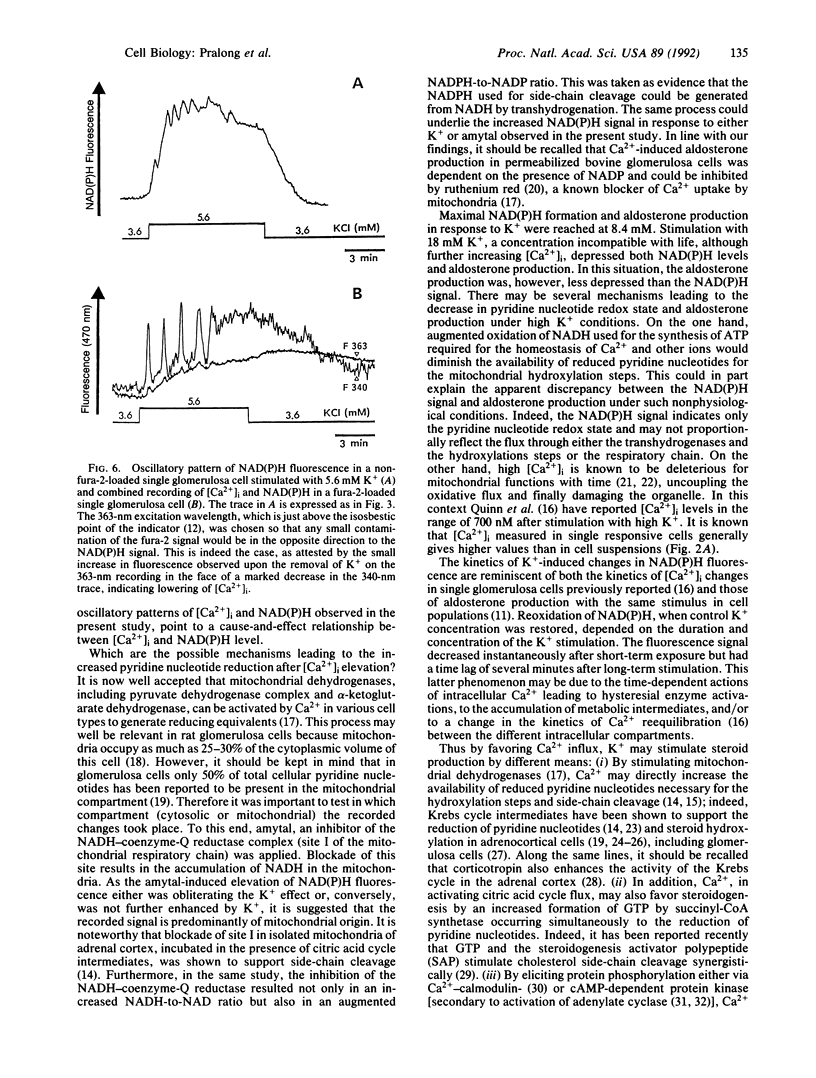
Extracellular potassium ions (K+) raise the intracellular concentration of free Ca2+ ([Ca2+]i) by gating voltage-dependent Ca2+ channels and stimulate aldosterone production in adrenal glomerulosa cells. The pathway leading from calcium influx to increased steroid synthesis has not been completely elucidated. In the present study we demonstrate that the reduction of pyridine nucleotides known to be required for steroid hydroxylation is enhanced by K+ (4.1-8.4 mM) in single rat glomerulosa cells. The action of K+ was strictly dependent on the presence of extracellular Ca2+. Amytal, a blocker of site I of the mitochondrial respiratory chain, abolished the K+ effect, indicating a mitochondrial origin for the recorded changes. Supraphysiological K+ concentration (18 mM) resulted in a further increase in [Ca2+]i, while steroidogenesis was decreased as measured in cell suspensions. However, a possible explanation for this dichotomy is provided by the finding that the level of reduced pyridine nucleotides also decreased at supraphysiological K+ concentration.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albano J. D., Brown B. L., Ekins R. P., Tait S. A., Tait J. F. The effects of potassium, 5-hydrocytryptamine, adrenocorticotrophin and angiotensin II on the concentration of adenosine 3':5'-cyclic monophosphate in suspensions of dispersed rat adrenal zona glomerulosa and zona fasciculata cells. Biochem J. 1974 Aug;142(2):391–400. doi: 10.1042/bj1420391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balla T., Hunyady L., Spät A. Possible role of calcium uptake and calmodulin in adrenal glomerulosa cells: effects of verapamil and trifluoperazine. Biochem Pharmacol. 1982 Apr 1;31(7):1267–1271. doi: 10.1016/0006-2952(82)90014-4. [DOI] [PubMed] [Google Scholar]
- Balla T., Várnai P., Holló Z., Spät A. Effects of high potassium concentration and dihydropyridine Ca2(+)-channel agonists on cytoplasmic Ca2+ and aldosterone production in rat adrenal glomerulosa cells. Endocrinology. 1990 Aug;127(2):815–822. doi: 10.1210/endo-127-2-815. [DOI] [PubMed] [Google Scholar]
- Boyd J. E., Palmore W. P., Mulrow P. J. Role of potassium in the control of aldosterone secretion in the rat. Endocrinology. 1971 Mar;88(3):556–565. doi: 10.1210/endo-88-3-556. [DOI] [PubMed] [Google Scholar]
- Braley L. M., Williams G. H. Rat adrenal cell sensitivity to angiotensin II, alpha-1-24-ACTH, and potassium: a comparative study. Am J Physiol. 1977 Nov;233(5):E402–E406. doi: 10.1152/ajpendo.1977.233.5.E402. [DOI] [PubMed] [Google Scholar]
- Capponi A. M., Rossier M. F., Davies E., Vallotton M. B. Calcium stimulates steroidogenesis in permeabilized bovine adrenal cortical cells. J Biol Chem. 1988 Nov 5;263(31):16113–16117. [PubMed] [Google Scholar]
- Cohen C. J., McCarthy R. T., Barrett P. Q., Rasmussen H. Ca channels in adrenal glomerulosa cells: K+ and angiotensin II increase T-type Ca channel current. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2412–2416. doi: 10.1073/pnas.85.7.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hall P. F. A possible role for transhydrogenation in side-chain cleavage of cholesterol. Biochemistry. 1972 Jul 18;11(15):2891–2897. doi: 10.1021/bi00765a023. [DOI] [PubMed] [Google Scholar]
- Haning R., Tait S. A., Tait J. F. In vitro effects of ACTH, angiotensins, serotonin and potassium on steroid output and conversion of corticosterone to aldosterone by isolated adrenal cells. Endocrinology. 1970 Dec;87(6):1147–1167. doi: 10.1210/endo-87-6-1147. [DOI] [PubMed] [Google Scholar]
- Hoek J. B., Rydström J. Physiological roles of nicotinamide nucleotide transhydrogenase. Biochem J. 1988 Aug 15;254(1):1–10. doi: 10.1042/bj2540001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima I., Kojima K., Rasmussen H. Intracellular calcium and adenosine 3',5'-cyclic monophosphate as mediators of potassium-induced aldosterone secretion. Biochem J. 1985 May 15;228(1):69–76. doi: 10.1042/bj2280069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laury L. W., McCarthy J. L. In vitro adrenal mitochondrial 11 beta-hydroxylation following in vivo adrenal stimualtion or inhibition: enhanced substrate utilization. Endocrinology. 1970 Dec;87(6):1380–1385. doi: 10.1210/endo-87-6-1380. [DOI] [PubMed] [Google Scholar]
- Lin M. T., Haksar A., Peron F. G. The role of the Krebs cycle in the generation of intramitochondrial reducing equivalents for the 11 beta-hydroxylation of deoxycorticosterone in isolated rat adrenal cells. Arch Biochem Biophys. 1974 Oct;164(2):429–439. doi: 10.1016/0003-9861(74)90052-6. [DOI] [PubMed] [Google Scholar]
- Lin M., Haksar A., Peron F. G. A possible mechanism for the stimulation of Krebs cycle activity by ACTH in isolated rat adrenal cells. Biochem Biophys Res Commun. 1974 Jun 18;58(4):983–989. doi: 10.1016/s0006-291x(74)80240-8. [DOI] [PubMed] [Google Scholar]
- Matsunaga H., Maruyama Y., Kojima I., Hoshi T. Transient Ca2+-channel current characterized by a low-threshold voltage in zona glomerulosa cells of rat adrenal cortex. Pflugers Arch. 1987 Apr;408(4):351–355. doi: 10.1007/BF00581128. [DOI] [PubMed] [Google Scholar]
- McCormack J. G. Characterization of the effects of Ca2+ on the intramitochondrial Ca2+-sensitive enzymes from rat liver and within intact rat liver mitochondria. Biochem J. 1985 Nov 1;231(3):581–595. doi: 10.1042/bj2310581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack J. G., Halestrap A. P., Denton R. M. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol Rev. 1990 Apr;70(2):391–425. doi: 10.1152/physrev.1990.70.2.391. [DOI] [PubMed] [Google Scholar]
- Mertz L. M., Pedersen R. C. The kinetics of steroidogenesis activator polypeptide in the rat adrenal cortex. Effects of adrenocorticotropin, cyclic adenosine 3':5'-monophosphate, cycloheximide, and circadian rhythm. J Biol Chem. 1989 Sep 15;264(26):15274–15279. [PubMed] [Google Scholar]
- Müller J. Aldosterone stimulation in vitro. II. Stimulation of aldosterone production by monovalent cations. Acta Endocrinol (Copenh) 1965 Oct;50(2):301–309. [PubMed] [Google Scholar]
- Orrenius S., McConkey D. J., Bellomo G., Nicotera P. Role of Ca2+ in toxic cell killing. Trends Pharmacol Sci. 1989 Jul;10(7):281–285. doi: 10.1016/0165-6147(89)90029-1. [DOI] [PubMed] [Google Scholar]
- Payet M. D., Benabderrazik M., Gallo-Payet N. Excitation-secretion coupling: ionic currents in glomerulosa cells: effects of adrenocorticotropin and K+ channel blockers. Endocrinology. 1987 Sep;121(3):875–882. doi: 10.1210/endo-121-3-875. [DOI] [PubMed] [Google Scholar]
- Pralong W. F., Bartley C., Wollheim C. B. Single islet beta-cell stimulation by nutrients: relationship between pyridine nucleotides, cytosolic Ca2+ and secretion. EMBO J. 1990 Jan;9(1):53–60. doi: 10.1002/j.1460-2075.1990.tb08079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn S. J., Williams G. H., Tillotson D. L. Calcium response of single adrenal glomerulosa cells to external potassium. Am J Physiol. 1988 Oct;255(4 Pt 1):E488–E495. doi: 10.1152/ajpendo.1988.255.4.E488. [DOI] [PubMed] [Google Scholar]
- Simpson E. R., Boyd G. S. The metabolism of pyruvate by bovine-adrenal-cortex mitochondria. Eur J Biochem. 1971 Oct 26;22(4):489–499. doi: 10.1111/j.1432-1033.1971.tb01568.x. [DOI] [PubMed] [Google Scholar]
- Spät A., Balla I., Balla T., Cragoe E. J., Jr, Hajnóczky G., Hunyady L. Angiotensin II and potassium activate different calcium entry mechanisms in rat adrenal glomerulosa cells. J Endocrinol. 1989 Jul;122(1):361–370. doi: 10.1677/joe.0.1220361. [DOI] [PubMed] [Google Scholar]
- Spät A., Nagy K., Tarján E. Hyperaldosteronism in the sodium-depleted rat: mechanism of aldosterone stimulation by peritoneal dialysis with glucose solution. J Endocrinol. 1979 Jul;82(1):17–25. doi: 10.1677/joe.0.0820017. [DOI] [PubMed] [Google Scholar]
- Xu T. S., Bowman E. P., Glass D. B., Lambeth J. D. Stimulation of adrenal mitochondrial cholesterol side-chain cleavage by GTP, steroidogenesis activator polypeptide (SAP), and sterol carrier protein2. GTP and SAP act synergistically. J Biol Chem. 1991 Apr 15;266(11):6801–6807. [PubMed] [Google Scholar]


