Abstract
Rationale
Manipulations of the endocannabinoid system could potentially produce therapeutic effects with minimal risk of adverse cannabis-like side effects. Inhibitors of fatty acid amide hydrolase (FAAH) increase endogenous levels of the cannabinoid-receptor agonist, anandamide, and show promise for treating a wide range of disorders. However, their effects on learning and memory have not been fully characterized.
Objectives
We determined the effects of five structurally different FAAH inhibitors in an animal model of working memory known to be sensitive to impairment by delta-9 tetrahydrocannabinol (THC).
Methods
A delayed nonmatching-to-position procedure was used in rats. Illuminated nosepoke holes were used to provide sample cues (left versus right) and record responses (correct versus incorrect) after delays ranging from 0-28 seconds. Various test drugs were given acutely up to two times per week before daily sessions.
Results
One FAAH inhibitor, AM3506 (3 mg/kg), decreased accuracy in the memory task. Four other FAAH inhibitors (URB597, URB694, PF-04457845, and ARN14633) and a monoacylglycerol lipase inhibitor (JZL184, which blocks the degradation of the endocannabinoid 2-arachidonoylglycerol) had no effect. Testing of AM3506 in combination with antagonists for receptors known to be affected by anandamide and other fatty-acid amides indicated that the impairment induced by AM3506 was mediated by cannabinoid CB1 receptors, and not by alpha-type peroxisome proliferator-activated receptors (PPAR-alpha) or vanilloid transient receptor potential cation channels (TRPV1).
Conclusions
FAAH inhibitors differ with respect to their potential for memory impairment, abuse liability, and probably other cannabis-like effects, and they should be evaluated individually for specific therapeutic and adverse effects.
Keywords: delayed spatial matching, working memory, endocannabinoids, FAAH inhibition, monoacylglycerol lipase inhibition
Introduction
Cannabis and synthetic cannabinoid agonists can produce certain therapeutic effects, but they can also produce adverse side effects including dependence and memory impairment. They produce these effects by activating cannabinoid CB1 receptors, mimicking the effects of endogenous cannabinoid substances (endocannabinoids). The two main endocannabinoids, anandamide and 2-arachidonoylglycerol (2-AG), are produced on demand and are rapidly degraded by fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MGL), respectively. Since CB1 receptors have two separate endogenous ligands, it is likely that the brain circuits involving anandamide and 2-AG underlie distinct sets of neurobehavioral processes that can be selectively _targeted for therapeutic purposes. This can be accomplished by administering inhibitors of FAAH or MGL, thereby increasing the effects of anandamide or 2-AG when and where they are released. This amplification of natural endocannabinoid signaling could potentially produce beneficial effects without the adverse side effects associated with exogenous cannabinoid agonists, which directly activate CB1 receptors throughout the brain (see reviews by Blankman and Cravatt 2013; Clapper et al. 2009a; Hwang et al. 2010; Panlilio et al. 2013; Pertwee 2014; Schlosburg et al. 2009; Zanettini et al. 2011).
The FAAH inhibitor that has been studied most intensively is URB597 (Piomelli et al. 2006). In preclinical testing, URB597 does not produce classical THC-like effects such as catalepsy, hypothermia, and hyperphagia (Kathuria et al. 2003). URB597 also shows no signs of abuse potential in animal models of cannabis abuse; it does not have THC-like in rats trained to detect the interoceptive effects of THC (Gobbi et al. 2005), and it is not self-administered by squirrel monkeys that have extensive experience self-administering anandamide and other cannabinoid agonists (Justinova et al. 2008). However, other FAAH inhibitors, including URB694 (Justinova et al. 2015), PF-04457845 (Justinova et al. 2014) and AM3506 (Bergman et al. 2011), have shown moderate to strong reinforcing effects when offered as an intravenous solution to squirrel monkeys. These findings indicate that FAAH inhibitors can vary considerably in their effect profiles and should be evaluated individually for specific therapeutic and adverse effects.
Delta-9-tetrahydracannabinol (THC) impairs learning and memory in humans (Ranganathan and D'Souza 2006) and animals (Zanettini et al. 2011), with working memory being particularly sensitive. In rodents, memory has also been shown to be impaired by administration of exogenous anandamide, but only when its degradation by FAAH is prevented (Goonawardena et al. 2011; Lichtman et al. 1995; Mallet and Beninger 1996; 1998; Varvel et al. 2006). Surprisingly, inhibition or genetic deletion of FAAH, which substantially increases endogenous levels of anandamide, has been found to enhance rather than impair memory in rodents trained with procedures involving aversively-motivated behavior (i.e., water maze: Varvel et al., 2006, 2007; or passive-avoidance of a context associated with footshock: Hasanein and Teimuri Far 2015; Mazzola et al. 2009; Morena et al. 2014). However, memory-related studies with appetitively-motivated procedures have mostly shown impairment rather than enhancement after treatment with a FAAH inhibitor (Basavarajappa et al. 2014; Busquets-Garcia et al. 2011; Goonawardena et al. 2011; Seillier et al. 2010; these studies all used URB597). There have been fewer studies involving MGL inhibition. The MGL inhibitor JZL184 did not affect memory in an object-recognition procedure (Busquets-Garcia et al. 2011), but JZL184 and a dual FAAH-MGL inhibitor (JZL195) both impaired memory in a repeated-acquisition water-maze procedure in mice (Wise et al. 2012).
In the present study, we focused on the effects of FAAH inhibitors on working memory in rats, using a food-based procedure known to be sensitive to impairment by THC (Justinova et al. 2013; Panlilio et al. 2012; Panlilio et al. 2011). We tested five different FAAH inhibitors (and one MGL inhibitor) at doses sufficient to substantially increase levels of anandamide (or 2-AG). We found that only one of these compounds, the FAAH inhibitor AM3506, impaired working memory at the doses tested. Since pharmacological doses of anandamide may activate alpha-type peroxisome proliferator-activated receptors (PPAR-alpha) and vanilloid transient receptor potential cation channels (TRPV1), and since FAAH inhibition increases endogenous levels of not only anandamide but also other fatty acid amides that are ligands for PPAR-alpha and TRPV1, we explored the mechanism of AM3506's effects by giving AM3506 in combination with a CB1 antagonist (rimonabant), a PPAR-alpha antagonist (MK886), or a TRPV1 antagonist (capsazepine). These tests indicated that the memory impairment induced by AM3506 was mediated by CB1 receptors.
Materials and methods
Subjects
Twelve experimentally naive male Sprague-Dawley rats were maintained in individual cages on a 12-hr light/dark cycle with lights on starting at 0645 hrs. Procedures were conducted Monday through Friday between 1000 and 1400 hrs. Rats were fed approximately 15 g of food per day to maintain stable body weights. The facilities were fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC), and all experiments were conducted in accordance with the guidelines of the Animal Care and Use Committee of the National Institute on Drug Abuse Intramural Research Program and the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Research Council 2011).
Apparatus
The apparatus has been described in detail previously (Panlilio et al. 2011). Briefly, test chambers (model MED-NPW-9L; MED Associates, St. Albans, VT) had 3 response holes in a horizontal array on one wall. The holes could be individually illuminated from within by LED to provide samples for the nonmatching task. Food pellets (45-mg; type F0021; Bio-Serv, Frenchtown, NJ, USA) were dispensed into a trough mounted on the opposite wall.
Drugs
The FAAH inhibitors URB597 (cyclohexyl carbamic acid 3′-carbamoyl-3-yl ester), URB694 (6-hydroxy-[1,1′-biphenyl]-3-yl-cyclohexylcarbamate), AM3506 (5-(4-hydroxyphenyl)pentanesulfonyl fluoride), PF-04457845 (N-pyridazin-3-yl-4-[(3-[5-(trifluoromethyl)pyridin-2-yl]oxyphenyl)methylidene]piperidine-1-carboxamide), and ARN14633 ([4-fluoro-3-[3-(methylcarbamoyl)phenyl]phenyl] N-cyclohexylcarbamate), and the monoacylglycerol lipase inhibitor JZL184 (4-[Bis(1,3-benzodioxol-5-yl)hydroxymethyl]-1-piperidinecarboxylic acid 4-nitrophenyl ester) were given 40 minutes before the session. URB597, URB694, and ARN14633 were synthesized at the Istituto Italiano di Tecnologia. AM3506 was synthesized at the Center for Drug Discovery, Northeastern University. JZL184 was synthesized at the Scripps Research Institute (La Jolla, CA). These FAAH inhibitors do not differ greatly in potency, and the available information indicates that each would be expected to produce substantial, selective effects on FAAH versus MGL within the range of 1-3 mg/kg (URB597: Fegley et al. 2005; URB694: Clapper et al. 2009b; AM3506: Godlewski et al. 2010; PF-04457845: Hicks et al. 2013). ARN14633 is a novel analog of URB597 with improved oral bioavailability. The MGL inhibitor JZL184 is less potent that the other drugs and was expected to produce selective effects on MGL versus FAAH at doses of 10 and 30 mg/kg (Seillier et al. 2014). The FAAH inhibitors were dissolved in vehicle containing 5% dimethylsulfoxide (DMSO), 5% Tween 80, and saline. JZL184 was dissolved in vehicle containing 6% ethanol, 6% Cremophor EL, and saline. During testing of drug combinations, the CB1-receptor antagonist rimonabant, the PPAR-alpha antagonist MK886, or the TRPV1 antagonist capsazepine were given 60 minutes before the session; the doses of these pretreatments were chosen based on previous studies in which they blocked the effects of endocannabinoid-related treatments (Mascia et al. 2011; Mazzola et al. 2009; Panlilio et al. 2009; Solinas et al. 2007). Rimonabant [SR141716; N-piperidino-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methylpyrazole-3-carboxamide)] (NIDA Drug Supply Program, Bethesda, MD, USA) was dissolved in 2% Tween 80, 2% ethanol, and saline. MK886 (1-[(4-Chlorophenyl)methyl]-3-[(1,1-dimethylethyl)thio]-α,α-dimethyl-5-(1-methylethyl)-1H-Indole-2-propanoic acid; Tocris) was dissolved in 4% Tween 80, 4% DMSO, and sterile water. Capsazepine (N-[2-(4-Chlorophenyl)ethyl]-1,3,4,5-tetrahydro-7,8-dihydroxy-2H-2-benzazepine-2-carbothioamide; Tocris) was dissolved in 5% Tween 80, 5% DMSO, and saline. For vehicle-plus-vehicle testing during the blocking experiments, both injections consisted of 5% Tween 80 and 5% DMSO in saline. All drugs were given as single i.p injections with a volume of 1 ml/kg, except JZL184 at the 30 mg/kg dose, which was given as 2 injections of 1 ml/kg each.
Procedure
Delayed nonmatching-to-position procedure
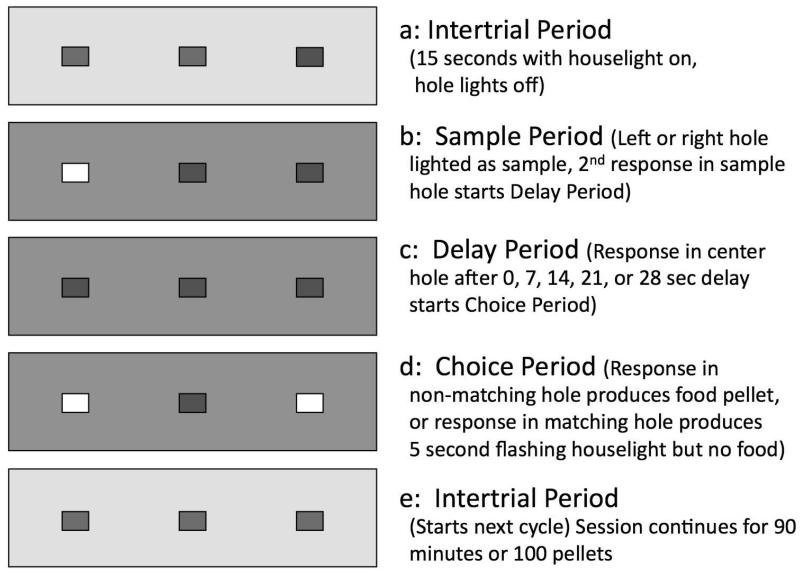
The preliminary training procedures, including magazine training with food, shaping of nosepoke responding, and response-chain training were described in detail previously (Panlilio et al. 2011). Under the nonmatching-to-position task used for baseline and test sessions in the present study (see Figure 1), there were repeated trials in which: 1) either the left or right nosepoke hole was illuminated as a sample; 2) two responses in the sample hole extinguished the sample-hole light and turned on the center-hole light, starting the delay period; 3) after a delay of 0, 7, 14, 21, or 28 s, the next response in the center hole extinguished the center-hole light and illuminated both side holes, starting the choice phase of the trial; 4) during the choice phase, a response in the side hole opposite to the sample constituted a correct (i.e., nonmatching) response and produced a food pellet, extinguished the hole lights, and started a 15-second intertrial period with only the houselight on; 5) alternatively, during the choice phase, a response in the same hole in which the sample had been presented constituted an incorrect response and did not produce a food pellet, but extinguished the hole lights and caused the houselight to flash at 5 Hz for 5 seconds, followed by a 15-second intertrial period with only the houselight on; 5) regardless of whether the choice response had been correct or incorrect, the houselight was extinguished and a sample hole was illuminated after the intertrial period, starting a new trial. The side of the sample hole (left or right) in each trial was drawn without replacement from a list in which each side appeared twice. Similarly, the value of the delay was drawn without replacement from a list in which in which each of the five possible values appeared once. When either list was depleted, it was replenished before the next trial. Sessions were conducted Monday through Friday and lasted for 90 minutes or until 100 food pellets had been delivered.
Fig 1.
Schematic representation of the delayed nonmatching-to-position procedure, showing the state of the three panel lights during each period of a trial in which the left side-hole was randomly selected as the sample, making the right side-hole the correct (nonmatching) hole during the choice period. The Intertrial period (a) precedes each trial. The Sample period (b) requires two responses in the sample hole to proceed to the Delay Period. During the Delay period (c), responding in the center hole is required to enter the Choice period. During the Choice Period (d), a nonmatching response produces food and turns off all lights, or a matching response turns off the hole lights and produces 5 seconds of flashing houselight. After food delivery or flashing of the houselight, the next Intertrial Period (e) begins.
Drug testing
Tests were conducted up to two times per week, usually on Tuesday and Friday, if the accuracy of choice responding was over 90% correct at the 0-s delay and there was <10 percentage-points difference in accuracy at a given delay over the two previous baseline sessions. The FAAH inhibitors were first tested in the following order: URB694, AM3506, URB597, PF-04457845, ARN14633. The monoacylglycerol lipase inhibitor, JZL184, was tested after ARN14633. For each test drug, the vehicle and two doses were tested in counterbalanced order across subjects. This counterbalancing was intended to avoid artifacts due to potential confounding of shifts in baseline performance and the order in which the drugs were tested, by allowing each drug treatment to be compared to a contemporaneous vehicle control session. After this single-drug testing, the effects of treatment with AM3506 (3 mg/kg) and its vehicle were tested in combination with a pretreatment injection of rimonabant (1 mg/kg), MK886 (3 mg/kg), capsazepine (10 mg/kg), or vehicle, with the order of combinations counterbalanced across subjects.
Data analysis
Analyses were performed with Proc Mixed (SAS Institute, Cary, NC), using the Tukey-Kramer procedure to maintain a 0.05 significance level for paired comparisons. For figures showing delay curves, simultaneous confidence intervals with a Bonferroni-corrected 95% confidence level were determined for all points within each experiment, and gray bands were included in the figures such that points falling outside the band were significantly higher than 50% (chance level). The percentage of trials with a correct response (i.e., accuracy) was analyzed as a function of the pretreatment dose (if used), the treatment dose, and the delay value. All percentage measures were arcsine-root transformed for analysis. Responding during the delay period was also analyzed using procedures (described in detail by Panlilio et al. 2011) to assess the role of mediating behavior in performance of the matching task. Briefly: 1) logistic regression was used for each subject to determine whether responding in either the to-be-correct hole or the to-be-incorrect hole during the delay period influenced the accuracy of the choice response; 3) based on this regression, each rat was categorized according to whether responding in the to-be-correct hole or the to-be-incorrect hole was “appropriate” (i.e., predictive of a correct choice response); 4) each trial from each test session was then categorized according to whether side-hole responding occurred during the delay period only in the appropriate hole, only in the inappropriate hole, both, or neither. To obtain sufficient samples for the logistic regression used to the categorize each rat, data were combined from all the baseline sessions that preceded treatment sessions.
Results
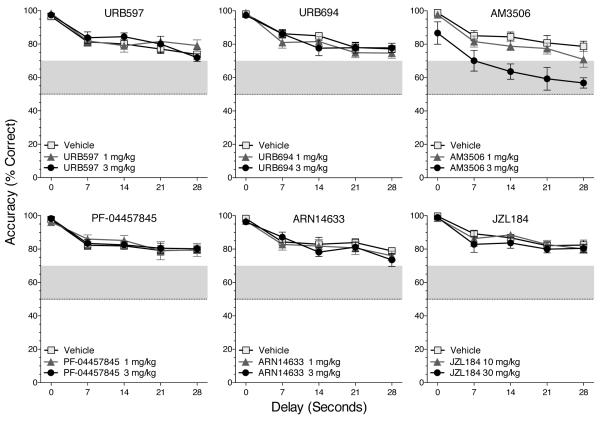
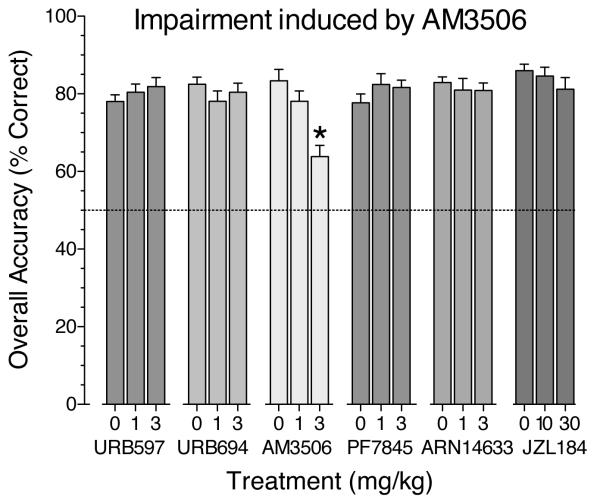
Accuracy (i.e., percentage of trials with a correct response) under the nonmatching-to-sample task was high at the 0-second delay and decreased monotonically as a function of delay under baseline conditions (not shown) and after treatment with vehicle (see Figure 2). Even at the longest delay, accuracy was well above chance level (50%) after treatment with vehicle. During drug testing (Figure 2), the accuracy curves continued to show a general downward slope. The data in each frame of Figure 2 were analyzed separately, and in each case the main effect of delay was highly significant [F(4,44) ranging from 24.0 to 46.0, all p's<.0001]. The main effect of AM3506 on accuracy was significant [F(2,22)=30.2, p<.0001], but the other treatment drugs had no significant main effects or interaction effects (Figure 2). The dose-effect functions for all treatments are summarized in Figure 3, collapsing across delays; only AM3506 had a significant effect, and this was only at the highest dose.
Fig 2.
Accuracy (i.e., percentage of trials with a correct nonmatching response) after treatment with vehicle, a FAAH inhibitor (URB597, URB694, AM3506, PF-04457845, or ARN14633) or an MGL inhibitor (JZL184). Data represent mean (± s.e.m.) accuracy as a function of dose and delay value. Dashed lines in all figures indicate the expected level if responses were completely random (i.e., chance level). Points outside the gray bands have Bonferroni-corrected 95% confidence intervals that do not include 50%.
Fig 3.
Overall accuracy (averaged across delay values) for each treatment shown in Figure 1. Data represent mean (± s.e.m.). * indicates significant impairment under AM3506 (3 mg/kg) versus vehicle (0 mg/kg), p<.0001.
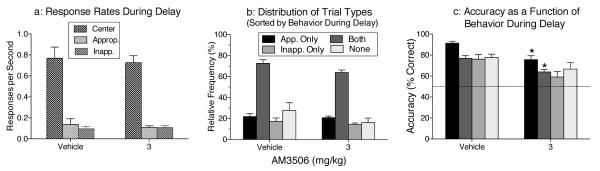
Responding in the side holes is not explicitly required during the delay period, but it appears to serve a rehearsal-like mediating function, enhancing the accuracy of the nonmatching response (Panlilio et al. 2011, 2012). Rats show individual differences in whether their mediating behavior during the delay occurs in the matching or nonmatching hole. Logistic regression indicated that six of the rats in the present study were more likely to choose the correct hole if at least one response had occurred in the to-be-correct hole (i.e., the nonmatching hole) during the delay, while the other five rats were more likely to choose the correct hole if at least one response occurred in the other side hole (i.e., the matching hole) during the delay; this analysis identified the “appropriate” side hole for each rat. Neither AM3506 nor any of the other treatments had a significant effect on 1) the rate of responding in the side holes or center hole during the delay (data shown for AM3506; Figure 4a); or 2) the relative frequency of the four types of trials (i.e., trials with responding in only the appropriate side hole, only the inappropriate side hole, neither side hole, or both side holes; Figure 4b). However, AM3506 treatment [F(1,11)=33.6, p<.0001] and trial type [F(3,33)=12.96, p<.0001] had significant effects on accuracy (Figure 4c), and paired comparisons revealed that the 3 mg/kg dose of AM3506 significantly decreased accuracy of the nonmatching response specifically in trials where at least one response occurred in the appropriate hole (i.e., in “Appropriate Only” trials and “Both” trials).
Fig 4.
Analysis of behavior during the delay period after treatment with vehicle or 3 mg/kg AM3506. (a) Response rates in the center hole, the “appropriate” hole and the “inappropriate” hole during the delay. Responding in the center hole was required to end the delay. Responding in the side holes during the delay was not explicitly reinforced, but presumably serves a rehearsal-like function in performance of the memory task. The appropriate hole for responding during the delay was defined for each rat as either the matching hole or the nonmatching hole, based on which kind of side-hole responding increased the odds of a correct nonmatching response at the end of the trial in baseline sessions. (b) Relative frequency of trials that included only an appropriate response, only an inappropriate response, both, or neither during the delay period. (c) Accuracy of the nonmatching response as a function of whether the trial included only an appropriate response, only an inappropriate response, both, or neither during the delay period. Data represent mean (± s.e.m.). * indicates significant impairment under AM3506 versus vehicle in the same type of trial, p<.003.
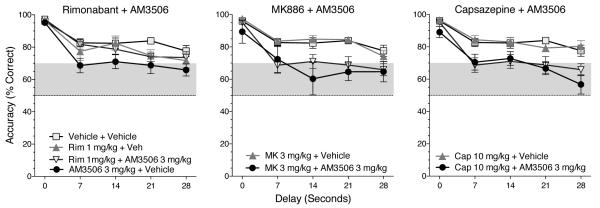
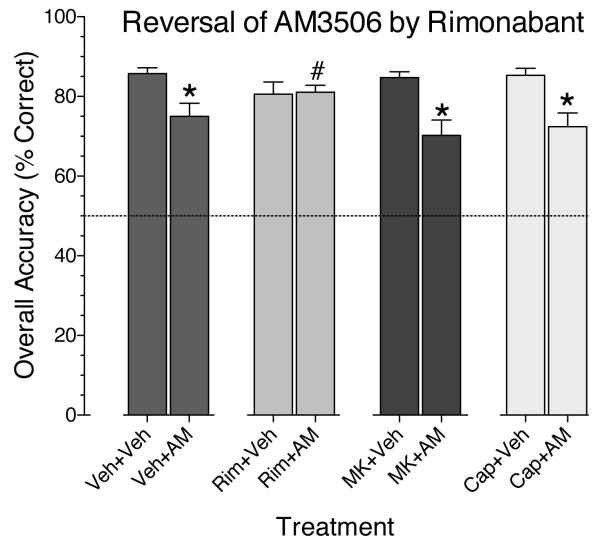
To explore the mechanism of the AM3506 effect, we attempted to block it with: 1) the CB1-receptor antagonist, rimonabant; 2) the PPAR-alpha antagonist, MK886; and 3) the TRPV1 antagonist, capsazepine. As in the single-injection experiments (Figure 2), the 3 mg/kg dose of AM3506 was again found to impair accuracy during the antagonist experiments (Figure 5). This replication of the AM3506-induced impairment —obtained after all six inhibitors had been tested under the single-injection procedure— indicates that the effect was reliable and not dependent on the order in which the inhibitors were tested. Accuracy decreased as a function of delay, and the main effect of delay was significant in each frame of Figure 5 [F(4,44) ranging from 34.7 to 68.1, all p's<.0001]. Rimonabant produced a statistically significantly blockade of the AM3506 effect, but the other antagonists did not. For the data in the rimonabant frame of Figure 5, the interaction of pretreatment and treatment was significant [F(1,11)=12.1, p<.005]. In contrast, while the main effect of AM3506 treatment was significant for the data in the MK886 frame [F(1,11)=44.4, p<.0001] and the capsazepine frame [F(1,11)=62.3, p<.0001] of Figure 5, all main effects and interactions involving pretreatment with MK886 or capsazepine were nonsignificant. The results of the antagonist tests are summarized in Figure 6, with the accuracy data collapsed across delays. The 3 mg/kg dose of AM3506 significantly impaired accuracy when it was given in combination with an injection of vehicle, MK886 or capsazepine. However, when AM3506 was combined with rimonabant, accuracy was significantly improved compared to when AM3506 was combined with vehicle. Accuracy was not significantly affected by any of the antagonists when given alone (p's>.3).
Fig 5.
Accuracy after treatment with AM3506 (3 mg/kg) or vehicle in combination with the cannabinoid-receptor antagonist/inverse agonist rimonabant (1 mg/kg), the PPAR-alpha antagonist MK886 (3 mg/kg), or the TRPV1 antagonist capsazepine (10 mg/kg). Data represent mean (± s.e.m.) accuracy as a function of drug treatment and delay value. Points outside the gray bands have Bonferroni-corrected 95% confidence intervals that do not include 50%.
Fig 6.
Overall accuracy (averaged across delay values) for each treatment shown in Figure 3. Data represent mean (± s.e.m.). * indicates significant impairments under vehicle + AM3506, MK886 + AM3506, and capsazapine + AM3506, each versus vehicle + vehicle, all P's<.0002. # indicates significant difference between vehicle + AM3506 versus rimonabant + AM3506, P<.03.
Discussion
We tested several compounds that enhance endogenous levels of endocannabinoids and found that one compound, AM3506, produced THC-like impairments in a rodent model of working memory. The level of accuracy in the memory task after treatment with a 3-mg/kg dose of AM3506 in this study was comparable to the levels we observed in earlier studies after treatment with a 3-mg/kg dose of THC (Panlilio et al. 2011, 2012; Justinova et al. 2013). Further testing with receptor-specific antagonists indicated that these impairments were mediated by cannabinoid CB1 receptors, but not by PPAR-alpha or TRPV1, which are known to be affected by anandamide and other fatty acid amides. These findings indicate that the impairments induced by AM3506 were due to the activation of CB1 receptors by anandamide, possibly in combination with slightly increased levels of 2-AG.
A feature of the nonmatching-to-position procedure used here is that it allows automated recording of the rehearsal-like mediating behavior that occurs during the delay period (Panlilio et al. 2011, 2012). Like THC in earlier studies with this procedure, AM3506 did not change the rate or distribution of behavior during the delay period, but it seemed to make the mediating response less effective, impairing accuracy even when the most propitious behavior occurred during the delay. That is, AM3506 had significant effects on accuracy specifically in the trials in which the appropriate mediating response did occur. In this respect, THC and AM3506 clearly differ from scopolamine, which disrupts performance of the mediating response (specifically decreasing the proportion of trials in which the rat responds only in the appropriate side hole during the delay; Panlilio et al. 2011).
FAAH and MGL are members of the serine hydrolase family, which includes over 200 members, many of which are not well characterized (Bachovchin and Cravatt 2012). AM3506 is a sulfonylfluoride inhibitor and might show substantial differences in cross-reactivity with serine hydrolases compared to the more typical carbamate/urea inhibitors of FAAH like URB597 and PF-04457845. AM3506 mainly affects FAAH and to a lesser extent MGL, and did not have “off _target” effects when tested against a large number of serine hydrolases using activity-based proteomic methods (Godlewski et al. 2010). However, the differences in the effects of AM3506 and the other test compounds on memory might reflect inhibition of other serine hydrolases in addition to FAAH. Alternatively, in vivo AM3506 might irreversibly deactivate a significantly larger FAAH population compared to the other inhibitors.
Squirrel monkeys are sensitive to the reinforcing effects of CB1 agonists and will intravenously self-administer solutions of THC (Justinova et al. 2003), anandamide (Justinova et al. 2005) or 2-AG (Justinova et al. 2011). The FAAH and MGL inhibitors tested in the present study have all been assessed for reinforcing effects in squirrel monkeys (Bergman et al. 2011; Justinova et al. 2008; Justinova et al. 2014; Justinova et al. 2015), and it is interesting to note that there is only a partial correspondence between their effect in the memory and reinforcement models. That is: 1) URB597 was not self-administered and did not impair memory; 2) AM3506 was self-administered and did impair memory; but 3) the other inhibitors were all self-administered at moderate to high levels, and did not affect memory.
It is possible that the inhibitors that did not disrupt memory in these tests might do so at higher doses. Although it is not clear how doses compare across species, the lowest doses of AM3506, URB 694 and ARN14633 that maintained significant rates of self-administration in squirrel monkeys were the same across drugs (1 g/kg), and these were three times more potent than PF-04457845; thus, there was no indication that AM3506 was more potent than the other inhibitors. The doses selected for the present study were intended to be high enough to produce near-maximal inhibition of FAAH while maintaining selectivity for FAAH versus MGL. However, there has been no study in which the selectivity of all or even most of these drugs have been measured under the same conditions in any species, and it is most parsimonious at this point to assume that they all have the potential to inhibit both FAAH and MGL to some extent. For example, Seillier et al. (2014) found that doses of JZL184 ranging from 5-30 mg/kg increased 2-AG levels 3-5 fold in rat hippocampus, but doses of 15 or 30 mg/kg also increased anandamide levels more than 2 fold. Godlewski et al. (2010) found that a 1 mg/kg i.p. dose of AM3506 fully inhibited FAAH and increased anandamide in rat brain, but did not increase 2-AG; higher doses were not tested in rats, but a 3 mg/kg dose produced a moderate (-39%) inhibition of MGL in mice. One reason that selectivity is important is that treatment with a dual inhibitor of FAAH and MGL or with a selective FAAH inhibitor combined with a selective MGL inhibitor can produce THC-like effects in drug discrimination (Hruba et al. 2015) and short-term memory tests (Wise et al. 2012). Interestingly, Hruba et al. (2015) found that combined treatment with the FAAH inhibitor PF3845 plus the MGL inhibitor JZL184 produced THC-like discriminative effects in mice, but combined treatment with the FAAH inhibitor URB597 plus JZL184 did not. They suggest that this lack of interaction between URB597 and JZL184 might be due to URB597 decreasing levels of 2-AG.
Goonawardena et al. (2011) used a delayed nonmatching-to-position procedure that was similar to the one used in the present study, but used retractable levers instead of lighted nosepoke holes to present the samples and record the responses. Their behavioral procedure is sensitive to impairment by THC, and these impairments correlate with suppressed hippocampal cell firing around the time of the sample response, presumably representing disrupted encoding of the sample (Hampson and Deadwyler 2000). Goonawardena et al. (2011) found that treatment with methanandamide or URB597 (3 mg/kg) produced THC-like effects on memory and hippocampal cell firing. It is unclear why URB597 produced impairments in their procedure but not ours, but one possibility is the use of Long-Evans rats in their study versus Sprague-Dawley rats in ours. They did not test other FAAH inhibitors, but they did find that the anandamide transport inhibitor AM404 only decreased accuracy of nonmatching at the longest delay and did not affect hippocampal firing.
As noted above, FAAH inhibition and FAAH deletion have produced learning enhancements rather than impairments in some previous studies. It appears that aversively-motivated learning is most sensitive to enhancement by FAAH manipulations, possibly due to effects of FAAH inhibition on anxiety (Haller et al. 2009) or coping behavior (Haller et al. 2013). FAAH inhibition also increases endogenous levels of PPAR-alpha ligands that might play a role in these memory enhancements (Mazzola et al. 2009; Panlilio et al. 2013). With respect to the present study, it is worth noting that our delayed nonmatching-to-position procedure is not sensitive to enhancement effects. In past experiments, we did not observe memory enhancement with nicotine, caffeine, galantamine, or rimonabant, all of which have produced cognitive-enhancing effects in other procedures. We also did not observe either enhancement or impairment of memory in an earlier study (unpublished) when —in the same nonmatching task used in the present study— URB597 was tested in Long-Evans rats at doses (0.1 and 0.3 mg/kg) that enhance passive-avoidance learning (Mazzola et al. 2009); the accuracy curves obtained at these lower doses closely resemble the curves for higher doses of URB597 shown in Figure 2 of the present study. The insensitivity of our delayed nonmatching to position procedure to enhancement might be due to the fact that the rats are highly trained prior to drug testing, producing a ceiling effect. Consistent with this possibility, rimonabant produced a more robust enhancement in a delayed nonmatching procedure when extra-long delays were included during the test (up to 80 s; Deadwyler and Hampson 2008) than when the tests used the same delay values as the baseline training schedule (up to 30 s; Deadwyler et al. 2007; Hampson and Deadwyler 2000).
In conclusion, we find that FAAH inhibitors vary in whether they produce THC-like amnestic effects in a rodent model of working memory. In other experiments, we have found that these same FAAH inhibitors vary in whether they produce THC-like reinforcing effects in the squirrel monkey model of cannabinoid self-administration. Puzzlingly, the profiles exhibited by these drugs in the memory-impairment and abuse-potential models do not fully match. These findings strongly suggest that FAAH inhibitors need to be evaluated on a case-by-case basis for different kinds of adverse effects.
Acknowledgements
This research was supported by the Intramural Research Program of the NIH, National Institute on Drug Abuse (LVP, EBT, SRG, ZJ), by grant 5 DP1 DA031387 (DP), and by grants DA031020, DA09158, and DA3801 (AM).
Footnotes
Disclosure Statement DP and TB are inventors in patent applications filed by the University of California and the Fondazione Istituto Italiano di Tecnologia, which protect composition and use of chemicals described in the present study. All other authors declare that there is no actual or potential conflict of interest related to this manuscript.
Contributor Information
Leigh V. Panlilio, Preclinical Pharmacology Section, Behavioral Neuroscience Research Branch, Intramural Research Program, National Institute on Drug Abuse, National Institutes of Health, Baltimore, MD, 21224, USA
Eric B. Thorndike, Preclinical Pharmacology Section, Behavioral Neuroscience Research Branch, Intramural Research Program, National Institute on Drug Abuse, National Institutes of Health, Baltimore, MD, 21224, USA
Spyros P. Nikas, Center for Drug Discovery and Department of Pharmaceutical Sciences, Northeastern University, Boston, MA, USA
Shakiru O. Alapafuja, MAKScientific, LLC, Boston, MA, USA
Tiziano Bandiera, Drug Discovery and Development, Istituto Italiano di Tecnologia, Genoa, Italy.
Benjamin F. Cravatt, The Skaggs Institute for Chemical Biology, The Scripps Research Institute, La Jolla, California, USA; Department of Chemical Physiology, The Scripps Research Institute, La Jolla, California 92037, USA
Alexandros Makriyannis, Center for Drug Discovery, Department of Pharmaceutical Sciences and Chemistry and Chemical Biology, Northeastern University, Boston, MA, USA.
Daniele Piomelli, Drug Discovery and Development, Istituto Italiano di Tecnologia, Genoa, Italy; Department of Anatomy and Neurobiology, University of California Irvine, Irvine, California, USA.
Steven R. Goldberg, Preclinical Pharmacology Section, Behavioral Neuroscience Research Branch, Intramural Research Program, National Institute on Drug Abuse, National Institutes of Health, Baltimore, MD, 21224, USA
Zuzana Justinova, Preclinical Pharmacology Section, Behavioral Neuroscience Research Branch, Intramural Research Program, National Institute on Drug Abuse, National Institutes of Health, Baltimore, MD, 21224, USA.
References
- Bachovchin DA, Cravatt BF. The pharmacological landscape and therapeutic potential of serine hydrolases. Nature reviews Drug discovery. 2012;11:52–68. doi: 10.1038/nrd3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman J, Olajire S, Makriyannnis A, Justinova Z, Goldberg SR. Discriminative-stimulus and reinforcing effects of FAAH inhibitors in CB-1 trained subjects. FASEB J. 2011;25:796.4. [Google Scholar]
- Busquets-Garcia A, Puighermanal E, Pastor A, de la Torre R, Maldonado R, Ozaita A. Differential role of anandamide and 2-arachidonoylglycerol in memory and anxiety-like responses. Biological psychiatry. 2011;70:479–86. doi: 10.1016/j.biopsych.2011.04.022. [DOI] [PubMed] [Google Scholar]
- Clapper JR, Mangieri RA, Piomelli D. The endocannabinoid system as a _target for the treatment of cannabis dependence. Neuropharmacology. 2009a;56(Suppl 1):235–43. doi: 10.1016/j.neuropharm.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapper JR, Vacondio F, King AR, Duranti A, Tontini A, Silva C, Sanchini S, Tarzia G, Mor M, Piomelli D. A second generation of carbamate-based fatty acid amide hydrolase inhibitors with improved activity in vivo. ChemMedChem. 2009b;4:1505–13. doi: 10.1002/cmdc.200900210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deadwyler SA, Goonawardena AV, Hampson RE. Short-term memory is modulated by the spontaneous release of endocannabinoids: evidence from hippocampal population codes. Behavioural pharmacology. 2007;18:571–80. doi: 10.1097/FBP.0b013e3282ee2adb. [DOI] [PubMed] [Google Scholar]
- Deadwyler SA, Hampson RE. Endocannabinoids modulate encoding of sequential memory in the rat hippocampus. Psychopharmacology. 2008;198:577–86. doi: 10.1007/s00213-007-1055-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fegley D, Gaetani S, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. Characterization of the fatty acid amide hydrolase inhibitor cyclohexyl carbamic acid 3′-carbamoyl-biphenyl-3-yl ester (URB597): effects on anandamide and oleoylethanolamide deactivation. The Journal of pharmacology and experimental therapeutics. 2005;313:352–8. doi: 10.1124/jpet.104.078980. [DOI] [PubMed] [Google Scholar]
- Gobbi G, Bambico FR, Mangieri R, Bortolato M, Campolongo P, Solinas M, Cassano T, Morgese MG, Debonnel G, Duranti A, Tontini A, Tarzia G, Mor M, Trezza V, Goldberg SR, Cuomo V, Piomelli D. Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:18620–5. doi: 10.1073/pnas.0509591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godlewski G, Alapafuja SO, Batkai S, Nikas SP, Cinar R, Offertaler L, Osei-Hyiaman D, Liu J, Mukhopadhyay B, Harvey-White J, Tam J, Pacak K, Blankman JL, Cravatt BF, Makriyannis A, Kunos G. Inhibitor of fatty acid amide hydrolase normalizes cardiovascular function in hypertension without adverse metabolic effects. Chem Biol. 2010;17:1256–66. doi: 10.1016/j.chembiol.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goonawardena AV, Sesay J, Sexton CA, Riedel G, Hampson RE. Pharmacological elevation of anandamide impairs short-term memory by altering the neurophysiology in the hippocampus. Neuropharmacology. 2011;61:1016–25. doi: 10.1016/j.neuropharm.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller J, Barna I, Barsvari B, Gyimesi Pelczer K, Yasar S, Panlilio LV, Goldberg S. Interactions between environmental aversiveness and the anxiolytic effects of enhanced cannabinoid signaling by FAAH inhibition in rats. Psychopharmacology. 2009;204:607–16. doi: 10.1007/s00213-009-1494-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller J, Goldberg SR, Pelczer KG, Aliczki M, Panlilio LV. The effects of anandamide signaling enhanced by the FAAH inhibitor URB597 on coping styles in rats. Psychopharmacology. 2013;230:353–62. doi: 10.1007/s00213-013-3161-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson RE, Deadwyler SA. Cannabinoids reveal the necessity of hippocampal neural encoding for short-term memory in rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:8932–42. doi: 10.1523/JNEUROSCI.20-23-08932.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruba L, Seillier A, Zaki A, Cravatt BF, Lichtman AH, Giuffrida A, McMahon LR. Simultaneous inhibition of fatty acid amide hydrolase and monoacylglycerol lipase shares discriminative stimulus effects with Delta9-tetrahydrocannabinol in mice. The Journal of pharmacology and experimental therapeutics. 2015;353:261–8. doi: 10.1124/jpet.115.222836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova Z, Mangieri RA, Bortolato M, Chefer SI, Mukhin AG, Clapper JR, King AR, Redhi GH, Yasar S, Piomelli D, Goldberg SR. Fatty acid amide hydrolase inhibition heightens anandamide signaling without producing reinforcing effects in primates. Biological psychiatry. 2008;64:930–7. doi: 10.1016/j.biopsych.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova Z, Mascia P, Secci ME, Redhi GH, Piomelli D, Goldberg SR. The FAAH inhibitor PF-04457845 has THC-like rewarding and reinstatement effects in squirrel monkeys and increases dopamine levels in the nucleus accumbens shell in rats. FASEB J. 2014;28:838.6. [Google Scholar]
- Justinova Z, Mascia P, Wu HQ, Secci ME, Redhi GH, Panlilio LV, Scherma M, Barnes C, Parashos A, Zara T, Fratta W, Solinas M, Pistis M, Bergman J, Kangas BD, Ferre S, Tanda G, Schwarcz R, Goldberg SR. Reducing cannabinoid abuse and preventing relapse by enhancing endogenous brain levels of kynurenic acid. Nature neuroscience. 2013;16:1652–61. doi: 10.1038/nn.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova Z, Panlilio LV, Moreno-Sanz G, Redhi GH, Auber A, Secci ME, Mascia P, Bandiera T, Armirotti A, Bertorelli R, Chefer SI, Barnes C, Yasar S, Piomelli D, Goldberg SR. Effects of Fatty Acid Amide Hydrolase (FAAH) Inhibitors in Non-Human Primate Models of Nicotine Reward and Relapse. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2015;40:2185–97. doi: 10.1038/npp.2015.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova Z, Solinas M, Tanda G, Redhi GH, Goldberg SR. The endogenous cannabinoid anandamide and its synthetic analog R(+)-methanandamide are intravenously self-administered by squirrel monkeys. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:5645–50. doi: 10.1523/JNEUROSCI.0951-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova Z, Tanda G, Redhi GH, Goldberg SR. Self-administration of delta9-tetrahydrocannabinol (THC) by drug naive squirrel monkeys. Psychopharmacology. 2003;169:135–40. doi: 10.1007/s00213-003-1484-0. [DOI] [PubMed] [Google Scholar]
- Justinova Z, Yasar S, Redhi GH, Goldberg SR. The endogenous cannabinoid 2-arachidonoylglycerol is intravenously self-administered by squirrel monkeys. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:7043–8. doi: 10.1523/JNEUROSCI.6058-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathuria S, Gaetani S, Fegley D, Valino F, Duranti A, Tontini A, Mor M, Tarzia G, La Rana G, Calignano A, Giustino A, Tattoli M, Palmery M, Cuomo V, Piomelli D. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Dimen KR, Martin BR. Systemic or intrahippocampal cannabinoid administration impairs spatial memory in rats. Psychopharmacology. 1995;119:282–90. doi: 10.1007/BF02246292. [DOI] [PubMed] [Google Scholar]
- Mallet PE, Beninger RJ. The endogenous cannabinoid receptor agonist anandamide impairs memory in rats. Behavioural pharmacology. 1996;7:276–284. [Google Scholar]
- Mallet PE, Beninger RJ. The cannabinoid CB1 receptor antagonist SR141716A attenuates the memory impairment produced by delta9-tetrahydrocannabinol or anandamide. Psychopharmacology. 1998;140:11–9. doi: 10.1007/s002130050733. [DOI] [PubMed] [Google Scholar]
- Mascia P, Pistis M, Justinova Z, Panlilio LV, Luchicchi A, Lecca S, Scherma M, Fratta W, Fadda P, Barnes C, Redhi GH, Yasar S, Le Foll B, Tanda G, Piomelli D, Goldberg SR. Blockade of nicotine reward and reinstatement by activation of alpha-type peroxisome proliferator-activated receptors. Biological psychiatry. 2011;69:633–41. doi: 10.1016/j.biopsych.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzola C, Medalie J, Scherma M, Panlilio LV, Solinas M, Tanda G, Drago F, Cadet JL, Goldberg SR, Yasar S. Fatty acid amide hydrolase (FAAH) inhibition enhances memory acquisition through activation of PPAR-alpha nuclear receptors. Learn Mem. 2009;16:332–7. doi: 10.1101/lm.1145209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morena M, Roozendaal B, Trezza V, Ratano P, Peloso A, Hauer D, Atsak P, Trabace L, Cuomo V, McGaugh JL, Schelling G, Campolongo P. Endogenous cannabinoid release within prefrontal-limbic pathways affects memory consolidation of emotional training. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:18333–8. doi: 10.1073/pnas.1420285111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panlilio LV, Ferre S, Yasar S, Thorndike EB, Schindler CW, Goldberg SR. Combined effects of THC and caffeine on working memory in rats. Br J Pharmacol. 2012;165:2529–38. doi: 10.1111/j.1476-5381.2011.01554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panlilio LV, Justinova Z, Goldberg SR. Inhibition of FAAH and activation of PPAR: new approaches to the treatment of cognitive dysfunction and drug addiction. Pharmacology & therapeutics. 2013;138:84–102. doi: 10.1016/j.pharmthera.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panlilio LV, Mazzola C, Medalie J, Hahn B, Justinova Z, Drago F, Cadet JL, Yasar S, Goldberg SR. Anandamide-induced behavioral disruption through a vanilloid-dependent mechanism in rats. Psychopharmacology. 2009;203:529–38. doi: 10.1007/s00213-008-1399-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panlilio LV, Yasar S, Thorndike EB, Goldberg SR, Schindler CW. Automatic recording of mediating behavior in delayed matching- and nonmatching-to-position procedures in rats. Psychopharmacology. 2011;214:495–504. doi: 10.1007/s00213-010-2057-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piomelli D, Tarzia G, Duranti A, Tontini A, Mor M, Compton TR, Dasse O, Monaghan EP, Parrott JA, Putman D. Pharmacological profile of the selective FAAH inhibitor KDS-4103 (URB597) CNS drug reviews. 2006;12:21–38. doi: 10.1111/j.1527-3458.2006.00021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan M, D'Souza DC. The acute effects of cannabinoids on memory in humans: a review. Psychopharmacology. 2006;188:425–44. doi: 10.1007/s00213-006-0508-y. [DOI] [PubMed] [Google Scholar]
- Seillier A, Dominguez Aguilar D, Giuffrida A. The dual FAAH/MAGL inhibitor JZL195 has enhanced effects on endocannabinoid transmission and motor behavior in rats as compared to those of the MAGL inhibitor JZL184. Pharmacology, biochemistry, and behavior. 2014;124:153–9. doi: 10.1016/j.pbb.2014.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Tanda G, Justinova Z, Wertheim CE, Yasar S, Piomelli D, Vadivel SK, Makriyannis A, Goldberg SR. The endogenous cannabinoid anandamide produces delta-9-tetrahydrocannabinol-like discriminative and neurochemical effects that are enhanced by inhibition of fatty acid amide hydrolase but not by inhibition of anandamide transport. The Journal of pharmacology and experimental therapeutics. 2007;321:370–80. doi: 10.1124/jpet.106.114124. [DOI] [PubMed] [Google Scholar]
- Varvel SA, Cravatt BF, Engram AE, Lichtman AH. Fatty acid amide hydrolase (-/-) mice exhibit an increased sensitivity to the disruptive effects of anandamide or oleamide in a working memory water maze task. The Journal of pharmacology and experimental therapeutics. 2006;317:251–7. doi: 10.1124/jpet.105.095059. [DOI] [PubMed] [Google Scholar]
- Wise LE, Long KA, Abdullah RA, Long JZ, Cravatt BF, Lichtman AH. Dual fatty acid amide hydrolase and monoacylglycerol lipase blockade produces THC-like Morris water maze deficits in mice. ACS chemical neuroscience. 2012;3:369–78. doi: 10.1021/cn200130s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanettini C, Panlilio LV, Alicki M, Goldberg SR, Haller J, Yasar S. Effects of endocannabinoid system modulation on cognitive and emotional behavior. Front Behav Neurosci. 2011;5:57. doi: 10.3389/fnbeh.2011.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]