Abstract
Mitochondria are ancient endosymbiotic guests that joined the cells in the evolution of complex life. While the unique ability of mitochondria to produce adenosine triphosphate (ATP) and their contribution to cellular nutrition metabolism received condign attention, our understanding of the organelle’s contribution to Ca2+ homeostasis was restricted to serve as passive Ca2+ sinks that accumulate Ca2+ along the organelle’s negative membrane potential. This paradigm has changed radically. Nowadays, mitochondria are known to respond to environmental Ca2+ and to contribute actively to the regulation of spatial and temporal patterns of intracellular Ca2+ signaling. Accordingly, mitochondria contribute to many signal transduction pathways and are actively involved in the maintenance of capacitative Ca2+ entry, the accomplishment of Ca2+ refilling of the endoplasmic reticulum and Ca2+-dependent protein folding. Mitochondrial Ca2+ homeostasis is complex and regulated by numerous, so far, genetically unidentified Ca2+ channels, pumps and exchangers that concertedly accomplish the organelle’s Ca2+ demand. Notably, mitochondrial Ca2+ homeostasis and functions are crucially influenced by the organelle’s structural organization and motility that, in turn, is controlled by matrix/cytosolic Ca2+. This review intends to provide a condensed overview on the molecular mechanisms of mitochondrial Ca2+ homeostasis (uptake, buffering and storage, extrusion), its modulation by other ions, kinases and small molecules, and its contribution to cellular processes as fundamental basis for the organelle’s contribution to signaling pathways. Hence, emphasis is given to the structure-to-function and mobility-to-function relationship of the mitochondria and, thereby, bridging our most recent knowledge on mitochondria with the best-established mitochondrial function: metabolism and ATP production.
Keywords: Mitochondrial Ca2+, Mitochondrial Ca2+ uniporter, Mitochondrial ion transporters, ROS, Store operated Ca2+entry, Uncoupling proteins, ER refilling, Mitochondrial structure
Introduction
Right from its beginning until our days, the history of mitochondria has been a story of visionary suggestions, wrong assumptions, and reconsiderations. Following the visionary endosymbiotic theory, proposed by Andreas Schimper in 1883 and Konstantin Mereschkowsky in 1905, Ivan Wallin proposed in the 1920s that mitochondria are bacteria that have joined preeukaryotic cells. However, based on the false assumption that mitochondria do not contain DNA, this hypothesis was discontinued but became reestablished after mitochondrial DNA was identified in the 1960s. Today, the endosymbiotic theory is widely accepted, and based on similarities in their genomes, rickettsias are thought to be the closest relatives to the ancestors of mitochondria. However, mitochondrial DNA only encodes for 13 proteins of the respiratory chain, while all other mitochondria-localized proteins are encoded by the nuclear DNA and get imported into the organelle by the very sophisticated TIM/TOM machinery (for review, see [146]).
Beyond any doubt, the key role of mitochondria is to provide energy in the form of adenosine triphosphate (ATP), which is continuously necessary in the struggle against entropy to abide life. Additionally, in recent years, evidence has accumulated that mitochondria are much more than efficient suppliers of energy but are elementarily involved in virtually every signaling cascade and metabolic process that has been described to date. Therefore, it is not surprising that mitochondrial dysfunctions have been found to be involved in many different diseases, which, in turn, brings this organelle into the focus of (patho-)physiologists as well as pharmaceutical industry.
Accordingly, mitochondria play a crucial role in the initiation of apoptosis. Therein, proteins of the BCL-2 family interfere with mitochondrial carriers and poreforming proteins of the inner and outer mitochondrial membranes [59, 195]. The interrelation between mitochondrial Ca2+ and apoptosis has been convincingly reported [48, 196] and was very recently excellently reviewed by Hajnoczky et al. [80], Armstrong [4], or Chan [32]. Consequently, we decided to exclude the apoptotic branch of the mitochondria from this review and refer on the intriguing work of others.
Strikingly, the property of mitochondria to contribute to manifold physiological processes other than apoptosis is also tightly linked to the organelle’s Ca2+ homeostasis. The positive charged calcium ions are of crucial importance in many cellular physiological processes as they specifically govern versatile processes within almost all different cells to induce intrinsic functions. Ca2+ signal transduction is accomplished by a reversible binding of Ca2+ to specific different Ca2+ binding sites, such as EF-hands, C2-domains, or annexin-domains. Such Ca2+ binding sites have been found in numerous signaling-proteins, which alter their enzyme activity, cellular distribution, binding affinity to other proteins or heterologous biomolecules upon a Ca2+ induced steric rearrangement.
The versatility of Ca2+ as a second messenger is accomplished by a sophisticated machinery of an impressive number of Ca2+ shuttling proteins that include ion channels, transporters, pumps, exchangers, and buffer proteins that concertedly control the spatiotemporal Ca2+ signaling [16]. Notably, mitochondria play an ambiguous role in the regulation of Ca2+ homeostasis as they house _targets for signaling Ca2+ but contribute to local and global Ca2+ signaling also. Accordingly, mitochondria not only act as simple Ca2+ sinks, but also specifically respond to this universal messenger.
In this review, we attempt to give a condensed overview on the molecular aspects of mitochondrial Ca2+ homeostasis and its regulatory potential as fundamental basis for the organelle’s contribution to signaling pathways. This review does not claim to be complete but represents the authors’ subjective assortment on mitochondrial Ca2+ signaling and Ca2+ function in these old guests that joined the cells in the evolution of complex life.
Mitochondrial Ca2+ handling
Mitochondrial Ca2+ uptake
The phenomenon of mitochondrial Ca2+ uptake basically builds on two landmark observations that lay 30 years apart from each other. In the late 1960s, Naranjan S. Dhalla described for the first time strong Ca2+ accumulation in isolated respiring mitochondria [49]. However, due to the low sensitivity of the mitochondrial Ca2+ uptake machinery, it was assumed that Ca2+ sequestration by mitochondria in living cells is physiologically irrelevant. This false paradigm lasted until reliable measurements of mitochondrial Ca2+ uptake with _targeted protein based Ca2+ sensors clearly demonstrated that mitochondria in intact cells promptly sequester Ca2+ upon cell stimulation under physiological condition [184]. The apparent discrepancy between the striking mitochondrial Ca2+ response in intact cells and the low affinity of the mitochondrial Ca2+ uptake system of isolated mitochondria has not been solved entirely, but is most likely due to the exposure of the mitochondria to microdomains of high Ca2+ that meet the low Ca2+ affinity of the mitochondrial Ca2+ uptake system [187]. Such microdomains of high Ca2+ are thought to be achieved by a close proximity of sites of mitochondrial Ca2+ uptake with those of Ca2+ release at the endoplasmic reticulum (ER) and/or by functional coupling with Ca2+ entry channels at the plasma membrane [44, 126, 186]. Another aspect, which has been barely considered so far, but may also explain the obvious discrepancy between the prompt mitochondrial Ca2+ response in intact cells and the rather ponderous Ca2+ sequestration of isolated mitochondria, is that mitochondrial Ca2+ uptake in intact cells might be modulated by soluble proteins, which trail away upon cell permeabilization and/or isolation of the organelles. In line with this suggestion, mitochondrial Ca2+ homeostasis has been linked to protein kinase C family members [171], although the final proof is still missing.
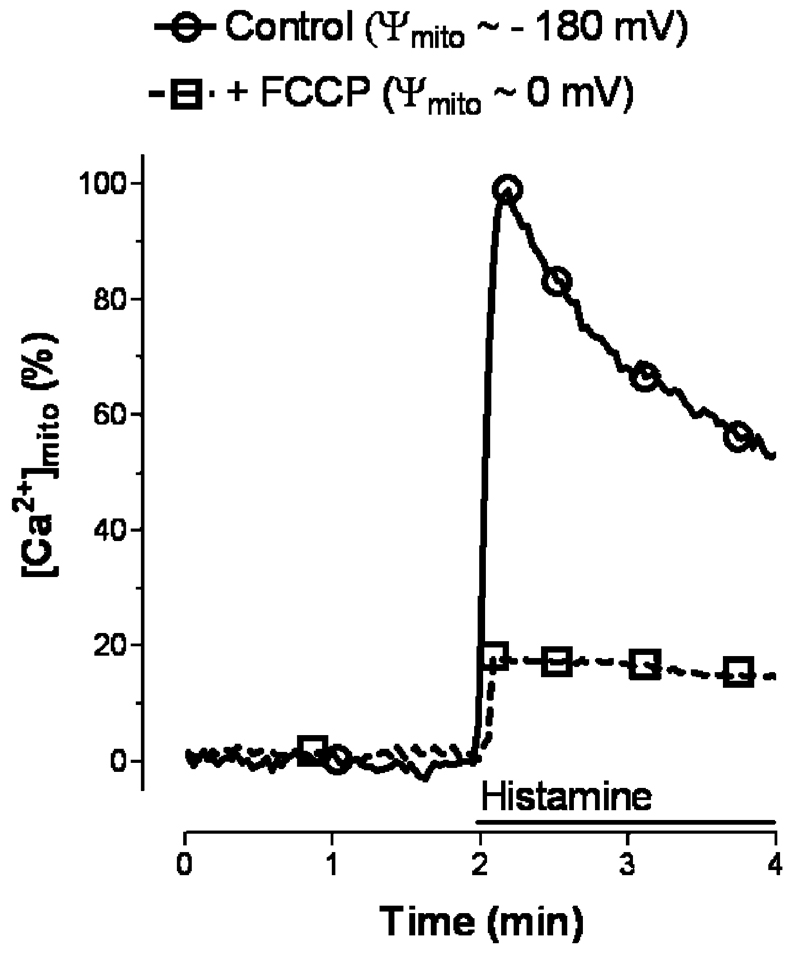
Regardless of whether the organelles are isolated or reside within a cell, Ca2+ uptake into mitochondria critically depends on the electrical driving force for Ca2+ influx that is established by a huge negative membrane potential across the inner mitochondrial membrane (IMM). In respiring, energized mitochondria, a membrane potential of approximately −180 mV is generated by a large H+ gradient at the IMM that is established by continuous translocation of H+ from the matrix into the intermembrane space, which is accompanied by electron fluxes between the complexes of the respiratory chain [140]. According to Nernst equation, equilibrium would be reached only if Ca2+ inside the mitochondria reaches values 106 higher than in the extramitochondrial area. Dissipation of the proton gradient across the IMM by chemical uncouplers such as carbonyl cyanide 4-(trifluoromethoxyl)s-phenyl-hydrazone (FCCP), which immediately causes a depolarization of the IMM, reduces the driving force for Ca2+ entry and thus strongly diminishes mitochondrial Ca2+ uptake (Fig. 1).
Fig. 1.
Effect of membrane depolarization of the mitochondria on the organelle’s Ca2+ sequestration upon cell stimulation with the IP3-generating agonist histamine. Mitochondrial Ca2+ signaling was measured in single endothelial cells that expressed mitochondrial _targeted ratiometric pericam using a high resolution fluorescence microscope as described previously [127, 128]. In Ca2+ containing solution, mitochondria were depolarized by 2 μM FCCP. Changes of the fluorescence intensity at 430 nm excitation and 535 nm emission are shown in percent of the maximal effect of histamine under control conditions (i.e., in the absence of the chemical uncoupler)
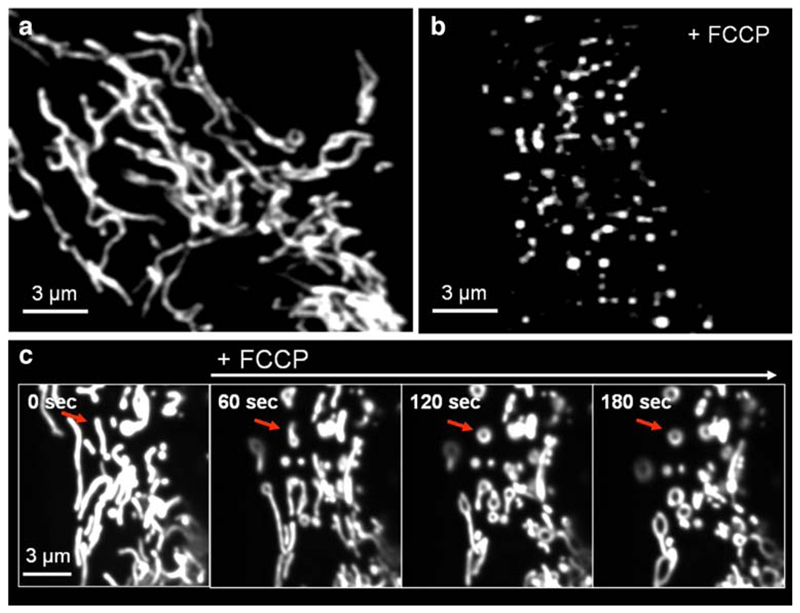
At this point it should be mentioned that protonophores like FCCP rapidly provoke a severe morphological alteration of the structural organization of the mitochondria in living cells. Notably, under physiological conditions mitochondria form a tubular highly interconnected network while mitochondrial structure turns toward disconnected, spherical, singular mitochondria upon FCCP (Fig. 2). Notably, such structural changes of mitochondria are accompanied with alterations in mitochondrial Ca2+ homeostasis independently of the initiator of such structural reorganization of this organelle [160].
Fig. 2.
Effect of membrane depolarization of the mitochondria on the organelle’s morphology and structural integrity. The architectural organization of mitochondria was visualized in human endothelial cells, which transiently expressed mitochondrial-_targeted DsRed using an array confocal laser scanning microscope as described previously [160, 215]. a Under basal conditions, mitochondria consist as tubular, highly interconnected network. b After treatment with 2 μM FCCP for 10 min, mitochondria fragment and form singular round mitochondria. c Time course of FCCP induced fragmentation of tubular mitochondria. Upon membrane depolarization by the chemical uncoupler, mitochondrial fragmentation is preluded by the formation of ring-like structures
Ca2+ transit through the outer mitochondrial membrane
The access of cytosolic Ca2+ to the mitochondrial matrix is restricted by the outer mitochondrial membrane (OMM) and by the IMM. Notably, for a long time, the OMM that contains various large pored porines like the voltage-dependent anion-selective channel (VDAC) [198] was thought to be freely permeable for Ca2+. However, this assumption has changed by a report in which the overexpression of VDAC1 was found to facilitate the transfer of Ca2+ from the ER into mitochondria upon cell stimulation, thus indicating that the number of porines in the OMM correlates with mitochondrial Ca2+ sequestration [177]. Complement findings were obtained with tcBid, a proapoptotic protein, that increases the permeability of the OMM [42]. Very recently, the group of György Hajnóczky demonstrated an enhanced Ca2+ conductance through the OMM by Ca2+ when the cytosolic Ca2+ concentration was raised from ~0 to 2 mM or above. While this effect may have significance during IP3-induced Ca2+ release in the microdomain between ER and mitochondria however, these data do not indicate whether Ca2+ concentrations out of these microdomains may have any similar effect [12]. Further to this limited permeability to cytosolic Ca2+, the OMM, by housing certain adaptor/scaffold proteins, essentially contributes to the establishment of junction with other organelles or the plasma membrane (see: Endoplasmic reticulum—mitochondria Ca2+ crosstalk) and organelle motility (see: Effect of Ca2+ on mitochondrial motility and morphology).
Ca2+ transport through the inner mitochondrial membrane
In contrast to the OMM, the IMM was very early expected to be impermeable for ions to maintain the membrane potential and to run the endoergonic generation of ATP. Interestingly, despite the molecular nature of all Ca2+ shuttling proteins in the IMM are unknown or a matter of intensive debate, various phenomena of the Ca2+ transport through the IMM have been carefully characterized, and pharmacological tools were developed. In the following, the most frequent Ca2+ shuttling phenomena across the IMM are listed:
The mitochondrial Ca2+ uniporter
Most of Ca2+ flux across the IMM is postulated to be accomplished by the so-called mitochondrial Ca2+ uniporter (MCU), which represents a gated, highly selective ion channel [106, 192] and allows Ca2+ influx along the electrochemical gradient without any accompanying other ion or the need for ATP hydrolysis [77]. Based on carefully elaborated experiments using the patch clamp technique on so-called mitoblasts (isolated swollen mitochondria lacking the OMM), David Clapham’s group emphasized that the MCU is a highly selective Ca2+ channel, which shows a second-order kinetics with both an activation domain as well as a transport site [106]. Notably, these electrophysiological data revealed that the MCU is hardly saturable by Ca2+ and exhibits a half-activation constant, K0.5 of 19 mM Ca2+. However, previous data obtained in suspended isolated mitochondria reported a K0.5 of 10 μM Ca2+ [76, 78]. Since in whole-cell recordings the membrane potential is imposed, whereas in mitochondria suspension, the membrane potential rapidly depolarized upon Ca2+ entry, and thus, the MCU gets saturated at lower Ca2+ concentration, this difference was suggested to be due to the different techniques used [106].
Pharmacology of the MCU
While Ca2+ itself is thought to activate MCU, other ions like the lanthanides, ruthenium red [178], its derivate Ru360 [131], diamino-pentane pentamic acid [40] and the plasma membrane Na+/Ca2+ exchange inhibitor KB-R7943 [191] inhibit MCU. However, none of these inhibitors is known to act selectively on MCU and many exhibit no or very limited membranepermeability. Furthermore, Mg2+ as well as nucleotides were shown to either inhibit or stimulate the MCU [115, 120]. In the 1980s, Nicchitta and Williamson reported that spermine activates mitochondrial Ca2+ uniport, suggesting that polyamines may have an important physiological role in intracellular Ca2+ handling [147]. A similar effect of activation on the transport of Ca2+ by isolated mitochondria from rat liver was described for 2-aminoethanesulfonic acid (taurine) [157]. Very recently, Montero et al. [143] found out that the p38 MAPK inhibitor SB202190, estrogen receptor agonists and antagonists [121] as well as several plant flavonoides [142] accelerate mitochondrial Ca2+ sequestration in intact as well as permeabilized cells, suggesting that the MCU can be activated selectively by certain pharmacological compounds. Whether or not pharmacological modulation of MCU activity can have some therapeutic potential is currently unknown. However, in view of the overwhelming reports that point to the crucial involvement of mitochondria in the development of numerous diseases (for review, see [55, 56]), it seems imperative that respective tests are performed in the near future.
Identification of components of the MCU
Although the phenomenon of mitochondrial Ca2+ sequestration has been convincingly reported and explicitly characterized by numerous sophisticated approaches, all efforts to genetically identify the protein(s) that actually account for the MCU have been unsuccessful until now [193].
We have recently demonstrated that UCP2 and UCP3 are essentially involved in MCU [215]. Prima facie, these findings seem to be misleading as one would expect UCPs to depolarize mitochondria and, thus, to attenuate the electrical driving force for mitochondrial Ca2+ uptake. However, a closer examination reveals that the physiological function of UCP2 and UCP3, which are embedded in the inner mitochondrial membrane and belong to the superfamily of mitochondrial ion transporters [181], is still a matter of debate. In a recent review, Michael Duchen wrote “the uncoupling proteins, or UCPs, are at present a rather mysterious group of proteins waiting for a role” [55]. Indeed, the many reported functions such as modulation of free radical formation, apoptosis, regulation of hormone secretion and glucose and fatty acid metabolism that have been suggested for UCP2/3 [26] can hardly be explained by a smooth uncoupling function of these proteins [102]. In contrast, in view of the versatility of Ca2+ as a multifactorial second messenger, UCP2/3 facilitated Ca2+ fluxes across the IMM might represent an attractive explanation of the molecular mechanisms behind the diverse biological processes that were described to be affected by UCP2 and UCP3. Notably, there is a wide consensus that these orthologs do not exhibit a thermogenetic function like UCP1. UCP2 and UCP3 have been identified in many tissues [181] and promote H+ influx and smooth uncoupling in isolated mitochondria only under specific conditions [25], while their involvement in heat production could not be confirmed so far (for review, see [109]). Moreover, since UCP2 and UCP3 related proteins also exist in ectothermic fish and plants that do not require thermogenesis, further/alternative functions of these UCPs were emphasized. A contribution of either/both proteins was described in mitochondrial free radical production [25], apoptosis [46], the regulation of hormone secretion (UCP2) [110], and glucose and fatty acid metabolism (UCP3) [86].
Our assumption that UCP2 and UCP3 are essentially involved in the MCU is based on experiments using overexpression and knock-down (siRNA) experiments in single endothelial, HeLa, HEK293, and AtT20 cells [215]. In all cells tested, overexpression of UCP2 or UCP3 resulted in enhanced Ca2+ sequestration into mitochondria upon cell stimulation with an IP3-generating agonist. Notably, mitochondrial Ca2+ extrusion remained unchanged, and no significant differences in mitochondrial pH compared to wild type cells were obtained. Notably, UCP2- and UCP3-mediated enhanced mitochondrial Ca2+ elevation occurred independently of the source of Ca2+ (i.e., IP3-initiated intracellular Ca2+ release and entering Ca2+). In line with these findings, mitochondrial Ca2+ uptake was strongly reduced in cells treated with siRNA against either UCP2 or UCP3 and was almost prevented if both siRNAs were combined. Convincingly, in HeLa cells that only express UCP3, expression of UCP2 could rescue the abolished mitochondrial Ca2+ sequestration in cells treated with siRNA against UCP3. Supportingly, single isolated liver mitochondria of UCP2–/– mice lacked MCU, while a ruthenium red-insensitive Ca2+ uptake, which was responsible for approximately 50% of mitochondrial Ca2+ accumulation in liver mitochondria of wild type animals, remained. In line with our data, a role of UCP2/3 as carriers of ions other than H+ has been recently postulated in CHO cells in which UCP3 did not cause uncoupling, but controls mitochondrial metabolic activity [145]. Additional analogies of UCP2/3 and the MCU concern their sensitivity to fatty acids and nucleotides. UCP2 and UCP3 were described to be activated by fatty acids and blocked by nucleotides [26, 99, 100], a sensitivity that was also recently reported for ruthenium red-sensitive MCU [120, 229]. These data and reports conclusively point to an elementary contribution of UCP2 and UCP3 to MCU in response to cell stimulation under physiological conditions and may explain the molecular mechanism beyond the reported effects of UCP2 and UCP3 [26, 101, 109].
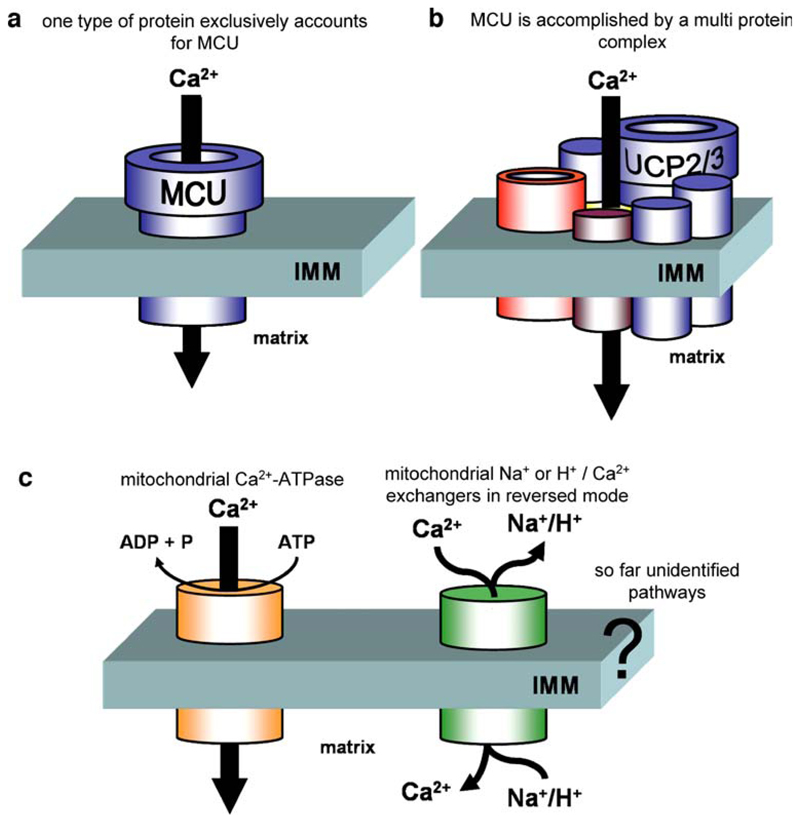
In recent experiments, MCU could not be detected in isolated single yeast mitochondria, despite there was a significant, ruthenium red-insensitive Ca2+ accumulation. Since there is no UCP-homolog in yeast, these data may indicate that the MCU phenomenon is causally linked to the expression of UCP2 or 3. However, a heterologous expression of human UCP2 or UCP3 in this system failed to establish ruthenium red-sensitive MCU in yeast. Accordingly, and in view of the MCU rescue by UCP2 in siRNA-treated HeLa cells mentioned above, we speculate that UCP2/3 alone are not sufficient to establish MCU in yeast due to the lack of so far unknown mammalian constituent/s (e.g., VDAC) that is/are essential to assemble an active MCU [215] (Fig. 3).
Fig. 3.
Schematic illustration of putative pathways for mitochondrial Ca2+ fluxes across the inner mitochondrial membrane (IMM; a–b ruthenium red-sensitive MCU). a The ruthenium red-sensitive MCU might consist of just one type of protein (s). Probably UCP2/UCP3 or some other, so far unidentified, protein works alone or forms homomultimeres to establish MCU. However, because of our recent work, this possibility seems unlikely at least for UCP2/UCP3 [215]. b Alternatively, MCU is accomplished by the assembly of a multi protein complex that may form a Ca2+ permeable channel similar to what has been described for the mitochondrial permeability transition pore. Our recent work favors this possibility and points to a fundamental contribution of UCP2/UCP3 in this process [215]. c The ruthenium red-insensitive mitochondrial Ca2+ uptake pathways that either depend on ATP or other ions (Na+, H+) might also contribute to mitochondrial Ca2+ signaling under certain conditions and tissues
Alternative routes of mitochondrial Ca2+ uptake
Along with our recent findings on the involvement of UCP2 and UCP3 in the MCU phenomenon, it remains important to elucidate the involvement of the two more recently described UCP homologous [60] UCP4 and UCP5 in mitochondrial ion homeostasis. Notably, UCP5 is the closest family member to UCP1/2/3. Considering our findings that UCP2 and UCP3 can substitute each other for MCU, an assessment of the involvement of UCP5 in mitochondrial Ca2+/ion homeostasis remains mandatory. In regard to UCP4, recent data in which expression of UCP4 attenuated store-operated Ca2+ influx in PC12 cells tend to support an uncoupling function of this homolog even in intact cells [33]. Although it has not been measured in this study, mitochondrial depolarization as a result of a still-to-be-approved UCP4 uncoupling activity in intact cells would reduce the mitochondrial capacity to buffer entering Ca2+. Consequently to the lack of subplasmalemmal Ca2+ buffering by the mitochondria, one can expect Ca2+-inhibitable entry pathway to be reduced (see: Ca2+ entry pathways).
In heart as well as in liver, an alternative mitochondrial Ca2+ uptake pathway has been described that shows distinct properties that do not apply for the MCU described above. This pathway has the feature to transfer Ca2+ very rapidly into mitochondria during brief Ca2+ pulses and is therefore termed the rapid uptake mode (RaM, 77). So far, the physiological function of RaM is unknown, and whether RaM is molecularly distinct from the MCU or exhibits a certain state of the MCU awaits further investigation. However, the sensitivity of RaM to ruthenium red fuels the latter concept. In chicken neurons, a ruthenium red-insensitive, electroneutral Na+- and H+-independent Ca2+ antiporter has been described that accounts for spatially organized mitochondrial Ca2+ uptake [37], thus indicating that depending on species and/or tissue, there might be various mitochondrial Ca2+ uptake pathways.
In line with these assumptions, we have recently reported that in liver mitochondria isolated from wild type as well as UCP2–/– mice, a ruthenium red-insensitive Ca2+ sequestration mechanism exists besides the classical MCU [215]. These data are in agreement with our findings in yeast in which mitochondrial Ca2+ sequestration was found to be rather insensitive to this blocker of MCU [215]. Alternatively, since higher concentrations of ruthenium red (>1 μM) diminished these non-MCU typed Ca2+ uptake into mammalian mitochondria, it remains possible that either ruthenium red exhibits additional inhibitory properties on various mitochondrial Ca2+ uptake pathways or the sensitivity of MCU to ruthenium red depends on the protein composition of a multiprotein signalplex that accomplishes mitochondrial Ca2+ sequestration (Fig. 3).
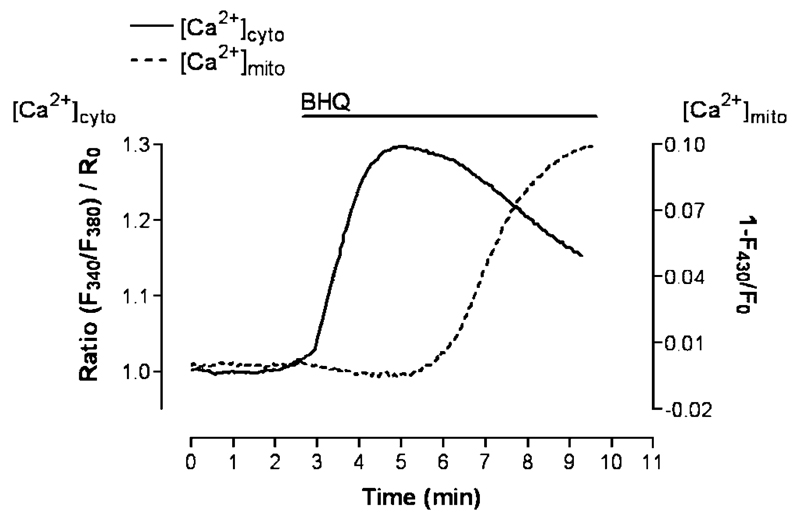
However, there are some additional candidates of putative ion carriers described in mitochondria that are most likely not involved in MCU and may be responsible for ruthenium red-insensitive Ca2+ uptake into the mitochondria. The formation of the mitochondrial permeability transition pore has been described to be involved in mitochondrial Ca2+ sequestration upon agonist-induced Ca2+ mobilization from the ER [222]. Despite these convincing data, the circumstances that lead to such transient formation of the mitochondrial permeability transition pore upon physiological cell stimulation and the modulation of its gating remains unclear. Moreover, a mitochondrial Ca2+-ATPase, that belongs to the SERCA family, has been identified in brown adipose tissue and described to account for heat/ATP production even under conditions in which mitochondria were depolarized [45]. In endothelial cells, a long-lasting incubation of the SERCA inhibitor, BHQ, results in slow accumulation of Ca2+ in the mitochondria (Fig. 4). Since this effect occurs far later than ER depletion by BHQ [127, 154], it is unlikely that this increase in mitochondrial Ca2+ by BHQ is due to ER depletion. Among other possible explanations of this slow mitochondrial Ca2+ accumulation induced by SERCA inhibitors, the existence of mitochondria-localized Ca2+-ATPase needs to be evaluated.
Fig. 4.
Correlation between the effect of an inhibition of SERCA on the cytosolic and mitochondrial Ca2+ concentration. Cytosolic free Ca2+ concentration ([Ca2+]cyto) was measured in single human endothelial cells using fura-2 in a high resolution fluorescence microscope as described previously [73, 74, 125]. Analog experiments were performed with cells, which stably expressed mitochondrial _targeted ratiometric pericam, to monitor mitochondrial free Ca2+ content ([Ca2+]mito). Changes in [Ca2+]mito are expressed as 1-F430/F0, as at 430 nm excitation the fluorescence intensity reflects the Ca2+ sensitivity of this sensor [128]. As indicated, SERCA was inhibited by the addition of 2,5-di-tert-butylhydroquinone (BHQ, 15 μM)
Beside the UCPs, another interesting protein of the solute carrier family 25 (SLC25) that may be involved in mitochondrial ion homeostasis is the human mitochondrial carrier CGI-69. This protein has two predicted transmembrane domains and shares high homology in the mitochondrial energy-transfer signature motifs found in UCPs [228]. However, just like UCP2 and UCP3 [26, 215], overexpression of CGI-69 did not initiate depolarization of mitochondria in HEK293 cells [228]. Like most other members of the SLC25 family that do not contain an EF-hand motif for Ca2+ binding, the physiological function of CGI-69 is unknown. Accordingly, an assessment of the involvement of these SLC25 family members in mitochondrial Ca2+ signaling is necessary for a better understanding of the molecular nature of mitochondrial Ca2+ transporters.
While there is overwhelming evidence that the mitochondrial Na+/Ca2+ exchanger (NCXmito) accounts for mitochondrial Ca2+ extrusion in most cells (see: Mitochondrial Ca2+ extrusion processes), under certain conditions, this exchanger might work in the opposite direction resulting in Ca2+ uptake and Na+ extrusion [200]. This feature, which NCXmito shares with NCXpm and other antiporters, may gain particular importance under conditions in which the cellular Na+ homeostasis is disturbed. Accordingly, it remains to be resolved whether or not NCXmito is functionally linked with other, nonmitochondrial ion carriers/channels (e.g., TRPC3 [58]), and if so, what might be the contribution of such arrangement to mitochondrial Ca2+ homeostasis and organelle function. Importantly, the switch between the modes of the NCXmito critically depends on its electrogenity and Na+:Ca2+ stoichiometry that is not entirely clear so far. Nevertheless, even a 2 Na+:1 Ca2+ stoichiometry like the NCXpm would allow switching this antiporter to the reversed mode under conditions where microdomains of Na+ and or Ca2+ are established.
In heart mitochondria, besides the ruthenium red-sensitive MCU and RaM, ryanodine receptors (mRyR) that are localized in the IMM contribute to mitochondrial Ca2+ uptake [18]. Pharmacologically, mRyR have been characterized as type 1 RyR and their contribution to mitochondrial Ca2+ sequestration during excitation–contraction coupling to stimulate oxidative phosphorylation for ATP production to meet metabolic demands has been described [19].
Mitochondrial Ca2+ buffering and Ca2+ storage
The ability of isolated mitochondria to buffer and store large amounts of Ca2+ depends on phosphate and physiological concentrations of adenine nucleotides [148, 150], and the level of the free matrix Ca2+ concentration is controlled by the formation of an instantly reversible Ca2+-phosphate complex in the mitochondrial matrix [232]. In the presence of sufficient phosphate concentrations in the buffer, the free matrix Ca2+ concentration of isolated mitochondria is surprisingly invariant from Ca2+ load and was found to change less than 50% when total matrix Ca2+ was increased 50-fold [31]. While there is considerable uncertainty on the actual structure of the Ca2+-phosphate complex formed, the importance of matrix pH in dissolving this complex is known. Notably, matrix acidification induced by protonophores counteract the formation of Ca2+-phosphate complexes within mitochondria and thus reduces the mitochondrial Ca2+ buffer capacity while initiating large mitochondrial Ca2+ release [15, 149]. The formation of Ca2+-phosphate complexes as a high capacity mechanism of strong mitochondrial Ca2+ buffering could also be observed in intact bovine adrenal chromaffin cells [53].
On the contrary, in intact human endothelial cells, no large mitochondrial Ca2+ release is observed upon mitochondrial acidification, and free matrix Ca2+ concentration is more likely under the control of the balance between the amount of Ca2+ uptake and the capacity of its extrusion [125–128, 215]. These data may suggest that the formation of Ca2+-phosphate complexes, at least in endothelial cells , is marginally involved in mitochondrial Ca2+ homeostasis under physiological conditions. In view of the signaling function of matrix Ca2+ to stimulate various metabolic and housekeeping enzymes and to facilitate a fast regulator and tunable regulatory function of mitochondrial Ca2+, initial mitochondrial Ca2+ elevations up to 1 to 2 μM free Ca2+ might not be buffered by the formation of Ca2+-phosphate. Nevertheless, it is reasonable to assume that under conditions of altered mitochondrial/cellular Ca2+ homeostasis, the formation of Ca2+-phosphate complex occurs and may counteract massive accumulation of free matrix Ca2+ that would initiate apoptosis. Notably, such mitochondrial Ca2+ buffer capacity via formation of Ca2+-phosphate critically depends on the availability of phosphate that enters the mitochondria as H3PO4 (antiport) via the electroneutral phosphate transporter [149]. Once the Ca2+-phosphate complex is formed, the H+ release needs to be compensated by the respiratory chain. A limited disposal of phosphate under pathological conditions of mitochondrial Ca2+ overload might explain the brake of mitochondrial Ca2+ buffer capacity and initiation of Ca2+-triggered apoptosis. In line with this assumption, the environmental buffer phosphate concentration was described to enhance Ca2+ buffer capacity of isolated mitochondria [232].
Whether the appearance of H+ in the matrix that is associated with the deprotonation of H3PO4 to form prior complexing with Ca2+ exhibits a beneficial smooth uncoupling that might decrease excessive ROS production is unknown but would be a further possibility how UCP2 and UCP3, which facilitate Ca2+ entry into the mitochondria, trigger smooth uncoupling and attenuation of mitochondrial ROS production [25, 26].
Mitochondrial Ca2+ efflux and functional coupling with other organelles
Mitochondrial Ca2+ extrusion processes
In most cells, the main mechanism of Ca2+ extrusion from the mitochondria is the NCXmito. The postulation of the existence of a Na+/Ca2+ carrier in mammalian mitochondria is based on findings of a Na+-dependent Ca2+ flux across the inner mitochondrial membrane [41] and on pharmacological characterization using the quite selective inhibitor of NCXmito, chloro-5-(2-chlorophenyl)-1,5-dihydro-4,1-benzothiazepin-2(3H)-one (CGP 37157 [11]). There is a broad consensus that NCXmito is responsible for mitochondrial Ca2+ extrusion in most cells [77] and thus represents the physiological counterpart to the MCU. In line with these reports, in endothelial cells, mitochondrial Ca2+ extrusion after agonist-induced elevation in [Ca2+]mito highly depends on cytosolic Na+ [127, 197] and is prevented by CGP 37157 [127, 128]. Although the existence of a Na+-dependent Ca2+ efflux through the IMM has been experimentally confirmed, the actual protein(s) that account(s) for NCXmito is/are still unknown and await(s) further investigation.
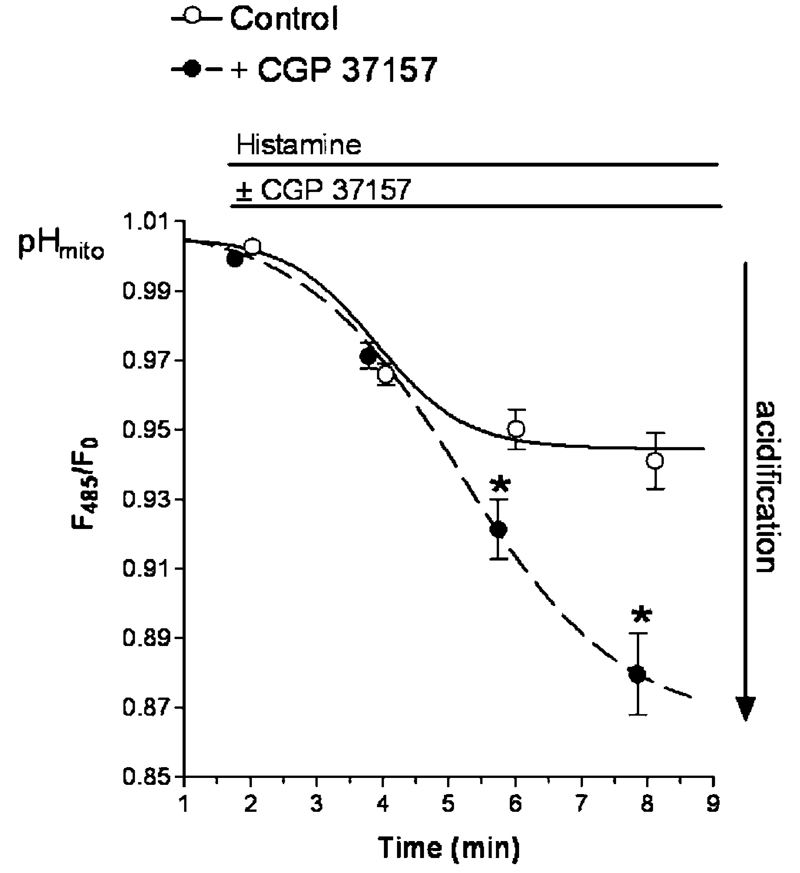
In liver mitochondria a Ca2+/H+ exchanger was described to be responsible for mitochondrial Ca2+ extrusion [79, 170]. So far, it is not clear whether this phenomenon is specific for liver mitochondria, but the large acidification of mitochondria in intact endothelial cells, Hek293 cells, or HeLa cells upon Ca2+ sequestration in the presence of the NCXmito inhibitor CGP37157 (Fig. 5) may point to its ubiquitous existence. In addition to the ion exchanger, a transient formation of the mitochondrial permeability transition pore may be an alternative mechanism of mitochondrial Ca2+ extrusion [61, 139].
Fig. 5.
Effect of an inhibition of the mitochondrial Na+/Ca2+ exchanger on histamine-induced changes in the pH of the mitochondrial matrix. Endothelial cells, which stably express mitochondrial _targeted ratiometric pericam were used to monitor changes of the matrix pH by following the pH sensitive wavelength from the sensor. Cells were illuminated at 480 nm and emission was collected at 535 nm on a high-resolution fluorescence microscope [127, 128]. As indicated cells were stimulated with 100 μM histamine in the absence (continous line, open circles) or in the presence of 20 μM CGP 37157 (dotted line, filled circles)
Endoplasmic reticulum—mitochondria Ca2+ crosstalk
In living cells, the mitochondria were found to be in close proximity (~10–100 nM) to the ER [44, 183, 187]. Although mitochondria exhibit high motility in living cells, in cultured human endothelial cells, which expressed organelle-_targeted fluorescent proteins, the proportion of mitochondria that colocalizes with the ER and vice versa is remarkably constant at approximately 50–55 and 22–27%, respectively [215]. This tight vicinity is actually the basis to explain how mitochondria are able to accumulate Ca2+ efficiently despite the low affinity of the uniporter for Ca2+ [185]. In line with this report, mitochondrial Ca2+ uptake was found to depend on the establishment of high Ca2+ microdomains by ER Ca2+ release sites close to mitochondria [183, 205]. Notably, the maintenance of such high Ca2+ gradients essentially rely on a continuous Ca2+ cycling through the ER and is maintained by Ca2+ influx from the extracellular space [205].
Filippin et al. [63] also proposed that the interaction between the mitochondria and the ER is stable over time, suggesting a specific interaction between both organelles that might include a physical linkage between the ER and the mitochondria. Morphological evidence was reported by Mannella et al. [129, 130], and recently, the group of Hajnoczky [43] showed, using electron tomography, a visible linker between mitochondria and the ER. In line with this, they construct a synthetic linker and reported an increased mitochondrial Ca2+ uptake, which eventually led to Ca2+ overload. In line with these findings, a chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels has been described [203] and strongly point to a possibly reversible organelle junction that allows rapid and very efficient Ca2+ transfer from the ER to the mitochondria and vice versa [5, 125, 127, 128].
However, in view of the high mobility of the mitochondria and their constant fusion/fission processes [14, 64], the kinetics and duration of the establishment of a physical linker that attach mitochondria to the ER as well as the molecular initiators of organelle linkage need further attention. Most reports indicate that mitochondria are more efficient in accumulating Ca2+ when the Ca2+ concentration is locally high like in hotspots and thus support the concept of the formation of a physical organelle junction. Besides the importance of such Ca2+ hotspots for the Ca2+ transport via a low Ca2+-sensitive uptake machinery, spatial Ca2+ elevation might also be responsible for initiating the linkage between mitochondria and the ER [220]. However, it was also shown that mitochondria are able to take up Ca2+ at low Ca2+ concentration range [39, 105, 172, 207], and thus such physical linkage might not be prerequisite for all mitochondrial Ca2+ uptake processes.
ER Ca2+ refilling A reverse Ca2+ flux from mitochondrial Ca2+ toward the ER fuels SERCA-mediated ER-refilling [128]. Since in the presence of the agonist Ca2+ refilling of the ER essentially depends on Ca2+ that enters the cell through CCE and is rapidly sequestered by subplasmalemmal mitochondria [126], a coupling, at least functional, between the mitochondria and the plasma membrane can be assumed (see: Plasma membrane—mitochondria Ca2+ connection).
Such reverse Ca2+ flux from the mitochondria toward the ER was found to be important for the ER Ca2+ refilling process. In HeLa cells, depolarizing mitochondria lead to a more profound Ca2+ store depletion during cell stimulation, showing that part of the Ca2+ accumulated by mitochondria during the release phase is returned back to the ER, thus limiting the level of depletion [5]. In endothelial cells, the same effect was observed and, in addition, mitochondria were shown to relay Ca2+ entering the cells through the plasma membrane toward the ER [128]. This effect gained even more importance under conditions of reduced Ca2+ entry by plasma membrane depolarization [125]. In agreement with these reports that point to an important contribution of mitochondria to ER Ca2+ refilling, the refilling process of the ER following bradykinin stimulation is slowed down after mitochondria were uncoupled by FCCP in BHK-21 cells [112].
Another reflection of the contribution of mitochondria to ER Ca2+ refilling has been described under conditions of mitochondrial fragmentation by exposure to elevated D-glucose in which fragmentation of mitochondria yielded delayed mitochondrial Ca2+ extrusion [160] and diminished ER Ca2+ refilling (Graier and Malli, unpublished data).
Maintenance of protein folding processes in the ER Causally linked to the mitochondrial contribution to ER Ca2+ refilling, the Ca2+ transfer from the mitochondria toward the ER should be considered of particular importance for Ca2+-dependent protein folding in the lumen of the ER [136–138]. Accordingly, mitochondrial Ca2+ homeostasis and especially the Ca2+ transfer from the mitochondria toward the ER via the NCXmito have been found to be prerequisite to maintain calreticulin/calnexin-mediated protein folding in the ER [153]. Additionally, ER protein folding is attenuated by mitochondrial ROS production that evokes sustained ER Ca2+ depletion in leukemia cells [230] and thus induces activation-enhanced cell death [201]. Thus, convincing evidence on the contribution of a pathological, altered mitochondrial ion homeostasis to protein dysfunction/ER stress exist [72] and further point to a considerable new function of mitochondrial Ca2+ homeostasis in cellular metabolism.
Plasma membrane—mitochondria Ca2+ connection
In view of the mitochondrial contribution to subplasmalemmal Ca2+ buffering to maintain the activity of Ca2+-sensitive store-depletion activated Ca2+ entry pathways [69, 70, 93, 94, 126], a functional interplay that might build on actual junctions between the mitochondrial Ca2+ uptake machinery and plasma membrane Ca2+ channels seems possible. Moreover, in view of the recent data that indicate Ca2+-triggered mitochondrial moving arrest [43], such junction might get established on demand in a Ca2+ dependent manner. In line with such expectations, the molecular mechanisms of the development of Ca2+ current in response to Ca2+ store depletion represents a dynamic assembly of various TRPC family members, STIM1, and Orai1 [117, 152] and might be extended to a so far unknown linkage protein between the OMM and the TRPC/STIM1/Orai complex or a protein of the OMM that couples to the store-operated Ca2+ channel complex and allows mitochondrial Ca2+ buffering of entering Ca2+. Additionally, in view of the strict dependency of the main mitochondrial Ca2+ extrusion pathway from environmental/cytosolic Na+, one might speculate that Na+ permeable plasma membrane ion channels (e.g., TRPC3, [141]) and/or the plasma membrane Na+/Ca2+ exchanger (NCXpm) are, at least functionally, linked to mitochondrial Na+ carriers.
While a physical linkage between mitochondria and the plasma membrane can be conceptionally anticipated, further studies are essential to identify proteins in the OMM that serve as anchors for such junction.
Tuning mitochondrial Ca2+ signaling
Mutual regulation of mitochondrial ions
Mitochondrial protonophores like FCCP, which equalize H+ concentration in the matrix and the intermembrane space/cytosol, strongly diminish mitochondrial Ca2+ uptake due to their mitochondria-depolarizing property [215]. Besides this electrophysical importance of H+ to establish the negative membrane potential, the homeostasis of Ca2+ is closely linked to that of H+. Notably, Ca2+ indirectly affects H+ homeostasis by its effect on metabolic enzymes, which, in turn, fuel the respiratory chain resulting in enhanced H+ flux [134] (see: Effect of Ca2+ on mitochondrial metabolism).
Besides H+, there are at least three other ions that need to be considered as to be involved in mitochondrial Ca2+ homeostasis: Na+, K+, and Mg2+. For all three cations, mitochondrial carriers/channels exist [53] and preliminary data point to a mutual regulation of these ions with mitochondrial Ca2+. In case of Na+, the importance of cytosolic/mitochondrial Na+ for mitochondrial Ca2+ homeostasis has been already indicated above (see: Mitochondrial Ca2+ extrusion processes). However, in regard of the role of Na+ for mitochondrial Ca2+ homeostasis, one needs to consider the possibility of functional interaction between mitochondrial Na+ carriers and plasma membrane Na+ fluxes. In endothelial cells, the occurrence of subplasmalemmal Na+ gradients, which are generated by activation of Na+-permeable ion channels, most likely TRPC3 [58], affect NCXpm [159, 212]. Whether or not mitochondrial Ca2+ homeostasis is regulated by plasma membrane Na+ currents is unclear and deserves more attention particularly considering the pathological consequences of altered Na+ signaling on mitochondrial ion homeostasis.
While the molecular nature of mitochondrial transporters for Na+ is unknown, putative carriers for K+ and Mg2+ in this organelle have already been identified. Three classes of mitochondrial K+ channels have been pharmacologically and functionally characterized and were found to be similar to some of the K+ channels present in the plasma membrane [208]: the ATP-regulated K+ channel (mitoKATP; apparently Kir6.1 and Kir6.2 [111]), the large conductance Ca2+-activated K+ channel (mitoBKCa; apparently Slo1 [223]) and the voltage-dependent K+ channel (mitoKv1.3 [206]). Whereas the physiological function of the latter one has not been well characterized so far, mitoKATP and mitoBKCa are described to be involved in the regulation of mitochondrial membrane potential, ROS production, mitochondrial transition pore and oxidative phosphorylation [208]. Moreover, the KATP channel opener diazoxide was found to be cardioprotective presumably due to its mitochondrial depolarizing effect [67]. However, although the molecular structure of the channel is often described as Kir6.1/2 and SUR2, this assumption has been challenged [30, 217] and the respective current has also been attributed to the function of a complex of five proteins not involving Kir6.1/2 or SUR2 [3]. In line with these reports, this drug has been described to act directly on mitochondrial K+ influx [28, 51, 83, 84, 90, 155].
Besides mitochondrial K+ channels, a mitochondrial K+/H+ carrier has been described [66] of which the contribution to mitochondrial Ca2+ is unknown but may influence mitochondrial Ca2+ homeostasis via its impact on matrix H+ concentration.
For mitochondrial Mg2+, the human mitochondrial Mrs2 protein [233] was recently described to substitute functionally its yeast homologue for the mitochondrial Mg2+ carrier [108]. The contribution of mitochondrial Mg2+ and its carrier for mitochondrial Ca2+/ion homeostasis has not been evaluated so far.
Additionally, to H+, Na+, K+, and Mg2+, cytosolic Ca2+ itself may regulate mitochondrial Ca2+ homeostasis by its regulatory effect on proteins in the outer or inner mitochondrial membranes. Recently, cellular Ca2+ has been shown to modulate activity of VDAC, which may tune mitochondrial Ca2+ sequestration and facilitate mitochondrial overload upon huge cytosolic Ca2+ elevation [12]. Additionally, there are several members of the mitochondrial SLC25 family that contain one or more EF-hand motifs for Ca2+ binding (e.g., Aralar, short Ca2+-binding mitochondrial carriers) and thus may serve as transducers of outer Ca2+ into the mitochondria and/or adapt ion carrier activities to the existing Ca2+ concentration outside the organelle. The family of short Ca2+-binding mitochondrial carriers (SCaMC) consists of three genes in the human genome that code for highly conserved proteins (70–80% homology) with a characteristic mitochondrial carrier domain and EF-hand Ca2+-binding motifs at either the C-(SCaMC-1 & 2) or N-terminal side [47].
Protein phosphorylation for regulation of mitochondrial Ca2+ homeostasis
Mitochondrial protein phosphorylation is a largely unresolved and complex phenomenon (Ca2+ as regulator of mitochondrial phosphoproteom). A bidirectional modulation of mitochondrial Ca2+ uptake was described for the protein kinase C family, of which protein kinase Cβ diminished and protein kinase Cζ enhanced mitochondrial Ca2+ accumulation upon cell stimulation [171]. Considering the existence of many kinases and phosphatases in the mitochondrial matrix [92], an assessment whether or not reversible protein phosphorylation represents a regulatory mechanism in mitochondrial Ca2+ homeostasis is mandatory.
Second messenger-modulation of mitochondrial Ca2+ signaling
There is already strong evidence of a modulation of mitochondrial Ca2+/ion carrier by intracellular messengers/small molecules. Among them, NO• has been described to diminish mitochondrial Ca2+ accumulation [213], whereas peroxynitrite and other reactive oxygen species (;H2O2) were found to yield accumulation of Ca2+ in the mitochondria [75]. Additionally, nucleotides regulate mitochondrial Ca2+ transport [120] by P2Y1- and P2Y2-like mitochondrial receptors [13]. On the other hand, polyunsaturated fatty acids cause Ca2+ release from mitochondria [229].
Overall, there is strong evidence that mitochondrial Ca2+ homeostasis represents a _target of multiple modulator effects of intracellular messengers/small molecules. The actual molecular mechanisms and proteins involved in these regulatory processes are so far unknown and deserve a detailed assessment.
Contribution of mitochondria to cytosolic Ca2+ homeostasis
Shaping cytosolic Ca2+ elevation
Ca2+ mobilization from the internal Ca2+ stores occurs primarily from the ER upon activation of the IP3 receptor (IP3R) or the RyR. IP3R is activated following cell stimulation by extracellular agonists, activation of the phospholipase C and production of IP3. On the other hand, the RyR can be activated by a conformational change of the plasma membrane voltage-operated Ca2+ channels in skeletal muscle, by Ca2+ itself leading to Ca2+-induced Ca2+-release or by cADP ribose or NAADP [16]. The latter two mechanisms are less well documented at present. Once Ca2+ is elevated in the cytosol as a result of Ca2+ mobilization, it is immediately taken up by several pumps or exchangers to counteract the Ca2+ rise.
In excitable cells, in which cytosolic Ca2+ elevation is due to Ca2+ entry through voltage-gated Ca2+channels, the impact of mitochondria is rather clear and consistent. For instance, in chromaffin cells, mitochondria rapidly accumulate Ca2+ in an initial phase and thus delivers Ca2+ back to the cytosol, thus prolonging the low plateau phase of the Ca2+ elevation [8, 9, 54, 89]. However, if the ER represents the source of Ca2+, the way how mitochondria shape the cytosolic Ca2+ is less clear. In that case, the release of Ca2+ is accompanied by v entry through store-operated Ca2+ channels, which are also modulated by mitochondria (see: Ca2+ entry pathways). Thus, to precisely assess the role of the mitochondria, experiments should be performed in the absence of extracellular Ca2+. In such circumstances, preventing mitochondrial Ca2+ uptake results in an increased cytosolic Ca2+ signal [5]. In case of intracellular Ca2+ wave propagation, mitochondria were shown to accelerate or slow down the wave, depending most likely on the IP3R subtype [54], which exhibit isoform specific Ca2+ dependence. Thus, in case of a linear Ca2+ activation of IP3R, mitochondria slowed down the wave propagation [20], whereas in case of a bell shape Ca2+ activation, mitochondria accelerated the process by preventing Ca2+-dependent inhibition of the IP3R [103].
The inhibition of local Ca2+ elevation by mitochondrial Ca2+ uptake that yields suppression of Ca2+-triggered feedback to IP3R has also been found to contribute to cytosolic Ca2+ oscillation [8, 62, 81, 103, 112, 182, 187]. Recent data show that Ca2+ concentrations in the mitochondria and the ER oscillate concomitantly with cytosolic Ca2+ oscillations and that the Ca2+ shuttling between these organelles exhibits a pacemaker role in cytosolic Ca2+ oscillation [96].
Another important aspect in modulating cytosolic Ca2+ signaling by mitochondria is their capacity to exhibit spatial Ca2+ buffering in distinct area of the cytosol. Notably, the property of mitochondria to act as an intracellular Ca2+ buffering mechanism in contractile tissue with regard to the organelle’s intracellular location was already described in 1980 [135]. Subsequently, numerous reports approved these early findings in many excitable cells, such like smooth muscle cells, in which mitochondria were found to modulate Ca2+ sparks and Ca2+-activated K+ currents [35]. In chromaffin cells mitochondria were found to modulate Ca2+ inhibition of various Ca2+ channels [88]. Moreover, mitochondria may also be responsible for the difference in Ca2+ signaling pattern in the perinuclear and subplasmalemmal cytosol [207]. In cardiac myocytes, mitochondrial Ca2+ buffering was found to be responsible for the delay between the peak of the Ca2+ transient and the peak shortening in myocytes [209]. Intriguingly, in myocytes as well as smooth muscle cells, mitochondrial Ca2+ buffering was found to be linked to the cellular Na+ homeostasis [123, 173], thus pointing to the strong inter-dependence of mitochondrial Ca2+ homeostasis with that of other ions.
In respect of spatial mitochondrial Ca2+ buffering, the group of Ole Petersen has conducted pioneer work in pancreatic acinar cells in which the mitochondria are distinctively located around the granular pole and form a belt that retains the Ca2+ signal in the granular pole [162, 166–168, 214]. This landmark observation was complemented by their findings on the focal Ca2+ delivery function of the ER in this cell type [22, 68, 162, 166, 167, 169].
This overview on the aspect of spatial mitochondrial Ca2+ buffering only merely touches the role of mitochondria as a local and/or global regulator of cytosolic Ca2+ signal, but indicates that the quality of the modification of the cytosolic Ca2+ signal by mitochondria is complex and highly depends on the cellular system investigated.
Ca2+ entry pathways
The role of mitochondria as a regulator of Ca2+ entry has been mainly studied in the particular context of the activation of store-operated Ca2+ entry. This mechanism, originally described by Putney [175], is now evident in virtually every cell type. Upon store depletion, Ca2+ permeable channels open at the plasma membrane and allow Ca2+ entry that eventually refilled the store. The best characterized current supporting this influx was described in blood cells and termed CRAC for Ca2+ release activated Ca2+ current. One of the characteristics of this current is a feedback inhibition due to rise in intracellular Ca2+ concentration [161]. Mitochondria, by their ability to take up Ca2+, were shown to prevent Ca2+-dependent inhibition of the current, and thus allow a sustained Ca2+ entry through CRAC [94]. This mechanism of SOCE was also reported in other cell types, and mitochondria were shown to have the same effect. In endothelial cells, it was clearly shown that mitochondria are able to maintain low level of Ca2+ concentration in their vicinity, thus preventing Ca2+-depedent inhibition of SOCE [126].
In the case of Ca2+ entry, it is also postulated that mitochondria have to be located close to the membrane to efficiently buffer entering Ca2+. This was indeed confirmed by experiments where mitochondria were relocalized more distantly from the membrane after overexpression of dynamitin [216]. Intriguingly, a sustained activity of CRAC requires translocation of mitochondria to the plasma membrane [176]. In that particular case, the SOCE was significantly reduced. However, overexpression of hFis1 that also relocated mitochondria away from the plasma membrane, did not significantly impact on SOCE [65]. In this condition, mitochondria were still able to accumulate entering Ca2+ (even with a slower time course), and depolarizing mitochondria reduced the SOCE. Thus, mitochondria prevent Ca2+-dependent inhibition of SOCE by local Ca2+ buffering, but they may also release a diffusible compound that activates Ca2+ entry as shown in RBL cells [71], as well as in immune cells [6].
Until recently, neither the mechanism of SOCE activation nor the molecular identity of the channel was known. During the last 2 years, major advances in the field have been obtained with the identification of STIM 1 as a key regulator of SOCE [119, 189] and Orai1 as the channel supporting the CRAC current [218, 226]. With this in hand, the role of mitochondria in SOCE regulation will probably be reassessed and some remaining controversies clarified.
Ca2+ entry does not exclusively rely on SOCE. The arachidonic-activated Ca2+ entry pathway is a well-documented route of Ca2+ influx that is distinct from SOCE [199, 221]. In addition, the large family of TRP cation channels also constitute an important way of Ca2+ influx [141]. However, the role of mitochondria in these pathways has not been investigated so far. An interesting exception is the TRPM2 channel. This nonselective cation channel is Ca2+-permeable and activated by different compounds, among them oxidative stress and ADP-ribose. Recently, it was shown that oxidative stress stimulates mitochondria to produce ADP-ribose that subsequently activates TRPM2 [165]. Whether mitochondria are also involved in modulating the activation of other TRP channels is currently unknown.
Contribution of Ca2+ to mitochondrial function
Effect of Ca2+ on mitochondrial metabolism
In most cells, mitochondria provide most of the cell’s energy as ATP of which the synthesis from ADP is linked to a series of electron transport through the IMM. This complex endergonic reaction is powered by the oxidation of reduced cofactors (NADH++H+, FADH2), which are generated during nutrition catabolism via, e.g., the Krebs cycle, and deliver electrons to complex I and complex II of the respiratory chain in the IMM. The subsequent electron transfer to complex III and further to complex IV of the electron transport chain supplies energy to establish a proton gradient across the IMM, which is finally converted into the synthesis of ATP molecules at the ATP synthase complex (complex V). This vital process leading to mitochondrial ATP production has to be up- or downregulated according to the cellular needs of energy and the fluctuating supply of nutrition as well [21].
Mitochondrial Ca2+ signaling appears to be fundamental in the control of mitochondrial metabolism. Forty years ago, Hansford and Chappell reported that the oxidation of glycerol phosphate is activated by Ca2+ [85] and later on, McCormack and Denton precisely described that pyruvate dehydrogenase is activated by Ca2+-dependent dephosphorylation, while the NAD+-isocitrate deyhydrogenase and the 2-oxoglutarate dehydrogenase are directly activated by Ca2+ [133]. Thus, an increase in matrix Ca2+ concentration in the mitochondria accelerates the enzymatic activities of these three dehydrogenases leading to increased NADH levels [52, 82, 174], and subsequently, this Ca2+-triggered activation of Krebs cycle dehydrogenases actually culminates in an augmentation of the mitochondrial ATP production in intact living cells. That was shown in elegant experiments using the luciferin luciferase reaction [104]. Moreover, they could also describe a so far not further characterized mitochondrial memory that allows a long-term activation of mitochondrial ATP production up to 60 min after agonist-induced Ca2+ signal elevation. In line with these reports, we could demonstrate that an augmentation of mitochondrial Ca2+ uptake capacity upon cell stimulation with an IP3 generating agonist by overexpression of UCP2 or UCP3 resulted in a significant enhancement of the agonist-induced mitochondrial ATP generation in endothelial cells [215]. In addition, there are several reports indicating that the Ca2+-sensitive dehydrogenases are not the only _targets of the mitochondrial metabolic pathways for Ca2+-dependent activation of mitochondrial ATP production. Ca2+-sensitivity was also reported for the F0/F1-ATPase (heart, [87, 211]), the adenine nucleotide translocase (liver, [144]), complexes of the respiratory chain [188], and EF-hand containing substrate carriers of the IMM [113, 158].
All these reports on the stimulatory effect of Ca2+ for mitochondrial ATP production illustrate the functional significance of mitochondrial Ca2+ sequestration and explain how a cell can accommodate the increasing demand of energy during stimulated states. However, the effects of matrix Ca2+ that exceed its regulatory function on oxidative phosphorylation might be multiple and await further investigation.
Interestingly, there is a limited number of reports that are contrary to the dogma that mitochondrial Ca2+ accumulation accelerates mitochondrial metabolism [17, 122]. In these reports, conditions are described in which the depolarizing effect of Ca2+ on the IMM exceeds its stimulatory effect on the respiratory chain/dehydrogenases, and consequently leads to a Ca2+-induced decrease in NADH levels. Such a scenario seems conceivable under conditions of excessive activation of respiration by substrate overload and/or under conditions of pathologically increased mitochondrial Ca2+ sequestration as elaborated in a mathematical model by Bertram et al. [17]. Notably, under conditions of substrate or Ca2+ overload of the mitochondria the formation of reactive oxygen species (ROS) is assumed to play an important role in mitochondrial degeneration/dysfunction.
Impact of mitochondrial Ca2+ on ROS generation and ROS defense
In most cells mitochondria represent the main source of the physiological production of ROS. Basically, mitochondrial-generated ROS come from an interaction between oxygen (O2) with unpaired electrons, which occur along the respiration. In average 1 to 2% of the total electrons transported through the respiratory chain leak to produce superoxide anions [24], which can be rapidly converted into the more reactive H2O2 and its aggressive derivative the hydroxyl radicals (•OH–). Among the actual sites of ROS generation in mitochondria complex I and complex III have attracted most attention [10, 114]. However, very recently, matrix enzymes such as the alpha-ketoglutarate dehydrogenase and other components of dehydrogenase complexes of the Krebs cycle have been also considered as important sources of ROS within mitochondria [36]. To balance the permanent production of ROS, mitochondria also house a sophisticated defense network of enzymatic and nonenzymatic antioxidants against ROS [2]. However, although ROS have been accused to exhibit numerous pathological effects in mitochondria and can lead to lipid peroxidation, which subsequently causes oxidative damage of mitochondrial DNA, RNA and enzymes, mitochondrial ROS generation might constitute an important signaling molecule to modulate cellular signal transduction in health and disease.
The impact of mitochondrial Ca2+ signaling on mitochondrial ROS homeostasis is in most instances ambiguous. There are various reports implying that an excessive mitochondrial Ca2+ sequestration facilitate the generation of ROS within mitochondria [57, 179, 180]. However, the underlying mechanisms for Ca2+-induced mitochondrial ROS production has not been clarified entirely but might include activation of the electron transport chain as well as Ca2+-induced opening of the mitochondrial permeability transition pore (PTP).
A different conclusion of the consequence of mitochondrial Ca2+ uptake for mitochondrial ROS generation can be obtained if the electrical effect of Ca2+ on the potential of the IMM (Δψmito) is considered. Ca2+ uptake into mitochondria reduces Δψmito, which has been shown to counteract generation of during oxidative phosphorylation [202, 219]. Moreover, Ca2+ directly or via calmodulin modulates the activity of enzymes of the antioxidant defense systems (e,g, MnSOD), indicating that Ca2+ exhibits dual contribution in the regulation and control of the mitochondrial ROS homeostasis [91, 225]. Additionally, mitochondrial Ca2+ inhibits the removal of hydrogen peroxide and its succinate-fueled production, thus indicating that mitochondria function as intracellular Ca2+-modulated peroxide sinks [231].
Our understanding on mitochondrial ROS production is controversial and rather confused. This is not surprising as the methods to measure ROS production lack specificity and mitochondria from different tissue origin might metabolically not be comparable. Notably, UCP2 and UCP3 have been shown to suppress mitochondrial ROS production under various experimental conditions presumably via their uncoupling function (for a recent review see Dlaskova et al. [50]). We have recently demonstrated in intact as well as isolated mitochondria that UCP2 and UCP3 are fundamental for mitochondrial Ca2+ uniport while no obvious evidence for H+ conductance was found [215]. Consequently, in addition to the dogma of smooth uncoupling via UCP2/UCP3-mediated H+ flux, the discovered Ca2+ function of these proteins might be, at least partly, responsible for the reported reduction in mitochondrial ROS production by these UCP proteins. Since the correlation of UCP2/3-dependent mitochondrial Ca2+ fluxes with mitochondrial ROS production has not been elucidated so far, the molecular mechanisms beyond UCP2/UCP3-associated attenuation of mitochondrial ROS production awaits further investigation.
On the other hand, extramitochondrial-produced ROS might affect mitochondria as well. In recent studies, exposure of mitochondria to ROS have been shown to affect the structural organization and Ca2+ homeostasis [160] and trigger IP3-linked apoptotic cascade [124], thus indicating that this organelle can be affected by cytosolic-/plasma membrane-originated ROS also.
Ca2+ as regulator of mitochondrial phosphoproteom
Reversible phosphorylation of enzymes, receptors, ion channels, transcription factors and cytoskeletal elements is a hallmark of cell signaling (Greengard, Science, 1976, 146–152). It has recently emerged that the role of phosphorylated proteins as physiological effectors is not restricted to the cytosol, but is also fundamentally involved in the homeostasis of mitochondrial functions. With the development of improved phosphoproteomic screens, the existence of multiple phosphoproteins in mitochondria could be confirmed [91]. Importantly, there are protein kinases and phosphatases that are exclusively located within the mitochondrial matrix [132], in the IMM [107], the intermembrane space [194], and at the cytoplasmic surface of mitochondria [34]. Moreover, cytosolic protein kinases are known to be imported into mitochondria by a yet not fully understood mechanisms [97, 156].
Although the Ca2+ dependent dephosphorylation of the pyruvate dehydrogenase complex within mitochondria was already described in the late 1960s [118], only limited knowledge is available about the impact of Ca2+ on posttranslational modification of mitochondrial proteins. Very recently, a dynamic change in the phosphorylation state of multiple mitochondrial proteins has been shown in porcine heart mitochondria in response to Ca2+ elevation [91]. The mitochondrial proteins that got phosphorylated in response to matrix Ca2+ elevation included components of the electron transport chain and the Krebs cycle, indicating that Ca2+ impacts mitochondrial bioenergetics on various levels. Moreover, a Ca2+-induced dephosphorylation of the MnSOD was also described, which was associated with an increase in its enzymatic activity, while neither the kinase(s) nor the phosphatase(s) involved in this process has been identified so far. Similar findings were obtained in rat brain mitochondria, in which several, so far unidentified, low molecular mass proteins were found to be phosphorylated in a Ca2+-dependent manner [7]. Interestingly, this Ca2+-induced protein phosphorylation depended on the opening of the mitochondrial PTP while its particular function in Ca2+-activated phosphorylation of mitochondrial proteins remained unresolved.
In summary, convincing evidence points to an importance of mitochondrial Ca2+ fluxes for protein phosphorylation and dephosphorylation as a crucial mechanism in the dynamic regulation of various mitochondrial functions. However, this remains an emerging field, which requires further investigation to be fully integrated into a complex understanding of the molecular mechanisms of the regulation of mitochondrial functions.
Effect of Ca2+ on mitochondrial motility and morphology
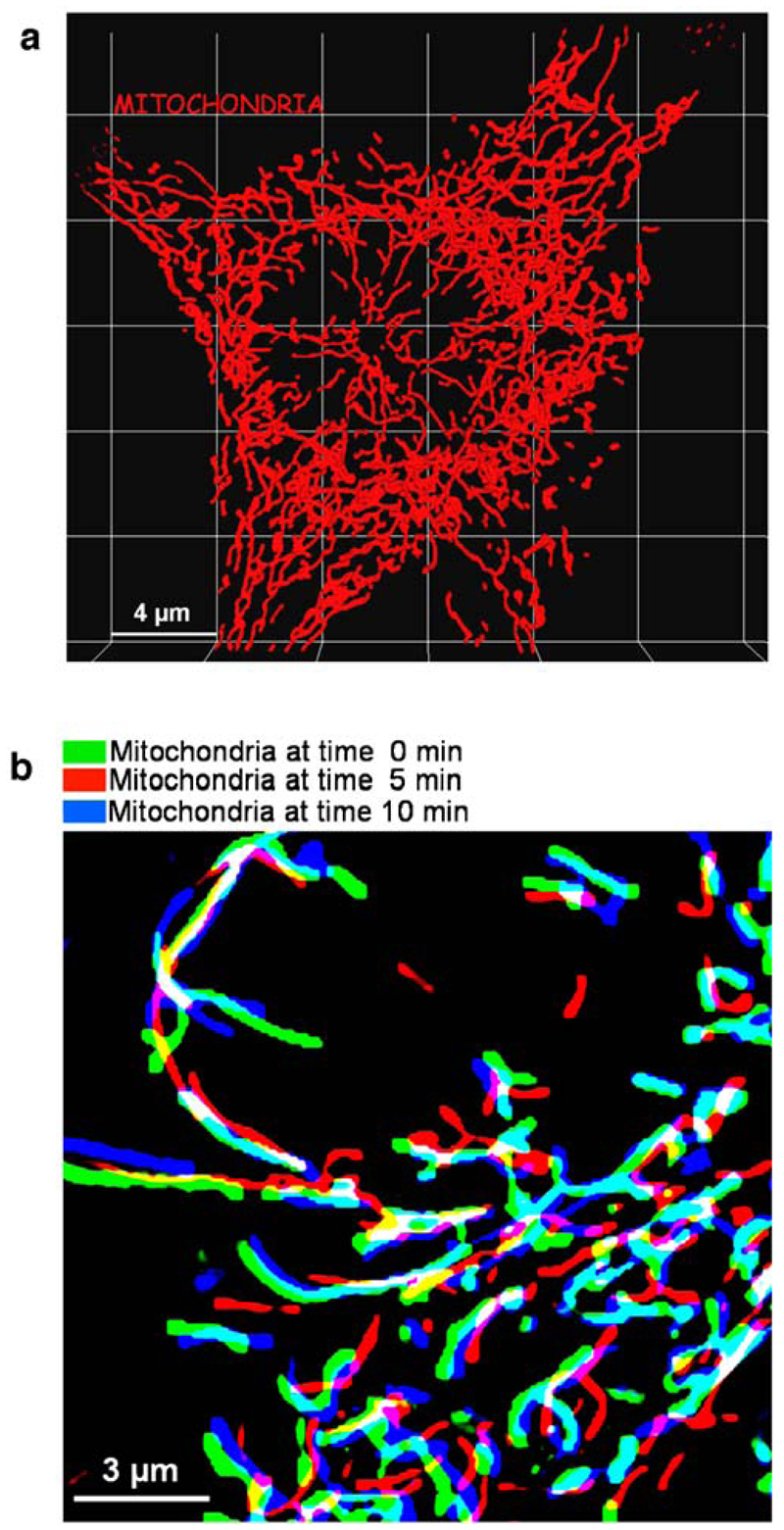
Mitochondria display a very complex architectural organization that varies continuously over time depending on their metabolic activity, the level of cell activation [224], the cell cycle status [14, 64], and the impact of physical or chemical environmental signals. In most cells, mitochondria are largely interconnected tubular structures [190] (Fig. 6a), whereas mitochondria with round punctuated morphology are less frequently described [38] and may represent an early indication of mitochondrial stress initiated by either metabolic overload [160], chemical depolarization (Fig. 2), uncontrolled fission processes [1, 163, 164], or physical disturbance (R. Malli and W.F. Graier, unpublished observations). In addition, in certain cell types, mitochondrial structure reflects their function, such like spatial Ca2+ buffering in pancreas acinar cells [22, 68, 162, 166, 167, 169], cardiac muscle [135, 209], chromaffin cells [88], or nerves [29, 32, 151].
Fig. 6.
Mitochondrial morphology and motility. a Highly interconnected mitochondria in a single living HeLa cell that transiently expressed mitochondria-_targeted DsRed. Z-Scans were performed on an array confocal laser scanning microscope applying 514 nm excitation and measuring 570 nm emission [160]. 3-D reconstruction of the mitochondrial network was performed using Imaris 4.2 software. b Overlay of mitochondrial structures in a single endothelial cell expressing mitochondria-_targeted DsRed at 0, 5 and 10 min
Besides the complexity of their architectural organization, mitochondria exhibit complex dynamics (Fig. 6b) that have been shown to depend on cytoskeleton-based transport mainly along microtubule via motor proteins such as the mitochondria linked kinesin Kif1B [210]. Hence, mitochondrial structure continuously changes by processes of fusion, branching, or fission [14, 224] that results in a permanent rearrangement of the mitochondria (Fig. 6b). In contrast to the molecular mechanisms responsible for mitochondrial motility that are poorly understood so far, major proteins that participate in the regulation of the organelles fission, fusion, and transport along cytoskeleton elements have already been identified (for review, see [190, 224]). The processes of fission and fusion are mainly under the control of guanosine triphosphatases (GTPases) such Drp1, Mitofusins (Mfn) and OPA-1 [23], and their associated adaptor proteins like hFis1 [98] as well as Rho family proteins [95, 204].
Both, mitochondrial motility and morphology are significantly affected by Ca2+. Particularly, mitochondrial motility is regulated by Ca2+ in the physiological range with maximal movement at low resting cytosolic Ca2+ levels and a complete arrest at 1–2 μM free Ca2+ in the cytosol [227]. The molecular mechanism behind this Ca2+-triggered arresting of mitochondria is unknown, but indicates that mitochondria might be recruited in a Ca2+-dependent manner to enhance local Ca2+ buffering and/or ATP supply on distinct cellular demands. Hence, the elevation of intracellular Ca2+ concentration initiates the translocation of Drp1 to the mitochondria and thus initiation of fission processes that consequently results in mitochondrial fragmentation [27]. In this regard, a reversible Ca2+-dependent fragmentation of interconnected tubular mitochondria upon stimulation with an IP3-generating agonist was described in HeLa cells [204]. Whether this Ca2+ induced mitochondrial fission is due to alterations of the potential of the inner mitochondrial membrane and alteration of the matrix pH or depends on an activation of GTPases most likely of Drp-1 [116] remains however elusive.
In summary, mitochondria are very mobile organelles, which move, fuse, branch, and divide to create a flexible and highly dynamic tubular network, whose morphology, motility, and intracellular distribution is evidently affected by Ca2+. Although the molecular processes, pathways of regulation as well as the physiological function of this striking aspect of mitochondrial Ca2+ signaling are not fully understood yet, one can expect that along their exploration we will learn further exciting and so far unknown functions of these old guests.
Conclusion
During recent years, mitochondria have been discovered to represent much more than the cell’s power plants that are crucially involved in nutrition metabolism and energy production. Utilizing newly developed techniques and instrumentations we started to explore the contribution of this fascinating organelle to signal transduction pathways, regulation, and tuning of multiple cell functions and ion homeostasis. Due to the versatile signaling properties of Ca2+, mitochondrial Ca2+ homeostasis, which was proved to represent an active and complexly regulated process, obviously plays a crucial role in most already established as well as newly discovered mitochondrial functions. In spite of many outstanding contributions regarding the regulation and function of mitochondrial Ca2+ most of the molecular contributors to mitochondrial Ca2+ homeostasis urgently await identification. Complementing this task will be prerequisite for further exploration of new Ca2+-dependent functions of these old guests.
Acknowledgement
The scientific work of the authors is supported by the Austrian Funds (P16860-B09 and SFB F3010-B05; WFG), the Franz Lanyar Foundation (WFG), the Swiss National Science Foundation (#320000-107622; MF), and the Foundation Carlos and Elsie de Reuter (MF).
Contributor Information
Wolfgang F. Graier, Molecular and Cellular Physiology Research Unit, MCPRU, Institute of Molecular Biology and Biochemistry, Center of Molecular Medicine, Medical University of Graz, Harrachgasse 21/III, A-8010 Graz, Austria, wolfgang.graier@meduni-graz.at URL: http://user.meduni-graz.at/wolfgang.graier/graier.htm
Maud Frieden, Department of Cell Physiology and Metabolism, Geneva Medical Center, 1211 Geneva 4, Switzerland.
Roland Malli, Molecular and Cellular Physiology Research Unit, MCPRU, Institute of Molecular Biology and Biochemistry, Center of Molecular Medicine, Medical University of Graz, Harrachgasse 21/III, A-8010 Graz, Austria.
References
- 1.Alirol E, Martinou JC. Mitochondria and cancer: is there a morphological connection? Oncogene. 2006;25:4706–4716. doi: 10.1038/sj.onc.1209600. [DOI] [PubMed] [Google Scholar]
- 2.Andreyev AY, Kushnareva YE, Starkov AA. Mitochondrial metabolism of reactive oxygen species. Biochemistry (Mosc) 2005;70:200–214. doi: 10.1007/s10541-005-0102-7. [DOI] [PubMed] [Google Scholar]
- 3.Ardehali H, Chen Z, Ko Y, Mejia-Alvarez R, Marban E. Multiprotein complex containing succinate dehydrogenase confers mitochondrial ATP-sensitive K+ channel activity. Proc Natl Acad Sci USA. 2004;101:11880–11885. doi: 10.1073/pnas.0401703101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armstrong JS. The role of the mitochondrial permeability transition in cell death. Mitochondrion. 2006;6:225–234. doi: 10.1016/j.mito.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 5.Arnaudeau S, Kelley WL, Walsh JV, Jr, Demaurex N. Mitochondria recycle Ca2+ to the endoplasmic reticulum and prevent the depletion of neighboring endoplasmic reticulum regions. J Biol Chem. 2001;276:29430–29439. doi: 10.1074/jbc.M103274200. [DOI] [PubMed] [Google Scholar]
- 6.Ayub K, Hallett MB. The mitochondrial ADPR link between Ca2+ store release and Ca2+ influx channel opening in immune cells. Faseb J. 2004;18:1335–1338. doi: 10.1096/fj.04-1888hyp. [DOI] [PubMed] [Google Scholar]
- 7.Azarashvili T, Krestinina O, Odinokova I, Evtodienko Y, Reiser G. Physiological Ca2+ level and Ca2+-induced Permeability Transition Pore control protein phosphorylation in rat brain mitochondria. Cell Calcium. 2003;34:253–259. doi: 10.1016/s0143-4160(03)00107-6. [DOI] [PubMed] [Google Scholar]
- 8.Babcock DF, Herrington J, Goodwin PC, Park YB, Hille B. Mitochondrial participation in the intracellular Ca2+ network. J Cell Biol. 1997;136:833–844. doi: 10.1083/jcb.136.4.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Babcock DF, Hille B. Mitochondrial oversight of cellular Ca2+ signaling. Curr Opin Neurobiol. 1998;8:398–404. doi: 10.1016/s0959-4388(98)80067-6. [DOI] [PubMed] [Google Scholar]
- 10.Barja G. Mitochondrial oxygen radical generation and leak: sites of production in states 4 and 3, organ specificity, and relation to aging and longevity. J Bioenerg Biomembr. 1999;31:347–366. doi: 10.1023/a:1005427919188. [DOI] [PubMed] [Google Scholar]
- 11.Baron KT, Thayer SA. CGP37157 modulates mitochondrial Ca2+ homeostasis in cultured rat dorsal root ganglion neurons. Eur J Pharmacol. 1997;340:295–300. doi: 10.1016/s0014-2999(97)01433-7. [DOI] [PubMed] [Google Scholar]
- 12.Bathori G, Csordas G, Garcia-Perez C, Davies E, Hajnoczky G. Ca2+-dependent control of the permeability properties of the mitochondrial outer membrane and voltage-dependent anion-selective channel (VDAC) J Biol Chem. 2006;281:17347–17358. doi: 10.1074/jbc.M600906200. [DOI] [PubMed] [Google Scholar]
- 13.Belous AE, Jones CM, Wakata A, Knox CD, Nicoud IB, Pierce J, Chari RS. Mitochondrial calcium transport is regulated by P2Y1- and P2Y2-like mitochondrial receptors. J Cell Biochem. 2006;22:1165–1174. doi: 10.1002/jcb.20985. [DOI] [PubMed] [Google Scholar]
- 14.Bereiter-Hahn J, Voth M. Dynamics of mitochondria in living cells: shape changes, dislocations, fusion, and fission of mitochondria. Microsc Res Tech. 1994;27:198–219. doi: 10.1002/jemt.1070270303. [DOI] [PubMed] [Google Scholar]
- 15.Bernardi P. Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiol Rev. 1999;79:1127–1155. doi: 10.1152/physrev.1999.79.4.1127. [DOI] [PubMed] [Google Scholar]
- 16.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 17.Bertram R, Gram Pedersen M, Luciani DS, Sherman A. A simplified model for mitochondrial ATP production. J Theor Biol. 2006;243:575–586. doi: 10.1016/j.jtbi.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 18.Beutner G, Sharma VK, Giovannucci DR, Yule DI, Sheu SS. Identification of a ryanodine receptor in rat heart mitochondria. J Biol Chem. 2001;276:21482–21488. doi: 10.1074/jbc.M101486200. [DOI] [PubMed] [Google Scholar]
- 19.Beutner G, Sharma VK, Lin L, Ryu SY, Dirksen RT, Sheu SS. Type 1 ryanodine receptor in cardiac mitochondria: transducer of excitation-metabolism coupling. Biochim Biophys Acta. 2005;1717:1–10. doi: 10.1016/j.bbamem.2005.09.016. (Epub 2005 Oct 2011) [DOI] [PubMed] [Google Scholar]
- 20.Boitier E, Rea R, Duchen MR. Mitochondria exert a negative feedback on the propagation of intracellular Ca2+ waves in rat cortical astrocytes. J Cell Biol. 1999;145:795–808. doi: 10.1083/jcb.145.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boneh A. Regulation of mitochondrial oxidative phosphorylation by second messenger-mediated signal transduction mechanisms. Cell Mol Life Sci. 2006;63:1236–1248. doi: 10.1007/s00018-005-5585-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bootman MD, Petersen OH, Verkhratsky A. The endoplasmic reticulum is a focal point for co-ordination of cellular activity. Cell Calcium. 2002;32:231–234. doi: 10.1016/s0143416002002002. [DOI] [PubMed] [Google Scholar]
- 23.Bossy-Wetzel E, Barsoum MJ, Godzik A, Schwarzenbacher R, Lipton SA. Mitochondrial fission in apoptosis, neurodegeneration and aging. Curr Opin Cell Biol. 2003;15:706–716. doi: 10.1016/j.ceb.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 24.Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J. 1973;134:707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brand MD, Affourtit C, Esteves TC, Green K, Lambert AJ, Miwa S, Pakay JL, Parker N. Mitochondrial superoxide: production, biological effects, and activation of uncoupling proteins. Free Radic Biol Med. 2004;37:755–767. doi: 10.1016/j.freeradbiomed.2004.05.034. [DOI] [PubMed] [Google Scholar]
- 26.Brand MD, Esteves TC. Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3. Cell Metab. 2005;2:85–93. doi: 10.1016/j.cmet.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 27.Breckenridge DG, Stojanovic M, Marcellus RC, Shore GC. Caspase cleavage product of BAP31 induces mitochondrial fission through endoplasmic reticulum calcium signals, enhancing cytochrome c release to the cytosol. J Cell Biol. 2003;160:1115–1127. doi: 10.1083/jcb.200212059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brennan JP, Southworth R, Medina RA, Davidson SM, Duchen MR, Shattock MJ. Mitochondrial uncoupling, with low concentration FCCP, induces ROS-dependent cardioprotection independent of KATP channel activation. Cardiovasc Res. 2006;72:313–321. doi: 10.1016/j.cardiores.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 29.Brown MR, Sullivan PG, Geddes JW. Synaptic mitochondria are more susceptible to Ca2+ overload than nonsynaptic mitochondria. J Biol Chem. 2006;281:11658–11668. doi: 10.1074/jbc.M510303200. [DOI] [PubMed] [Google Scholar]
- 30.Brustovetsky T, Shalbuyeva N, Brustovetsky N. Lack of manifestations of diazoxide/5-hydroxydecanoate-sensitive KATP channel in rat brain nonsynaptosomal mitochondria. J Physiol. 2005;568:47–59. doi: 10.1113/jphysiol.2005.091199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chalmers S, Nicholls DG. The relationship between free and total calcium concentrations in the matrix of liver and brain mitochondria. J Biol Chem. 2003;278:19062–19070. doi: 10.1074/jbc.M212661200. [DOI] [PubMed] [Google Scholar]
- 32.Chan DC. Mitochondria: dynamic organelles in disease, aging, and development. Cell. 2006;125:1241–1252. doi: 10.1016/j.cell.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 33.Chan SL, Liu D, Kyriazis GA, Bagsiyao P, Ouyang X, Mattson MP. Mitochondrial uncoupling protein-4 regulates calcium homeostasis and sensitivity to store depletion-induced apoptosis in neural cells. J Biol Chem. 2006;281:37391–37403. doi: 10.1074/jbc.M605552200. [DOI] [PubMed] [Google Scholar]
- 34.Chen Q, Lin RY, Rubin CS. Organelle-specific _targeting of protein kinase AII (PKAII). Molecular and in situ characterization of murine A kinase anchor proteins that recruit regulatory subunits of PKAII to the cytoplasmic surface of mitochondria. J Biol Chem. 1997;272:15247–15257. doi: 10.1074/jbc.272.24.15247. [DOI] [PubMed] [Google Scholar]
- 35.Cheranov SY, Jaggar JH. Mitochondrial modulation of Ca2+ sparks and transient KCa currents in smooth muscle cells of rat cerebral arteries. J Physiol. 2004;556:755–771. doi: 10.1113/jphysiol.2003.059568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chinopoulos C, Adam-Vizi V. Calcium, mitochondria and oxidative stress in neuronal pathology. Novel aspects of an enduring theme. Febs J. 2006;273:433–450. doi: 10.1111/j.1742-4658.2005.05103.x. [DOI] [PubMed] [Google Scholar]
- 37.Coatesworth W, Bolsover S. Spatially organised mitochondrial calcium uptake through a novel pathway in chick neurones. Cell Calcium. 2006;39:217–225. doi: 10.1016/j.ceca.2005.10.012. (Epub 2005 Dec 2009) [DOI] [PubMed] [Google Scholar]
- 38.Collins TJ, Berridge MJ, Lipp P, Bootman MD. Mitochondria are morphologically and functionally heterogeneous within cells. EMBO J. 2002;21:1616–1627. doi: 10.1093/emboj/21.7.1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Collins TJ, Lipp P, Berridge MJ, Bootman MD. Mitochondrial Ca2+ uptake depends on the spatial and temporal profile of cytosolic Ca2+ signals. J Biol Chem. 2001;276:26411–26420. doi: 10.1074/jbc.M101101200. [DOI] [PubMed] [Google Scholar]
- 40.Crompton M, Andreeva L. On the interactions of Ca2+ and cyclosporin A with a mitochondrial inner membrane pore: a study using cobaltammine complex inhibitors of the Ca2+ uniporter. Biochem J. 1994;302:181–185. doi: 10.1042/bj3020181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crompton M, Kunzi M, Carafoli E. The calcium-induced and sodium-induced effluxes of calcium from heart mitochondria. Evidence for a sodium–calcium carrier. Eur J Biochem. 1977;79:549–558. doi: 10.1111/j.1432-1033.1977.tb11839.x. [DOI] [PubMed] [Google Scholar]
- 42.Csordas G, Madesh M, Antonsson B, Hajnoczky G. tcBid promotes Ca2+ signal propagation to the mitochondria: control of Ca2+ permeation through the outer mitochondrial membrane. EMBO J. 2002;21:2198–2206. doi: 10.1093/emboj/21.9.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Csordas G, Renken C, Varnai P, Walter L, Weaver D, Buttle KF, Balla T, Mannella CA, Hajnoczky G. Structural and functional features and significance of the physical linkage between ER and mitochondria. J Cell Biol. 2006;174:915–921. doi: 10.1083/jcb.200604016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Csordas G, Thomas AP, Hajnoczky G. Quasi-synaptic calcium signal transmission between endoplasmic reticulum and mitochondria. EMBO J. 1999;18:96–108. doi: 10.1093/emboj/18.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Meis L, Arruda AP, da Costa RM, Benchimol M. Identification of a Ca2+-ATPase in brown adipose tissue mitochondria: regulation of thermogenesis by ATP and Ca2+ J Biol Chem. 2006;281:16384–16390. doi: 10.1074/jbc.M600678200. [DOI] [PubMed] [Google Scholar]
- 46.Dejean L, Camara Y, Sibille B, Solanes G, Villarroya F. Uncoupling protein-3 sensitizes cells to mitochondrial-dependent stimulus of apoptosis. J Cell Physiol. 2004;201:294–304. doi: 10.1002/jcp.20048. [DOI] [PubMed] [Google Scholar]
- 47.Del Arco A, Satrustegui J. Identification of a novel human subfamily of mitochondrial carriers with calcium-binding domains. J Biol Chem. 2004;279:24701–24713. doi: 10.1074/jbc.M401417200. [DOI] [PubMed] [Google Scholar]
- 48.Demaurex N, Distelhorst C. Cell biology. Apoptosis—the calcium connection. Science. 2003;300:65–67. doi: 10.1126/science.1083628. [DOI] [PubMed] [Google Scholar]
- 49.Dhalla NS. Excitation–contraction coupling in heart. I. Comparison of calcium uptake by the sarcoplasmic reticulum and mitochondria of the rat heart. Arch Int Physiol Biochim. 1969;77:916–934. doi: 10.3109/13813456909059804. [DOI] [PubMed] [Google Scholar]
- 50.Dlaskova A, Spacek T, Skobisova E, Santorova J, Jezek P. Certain aspects of uncoupling due to mitochondrial uncoupling proteins in vitro and in vivo. Biochim Biophys Acta. 2006;1757:467–473. doi: 10.1016/j.bbabio.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 51.Drose S, Brandt U, Hanley PJ. K+-independent actions of diazoxide question the role of inner membrane KATP channels in mitochondrial cytoprotective signaling. J Biol Chem. 2006;281:23733–23739. doi: 10.1074/jbc.M602570200. [DOI] [PubMed] [Google Scholar]
- 52.Duchen MR. Ca2+-dependent changes in the mitochondrial energetics in single dissociated mouse sensory neurons. Biochem J. 1992;283:41–50. doi: 10.1042/bj2830041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Duchen MR. Contributions of mitochondria to animal physiology: from homeostatic sensor to calcium signalling and cell death. J Physiol. 1999;516:1–17. doi: 10.1111/j.1469-7793.1999.001aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Duchen MR. Mitochondria and calcium: from cell signalling to cell death. J Physiol. 2000;529:57–68. doi: 10.1111/j.1469-7793.2000.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Duchen MR. Mitochondria in health and disease: perspectives on a new mitochondrial biology. Mol Aspects Med. 2004;25:365–451. doi: 10.1016/j.mam.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 56.Duchen MR. Roles of mitochondria in health and disease. Diabetes. 2004;53:S96–S102. doi: 10.2337/diabetes.53.2007.s96. [DOI] [PubMed] [Google Scholar]
- 57.Dykens JA. Isolated cerebral and cerebellar mitochondria produce free radicals when exposed to elevated Ca2+ and Na+: implications for neurodegeneration. J Neurochem. 1994;63:584–591. doi: 10.1046/j.1471-4159.1994.63020584.x. [DOI] [PubMed] [Google Scholar]
- 58.Eder P, Poteser M, Romanin C, Groschner K. Na+ entry and modulation of Na+/Ca2+ exchange as a key mechanism of TRPC signaling. Pflugers Arch. 2005;451:99–104. doi: 10.1007/s00424-005-1434-2. [DOI] [PubMed] [Google Scholar]
- 59.Er E, Oliver L, Cartron PF, Juin P, Manon S, Vallette FM. Mitochondria as the _target of the pro-apoptotic protein Bax. Biochim Biophys Acta. 2006;1757:1301–1311. doi: 10.1016/j.bbabio.2006.05.032. [DOI] [PubMed] [Google Scholar]
- 60.Erlanson-Albertsson C. Uncoupling proteins—a new family of proteins with unknown function. Nutr Neurosci. 2002;5:1–11. doi: 10.1080/10284150290007038. [DOI] [PubMed] [Google Scholar]
- 61.Evtodienko YV. Sustained oscillations of transmembrane Ca2+ fluxes in mitochondria and their possible biological significance. Membr Cell Biol. 2000;14:1–17. [PubMed] [Google Scholar]
- 62.Falcke M, Hudson JL, Camacho P, Lechleiter JD. Impact of mitochondrial Ca2+ cycling on pattern formation and stability. Biophys J. 1999;77:37–44. doi: 10.1016/S0006-3495(99)76870-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Filippin L, Magalhaes PJ, Di Benedetto G, Colella M, Pozzan T. Stable interactions between mitochondria and endoplasmic reticulum allow rapid accumulation of calcium in a subpopulation of mitochondria. J Biol Chem. 2003;31:31. doi: 10.1074/jbc.M302301200. [DOI] [PubMed] [Google Scholar]
- 64.Frank S, Gaume B, Bergmann-Leitner ES, Leitner WW, Robert EG, Catez F, Smith CL, Youle RJ. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell. 2001;1:515–525. doi: 10.1016/s1534-5807(01)00055-7. [DOI] [PubMed] [Google Scholar]
- 65.Frieden M, James D, Castelbou C, Danckaert A, Martinou JC, Demaurex N. Ca2+ homeostasis during mitochondrial fragmentation and perinuclear clustering induced by hFis1. J Biol Chem. 2004;279:22704–22714. doi: 10.1074/jbc.M312366200. [DOI] [PubMed] [Google Scholar]
- 66.Garlid KD. On the mechanism of regulation of the mitochondrial K+/H+ exchanger. J Biol Chem. 1980;255:11273–11279. [PubMed] [Google Scholar]
- 67.Garlid KD, Paucek P, Yarov-Yarovoy V, Murray HN, Darbenzio RB, D'Alonzo AJ, Lodge NJ, Smith MA, Grover GJ. Cardioprotective effect of diazoxide and its interaction with mitochondrial ATP-sensitive K+ channels. Possible mechanism of cardioprotection. Circ Res. 1997;81:1072–1082. doi: 10.1161/01.res.81.6.1072. [DOI] [PubMed] [Google Scholar]
- 68.Gerasimenko OV, Gerasimenko JV, Rizzuto RR, Treiman M, Tepikin AV, Petersen OH. The distribution of the endoplasmic reticulum in living pancreatic acinar cells. Cell Calcium. 2002;32:261–268. doi: 10.1016/s0143416002001938. [DOI] [PubMed] [Google Scholar]
- 69.Gilabert JA, Bakowski D, Parekh AB. Energized mitochondria increase the dynamic range over which inositol 1,4,5-trisphosphate activates store-operated calcium influx. EMBO J. 2001;20:2672–2679. doi: 10.1093/emboj/20.11.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gilabert JA, Parekh AB. Respiring mitochondria determine the pattern of activation and inactivation of the store-operated Ca2+ current ICRAC. EMBO J. 2000;19:6401–6407. doi: 10.1093/emboj/19.23.6401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Glitsch MD, Bakowski D, Parekh AB. Store-operated Ca2+ entry depends on mitochondrial Ca2+ uptake. EMBO J. 2002;21:6744–6754. doi: 10.1093/emboj/cdf675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gorlach A, Klappa P, Kietzmann T. The endoplasmic reticulum: folding, calcium homeostasis, signaling, and redox control. Antioxid Redox Signal. 2006;8:1391–1418. doi: 10.1089/ars.2006.8.1391. [DOI] [PubMed] [Google Scholar]
- 73.Graier WF, Paltauf-Doburzynska J, Hill BJ, Fleischhacker E, Hoebel BG, Kostner GM, Sturek M. Submaximal stimulation of porcine endothelial cells causes focal Ca2+ elevation beneath the cell membrane. J Physiol. 1998;506:109–125. doi: 10.1111/j.1469-7793.1998.109bx.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Graier WF, Simecek S, Sturek M. Cytochrome P450 mono-oxygenase-regulated signalling of Ca2+ entry in human and bovine endothelial cells. J Physiol. 1995;482:259–274. doi: 10.1113/jphysiol.1995.sp020515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guidarelli A, Sciorati C, Clementi E, Cantoni O. Peroxynitrite mobilizes calcium ions from ryanodine-sensitive stores, a process associated with the mitochondrial accumulation of the cation and the enforced formation of species mediating cleavage of genomic DNA. Free Radic Biol Med. 2006;41:154–164. doi: 10.1016/j.freeradbiomed.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 76.Gunter KK, Gunter TE. Transport of calcium by mitochondria. J Bioenerg Biomembr. 1994;26:471–485. doi: 10.1007/BF00762732. [DOI] [PubMed] [Google Scholar]
- 77.Gunter TE, Buntinas L, Sparagna G, Eliseev R, Gunter K. Mitochondrial calcium transport: mechanisms and functions. Cell Calcium. 2000;28:285–296. doi: 10.1054/ceca.2000.0168. [DOI] [PubMed] [Google Scholar]
- 78.Gunter TE, Pfeiffer DR. Mechanisms by which mitochondria transport calcium. Am J Physiol. 1990;258:C755–C786. doi: 10.1152/ajpcell.1990.258.5.C755. [DOI] [PubMed] [Google Scholar]
- 79.Gunter TE, Yule DI, Gunter KK, Eliseev RA, Salter JD. Calcium and mitochondria. FEBS Lett. 2004;567:96–102. doi: 10.1016/j.febslet.2004.03.071. [DOI] [PubMed] [Google Scholar]
- 80.Hajnoczky G, Csordas G, Das S, Garcia-Perez C, Saotome M, Sinha Roy S, Yi M. Mitochondrial calcium signalling and cell death: approaches for assessing the role of mitochondrial Ca2+ uptake in apoptosis. Cell Calcium. 2006;40:553–560. doi: 10.1016/j.ceca.2006.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hajnoczky G, Hager R, Thomas AP. Mitochondria suppress local feedback activation of inositol 1,4, 5-trisphosphate receptors by Ca2+ J Biol Chem. 1999;274:14157–14162. doi: 10.1074/jbc.274.20.14157. [DOI] [PubMed] [Google Scholar]
- 82.Hajnoczky G, Robb-Gaspers LD, Seitz MB, Thomas AP. Decoding of cytosolic calcium oscillations in the mitochondria. Cell. 1995;82:415–424. doi: 10.1016/0092-8674(95)90430-1. [DOI] [PubMed] [Google Scholar]
- 83.Hanley PJ, Daut J. KATP channels and preconditioning: a re-examination of the role of mitochondrial KATP channels and an overview of alternative mechanisms. J Mol Cell Cardiol. 2005;39:17–50. doi: 10.1016/j.yjmcc.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 84.Hanley PJ, Mickel M, Loffler M, Brandt U, Daut J. KATP channel-independent _targets of diazoxide and 5-hydroxydecanoate in the heart. J Physiol. 2002;542:735–741. doi: 10.1113/jphysiol.2002.023960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hansford RG, Chappell JB. The effect of Ca2+ on the oxidation of glycerol phosphate by blowfly flight-muscle mitochondria. Biochem Biophys Res Commun. 1967;27:686–692. doi: 10.1016/s0006-291x(67)80090-1. [DOI] [PubMed] [Google Scholar]
- 86.Harper ME, Dent R, Monemdjou S, Bezaire V, Van Wyck L, Wells G, Kavaslar GN, Gauthier A, Tesson F, McPherson R. Decreased mitochondrial proton leak and reduced expression of uncoupling protein 3 in skeletal muscle of obese diet-resistant women. Diabetes. 2001;51:2459–2466. doi: 10.2337/diabetes.51.8.2459. [DOI] [PubMed] [Google Scholar]
- 87.Harris DA, Das AM. Control of mitochondrial ATP synthesis in the heart. Biochem J. 1991;280:561–573. doi: 10.1042/bj2800561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hernandez-Guijo JM, Maneu-Flores VE, Ruiz-Nuno A, Villarroya M, Garcia AG, Gandia L. Calcium-dependent inhibition of L, N, and P/Q Ca2+ channels in chromaffin cells: role of mitochondria. J Neurosci. 2001;21:2553–2560. doi: 10.1523/JNEUROSCI.21-08-02553.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Herrington J, Park YB, Babcock DF, Hille B. Dominant role of mitochondria in clearance of large Ca2+ loads from rat adrenal chromaffin cells. Neuron. 1996;16:219–228. doi: 10.1016/s0896-6273(00)80038-0. [DOI] [PubMed] [Google Scholar]
- 90.Holmuhamedov EL, Jahangir A, Oberlin A, Komarov A, Colombini M, Terzic A. Potassium channel openers are uncoupling protonophores: implication in cardioprotection. FEBS Lett. 2004;568:167–170. doi: 10.1016/j.febslet.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 91.Hopper RK, Carroll S, Aponte AM, Johnson DT, French S, Shen RF, Witzmann FA, Harris RA, Balaban RS. Mitochondrial matrix phosphoproteome: effect of extra mitochondrial calcium. Biochemistry. 2006;45:2524–2536. doi: 10.1021/bi052475e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Horbinski C, Chu CT. Kinase signaling cascades in the mitochondrion: a matter of life or death. Free Radic Biol Med. 2005;38:2–11. doi: 10.1016/j.freeradbiomed.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 93.Hoth M, Button DC, Lewis RS. Mitochondrial control of calcium-channel gating: a mechanism for sustained signaling and transcriptional activation in T lymphocytes. Proc Natl Acad Sci USA. 2000;97:10607–10612. doi: 10.1073/pnas.180143997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hoth M, Fanger CM, Lewis RS. Mitochondrial regulation of store-operated calcium signaling in T lymphocytes. J Cell Biol. 1997;137:633–648. doi: 10.1083/jcb.137.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ishihara N, Jofuku A, Eura Y, Mihara K. Regulation of mitochondrial morphology by membrane potential, and DRP1-dependent division and FZO1-dependent fusion reaction in mammalian cells. Biochem Biophys Res Commun. 2003;301:891–898. doi: 10.1016/s0006-291x(03)00050-0. [DOI] [PubMed] [Google Scholar]
- 96.Ishii K, Hirose K, Iino M. Ca2+ shuttling between endoplasmic reticulum and mitochondria underlying Ca2+ oscillations. EMBO Rep. 2006;7:390–396. doi: 10.1038/sj.embor.7400620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Itoh S, Lemay S, Osawa M, Che W, Duan Y, Tompkins A, Brookes PS, Sheu SS, Abe J. Mitochondrial Dok-4 recruits Src kinase and regulates NF-kappaB activation in endothelial cells. J Biol Chem. 2005;280:26383–26396. doi: 10.1074/jbc.M410262200. [DOI] [PubMed] [Google Scholar]
- 98.James DI, Parone PA, Mattenberger Y, Martinou JC. hFis1, a novel component of the mammalian mitochondrial fission machinery. J Biol Chem. 2003;278:36373–36379. doi: 10.1074/jbc.M303758200. [DOI] [PubMed] [Google Scholar]
- 99.Jezek P. Fatty acid interaction with mitochondrial uncoupling proteins. J Bioenerg Biomembr. 1999;31:457–466. doi: 10.1023/a:1005496306893. [DOI] [PubMed] [Google Scholar]
- 100.Jezek P, Garlid KD, Jaburek M. Possible physiological roles of mitochondrial uncoupling proteins-UCPn: Mechanism of uncoupling protein action. Int J Biochem Cell Biol. 2002;34:1190–1206. doi: 10.1016/s1357-2725(02)00061-4. [DOI] [PubMed] [Google Scholar]
- 101.Jezek P, Zackova M, Ruzicka M, Skobisova E, Jaburek M. Mitochondrial uncoupling proteins—facts and fantasies. Physiol Res. 2004;53:S199–S211. [PubMed] [Google Scholar]
- 102.Johnson-Cadwell LI, Jekabsons MB, Wang A, Polster BM, Nicholls DG. ‘Mild Uncoupling’ does not decrease mitochondrial superoxide levels in cultured cerebellar granule neurons but decreases spare respiratory capacity and increases toxicity to glutamate and oxidative stress. J Neurochem. 2007;10:1619–1631. doi: 10.1111/j.1471-4159.2007.04516.x. [DOI] [PubMed] [Google Scholar]
- 103.Jouaville LS, Ichas F, Holmuhamedov EL, Camacho P, Lechleiter JD. Synchronization of calcium waves by mitochondrial substrates in Xenopus laevis oocytes. Nature. 1995;377:438–441. doi: 10.1038/377438a0. [DOI] [PubMed] [Google Scholar]
- 104.Jouaville LS, Pinton P, Bastianutto C, Rutter GA, Rizzuto R. Regulation of mitochondrial ATP synthesis by calcium: evidence for a long-term metabolic priming. Proc Natl Acad Sci USA. 1999;96:13807–13812. doi: 10.1073/pnas.96.24.13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kamishima T, Quayle JM. Mitochondrial Ca2+ uptake is important over low [Ca2+]i range in arterial smooth muscle. Am J Physiol. 2002;283:H2431–H2439. doi: 10.1152/ajpheart.00865.2001. [DOI] [PubMed] [Google Scholar]
- 106.Kirichok Y, Krapivinsky G, Clapham DE. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004;427:360–364. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- 107.Kitagawa Y, Racker E. Purification and characterization of two protein kinases from bovine heart mitochondrial membrane. J Biol Chem. 1982;257:4547–4551. [PubMed] [Google Scholar]
- 108.Kolisek M, Zsurka G, Samaj J, Weghuber J, Schweyen RJ, Schweigel M. Mrs2p is an essential component of the major electrophoretic Mg2+ influx system in mitochondria. EMBO J. 2003;22:1235–1244. doi: 10.1093/emboj/cdg122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Krauss S, Zhang CY, Lowell BB. The mitochondrial uncoupling-protein homologues. Nat Rev Mol Cell Biol. 2005;6:248–261. doi: 10.1038/nrm1592. [DOI] [PubMed] [Google Scholar]
- 110.Krauss S, Zhang CY, Scorrano L, Dalgaard LT, St-Pierre J, Grey ST, Lowell BB. Superoxide-mediated activation of uncoupling protein 2 causes pancreatic {beta} cell dysfunction. J Clin Invest. 2003;112:1831–1842. doi: 10.1172/JCI19774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lacza Z, Snipes JA, Kis B, Szabo C, Grover G, Busija DW. Investigation of the subunit composition and the pharmacology of the mitochondrial ATP-dependent K+ channel in the brain. Brain Res. 2003;994:27–36. doi: 10.1016/j.brainres.2003.09.046. [DOI] [PubMed] [Google Scholar]
- 112.Landolfi B, Curci S, Debellis L, Pozzan T, Hofer AM. Ca2+ homeostasis in the agonist-sensitive internal store: functional interactions between mitochondria and the ER measured In situ in intact cells. J Cell Biol. 1998;142:1235–1243. doi: 10.1083/jcb.142.5.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lasorsa FM, Pinton P, Palmieri L, Fiermonte G, Rizzuto R, Palmieri F. Recombinant expression of the Ca2+-sensitive aspartate/glutamate carrier increases mitochondrial ATP production in agonist-stimulated Chinese hamster ovary cells. J Biol Chem. 2003;278:38686–38692. doi: 10.1074/jbc.M304988200. [DOI] [PubMed] [Google Scholar]
- 114.Lenaz G. The mitochondrial production of reactive oxygen species: mechanisms and implications in human pathology. IUBMB Life. 2001;52:159–164. doi: 10.1080/15216540152845957. [DOI] [PubMed] [Google Scholar]
- 115.Lenzen S, Hickethier R, Panten U. Interactions between spermine and Mg2+ on mitochondrial Ca2+ transport. J Biol Chem. 1986;261:16478–16483. [PubMed] [Google Scholar]
- 116.Li Z, Okamoto K, Hayashi Y, Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119:873–887. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 117.Liao Y, Erxleben C, Yildirim E, Abramowitz J, Armstrong DL, Birnbaumer L. Orai proteins interact with TRPC channels and confer responsiveness to store depletion. Proc Natl Acad Sci USA. 2007;104:4682–4687. doi: 10.1073/pnas.0611692104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Linn TC, Pettit FH, Reed LJ. Alpha-keto acid dehydrogenase complexes. X. Regulation of the activity of the pyruvate dehydrogenase complex from beef kidney mitochondria by phosphorylation and dephosphorylation. Proc Natl Acad Sci USA. 1969;62:234–241. doi: 10.1073/pnas.62.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Litsky ML, Pfeiffer DR. Regulation of the mitochondrial Ca2+ uniporter by external adenine nucleotides: the uniporter behaves like a gated channel which is regulated by nucleotides and divalent cations. Biochemistry. 1997;36:7071–7080. doi: 10.1021/bi970180y. [DOI] [PubMed] [Google Scholar]
- 121.Lobaton CD, Vay L, Hernandez-Sanmiguel E, Santodomingo J, Moreno A, Montero M, Alvarez J. Modulation of mitochondrial Ca2+ uptake by estrogen receptor agonists and antagonists. Br J Pharmacol. 2005;145:862–871. doi: 10.1038/sj.bjp.0706265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Luciani DS, Misler S, Polonsky KS. Ca2+ controls slow NAD(P)H oscillations in glucose-stimulated mouse pancreatic islets. J Physiol. 2006;572:379–392. doi: 10.1113/jphysiol.2005.101766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Maack C, Cortassa S, Aon MA, Ganesan AN, Liu T, O'Rourke B. Elevated cytosolic Na+ decreases mitochondrial Ca2+ uptake during excitation-contraction coupling and impairs energetic adaptation in cardiac myocytes. Circ Res. 2006;99:172–182. doi: 10.1161/01.RES.0000232546.92777.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Madesh M, Hawkins BJ, Milovanova T, Bhanumathy CD, Joseph SK, RamachandraRao SP, Sharma K, Kurosaki T, Fisher AB. Selective role for superoxide in InsP3 receptor-mediated mitochondrial dysfunction and endothelial apoptosis. J Cell Biol. 2005;170:1079–1090. doi: 10.1083/jcb.200505022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Malli R, Frieden M, Hunkova M, Trenker M, Graier WF. Ca2+ refilling of the endoplasmic reticulum is largely preserved albeit reduced Ca2+ entry in endothelial cells. Cell Calcium. 2007;41:63–76. doi: 10.1016/j.ceca.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Malli R, Frieden M, Osibow K, Graier WF. Mitochondria efficiently buffer subplasmalemmal Ca2+ elevation during agonist stimulation. J Biol Chem. 2003;278:10807–10815. doi: 10.1074/jbc.M212971200. [DOI] [PubMed] [Google Scholar]
- 127.Malli R, Frieden M, Osibow K, Zoratti C, Mayer M, Demaurex N, Graier WF. Sustained Ca2+ transfer across mitochondria is essential for mitochondrial Ca2+ buffering, store-operated Ca2+ entry, and Ca2+ store refilling. J Biol Chem. 2003;278:44769–44779. doi: 10.1074/jbc.M302511200. [DOI] [PubMed] [Google Scholar]
- 128.Malli R, Frieden M, Trenker M, Graier WF. The role of mitochondria for Ca2+ refilling of the ER. J Biol Chem. 2005;280:12114–12122. doi: 10.1074/jbc.M409353200. [DOI] [PubMed] [Google Scholar]
- 129.Mannella CA. Conformational changes in the mitochon-drial channel protein, VDAC, and their functional implications. J Struct Biol. 1998;121:207–218. doi: 10.1006/jsbi.1997.3954. [DOI] [PubMed] [Google Scholar]
- 130.Mannella CA, Pfeiffer DR, Bradshaw PC, Moraru II, Slepchenko B, Loew LM, Hsieh CE, Buttle K, Marko M. Topology of the mitochondrial inner membrane: dynamics and bioenergetic implications. IUBMB Life. 2001;52:93–100. doi: 10.1080/15216540152845885. [DOI] [PubMed] [Google Scholar]
- 131.Matlib MA, Zhou Z, Knight S, Ahmed S, Choi KM, Krause-Bauer J, Phillips R, Altschuld R, Katsube Y, Sperelakis N, Bers DM. Oxygen-bridged dinuclear ruthenium amine complex specifically inhibits Ca2+ uptake into mitochondria in vitro and in situ in single cardiac myocytes. J Biol Chem. 1998;273:10223–10231. doi: 10.1074/jbc.273.17.10223. [DOI] [PubMed] [Google Scholar]
- 132.McBride HM, Neuspiel M, Wasiak S. Mitochondria: more than just a powerhouse. Curr Biol. 2006;16:R551–R560. doi: 10.1016/j.cub.2006.06.054. [DOI] [PubMed] [Google Scholar]
- 133.McCormack JG, Denton RM. The effects of calcium ions and adenine nucleotides on the activity of pig heart 2-oxoglutarate dehydrogenase complex. Biochem J. 1979;180:533–544. doi: 10.1042/bj1800533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.McCormack JG, Halestrap AP, Denton RM. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol Rev. 1990;70:391–425. doi: 10.1152/physrev.1990.70.2.391. [DOI] [PubMed] [Google Scholar]
- 135.McMillin-Wood J, Wolkowicz PE, Chu A, Tate CA, Goldstein MA, Entman ML. Calcium uptake by two preparations of mitochondria from heart. Biochim Biophys Acta. 1980;591:251–265. doi: 10.1016/0005-2728(80)90157-7. [DOI] [PubMed] [Google Scholar]
- 136.Michalak M, Burns K, Andrin C, Mesaeli N, Jass GH, Busaan JL, Opas M. Endoplasmic reticulum form of calreticulin modulates glucocorticoid-sensitive gene expression. J Biol Chem. 1996;271:29436–29445. doi: 10.1074/jbc.271.46.29436. [DOI] [PubMed] [Google Scholar]
- 137.Michalak M, Lynch J, Groenendyk J, Guo L, Robert Parker JM, Opas M. Calreticulin in cardiac development and pathology. Biochim Biophys Acta. 2002;1600:32–37. doi: 10.1016/s1570-9639(02)00441-7. [DOI] [PubMed] [Google Scholar]
- 138.Michalak M, Robert Parker JM, Opas M. Ca2+ signaling and calcium binding chaperones of the endoplasmic reticulum. Cell Calcium. 2002;32:269–278. doi: 10.1016/s0143416002001884. [DOI] [PubMed] [Google Scholar]
- 139.Mironov SL, Ivannikov MV, Johansson M. [Ca2+]i signaling between mitochondria and endoplasmic reticulum in neurons is regulated by microtubules: From mitochondrial permeability transition pore to Ca2+-induced Ca2+ release. J Biol Chem. 2005;280:715–721. doi: 10.1074/jbc.M409819200. [DOI] [PubMed] [Google Scholar]
- 140.Mitchell P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961;191:144–148. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- 141.Montell C. The TRP superfamily of cation channels. Sci STKE. 2005;2005:re3. doi: 10.1126/stke.2722005re3. [DOI] [PubMed] [Google Scholar]
- 142.Montero M, Lobaton CD, Hernandez-Sanmiguel E, Santodomingo J, Vay L, Moreno A, Alvarez J. Direct activation of the mitochondrial calcium uniporter by natural plant flavonoids. Biochem J. 2004;384:19–24. doi: 10.1042/BJ20040990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Montero M, Lobaton CD, Moreno A, Alvarez J. A novel regulatory mechanism of the mitochondrial Ca2+ uniporter revealed by the p38 mitogen-activated protein kinase inhibitor SB202190. FASEB J. 2002;16:1955–1957. doi: 10.1096/fj.02-0553fje. [DOI] [PubMed] [Google Scholar]
- 144.Moreno-Sanchez R. Contribution of the translocator of adenine nucleotides and the ATP synthase to the control of oxidative phosphorylation and arsenylation in liver mitochondria. J Biol Chem. 1985;260:12554–12560. [PubMed] [Google Scholar]
- 145.Mozo J, Ferry G, Studeny A, Pecqueur C, Rodriguez M, Boutin JA, Bouillaud F. Expression of UCP3 in CHO cells does not cause uncoupling, but controls mitochondrial activity in the presence of glucose. Biochem J. 2006;393:431–439. doi: 10.1042/BJ20050494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Neupert W. Protein import into mitochondria. Annu Rev Biochem. 1997;66:863–917. doi: 10.1146/annurev.biochem.66.1.863. [DOI] [PubMed] [Google Scholar]
- 147.Nicchitta CV, Williamson JR. Spermine. A regulator of mitochondrial calcium cycling. J Biol Chem. 1984;259:12978–12983. [PubMed] [Google Scholar]
- 148.Nicholls DG. The regulation of extramitochondrial free calcium ion concentration by rat liver mitochondria. Biochem J. 1978;176:463–474. doi: 10.1042/bj1760463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Nicholls DG. Mitochondria and calcium signaling. Cell Calcium. 2005;38:311–317. doi: 10.1016/j.ceca.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 150.Nicholls DG, Scott ID. The regulation of brain mitochondrial calcium-ion transport. The role of ATP in the discrimination between kinetic and membrane-potential-dependent calcium-ion efflux mechanisms. Biochem J. 1980;186:833–839. doi: 10.1042/bj1860833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Nunez L, Senovilla L, Sanz-Blasco S, Chamero P, Alonso MT, Villalobos C, Garcia-Sancho J. Bioluminescence imaging of mitochondrial Ca2+ dynamics in soma and neurites of individual adult mouse sympathetic neurons. J Physiol. 2007;580:385–395. doi: 10.1113/jphysiol.2006.126524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ong HL, Cheng KT, Liu X, Bandyopadhyay BC, Paria BC, Soboloff J, Pani B, Gwack Y, Srikanth S, Singh BB, Gill D, et al. Dynamic assembly of TRPC1-STIM1-Orai1 ternary complex is involved in store-operated calcium influx. Evidence for similarities in store-operated and calcium release-activated calcium channel components. J Biol Chem. 2007;282:9105–9116. doi: 10.1074/jbc.M608942200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Osibow K, Frank S, Malli R, Zechner R, Graier WF. Mitochondria maintain maturation and secretion of lipoprotein lipase in the endoplasmic reticulum. Biochem J. 2006;396:173–182. doi: 10.1042/BJ20060099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Osibow K, Malli R, Kostner GM, Graier WF. A new type of non-Ca2+-buffering apo(a)-based fluorescent indicator for intraluminal Ca2+ in the endoplasmic reticulum. J Biol Chem. 2006;281:5017–5025. doi: 10.1074/jbc.M508583200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ovide-Bordeaux S, Ventura-Clapier R, Veksler V. Do modulators of the mitochondrial KATP channel change the function of mitochondria in situ? J Biol Chem. 2000;275:37291–37295. doi: 10.1074/jbc.M005772200. [DOI] [PubMed] [Google Scholar]
- 156.Pagliarini DJ, Dixon JE. Mitochondrial modulation: reversible phosphorylation takes center stage? Trends Biochem Sci. 2006;31:26–34. doi: 10.1016/j.tibs.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 157.Palmi M, Youmbi GT, Fusi F, Sgaragli GP, Dixon HB, Frosini M, Tipton KF. Potentiation of mitochondrial Ca2+ sequestration by taurine. Biochem Pharmacol. 1999;58:1123–1131. doi: 10.1016/s0006-2952(99)00183-5. [DOI] [PubMed] [Google Scholar]
- 158.Palmieri L, Pardo B, Lasorsa FM, del Arco A, Kobayashi K, Iijima M, Runswick MJ, Walker JE, Saheki T, Satrustegui J, Palmieri F. Citrin and aralar1 are Ca2+-stimulated aspartate/glutamate transporters in mitochondria. EMBO J. 2001;20:5060–5069. doi: 10.1093/emboj/20.18.5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Paltauf-Doburzynska J, Frieden M, Spitaler M, Graier WF. Histamine-induced Ca2+ oscillations in a human endothelial cell line depend on transmembrane ion flux, ryanodine receptors and endoplasmic reticulum Ca2+–ATPase. J Physiol. 2000;524:701–713. doi: 10.1111/j.1469-7793.2000.00701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Paltauf-Doburzynska J, Malli R, Graier WF. Hyperglycemic conditions affect shape and Ca2+ homeostasis of mitochondria in endothelial cells. J Cardiovasc Pharmacol. 2004;44:424–437. doi: 10.1097/01.fjc.0000139449.64337.1b. [DOI] [PubMed] [Google Scholar]
- 161.Parekh AB, Putney JWJ. Store-operated calcium channels. Physiol Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 162.Park MK, Ashby MC, Erdemli G, Petersen OH, Tepikin AV. Perinuclear, perigranular and sub-plasmalemmal mitochondria have distinct functions in the regulation of cellular calcium transport. EMBO J. 2001;20:1863–1874. doi: 10.1093/emboj/20.8.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Parone PA, James D, Martinou JC. Mitochondria: regulating the inevitable. Biochimie. 2002;84:105–111. doi: 10.1016/s0300-9084(02)01380-9. [DOI] [PubMed] [Google Scholar]
- 164.Parone PA, Martinou JC. Mitochondrial fission and apoptosis: an ongoing trial. Biochim Biophys Acta. 2006;1763:522–530. doi: 10.1016/j.bbamcr.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 165.Perraud AL, Takanishi CL, Shen B, Kang S, Smith MK, Schmitz C, Knowles HM, Ferraris D, Li W, Zhang J, Stoddard BL, et al. Accumulation of free ADP-ribose from mitochondria mediates oxidative stress-induced gating of TRPM2 cation channels. J Biol Chem. 2005;280:6138–6148. doi: 10.1074/jbc.M411446200. [DOI] [PubMed] [Google Scholar]
- 166.Petersen OH. Calcium signal compartmentalization. Biol Res. 2002;35:177–182. doi: 10.4067/s0716-97602002000200008. [DOI] [PubMed] [Google Scholar]
- 167.Petersen OH. Localization and regulation of Ca2+ entry and exit pathways in exocrine gland cells. Cell Calcium. 2003;33:337–344. doi: 10.1016/s0143-4160(03)00047-2. [DOI] [PubMed] [Google Scholar]
- 168.Petersen OH, Burdakov D, Tepikin AV. Polarity in intracellular calcium signaling. Bioessays. 1999;21:851–860. doi: 10.1002/(SICI)1521-1878(199910)21:10<851::AID-BIES7>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 169.Petersen OH, Tepikin A, Park MK. The endoplasmic reticulum: one continuous or several separate Ca2+ stores? Trends Neurosci. 2001;24:271–276. doi: 10.1016/s0166-2236(00)01787-2. [DOI] [PubMed] [Google Scholar]
- 170.Pfeiffer DR, Gunter TE, Eliseev R, Broekemeier KM, Gunter KK. Release of Ca2+ from mitochondria via the saturable mechanisms and the permeability transition. IUBMB Life. 2001;52:205–212. doi: 10.1080/15216540152846019. [DOI] [PubMed] [Google Scholar]
- 171.Pinton P, Leo S, Wieckowski MR, Di Benedetto G, Rizzuto R. Long-term modulation of mitochondrial Ca2+ signals by protein kinase C isozymes. J Cell Biol. 2004;165:223–232. doi: 10.1083/jcb.200311061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Pitter JG, Maechler P, Wollheim CB, Spat A. Mitochondria respond to Ca2+ already in the submicromolar range: correlation with redox state. Cell Calcium. 2002;31:97–104. doi: 10.1054/ceca.2001.0264. [DOI] [PubMed] [Google Scholar]
- 173.Poburko D, Potter K, van Breemen E, Fameli N, Liao CH, Basset O, Ruegg UT, van Breemen C. Mitochondria buffer NCX-mediated Ca2+-entry and limit its diffusion into vascular smooth muscle cells. Cell Calcium. 2006;40:359–371. doi: 10.1016/j.ceca.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 174.Pralong WF, Hunyady L, Varnai P, Wollheim CB, Spat A. Pyridine nucleotide redox state parallels production of aldosterone in potassium-stimulated adrenal glomerulosa cells. Proc Natl Acad Sci USA. 1992;89:132–136. doi: 10.1073/pnas.89.1.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Putney JW., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 176.Quintana A, Schwarz EC, Schwindling C, Lipp P, Kaestner L, Hoth M. Sustained activity of calcium release-activated calcium channels requires translocation of mitochondria to the plasma membrane. J Biol Chem. 2006;281:40302–40309. doi: 10.1074/jbc.M607896200. [DOI] [PubMed] [Google Scholar]
- 177.Rapizzi E, Pinton P, Szabadkai G, Wieckowski MR, Vandecasteele G, Baird G, Tuft RA, Fogarty KE, Rizzuto R. Recombinant expression of the voltage-dependent anion channel enhances the transfer of Ca2+ microdomains to mitochondria. J Cell Biol. 2002;159:613–624. doi: 10.1083/jcb.200205091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Reed KC, Bygrave FL. The inhibition of mitochondrial calcium transport by lanthanides and ruthenium red. Biochem J. 1974;140:143–155. doi: 10.1042/bj1400143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Rego AC, Oliveira CR. Mitochondrial dysfunction and reactive oxygen species in excitotoxicity and apoptosis: implications for the pathogenesis of neurodegenerative diseases. Neurochem Res. 2003;28:1563–1574. doi: 10.1023/a:1025682611389. [DOI] [PubMed] [Google Scholar]
- 180.Reynolds IJ, Hastings TG. Glutamate induces the production of reactive oxygen species in cultured forebrain neurons following NMDA receptor activation. J Neurosci. 1995;15:3318–3327. doi: 10.1523/JNEUROSCI.15-05-03318.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ricquier D, Bouillaud F. The uncoupling protein homologues: UCP1, UCP2, UCP3, StUCP and AtUCP. Biochem J. 2000;345:161–179. [PMC free article] [PubMed] [Google Scholar]
- 182.Rizzuto R, Bastianutto C, Brini M, Murgia M, Pozzan T. Mitochondrial Ca2+ homeostasis in intact cells. J Cell Biol. 1994;126:1183–1194. doi: 10.1083/jcb.126.5.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Rizzuto R, Brini M, Murgia M, Pozzan T. Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science. 1993;262:744–747. doi: 10.1126/science.8235595. [DOI] [PubMed] [Google Scholar]
- 184.Rizzuto R, Brini M, Pizzo P, Murgia M, Pozzan T. Chimeric green fluorescent protein as a tool for visualizing subcellular organelles in living cells. Curr Biol. 1995;5:635–642. doi: 10.1016/s0960-9822(95)00128-x. [DOI] [PubMed] [Google Scholar]
- 185.Rizzuto R, Duchen MR, Pozzan T. Flirting in little space: the ER/mitochondria Ca2+ liaison. Sci STKE. 2004;2004:re1. doi: 10.1126/stke.2152004re1. [DOI] [PubMed] [Google Scholar]
- 186.Rizzuto R, Pinton P, Brini M, Chiesa A, Filippin L, Pozzan T. Mitochondria as biosensors of calcium microdomains. Cell Calcium. 1999;26:193–199. doi: 10.1054/ceca.1999.0076. [DOI] [PubMed] [Google Scholar]
- 187.Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- 188.Robb-Gaspers LD, Burnett P, Rutter GA, Denton RM, Rizzuto R, Thomas AP. Integrating cytosolic calcium signals into mitochondrial metabolic responses. EMBO J. 1998;17:4987–5000. doi: 10.1093/emboj/17.17.4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Velicelebi G, et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Rube DA, van der Bliek AM. Mitochondrial morphology is dynamic and varied. Mol Cell Biochem. 2004:256–257. 331–339. doi: 10.1023/b:mcbi.0000009879.01256.f6. [DOI] [PubMed] [Google Scholar]
- 191.Santo-Domingo J, Vay L, Hernandez-Sanmiguel E, Lobaton CD, Moreno A, Montero M, Alvarez J. The plasma membrane Na+/Ca2+ exchange inhibitor KB-R7943 is also a potent inhibitor of the mitochondrial Ca2+ uniporter. Br J Pharmacol. 2007 doi: 10.1038/sj.bjp.0707260. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Saris NE, Allshire A. Calcium ion transport in mitochondria. Methods Enzymol. 1989;174:68–85. doi: 10.1016/0076-6879(89)74011-8. [DOI] [PubMed] [Google Scholar]
- 193.Saris NE, Carafoli E. A historical review of cellular calcium handling, with emphasis on mitochondria. Biochemistry (Mosc) 2005;70:187–194. doi: 10.1007/s10541-005-0100-9. [DOI] [PubMed] [Google Scholar]
- 194.Sarrouilhe D, Baudry M. Evidence of true protein kinase CKII activity in mitochondria and its spermine-mediated translocation to inner membrane. Cell Mol Biol (Noisy-le-grand) 1996;42:189–197. [PubMed] [Google Scholar]
- 195.Schwarz M, Andrade-Navarro MA, Gross A. Mitochondrial carriers and pores: Key regulators of the mitochondrial apoptotic program? Apoptosis. 2007;12:869–876. doi: 10.1007/s10495-007-0748-2. [DOI] [PubMed] [Google Scholar]
- 196.Scorrano L, Oakes SA, Opferman JT, Cheng EH, Sorcinelli MD, Pozzan T, Korsmeyer SJ. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science. 2003;300:135–139. doi: 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- 197.Sedova M, Blatter LA. Intracellular sodium modulates mitochondrial calcium signaling in vascular endothelial cells. J Biol Chem. 2000;275:35402–35407. doi: 10.1074/jbc.M006058200. [DOI] [PubMed] [Google Scholar]
- 198.Shoshan-Barmatz V, Israelson A, Brdiczka D, Sheu SS. The voltage-dependent anion channel (VDAC): function in intracellular signalling, cell life and cell death. Curr Pharm Des. 2006;12:2249–2270. doi: 10.2174/138161206777585111. [DOI] [PubMed] [Google Scholar]
- 199.Shuttleworth TJ, Thompson JL, Mignen O. ARC channels: a novel pathway for receptor-activated calcium entry. Physiology (Bethesda) 2004;19:355–361. doi: 10.1152/physiol.00018.2004. [DOI] [PubMed] [Google Scholar]
- 200.Smets I, Caplanusi A, Despa S, Molnar Z, Radu M, VandeVen M, Ameloot M, Steels P. Ca2+ uptake in mitochondria occurs via the reverse action of the Na+/Ca2+ exchanger in metabolically inhibited MDCK cells. Am J Physiol. 2004;286:F784–F794. doi: 10.1152/ajprenal.00284.2003. [DOI] [PubMed] [Google Scholar]
- 201.Soboloff J, Berger SA. Sustained ER Ca2+ depletion suppresses protein synthesis and induces activation-enhanced cell death in mast cells. J Biol Chem. 2002;277:13812–13820. doi: 10.1074/jbc.M112129200. [DOI] [PubMed] [Google Scholar]
- 202.Starkov AA, Polster BM, Fiskum G. Regulation of hydrogen peroxide production by brain mitochondria by calcium and Bax. J Neurochem. 2002;83:220–228. doi: 10.1046/j.1471-4159.2002.01153.x. [DOI] [PubMed] [Google Scholar]
- 203.Szabadkai G, Bianchi K, Varnai P, De Stefani D, Wieckowski MR, Cavagna D, Nagy AI, Balla T, Rizzuto R. Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J Cell Biol. 2006;175:901–911. doi: 10.1083/jcb.200608073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Szabadkai G, Simoni AM, Bianchi K, De Stefani D, Leo S, Wieckowski MR, Rizzuto R. Mitochondrial dynamics and Ca2+ signaling. Biochim Biophys Acta. 2006;1763:442–449. doi: 10.1016/j.bbamcr.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 205.Szabadkai G, Simoni AM, Rizzuto R. Mitochondrial Ca2+ uptake requires sustained Ca2+ release from the endoplasmic reticulum. J Biol Chem. 2003;278:15153–15161. doi: 10.1074/jbc.M300180200. [DOI] [PubMed] [Google Scholar]
- 206.Szabo I, Bock J, Jekle A, Soddemann M, Adams C, Lang F, Zoratti M, Gulbins E. A novel potassium channel in lymphocyte mitochondria. J Biol Chem. 2005;280:12790–12798. doi: 10.1074/jbc.M413548200. [DOI] [PubMed] [Google Scholar]
- 207.Szanda G, Koncz P, Varnai P, Spat A. Mitochondrial Ca2+ uptake with and without the formation of high-Ca2+ microdomains. Cell Calcium. 2006;40:527–537. doi: 10.1016/j.ceca.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 208.Szewczyk A, Skalska J, Glab M, Kulawiak B, Malinska D, Koszela-Piotrowska I, Kunz WS. Mitochondrial potassium channels: from pharmacology to function. Biochim Biophys Acta. 2006;1757:715–720. doi: 10.1016/j.bbabio.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 209.Tanaami T, Ishida H, Seguchi H, Hirota Y, Kadono T, Genka C, Nakazawa H, Barry WH. Difference in propagation of Ca2+ release in atrial and ventricular myocytes. Jpn J Physiol. 2005;55:81–91. doi: 10.2170/jjphysiol.R2077. [DOI] [PubMed] [Google Scholar]
- 210.Tanaka Y, Kanai Y, Okada Y, Nonaka S, Takeda S, Harada A, Hirokawa N. _targeted disruption of mouse conventional kinesin heavy chain, kif5B, results in abnormal perinuclear clustering of mitochondria. Cell. 1998;93:1147–1158. doi: 10.1016/s0092-8674(00)81459-2. [DOI] [PubMed] [Google Scholar]
- 211.Territo PR, Mootha VK, French SA, Balaban RS. Ca2+ activation of heart mitochondrial oxidative phosphorylation: role of the F0/F1-ATPase. Am J Physiol Cell Physiol. 2000;278:C423–C435. doi: 10.1152/ajpcell.2000.278.2.C423. [DOI] [PubMed] [Google Scholar]
- 212.Teubl M, Groschner K, Kohlwein SD, Mayer B, Schmidt K. Na+/Ca2+ exchange facilitates Ca2+-dependent activation of endothelial nitric-oxide synthase. J Biol Chem. 1999;274:29529–29535. doi: 10.1074/jbc.274.41.29529. [DOI] [PubMed] [Google Scholar]
- 213.Thyagarajan B, Malli R, Schmidt K, Graier WF, Groschner K. Nitric oxide inhibits capacitative Ca2+ entry by suppression of mitochondrial Ca2+ handling. Br J Pharmacol. 2002;137:821–830. doi: 10.1038/sj.bjp.0704949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Tinel H, Cancela JM, Mogami H, Gerasimenko JV, Gerasimenko OV, Tepikin AV, Petersen OH. Active mitochondria surrounding the pancreatic acinar granule region prevent spreading of inositol trisphosphate-evoked local cytosolic Ca2+ signals. EMBO J. 1999;18:4999–5008. doi: 10.1093/emboj/18.18.4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Trenker M, Malli R, Fertschai I, Levak-Frank S, Graier WF. Uncoupling-proteins 2 and 3 are elementary for mitochondrial Ca2+ uniport. Nat Cell Biol. 2007;9:445–452. doi: 10.1038/ncb1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Varadi A, Cirulli V, Rutter GA. Mitochondrial localization as a determinant of capacitative Ca2+ entry in HeLa cells. Cell Calcium. 2004;36:499–508. doi: 10.1016/j.ceca.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 217.Varadi A, Grant A, McCormack M, Nicolson T, Magistri M, Mitchell KJ, Halestrap AP, Yuan H, Schwappach B, Rutter GA. Intracellular ATP-sensitive K+ channels in mouse pancreatic beta cells: against a role in organelle cation homeostasis. Diabetologia. 2006;49:1567–1577. doi: 10.1007/s00125-006-0257-9. [DOI] [PubMed] [Google Scholar]
- 218.Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, Kinet JP. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006;312:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Votyakova TV, Reynolds IJ. DeltaPsi(m)-dependent and - independent production of reactive oxygen species by rat brain mitochondria. J Neurochem. 2001;79:266–277. doi: 10.1046/j.1471-4159.2001.00548.x. [DOI] [PubMed] [Google Scholar]
- 220.Wang HJ, Guay G, Pogan L, Sauve R, Nabi IR. Calcium regulates the association between mitochondria and a smooth subdomain of the endoplasmic reticulum. J Cell Biol. 2000;150:1489–1498. doi: 10.1083/jcb.150.6.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Watanabe H, Vriens J, Janssens A, Wondergem R, Droogmans G, Nilius B. Modulation of TRPV4 gating by intra- and extracellular Ca2+ Cell Calcium. 2003;33:489–495. doi: 10.1016/s0143-4160(03)00064-2. [DOI] [PubMed] [Google Scholar]
- 222.Wieckowski MR, Szabadkai G, Wasilewski M, Pinton P, Duszynski J, Rizzuto R. Overexpression of adenine nucleotide translocase reduces Ca(2+) signal transmission between the ER and mitochondria. Biochem Biophys Res Commun. 2006;348:393–399. doi: 10.1016/j.bbrc.2006.07.072. [DOI] [PubMed] [Google Scholar]
- 223.Wu SN. Large-conductance Ca2+- activated K+ channels: physiological role and pharmacology. Curr Med Chem. 2003;10:649–661. doi: 10.2174/0929867033457863. [DOI] [PubMed] [Google Scholar]
- 224.Yaffe MP. The machinery of mitochondrial inheritance and behavior. Science. 1999;283:1493–1497. doi: 10.1126/science.283.5407.1493. [DOI] [PubMed] [Google Scholar]
- 225.Yan Y, Wei CL, Zhang WR, Cheng HP, Liu J. Cross-talk between calcium and reactive oxygen species signaling. Acta Pharmacol Sin. 2006;27:821–826. doi: 10.1111/j.1745-7254.2006.00390.x. [DOI] [PubMed] [Google Scholar]
- 226.Yeromin AV, Zhang SL, Jiang W, Yu Y, Safrina O, Cahalan MD. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature. 2006;443:226–229. doi: 10.1038/nature05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Yi M, Weaver D, Hajnoczky G. Control of mitochondrial motility and distribution by the calcium signal: a homeostatic circuit. J Cell Biol. 2004;167:661–672. doi: 10.1083/jcb.200406038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Yu XX, Lewin DA, Zhong A, Brush J, Schow PW, Sherwood SW, Pan G, Adams SH. Overexpression of the human 2-oxoglutarate carrier lowers mitochondrial membrane potential in HEK-293 cells: contrast with the unique cold-induced mitochondrial carrier CGI-69. Biochem J. 2001;353:369–375. doi: 10.1042/0264-6021:3530369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Zhang BX, Ma X, Zhang W, Yeh CK, Lin A, Luo J, Sprague EA, Swerdlow RH, Katz MS. Polyunsaturated fatty acids mobilize intracellular Ca2+ in NT2 human teratocarcinoma cells by causing release of Ca2+ from mitochondria. Am J Physiol. 2006;290:C1321–C1333. doi: 10.1152/ajpcell.00335.2005. [DOI] [PubMed] [Google Scholar]
- 230.Zhang Y, Soboloff J, Zhu Z, Berger SA. Inhibition of Ca2+ influx is required for mitochondrial reactive oxygen species-induced endoplasmic reticulum Ca2+ depletion and cell death in leukemia cells. Mol Pharmacol. 2006;70:1424–1434. doi: 10.1124/mol.106.024323. [DOI] [PubMed] [Google Scholar]
- 231.Zoccarato F, Cavallini L, Alexandre A. Respiration-dependent removal of exogenous H2O2 in brain mitochondria: inhibition by Ca2+ J Biol Chem. 2004;279:4166–4174. doi: 10.1074/jbc.M308143200. [DOI] [PubMed] [Google Scholar]
- 232.Zoccarato F, Nicholls D. The role of phosphate in the regulation of the independent calcium-efflux pathway of liver mitochondria. Eur J Biochem. 1982;127:333–338. doi: 10.1111/j.1432-1033.1982.tb06875.x. [DOI] [PubMed] [Google Scholar]
- 233.Zsurka G, Gregan J, Schweyen RJ. The human mitochondrial Mrs2 protein functionally substitutes for its yeast homologue, a candidate magnesium transporter. Genomics. 2001;72:158–168. doi: 10.1006/geno.2000.6407. [DOI] [PubMed] [Google Scholar]