Abstract
Background:
Magnetic resonance imaging-estimated proton-density-fat-fraction (MRI-PDFF) has been shown to be a noninvasive, accurate and reproducible imaging-based biomarker for assessing steatosis and treatment response in nonalcoholic steatohepatitis (NASH) clinical trials. However, there are no data on the magnitude of MRI-PDFF reduction corresponding to histologic response in the setting of a NASH clinical trial. The aim of this study was to quantitatively compare the magnitude of MRI-PDFF reduction between histologic responders versus histologic nonresponders in NASH patients.
Methods:
This study is a secondary analysis of the MOZART trial, which included 50 patients with biopsy-proven NASH randomized to ezetimibe 10 mg/day orally or placebo for 24 weeks. The primary aim was to perform a head-to-head comparative analysis of histologic responders [defined as a ⩾2-point reduction in the nonalcoholic fatty liver disease (NAFLD) Activity Score (NAS) without worsening fibrosis] versus nonresponders, and the corresponding quantitative change in liver fat content measured via MRI-PDFF.
Results:
Of the 35 patients who underwent paired liver biopsy and MRI-PDFF assessment at the beginning and end of treatment, 10 demonstrated a histologic response. Compared with histologic nonresponders, histologic responders had a statistically significant reduction in MRI-PDFF of −4.1% ± 4.9 versus −0.6 ± 4.1 (p < 0.04) with a mean relative percent change of −29.3% ± 33.0 versus +2.0% ± 24.0 (p < 0.004), respectively.
Conclusions:
Utilizing paired MRI-PDFF and liver histology data, we demonstrate that a relative reduction of 29% in liver fat on MRI-PDFF is associated with a histologic response in NASH. After external validation by independent research groups, these results can be incorporated into designing future NASH clinical trials, especially those utilizing change in hepatic fat quantified by MRI-PDFF, as a treatment endpoint.
Keywords: advanced fibrosis, biomarker, fat mapping, magnetic resonance imaging (MRI), MRI-proton-density-fat-fraction (PDFF), nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, noninvasive assessment
Introduction
Nonalcoholic steatohepatitis (NASH) has become one of the most common causes of liver disease in Western countries [Chalasani et al. 2012; Loomba and Sanyal, 2013] and an important indication for liver transplantation [Charlton et al. 2011]. Nonalcoholic fatty liver disease (NAFLD) afflicts 80–100 million Americans [Browning et al. 2004; Vernon et al. 2011; Williams et al. 2011]. Approximately 30% of these individuals may have the progressive form of NAFLD, termed as NASH. It is associated with insulin resistance, diabetes mellitus type 2, and dyslipidemia [Neuschwander-Tetri et al. 2010; Chalasani et al. 2012; Loomba et al. 2012]. Liver histology findings associated with NASH include macrovesicular steatosis in acinar zone 3, lobular inflammation, and hepatocyte ballooning, all of these with or without perisinusoidal fibrosis [Matteoni et al. 1999]. NASH can further progress to advanced fibrosis, cirrhosis and hepatocellular carcinoma (HCC) [Matteoni et al. 1999; Adams et al. 2005; Vernon et al. 2011; Singh et al. 2014]. Liver biopsy is the current gold standard for diagnosing NASH, but it is an expensive, invasive procedure with high sampling error and is associated with complications such as pain, bleeding and death [Bravo et al. 2001; Ratziu et al. 2005; Chalasani et al. 2012; Sumida et al. 2014]. It is not feasible to perform liver biopsy assessment for all NASH patients and given its subjective nature, it often requires a large number of patients in clinical trials to show treatment response; thus noninvasive objective biomarkers are needed.
Imaging modalities are increasingly used as noninvasive methods for diagnosis and assessment of NASH. Ultrasound and computed tomography (CT) are convenient, widely available and safe but have their limitations [Saadeh et al. 2002; Mishra and Younossi, 2007]. In a meta-analysis, ultrasound studies had limited sensitivity with mild hepatic steatosis <30% and were found to be an unreliable and operator-dependent imaging technique [Bohte et al. 2011]. CT also has low sensitivity for mild steatosis, along with costs and radiation exposure. More importantly neither of these studies can quantify liver fat content [Bohte et al. 2011]. Magnetic resonance spectroscopy (MRS) is a safe, noninvasive and accurate method for hepatic fat quantification with high sensitivity and specificity but remains primarily a research tool due to its low availability and limited clinical application [Szczepaniak et al. 2005; Schwenzer et al. 2009; Wong et al. 2012]. Furthermore, MRS has been used for assessment of treatment response [Keating et al. 2015] but the precise decline in hepatic fat associated with histologic change remains unknown. Advanced magnetic resonance imaging (MRI) can measure the proton density fat fraction (PDFF), an objective and quantitative indicator of hepatic fat content, across the entire liver in an accurate, reproducible manner [Yokoo et al. 2009; Kang et al. 2011; Tang et al. 2013; Negrete et al. 2014] while minimizing errors due to factors such as T1 bias, T2* decay, and multifrequency signal interference effects of protons in fat that confound fat quantification with conventional MRI [Liu et al. 2007; Reeder and Sirlin, 2010]. MRI-estimated PDFF has been validated against MRS [Yokoo et al. 2009, 2011; Kang et al. 2011] as well as liver biopsy histological data [Tang et al. 2013, 2015] and was found to be more sensitive in detecting changes in hepatic fat content than histology-determined steatosis grade [Noureddin et al. 2013]. MRI-PDFF is emerging as the leading biomarker for assessing treatment response in NASH trials and has been utilized in several early phase II studies to monitor longitudinal changes in hepatic fat [Le et al. 2012; Patel et al. 2014].
Despite the growing need for noninvasive methods of screening and evaluation of NASH progression, along with the increasing use of MRI-PDFF in clinical trials for hepatic fat assessment, there are no data on the magnitude of MRI-PDFF reduction corresponding to histologic response in the setting of a NASH clinical trial. Given the emergence of MRI and MRS-PDFF as the leading biomarkers for hepatic steatosis, these data are of paramount importance for the design of future and ongoing clinical trials. In this study, we aimed to quantitatively compare MRI-PDFF change between histologic responders versus histologic nonresponders in patients with biopsy-proven NASH participating in a randomized clinical trial.
Methods
Study design and patient population
This is a secondary longitudinal analysis of the MOZART trial, a randomized, placebo-controlled, double-blind study of the efficacy of ezetimibe versus placebo in reducing hepatic fat as measured by MRI-PDFF technique in biopsy-proven NASH patients [Loomba et al. 2014]. In the MOZART trial, 50 patients were randomized to receive ezetimibe 10 mg orally daily or placebo for 24 weeks while also undergoing biochemical testing, liver biopsy, and MRI at baseline and at completion of therapy. In this trial, patients were enrolled from San Diego Integrated NAFLD Research Consortium (SINC) cohort, a collaboration between the University of California San Diego (UCSD) Medical Center, Sharp Health System, Naval Medical Center San Diego, and Kaiser Permanente Southern California to study NAFLD and led by the principal investigator (Rohit Loomba). Clinical research assessments were performed at the UCSD NAFLD Translational Research Unit and MRI-PDFF was performed by the UCSD Liver Imaging Group. All enrolled patients provided written informed consent for their participation in the MOZART trial. The study protocol was approved by the UCSD Institutional Review Board.
Patients in the MOZART trial who underwent both liver biopsy and MRI-PDFF at weeks 0 and 24 were included in this secondary analysis (Figure 1). Patients were classified as ‘histologic responders’ if they had ⩾2 point reduction in NAFLD Activity Score (NAS) without any increase in fibrosis stage and as ‘histologic nonresponders’ if they did not meet this criterion. As previously shown in a meta-analysis of NASH clinical trials, a 2-point change in histologic parameters is rarely seen in placebo patient groups and is a reliable indicator for treatment-related changes [Loomba et al. 2008].
Figure 1.
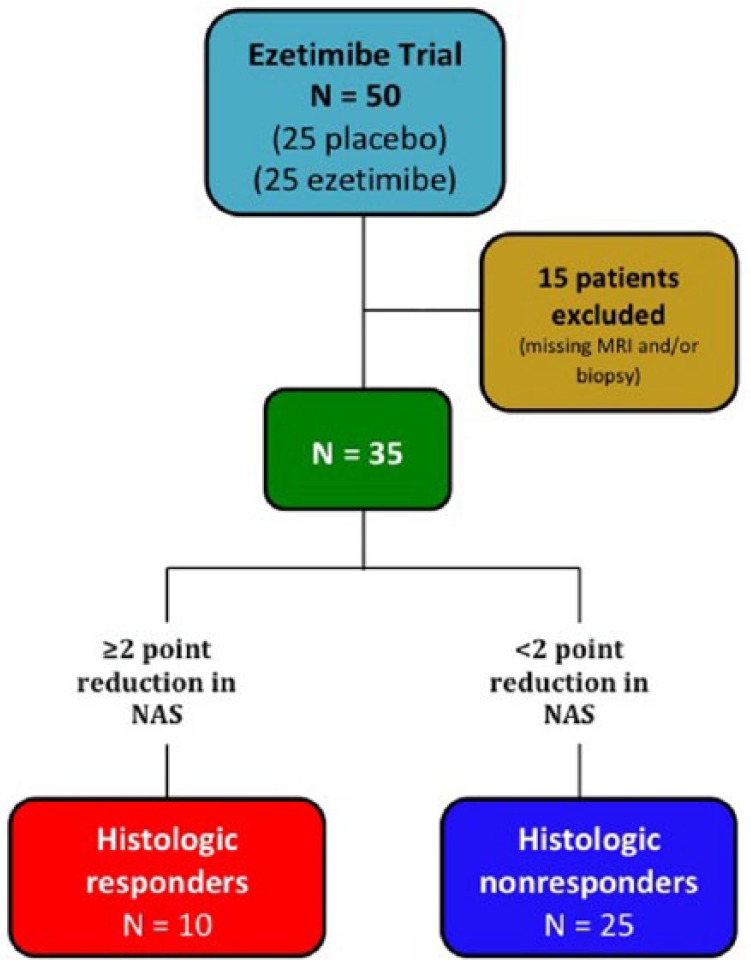
Study design and derivation of cohort for this secondary analysis.
NAFLD, nonalcoholic fatty liver disease; NAS, NAFLD Activity Score.
Inclusion and exclusion criteria
Inclusion criteria included: patients aged ⩾18 years with liver biopsy-confirmed NASH as defined by the Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN) histologic scoring system, MRI with evidence of hepatic steatosis with MRI-PDFF ⩾5%, liver biopsy and MRI-PDFF performed at week 0 and week 24 of MOZART trial, and serum alanine aminotransferase (ALT) or aspartate aminotransferase (AST) levels above the upper limit of normal (19 U/l for women and 30 U/l for men). The criteria included AST and ALT values that were lower than standard upper limits of normal at most laboratories since they were determined from prior studies to have higher sensitivities for NAFLD [Prati et al. 2002]. Exclusion criteria included the following: (1) evidence of other forms of liver disease based on liver biopsy findings or laboratory findings of positive hepatitis B surface antigen, hepatitis C viral RNA, autoimmune hepatitis, hemochromatosis, Wilson’s disease, and alpha-1-antitrypsin deficiency, (2) alcohol intake above 30 g per day in the prior 10 years or above 10 g per day in the previous 1 year, (3) Child-Pugh score >7 points, and (4) active substance abuse. Patients were also excluded if they had severe systemic illnesses including renal insufficiency, human immunodeficiency virus infection, positive pregnancy test, evidence of hepatocellular carcinoma, contraindications to liver biopsy or MRI exam, or any use of steatogenic medications or drug agents such as vitamin E or pioglitazone, known to improve NASH.
Primary outcome measurement and rationale for study
The primary aim was to perform a head-to-head comparative analysis of histologic responders, defined as a ⩾2-point reduction in the NAS (please see liver histologic assessment section) without worsening fibrosis, versus nonresponders, and the corresponding quantitative change in liver fat content measured via MRI-PDFF. These data are needed for future NASH phase IIa and IIb clinical trial design utilizing MRI-PDFF as a biomarker for treatment response.
Baseline clinical evaluation
All patients were screened with history, physical examination, an alcohol history assessment at the Clinical and Translational Research Institute at UCSD. The Alcohol Use Disorders Identification Test and the Skinner Lifetime Drinking Questionnaire were used to assess alcohol use history. All patients prior to treatment initiation underwent an evaluation, including routine history and physical exam, vital sign measurements, body height and weight measurements, and body mass index (BMI) calculation performed by a trained investigator. All subjects had blood tests for measurement of AST, ALT, gamma-glutamyl transpeptidase (GGT), alkaline phosphatase, albumin, prothrombin time, lipid panel, hemoglobin A1c (HbA1c), fasting glucose, insulin, total bilirubin, and free fatty acids.
Liver histology assessment
Liver biopsy was performed on all patients prior to inclusion in the trial and at the conclusion of the trial at week 24 on patients that consented for repeat biopsy. The liver biopsy specimens obtained had an average length of 17.0 mm (SD ± 6.6) with average number of 14.9 (SD ± 7.9) portal tracts, which are characteristic of acceptable liver biopsy specimens performed for NASH clinical trials [Neuschwander-Tetri et al. 2010; Le et al. 2012]. All liver histology assessments for the study were interpreted by a single hepatopathologist (Mark Valasek) blinded to clinical and imaging data, prior liver biopsy data and order of liver biopsy. The biopsies were scored using NASH-CRN histologic scoring system [Kleiner et al. 2005]. The NAS ranges from 0–8 and is the sum of scores of histologic variables of steatosis (0–3), lobular inflammation (0–3), and hepatocellular ballooning (0–2). Liver fibrosis was scored as stage 0–4 using the NASH-CRN scoring system, with stage 4 indicating cirrhosis.
Magnetic resonance imaging protocol
MRI exams were performed at baseline and at week 24 to measure PDFF as a noninvasive indicator of liver fat content using a previously described, validated and reproducible technique that estimates PDFF [Reeder et al. 2009; Hines et al. 2011; Permutt et al. 2012; Tang et al. 2013, 2015]. In brief, this advanced technique utilizes a gradient echo sequence with low flip angle to minimize T1 bias and acquires multiple echoes at echo times at which fat and water signals are nominally in phase or out of phase with each other. The acquired source images are processed through an algorithm that, pixel by pixel, corrects for the T2* signal decay and multi-fat-peak spectral interference effects that confound results of conventional MRI examinations. The resulting parametric PDFF map illustrates fat distribution and quantity across the entire liver. Hepatic fat quantification via MRI-PDFF has been previously shown to be sensitive in detecting liver fat changes and has been utilized in NASH clinical trials for quantitative fat assessment [Le et al. 2012; Permutt et al. 2012; Doycheva et al. 2015; Loomba et al. 2015; Zarrinpar et al. 2015].
All MRI-PDFF parametric maps were analyzed under the supervision of an experienced hepatoradiologist (Claude Sirlin) [Hines et al. 2011; Kang et al. 2011] who was blinded to clinical and histological data, and order of scan. Trained image analysts placed regions of interest (ROIs) about 300–400 mm2 in area in each of the nine liver segments while carefully evading blood vessels, bile ducts and artifacts in the region. To quantify longitudinal hepatic fat content changes between the baseline exam and follow-up exam, ROIs in each segment were colocalized using anatomic landmarks on maps obtained at each time point, and PDFF change in each segment was calculated.
Statistical analysis
Patients with NAS score reduction ⩾2 points were classified as histologic responders and the remaining patients were classified as histologic nonresponders. For categorical variables, the Chi-square test or Fisher’s exact test was used and for continuous variables, the Wilcoxon-Mann-Whitney test or independent samples Student’s t-test was used to compare histologic responder and nonresponder groups. For the comparative analyses within groups, we utilized the Wilcoxon signed rank sum test or paired samples Student’s t-test as appropriate. A p-value < 0.05 was considered statistically significant. All statistical analyses were performed using SAS version 9.4 (Cary, NC, USA).
Results
Demographic, biochemical, and histological baseline characteristics of patients
Of the 50 patients randomized into the MOZART Trial, 35 patients were included in this secondary analysis with 10 patients in the histologic responders group and 25 patients in the histologic nonresponders group (Figure 1). Histologic responders and nonresponders had similar baseline demographics and histological characteristics, with a median age of 60.5 years and 70% female patients for histologic responders, and a median age of 49 years and 64% female patients for histologic nonresponders (Table 1). There was a significant difference in BMI between the two groups with a median BMI of 30.6 in the histologic responder group versus 35.0 in the histologic nonresponder group (p = 0.01), although no significant difference was observed for weight and height (Table 1). Biochemical profiles of both groups were comparable with the exception of the homeostatic model assessment of insulin resistance (HOMA-IR) (p = 0.02; Table 1).
Table 1.
Baseline demographic, biochemical, and histological characteristics of patients.
| Histologic responders (n = 10) |
Histologic nonresponders (n = 25) |
p-value | |
|---|---|---|---|
| Demographics | |||
| Age (years) | 60.5 (15.0) | 49.0 (22.0) | 0.23 |
| Male (%) | 3 (30.0%) | 9 (36.0%) | 1.00 |
| Weight (kg) | 84.8 (18.4) | 93.6 (25.0) | 0.07 |
| Height (m) | 1.7 (0.1) | 1.6 (0.2) | 0.70 |
| BMI (kg/m2) | 30.6 (5.1) | 35.0 (6.2) | 0.01 |
| Race and ethnicity | |||
| White (versus non-White) | 9 (90.0%) | 20 (80.0%) | 0.65 |
| Hispanic (versus non-Hispanic) | 2 (20.0%) | 12 (48.0%) | 0.25 |
| Biochemical profile | |||
| AST (U/l) | 31.0 (14.0) | 32.0 (28.0) | 0.77 |
| ALT (U/l) | 44.5 (15.0) | 50.0 (32.0) | 0.53 |
| AST/ALT ratio | 0.8 (0.3) | 0.8 (0.5) | 0.87 |
| Alk Phos (U/l) | 72.0 (30.0) | 74.0 (27.0) | 0.53 |
| GGT (U/l) | 33.0 (21.0) | 37.0 (36.0) | 0.37 |
| Total bilirubin (mg/dl) | 0.4 (0.2) | 0.4 (0.2) | 0.96 |
| Direct bilirubin (mg/dl) | 0.1 (0.0) | 0.1 (0.1) | 0.65 |
| Albumin (g/dl) | 4.6 (0.4) | 4.4 (0.3) | 0.57 |
| Glucose (mg/dl) | 85.0 (27.0) | 106.0 (33.0) | 0.06 |
| HbA1c (%) | 6.1 (0.7) | 6.1 (0.9) | 0.60 |
| Triglycerides (mg/dl) | 117.5 (61.0) | 149.0 (96.0) | 0.65 |
| Total cholesterol (mg/dl) | 175.0 (66.0) | 171.0 (38.0) | 0.45 |
| LDL (mg/dl) | 92.0 (47.0) | 90.5 (29.0) | 0.93 |
| Free fatty acids (mg/dl) | 0.5 (0.3) | 0.6 (0.3) | 0.78 |
| HOMA-IR | 4.3 (4.5) | 6.8 (5.6) | 0.02 |
| Platelet count (109/l) | 242.5 (62.0) | 252.0 (68.0) | 0.73 |
| Prothrombin time | 10.9 (1.5) | 10.7 (1.2) | 0.30 |
| Diabetes | 1 (10.0%) | 10 (40.0%) | 0.12 |
| Histology | |||
| Steatosis | 2.0 (1.0) | 2.0 (2.0) | 0.98 |
| Lobular inflammation | 2.0 (1.0) | 2.0 (1.0) | 0.85 |
| Ballooning | 1.0 (1.0) | 1.0 (1.0) | 0.54 |
| Fibrosis | 0.5 (3.0) | 1.0 (2.0) | 0.41 |
| NAS | 5.5 (2.0) | 5.0 (2.0) | 0.37 |
All above values are reported as median (interquartile range) or n (%). p-values were determined using Wilcoxon-Mann-Whitney test for continuous variables and Chi-square test or Fisher’s Exact test for categorical variables as appropriate.
ALT, alanine aminotransferase; Alk Phos, alkaline phosphatase; AST, aspartate aminotransferase; BMI, body mass index; GGT, gamma-glutamyl transferase; HbA1c, glycated hemoglobin; HOMA-IR, homeostatic model assessment – insulin resistance; LDL, low-density lipoprotein; NAFLD, nonalcoholic fatty liver disease; NAS, NAFLD activity score.
Primary outcome: change in MRI-PDFF estimated hepatic fat associated with histologic response
There was a significant reduction in MRI-PDFF in the histologic responder group (−4.1% ± 4.9) compared with nonresponder group (−0.6% ± 4.1), (p = 0.04; Table 2). The significant decline in MRI-PDFF was consistently seen across most liver segments in the histologic responder group (Table 2). Compared with histologic nonresponders, histologic responders had a statistically significant proportional reduction in MRI-PDFF relative to baseline with a mean percent change of −29.3% ± 33.0 versus 2.0% ± 24.0, (p < 0.004; Figure 2).
Table 2.
MRI-PDFF-estimated liver fat by segments in histologic responders and histologic nonresponders.
| Liver segments |
Histologic responders
(n = 10) |
Histologic nonresponders (n = 25)
|
p-value difference | ||||
|---|---|---|---|---|---|---|---|
| Week 0 | Week 24 | p-value | Week 0 | Week 24 | p-value | ||
| 1 | 10.9 (7.0) | 9.5 (7.0) | 0.07 | 17.2 (11.2) | 18.5 (8.7) | 0.74 | 0.05 |
| 2 | 11.3 (7.2) | 8.0 (6.4) | 0.02 | 16.1 (12.2) | 16.7 (9.0) | 0.80 | 0.03 |
| 3 | 13.0 (5.9) | 7.9 (8.5) | 0.08 | 18.5 (14.1) | 17.1 (12.0) | 0.74 | 0.12 |
| 4a | 12.7 (8.6) | 10.0 (8.0) | 0.04 | 17.2 (12.7) | 17.5 (11.4) | 0.21 | 0.10 |
| 4b | 12.8 (6.2) | 8.2 (7.2) | 0.02 | 17.6 (13.3) | 17.2 (10.2) | 0.72 | 0.04 |
| 5 | 14.2 (9.7) | 8.3 (6.2) | 0.05 | 16.8 (12.8) | 17.3 (12.9) | 0.76 | 0.05 |
| 6 | 13.2 (4.8) | 7.7 (5.3) | 0.02 | 17.7 (11.2) | 18.9 (10.9) | 0.54 | 0.05 |
| 7 | 13.0 (6.3) | 8.6 (4.2) | 0.03 | 17.6 (11.4) | 17.9 (9.2) | 0.61 | 0.06 |
| 8 | 14.1 (6.3) | 9.5 (6.5) | 0.03 | 18.1 (12.0) | 18.5 (11.4) | 0.50 | 0.09 |
| MRI-PDFF (%) | 13.4 (6.8) | 8.8 (6.5) | 0.03 | 18.2 (12.0) | 18.7 (10.2) | 0.76 | 0.06 |
| Absolute change in MRI-PDFF (%) | −4.1 ± 4.9 | 0.02 | −0.6 ± 4.1 | 0.46 | 0.04 | ||
| Percent change in MRI-PDFF (%) | −29.3% ± 33.0 | 0.02 | 2.0 ± 24.0 | 0.67 | 0.004 | ||
Data values are reported as median (interquartile range) or mean ± standard deviation. p-values were determined using Wilcoxon signed rank sum test or paired t-test. p-value difference was determined using Wilcoxon-Mann-Whitney test or independent samples t-test.
MRI, magnetic resonance imaging; PDFF, proton density fat fraction.
Figure 2.
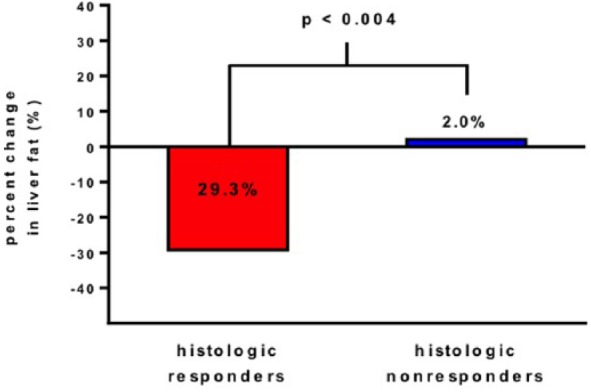
Overall mean % change in MRI-PDFF estimated liver fat content between week 0 and week 24 in two groups with histologic responders on left (shown in red color bar), and histologic nonresponders (shown in blue color bar). There was a significant difference in fat change % from baseline between two groups (p < 0.004).
MRI-PDFF, magnetic resonance imaging-estimated proton-density-fat-fraction.
The histologic responders had a median decrease in hepatic steatosis grade of 1, which was significant compared with histologic nonresponders with no overall net change over 24 weeks (p = 0.01; supplementary online Table A). Similar decline was seen in hepatocellular ballooning median grade in histologic responders compared with nonresponders (p = 0.03; supplementary online Table A). There was no significant change in lobular inflammation scores and fibrosis scores over 24 weeks within groups as well as between groups (supplementary online Table A).
Secondary outcome: change in biochemical characteristics associated with histologic change
We compared the weight and biochemical profiles during the study time period between the two groups. The median weight decreased more in the histologic responders group compared with the nonresponders group but did not reach statistical significance (supplementary online Table B). Similarly, patients with a NAS change ⩾2 points had no significant changes in commonly tested laboratory values of AST, ALT and GGT (supplementary online Table B). Fatty liver index was calculated for the two groups and no significant difference was seen between two groups.
Discussion
In this secondary analysis of a randomized, double-blinded, placebo-controlled clinical trial with biopsy-proven NASH patients, we showed that patients with ⩾2-point reduction in NAS, without any worsening of fibrosis on liver biopsy, had a proportional reduction in MRI-PDFF of 29.3% from baseline which was statistically significant when compared with histologic nonresponders. Thus, this longitudinal study provides quantitative MRI-PDFF change data that are associated with changes in liver histologic parameters, which can be incorporated into the design of future trials as well as to assess for intervention effect in early phase NASH trial in which MRI- related fat change is being used as a treatment endpoint.
This study utilized an advanced, noninvasive, accurate MRI-based biomarker for liver fat quantification that has been shown to be superior to other noninvasive imaging biomarkers that are currently available such as ultrasound or CT scan-derived fat assessment. Liver biopsy remains the gold standard for diagnosis, classification, and monitoring of NASH. However, it has its limitations due to sampling variability, inter-observer rating variability and procedure-related risks and complications [Bravo et al. 2001; Ratziu et al. 2005]. Also, since liver biopsy only examines a small portion of liver, and fat distribution can be uneven in NASH, this can lead to errors in diagnosis as well as not detecting longitudinal changes in liver fat when utilized in clinical trials. Ultrasound imaging is low in cost and risks, with wide availability but it is limited due to operator-dependency and inability to quantify hepatic fat. CT imaging has associated risks of ionizing radiation exposure and lacks accuracy and precision for diagnosis of hepatic steatosis compared with MRI. Although MR spectroscopy is considered a reference standard for liver fat quantification, it is limited in applicability due to its requirements for expert analysis and low availability compared with MRI. MRS is also limited due to its sampling error since only a single, small voxel (2 × 2 × 2 cm3) is utilized for fat quantification whereas the MRI-PDFF technique provides fat mapping for the entire liver.
In prior studies conducted at our center and by others, the MRI-PDFF technique has been validated against both histology-determined steatosis grade data and MRS fat quantification and shown to be an accurate and precise method for hepatic fat quantification [Meisamy et al. 2011; Permutt et al. 2012; Idilman et al. 2013; Tang et al. 2013, 2015]. We have also shown that the MRI-PDFF-estimated liver fat technique is more sensitive in detecting longitudinal fat changes compared with liver histology, which supports its use in NASH clinical trials [Le et al. 2012; Noureddin et al. 2013].
Strengths and limitations
The study’s major strengths include: (1) the use of a cohort derived from a randomized, placebo-controlled, double-blinded study, the MOZART trial, which allowed for prospective, systematic longitudinal assessment of biopsy-proven NASH patients at baseline and 24 weeks, (2) the MR imaging performed under standardized protocol and processed under the supervision of a single, blinded radiologist, (3) similarly, the liver biopsies were reviewed using NASH-CRN histological scoring system by a single, blinded hepatopathologist, (4) extensive MR assessment of liver fat including precise quantification and fat mapping of the entire liver, and (5) comparison of colocalized ROIs for fat changes in each of nine liver segments between week 0 and week 24. Despite these strengths of our study, we recognize several limitations. This study was performed at a single site, the UCSD NAFLD Translational Research Unit, and validation at other clinical sites will be needed for wide generalizability. Despite the excellent precision and sensitivity of MRI-PDFF to detect hepatic steatosis, its utility in future clinical trials especially those including patients with advanced fibrosis may be limited and require further investigation in this specific population [Idilman et al. 2013]. Although histologic responders in the study experienced reduction in lobular inflammation scores, they did not reach statistical significance and it remains to be validated whether a 29% reduction in liver fat by MRI-PDFF could serve as marker of treatment response in NASH. In the current state, the application of MRI-PDFF will be limited by its cost and requirement of expert analysis. Furthermore, our study is limited by a small sample size and our results would need further validation by larger, multicenter cohort studies prior to utilization of MRI-PDFF technique in future NASH clinical trials.
Implications
MRI-PDFF is a novel and accurate biomarker to quantify liver fat. These data suggest that a relative reduction of 29% in MRI-PDFF may be associated with a clinically important improvement in liver histology in the setting of a clinical trial in NASH. MRI-PDFF has the potential to be a cost-effective and convenient method for liver fat quantification in future clinical trials, as it only requires a single 20-second breath hold and an estimated time of about 5 minutes in an MRI scanner suite. This study has implications for the design of future NASH clinical trials.
Supplementary Material
Acknowledgments
Janki Patel provided analysis and interpretation of data, statistical analysis, drafting of the manuscript, critical revision of the manuscript, and approved the final submission.
Ricki Bettencourt provided data analysis, critical revision of the manuscript, and approved the final submission.
Jeffrey Cui, Hamed Aryafar and Mark Valasek provided a critical revision of the manuscript and approved the final submission.
Jonathan Hooker, William Haufe and Catherine Hooker conducted analysis of MRI data, critically reviewed the manuscript, and approved the final submission.
Joanie Salotti, Archana Bhatt, Carolyn Hernandez, Lisa Richards and Phirum Nguyen conducted patient visits, critically reviewed the manuscript, and approved the final submission.
Claude Sirlin provided analysis and interpretation of data, drafting of the manuscript, MRI analysis, critical revision of the manuscript, and approved the final submission.
Rohit Loomba provided the study concept and design, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript, obtained funding, study supervision, and approved the final submission.
Footnotes
Funding: This work was supported by an investigator-initiated study grant to Dr Rohit Loomba by Merck & Co. Inc., USA. The study was conducted at the Clinical and Translational Research Institute, UCSD. Dr Rohit Loomba is supported, in part, by the American Gastroenterological Association (AGA) Foundation, Sucampo, ASP Designated Research Award in Geriatric Gastroenterology and by a T. Franklin Williams Scholarship Award. Funding was provided by Atlantic Philanthropies, Inc., the John A. Hartford Foundation, OM, the Association of Specialty Professors, and the American Gastroenterological Association and the National Institutes of Health grant, K23-DK090303.
Conflict of interest statement: The study sponsors had no role in the study design, collection, analysis, interpretation of the data or drafting of the manuscript. All authors report that no conflicts of interest exist.
Contributor Information
Janki Patel, Department of Internal Medicine, University of California at San Diego, La Jolla, CA, USA.
Ricki Bettencourt, NAFLD Translational Research Unit, Department of Medicine, University of California at San Diego, La Jolla, CA, USA Division of Epidemiology, Department of Family and Preventive Medicine, University of California at San Diego, La Jolla, CA, USA.
Jeffrey Cui, NAFLD Translational Research Unit, Department of Medicine, University of California at San Diego, La Jolla, CA, USA.
Joanie Salotti, NAFLD Translational Research Unit, Department of Medicine, University of California at San Diego, La Jolla, CA, USA Division of Gastroenterology, Department of Medicine, University of California at San Diego, La Jolla, CA, USA.
Jonathan Hooker, Liver Imaging Group, Department of Radiology, University of California at San Diego, La Jolla, CA, USA.
Archana Bhatt, NAFLD Translational Research Unit, Department of Medicine, University of California at San Diego, La Jolla, CA, USA.
Carolyn Hernandez, NAFLD Translational Research Unit, Department of Medicine, University of California at San Diego, La Jolla, CA, USA.
Phirum Nguyen, NAFLD Translational Research Unit, Department of Medicine, University of California at San Diego, La Jolla, CA, USA Division of Gastroenterology, Department of Medicine, University of California at San Diego, La Jolla, CA, USA.
Hamed Aryafar, Liver Imaging Group, Department of Radiology, University of California at San Diego, La Jolla, CA, USA.
Mark Valasek, Department of Pathology, University of California at San Diego, La Jolla, CA, USA.
William Haufe, Liver Imaging Group, Department of Radiology, University of California at San Diego, La Jolla, CA, USA.
Catherine Hooker, Liver Imaging Group, Department of Radiology, University of California at San Diego, La Jolla, CA, USA.
Lisa Richards, NAFLD Translational Research Unit, Department of Medicine, University of California at San Diego, La Jolla, CA, USA Division of Gastroenterology, Department of Medicine, University of California at San Diego, La Jolla, CA, USA.
Claude B. Sirlin, Liver Imaging Group, Department of Radiology, University of California at San Diego, La Jolla, CA, USA
Rohit Loomba, NAFLD Translational Research Unit, Division of Gastroenterology and Epidemiology, University of California at San Diego, 9500 Gilman Drive, MC 0063, La Jolla, CA 92093, USA.
References
- Adams L., Lymp J., St Sauver J., Sanderson S., Lindor K., Feldstein A., et al. (2005) The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology 129: 113–121. [DOI] [PubMed] [Google Scholar]
- Bohte A., van Werven J., Bipat S., Stoker J. (2011) The diagnostic accuracy of US, CT, MRI and 1h-MRS for the evaluation of hepatic steatosis compared with liver biopsy: a meta-analysis. Eur Radiol 21: 87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo A., Sheth S., Chopra S. (2001) Liver biopsy. N Engl J Med 344: 495–500. [DOI] [PubMed] [Google Scholar]
- Browning J., Szczepaniak L., Dobbins R., Nuremberg P., Horton J., Cohen J., et al. (2004) Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology 40: 1387–1395. [DOI] [PubMed] [Google Scholar]
- Chalasani N., Younossi Z., Lavine J., Diehl A., Brunt E., Cusi K., et al. (2012) The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 55: 2005–2023. [DOI] [PubMed] [Google Scholar]
- Charlton M., Burns J., Pedersen R., Watt K., Heimbach J., Dierkhising R. (2011) Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology 141: 1249–1253. [DOI] [PubMed] [Google Scholar]
- Doycheva I., Cui J., Nguyen P., Costa E., Hooker J., Hofflich H., et al. (2015) Noninvasive screening of diabetics in primary care for NAFLD and advanced fibrosis by MRI and MRE. Aliment Pharmacol Ther 43: 83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines C., Frydrychowicz A., Hamilton G., Tudorascu D., Vigen K., Yu H., et al. (2011) T(1) independent, T(2) (*) corrected chemical shift based fat-water separation with multi-peak fat spectral modeling is an accurate and precise measure of hepatic steatosis. J Magn Reson Imaging 33: 873–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idilman I., Aniktar H., Idilman R., Kabacam G., Savas B., Elhan A., et al. (2013) Hepatic steatosis: quantification by proton density fat fraction with MR imaging versus liver biopsy. Radiology 267: 767–775. [DOI] [PubMed] [Google Scholar]
- Kang G., Cruite I., Shiehmorteza M., Wolfson T., Gamst A., Hamilton G., et al. (2011) Reproducibility of MRI-determined proton density fat fraction across two different MR scanner platforms. J Magn Reson Imaging 34: 928–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating S., Hackett D., Parker H., O’Connor H., Gerofi J., Sainsbury A., et al. (2015) Effect of aerobic exercise training dose on liver fat and visceral adiposity. J Hepatol 63: 174–182. [DOI] [PubMed] [Google Scholar]
- Kleiner D., Brunt E., van Natta M., Behling C., Contos M., Cummings O., et al. (2005) Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41: 1313–1321. [DOI] [PubMed] [Google Scholar]
- Le T., Chen J., Changchien C., Peterson M., Kono Y., Patton H., et al. (2012) Effect of colesevelam on liver fat quantified by magnetic resonance in nonalcoholic steatohepatitis: a randomized controlled trial. Hepatology 56: 922–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., McKenzie C., Yu H., Brittain J., Reeder S. (2007) Fat quantification with ideal gradient echo imaging: correction of bias from T(1) and noise. Magn Reson Med 58: 354–364. [DOI] [PubMed] [Google Scholar]
- Loomba R., Abraham M., Unalp A., Wilson L., Lavine J., Doo E., et al. (2012) Association between Diabetes, family history of diabetes, and risk of nonalcoholic steatohepatitis and fibrosis. Hepatology 56: 943–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomba R., Sanyal A. (2013) The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol 10: 686–690. [DOI] [PubMed] [Google Scholar]
- Loomba R., Schork N., Chen C., Bettencourt R., Bhatt A., Ang B., et al. (2015) Heritability of hepatic fibrosis and steatosis based on a prospective twin study. Gastroenterology 149: 1784–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomba R., Sirlin C., Ang B., Bettencourt R., Jain R., Salotti J., et al. (2014) Ezetimibe for the treatment of nonalcoholic steatohepatitis: assessment by novel MRI and MRE in a randomized trial (Mozart Trial). Hepatology 61: 1239–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomba R., Wesley R., Pucino F., Liang T., Kleiner D., Lavine J. (2008) Placebo in nonalcoholic steatohepatitis: insight into natural history and implications for future clinical trials. Clin Gastroenterol Hepatol 6: 1243–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteoni C., Younossi Z., Gramlich T., Boparai N., Liu Y., McCullough A. (1999) Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology 116:1413–1419. [DOI] [PubMed] [Google Scholar]
- Meisamy S., Hines C., Hamilton G., Sirlin C., Mckenzie C., Yu H., et al. (2011) Quantification of hepatic steatosis with T1-independent, T2-corrected MR imaging with spectral modeling of fat: blinded comparison with MR spectroscopy. Radiology 258: 767–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra P., Younossi Z. (2007) Abdominal ultrasound for diagnosis of nonalcoholic fatty liver disease (NAFLD). Am J Gastroenterol 102: 2716–2717. [DOI] [PubMed] [Google Scholar]
- Negrete L., Middleton M., Clark L., Wolfson T., Gamst A., Lam J., et al. (2014) Inter-examination precision of magnitude-based MRI for estimation of segmental hepatic proton density fat fraction in obese subjects. J Magn Reson Imaging 39: 1265–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuschwander-Tetri B., Clark J., Bass N., van Natta M., Unalp-Arida A., Tonascia J., et al. (2010) Clinical, laboratory and histological associations in adults with nonalcoholic fatty liver disease. Hepatology 52: 913–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noureddin M., Lam J., Peterson M., Middleton M., Hamilton G., Le T., et al. (2013) Utility of magnetic resonance imaging versus histology for quantifying changes in liver fat in nonalcoholic fatty liver disease trials. Hepatology 58: 1930–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel N., Doycheva I., Peterson M., Hooker J., Kisselva T., Schnabl B., et al. (2014) Effect of weight loss on magnetic resonance imaging estimation of liver fat and volume in patients with nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol 13: 561–568.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Permutt Z., Le T., Peterson M., Seki E., Brenner D., Sirlin C., et al. (2012) Correlation between liver histology and novel magnetic resonance imaging in adult patients with non-alcoholic fatty liver disease - MRI accurately quantifies hepatic steatosis in NAFLD. Aliment Pharmacol Ther 36: 22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prati D., Taioli E., Zanella A., Della Torre E., Butelli S., Del Vecchio E., et al. (2002) Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med 137: 1–10. [DOI] [PubMed] [Google Scholar]
- Ratziu V., Charlotte F., Heurtier A., Gombert S., Giral P., Bruckert E., et al. (2005) Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology 128: 1898–1906. [DOI] [PubMed] [Google Scholar]
- Reeder S., Robson P., Yu H., Shimakawa A., Hines C., Mckenzie C., et al. (2009) Quantification of hepatic steatosis with mri: the effects of accurate fat spectral modeling. J Magn Reson Imaging 29: 1332–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeder S., Sirlin C. (2010) Quantification of liver fat with magnetic resonance imaging. Magn Reson Imaging Clin N Am 18: 337–357, ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadeh S., Younossi Z., Remer E., Gramlich T., Ong J., Hurley M., et al. (2002) The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology 123: 745–750. [DOI] [PubMed] [Google Scholar]
- Schwenzer N., Springer F., Schraml C., Stefan N., Machann J., Schick F. (2009) Noninvasive assessment and quantification of liver steatosis by ultrasound, computed tomography and magnetic resonance. J Hepatol 51: 433–445. [DOI] [PubMed] [Google Scholar]
- Singh S., Allen A., Wang Z., Prokop L., Murad M., Loomba R. (2014) Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol 13: 643–654.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumida Y., Nakajima A., Itoh Y. (2014) Limitations of liver biopsy and noninvasive diagnostic tests for the diagnosis of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol 20: 475–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczepaniak L., Nurenberg P., Leonard D., Browning J., Reingold J., Grundy S., et al. (2005) Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab 288: e462–e468. [DOI] [PubMed] [Google Scholar]
- Tang A., Desai A., Hamilton G., Wolfson T., Gamst A., Lam J., et al. (2015) Accuracy of MR imaging-estimated proton density fat fraction for classification of dichotomized histologic steatosis grades in nonalcoholic fatty liver disease. Radiology 274: 416–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang A., Tan J., Sun M., Hamilton G., Bydder M., Wolfson T., et al. (2013) Nonalcoholic fatty liver disease: MR imaging of liver proton density fat fraction to assess hepatic steatosis. Radiology 267: 422–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon G., Baranova A., Younossi Z. (2011) Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 34: 274–285. [DOI] [PubMed] [Google Scholar]
- Williams C., Stengel J., Asike M., Torres D., Shaw J., Contreras M., et al. (2011) Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology 140: 124–131. [DOI] [PubMed] [Google Scholar]
- Wong V., Chu W., Wong G., Chan R., Chim A., Ong A., et al. (2012) Prevalence of non-alcoholic fatty liver disease and advanced fibrosis in Hong Kong Chinese: a population study using proton-magnetic resonance spectroscopy and transient elastography. Gut 61: 409–415. [DOI] [PubMed] [Google Scholar]
- Yokoo T., Bydder M., Hamilton G., Middleton M., Gamst A., Wolfson T., et al. (2009) Nonalcoholic fatty liver disease: diagnostic and fat-grading accuracy of low-flip-angle multiecho gradient-recalled-echo MR imaging at 1.5 T. Radiology 251: 67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoo T., Shiehmorteza M., Hamilton G., Wolfson T., Schroeder M., Middleton M., et al. (2011) Estimation of hepatic proton-density fat fraction by using MR imaging at 3.0 T. Radiology 258: 749–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarrinpar A., Gupta S., Maurya M., Subramaniam S., Loomba R. (2015) Serum micrornas explain discordance of non-alcoholic fatty liver disease in monozygotic and dizygotic twins: a prospective study. Gut. doi: 10.1136/gutjnl-2015-309456 22 May 2015. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


