Abstract
Chronic liver disease (CLD) and cirrhosis are major sources of morbidity and mortality in the United States. Little is known about the epidemiology of these two diseases in ethnic minority populations in the United States. We examined the prevalence of CLD and cirrhosis by underlying etiologies among African Americans, Native Hawaiians, Japanese Americans, Latinos and whites in the Multiethnic Cohort. CLD and cirrhosis cases were identified using Medicare claims between 1999 and 2012 among the fee-for-service participants (n=106,458). We used ICD-9 codes, body mass index, history of diabetes mellitus and alcohol consumption from questionnaires to identify underlying etiologies. A total of 5,783 CLD (3,575 CLD without cirrhosis and 2,208 cirrhosis) cases were identified. The prevalence of CLD ranged from 3.9% in African Americans and Native Hawaiians to 4.1% in whites, 6.7% in Latinos and 6.9% in Japanese. Nonalcoholic fatty liver disease (NAFLD) was the most common cause of CLD in all ethnic groups combined (52%), followed by alcoholic liver disease (ALD) (21%). NAFLD was the most common cause of cirrhosis in the entire cohort. By ethnicity, NALFD was the most common cause of cirrhosis in Japanese Americans, Native Hawaiians, and Latinos, accounting for 32% of cases. ALD was the most common cause of cirrhosis in whites (38.2%), while hepatitis C virus was the most common cause in African Americans (29.8%).
Conclusions
We showed racial/ethnic variations in the prevalence of CLD and cirrhosis by underlying etiology. NAFLD is the most common cause of CLD and cirrhosis in the entire cohort. The high prevalence of NAFLD among Japanese Americans and Native Hawaiians are novel findings and studies elucidating the causes are warranted.
Keywords: fatty liver, steatosis, Hepatitis C Virus, minority populations
Liver disease is a major health problem in the United States (US). According to the Centers for Disease Control and Prevention (CDC), chronic liver disease (CLD) and cirrhosis were the 12th leading cause of death in the US in 2013, accounting for more than 36,000 deaths (1). In a recent analysis, however, Mayo Clinic researchers showed that liver disease-related mortality in the US has been underestimated during the past two decades, and the figure was closer to 66,000 deaths annually (2).
Although chronic infection with hepatitis C virus (HCV) and alcoholic liver disease are major causes of CLD in the US, nonalcoholic fatty liver disease (NAFLD) has become the most common cause (3–6). Data from the National Health and Nutrition Examination Survey (NHANES) showed that while the prevalence of most major causes of CLD was stable, the prevalence of NAFLD increased steadily from 1988 to 2008 (4). A recent study from the United Network for Organ Sharing (UNOS) database showed that, between 2004 and 2013, the number of new waitlist liver transplant registrants with NAFLD increased by 170%, compared with 45% for ALD and 14% for HCV, making NALFD now the second most common cause of cirrhosis leading to liver transplant after HCV (7).
It has been shown that there are racial/ethnic differences in the prevalence of CLD and cirrhosis in the US (3, 8), however, the data are limited and restricted to a few ethnic/racial populations (3). A recent study suggested that some ethnic/racial groups that are not well studied could have a higher burden of liver disease; the researchers suggested conducting more detailed research in other ethnicities (9). Moreover, the prevalence of etiologies and common causes of CLD and cirrhosis within each ethnic/racial group have not been fully investigated. For instance, while it is known that the prevalence of HCV infection in African Americans is higher compared to other ethnic/racial groups (10), it is unknown if it is the most common cause of CLD and cirrhosis in this population.
In this study we examined the prevalence of both CLD and cirrhosis by underlying etiology in a large cohort of African Americans, Native Hawaiians, Japanese Americans, Latinos and Whites participating in the Multiethnic Cohort (MEC) study. Our data highlight the detailed etiologies of CLD and cirrhosis in each racial group and provide new information for understudied ethnic groups, such as Native Hawaiians and Japanese Americans.
Methods
Study population
The MEC is a prospective cohort of more than 215,000 men and women, aged 45–75 years, enrolled between 1993 and 1996. The MEC study design and baseline characteristics have been described in detail previously (11). Japanese Americans comprised the largest subgroup within each sex (28% of men and 25% of women), followed by whites (24% and 22%), Latinos (24% and 21%), African Americans (13% and 19%), and Native Hawaiians (6% and 7%). The baseline mailed questionnaire assessed diet, lifestyle, weight and height, family and personal medical history and, for women, menstrual and reproductive history and hormone use. Since baseline, there have been four follow-up questionnaires, and between 1995 and 2006, blood and urine specimens were collected from ~70,000 participants for biomarker and genetic studies. Incident cancers in the cohort are identified through annual linkage to the Hawaii Tumor Registry, the Cancer Surveillance Program for Los Angeles County, and the California State Cancer Registry; these cancer registries are part of the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) program. Deaths are determined through annual linkage to state death certificate files in California and Hawaii, and periodic linkage to the National Death Index. The MEC participants older than 65 years were linked to Centers for Medicare Services (CMS) claims (1999–2012) using Social Security number, sex, and date of birth; 93% of these participants were successfully linked (12). For this analysis, we excluded participants who were not from the five major ethnic groups (N=12,008) or who were not fee-for-service (FFS) members (N=64,904). The characteristics of FFS vs. non FFS members are shown in Supplemental Table 1; the proportion of FFS participants was higher in Hawaii than in California, higher among whites, Japanese Americans and Hawaiians than among African Americans and Latinos, and higher among participants with a higher educational level. A total of 106,458 eligible participants were available for analysis. The average length of FFS enrollment was 6.7 years, ranging from 5.9 years for Latinos to 7.8 years for Japanese Americans. The Institutional Review Boards for the University of Southern California and the University of Hawaii approved this study.
Case ascertainment
We identified CLD and cirrhosis cases using 1 inpatient or ≥ 2 outpatient/carrier qualifying claims during a one-year period between 1999 and 2012 using International Classification of Diseases (ICD) 9th revision codes. CLD was identified using the ICD-9 codes: 571.0–571.9. Cirrhosis was identified using the following codes: 571.2 (cirrhosis with alcoholism); 571.5 (cirrhosis no mention of alcohol); 456.0, 456.1, 456.20, and 456.21 (esophageal varices); 567.23 (spontaneous bacterial peritonitis); 572.2 (hepatic encephalopathy); and 572.4 (hepatorenal syndrome). A total of 5,783 CLD cases (3,575 without cirrhosis and 2,208 with cirrhosis) were identified among the study population.
Etiology of CLD and cirrhosis
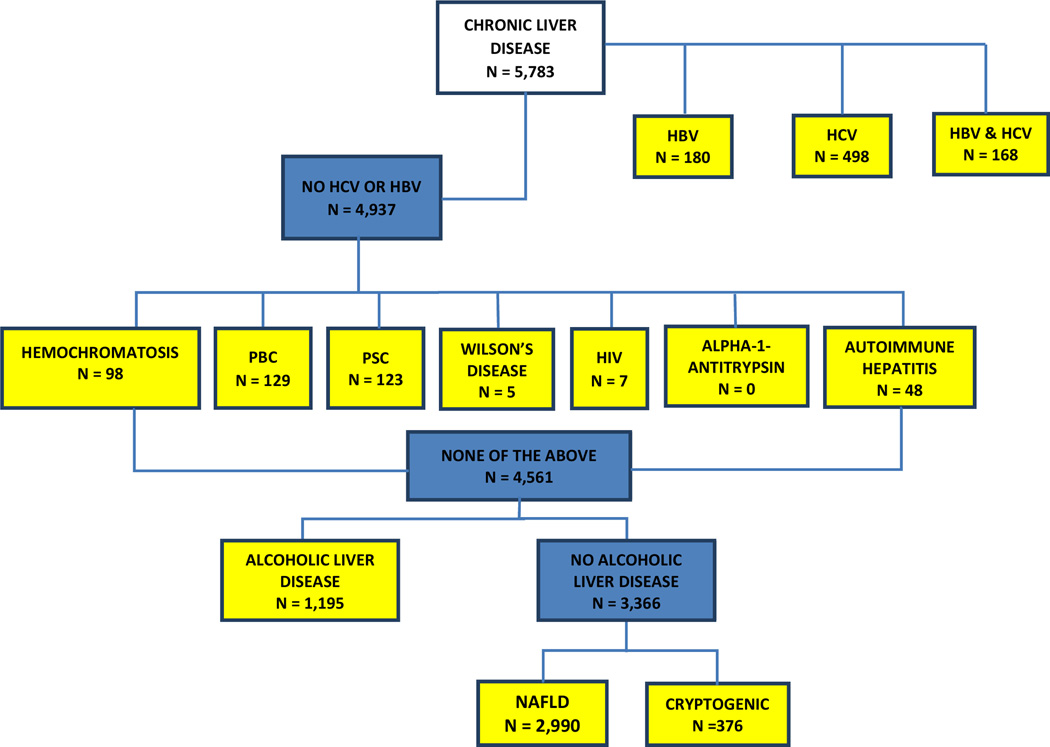
We followed published criteria (13) to define the underlying liver disease of CLD and cirrhosis cases in the MEC (Figure 1). We first identified cases with HCV and/or hepatitis B virus (HBV). These cases were categorized as of HCV, HBV, or HCV and HBV etiology regardless of any additional etiologies. Alcoholic liver disease (ALD) was identified as the cause in cases with alcohol use disorders (alcohol-related CLD, alcohol dependence, alcohol abuse, alcohol mental disorders, alcoholic polyneuropathy, alcoholic cardiomyopathy, and alcoholic pancreatitis). NAFLD was identified as etiologic for cases without any other cause (including HCV, HBV, hemochromatosis, primary biliary cirrhosis, primary sclerosing cholangitis, Wilson’s disease, HIV, Alpha-1-Antitrypsin deficiency and autoimmune hepatitis) and with a baseline BMI ≥30 kg/m2, diabetes mellitus or NAFLD ICD-9 code 571.8. Diabetes was identified via Medicare claims, as well as from baseline and follow up questionnaires. Using the American Association for the Study of Liver Diseases (AASLD) guidelines (14), women with NAFLD who reported >14 drinks/week and men with NAFLD who reported >21 drinks/week on the alcohol baseline questionnaire were reclassified to ALD. If cases did not satisfy any of the above criteria, they were classified as cryptogenic.
Figure 1. Identification of the cause of liver disease.
Alcoholic Liver Disease: alcohol dependence (303.00–303.93), alcohol abuse (305.00–305.03), alcohol mental disorders (291.0–291.9), alcoholic polyneuropathy (357.5), cardiomyopathy (425.5), pancreatitis (577.0, 577.1), and alcoholic-related CLD (571.0–571.3); Alpha-1-Antitrypsin: 273.4; Autoimmune Hepatitis: 571.42; Cirrhosis: 571.2, 571.5, hepatic encephalopathy (572.2), hepatorenal syndrome (572.4), esophageal varices (456.0, 456.1, 456.20, 456.21), and spontaneous bacterial peritonitis (567.23); CLD: 571.0–571.9; Hemochromatosis: 275.0; Hepatitis B: 0702, 07020, 07021, 07022, 07023, 0703, 07030, 07031, 07032, 07033, V0261; Hepatitis C: 07041, 07044, 07051, 07054, 07070, 07071, V0262; HIV: 042; NAFLD: Diabetic or BMI ≥ 30 kg/m2 or 571.8. Women with NAFLD who drank >14 drinks/week and men with NAFLD who drank >21 drinks/week were reclassified to alcoholic liver disease; Primary Biliary Cirrhosis (PBC): 571.6; Primary Sclerosing Cholangitis (PSC): 576.1; Wilson’s Disease: 275.1
Statistical analysis
We determined the number of cases with CLD or cirrhosis among MEC participants who were enrolled in the Medicare-FFS between 1999 and 2012. We examined the prevalence of CLD and cirrhosis by underlying etiologies across race/ethnicity by dividing the number of cases with the population size. We also examined whether the prevalence of CLD types differed between whites and the other groups using logistic regression adjusting for sex and duration of Medicare enrollment (in years). All analyses were conducted using SAS version 9.3 (SAS Institute, Inc., Cary, NC). All P values were two-sided.
Results
The characteristics of CLD cases in the MEC
We identified 5,783 cases of CLD with and without cirrhosis among the study population between 1999 and 2012 (Table 1). Among these cases, 2,990 (51.7%) had NAFLD; 1,195 (20.7%) had ALD; 498 (8.6%) had HCV; 180 (3.1%) had HBV; 168 (2.9%) had HCV and HBV; 376 (6.5%) were classified as cryptogenic; and the rest of the cases were due to other causes of liver disease. The average age at the first CLD claim was 72.9 years, with the oldest found among cryptogenic cases (74.4 years). The ethnicity breakdown was 39.5% Japanese Americans, 24.9% Latinos, 19.9% whites, 10.5% African Americans, and 5.2% Native Hawaiians. There were more women (56.6%) than men (43.3%). The average alcohol intake (ethanol g/day) was highest among ALD cases (36.5 g/day) and lowest among NAFLD cases (5.1 g/day). The average BMI was highest among NAFLD cases (28.7 kg/m2) and lowest among cryptogenic cases (25.3 kg/m2).
Table 1.
Characteristics of Chronic Liver Disease Cases, Overall and by Etiologies in the Multiethnic Cohort, 1999–2012
| HBV | HBV & HCV |
HCV | ALD | NAFLD | Other | Cryptogenic | All Cases | |
|---|---|---|---|---|---|---|---|---|
| No. of cases, N (%) | 180 (3.1%) | 168 (2.9%) | 498 (8.6%) | 1,195 (20.7%) | 2,990 (51.7%) | 376 (6.5%) | 376 (6.5%) | 5,783 |
|
Age at date of 1st claim, mean (SD) |
72.0 (6.8) | 70.6 (6.0) | 71.4 (6.3) | 72.9 (6.3) | 73.2 (6.6) | 73.1 (6.5) | 74.4 (6.7) | 72.9 (6.5) |
| Race/Ethnicity, N (row %) | ||||||||
| White | 31 (2.7%) | 24 (2.1%) | 98 (8.5%) | 345 (30.1%) | 467 (40.7%) | 80 (7.0%) | 103 (9.0%) | 1,148 |
| African American | 19 (3.1%) | 28 (4.6%) | 102 (16.7%) | 160 (26.3%) | 239 (39.2%) | 23 (3.8%) | 38 (6.2%) | 609 |
| Native Hawaiian | 9 (3.0%) | 2 (0.7%) | 8 (2.7%) | 67 (22.3%) | 174 (57.8%) | 21 (7.0%) | 20 (6.6%) | 301 |
| Japanese American | 79 (3.5%) | 55 (2.4%) | 99 (4.3%) | 276 (12.1%) | 1,452 (63.6%) | 171 (7.5%) | 151 (6.6%) | 2,283 |
| Latino | 42 (2.9%) | 59 (4.1%) | 191 (13.2%) | 347 (24.1%) | 658 (45.6%) | 81 (5.6%) | 64 (4.4%) | 1,442 |
| Sex, N (row %) | ||||||||
| Male | 73 (2.9%) | 78 (3.1%) | 218 (8.7%) | 741 (29.5%) | 1,097 (43.7%) | 144 (5.7%) | 159 (6.3%) | 2,510 |
| Female | 107 (3.3%) | 90 (2.7%) | 280 (8.6%) | 454 (13.9%) | 1,893 (57.8%) | 232 (7.1%) | 217 (6.6%) | 3,273 |
| Alcohol intake (g/day) | ||||||||
| Mean† | 11.6 | 7.8 | 13.1 | 36.5 | 5.1 | 13.0 | 15.5 | 14.8 |
| Body mass index (kg/m2) | ||||||||
| Mean† | 28.2 | 27.3 | 27.2 | 27.2 | 28.7 | 27.8 | 25.3 | 27.9 |
Adjusted for sex and race/ethnicity distribution.
Prevalence of CLD by underlying etiology in the MEC
Table 2 shows the prevalence of CLD with and without cirrhosis in the MEC by underlying cause and race/ethnicity. We found that Japanese Americans (6.9%) and Latinos (6.7%) had the highest prevalence of CLD, while the prevalence was lower in whites (4.1%) and in African Americans and Native Hawaiians (3.9% each). The highest prevalence of NAFLD was observed in Japanese Americans (4.4%), followed by Latinos (3.1%), Native Hawaiians (2.3%), whites (1.7%) and African Americans (1.5%). The highest prevalence of ALD was observed in Latinos (1.6%), followed by whites (1.2%), African Americans (1.0%), Native Hawaiians (0.9%) and Japanese Americans (0.8%). The patterns were similar when analysis was restricted to participants with at least 12 months of FFS enrollment (data not shown). In a time trend analysis based on 3 periods (1999–2003, 2004–2008, 2009–2012), the proportion of NAFLD increased over time in all racial/ethnic groups (Supplemental Tables 2–3).
Table 2.
Prevalence of Chronic Liver Disease and Cirrhosis by Etiologies in the Multiethnic Cohort, 1999–2012
| All | HBV n=180 |
HBV & HCV n=168 |
HCV n=498 |
ALD n=1,195 |
NAFLD n=2,990 |
Others n=376 |
Cryptogenic n=376 |
Total Cases n=5,783 |
Base Population n = 106,458 |
|---|---|---|---|---|---|---|---|---|---|
| White | 31 (0.1 %) | 24 (0.1 %) | 98 (0.3%) | 345 (1.2%) | 467 (1.7 %) | 80 (0.3 %) | 103 (0.4 %) | 1,148 (4.1 %) | 28,225 |
| African American | 19 (0.1 %) | 28 (0.2 %) | 102 (0.6 %) | 160 (1.0 %) | 239 (1.5 %) | 23 (0.1 %) | 38 (0.2 %) | 609 (3.9 %) | 15,782 |
| Native Hawaiian | 9 (0.1 %) | 2 (0.0 %) | 8 (0.1 %) | 67 (0.9 %) | 174 (2.3 %) | 21 (0.3 %) | 20 (0.3 %) | 301 (3.9 %) | 7,647 |
| Japanese American | 79 (0.2 %) | 55 (0.2 %) | 99 (0.3 %) | 276 (0.8 %) | 1,452 (4.4 %) | 171 (0.5 %) | 151 (0.5 %) | 2,283 (6.9 %) | 33,312 |
| Latino | 42 (0.2 %) | 59 (0.3 %) | 191 (0.9 %) | 347 (1.6 %) | 658 (3.1 %) | 81 (0.4 %) | 64 (0.3 %) | 1,442 (6.7 %) | 21,492 |
Using the following ICD-9 diagnosis codes for CLD: 571.0–571.9, we selected any 1 inpatient claim or 2 outpatient/carrier claims during a 1 year period for years 1999–2012 to identify CLD cases. Cirrhosis include cirrhosis with alcoholism (571.2), cirrhosis no mention of alcohol (571.5), esophageal varices (456.0, 456.1, 456.20, 456.21), spontaneous bacterial peritonitis (567.23), hepatic encephalopathy (572.2), and hepatorenal syndrome (572.4).
Analysis of Causes of CLD by Cirrhosis Status
We investigated the underlying cause of CLD without cirrhosis (N=3,575 cases) and with cirrhosis (N=2,208 cases) across race/ethnicity (Table 3). Because of the small numbers, we combined HCV cases with those with both HCV and HBV. NAFLD was the most common cause of CLD without cirrhosis (65.6%) in all racial/ethnic groups, followed by alcoholic liver disease (ALD) (16.5%), HCV (5.3%) and others (5.3%). Interestingly, CLD without cirrhosis due to NAFLD was highest in Japanese Americans (72.9%) and Native Hawaiians (72.5%), followed by Latinos (61.1%) and whites (55.8%), and lowest in African Americans (49.3%). Compared to whites, the differences in Japanese Americans (P ≤0.0001), Native Hawaiians (P ≤0.0001) and African Americans (P <0.05) were statistically significant. CLD without cirrhosis due to ALD was highest in African Americans (27.0%), followed by Latinos (20.8%), whites (23.6%), Native Hawaiians (17.6%) and Japanese Americans (10.4%). CLD without cirrhosis due to HCV was highest in African Americans (12.8%), followed by Latinos (7.7%), whites (5.9%), Japanese Americans (3.2%) and Native Hawaiians (2.6%).
Table 3.
Distribution of Chronic Liver Disease and Cirrhosis by Etiologies and Race/Ethnicity in the Multiethnic Cohort, 1999–2012
| CLD without cirrhosis | HBV n=98 |
HCV† n=190 |
ALD n=591 |
NAFLD n=2,344 |
Others n=191 |
Cryptogenic n=161 |
All Cases n=3,575 |
|---|---|---|---|---|---|---|---|
| White | 15 (2.3%) | 38 (5.9%) | 151 (23.6%) | 357 (55.8%) | 41 (6.4%) | 38 (5.9%) | 640 |
| African American | 11 (3.6%) | 39 (12.8%)** | 82 (27.0%) | 150 (49.3%)** | 8 (2.6%)** | 14 (4.6%) | 304 |
| Native Hawaiian | 2 (1.0%) | 5 (2.6%) | 34 (17.6%) | 140 (72.5%)* | 7 (3.6%) | 5 (2.6%) | 193 |
| Japanese American | 50 (2.8%) | 56 (3.2%)** | 183 (10.4%)* | 1,283 (72.9%)* | 100 (5.7%) | 88 (5.0%) | 1,760 |
| Latino | 20 (2.9%) | 52 (7.7%) | 141 (20.8%) | 414 (61.1%) | 35 (5.2%) | 16 (2.4%)** | 678 |
| Cirrhosis |
HBV n=82 |
HCV† n=476 |
ALD n=604 |
NAFLD n=646 |
Others n=185 |
Cryptogenic n=215 |
All Cases n=2,208 |
| White | 16 (3.1%) | 84 (16.5%) | 194 (38.2%) | 110 (21.7%) | 39 (7.7%) | 65 (12.8%) | 508 |
| African American | 8 (2.6%) | 91 (29.8%)* | 78 (25.6%)** | 89 (29.2%)** | 15 (4.9 %) | 24 (7.9%)** | 305 |
| Native Hawaiian | 7 (6.5%) | 5 (4.6%)** | 33 (30.6%) | 34 (31.5%)** | 14 (13.0 %) | 15 (13.9%) | 108 |
| Japanese American | 29 (5.5%) | 98 (18.7%) | 93 (17.8%)* | 169 (32.3%)** | 71 (13.6%)** | 63 (12.0%) | 523 |
| Latino | 22 (2.9%) | 198 (25.9%)* | 206 (27.0%)* | 244 (31.9%)* | 46 (6.0%) | 48 (6.3%)* | 764 |
HCV cases with HBV were also included in this group.
P≤0.0001 compared with Whites and adjusted for sex and duration of Medicare enrollment.
P<0.05 compared with Whites and adjusted for sex and duration of Medicare enrollment.
NALFD was the most common cause of cirrhosis in the entire cohort (29.3%). When stratified by race/ethnicity, NAFLD was the most common cause of cirrhosis in Japanese Americans (32.3%), Native Hawaiians (31.5%), and Latinos (31.9%); these numbers were significantly different from whites (21.7%) (Table 3). ALD was the most common cause of cirrhosis in whites (38.2%), while HCV and NAFLD were the most common causes of cirrhosis in African Americans (29.8% and 29.2%), respectively.
Discussion
In this large cohort study, we showed racial/ethnic variations in the prevalence of CLD and cirrhosis by underlying etiology. NAFLD was the most common cause of CLD and cirrhosis in the entire cohort. When analyzed by race/ethnicity, NAFLD was the most common cause of CLD without cirrhosis in all ethnic groups and of CLD with cirrhosis in Japanese Americans, Latinos and Native Hawaiians. HCV and ALD were the most common causes of cirrhosis in African Americans and whites, respectively. Our study reports several novel findings: (1) NAFLD is the most common cause of cirrhosis in this US multiethnic cohort; (2) although the prevalence of NAFLD is lowest in African Americans, it is the most common cause of CLD and second most common cause of cirrhosis in this group; and (3) the prevalence of NAFLD in Japanese Americans is higher than in Latinos and other ethnic groups.
With the striking increase in the prevalence of obesity, NAFLD has become the most common cause of CLD (15). Our results underscored the importance of NAFLD as the major cause of CLD across all ethnic groups in the MEC. However, our data likely underestimated the prevalence of NAFLD although it is consistent with other epidemiological studies that did not include imaging studies (16, 17). Studies that used imaging modalities or liver biopsy have shown higher prevalence of NAFLD which can be up to 45% in the US population (18–21). In the US, the prevalence of NAFLD has been reported to be higher among Latinos than in other racial/ethnic groups and lowest among African Americans (20, 22–25). We found that the lowest prevalence of NAFLD was observed in African Americans but, surprisingly, the prevalence in Japanese Americans was higher than that of Latinos in our cohort.
Data on the prevalence of NAFLD in Japanese Americans are scant; most published data on this population originated from Japan. The prevalence of NAFLD in the general population in Japan was estimated to be 9–14% in 1988 (26). During the past 20 to 30 years, the prevalence of NAFLD in Japan has increased gradually (27). Recent data from Japan showed that the prevalence of fatty liver based on ultrasonography among healthy adults was 30% (28), and as high as 72% when at least two of the risk factors (obesity, diabetes or hyperlipidemia) were present (29, 30). In a prospective study, metabolic syndrome was shown to be a strong predictor of NAFLD in apparently healthy Japanese men and women (31).
Despite the relatively low prevalence of NAFLD in African Americans, NAFLD was the most common cause of CLD in this group. Browning et al. and others showed that African Americans have significantly lower NAFLD prevalence compared to Latinos and non-Hispanic whites after adjusting for obesity and diabetes (22, 32). One explanation for these ethnic differences in NAFLD prevalence may be due to ethnic differences in body fat distribution (33–35), fat metabolism (15) and/or genetics (e.g. a genetic variant in PNPLA3 (rs738409) associated with hepatic fat content and hepatic inflammation was found to be less prevalent in African Americans) (36–38). Compared to whites with similar total adiposity, Latinos and Asians are more likely, and African Americans less likely, to accumulate fat in the abdominal visceral compartment and in the liver (22, 39, 40). In the MEC, we previously reported that compared to white women with comparable total adiposity, Japanese-American women had more visceral and liver fat as measured by MRI (41). It also has been suggested that African Americans have a different lipoprotein metabolism (42). One can argue that the diagnosis of NAFLD was possibly overestimated in African Americans in our cohort using the definition criteria. However, all of our subjects had a diagnosis of CLD and NAFLD cases were classified as such after ruling out other causes of liver disease. Indeed, in our study, CLD and cirrhosis due to HCV were higher in African Americans compared to others. HBV was also prevalent in in African Americans in our cohort. These findings are consistent with HCV and HBV data in African Americans shown in other studies supporting the methodology we have used (3, 43, 44).
Our study shows that NAFLD was the most common cause of cirrhosis in the entire MEC cohort. When we stratified by race/ethnicity, NAFLD was the most common cause in Japanese, Latinos and Native Hawaiians, while ALD and HCV were the most common causes of cirrhosis in whites and African Americans, respectively. However, a recent report based on a transplant database (2004 to 2013) (7) showed that NAFLD was the second leading indication for liver transplant among cirrhosis patients after HCV. It is possible that because NAFLD is a silent disease, underdiagnosed and associated with many comorbidities, NAFLD patients are not eligible for the transplant list (45). It is likely that ALD cases were excluded from the transplant list. A study among US Veterans (2001–2013) reported that HCV was the most common cause of cirrhosis (13). The most likely explanation of the discrepancy between the VA results and ours is that the VA participants were predominantly men and the prevalence of HCV in this population was approximately double that of comparable age and sex groups of US population (13). Our data highlights the importance of NAFLD as a leading cause of cirrhosis, especially in certain ethnic groups.
Alcohol continues to be one of the major contributors to liver disease in the US. Indeed, we found that ALD is the most common cause of cirrhosis in whites. While the prevalence of alcohol abuse among racial/ethnic groups have been characterized, data on racial disparities in CLD and cirrhosis due to alcohol are scant. Older data described higher heavy drinking in African Americans and Latinos (46, 47). However, recent data from the National Institute on Alcohol Abuse and Alcoholism (NIAAA) showed that whites are more likely to die from alcoholic liver cirrhosis than blacks (http://pubs.niaaa.nih.gov/publications/Surveillance100/Cirr11.htm). Our data are consistent with the NIAAA findings.
The limitations of our study include case identification based on Medicare claim files, probable underestimation of the true prevalence of NAFLD given the lack of biochemical and imaging testing and possibly other etiologies (e.g. HCV and HBV related diseases due to under diagnosis), and restriction to FFS participants. Our data included mostly people aged on average 60 years and older when liver diseases are usually more severe (48, 49). The strengths of our study include its large size and population-based design, longitudinal follow up, inclusion of five racial/ethnic populations, including understudied populations, and information on several factors including alcohol intake, BMI, and diabetes status. Our study has investigated all major causes of liver diseases in these racial groups using a published method (13). To our knowledge, our study is the largest detailed study with multiple ethnic groups to date on racial disparities in liver diseases.
In conclusion, in this large multiethnic cohort, we found NAFLD to be the most important cause of CLD and cirrhosis. The high prevalence of NAFLD among Japanese Americans and Native Hawaiians is a novel finding and studies elucidating the causes of this are warranted. Better screening, diagnostic and management approaches need to be implemented to face this growing epidemic. Further studies are needed to confirm our findings and to investigate underlying genetic, metabolic and nutritional causes.
Supplementary Material
Acknowledgments
We thank the MEC participants for their participation and commitment.
Grant support: National Cancer Institute grants CA164973 and CA186203
Abbreviations
- ALD
alcoholic liver disease
- BMI
body mass index
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- NAFLD
nonalcoholic fatty liver disease
- SD
standard deviation
Footnotes
Conflicts of interest: the authors disclose no conflicts
References
- 1.National Vital Statistics Reports 1999–2013: Centers for Disease Control and Prevention
- 2.Asrani SK, Larson JJ, Yawn B, Therneau TM, Kim WR. Underestimation of liver-related mortality in the United States. Gastroenterology. 2013;145:375–382. e371–e372. doi: 10.1053/j.gastro.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nguyen GC, Thuluvath PJ. Racial disparity in liver disease: Biological, cultural, or socioeconomic factors. Hepatology. 2008;47:1058–1066. doi: 10.1002/hep.22223. [DOI] [PubMed] [Google Scholar]
- 4.Younossi ZM, Stepanova M, Afendy M, Fang Y, Younossi Y, Mir H, Srishord M. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol. 2011;9:524–530. e521. doi: 10.1016/j.cgh.2011.03.020. quiz e560. [DOI] [PubMed] [Google Scholar]
- 5.Vuppalanchi R, Chalasani N. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: Selected practical issues in their evaluation and management. Hepatology. 2009;49:306–317. doi: 10.1002/hep.22603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313:2263–2273. doi: 10.1001/jama.2015.5370. [DOI] [PubMed] [Google Scholar]
- 7.Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, Ahmed A. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148:547–555. doi: 10.1053/j.gastro.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 8.Kemmer N, Neff GW. Ethnic variations in chronic liver diseases. Dig Dis Sci. 2008;53:1339–1344. doi: 10.1007/s10620-007-9992-0. [DOI] [PubMed] [Google Scholar]
- 9.Suryaprasad A, Byrd KK, Redd JT, Perdue DG, Manos MM, McMahon BJ. Mortality caused by chronic liver disease among American Indians and Alaska Natives in the United States, 1999–2009. Am J Public Health. 2014;104(Suppl 3):S350–S358. doi: 10.2105/AJPH.2013.301645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forde KA, Tanapanpanit O, Reddy KR. Hepatitis B and C in African Americans: current status and continued challenges. Clin Gastroenterol Hepatol. 2014;12:738–748. doi: 10.1016/j.cgh.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kolonel LN, Henderson BE, Hankin JH, Nomura AM, Wilkens LR, Pike MC, Stram DO, et al. A multiethnic cohort in Hawaii and Los Angeles: baseline characteristics. Am J Epidemiol. 2000;151:346–357. doi: 10.1093/oxfordjournals.aje.a010213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Setiawan VW, Virnig BA, Porcel J, Henderson BE, Le Marchand L, Wilkens LR, Monroe KR. Linking Data from the Multiethnic Cohort Study to Medicare Data: Linkage Results and Application to Chronic Disease Research. Am J Epidemiol. 2015 doi: 10.1093/aje/kwv055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in Burden of Cirrhosis and Hepatocellular Carcinoma by Underlying Liver Disease in US Veterans, 2001–2013. Gastroenterology. 2015;149:1471–1482. e1475. doi: 10.1053/j.gastro.2015.07.056. [DOI] [PubMed] [Google Scholar]
- 14.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–2023. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 15.Lazo M, Clark JM. The epidemiology of nonalcoholic fatty liver disease: a global perspective. Semin Liver Dis. 2008;28:339–350. doi: 10.1055/s-0028-1091978. [DOI] [PubMed] [Google Scholar]
- 16.Ruhl CE, Everhart JE. Determinants of the association of overweight with elevated serum alanine aminotransferase activity in the United States. Gastroenterology. 2003;124:71–79. doi: 10.1053/gast.2003.50004. [DOI] [PubMed] [Google Scholar]
- 17.Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003;98:960–967. doi: 10.1111/j.1572-0241.2003.07486.x. [DOI] [PubMed] [Google Scholar]
- 18.Szczepaniak LS, Nurenberg P, Leonard D, Browning JD, Reingold JS, Grundy S, Hobbs HH, et al. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288:E462–E468. doi: 10.1152/ajpendo.00064.2004. [DOI] [PubMed] [Google Scholar]
- 19.Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124–131. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 20.Lazo M, Hernaez R, Eberhardt MS, Bonekamp S, Kamel I, Guallar E, Koteish A, et al. Prevalence of nonalcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988–1994. Am J Epidemiol. 2013;178:38–45. doi: 10.1093/aje/kws448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Younossi ZM, Stepanova M, Negro F, Hallaji S, Younossi Y, Lam B, Srishord M. Nonalcoholic fatty liver disease in lean individuals in the United States. Medicine (Baltimore) 2012;91:319–327. doi: 10.1097/MD.0b013e3182779d49. [DOI] [PubMed] [Google Scholar]
- 22.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 23.Weston SR, Leyden W, Murphy R, Bass NM, Bell BP, Manos MM, Terrault NA. Racial and ethnic distribution of nonalcoholic fatty liver in persons with newly diagnosed chronic liver disease. Hepatology. 2005;41:372–379. doi: 10.1002/hep.20554. [DOI] [PubMed] [Google Scholar]
- 24.Pan JJ, Fallon MB. Gender and racial differences in nonalcoholic fatty liver disease. World J Hepatol. 2014;6:274–283. doi: 10.4254/wjh.v6.i5.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saab S, Manne V, Nieto J, Schwimmer JB, Chalasani NP. Nonalcoholic Fatty Liver Disease in Latinos. Clin Gastroenterol Hepatol. 2016;14:5–12. doi: 10.1016/j.cgh.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 26.Nomura H, Kashiwagi S, Hayashi J, Kajiyama W, Tani S, Goto M. Prevalence of fatty liver in a general population of Okinawa, Japan. Jpn J Med. 1988;27:142–149. doi: 10.2169/internalmedicine1962.27.142. [DOI] [PubMed] [Google Scholar]
- 27.Okanoue T, Umemura A, Yasui K, Itoh Y. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis in Japan. J Gastroenterol Hepatol. 2011;26(Suppl 1):153–162. doi: 10.1111/j.1440-1746.2010.06547.x. [DOI] [PubMed] [Google Scholar]
- 28.Jimba S, Nakagami T, Takahashi M, Wakamatsu T, Hirota Y, Iwamoto Y, Wasada T. Prevalence of non-alcoholic fatty liver disease and its association with impaired glucose metabolism in Japanese adults. Diabet Med. 2005;22:1141–1145. doi: 10.1111/j.1464-5491.2005.01582.x. [DOI] [PubMed] [Google Scholar]
- 29.Omagari K, Kadokawa Y, Masuda J, Egawa I, Sawa T, Hazama H, Ohba K, et al. Fatty liver in non-alcoholic non-overweight Japanese adults: incidence and clinical characteristics. J Gastroenterol Hepatol. 2002;17:1098–1105. doi: 10.1046/j.1440-1746.2002.02846.x. [DOI] [PubMed] [Google Scholar]
- 30.Kojima S, Watanabe N, Numata M, Ogawa T, Matsuzaki S. Increase in the prevalence of fatty liver in Japan over the past 12 years: analysis of clinical background. J Gastroenterol. 2003;38:954–961. doi: 10.1007/s00535-003-1178-8. [DOI] [PubMed] [Google Scholar]
- 31.Hamaguchi M, Kojima T, Takeda N, Nakagawa T, Taniguchi H, Fujii K, Omatsu T, et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med. 2005;143:722–728. doi: 10.7326/0003-4819-143-10-200511150-00009. [DOI] [PubMed] [Google Scholar]
- 32.Schneider AL, Lazo M, Selvin E, Clark JM. Racial differences in nonalcoholic fatty liver disease in the U.S. population. Obesity (Silver Spring) 2014;22:292–299. doi: 10.1002/oby.20426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Araneta MR, Barrett-Connor E. Ethnic differences in visceral adipose tissue and type 2 diabetes: Filipino, African-American, and white women. Obes Res. 2005;13:1458–1465. doi: 10.1038/oby.2005.176. [DOI] [PubMed] [Google Scholar]
- 34.Lear SA, Humphries KH, Kohli S, Chockalingam A, Frohlich JJ, Birmingham CL. Visceral adipose tissue accumulation differs according to ethnic background: results of the Multicultural Community Health Assessment Trial (M-CHAT) Am J Clin Nutr. 2007;86:353–359. doi: 10.1093/ajcn/86.2.353. [DOI] [PubMed] [Google Scholar]
- 35.Azuma K, Kadowaki T, Cetinel C, Kadota A, El-Saed A, Kadowaki S, Edmundowicz D, et al. Higher liver fat content among Japanese in Japan compared with non-Hispanic whites in the United States. Metabolism. 2009;58:1200–1207. doi: 10.1016/j.metabol.2009.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hernaez R, McLean J, Lazo M, Brancati FL, Hirschhorn JN, Borecki IB, Harris TB, et al. Association between variants in or near PNPLA3, GCKR, and PPP1R3B with ultrasound-defined steatosis based on data from the third National Health and Nutrition Examination Survey. Clin Gastroenterol Hepatol. 2013;11:1183–1190. e1182. doi: 10.1016/j.cgh.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wagenknecht LE, Palmer ND, Bowden DW, Rotter JI, Norris JM, Ziegler J, Chen YD, et al. Association of PNPLA3 with non-alcoholic fatty liver disease in a minority cohort: the Insulin Resistance Atherosclerosis Family Study. Liver Int. 2011;31:412–416. doi: 10.1111/j.1478-3231.2010.02444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, Boerwinkle E, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guerrero R, Vega GL, Grundy SM, Browning JD. Ethnic differences in hepatic steatosis: an insulin resistance paradox? Hepatology. 2009;49:791–801. doi: 10.1002/hep.22726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wagenknecht LE, Scherzinger AL, Stamm ER, Hanley AJ, Norris JM, Chen YD, Bryer-Ash M, et al. Correlates and heritability of nonalcoholic fatty liver disease in a minority cohort. Obesity (Silver Spring) 2009;17:1240–1246. doi: 10.1038/oby.2009.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lim U, Ernst T, Buchthal SD, Latch M, Albright CL, Wilkens LR, Kolonel LN, et al. Asian women have greater abdominal and visceral adiposity than Caucasian women with similar body mass index. Nutr Diabetes. 2011;1:e6. doi: 10.1038/nutd.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Caldwell SH, Ikura Y, Iezzoni JC, Liu Z. Has natural selection in human populations produced two types of metabolic syndrome (with and without fatty liver)? J Gastroenterol Hepatol. 2007;22(Suppl 1):S11–S19. doi: 10.1111/j.1440-1746.2006.04639.x. [DOI] [PubMed] [Google Scholar]
- 43.Seeff LB, Miller RN, Rabkin CS, Buskell-Bales Z, Straley-Eason KD, Smoak BL, Johnson LD, et al. 45-year follow-up of hepatitis C virus infection in healthy young adults. Ann Intern Med. 2000;132:105–111. doi: 10.7326/0003-4819-132-2-200001180-00003. [DOI] [PubMed] [Google Scholar]
- 44.McQuillan GM, Coleman PJ, Kruszon-Moran D, Moyer LA, Lambert SB, Margolis HS. Prevalence of hepatitis B virus infection in the United States: the National Health and Nutrition Examination Surveys, 1976 through 1994. Am J Public Health. 1999;89:14–18. doi: 10.2105/ajph.89.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O'Leary JG, Landaverde C, Jennings L, Goldstein RM, Davis GL. Patients with NASH and cryptogenic cirrhosis are less likely than those with hepatitis C to receive liver transplants. Clin Gastroenterol Hepatol. 2011;9:700–704. e701. doi: 10.1016/j.cgh.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caetano R, Clark CL. Trends in alcohol consumption patterns among whites, blacks and Hispanics, 1984 and 1995. J Stud Alcohol. 1998;59:659–668. doi: 10.15288/jsa.1998.59.659. [DOI] [PubMed] [Google Scholar]
- 47.Dawson DA, Grant BF, Chou SP, Pickering RP. Subgroup variation in US drinking patterns: results of the 1992 national longitudinal alcohol epidemiologic study. J Subst Abuse. 1995;7:331–344. doi: 10.1016/0899-3289(95)90026-8. [DOI] [PubMed] [Google Scholar]
- 48.Noureddin M, Yates KP, Vaughn IA, Neuschwander-Tetri BA, Sanyal AJ, McCullough A, Merriman R, et al. Clinical and histological determinants of nonalcoholic steatohepatitis and advanced fibrosis in elderly patients. Hepatology. 2013;58:1644–1654. doi: 10.1002/hep.26465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim IH, Kisseleva T, Brenner DA. Aging and liver disease. Curr Opin Gastroenterol. 2015;31:184–191. doi: 10.1097/MOG.0000000000000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.