Abstract
Associations between behavioural and other personal factors and colorectal cancer risk have been reported to vary by tumour characteristics, but evidence is inconsistent. In a large UK‐based prospective study we examined associations of 14 postulated risk factors with colorectal cancer risk overall, and across three anatomical sites and four morphological subtypes. Among 1.3 million women, 18,518 incident colorectal cancers were identified during 13.8 (SD 3.4) years follow‐up via record linkage to national cancer registry data. Cox regression yielded adjusted relative risks. Statistical significance was assessed using correction for multiple testing. Overall, colorectal cancer risk was significantly associated with height, body mass index (BMI), smoking, alcohol intake, physical activity, parity and menopausal hormone therapy use. For smoking there was substantial heterogeneity across morphological types; relative risks around two or greater were seen in current smokers both for signet ring cell and for neuroendocrine tumours. Obese women were also at higher risk for signet ring cell tumours. For adenocarcinomas, the large majority of colorectal cancers in the cohort, all risk factor associations were weak. There was little or no heterogeneity in risk between tumours of the right colon, left colon and rectum for any of the 14 factors examined. These epidemiological findings complement an emerging picture from molecular studies of possible different developmental pathways for different tumour types.
Keywords: colorectal cancer, risk factors, subtype, smoking
Short abstract
What's new?
Evidence suggests that risk factors are differentially linked to colorectal cancer type and tumor site, suggesting that the disease can arise via distinct pathways, likely depending on the type of precancerous lesion. To explore risk factor associations by anatomical site and tumor morphology, the authors of this study examined data from the Million Women Study cohort, with more than 18,500 incident colorectal cancer cases. While there was little evidence for risk differences by tumor site, substantial differences were found for tumor morphology, particularly for smoking, which showed relative risks of twofold or more for some rare colorectal tumors.
It has been suggested that risk factors for colorectal cancer may vary by tumour site, and by morphological type, possibly reflecting different pathways by which tumours develop from conventional adenomas or serrated bowel polyps.1, 2, 3, 4 Much existing evidence is inconsistent and limited by small numbers of cases in most individual studies. Studies need to be very large to have enough power to detect reliably heterogeneity in risk by colorectal cancer site or by tumour subtype.
We report here on associations observed between certain behavioural and personal characteristics and incident colorectal cancer risk, comparing risks across anatomical site and across morphological subtype, in a prospective study of over 1 million UK women.
Methods
Study population, data collection and follow‐up
Between 1996 and 2001, around 1.3 million women aged on average 56 years (SD 5) joined the Million Women Study through National Health Service (NHS) breast screening clinics in England and Scotland, completing a questionnaire about anthropometric, social and demographic factors, and other personal characteristics. The cohort has been resurveyed every 3 to 5 years since then. The study design and methods are described in detail elsewhere,5, 6 and questionnaires can be viewed online at http://www.millionwomenstudy.org.
All participants gave consent for follow‐up through their medical records, and have been linked to the NHS Central Register; study investigators are routinely notified of deaths, emigration and cancer registrations through the Health and Social Care Information Centre in England, and the Information Services Division in Scotland. Follow‐up data were available for England to December 31, 2013, and for Scotland to December 31, 2008.
To date, only about 1% of the cohort has been lost to follow‐up, mainly by emigration and cessation of registration with the NHS; such women contribute person‐years and events up to the date when follow‐up ceased.
The information provided for cancer includes the date of diagnosis together with the cancer site (coded using the 10th revision of the International Classification of Diseases, ICD‐10)7 and tumour morphology (coded using the second and third editions of the International Classification of Diseases for Oncology, ICD‐O).8, 9
Outcomes
The outcome of interest was incident primary invasive colorectal cancer (ICD‐10 C18‐20). For analyses by tumour site, cancers were assigned to the following groups: colon, ICD‐10 C18.0‐18.9; colon right (proximal), C18.0 caecum to C18.4 transverse colon; colon left (distal), C18.5 splenic flexure to C18.7 sigmoid colon; rectum, C19‐C20. For analyses by morphological type, cancers were divided into five groups based on the WHO/IARC classification:10 (i) adenocarcinoma, ICD‐O code M8140/3 (and 12 related codes: Supporting Information Appendix Table 1); (ii) mucinous adenocarcinoma, ICD‐O M8480/3 (and three related codes); (iii) signet ring cell carcinoma, ICD‐O code M8490/3; (iv) neuroendocrine tumours, including carcinoid, ICD‐O codes M8246/3, M8240/3, M8243/3 and six related codes; and (v) other tumours: all remaining tumours, including other specified carcinoma, specified non‐carcinoma tumours, and cancers of unspecified morphology.
Table 1.
Characteristics of women at recruitment, and follow‐up for incident colorectal cancer
| All women in analysis |
All women with colorectal cancer
ICD‐10 C18‐C20 |
|
|---|---|---|
| n = 1,310,390 | n = 18,518 | |
| Age (yr) | ||
| Mean (SD) | 56.1 (4.6) | 57.6 (4.7) |
| Socioeconomic status | ||
| % Most deprived tertile | 33.3 | 33.4 |
| Height (cm) | ||
| Mean (SD) | 162.0 (6.7) | 162.5 (6.7) |
| Body mass index (kg/m2) | ||
| Mean (SD) | 26.2 (4.7) | 26.4(4.7) |
| Smoking | ||
| % Current | 20.6 | 19.9 |
| Alcohol | ||
| %15 + units/wk | 5.0 | 5.7 |
| Strenuous physical activity | ||
| %1 + /wk | 38.9 | 36.6 |
| Age at menarche | ||
| % 15+ yr | 17.0 | 17.9 |
| Parity | ||
| % Nulliparous | 10.8 | 11.7 |
| N Pregnanciesa | ||
| %3+ | 37.0 | 39.0 |
| Hysterectomy | ||
| % Yes | 25.0 | 24.3 |
| Sterilisation | ||
| % Yes | 23.2 | 21.7 |
| Age at menopause (yr)b | ||
| Mean (SD) | 47.5 (6.1) | 49.4 (4.7) |
| Oral contraceptive use | ||
| % Ever | 33.2 | 29.9 |
| Hormone therapy usec | ||
| % Ever | 50.5 | 46.9 |
| Years of follow‐up | ||
| Mean (SD) | 13.8 (3.4) | 8.8 (4.3) |
| Age at cancer diagnosis (yr) | ||
| Mean (SD) | – | 66.9 (6.2) |
In parous women.
In never HT users.
In postmenopausal women.
Exposures
All exposure variables were measured at study recruitment. The 14 exposures studied were: socioeconomic status (tertiles of the area‐based Townsend deprivation index);11 height (<160 cm, 160–165 cm, 165 + cm); body mass index (BMI) (<25, 25–29 and 30+ kg/m2, calculated from reported height and weight); smoking status (never smoker, past smoker, current smoker <15 cigarettes/day, current smoker 15+ cigarettes/day; for some analyses, the two current smoking categories were combined); alcohol intake (0–2, 3–14.9, 15+ units/week, with one unit = 10 g alcohol); strenuous physical activity (rarely/never, up to once a week, more than once a week); use of hormone replacement therapy for menopause (HT) (never, ever, within postmenopausal women); age at menarche (<13, 13–14, 15+ years); parity (parous, nulliparous); number of full‐term pregnancies (1,2,3+, within parous women); age at menopause (<45, 45–49, 50+ years, within postmenopausal never users of HT); hysterectomy (yes, no), sterilisation (tubal ligation) (yes, no) and past use of oral contraceptives (OCs) (never, for <5 years, for 5+ years).
Statistical analysis
Women were excluded from the analyses if they had been registered with any invasive cancer other than non‐melanoma skin cancer (ICD‐10 C44) prior to recruitment (n = 44,829). The remaining women contributed person‐years from the date of recruitment to the study until the date of registration for colorectal cancer, the date of death, or last date of follow‐up, whichever was soonest. Women were censored at diagnosis of any non‐colorectal cancer.
Cox (proportional hazards) regression models were used to estimate hazard ratios (referred to here as relative risks, RRs) of developing colorectal cancer in relation to the exposures of interest. Attained age was the underlying time variable. There was no evidence of significant violation of the proportional hazards assumption, as assessed by tests based on Schoenfeld residuals.
All analyses were stratified by geographical region (10 regions corresponding to the areas covered by the recruiting cancer registries), and mutually adjusted for other exposure variables as appropriate.
For adjustment and stratification variables, missing values (<6% for each variable) were assigned to a separate category. A sensitivity analysis was conducted excluding the 15% of women with any missing data on covariates within the model. A further sensitivity analysis was performed among the 1.2 million women in England, censoring at the date of first invitation to bowel cancer screening, (the NHS Bowel Cancer Screening Programme was introduced in England from 2006, and information on invitation date is available for study participants through linkage to screening data.12 No equivalent data are available for women recruited in Scotland.) Information on diet and on parental history of cancer is available for some 830,000 women in the study from the 3‐year re‐survey questionnaire. A sensitivity analysis was performed in these women, with follow‐up starting at the date of completion of the re‐survey questionnaire, and with additional adjustment for intake of red and processed meat (no red or processed meat, only red meat, red and processed meat) and dietary fibre (non‐starch polysaccharides [Englyst method], tertiles), and for history of colorectal cancer in mother and/or father (yes, no).
Tests of heterogeneity in the relationships between exposures and colorectal cancer risk by subsite and subtype were performed using a competing risks approach. We interpreted significance after correction of p values for multiple comparisons using the Holm‐Bonferroni method,13 describing corrected p < 0.05 as statistically significant. Analyses were performed in Stata‐14.14 Tests of statistical significance were two‐sided.
Results
Overall 1,310,390 women without prior cancer, with a mean age at recruitment of 56.1 (SD 4.6) years, were included in the analyses. Women were followed for incident colorectal cancer over 18.1 million person‐years, with a mean duration of follow‐up of 13.8 (SD 3.4) years per woman. During this period, 18,518 incident primary invasive colorectal cancers were registered, with a mean age at diagnosis of 66.9 years (SD 6.2). By site, 12,761 cancers were in the colon (6,278 specified in the right colon and 5,269 in the left colon), and 5,757 in the rectum. The remaining 1,214 tumours were of overlapping or unknown site. By morphology, 15,543 cancers (83.9%) were reported as adenocarcinoma, 1,270 (6.8%) as mucinous tumours, 107 (0.6%) as signet ring cell tumours, and 234 (1.3%) as neuroendocrine tumours (predominantly carcinoid); the remaining 1,364 cancers were of other or unspecified histological type.
Table 1 shows characteristics of women in the analysis. Women diagnosed with colorectal cancer were somewhat less likely to smoke, undertake strenuous physical activity, or ever to have taken HT, and more likely to drink 15 or more units of alcohol a week. Characteristics did not vary much by cancer site, but there were some differences between women by tumour type (Supporting Information Appendix Table 2). Those diagnosed with signet ring or neuroendocrine tumours were more likely to be current smokers than those diagnosed with other tumour types; they tended also to be younger at recruitment and at cancer diagnosis.
Table 2.
Distribution of colorectal cancers by site and by morphology
| Adenocarcinoma | Mucinous | Signet ring | Neuroendocrine | Other and unspecified | Total | |
|---|---|---|---|---|---|---|
| Colon, right | 4,892 | 695 | 65 | 159 | 467 | 6,278 |
| Caecum | 2,212 | 288 | 25 | 63 | 232 | 2,820 |
| Appendix | 39 | 85 | 8 | 87 | 14 | 233 |
| Ascending colon | 1,378 | 176 | 18 | 6 | 93 | 1,671 |
| Hepatic flexure/transverse colon | 1,263 | 146 | 14 | 3 | 128 | 1,554 |
| Colon, left | 4,692 | 254 | 10 | 10 | 303 | 5,269 |
| Splenic flexure/descending colon | 846 | 74 | 6 | 0 | 65 | 991 |
| Sigmoid colon | 3,846 | 180 | 4 | 10 | 238 | 4,278 |
| Colon, overlapping/unspecified | 881 | 86 | 10 | 15 | 222 | 1,214 |
| Rectum | 5,078 | 235 | 22 | 50 | 372 | 5,757 |
| Recto‐sigmoid | 1,228 | 47 | 8 | 2 | 84 | 1,369 |
| Rectum | 3,850 | 188 | 14 | 48 | 288 | 4,388 |
| Total | 15,543 | 1,270 | 107 | 234 | 1,364 | 18,518 |
Table 2 shows the distribution of colorectal cancers cross‐classified by site and type. While adenocarcinomas are approximately evenly distributed between right colon, left colon and rectum, mucinous, signet ring and (in particular) neuroendocrine tumours are predominantly found in the right colon.
Table 3 shows adjusted relative risks for incident colorectal cancer for the 14 exposures examined. Taking multiple testing into account, cancer risk was significantly increased (p < 0.05) in relation to taller height, greater BMI, smoking, higher alcohol intake, and in parous versus nulliparous women; and risk was significantly decreased in relation to higher levels of physical activity, and to ever use of hormone replacement therapy. No significant associations were seen for socioeconomic status or for the remaining reproductive factors examined (including number of children in parous women).
Table 3.
Relative risks (RRs) and 95% confidence intervals (CIs) for incident colorectal cancer in relation to 14 socioeconomic and behavioural factors
|
Colorectal cancers,
total = 18,518 |
RR (95% CI) |
χ
b
test for heterogeneity
(*p < 0.05 after Holm‐Bonferroni correction for multiple testing) |
|
|---|---|---|---|
| Socioeconomic status | |||
| Least deprived | 6,040 | Reference | |
| Mid tertile | 6,221 | 1.03 (0.99–1.07) | χ22 = 3.36 |
| Most deprived | 6,146 | 1.03 (0.99–1.07) | |
| Height (cm) | |||
| <160 | 5,438 | Reference | |
| 160–164.9 | 5,444 | 1.12 (1.08–1.16) | χ22 = 156.4* |
| 165+ | 7,333 | 1.25 (1.21–1.30) | |
| Body mass index (kg/m2) | |||
| <25 | 7,751 | Reference | |
| 25–29.9 | 6,523 | 1.07 (1.04–1.11) | χ22 = 27.5* |
| 30+ | 3,262 | 1.11 (1.06–1.15) | |
| Smoking | |||
| Never | 8,610 | Reference | |
| Past | 5,385 | 1.15 (1.11–1.19) | χ3 b = 85.5* |
| Current <15/d | 1,797 | 1.13 (1.07–1.19) | |
| Current 15+/d | 1,668 | 1.19 (1.13–1.26) | |
| Alcohol (units/wk) | |||
| 0–2 | 10,947 | Reference | |
| 3–14.9 | 6,389 | 1.04 (1.01–1.08) | χ22 = 44.9* |
| 15+ | 1,049 | 1.25 (1.17–1.33) | |
| Strenuous exercise | |||
| Rarely/never | 9,112 | Reference | |
| Once per week | 5,222 | 0.95 (0.91–0.98) | χ22 = 28.4* |
| >Once per week | 3,498 | 0.90 (0.87–0.94) | |
| Age at menarche (yr) | |||
| <13 | 6,833 | Reference | |
| 13–14 | 8,063 | 0.96 (0.93–0.99) | χ22 = 7.07 |
| 15+ | 3,238 | 0.98 (0.93–1.02) | |
| Parity | |||
| Nulliparous | 2,166 | Reference | χ1 b = 17.36* |
| Parous | 16,310 | 0.91 (0.87–0.95) | |
| Births (in parous women) | |||
| 1 | 2,612 | Reference | |
| 2 | 7,341 | 0.97 (0.92–1.01) | χ22 = 3.19 |
| 3+ | 6,357 | 0.99 (0.95–1.04) | |
| Hysterectomy | |||
| No | 13,916 | Reference | χ1 b = 4.72 |
| Yes | 4,461 | 0.96 (0.92–1.00) | |
| Sterilisation | |||
| No | 14,043 | Reference | χ1 b = 0.15 |
| Yes | 3,895 | 0.99 (0.96–1.03) | |
| Age at menopause a (yr) | |||
| <45 | 900 | Reference | |
| 45–49 | 1,949 | 0.93 (0.86–1.01) | χ22 = 3.23 |
| 50+ | 4,098 | 0.97 (0.90–1.04) | |
| Oral contraceptive use (yr) | |||
| Never | 8,422 | Reference | |
| <5 | 4,403 | 0.98 (0.94–1.02) | χ22 = 1.28 |
| 5+ | 5,479 | 0.99 (0.95–1.02) | |
| Hormone therapy use b | |||
| Never | 7,134 | Reference | χ1 b = 8.87* |
| Ever | 4,130 | 0.94 (0.90–0.98) | |
In postmenopausal never HT users.
In postmenopausal women.
Figures 1 and 2 show, respectively, results by site and by tumour morphology for six factors most strongly associated with risk of colorectal cancer. Details of the analyses for all exposures by site and by morphology are shown in the Supporting Information Appendix Tables 3 and 4. Results for parity versus nulliparity are not included in the figures (but are given in the Supporting Information Appendix) as the inconsistency with associations by number of births among parous women suggests that this finding may well be due to chance.
Figure 1.

Relative risks (RRs) and 95% confidence intervals (CIs) for incident colorectal cancer in relation to selected risk factors, by anatomic site. All tests for heterogeneity are non‐significant (p > 0.05) after Holm‐Bonferroni correction for multiple testing BMI, body mass index; HT, hormone therapy for menopause; pw, per week
Figure 2.
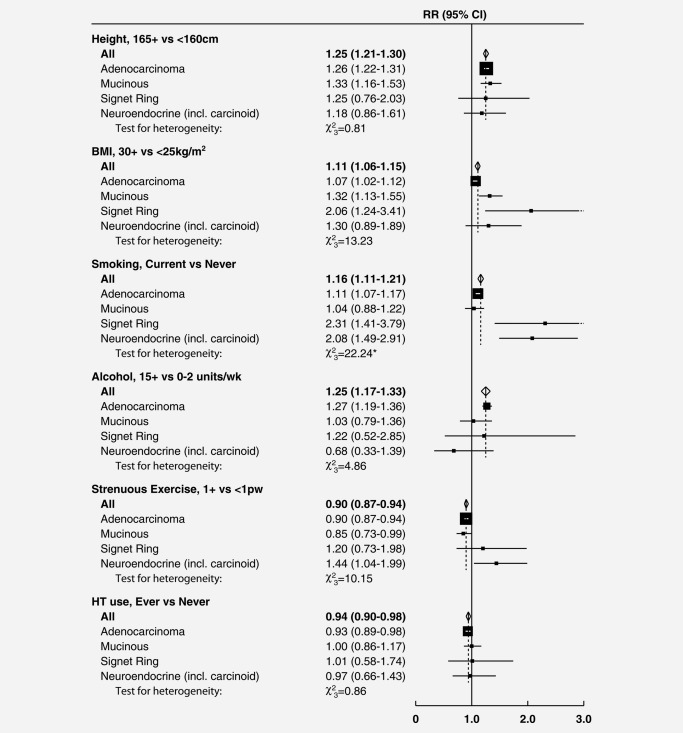
Relative risks (RRs) and 95% confidence intervals (CIs) for incident colorectal cancer in relation to selected risk factors, by morphology. Tests for heterogeneity are non‐significant (p > 0.05) after Holm‐Bonferroni correction for multiple testing, except where indicated by *p > 0.05. BMI, body mass index; HT, hormone therapy for menopause; pw, per week
There was no significant heterogeneity by tumour location for any of the 14 factors examined (Fig. 1 and Supporting Information Appendix Table 3). Associations for smoking varied substantially, however, by tumour morphology (Fig. 2). For two rare subtypes of colorectal cancer, signet ring cell and neuroendocrine tumours, risks were doubled in current versus never smokers (RRs = 2.31, 95% CI 1.41–3.79 and 2.08, 1.49–2.91 respectively); but for the most common subtype, adenocarcinoma, any association with smoking was weak (1.11, 1.07–1.17). In the more detailed analyses, risks for both signet ring cell and neuroendocrine tumours were substantially higher for current smokers of 15+ cigarettes per day (RRs around 3) than for current smokers of <15 cigarettes per day (RRs around 1.6), with p < 0.001 for heterogeneity by tumour morphology (Supporting Information Appendix Table 4). Obesity (BMI 30+ vs. <25 kg/m2) was more strongly associated with signet ring cell (RR 2.06, 1.24–3.41), mucinous (1.32, 1.13–1.55) and neuroendocrine tumours (1.30, 0.89–1.89) than with adenocarcinoma (1.07, 1.02–1.12); these differences were of borderline statistical significance (corrected p = 0.055). There was no significant heterogeneity by tumour morphology for height, alcohol intake, use of HT, physical activity or parity (Fig. 2, Supporting Information Appendix Table 4); nor for the other seven risk factors examined (Supporting Information Appendix Table 4).
Results from the three sensitivity analyses (restricting to women with no missing information on covariates; censoring at first invitation to bowel cancer screening; with additional adjustment for dietary factors and for parental history of colorectal cancer) did not differ substantially from those reported above (Supporting Information Appendix Table 5).
Discussion
In this large cohort of UK women, overall risk of incident invasive colorectal cancer was positively associated with height, body mass index, both past and current smoking, and alcohol intake; and inversely associated with physical activity and with use of hormone therapy for the menopause. The relative risk estimates for colorectal cancer were small, generally in the range 0.9 to 1.2. Colorectal cancer risk was lower in parous than in nulliparous women, but there was no trend in risk by number of births among parous women, and no associations were found with other reproductive factors, or with socioeconomic status. For smoking there was substantial heterogeneity across tumour morphological types; strong associations, with relative risks around 2 or greater were seen in current smokers for both signet ring cell and neuroendocrine tumours. Obese women were at similarly increased relative risk for signet ring cell tumours. For adenocarcinoma, the large majority of colorectal cancers in the cohort, the associations with BMI and with smoking were weak, with relative risks around 1.1. No significant differences in risk by tumour location were seen for any of the 14 factors examined.
This large prospective study, with 18,600 incident colorectal cancers, provides an opportunity to study risk factor associations in detail, taking account of potential confounding factors. In particular we were able to compare directly associations both by tumour site and by morphological type. For colorectal cancer overall, our results are consistent with past findings of small increases in risk of incident cancer in relation to taller height, adiposity, both past and current smoking, and moderately high alcohol intake; and of small decreases in risk associated with physical exercise, and with use of hormone therapy for menopause. Reproductive factors were in general not associated with risk of colorectal cancer; the lower risk in parous women is not accompanied by evidence for a trend in risk by number of births, and may be due to chance.
Existing evidence on risk factor associations by site of colorectal cancer is inconsistent.15, 16, 17 It has been suggested that risks may differ between colon and rectal cancers, or between right‐sided and left‐sided colon (or colorectal) cancers,18, 19 but results have been varied and interpretation often hindered by lack of statistical power to test reliably for differences between sites. We found little to suggest differences by tumour site for the 14 risk factors examined.
All the associations between smoking and cancer risk by site were modest. By contrast, the associations we found between smoking and risk of signet ring cell and neuroendocrine tumours are stronger and the results suggest a trend in risk with amount smoked (Supporting Information Appendix Table 3). While there is limited understanding of the relationship between morphological and molecular subtypes of colorectal cancer,20 our results on smoking appear consistent with recent evidence from molecular epidemiological studies, which have suggested a stronger association between smoking and risk of colorectal tumours21, 22, 23, 24, 25 and polyps26, 27 thought to arise from non‐conventional pathways (such as the serrated neoplasia pathway)2 than with adenocarcinomas arising from the conventional pathway typified by chromosomal instability. Less is known of possible differential risks for colorectal cancer by molecular (or morphological) type for other exposures. Our findings suggest that obesity may also be a stronger risk factor for the less common tumour types, in particular signet ring cell tumours, than for adenocarcinoma.
Strengths of this large study include prospectively‐collected information on potential risk and confounding factors, and virtually complete follow‐up through linkage to routinely‐collected, reliable, cancer registration data, giving sufficient power to compare risk associations directly both by site and by morphological type. The majority of colorectal cancers in this study were diagnosed in women who had not had access to bowel cancer screening, and results remained similar when women invited for screening were excluded from the analyses; screening acceptance has been shown in this cohort to be related to individual characteristics, such as smoking and BMI,12 and differential access to diagnosis could potentially bias studies of risk factors in screened populations. A limitation is that there was no independent assessment of the morphological classification of tumours, but misclassification (assumed to be unrelated to exposure status) would be likely to attenuate, rather than create, differences in risk associations by type. Even in this large study, we had limited numbers of tumours of rarer subtypes. The extent to which our findings are generalisable to men, and to other populations is not known.
We have shown in this large prospective study in UK women that associations of colorectal cancer risk with major lifestyle risk factors do not vary greatly by tumour site. There are however some substantial differences by morphological type, smoking in particular being associated with two to threefold increased risk of signet ring call carcinoma and of neuroendocrine tumours, but only weakly associated with risk of adenocarcinoma.
Abbreviations
- BMI
body mass index
- CI
confidence interval
- HT
hormone therapy for menopause
- ICD‐10
Tenth revision of the International Classification of Diseases
- ICD‐O
International Classification of Diseases for Oncology
- NHS
National Health Service
- OC
oral contraceptive
- RR
relative risk
- SD
standard deviation
Supporting information
Supporting Information
Acknowledgements
We thank all the women who participated in the Million Women Study, staff from NHS Breast Screening Centres; and Adrian Goodill for preparation of figures. Ethical approval for the study was provided by the Oxford and Anglia Multi‐centre Research Ethics Committee (now East of England Cambridge South Research Ethics Committee) and all participants gave signed consent for follow‐up. All authors contributed to study conception, design analysis and manuscript writing or revision. Million Women Study Co‐ordinating Centre staff: Hayley Abbiss, Simon Abbott, Rupert Alison, Naomi Allen, Miranda Armstrong, Krys Baker, Angela Balkwill, Emily Banks, Isobel Barnes, Valerie Beral, Judith Black, Roger Blanks, Kathryn Bradbury, Anna Brown, Benjamin Cairns, Karen Canfell, Dexter Canoy, Andrew Chadwick, Francesca Crowe, Dave Ewart, Sarah Ewart, Lee Fletcher, Sarah Floud, Toral Gathani, Laura Gerrard, Adrian Goodill, Jane Green, Lynden Guiver, Alicia Heath, Darren Hogg, Michal Hozak, Isobel Lingard, Sau Wan Kan, Nicky Langston, Bette Liu, Kath Moser, Kirstin Pirie, Gillian Reeves, Keith Shaw, Emma Sherman, Helena Strange, Sian Sweetland, Sarah Tipper, Ruth Travis, Lyndsey Trickett, Clare Wotton, Lucy Wright, Owen Yang, Heather Young. Million Women Study Advisory Committee: Emily Banks, Valerie Beral, Lucy Carpenter, Carol Dezateux, Jane Green, Julietta Patnick, Richard Peto, Cathie Sudlow. The NHS Breast Screening Centres which took part in the recruitment of participants were: Avon, Aylesbury, Barnsley, Basingstoke, Bedfordshire and Hertfordshire, Cambridge and Huntingdon, Chelmsford and Colchester, Chester, Cornwall, Crewe, Cumbria, Doncaster, Dorset, East Berkshire, East Cheshire, East Devon, East of Scotland, East Suffolk, East Sussex, Gateshead, Gloucestershire, Great Yarmouth, Hereford and Worcester, Kent, Kings Lynn, Leicestershire, Liverpool, Manchester, Milton Keynes, Newcastle, North Birmingham, North East Scotland, North Lancashire, North Middlesex, North Nottingham, North of Scotland, North Tees, North Yorkshire, Nottingham, Oxford, Portsmouth, Rotherham, Sheffield, Shropshire, Somerset, South Birmingham, South East Scotland, South East Staffordshire, South Derbyshire, South Essex, South Lancashire, South West Scotland, Surrey, Warrington Halton St Helens and Knowsley, Warwickshire Solihull and Coventry, West Berkshire, West Devon, West London, West Suffolk, West Sussex, Wiltshire, Winchester, Wirral, Wycombe.
The copyright line for this article was changed on 3 March 2017 after original online publication.
References
- 1. Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet 2014; 383:1490–502. doi: 10.1016/S0140-6736(13)61649-9. [DOI] [PubMed] [Google Scholar]
- 2. IJspeert JE, Vermeulen L, Meijer GA, et al. Serrated neoplasia‐role in colorectal carcinogenesis and clinical implications. Nat Rev Gastroenterol Hepatol 2015; 12:401–9. doi: 10.1038/nrgastro.2015.73. [DOI] [PubMed] [Google Scholar]
- 3. Guinney J, Dienstmann R, Wang X, et al. The consensus molecular subtypes of colorectal cancer. Nat Med 2015; 21:1350–6. doi: 10.1038/nm.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kocarnik JM, Shiovitz S, Phipps AI. Molecular phenotypes of colorectal cancer and potential clinical applications. Gastroenterol Rep (Oxf) 2015; 3:269–76. doi: 10.1093/gastro/gov046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Million Women Study Collaborative Group . The Million Women Study: design and characteristics of the study population. Breast Cancer Res 1999; 1:73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Million Women Study Collaborators . Breast cancer and hormone‐replacement therapy in the Million Women Study. Lancet 2003; 362:419–27. [DOI] [PubMed] [Google Scholar]
- 7. World Health Organization . International Statistical Classification of Diseases and related health problems, 10th revision. Geneva: World Health Organization, 1992. [Google Scholar]
- 8. Percy C, Van Holten V, Muir CS. International Classification of Diseases for Oncology: ICD‐O, 2nd edn Geneva: World Health Organization, 1990. [Google Scholar]
- 9. Fritz A, Percy C, Jack . International Classification of Diseases for Oncology: ICD‐O, 3rd edn Geneva: World Health Organization, 2000. [Google Scholar]
- 10. Bosman FT, Carneiro F, Hruban RH, Theise ND, eds. WHO Classification of Tumours of the Digestive System. Lyon: IARC, 2010. [Google Scholar]
- 11. Townsend P, Phillimore P, Beattie A. Health and Deprivation: inequality and the North. London: Croon Helm, 1988. [Google Scholar]
- 12. Blanks RG, Benson VS, Alison R, et al. Nationwide bowel cancer screening programme in England: cohort study of lifestyle factors affecting participation and outcomes in women. Br J Cancer 2015; 112:1562–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat 1979; 6:65–70. [Google Scholar]
- 14. StataCorp . Stata statistical software: release 14. College Station, TX: StataCorp LP, 2015. [Google Scholar]
- 15. Monographs on the evaluation of carcinogenic risks to humans, Vol. 100E. A review of human carcinogens. Part E: Personal habits and indoor combustions. Lyon, France: IARC, 2012. [PMC free article] [PubMed] [Google Scholar]
- 16. WCRF/AICR . Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Washington, DC: World Cancer Research Fund/American Institute of Cancer Research, 2007. [Google Scholar]
- 17. http://www.wcrf.org/int/research-we-fund/continuous-update-project-findings-reports/colorectal-bowel-cancer. Last accessed August 22, 2016.
- 18. Iacopetta B. Are there two sides to colorectal cancer? Int J Cancer 2002; 101:403–8. [DOI] [PubMed] [Google Scholar]
- 19. Yamauchi M, Lochhead P, Morikawa T, et al. Colorectal cancer: a tale of two sides or a continuum? Gut 2012; 61:794–7. doi:10.1136/gutjnl-2012-302014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jass JR. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology 2007; 50:113–30. [DOI] [PubMed] [Google Scholar]
- 21. Limsui D, Vierkant RA, Tillmans LS, et al. Cigarette smoking and colorectal cancer risk by molecularly defined subtypes. J Natl Cancer Inst 2010; 102:1012–22. doi: 10.1093/jnci/djq201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nishihara R, Morikawa T, Kuchiba A, et al. A prospective study of duration of smoking cessation and colorectal cancer risk by epigenetics‐related tumor classification. Am J Epidemiol 2013; 178:84–100. doi: 10.1093/aje/kws431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Weisenberger DJ, Levine AJ, Long TI, for the Colon Cancer Family Registry , et al. Association of the colorectal CpG island methylator phenotype with molecular features, risk factors, and family history. Cancer Epidemiol Biomarkers Prev 2015; 24:512–19. doi: 10.1158/1055-9965.EPI-14-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Slattery ML, Curtin K, Anderson K, et al. Associations between cigarette smoking, lifestyle factors, and microsatellite instability in colon tumors. J Natl Cancer Inst 2000; 92:1831–6. [DOI] [PubMed] [Google Scholar]
- 25. Samowitz WS, Albertsen H, Sweeney C, et al. Association of smoking, CpG island methylator phenotype, and V600E BRAF mutations in colon cancer. J Natl Cancer Inst 2006; 98:1731–8. [DOI] [PubMed] [Google Scholar]
- 26. Figueiredo JC, Crockett SD, Snover DC, et al. Smoking‐associated risks of conventional adenomas and serrated polyps in the colorectum. Cancer Causes Control 2015; 26:377–86. doi: 10.1007/s10552-014-0513-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. IJspeert JE, Bossuyt PM, Kuipers EJ, et al. Smoking status informs about the risk of advanced serrated polyps in a screening population. Endosc Int Open 2016; 4:E73–8. doi: 10.1055/s-0034-1393361. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


