Significance
This study demonstrates Pellino 1 (PELI1) as an important modulator that exerts opposite regulatory functions on apoptosis and necroptosis, two distinct forms of regulated cell death mechanisms. K63 ubiquitination of RIPK1 mediated by PELI1 depends on the kinase activity of RIPK1, which provides a mechanism to explain why inhibition of RIPK1 kinase activity by Nec-1s can also reduce its ubiquitination during necroptosis.
Keywords: necroptosis, apoptosis, Peli1, ubiquitination, TNF
Abstract
Apoptosis and necroptosis are two distinct cell death mechanisms that may be activated in cells on stimulation by TNFα. It is still unclear, however, how apoptosis and necroptosis may be differentially regulated. Here we screened for E3 ubiquitin ligases that could mediate necroptosis. We found that deficiency of Pellino 1 (PELI1), an E3 ubiquitin ligase, blocked necroptosis. We show that PELI1 mediates K63 ubiquitination on K115 of RIPK1 in a kinase-dependent manner during necroptosis. Ubiquitination of RIPK1 by PELI1 promotes the formation of necrosome and execution of necroptosis. Although PELI1 is not directly involved in mediating the activation of RIPK1, it is indispensable for promoting the binding of activated RIPK1 with its downstream mediator RIPK3 to promote the activation of RIPK3 and MLKL. Inhibition of RIPK1 kinase activity blocks PELI1-mediated ubiquitination of RIPK1 in necroptosis. However, we show that PELI1 deficiency sensitizes cells to both RIPK1-dependent and RIPK1-independent apoptosis as a result of down-regulated expression of c-FLIP, an inhibitor of caspase-8. Finally, we show that Peli1−/− mice are sensitized to TNFα-induced apoptosis. Thus, PELI1 is a key modulator of RIPK1 that differentially controls the activation of necroptosis and apoptosis.
Necroptosis and apoptosis are two distinct regulated cell death mechanisms involved in development, adult tissue homeostasis, and diseases (1, 2). Apoptosis is mediated by the activation of caspases, which controls the signal transduction and execution of apoptotic cell death (3). Under apoptotic-deficient conditions, for example, on inactivation of caspase-8 or the loss of its adaptor FADD (Fas-associated protein with death domain), necroptosis may be activated to lead to cell death and embryonic lethality. It is still not clear, however, how necroptosis and apoptosis might be differentially regulated under physiological or pathological conditions in cells.
TNFα, an important proinflammatory cytokine involved in mediating myriads of human diseases, can activate necroptosis or apoptosis in different conditions and cell types. Stimulation of TNFα promotes the rapid activation of TNFR1 to recruit RIPK1, TRADD, TRAF2, and cIAP1/2 to form a TNFR1 signaling complex (TNF-RSC, or complex I). cIAP1/2 is known to mediate K63 ubiquitination of RIPK1 to further recruit TAK1 and TAB1/TAB2 to promote the activation of TAK1 and subsequent phosphorylation of the IKK complex to induce the activation of NF-κB. TAK1 can also phosphorylate RIPK1 to inhibit its kinase activation; as a result, inhibition of TAK1 by 5z-7-oxozeaenol (5z7) combined with TNFα stimulation promotes RIPK1 kinase activity-dependent apoptosis (4, 5). In contrast, inhibition of protein synthesis by cycloheximide (CHX) to block NF-κB-mediated transcriptional response induced by TNFα leads to RIPK1-independent apoptosis. In the absence of caspase activity, RIPK1 is activated to promote the formation of complex IIb, which includes FADD, caspase-8, and RIPK3. The activated RIPK3 in turn mediates the phosphorylation and oligomerization of MLKL to promote the execution of necroptosis. Necroptosis induced by TNFα is effectively inhibited by RIPK1 inhibitor R-7-Cl-O-Nec-1 (Nec-1s), which keeps RIPK1 in an inactive conformation (6). The activation of RIPK1 has been implicated in human diseases by mediating cell death and inflammation, as Nec-1s has shown efficacy in ameliorating tissue injuries in animal models of diseases ranging from ischemic brain, kidney, and heart injuries to multiple sclerosis, amyotrophic lateral sclerosis, and Alzheimer’s disease (7–11). It is unclear, however, how inhibition of RIPK1 by Nec-1s prevents the interaction of RIPK1 and RIPK3, a decisive signaling event in necroptosis.
Pellino 1 (PELI1), a member of Pellino family, is an E3 ubiquitin ligase known to be involved in mediating TLR3/TLR4 signaling (12, 13). PELI1 has been shown to interact with RIPK1 and mediate K63 ubiquitination of RIPK1 to regulate the activation of NF-κB on stimulation of TLR4 in a TRIF-dependent manner. The role of PELI1 in TNFα signaling has not been investigated.
Here we report that PELI1 is an E3 ubiquitin ligase that mediates K63 ubiquitination of RIPK1 on K115 residue in a RIPK1 kinase activity-dependent manner to promote the formation of complex IIb and transduction of necroptotic signaling downstream of RIPK1 in cells stimulated by TNFα. PELI1-deficient cells are protected against necroptosis induced by TNFα under apoptosis-deficient conditions. In contrast, PELI1-deficient cells are sensitized to apoptosis through up-regulation of c-Myc expression and the suppression of c-FLIP expression. Thus, we conclude that PELI1 is a key modulator of TNFα-mediated cell death pathways that can promote necroptosis and inhibit apoptosis.
Results
PELI1 Deficiency Protects Against Necroptosis.
Because RIPK1 is subject to extensive ubiquitination modifications in TNF-RSC (complex I) and complex IIb in response to TNFα (14), we considered the possibility that RIPK1 might be modulated by additional E3 ubiquitin ligases beyond cIAP1/2, the only E3 ubiquitin ligase characterized as involved in regulating the activation of RIPK1. We conducted a _targeted screen of E3 ubiquitin ligases reported to interact with RIPK1 (Fig. S1A). In this screen, we identified PELI1 as an E3 ubiquitin ligase whose knockdown protected cells against necroptosis.
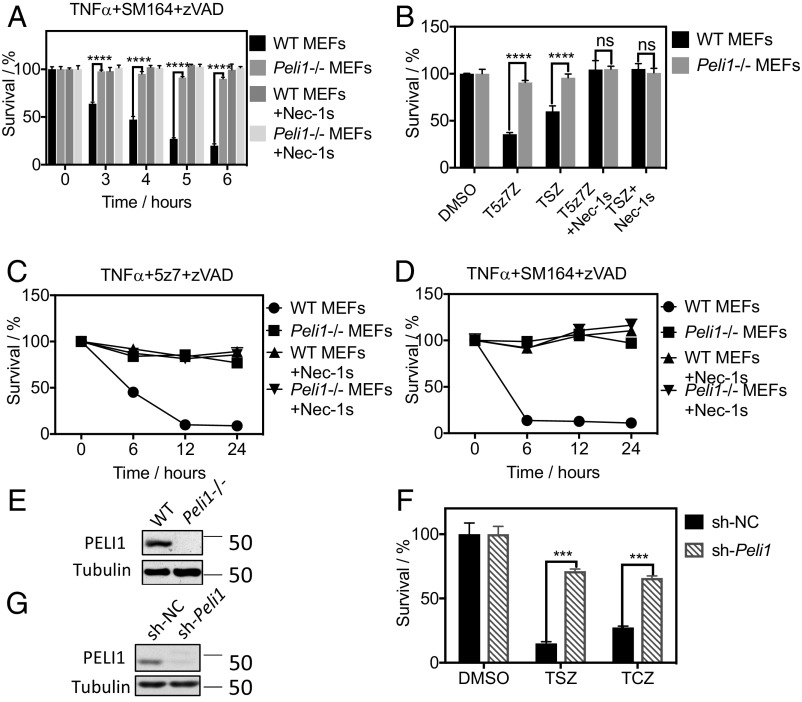
To confirm the role of PELI1 in mediating necroptosis, we generated WT and Peli1−/− MEFs (mouse embryonic fibroblasts cells) derived from WT and Peli1−/− mice (13) and tested their sensitivity to necroptosis. We found that Peli1−/− MEFs were highly resistant to necroptosis induced by TNFα/SM164/zVAD, TNFα/5z7/zVAD, or TNFα/CHX/zVAD (Fig. 1 A and B and Fig. S1B). The resistance of Peli1−/− MEFs to necroptosis induced by TNFα/SM164/zVAD and TNFα/5z7/zVAD was complete and could not be further enhanced by the treatment of Nec-1s (Fig. 1 C–E). We also tested the role of PELI1 in human HT29 cells and mouse RGC5 cells, using lentiviral vector-mediated shRNA _targeting Peli1. Knockdown of Peli1 in HT29 protected against TNFα/SM164/zVAD-induced or TNFα/CHX/zVAD-induced necroptosis (Fig. 1 F and G). Furthermore, knockdown of Peli1 in RGC5 cells protected against necroptosis induced by TNFα/SM164/zVAD (Fig. S1C). In addition, we checked the effect of knocking down Peli1 in murine fibrosarcoma L929 cells and found that knockdown of Peli1 protected L929 cells from necroptosis induced by zVAD (Fig. S1D).
Fig. 1.
PELI1 deficiency protects against necroptosis. (A) WT and Peli1−/− MEFs were pretreated with Nec-1s or DMSO for 1 h and then treated with TNFα + SM164 + zVAD for indicated periods of time. (B) WT and Peli1−/− MEFs were pretreated with Nec-1s or DMSO for 1 h and then treated with TNFα + 5z7 + zVAD (T5z7Z) or TNFα 10 ng/mL + SM164 + zVAD (TSZ) for 6 h. (C) WT and Peli1−/− MEFs were pretreated with Nec-1s or DMSO for 1 h and then treated with TNFα + 5z7 + zVAD for 6, 12, or 24 h. (D) WT and Peli1−/− MEFs were pretreated with Nec-1s or DMSO for 1 h and then treated with TNFα + SM164 + zVAD for 6, 12, or 24 h. (F and G) HT29 cells were transfected with shRNA _targeting Peli1 or Scrambled shRNA for 7 d and then treated with TNFα + SM164 + zVAD 25 (TSZ) or TNFα + CHX + zVAD (TCZ) for 24 h. The cell survival was measured with CellTiterGlo. (E and G) WT and Peli1−/− MEFs (E) or the control and Peli1 knockdown HT29 cells (G) were lysed with SDS buffer and the samples were analyzed by Western blotting with indicated antibodies (G). Concentrations of compounds used Nec-1s, 20 μM; TNFα, 10 ng/mL; SM164, 50 nM (A, B, D, and F), 500 nM (C); zVAD.fmk, 25 μM; CHX, 1 μg/mL.
Taken together, these data suggest that PELI1 deficiency protects against necroptosis.
PELI1 Is Indispensable for the Formation of RIPK1-RIPK3 Necrosome.
In WT MEFs, RIPK1 is activated after treatment of TNFα/SM164/zVAD to lead to the formation of complex IIb, also called necrosome, which includes FADD, caspase-8, and RIPK3, which mediates the activation of RIPK3 and the subsequent phosphorylation and activation of MLKL (14–18). The activated MLKL then oligomerizes to execute cell death by disrupting the integrity of plasma membranes (17, 19–21).
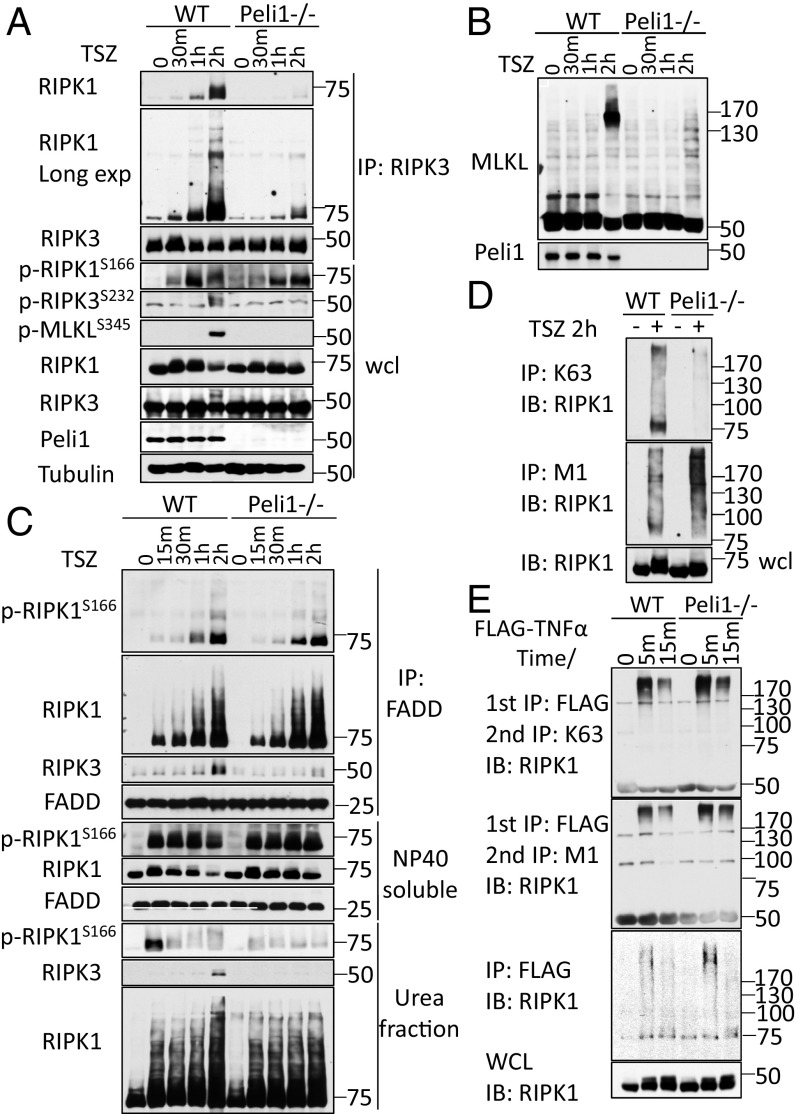
In Peli1−/− MEFs stimulated by TNFα/SM164/zVAD to induce necroptosis, the phosphorylation of S166 RIPK1, a marker for its activation (6, 8, 22), occurred on schedule as that of WT, suggesting PELI1 deficiency does not affect the activation of RIPK1 in MEFs (Fig. 2 A and B). In contrast, the phosphorylation of S232 RIPK3 and S345 MLKL, the markers for the activation of RIPK3 and MLKL, respectively, were blocked in Peli1−/− MEFs. Furthermore, the oligomerization of MLKL, detectable by Western blotting using nonreducing conditions, was also blocked in Peli1−/− MEFs (Fig. 2C). Thus, although PELI1 is important for the activation of RIPK3 and MLKL to promote necroptosis, it is not directly involved in mediating the activation of RIPK1.
Fig. 2.
PELI1 is indispensable for the formation of RIPK1-RIPK3 necrosome. (A and B) WT or Peli1−/− MEFs were pretreated with zVAD and SM164 for 30 min and then treated with TNFα (TSZ) for indicated periods of time. The cells were lysed with Nonidet P-40 buffer. The cell lysates were immunoprecipitated with anti-RIPK3 antibody (A) or anti-FADD antibody (B). (B) The Nonidet P-40 insoluble fraction was further extracted with 6M urea buffer. The whole-cell lysates and precipitated proteins were analyzed by Western blotting with indicated antibodies. (C) The cells were treated with same condition as A. The cells were lysed with Nonidet P-40 buffer without NEM and analyzed by Western blotting with anti-MLKL in nonreducing SDS/PAGE. (D) WT or Peli1−/− MEFs were pretreated with zVAD and SM164 for 30 min and then treated with TNFα 10 ng/mL (TSZ) for another 2 h. The cells were lysed with 3M urea buffer and immunoprecipitated with anti-K63 ubiquitin ab, or with 6M urea buffer and immunoprecipitated with anti-M1 ubiquitin antibody. (E) WT and Peli1−/− MEFs were treated with FLAG-TNFα 50 ng/mL for indicated periods of time and then lysed with Nonidet P-40 buffer. The cell lysates were immunoprecipitated with anti-FLAG antibody-conjugated agarose (first IP). The immunoprecipitated proteins were eluted with 6M urea buffer. The secondary immunoprecipitation (second IP) of eluted proteins with anti-M1 ubiquitin ab was performed in 6M urea; alternatively, the eluted proteins were diluted into 3M urea buffer and immunoprecipitated with anti-K63 ubiquitin antibody. All immunoprecipitated proteins and whole-cell lysates (WCL) were analyzed by Western blotting with indicated antibodies. Concentrations of compounds used: Nec-1s, 20 μM; TNFα, 10 ng/mL; SM164, 50 nM; zVAD.fmk, 25 μM.
We next analyzed the formation of complex IIb by immunoprecipitation, using an anti-FADD. We found that the binding of RIPK1, including p-S166 RIPK1, with FADD in cells stimulated by TNFα/SM164/zVAD was not affected by PELI1 deficiency (Fig. 2B). In contrast, the binding of RIPK3 with RIPK1 and FADD was blocked by PELI1 deficiency (Fig. 2 A and C).
Because the complex IIb/necrosome is known to be present in an insoluble amyloid-like conformation (23), we next analyzed the translocation of RIPK1 and RIPK3 into the insoluble fraction on induction of necroptosis. We found that although the translocation of modified RIPK1 was largely normal at early points (15 min−1⋅h) after induction of necroptosis, the presence of highly modified RIPK1 in insoluble fraction at 2 h, the time at which complex IIb formed, was reduced in Peli1−/− MEFs (Fig. 2C). Furthermore, the translocation of RIPK3 into the insoluble fraction was blocked by PELI1 deficiency. These data suggest PELI1 is involved in promoting the binding of RIPK1 to RIPK3 to mediate the formation of complex IIb.
We next analyzed the mechanism that might explain the inability of RIPK1 to bind to RIPK3 in TNFα/SM164/zVAD-treated Peli1−/− MEFs. Because PELI1 is an E3 ubiquitin ligase, we compared the ubiquitination patterns of RIPK1 in complex IIb in WT and Peli1−/− MEFs. Using anti-K63 and M1-ubiquitin chain-specific antibodies (24), we found that K63 ubiquitination of RIPK1 in complex IIb was inhibited, whereas M1 ubiquitination of RIPK1 increased in Peli1−/− MEFs after 2 h of treatment of TNFα/SM164/zVAD (Fig. 2D). In contrast, we found that PELI1 deficiency resulted in no difference in K63 or M1 ubiquitination of RIPK1 in the TNF-RSC (complex I) (Fig. 2E). These results suggest that PELI1 is involved in regulating RIPK1 ubiquitination in complex IIb.
PELI1 Is Recruited into TNF-RSC in a RIPK1-Dependent Manner.
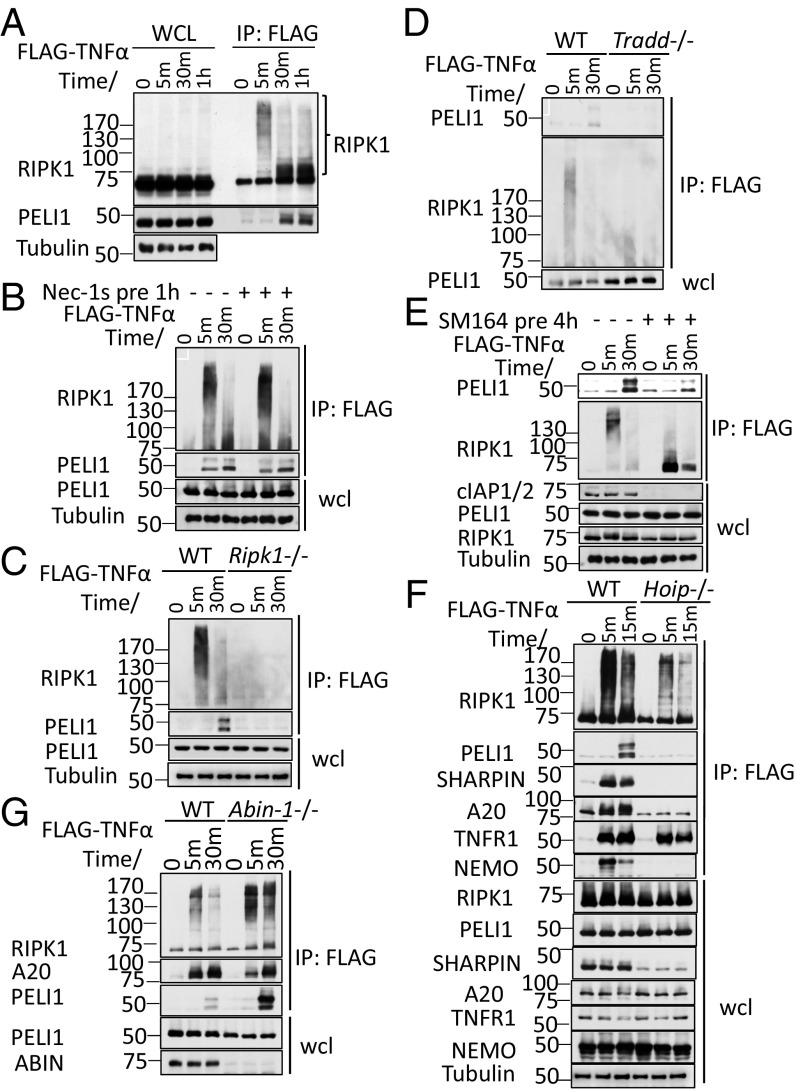
We next determined whether PELI1 might be recruited into TNF-RSC. We found that in cells treated with FLAG-TNFα for 30 min−1⋅h, but not at 5 min, immunoprecipitation by anti-FLAG revealed the presence of PELI1 (Fig. 3A). This recruitment of PELI1 into complex I was independent of RIPK1 kinase activity, as the pretreatment with RIPK1 kinase inhibitor Nec-1s had no effect on the recruitment of PELI1 into complex I (Fig. 3B). However, the recruitment of PELI1 into complex I was blocked in Ripk1−/− MEFs (Fig. 3C). Thus, the recruitment of PELI1 into complex I requires RIPK1, but not its kinase activity.
Fig. 3.
PELI1 is recruited into TNF-RSC in a RIPK1-dependent manner. (A) WT MEFs were treated with FLAG-TNFα for indicated periods of time and then lysed with Nonidet P-40 buffer. The cell lysates were immunoprecipitated with anti-FLAG antibody-conjugated agarose. (B) WT MEFs were pretreated with Nec-1s or vehicle control (0.01% DMSO) for 1 h and then treated with FLAG-TNFα for indicated periods of time. The cells were lysed with Nonidet P-40 buffer, and cell lysates were immunoprecipitated with anti-FLAG antibody-conjugated agarose. (C and D) WT and Ripk1−/− MEFs (C), or WT and Tradd−/− MEFs (D) were treated with FLAG-TNFα for indicated periods of time and then lysed with Nonidet P-40 buffer. The cell lysates were immunoprecipitated with anti-FLAG antibody-conjugated agarose. (E) WT MEFs were pretreated with SM164 or DMSO for 4 h and then treated with FLAG-TNFα for indicated periods of time. The cells were then lysed with Nonidet P-40 buffer, and cell lysates were immunoprecipitated with anti-FLAG antibody-conjugated agarose. (F) WT and Hoip−/− MEFs were treated with FLAG-TNFα for indicated periods of time and then lysed with Nonidet P-40 buffer. The cell lysates were immunoprecipitated with anti-FLAG antibody-conjugated agarose. (G) WT and Abin-1−/− MEFs were treated with FLAG-TNFα for indicated periods of time and then lysed with Nonidet P-40 buffer. The cell lysates were immunoprecipitated with anti-FLAG antibody-conjugated agarose. The whole cell lysates and immunoprecipitated proteins (A–F) were analyzed by Western blotting with indicated antibodies. Concentrations of compounds used: FLAG-TNFα, 50 ng/mL; Nec-1s, 20 μM; TNFα, 10 ng/mL; SM164, 50 nM.
The recruitment of PELI1 into complex I was also blocked in MEFs deficient for TRADD, a key adaptor protein that directly binds to the intracellular death domain of TNFR1 (25) (Fig. 3D). Because TRADD is involved in mediating the recruitment of cIAP1/2, we next pretreated cells with cIAP1/2 inhibitor SM164, which led to the degradation of cIAP1/2, and found that cIAP1/2 deficiency also inhibited the recruitment of PELI1 into complex I (Fig. 3E). The LUBAC complex, consisting of HOIP/HOIL/SHARPIN, is involved in mediating M1 ubiquitination of TNF-RSC, including RIPK1 (26, 27). In Hoip−/− cells stimulated by TNFα, no PELI1 was detected in complex I, suggesting that the recruitment of PELI1 into complex I requires M1 ubiquitination (Fig. 3F). In contrast, the recruitment of PELI1 into complex I in Abin-1−/− MEFs was increased (Fig. 3G). Because K63 ubiquitination of RIPK1 in complex I under ABIN-1-deficient conditions was increased (28), this result suggests that the recruitment of PELI1 into complex I is also regulated by K63 ubiquitination of RIPK1. However, the recruitment of PELI1 into complex I was not altered in Cyld−/− or Nemo−/− MEFs (Fig. S2).
Taken together, these results suggest that both K63 and M1 ubiquitination of RIPK1 are involved in regulating the recruitment of PELI1 into complex I.
PELI1 Mediates K63 Ubiquitination of RIPK1 in a Kinase Activity-Dependent Manner.
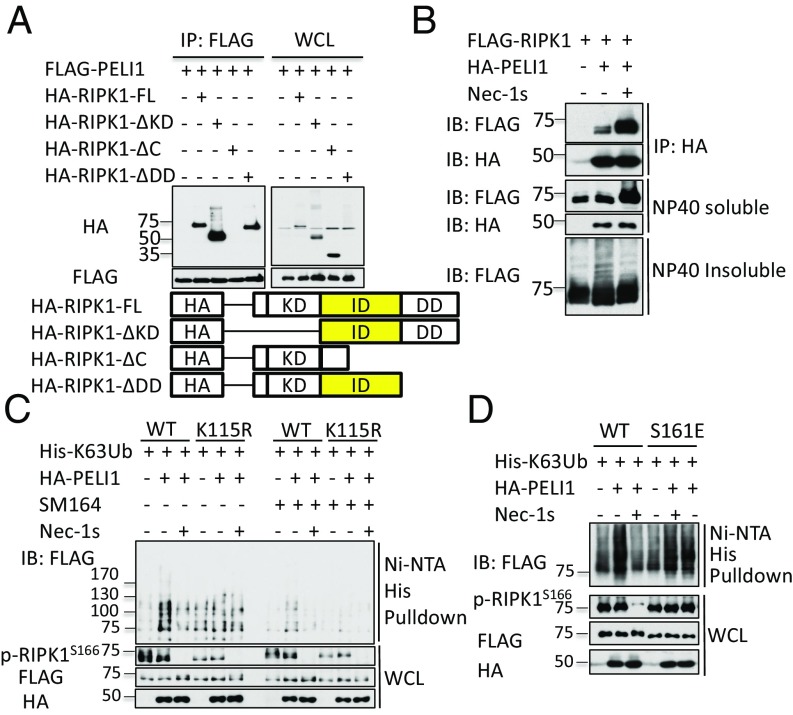
RIPK1 contains a kinase domain at N terminus, a death domain (DD) at its C terminus, and an intermediate domain. To investigate the domain of RIPK1 involved in mediating its binding with PELI1, we overexpressed full-length and truncation mutants of RIPK1, HA-RIPK1-ΔKD, HA-RIPK1-ΔC, and HA-RIPK1-ΔDD with that of FLAG-tagged PELI1 in 293T cells, and characterized their interactions by immunoprecipitation. We found that HA-RIPK1, HA-RIPK1-ΔKD, and HA-RIPK1-ΔDD, but not HA-RIPK1-ΔC, could bind to PELI1 (Fig. 4A). Thus, PELI1 interacts with the intermediate domain of RIPK1, and the interaction of RIPK1 and PELI1 was not inhibited by Nec-1s (Fig. 4B).
Fig. 4.
PELI1 mediates K63 ubiquitination of RIPK1 on K115 in a kinase activity-dependent manner. (A) 293T cells were transfected with expression vectors for FLAG-tagged PELI1 and HA-tagged full-length, ΔKD, ΔC, or ΔDD RIPK1 for 24 h and then lysed with Nonidet P-40 buffer. The cell lysates were immunoprecipitated with anti-FLAG antibody-conjugated agarose. (B) 293T cells were pretreated with Nec-1s (20 μM) or vehicle control (0.01% DMSO) for 1 h and then transfected with expression vectors for HA-tagged PELI1 and FLAG-tagged RIPK1 for 24 h. The cells were then lysed with Nonidet P-40 buffer, and cell lysates were immunoprecipitated with anti-HA antibody conjugated agarose. The Nonidet P-40 insoluble fraction was then lysed with 6M urea buffer. (C) 293T cells were transfected with expression vectors for His-K63 ubiquitin and WT or K115R FLAG-RIPK1 with or without that of HA-PELI1 for 24 h and then treated with SM164 or DMSO for 4 h before being lysed with 6M urea. His-tagged proteins were pulled down with Ni-NTA. (D) 293T cells were pretreated with Nec-1s or DMSO for 1 h and then transfected with expression vectors for His-K63 ubiquitin and WT or S161E RIPK1 with or without that of HA-PELI1 for 24 h. The cells were lysed with 6M urea buffer, and His-tagged proteins were pulled down with Ni-NTA. All cell lysates and immunoprecipitated or pulled down proteins were analyzed by Western blotting with indicated antibodies. Concentrations of compounds used: Nec-1s, 20 μM; SM164, 50 nM.
Because PELI1 was reported to be able to mediate K63 ubiquitination of RIPK1 to regulate the activation of NF-κB in response to TLR stimulation (13), and we found that K63 ubiquitination of RIPK1 in complex IIb in response to the treatment of TNFα/SM164/zVAD was strongly defective in PELI1-deficient cells (Fig. 2D), we next examined whether PELI1 was involved in mediating K63 ubiquitination of RIPK1 during necroptosis. Furthermore, as the K115 residue of RIPK1 was reported to be a dominant ubiquitination site of RIPK1 in necroptosis (29), we considered the possibility that PELI1 might ubiquitinate RIPK1 on K115.
We confirmed that overexpression of PELI1 can increase both WT and K63 ubiquitination of RIPK1 (Fig. S3A). Furthermore, the K63 ubiquitination of RIPK1 promoted by PELI1 was significantly reduced in the K115R mutant compared with that of WT, with or without the treatment of SM-164, which promotes the degradation of cIAP1/2 (30) (Fig. 4C). Interestingly, although the treatment of Nec-1s did not disrupt the binding of PELI1 and RIPK1 (Fig. 4B), it inhibited the K63 ubiquitination of RIPK1 mediated by PELI1 (Fig. 4C). However, Nec-1s did not reduce the PELI1-mediated K63 ubiquitination of S161E RIPK1, a mutation that keeps RIPK1 in constitutively active conformation and resistant to the inhibition by Nec-1s (6) (Fig. 4D). In contrast, the expression of PELI1 did not promote the ubiquitination of caspase-8, the other component of complex IIb that is also known to be subject to ubiquitination (31) (Fig. S3B). These results suggest that PELI1 is involved in mediating the K63 ubiquitination of activated RIPK1 on K115 in necroptotic cells induced by TNFα. Furthermore, as K63 ubiquitination of WT RIPK1, but not the S161E mutant of RIPK1 by PELI1, is blocked by Nec-1s, these results suggest that inhibition of PELI1-mediated K63 ubiquitination of RIPK1 by Nec-1s may, at least in part, explain the resistance of Nec-1s-treated cells to necroptosis, as PELI1 deficiency offers strong resistance to necroptosis.
PELI1 Inhibits RIPK1-Dependent and RIPK1-Independent Apoptosis by Regulating c-FLIP.
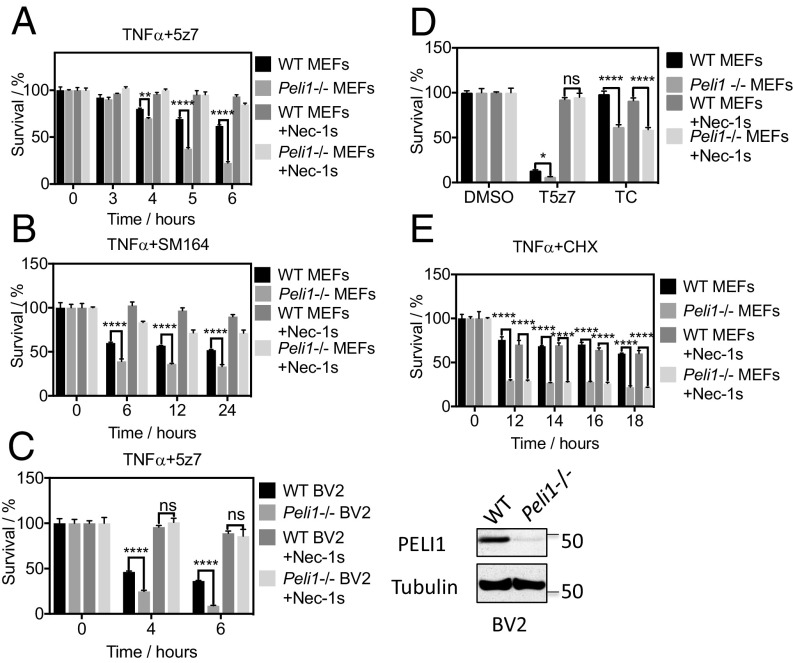
To investigate the role of PELI1 in apoptosis, we next investigated the sensitivity of PELI1 knockout cells to the induction of apoptosis. Although Peli1−/− MEFs were resistant to necroptosis, knockout of Peli1 in both MEFs and BV2 cells sensitized to apoptosis induced by TNFα/SM164 or TNFα/5z7 (Fig. 5 A–C). Apoptosis of Peli1−/− MEFs and Peli1−/− BV2 cells induced by TNFα/SM164 or TNFα/5z7 was fully blocked by Nec-1s (Fig. 5 A–C). In addition, Peli1−/− MEFs were also sensitized to apoptosis induced by TNFα/CHX, which was not inhibitable by Nec-1s (Fig. 5 D and E). Thus, PELI1 deficiency sensitizes to apoptosis mediated by both RIPK1-dependent and RIPK1-independent pathways.
Fig. 5.
PELI1 inhibits RIPK1-dependent and RIPK1-independent apoptosis. (A) WT and Peli1−/− MEFs were pretreated with Nec-1s or DMSO for 1 h and then treated with TNFα + 5z7 for the indicated periods. (B) WT and Peli1−/− MEFs were pretreated with Nec-1s or DMSO for 1 h and then treated with TNFα 10 ng/mL + SM164 for the indicated periods. (C) WT and Peli1−/− BV2 cells were pretreated with Nec-1s (20 μM) or DMSO for 1 h and then treated with TNFα 10 ng/mL + 5z7 for the indicated periods. (Right) WT and Peli1−/− BV2 cells were lysed with SDS buffer, and proteins were analyzed by Western blotting with indicated antibodies. (D) WT and Peli1−/− MEFs were pretreated with Nec-1s or DMSO for 1 h and then treated with TNFα + 5z7 or TNFα + CHX for 6 h. (E) WT and Peli1−/− MEFs were pretreated with Nec-1s or DMSO for 1 h and then treated with TNFα + CHX for the indicated periods. The cell survival was determined using CellTiterGlo. Concentrations of compounds used: Nec-1s, 20 μM; TNFα, 10 ng/mL; 5z7, 500 nM; SM164, 50 nM; zVAD.fmk, 25 μM; CHX, 1 μg/mL.
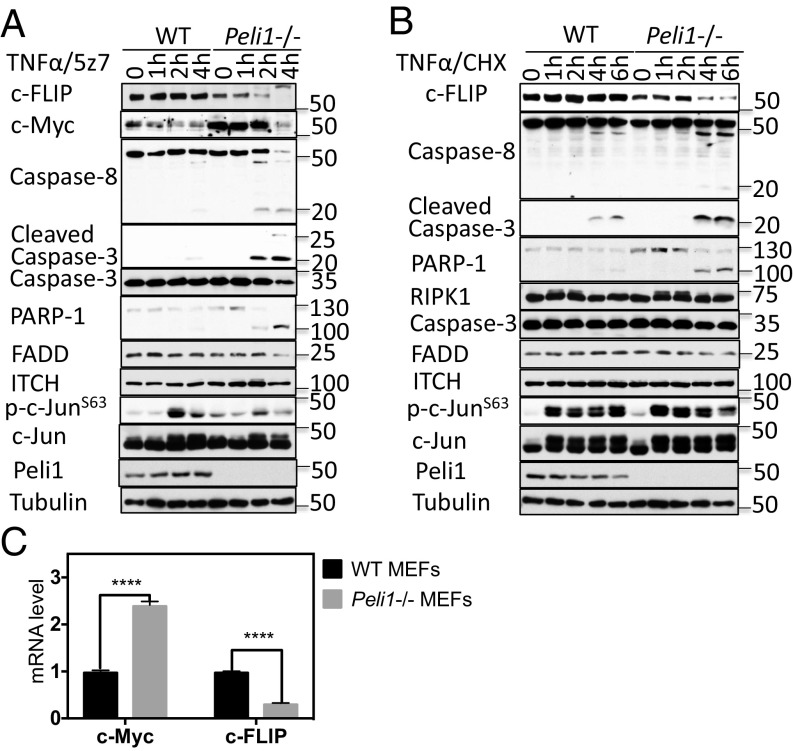
We characterized the expression of key genes involved in regulating TNFα-mediated apoptosis in WT and Peli1−/− cells. The cleavage of caspase-8, caspase-3, and PARP-1, the important hallmarks of apoptosis, induced by TNFα/5z7 and TNFα/CHX were elevated in Peli1−/− MEFs compared with that of WT (Fig. 6 A and B), consistent with increased sensitivity of Peli1−/− MEFs to both RIPK1-dependent and RIPK1-independent apoptosis (Fig. 5). In contrast, we found that the protein levels of caspase-8, caspase-3, FADD, ITCH, c-Jun, and PARP-1 were not different in Peli1−/− MEFs compared that of WT. Interestingly, the levels of c-FLIP, a key regulator of TNFα-mediated caspase-8 activation, were reduced in Peli1−/− MEFs (Fig. 6A). Consistent with the important role of c-FLIP in regulating both RIPK1-dependent and RIPK1-independent apoptosis, knockdown of c-FLIP sensitized cells to apoptosis induced by TNFα/CHX and TNFα/5z7 (Fig. S4).
Fig. 6.
PELI1 inhibits RIPK1-dependent and RIPK1-independent apoptosis by regulating c-FLIP. (A) WT and Peli1−/− MEFs were treated with TNFα + 5z7 for the indicated periods and then lysed with Nonidet P-40 buffer. The cell lysates were analyzed by Western blotting with the indicated antibodies. (B) WT and Peli1−/− MEFs were treated with TNFα + CHX for the indicated periods and then lysed with Nonidet P-40 buffer. The cell lysates were analyzed by Western blotting with indicated antibodies. (C) The mRNA was extracted from WT and Peli1−/− MEFs and reverse-transcribed into cDNA. The mRNA levels of c-Myc and c-FLIP were determined by quantitative real-time PCR with primers specific to corresponding genes. GAPDH was used as loading control. Concentrations of compounds used: Nec-1s, 20 μM; TNFα, 10 ng/mL; 5z7, 500 nM; CHX, 1 μg/mL.
We next explored the mechanism by which PELI1 might regulate the levels of c-FLIP. Because the expression of PELI1 has been found to be positively coregulated with that of c-Myc in diffuse large B-cell lymphoma (32) and c-Myc binds to and represses the c-FLIP promoter (33), we considered the possibility that PELI1 might regulate the levels of c-Myc. We found indeed that the levels of c-Myc were increased in Peli1−/− MEFs (Fig. 6A). To examine whether the regulation of c-FLIP and c-Myc by PELI1 might occur at the transcriptional levels, we measured and compared the mRNA levels of c-FLIP and c-Myc by qPCR. We found that, consistent with their protein levels, the levels of c-Myc mRNA were increased, whereas that of c-FLIP decreased, in Peli1−/− MEFs (Fig. 6C). These results suggest the possible contribution of elevated c-Myc to reduce the expression of c-FLIP in Peli1−/− MEFs.
Peli1−/− Mice Are Sensitized to TNFα-Induced SIRS.
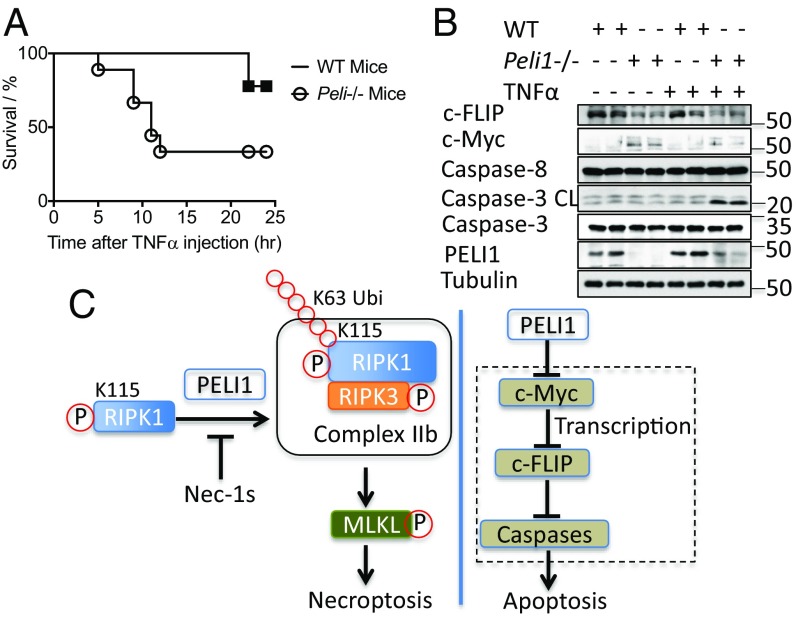
Because both necroptosis and apoptosis play important roles in TNFα injection-induced systemic inflammatory response syndrome (SIRS) in mice, we next investigated the response of Peli1−/− mice to SIRS. We found that Peli1−/− mice were sensitized to the lethality induced by i.v. injection of mTNFα. Peli1−/− mice died more and earlier than that of WT at 360 μg/kg of TNFα (Fig. 7A). Consistently, the levels of c-Myc were higher and those of c-FLIP were lower in the spleens of Peli1−/− mice (Fig. 7B). Furthermore, we detected increased cleavage of caspase-3 in the spleens of Peli1−/− mice injected with TNFα compared with that of WT. Thus, increased activation of apoptosis is sufficient to promote the lethality of PELI1-deficient mice in response to TNFα stimulation.
Fig. 7.
Peli1−/− mice are sensitized to TNFα-induced SIRS. (A) Survival curves of nine male WT and nine male Peli1−/− mice (8–12 wk) injected with mTNFα i.v. (mTNFα/mouse = 360 μg/kg). P = 0.0166 according to Gehan-Breslow-Wilcoxon test. (B) Two WT mice and two Peli1−/− mice were killed 6 h after injection with mTNFα and perfused. The spleens from these four mice were lysed and analyzed by Western blotting with indicated antibodies. (C) A model for the role of PELI1 in necroptosis and apoptosis. In necroptotic cells, PELI1 mediates K63 ubiquitination of activated RIPK1 on K115, which can be inhibited by Nec-1s. PELI1-mediated RIPK1 ubiquitination promotes the interaction of RIPK1 and RIPK3 to form necrosome and activation of MLKL to lead to necrosis.
Discussion
In this study, we demonstrate that PELI1 is a pronecroptosis E3 ubiquitin ligase that modulates K63 ubiquitination of RIPK1 to promote the interaction of activated RIPK1 with RIPK3 in necroptosis. We show that PELI1 mediates K63 ubiquitination of RIPK1 in kinase domain on K115, although our study does not rule out the involvement of Peli1 in mediating ubiquitination of RIPK1 on additional Lys residues. Because the hallmark of RIPK1 activation involves autophosphorylation on the kinase domain (6, 8), ubiquitination of RIPK1 by PELI1 might involve the recognition of phosphorylated RIPK1. In contrast, because PELI1 deficiency promotes strong resistance to necroptosis by blocking the formation of complex IIb, inhibition of PELI1-mediated K63 ubiquitination of RIPK1 in Nec-1s-treated cells might be important to explain the inhibition of complex IIb formation and necroptosis by Nec-1s, and why inhibition of RIPK1 prevents not only the phosphorylation of RIPK1 but also its ubiquitination in complex IIb (29).
We show that PELI1 inhibits apoptosis by modulating the expression levels of c-FLIP. PELI1-deficient cells are sensitized to apoptosis induced by TNFα in both an RIPK1-dependent and an RIPK1-independent manner because of the reduced expression levels of c-FLIP, a critical regulator of caspase-8 activation. Because the cleavage of RIPK1 during apoptosis separates its kinase domain from the intermediate domain (34), PELI1 cannot perform ubiquitination of RIPK1 during apoptosis, which might explain why increased sensitivity to apoptosis is dominant over the resistance to necroptosis in Peli1−/− mice on TNFα stimulation. We propose that increased c-Myc might suppress the expression of c-FLIP, as c-Myc can bind to and repress the activation of c-FLIP promoter (33). However, our study does not rule out the potential involvement of NF-κB in regulating the expression of c-FLIP. Because RIPK1 can also be activated by LPS (35), regulation of RIPK1 ubiquitination in its kinase-dependent manner by PELI1 may also contribute to the production of proinflammatory cytokines in response to certain TLR ligands.
Materials and Methods
Antibodies and Reagents.
The following commercial antibodies and reagents were used in this study: Pellino-1, Abcam (ab199336); TAK1, Cell Signaling Technology (5206); RIPK1, Cell Signaling Technology (3493) and BD Biosciences (610459); RIPK3, Biorad/Serotec (AHP1797); c-Myc, Santa Cruz (sc-764); c-FLIP, EnZo (ADI-AAP-440); NEMO, CST (2685); TBK1, Cell Signaling Technology (3504); FADD, Abcam (ab124812) and Santa Cruz (6036); α-Tubulin, Sigma-Aldrich (T9026); β-actin, Santa Cruz (81178); FLAG, CST (2368); 5Z-7 was from Sigma-Aldrich (O9890).
Statistics.
Data are expressed as the mean ± SEM. Pairwise comparisons between two groups were performed using the Student’s t test. Differences were considered statistically significant if *P < 0.05, **P < 0.01, ***P < 0.001 or not significant (n.s.). At least three independent repeats were included in each data point. Each experiment was repeated at least three times.
SI Materials and Methods
Cell Culture.
HEK293T, MEFs, RGC5, and L929 cells were cultured in DMEM (Gibco) with 10% (vol/vol) FBS (Gibco) and 100 U/mL penicillin/streptomycin and 1 mM sodium pyruvate (Invitrogen). HT29 cells were culture in McCoy’s 5A (Gibco) with 10% (vol/vol) FBS (Gibco) and 100 U/mL penicillin/streptomycin. All cells were cultured in 37 °C with 5% CO2.
Reagents.
CellTiter-Glo luminescent cell viability kit was from Promega. R-7-Cl-O-Nec-1 (Nec-1s) was made by custom synthesis. Cell lines used in this study were tested every 3 mo for mycoplasma contamination by the MycoAlert Mycoplasma Detection Kit from Lonza.
shRNA and siRNA Sequences.
The shRNA sense sequence used for knockdown of human Peli1 is 5′-CCATGTACATGGCTATCATAA-3′, mouse Peli1 are 5′-GCACCACAAGTATAGACAGTT-3′ and 5′-GCACTGTGCATATTGCTTGTA 3′, the siRNA sense sequence used for knockdown of c-FLIP is 5′-GAUGUAUCCUCCUUAGUUU-3′.
Plasmids.
CDS domain of human Peli1(NM_020651) was cloned with a FLAG or HA tag on the N-terminal into mammalian expression vector pRRL; sequence was confirmed by DNA sequencing. Primers containing shRNA _targeting implicated genes were cloned into PLKO.1 vector individually.
Lentivirus Production and Infection.
PLKO.1 vector plasmids carrying shRNA _targeting human Peli1 or scrambled sequence were transfected individually into 293T cells with VSVG, REV, and MDL plasmids for 8 h and then cell media was changed to remove transfection reagents and was replaced with fresh media. Harvest supernatant media from transfected 293T cells 48 h after transfection and filter with 45-μM membrane. Filtered media containing lentivirus particles was used to infect _target cells.
Real-Time Quantitative PCR.
RNA was purified from cells with RNeasy kit from Qiagen, and then cDNA was made from the RNA with TaKaRa EcoDry Premix. Primers used for detection of c-FLIP are 5′-GCT CCA GAA TGG GCG AAG TAA-3′, 5′-ACG GAT GTG CGG AGG TAA AAA-3′, primers used for detection of mRNA of c-Myc are 5′-CCCTATTTCATCTGCGACGAG-3′, 5′-GAGAAGGACGTAGCGACCG-3′.
Supplementary Material
Acknowledgments
We thank Dr. Vishva Dixit of Genentech for M1 and K63 ubiquitin-specific antibodies. This work was supported in part by Grants from the National Institute on Aging (1R01AG047231) and the National Institute of Neurological Disorders and Stroke (1R01NS082257), and Grants from the Chinese Academy of Sciences, the National Key R&D Program of China, the China Ministry of Science and Technology Program (2014ZX09102001-002), and the China National Natural Science Foundation (31530041), and the Chinese Academy of Sciences and the National Science Foundation in China (to J.Y.).
Footnotes
Conflict of interest statement: J.Y. and D.R.G. were coauthors on a 2016 nomenclature paper. There was no collaboration between them.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1715742114/-/DCSupplemental.
References
- 1.Christofferson DE, Yuan J. Necroptosis as an alternative form of programmed cell death. Curr Opin Cell Biol. 2010;22:263–268. doi: 10.1016/j.ceb.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weinlich R, Oberst A, Beere HM, Green DR. Necroptosis in development, inflammation and disease. Nat Rev Mol Cell Biol. 2017;18:127–136. doi: 10.1038/nrm.2016.149. [DOI] [PubMed] [Google Scholar]
- 3.Degterev A, Boyce M, Yuan J. A decade of caspases. Oncogene. 2003;22:8543–8567. doi: 10.1038/sj.onc.1207107. [DOI] [PubMed] [Google Scholar]
- 4.Geng J, et al. Regulation of RIPK1 activation by TAK1-mediated phosphorylation dictates apoptosis and necroptosis. Nat Commun. 2017;8:359. doi: 10.1038/s41467-017-00406-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morioka S, et al. TAK1 kinase switches cell fate from apoptosis to necrosis following TNF stimulation. J Cell Biol. 2014;204:607–623. doi: 10.1083/jcb.201305070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Degterev A, et al. Identification of RIP1 kinase as a specific cellular _target of necrostatins. Nat Chem Biol. 2008;4:313–321. doi: 10.1038/nchembio.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou W, Yuan J. Necroptosis in health and diseases. Semin Cell Dev Biol. 2014;35:14–23. doi: 10.1016/j.semcdb.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 8.Ofengeim D, et al. Activation of necroptosis in multiple sclerosis. Cell Rep. 2015;10:1836–1849. doi: 10.1016/j.celrep.2015.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ito Y, et al. RIPK1 mediates axonal degeneration by promoting inflammation and necroptosis in ALS. Science. 2016;353:603–608. doi: 10.1126/science.aaf6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caccamo A, et al. Necroptosis activation in Alzheimer’s disease. Nat Neurosci. 2017;20:1236–1246. doi: 10.1038/nn.4608. [DOI] [PubMed] [Google Scholar]
- 11.Ofengeim D, et al. RIPK1 mediates a disease-associated microglial response in Alzheimer’s disease. Proc Natl Acad Sci USA. September 13, 2017;114(41):E8788–E8797. doi: 10.1073/pnas.1714175114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang Z, et al. Pellino 1 is required for interleukin-1 (IL-1)-mediated signaling through its interaction with the IL-1 receptor-associated kinase 4 (IRAK4)-IRAK-tumor necrosis factor receptor-associated factor 6 (TRAF6) complex. J Biol Chem. 2003;278:10952–10956. doi: 10.1074/jbc.M212112200. [DOI] [PubMed] [Google Scholar]
- 13.Chang M, Jin W, Sun SC. Peli1 facilitates TRIF-dependent toll-like receptor signaling and proinflammatory cytokine production. Nat Immunol. 2009;10:1089–1095. doi: 10.1038/ni.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- 15.He S, et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137:1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 16.Cho YS, et al. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun L, et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148:213–227. doi: 10.1016/j.cell.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 18.Zhang DW, et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325:332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- 19.Murphy JM, et al. The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism. Immunity. 2013;39:443–453. doi: 10.1016/j.immuni.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 20.Wu J, et al. Mlkl knockout mice demonstrate the indispensable role of Mlkl in necroptosis. Cell Res. 2013;23:994–1006. doi: 10.1038/cr.2013.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei X, et al. Cationic nanocarriers induce cell necrosis through impairment of Na(+)/K(+)-ATPase and cause subsequent inflammatory response. Cell Res. 2015;25:237–253. doi: 10.1038/cr.2015.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berger SB, et al. Cutting edge: RIP1 kinase activity is dispensable for normal development but is a key regulator of inflammation in SHARPIN-deficient mice. J Immunol. 2014;192:5476–5480. doi: 10.4049/jimmunol.1400499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J, et al. The RIP1/RIP3 necrosome forms a functional amyloid signaling complex required for programmed necrosis. Cell. 2012;150:339–350. doi: 10.1016/j.cell.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Newton K, et al. Ubiquitin chain editing revealed by polyubiquitin linkage-specific antibodies. Cell. 2008;134:668–678. doi: 10.1016/j.cell.2008.07.039. [DOI] [PubMed] [Google Scholar]
- 25.Hsu H, Xiong J, Goeddel DV. The TNF receptor 1-associated protein TRADD signals cell death and NF-kappa B activation. Cell. 1995;81:495–504. doi: 10.1016/0092-8674(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 26.Rieser E, Cordier SM, Walczak H. Linear ubiquitination: A newly discovered regulator of cell signalling. Trends Biochem Sci. 2013;38:94–102. doi: 10.1016/j.tibs.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 27.Wei R, et al. SPATA2 regulates the activation of RIPK1 by modulating linear ubiquitination. Genes Dev. 2017;31:1162–1176. doi: 10.1101/gad.299776.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dziedzic SAS, et al. ABIN-1 regulates RIPK1 activation by bridging M1 ubiquitination with K63 deubiquitination in TNF-RSC. Nat Cell Biol. 2017 doi: 10.1038/ncb3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Almagro MC, et al. Coordinated ubiquitination and phosphorylation of RIP1 regulates necroptotic cell death. Cell Death Differ. 2017;24:26–37. doi: 10.1038/cdd.2016.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu J, et al. SM-164: A novel, bivalent Smac mimetic that induces apoptosis and tumor regression by concurrent removal of the blockade of cIAP-1/2 and XIAP. Cancer Res. 2008;68:9384–9393. doi: 10.1158/0008-5472.CAN-08-2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jin Z, et al. Cullin3-based polyubiquitination and p62-dependent aggregation of caspase-8 mediate extrinsic apoptosis signaling. Cell. 2009;137:721–735. doi: 10.1016/j.cell.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 32.Choe JY, et al. PELI1 expression is correlated with MYC and BCL6 expression and associated with poor prognosis in diffuse large B-cell lymphoma. Mod Pathol. 2016;29:1313–1323. doi: 10.1038/modpathol.2016.128. [DOI] [PubMed] [Google Scholar]
- 33.Ricci MS, et al. Direct repression of FLIP expression by c-myc is a major determinant of TRAIL sensitivity. Mol Cell Biol. 2004;24:8541–8555. doi: 10.1128/MCB.24.19.8541-8555.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin Y, Devin A, Rodriguez Y, Liu ZG. Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes Dev. 1999;13:2514–2526. doi: 10.1101/gad.13.19.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saleh D, et al. Kinase activities of RIPK1 and RIPK3 can direct IFN-β synthesis induced by lipopolysaccharide. J Immunol. 2017;198:4435–4447. doi: 10.4049/jimmunol.1601717. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.