Abstract
Liver fibrosis is a major health concern that results in significant morbidity and mortality. Up-to-date, there is no standard treatment for fibrosis because of its complex pathogenesis. Crocin is one of the main nutraceuticals isolated from the stigma of Crocus sativus with antioxidant and anti-inflammatory activities. The current study aimed at evaluating the potential antifibrotic activity of crocin against thioacetamide (TAA)-induced liver fibrosis in mice as well as the underlying mechanism using silymarin as a reference antifibrotic product. Crocin at two doses (25 and 100 mg/kg) significantly ameliorated the rise in serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities, and total bilirubin (TB). Further, the high dose significantly protected against the increase in serum total cholesterol (TC) and triglycerides (TG). These effects were confirmed by light microscopic examinations. Crocin antioxidant activities were confirmed by the observed inhibition of reduced glutathione depletion (GSH), super oxide dismutase (SOD) exhaustion and malondialdehyde (MDA) accumulation in liver tissue. The antifibrotic effects of crocin were explored by assessing fibrosis related gene expression. Administration of crocin (100 mg/kg) hampered expression of tumor growth factor-β (TGF-β), α alpha smooth muscle actin (α-SMA) and collagen 1-α expression and significantly raised gene expression of matrix metalloproteinase-2 (MMP-2). Further, it reduced protein expression of nuclear factor-κB (NF-κB) and cyclooxygenase-2 (COX-2) as assessed immunohistochemically. These anti-inflammatory effects were confirmed by the observed protein expression of interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α). Thus, it can be concluded that crocin protects against TAA-induced liver fibrosis in mice. This can be ascribed, at least partly, to its antioxidant and anti-inflammatory effects.
Keywords: Liver fibrosis, Thioacetamide, Crocin, Silymarin, Mice
1. Introduction
Liver fibrosis is a major health concern that results in significant morbidity and mortality (Sánchez-Valle et al., 2012). The pathogenesis of liver fibrosis is complex and involves deposition of extracellular matrix (ECM) by activated hepatic stellate cells (HSC) (Reeves and Friedman, 2002). Inflammation, induced by oxidative stress, is a key event in HSC activation (Greenwel et al., 2000). Several pro-inflammatory cytokines, chemokines and adhesion molecules initiate the activation of HSC (Pinzani and Macias-Barragan, 2010). There is no FDA approved medication for liver fibrosis (Bataller and Brenner, 2005). Several attempts have been made to combat liver fibrosis by inhibiting known molecular _targets or pathways that are critically involved in the pathogenesis of fibrogenesis.
Prevention or attenuation of the activation of hepatic stellate and Kupffer cells has been given much attention. Those two cells are responsible for propagation of oxidative and inflammatory responses, with subsequent activation of various fibrogenic mediators (Tacke and Weiskirchen, 2012). Several other _targets were proposed that are mainly part of the oxidative or the inflammatory signaling. Molecular _targets include nuclear factor-kappa B (NF-κB), tumor growth factor beta (TGF-β) and several inflammatory mediators (Popov and Schuppan, 2009). However, to protect against the development of fibrotic complications associated with the various hepatic injury, a long-term use of the medicaments is necessary. This fact limits the use of chemical products and gives a profound advantage to natural products.
In this regard, natural products have been suggested to combat human diseases including liver fibrosis. Crocin is one such nutraceutical isolated from the stigma of Crocus sativus commonly known as saffron which belongs to family Iridaceae. It is a common dietary colorant carotenoid with 14 free active functional OH groups. It has been reported to possess enormous therapeutic value. Clinically, saffron has shown antidepressant (Moshiri et al., 2006) and anti-Alzheimer activities (Akhondzadeh et al., 2010) as well as improvement of retinal function (Piccardi et al., 2012). Experimentally, crocin has shown anxiolytic (Pitsikas et al., 2008), antioxidant (El-Beshbishy et al., 2012), neuroprotective (Tamaddonfard et al., 2013), anti-inflammatory (Nam et al., 2010), hematoprotective and genoprotective (Hariri et al., 2011) and gastroprotective (El-Maraghy et al., 2015) activities. Further, crocin has been reported to ameliorate carbon tetrachloride-induced liver toxicity (Bahashwan et al., 2015) and inhibit stress-induced rise in serum transaminases in rats (Bandegi Ahmad et al., 2011). It protects against chemical insults as patulin and morphine (Boussabbeh et al., 2016, Salahshoor et al., 2016). At pharmacological and high doses, crocin did not exhibit marked damages to major organs of body and no mortality was seen by crocin in mice (Hosseinzadeh et al., 2010).
In a rat model of arthritis, crocin could inhibit the augmented serum levels of matrix metalloproteinases as well as several inflammatory mediators as TNF-α, NF-κB, and interleukins (IL) 1β and IL-6 (Hemshekhar et al., 2012). Crocin’s cytoprotective and anti-apoptotic effects in PC-12 cells have been reported to involve antagonism of TNF-α-induced expression of latent interleukin-1-converting enzyme (LICE), Bcl-XS, and Bcl-XL mRNAs. In addition, crocin suppressed the TNF-α-induced activation of caspase-3 (Soeda et al., 2001). Mechanistically, cytoprotective and anti-apoptotic activities of crocin in mammalian cells have been shown to involve reactive oxygen species (ROS)-mediated endoplasmic reticulum pathway (Boussabbeh et al., 2015). Therefore, the aim of current work was to investigate the potential antifibrotic effect of crocin against thioaetamide-induced liver fibrosis in mice and elucidate the possible underlying mechanisms.
2. Materials and methods
2.1. Chemicals
Crocin and thioacetamide were purchased from Sigma–Aldrich, St. Louis, MO, USA. Other chemicals were of the highest grade commercially available.
2.2. Animals
Experiments were performed on 25–35 g male SWR mice, supplied by the Animal Breeding Laboratory, King Fahd Medical Research Center. Animals were acclimatized for 7 days before the start of experiment in our animal facility. They were maintained on a 12-h light–dark cycle. Room temperature was kept at 22 ± 2 °C. Animal handling and experimental protocol were approved by the Unit of Biomedical Ethics, Faculty of Medicine, King Abdulaziz University (Reference # 157-14).
2.3. Experimental protocol
Mice were randomly divided into five groups (8 animals per group) and treated for six consecutive weeks. Group # 1 was considered as control group and given saline i.p. twice weekly. Group # 2 was given thioacetamide (100 mg/kg, i.p.), twice weekly to provoke liver fibrosis. Group # 3 was given both of thioacetamide (100 mh/kg, i.p.) and crocin (25 mg/kg). Group # 4 was given thioacetamide with crocin (100 mg/kg). Group # 5 was given thioacetamide with the positive standard “silymarin” (100 mg/kg). Crocin and silymarin treatments were given by oral gavage every day till day 42. Blood was withdrawn from the retro-orbital plexus. Then, mice were sacrificed. Liver from each animal was rapidly dissected out, washed and homogenized using phosphate-buffered saline (PBS; 50 mM potassium phosphate, pH 7.5) at 4 °C; producing a 20% homogenate. In addition to homogenates, representative liver specimens were kept in RNAlater storage solution (Sigma–Aldrich, St. Louis, MO, USA) for RNA extraction. Liver homogenates and liver tissues for RNA extraction were kept at −80 °C till time of analyses. Representative liver tissues were kept in 10% formalin-saline for histopathological and immunohistochemical examination.
2.4. Assessment of liver functions
Activities of serum ALT and AST, serum levels of TB, TC and TG were determined colorimetrically using commercially available kits (Biodiagnostic, Cairo, Egypt).
2.5. Histopathological examination
Liver tissues were fixed in 10% neutral buffered formalin for 24 h. This was followed by immersion in tap water and serial dilutions of alcohol. Specimens were cleared in xylene and embedded in paraffin at 56 °C in hot air oven for 24 h. Paraffin bees wax tissue blocks were prepared for sectioning at 4 μm thickness by slide microtome. Tissue sections were then processed and stained with hematoxylin and eosin for histopathological examinations as previously described (Bancroft and Layton, 2013).
2.6. Assessment of oxidative stress markers
Reduced glutathione (GSH), malondialdehyde (MDA) and protein content as well as superoxide dismutase (SOD) activity were determined in liver homogenates using appropriate commercial kits (Biodiagnostic, Cairo, Egypt).
2.7. Reverse transcriptase–polymerase chain reaction (RT-PCR) analyses of TGF-β, α-SMA, MMP2 and Collagen-1α gene expressions
Total RNA was extracted using PureLink® RNA Mini Kit assay kit. Reverse transcription was carried out using a High-Capacity cDNA Reverse Transcription Kit. Real time PCR was executed using the following primers: TGF-β sense primer, 5′-ACCAACTACTGCTTCAGCTCCACA-3′ and the corresponding antisense primer, 5′-TGTACTGTGTGTCCAGGCTCCAAA-3′. α-SMA sense primer, 5′-GGCTCTGGGCTCTGTAAGG-3′ and the corresponding antisense primer, 5′-CTCTTGCTCTGGGCTTCATC-3′. MMP2 sense primer, 5′-GAGATCTGCAAACAGGACAT-3′ and the corresponding antisense primer, 5′-GGTTCTCCAGCTTCAGGTAA-3′. Collagen-1α sense primer, 5′-GGGTCTAGACATGTTCAGCTTTGTG-3′ and the corresponding antisense primer, 5′-ACCCTTAGGCCATTGTGTATGC-3′. Beta-actin was used as reference housekeeping gene with sense primer, 5′-CCCAGCACAATGAAGATCAA GATCAT-3′ and the corresponding antisense primer, 5′-ATCTGCTGGAAGGTGGACA GCGA-3′. All the primers were obtained from Invitrogen (Camarillo, CA, USA). Relative gene expressions were evaluated by Quantitative real-time PCR using SYBR Green and Applied Biosystems Step One Real Time PCR System. After 10 min hot start at 95 °C, samples underwent denaturation at 95 °C for 15 s, annealing at 60 °C for 1 min for 45 cycles. Melting curve analysis was performed starting at 60 °C until 95 °C with stepwise temperature elevations. Fluorescent quantitative analysis was carried out with the thermal cycler’s software package to calculate the ΔCt value where the Ct value is the cycle number when the fluorescence curve crossed the baseline value. ΔCt value = _target gene Ct value − reference gene Ct value. ΔΔCt = experimental group ΔCt − control group ΔCt. Gene expression in the control group was given a value of unity and extent of gene expression in experimental groups were computed as 2−ΔΔCt and referred to as relative quantification (RQ).
2.8. Assessment of inflammatory markers
2.8.1. Immunohistochemical detection of cyclooxygenase-2 (COX-2) and NF-κB
Paraffin-embedded tissue sections of 4-μm thickness, were deparaffinized in xylene followed by hydration in graded ethanol solution and heated in citrate buffer (pH 6.0) for 5 min. Sections were then blocked with 5% bovine serum albumin (BSA) in Tris-buffered saline (TBS) for 2 h. Slides were then kept overnight at 4 °C with one of the following specific primary antibodies: COX-2 rabbit polyclonal antibody (cat#PA5–23638, Thermo Fischer Scientific), iNOS rabbit polyclonal antibody (cat#RB-9242-P, Thermo Fisher Scientific). Slides were washed with TBS and incubated for 15–20 min with the corresponding secondary antibody. Then, horseradish-peroxidase-conjugated streptavidin solution was added and incubated at room temperature for 15–20 min. The slides were then rinsed with TBS and incubated for 10 min in a solution of 0.02% diaminobenzidine containing 0.01% H2O2. Counter staining was carried out using hematoxylin, and the slides were examined under a light microscope. Immunohistochemical quantification was executed using image analysis software (ImageJ, 1.48a, NIH, USA).
2.8.2. Assessment of IL-1β and TNF-α levels
ELISA kits for IL-1β and TNF-α were obtained from Orgenium Laboratories’ (Vantaa, Finland). The quantitative sandwich immunoassay technique was applied. Briefly, each kit had a specific microplate pre-coated with monoclonal antibody against its analyte. After adding the standards and samples, the analyte was sandwiched by the immobilized antibody and a biotinylated polyclonal antibody specific for it. The later was recognized by a streptavidin–peroxidase conjugate. Unbound materials were then washed away. Thereafter, a peroxidase enzyme substrate was added followed by the stop solution. Finally, color intensity was determined at 450 nm using a microplate reader (ChroMate-4300, FL, USA).
2.9. Statistical analyses
Data were shown as mean ± SD values. Statistical tests were performed using ANOVA followed by Tukey multiple comparisons tests. The 0.05 level of probability or lower were considered the criterion for significance. GraphPad InStat software version 3 (La Jolla, CA, USA) was used to perform statistical tests. Graphs were drawn using GraphPad Prism software version 5 (ISI Software, La Jolla, CA, USA).
3. Results
3.1. Hepatic toxicity
After the TAA-administration, there was a leakage of hepatic enzymes ALT, AST from hepatic cells into the blood, manifested as significant elevation of ALT and AST serum activities compared to the corresponding control group, as shown in Table 1. In addition, TAA administration caused significant increases in TB, TC and TG, compared to the control group. Treatment with crocin (25 and 100 mg/kg) significantly reduced ALT and AST activities, as compared to the corresponding TAA-challenged group. Interestingly, treatment with crocin 25 mg/kg significantly reduced TB concentration but failed to induce any significant changes of TC and TG from the corresponding TAA-challenged group. However, crocin 100 mg/kg has shown significant decreases in TB, TC and TG levels from the corresponding TAA-challenged group. On the other hand, treatment with silymarin normalized the levels of all tested markers that were not statistically different from the corresponding control group.
Table 1.
Effect of crocin treatment on hepatotoxicity markers in a mouse model of TAA-induced liver fibrosis.
| Group | ALT | AST | TB | TC | TG |
|---|---|---|---|---|---|
| U/L | U/L | mg/dL | mg/dL | mg/dL | |
| Control | 16.02b ± 4.23 | 13.25b ± 2.04 | 0.23b ± 0.084 | 58.98b ± 3.06 | 73.13b ± 3.79 |
| Thioacetamide (TAA; 100 mg/kg) | 129.13a ± 17.09 | 95.54a ± 3.53 | 1.20a ± 0.249 | 126.53a ± 6.56 | 156.90a ± 8.14 |
| TAA + crocin 25 mg/kg | 52.86a,b ± 0.43 | 47.28a,b ± 3.22 | 0.83a,b ± 0.29 | 124.00a ± 6.43 | 153.76a ± 7.97 |
| TAA + crocin 100 mg/kg | 28.00a,b ± 0.50 | 29.14a,b ± 5.18 | 0.36a,b ± 0.129 | 69.45a,b ± 3.60 | 86.12a,b ± 4.46 |
| TAA + silymarin100 mg/kg | 22.17b ± 5.24 | 19.46b ± 4.32 | 0.29b ± 0.103 | 64.29b ± 3.33 | 79.71b ± 4.13 |
n = 6.
Statistical analysis was carried out by a one-way ANOVA followed by Tukey’s post hoc test.
Statistically significant from the corresponding control at p < 0.05.
Statistically significant from TAA-treated group at p < 0.05.
3.2. Histopathological examination
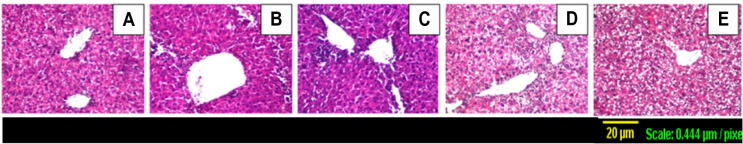
These hepatotoxicity findings were further confirmed by histopathological examination of representative liver tissues, as shown in Fig. 1. Liver sections from control mice showed normal architecture, hepatocyte structure and central vein (Fig. 1A), while TAA-intoxicated mouse livers exhibited focal inflammatory cells infiltration in the hepatic parenchyma surrounding the dilated central vein as well as in the portal area together with diffuse proliferation of kupffer cells between hepatocytes (Fig. 1B). Crocin treatment (25 mg/kg) has shown minimal protective effect as shown in (Fig. 1C) that was similar to sections from TAA-intoxicated liver sections. In contrary, sections taken from a mouse liver treated with crocin (100 mg/kg) and silymarin have provided observable protection with only some degenerative changes (Fig. 1D and E).
Figure 1.
Representative photomicrographs of liver sections stained by H&E (×100): (A) Control section with normal histological structure of the central vein and surrounding hepatocytes, (B) TAA-challenged group with focal inflammatory cells infiltration in the hepatic parenchyma surrounding the dilated central vein as well as in the portal area associated with diffuse kupffer cells proliferation in between the hepatocytes, (C) treatment with crocin 25 mg/kg showing focal inflammatory cells infiltration in the parenchyma surrounding the central vein with diffuse kupffer cells proliferation in between the hepatocytes, (D) treatment with crocin 100 mg/kg in which hepatocytes show degenerative change associated with focal inflammatory cell infiltration in the hepatic parenchyma. (E) treatment with silymarin 100 mg/kg showing degenerative change was diffuse manner all over the hepatocytes.
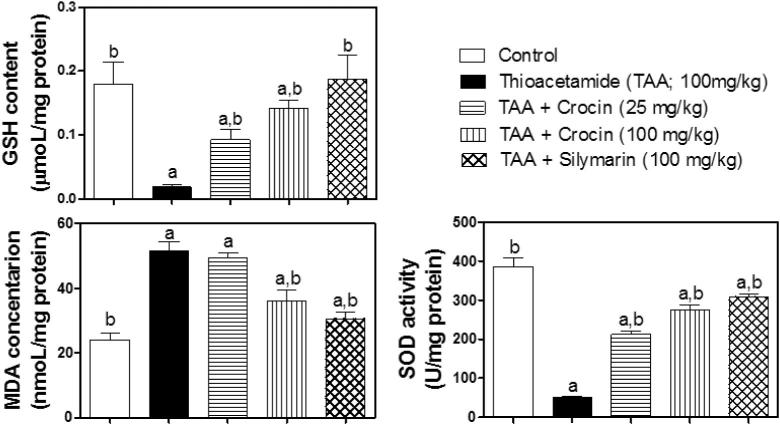
3.3. Assessment of oxidative stress
GSH content, MDA level and SOD activity were taken as markers of oxidative status. TAA significantly depleted GSH and caused more than 2-fold increase in MDA levels, compared to the corresponding control group (Fig. 2). These changes were successively restored by silymarin treatment. Besides, treatment with crocin (100 mg/kg) significantly enhanced GSH content and reduced MDA, compared to the corresponding TAA-challenged group. However, Crocin (25 mg/kg) treatment failed to show any significant change of MDA level, compared to TAA-challenged group. A similar pattern of biological activity was observed SOD activity that was significantly decreased to about 13% of the control by the TAA challenge. On the other hand, treatment with crocin (25 and 100 mg/kg) and silymarin significantly increased its activity, reaching 55%, 71% and 80% of the control activity, respectively (Fig. 2).
Figure 2.
Effect of crocin treatment (25 and 100 mg/kg) on oxidative stress parameters (GSA, MDA and SOD) in TAA-induced liver fibrosis in mice. n = 6. Statistical analysis was carried out by a one-way ANOVA followed by Tukey’s post hoc test. (a) Statistically significant from the corresponding control at p < 0.05. (b) Statistically significant from ATT-treated group at p < 0.05.
3.4. Assessment of liver fibrosis
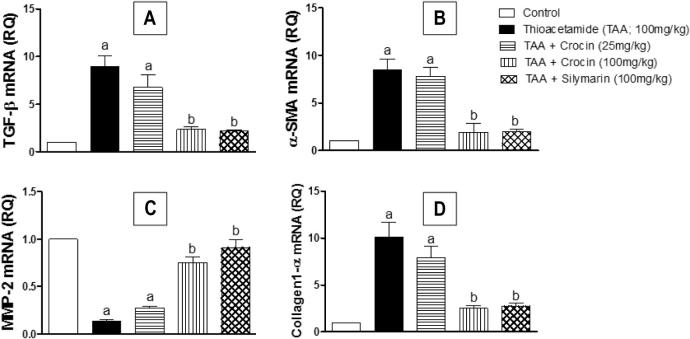
Gene expression analyses of fibrosis-related genes were carried out to evaluate the impact of crocin treatment on the extent of hepatic fibrosis. As shown in (Fig. 3), TAA-challenged mice showed significant up-regulation of TGF-β1, α-SMA and collagen-1 genes to 8.9, 8.4 and 10.1 folds, respectively. Meanwhile, MMP-2 gene expression was significantly decreased by about 87% of the control value. It is worthy to note that both crocin (100 mg/kg) and silymarin treatments significantly protected against such changes of gene expression, showing no significant differences from the corresponding control. However, treatment with crocin (25 mg/kg) was unable to demonstrate any significant difference from the corresponding TAA-challenged group.
Figure 3.
Effect of crocin treatment (25 and 100 mg/kg) on the expression of fibrosis-related genes (TGF-β, α-SMA, Collagen 1-α and MMP-2) in TAA-induced liver fibrosis in mice. n = 6. Statistical analysis was carried out by a one-way ANOVA followed by Tukey’s post hoc test. (a) Statistically significant from the corresponding control at p < 0.05. (b) Statistically significant from ATT-treated group at p < 0.05.
3.5. Assessment of inflammatory markers
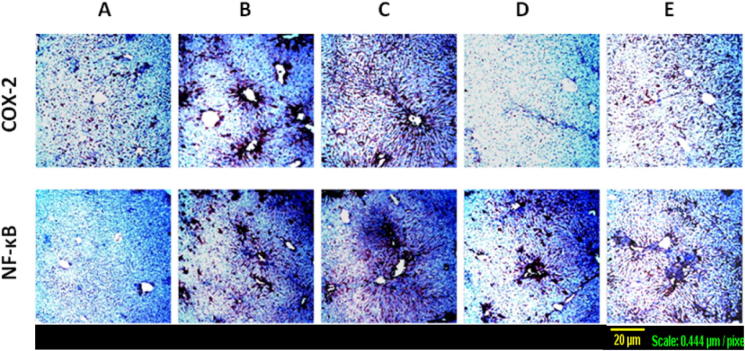
Expression of NF-κB activated subunit p65 in liver tissues and the proinflammatory enzyme COX-2 was assessed by immunohistochemical staining. As shown in (Fig. 4A), minimal immunostaining for both NF-κB and COX-2 was observed in sections from control group. On contrary, TAA elevated the expression of these markers as shown by the intense brown staining (Fig. 4B), especially around the central veins which correlates with the pathological centrilobular changes detected by H&E staining. Crocin (25 mg/kg) treatment did not alleviate such changes as shown in (Fig. 4C). However, treatment with crocin (100 mg/kg) and silymarin prevented these alterations to a large extent (Fig. 4D and E), showing only diffuse brown staining which again corresponds to the diffuse degeneration observed in H&E staining.
Figure 4.
Expression of COX-2 and NF-κB by immunohistochemical staining of liver sections (×100). (A) Control section with minimal expression of both COX-2 and NF-κB, (B) TAA-challenged group with intense brown staining surrounding the dilated central vein as well as in the portal area, (C) treatment with crocin 25 mg/kg showing similar effects to TAA-alone, (D) treatment with crocin 100 mg/kg with diffused brown staining. (E) treatment with silymarin 100 mg/kg showing diffuse brown staining.
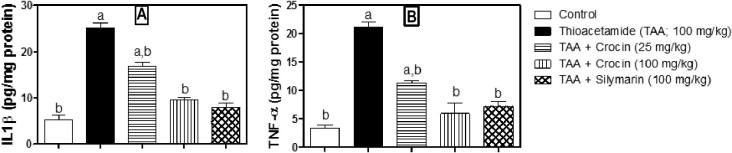
The previous effects of crocin treatment were further confirmed by assessing IL-1β and TNF-α using ELISA technique. As shown in (Fig. 5A and B), TAA caused significant 4.8 and 6.4 fold-increases in IL-1β and TNF-α, respectively. Treatment with crocin (25 mg/kg) significantly reduced their levels by 46.6% and 32.8% of the corresponding TAA group, respectively. The effect of crocin (100 mg/kg) was more pronounced, being able to cause significant reductions of IL-1β and TNF-α by 61.6% and 71.7% of the corresponding TAA group, respectively. It is worth mentioning that crocin (100 mg/kg) and silymarin did not show any significant difference of both parameters from the corresponding control group.
Figure 5.
Effect of crocin treatment (25 and 100 mg/kg) on the level of inflammatory cytokines (IL-1β and TNF-α) in TAA-induced liver fibrosis in mice. n = 6. Statistical analysis was carried out by a one-way ANOVA followed by Tukey’s post hoc test. (a) Statistically significant from the corresponding control at p < 0.05. (b) Statistically significant from TAA-treated group at p < 0.05.
4. Discussion
Liver fibrosis is a major health distress that causes significant morbidity and mortality. Up to date, there is no standard treatment for fibrosis because of its complex pathogenesis. Inflammation, induced by oxidative stress, is a key factor in HSC activation (Greenwel et al., 2000) which in turn involved in deposition of ECM (Reeves and Friedman, 2002). Pathological inflammation has been suggested to activate transcription of pro-apoptotic genes and damage hepatocytes. Also, pro-inflammatory cytokines have been shown to exacerbate oxidative damage of the liver (Mohamed et al., 2016). There is no FDA approved medication for liver fibrosis (Bataller and Brenner, 2005). Crocin is one of the main nutraceutical isolated from the stigma of C. sativus with antioxidant, anti-apoptotic and anti-inflammatory activities. Therefore, the current study aimed at evaluating the potential antifibrotic activities of crocin against thioacetamide-induced liver fibrosis in mice as well as the underlying mechanism.
The hepatotoxin TAA has been utilized in a multiplicity of studies evaluating the core mechanisms of liver fibrogenesis (Schnur et al., 2004, Sáez-Lara et al., 2006, Kornek et al., 2006). In comparison to other experimental models, TAA-induced liver fibrosis is intimately like to alcoholic liver fibrogenesis with similar histological and metabolic alterations commonly detected in the livers of afflicted humans (Nozu et al., 1992). In the present study, treatment with crocin (25 and 100 mg/kg) or silymarin significantly preserved hepatocyte integrity as indicated by reduced plasma activities of ALT and AST. It is noteworthy that the higher dose of crocin also improved the metabolic function as marked by plasma total bilirubin, cholesterol and triglycerides. These observations were confirmed histologically as crocin (100 mg/kg) mitigated hepatic injury and pericentral fibrosis and inflammatory cells induction. In addition, TAA-induced oxidative stress in hepatic tissues was alleviated by crocin treatment. This was evidenced by enhanced GSH content and reduced lipid peroxidation. Notably, crocin at 100 mg/kg was superior to its lower dose (25 mg/kg) in preserving SOD activity. The observed preserved integrity of hepatocyte membranes by crocin can be explained by its well-known antioxidant properties (El-Beshbishy et al., 2012). This antioxidant activity was in accordance with the previous work of Hemshekhar et al. (2012); in which crocin ameliorated the altered antioxidant status via enhancing GSH, SOD, CAT and GST in a rat model of arthritis.
Liver fibrosis is characterized by quantitative and qualitative amendments of hepatic ECM. The fundamental cellular event is HSC activation. Upon stimulation, quiescent HSCs are differentiated into myofibroblast-like cells with increased proliferation, accumulation of ECM – and expression of α-SMA, (Friedman, 2000). As a result, accumulation of collagen in the liver is considered as a mark of fibrosis (Bissell et al., 1990). In the current study, the antifibrotic activity of crocin was principally examined by assessing gene expression of fibrosis-related genes. Up-regulation of α-SMA and collagen 1-α genes is a fundamental step that convoys HSCs activation and liver fibrosis (Bataller and Brenner, 2005). The up-regulation of TGF-β as well as down-regulation of MMP-2 indicates the pathogenesis of fibrosis (Knittel et al., 2000, Guo and Friedman, 2007). Crocin (100 mg/kg) significantly inhibited over-expression of collagen-1α, α-SMA, TGF-β as well as the down-regulation of MMP-2 expression. It is worth mentioning that crocin (25 mg/kg) was unable to ameliorate TAA-induced modulation of fibrosis-related gene expression.
Subsequently, the antifibrotic mechanism of crocin was evaluated by studying different markers of inflammation. Assessment of inflammatory markers revealed that TAA induced a significant increase in hepatic NF-κBp65 and COX-2 expression together with significant elevation in tissue levels of the TNF-α and IL-1β. This indicates amplified inflammatory responses induced by TAA challenge. In this regard, kinases activation in NF-κB pathway is connected to oxidative stress (Baeuerle and Baichwal, 1997). However, antiangiogenic activities cannot be excluded based on those observed with crocetin (Umigai et al., 2012). ROS are shown to cause prolonged NF-κB DNA binding activity (Ripple et al., 1999). This resulted in enhanced production of proinflammatory cytokines such as TNF-α and IL-6 that are implicated in fibrosis (Racanelli and Rehermann, 2006). NF-κB also stimulates the expression of enzymes whose products participate in the pathogenesis of inflammation, including COX-2 (Pahl, 1999). COX-2 activity leads to high levels of eicosanoids resulting in cellular inflammation and necrosis (Hu, 2003). This was in line with the previous study of (Orfila et al. (2005), in which NF-κB induction during liver injury has been reported in hepatocytes in a model of CCl4-induced hepatotoxicity in rats. Thus, elevated levels of NF-κB promote the secretion of inflammatory and chemotactic factors in hepatocytes and worsen hepatic inflammation and fibrosis (Luedde and Schwabe, 2011). In the present study, treatment with crocin significantly reduced the expression of NF-κB and consequently inhibited the downstream inflammatory cascade. This is evidenced by decreasing the expression of COX-2 and levels of TNF-α and IL-1β. Thus, crocin proved to have reasonable anti-inflammatory activity. In conclusion, the present study indicates the potential antifibrotic activity of crocin, which can be attributed – at least partly – by its antioxidant and anti-inflammatory actions.
Footnotes
Peer review under responsibility of King Saud University.
References
- Akhondzadeh S., Sabet M.S., Harirchian M.H., Togha M., Cheraghmakani H., Razeghi S., Hejazi S.S., Yousefi M.H., Alimardani R., Jamshidi A., Zare F., Moradi A. Saffron in the treatment of patients with mild to moderate Alzheimer’s disease: a 16-week, randomized and placebo-controlled trial. J. Clin. Pharm. Ther. 2010;35:581–588. doi: 10.1111/j.1365-2710.2009.01133.x. [DOI] [PubMed] [Google Scholar]
- Baeuerle P.A., Baichwal V.R. NF-kappa B as a frequent _target for immunosuppressive and anti-inflammatory molecules. Adv. Immunol. 1997;65:111–137. [PubMed] [Google Scholar]
- Bahashwan S., Hassan M.H., Aly H., Ghobara M.M., El-Beshbishy H.A., Busati I. Crocin mitigates carbon tetrachloride-induced liver toxicity in rats. J. Taibah Univ. Med. Sci. 2015;10:140–149. [Google Scholar]
- Bancroft J., Layton C. The hematoxylins and eosin. In: Suvarna S.K., Layton C., Bancroft J., editors. Theory and Practice of Histological Techniques. Churchil Livingstone; New York, London, San Francisco, Tokyo: 2013. pp. 173–186. [Google Scholar]
- Bandegi Ahmad R., Vafaei Abbas A., Ghaderdoost B., Rashidy-Pour A.A. Assessment of protective affects of saffron and crocin on change of serum glucose, lipids and liver transaminase that induced by chronic stress in rat. Clin. Biochem. 2011;44:S112–S113. [Google Scholar]
- Bataller R., Brenner D.A. Liver fibrosis. J. Clin. Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell D.M., Friedman S.L., Maher J.J., Roll F.J. Connective tissue biology and hepatic fibrosis: report of a conference. Hepatology. 1990;11:488–498. doi: 10.1002/hep.1840110322. [DOI] [PubMed] [Google Scholar]
- Boussabbeh M., Ben Salem I., Belguesmi F., Neffati F., Najjar M.F., Abid-Essefi S., Bacha H. Crocin protects the liver and kidney from patulin-induced apoptosis in vivo. Environ. Sci. Pollut. Res. Int. 2016;23(10):9799–9808. doi: 10.1007/s11356-016-6195-2. [DOI] [PubMed] [Google Scholar]
- Boussabbeh M., Prola A., Ben Salem I., Guilbert A., Bacha H., Lemaire C., Abis-Essefi S. Crocin and quercetin prevent PAT-induced apoptosis in mammalian cells: INVOLVZEMENT of ROS-mediated ER stress pathway. Environ. Toxicol. 2015 doi: 10.1002/tox.22185. [DOI] [PubMed] [Google Scholar]
- El-Beshbishy H.A., Hassan M.H., Aly H.A.A., Doghish A.S., Alghaithy A.A.A. Crocin “saffron” protects against beryllium chloride toxicity in rats through diminution of oxidative stress and enhancing gene expression of antioxidant enzymes. Ecotoxicol. Environ. Saf. 2012;83:47–54. doi: 10.1016/j.ecoenv.2012.06.003. [DOI] [PubMed] [Google Scholar]
- El-Maraghy S.A., Rizk S.M., Shahin N.N. Gastroprotective effect of crocin in ethanol-induced gastric injury in rats. Chem. Biol. Interact. 2015;229:26–35. doi: 10.1016/j.cbi.2015.01.015. [DOI] [PubMed] [Google Scholar]
- Friedman S.L. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J. Biol. Chem. 2000;275:2247–2250. doi: 10.1074/jbc.275.4.2247. [DOI] [PubMed] [Google Scholar]
- Greenwel P., Domínguez-Rosales J.A., Mavi G., Rivas-Estilla A.M., Rojkind M. Hydrogen peroxide: a link between acetaldehyde-elicited alpha1(I) collagen gene up-regulation and oxidative stress in mouse hepatic stellate cells. Hepatology. 2000;31:109–116. doi: 10.1002/hep.510310118. [DOI] [PubMed] [Google Scholar]
- Guo J., Friedman S.L. Hepatic fibrogenesis. Semin. Liver Dis. 2007;27:413–426. doi: 10.1055/s-2007-991517. [DOI] [PubMed] [Google Scholar]
- Hariri A.T., Moallem S.A., Mahmoudi M., Hosseinzadeh H. The effect of crocin and safranal, constituents of saffron, against subacute effect of diazinon on hematological and genotoxicity indices in rats. Phytomedicine. 2011;18:499–504. doi: 10.1016/j.phymed.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Hemshekhar M., Sebastin Santhosh M., Sunitha K., Thushara R.M., Kemparaju K., Rangappa K.S., Girish K.S. A dietary colorant crocin mitigates arthritis and associated secondary complications by modulating cartilage deteriorating enzymes, inflammatory mediators and antioxidant status. Biochimie. 2012;94:2723–2733. doi: 10.1016/j.biochi.2012.08.013. [DOI] [PubMed] [Google Scholar]
- Hosseinzadeh H., Shariaty M.V., Sameni A.K., Vahabzadeh M. Acute and sub-acute toxicity of crocin, a constituent of Crocus sativus L. (saffron), in mice and rats. Pharmacologyonline. 2010;2:943–951. [Google Scholar]
- Hu K.-Q. Cyclooxygenase 2 (COX2)-prostanoid pathway and liver diseases. Prostaglandins Leukot. Essent. Fatty Acids. 2003;69:329–337. doi: 10.1016/j.plefa.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Knittel T., Mehde M., Grundmann A., Saile B., Scharf J.G., Ramadori G. Expression of matrix metalloproteinases and their inhibitors during hepatic tissue repair in the rat. Histochem. Cell Biol. 2000;113:443–453. doi: 10.1007/s004180000150. [DOI] [PubMed] [Google Scholar]
- Kornek M., Raskopf E., Guetgemann I., Ocker M., Gerceker S., Gonzalez-Carmona M.A., Rabe C., Sauerbruch T., Schmitz V. Combination of systemic thioacetamide (TAA) injections and ethanol feeding accelerates hepatic fibrosis in C3H/He mice and is associated with intrahepatic up regulation of MMP-2, VEGF and ICAM-1. J. Hepatol. 2006;45:370–376. doi: 10.1016/j.jhep.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Luedde T., Schwabe R.F. NF-κB in the liver–linking injury, fibrosis and hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2011;8:108–118. doi: 10.1038/nrgastro.2010.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed J., Nazratun Nafizah A.H., Zariyantey A.H., Budin S.B. Mechanisms of diabetes-induced liver damage: the role of oxidative stress and inflammation. Sultan Qaboos Univ. Med. J. 2016;16(2):e132–e141. doi: 10.18295/squmj.2016.16.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshiri E., Basti A.A., Noorbala A.-A., Jamshidi A.-H., Hesameddin Abbasi S., Akhondzadeh S. Crocus sativus L. (petal) in the treatment of mild-to-moderate depression: a double-blind, randomized and placebo-controlled trial. Phytomedicine. 2006;13:607–611. doi: 10.1016/j.phymed.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Nam K.N., Park Y.-M., Jung H.-J., Lee J.Y., Min B.D., Park S.-U., Jung W.-S., Cho K.-H., Park J.-H., Kang I., Hong J.-W., Lee E.H. Anti-inflammatory effects of crocin and crocetin in rat brain microglial cells. Eur. J. Pharmacol. 2010;648:110–116. doi: 10.1016/j.ejphar.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Nozu F., Takeyama N., Tanaka T. Changes of hepatic fatty acid metabolism produced by chronic thioacetamide administration in rats. Hepatology. 1992;15:1099–1106. doi: 10.1002/hep.1840150621. [DOI] [PubMed] [Google Scholar]
- Orfila C., Lepert J.-C., Alric L., Carrera G., Béraud M., Pipy B. Immunohistochemical distribution of activated nuclear factor kappaB and peroxisome proliferator-activated receptors in carbon tetrachloride-induced chronic liver injury in rats. Histochem. Cell Biol. 2005;123:585–593. doi: 10.1007/s00418-005-0785-2. [DOI] [PubMed] [Google Scholar]
- Pahl H.L. Activators and _target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6853–6866. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- Piccardi M., Marangoni D., Minnella A.M., Savastano M.C., Valentini P., Ambrosio L., Capoluongo E., Maccarone R., Bisti S., Falsini B. A longitudinal follow-up study of saffron supplementation in early age-related macular degeneration: sustained benefits to central retinal function. Evid. Based Complement. Alternat. Med. 2012;2012:429124. doi: 10.1155/2012/429124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinzani M., Macias-Barragan J. Update on the pathophysiology of liver fibrosis. Expert Rev. Gastroenterol. Hepatol. 2010;4:459–472. doi: 10.1586/egh.10.47. [DOI] [PubMed] [Google Scholar]
- Pitsikas N., Boultadakis A., Georgiadou G., Tarantilis P.A., Sakellaridis N. Effects of the active constituents of Crocus sativus L., crocins, in an animal model of anxiety. Phytomedicine. 2008;15:1135–1139. doi: 10.1016/j.phymed.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Popov Y., Schuppan D. _targeting liver fibrosis: strategies for development and validation of antifibrotic therapies. Hepatology. 2009;50:1294–1306. doi: 10.1002/hep.23123. [DOI] [PubMed] [Google Scholar]
- Racanelli V., Rehermann B. The liver as an immunological organ. Hepatology. 2006;43:S54–S62. doi: 10.1002/hep.21060. [DOI] [PubMed] [Google Scholar]
- Reeves H.L., Friedman S.L. Activation of hepatic stellate cells – a key issue in liver fibrosis. Front. Biosci. 2002;7:d808–d826. doi: 10.2741/reeves. [DOI] [PubMed] [Google Scholar]
- Ripple M.O., Henry W.F., Schwarze S.R., Wilding G., Weindruch R. Effect of antioxidants on androgen-induced AP-1 and NF-kappaB DNA-binding activity in prostate carcinoma cells. J. Natl. Cancer Inst. 1999;91:1227–1232. doi: 10.1093/jnci/91.14.1227. [DOI] [PubMed] [Google Scholar]
- Sáez-Lara M.J., Frecha C., Martín F., Abadía F., Toscano M., Gil A., Fontana L. Transplantation of human CD34+ stem cells from umbilical cord blood to rats with thioacetamide-induced liver cirrhosis. Xenotransplantation. 2006;13:529–535. doi: 10.1111/j.1399-3089.2006.00344.x. [DOI] [PubMed] [Google Scholar]
- Salahshoor M.R., Khashiadeh M., Roshankhah S., Kakabaraei S., Jalili C. Protective effect of crocin on liver toxicity induced by morphine. Res. Pharm. Sci. 2016;11(2):120–129. [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Valle V., Chávez-Tapia N.C., Uribe M., Méndez-Sánchez N. Role of oxidative stress and molecular changes in liver fibrosis: a review. Curr. Med. Chem. 2012;19:4850–4860. doi: 10.2174/092986712803341520. [DOI] [PubMed] [Google Scholar]
- Schnur J., Oláh J., Szepesi A., Nagy P., Thorgeirsson S.S. Thioacetamide-induced hepatic fibrosis in transforming growth factor beta-1 transgenic mice. Eur. J. Gastroenterol. Hepatol. 2004;16:127–133. doi: 10.1097/00042737-200402000-00002. [DOI] [PubMed] [Google Scholar]
- Soeda S., Ochiai T., Paopong L., Tanaka H., Shoyama Y., Shimeno H. Crocin suppresses tumor necrosis factor-alpha-induced cell death of neuronally differentiated PC-12 cells. Life Sci. 2001;69:2887–2898. doi: 10.1016/s0024-3205(01)01357-1. [DOI] [PubMed] [Google Scholar]
- Tacke F., Weiskirchen R. Update on hepatic stellate cells: pathogenic role in liver fibrosis and novel isolation techniques. Expert Rev. Gastroenterol. Hepatol. 2012;6:67–80. doi: 10.1586/egh.11.92. [DOI] [PubMed] [Google Scholar]
- Tamaddonfard E., Farshid A.A., Ahmadian E., Hamidhoseyni A. Crocin enhanced functional recovery after sciatic nerve crush injury in rats. Iran. J. Basic Med. Sci. 2013;16:83–90. [PMC free article] [PubMed] [Google Scholar]
- Umigai N., Tanaka J., Tsuruma K., Shimazawa M., Hara H. Crocetin, a carotenoid derivative, inhibits VEGF-induced angiogenesis via suppression of p38 phosphorylation. Curr. Neurovasc. Res. 2012;9(2):102–109. doi: 10.2174/156720212800410830. [DOI] [PubMed] [Google Scholar]