Abstract
Evidence regarding the prevalence of expiratory flow limitation (EFL) during exercise and the ventilatory response to exercise in children born preterm is limited. This study aimed to determine the prevalence of EFL as well as contributing factors to EFL and the ventilatory response to exercise in preterm children with and without bronchopulmonary dysplasia (BPD).
Preterm children (≤32 weeks gestational age) aged 9–12 years with (n=64) and without (n=42) BPD and term controls (n=43), performed an incremental treadmill exercise test with exercise tidal flow–volume loops.
More preterm children with BPD (53%) had EFL compared with preterm children without BPD (26%) or term controls (28%) (p<0.05). The presence of EFL was independently associated with decreased forced expiratory volume in 1 s/forced vital capacity z-score and lower gestational age (p<0.05). There was no difference in peak oxygen uptake between preterm children with BPD and term controls (48.0 versus 48.4 mL·kg−1·min−1; p=0.063); however, children with BPD had a lower tidal volume at peak exercise (mean difference −27 mL·kg−1, 95% CI −49– −5; p<0.05). Children born preterm without BPD had ventilatory responses to exercise similar to term controls.
Expiratory flow limitation is more prevalent in children born preterm with BPD and is associated with airway obstruction and a lower gestational age.
Short abstract
Children born preterm have an increased prevalence of expiratory limitation during exercise associated with reduced lung function and lower gestational age http://ow.ly/jLsk30leOVI
Introduction
Preterm birth (<37 weeks gestational age) constitutes ∼11% of live births worldwide [1]. Children born preterm, and in particular those born very preterm (<32 weeks gestational age), are born with an immature respiratory system characterised by few to no alveoli, insufficient surfactant production and gas exchange, and are at increased risk of developing bronchopulmonary dysplasia (BPD) [2]. Advances in neonatal care, including the widespread introduction of exogenous surfactant and more gentle forms of mechanical ventilation, have increased survival of children born at lower gestational age and birth weight [3]. Contemporary neonatal lung disease, termed “new BPD”, is characterised by peripheral lung abnormalities, failed alveolarisation and abnormal pulmonary vascularisation [3], which contribute to long-term respiratory sequelae including airway obstruction [4–6], impaired gas transfer [7], gas trapping [7, 8] and increased respiratory morbidity [5, 6, 9] throughout childhood.
The exercise capacity of children born preterm during the surfactant era is unclear; with some [8, 10–12], but not all [13–15], studies reporting reduced peak exercise capacity. In addition, a limited number of studies report an altered ventilatory response to exercise, including reduced tidal volume (VT) and increased respiratory frequency [10, 12, 16, 17]. However, the mechanisms underlying altered ventilatory responses to exercise in children with BPD remain unknown. One factor potentially contributing to altered ventilatory responses during exercise may be expiratory flow limitation (EFL). EFL is associated with an impaired ventilatory response to exercise in asthma, cystic fibrosis and chronic obstructive pulmonary disease [18–21]. The single study describing EFL after preterm birth reports an increased prevalence of EFL in children born at <29 weeks gestational age [12]. However, this study did not assess the impact of EFL on exercise capacity or explore risk factors associated with the presence of EFL and thus the broader consequence of their finding is unclear.
We aimed to investigate the ventilatory response to a maximal exercise test in school aged children born very preterm with and without a neonatal diagnosis of BPD. We also aimed to determine the prevalence of EFL and assess any contribution from neonatal exposures on the prevalence of EFL in these children. We hypothesised that children with a neonatal diagnosis of BPD would exhibit an altered ventilatory response to maximal exercise characterised by EFL and dynamic hyperinflation. Further, we hypothesised that the magnitude of the altered ventilatory response would be related to the severity of neonatal lung disease.
Methods
Full methodological details are provided in the online supplementary material.
Participants
Children were recruited to the study if they were aged 9–12 years and either born preterm (≤32 weeks completed gestational age) or were healthy term-born children as previously described [6]. Preterm children were classified as having BPD if they required at least 28 days of supplemental oxygen before 36 weeks postmenstrual age as per international guidelines [22]. Written informed consent from parents and assent from the child were obtained prior to study enrolment. Ethics approval was obtained from the Princess Margaret Hospital for Children (Perth, Australia) Human Ethics Committee (approval 1760EP).
Pulmonary function testing
Spirometry and lung volume measurements by multiple breath nitrogen washout (Sensormedics Encore 21–1A; Sensormedics, Yorba Linda, CA, USA) were performed in accordance with international guidelines [23, 24], and are reported as predicted z-scores [25, 26].
Peak exercise test
Participants performed an incremental treadmill exercise test (Marquette; Sensormedics, Yorba Linda, CA, USA) in accordance with a modified Balke protocol [27, 28] The testing was performed at ambient conditions within a laboratory and all results are reports at BTPS (body temperature, ambient pressure, saturated with water vapour). Briefly, baseline observations were obtained over 5 min. Subsequently, children ran at a comfortable pace on a gradient of 0% for 2 min after which the gradient was increased to 4% and then by 2% increments every 2 min until volitional exhaustion. A peak exercise test was defined as peak heart rate >90% predicted and physical signs of peak performance (sweating, flushed face and inability to maintain running speed). Peak metabolic (oxygen uptake (V′O2), carbon dioxide production) and ventilatory data (VT and breathing frequency) were recorded continuously using breath-by-breath analysis (SensorMedics 229 Metabolic Cart; SensorMedics). Breathing reserve was calculated as maximum minute ventilation − maximum voluntary ventilation (MVV), expressed as a percentage of MVV; MVV was calculated as forced expiratory volume in 1 s (FEV1) × 40 [29].
Tidal flow–volume loops
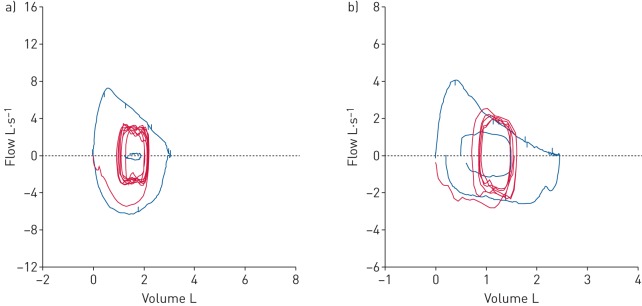
Tidal flow–volume loops were assessed as reported previously [30] and adapted by our group [31]. Briefly, 3–5 tidal breaths during exercise were recorded followed by a maximal inspiratory capacity (IC) manoeuvre to total lung capacity (TLC) which was recorded at the end of each exercise stage. Placement of the tidal flow–volume loop relative TLC was determined from the IC manoeuvre at the end of each exercise stage. Tidal flow–volume loops were set within the maximal flow–volume loop obtained during baseline spirometry based on IC. TLC was assumed to remain constant throughout the exercise. Dynamic flow limitation was determined if 5% or more of the tidal flow–volume loop tracked or exceeded the maximum flow–volume loop obtained prior to exercise (figure 1).
FIGURE 1.
Demonstration of the assessment of expiratory flow limitation (EFL). a) A subject with no EFL; b) a subject with EFL >5%.
Breathing strategy during exercise
End expiratory and inspiratory lung volume (EELV and EILV, respectively) were assessed at each stage as a measure of dynamic functional residual capacity (FRC) [31, 32] and expressed as a change from baseline (e.g. ΔEELV) and as a percentage of TLC (e.g. EELV%TLC). Full details are described in the online supplementary material.
Neonatal data and exercise symptoms
Neonatal variables including gestational age, days of supplemental oxygen and ventilatory support (mechanical ventilation and continuous positive airway pressure (CPAP)) were extracted from medical records and a prospectively maintained neonatal database. Parentally reported exercise symptoms within the preceding 3 months were recorded using a respiratory symptom questionnaire [33]. Children were classified as having current exercise-induced symptoms if parents reported cough, wheeze or shortness of breath on exertion, or symptoms that limited their child's physical activity within the preceding 3 months.
Statistical analysis
Data are reported as mean±sd for normally distributed data and median (interquartile range) for non-normally distributed data. Differences between groups were assessed by Mann–Whitney U-test or Kruskal–Wallis, as appropriate. Bonferroni correction was applied to account for possible type 1 errors due to multiple testing. Chi-squared analysis was used for differences in proportions between groups. This study was powered to detect a 25% difference in EFL to 80% power at a 0.05 significance level.
The relationships between neonatal factors, lung function and EFL were initially assessed using univariate regressions with EFL as a binary (yes/no) outcome. Factors with a significant univariate association (p<0.05) with the presence of EFL were included in subsequent stepwise binary logistic regressions. Multi-collinearities between the neonatal predictors were identified and adjusted for by using residuals of independent regressions of the collinear variables. For example, the independent impact of mechanical ventilation on EFL was determined from the residual of the regression between gestational age and mechanical ventilation.
The effect of lung development (gestational age and birth weight z-score), neonatal lung disease (days of supplemental oxygen, days of mechanical ventilation and days on CPAP), age, sex, height, weight and lung function (FEV1, forced vital capacity (FVC), TLC, residual volume and FRC z-scores) at the time of the exercise test were included in the logistic regression. Statistical analysis was performed using SPSS Version 22.0 (SPSS Inc., Chicago, IL, USA).
Results
Participants
221 (126 male) children were enrolled in the study including 99 with BPD, 64 without BPD and 58 healthy term-born controls. This cohort is representative of the broader preterm population in Perth during the same birth period, with no differences in neonatal characteristics of the recruited cohort, as reported by our group previously [6]. A valid maximal exercise test (n=171) was completed by 68 preterm children with BPD, 55 preterm children without BPD and 48 healthy term controls. Exercise tests (n=50) were determined invalid if there was a leak in the mask during testing (n=13), equipment malfunction (n=12), early termination of exercise (n=6), physical inability to perform exercise testing (n=13), poor baseline lung function (exercise testing deemed clinically inappropriate) (n=3) or consent not given (n=3). Of the 171 children completing a valid exercise test, 149 children successfully performed the flow–volume loop manoeuvres required for assessment of EFL (64 preterm children with BPD, 42 preterm children without BPD and 43 healthy term controls) and this population will form the basis of all analyses herein (figure 2). Table 1 shows the demographic details of the subjects who successfully completed the exercise test with matched flow–volume loops measurements.
FIGURE 2.
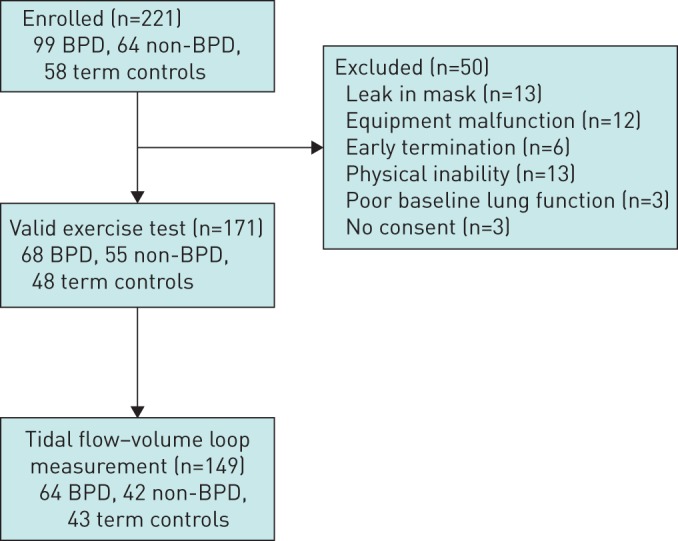
Flow diagram of enrolment for the study. BPD: bronchopulmonary dysplasia; non-BPD: preterm children without BPD.
TABLE 1.
Neonatal and demographic details of the study population
| BPD | Non-BPD | Term controls | |
| Subjects n | 64 | 42 | 43 |
| Male (%) | 42 (66%)#,¶ | 32 (76%)# | 18 (42%) |
| Gestational age (PMA) weeks | 26.0 (25–27.5)¶ | 30 (29.1–31.0) | |
| Birth weight g | 843 (709–993)¶ | 1420 (1238–1615) | |
| Birth weight z-score | −0.15±0.82 | −0.12±0.82 | |
| Mechanical ventilation days | 15.3 (4.4–32.8)¶ | 0.0 (0.0–1.0) | |
| CPAP days | 14.5 (6.6–24.0)¶ | 0.7 (0.0–3.6) | |
| Supplemental oxygen days | 86.5 (57.5–98.0)¶ | 1 (0.0–3.0) | |
| Recent exercise symptoms+ | 29 (46%) | 15 (38%) | |
| Doctor diagnosed asthma ever | 23 (36%)¶ | 17 (44%) | |
| Current asthma medication | 4 (6%)¶ | 10 (26%) | |
| Age at test years | 10.8±0.6 | 10.9±0.6 | 10.6±0.6 |
| Height at test cm | 141 (136–146)¶ | 142 (138–148) | 145.5 (138–153) |
| Weight at test kg | 32.4 (28.5–37.6)¶ | 36.5 (30.5–41.3) | 34.7 (30.0–42.2) |
Data are presented as mean±sd, median (interquartile range) or n (%), unless otherwise stated. Note the term controls did not have any neonatal intervention or respiratory symptoms. Not all children completed every question in the symptom questionnaire. BPD: bronchopulmonary dysplasia; non-BPD: preterm children without BPD; PMA: postmenstrual age; CPAP: continuous positive airway pressure. #: p<0.05 compared with healthy term controls; ¶: p<0.05 compared with non-BPD; +: exercise symptoms included parentally reported wheeze, cough and shortness of breath during exertion.
Children without a valid exercise test and matched flow–volume loop measurements had a lower FEV1 z-score (−0.78 versus −0.30) and had a higher prevalence of parentally reported exercise symptoms (65% versus 39%) (table E1). Children who performed acceptable exercise flow–volume loops had similar exercise outcomes as those who could not, although they had a lower peak VT (0.88 L versus 1.03 L) (table E1).
Table 2 shows the spirometry, lung volume and maximal exercise test results for the participants that performed a successful exercise test and flow–volume loop measurements. Preterm children with a neonatal diagnosis of BPD had a lower absolute V′O2 peak; however, V′O2 peak was not reduced when expressed relative to bodyweight (table 2). Children with BPD had a lower VT (mean difference= −27 mL·kg−1; (95% CI −49– −5); p<0.001) at peak exercise compared with the healthy term-born controls. Similarly, children with BPD had an increased respiratory rate (7 breaths·min−1 (95% CI 2–12); p<0.001), but minute ventilation and VT at peak exercise were not different from preterm children without BPD. Preterm children without BPD demonstrated no differences at peak exercise compared with term born controls (table 2).
TABLE 2.
Lung function and exercise variables for children who completed a successful maximal exercise test
| BPD | Non-BPD | Term controls | |
| Subjects n | 64 | 42 | 43 |
| FEV1 z-score | −0.83 (−1.57– −0.17)#,¶ | 0.09 (−0.91–0.37) | 0.04 (−0.57–0.61) |
| FVC z-score | −0.09 (−0.65–0.87)# | 0.37 (−0.25–0.97) | 0.24 (−0.65–0.86) |
| FEV1/FVC z-score | −1.35 (−2.59– −0.80)# | −0.87 (−1.52– −0.41)# | −0.42 (−1.06–0.48) |
| TLC z-score | −0.28 (−0.92–0.53) | 0.25 (−0.50–0.78) | −0.12 (−1.06–0.48) |
| FRC z-score | 0.29 (−0.64–0.87) | 0.71 (−0.19–1.91)# | −0.07 (−0.68–0.36) |
| RV z-score | −0.21 (−1.05–0.30) | 0.01 (−0.85–0.77) | −0.33 (−1.16–0.71) |
| V′O2 peak L·min−1 | 1.53 (1.40–1.76)#,¶ | 1.78 (1.49–1.95) | 1.69 (1.45–2.17) |
| V′O2 peak mL·kg−1·min−1 | 47.7 (42.8–53.2) | 46.1 (42.5–51.7) | 48.1 (45.5–52.4) |
| V′O2 at AT mL·kg−1·min−1 | 26.6 (13.9–47.2) | 31.0 (15.8–38.8) | 34.7 (13.6–45.3) |
| Peak RQ | 1.01 (0.98–1.02)# | 1.03 (1.01–1.06) | 1.04 (1.02–1.06) |
| Peak heart rate beats·min−1 | 196 (187–205) | 195 (185–202) | 200 (195–206) |
| Peak VT mL·kg−1 | 24 (21–27) | 27 (23–30) | 28 (24–31) |
| Peak fR breaths·min−1 | 64 (54–72)¶ | 54 (49–63) | 58 (54–68) |
| Peak V′E L·min−1·kg−1 | 1.53 (1.36–1.72) | 1.40 (1.30–1.68) | 1.55 (1.42–1.81) |
| Breathing reserve % | 34.0 (28.2–35.8) | 37.2 (27.0–38.3) | 34.4 (30.0–37.1) |
| EFL n (%) | 34 (53%)#,¶ | 11 (26%) | 12 (28%) |
| EFL%VT | 27.5 (0.0–60.0)#,¶ | 0.0 (0.0–26.5) | 0.0 (0.0–25.0) |
| ΔIC mL | 25 (−83–193) | 110 (−78–225) | 25 (−90–203) |
| ΔEELV mL | −30 (−145–200) | −50 (−180–170) | −15 (−175–137) |
| ΔEILV mL | 397 (146–557) | 371 (238–648) | 368 (157–723) |
| EELV %TLC rest | 32.0 (27.5–37.4) | 32.1 (28.0–35.1) | 30.1 (25.8–34.9) |
| EELV %TLC peak | 31.8 (28.4–35.9) | 31.9 (27.6–36.6) | 29.9 (27.0–33.7) |
Data are presented as median (interquartile range), unless otherwise stated. BPD: bronchopulmonary dysplasia; non-BPD: preterm children without BPD; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; TLC: total lung capacity; FRC: functional residual capacity; RV: residual volume; V′O2: oxygen uptake; AT: anaerobic threshold; RQ: respiratory quotient; VT: tidal volume; fR: respiratory frequency; V′E: minute ventilation; EFL%VT: percentage of tidal volume assessed as meeting or exceeding the maximum flow–volume loop; IC: inspiratory capacity; EELV: end expiratory lung volume; EILV: end inspiratory lung volume. #: p<0.05 compared with healthy term controls; ¶: p<0.05 compared with non-BPD.
Similarly, static lung volumes (TLC, FRC and residual volume) at rest and the change in EILV or EELV during exercise (expressed as either an absolute change or as a percentage of TLC), did not differ between preterm children with or without BPD and term-born controls (table 2). Children with BPD had a significantly lower FEV1 z-score compared with healthy controls (−0.98 (95% CI −1.5– −0.46); p<0.001) and non-BPD preterm children (−0.70 (95% CI −1.23– −0.17); p=0.005). The FEV1/FVC z-score of preterm children with (−1.06 (95% CI −1.61– −0.51); p<0.001) and without BPD (−0.71 (95% CI −1.31– −0.12); p=0.012) was lower compared with term-born controls.
Tidal flow–volume loop assessment
Approximately half of the children with BPD (53%) exhibited EFL during maximal exercise testing, which was significantly more prevalent than in the non-BPD and healthy term control groups (Chi-squared analysis p<0.01) (figure 3). The prevalence of EFL was not significantly different between the healthy term controls and the preterm children without BPD (26% and 28%, respectively).
FIGURE 3.
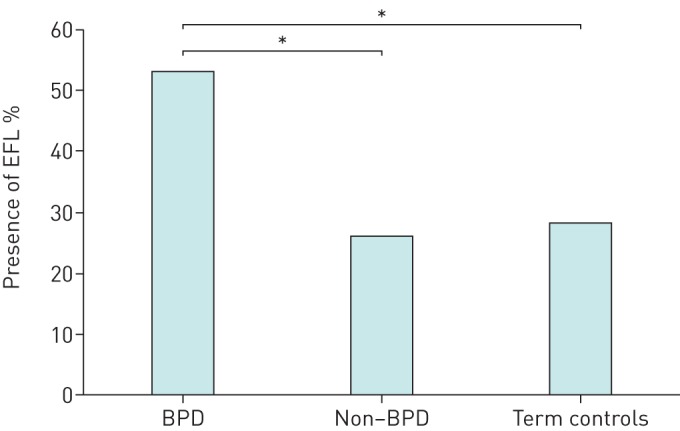
Prevalence of expiratory flow limitation (EFL). BPD: bronchopulmonary dysplasia; non-BPD: preterm children without BPD. *: p<0.05.
Differences in neonatal, spirometry and maximal exercise test outcomes in preterm children with and without EFL are presented in table 3. Preterm children with EFL had a significantly longer duration of supplemental oxygen and mechanical ventilation as well as a significantly worse baseline FEV1 and FEV1/FVC z-score compared with preterm children without EFL. VT, breathing frequency and breathing strategy at peak exercise and parentally reported frequency of symptoms during exercise were not significantly different between preterm children with EFL and those that did not experience EFL during exercise. Days of supplemental oxygen, FEV1 z-score and FEV1/FVC z-score were significantly associated with the presence of EFL on univariate analysis (table E2). FEV1/FVC z-score and days of supplemental oxygen after accounting for gestational age were subsequently included in the multivariate analysis; gestational age was also included to assess the impact of lung development. The impact of each of these in the multivariate model can be seen in table E3. Binary logistic regression analysis showed that a reduced FEV1/FVC z-score and lower gestational age were independent predictors of EFL developing during a maximal exercise test (table 4).
TABLE 3.
Differences between preterm participants with and without expiratory flow limitation (EFL)
| With EFL | Without EFL | |
| Subjects n | 45 | 61 |
| BPD | 34 (76%)* | 30 (49%) |
| Male | 28 (62%) | 43 (70%) |
| Gestation PMA weeks | 27.0 (25.0–29.2) | 28.6 (25.3–30.2) |
| Mechanical ventilation days | 5.0 (1.3–30.1) | 2.0 (0.0–13.5) |
| CPAP days | 6.5 (1.0–24.9) | 5.6 (0.6–15.7) |
| Supplemental oxygen days | 74.0 (28.0–94.5)* | 22.0 (1.0–83.5) |
| FEV1 z-score | −1.31±0.96* | −0.12±0.83 |
| FVC z-score | −0.40 (−0.57–0.78) | 0.22 (−0.51–1.13) |
| FEV1/FVC z score | −1.85±0.89* | −0.71±0.83 |
| V′O2 peak L·min−1 | 1.54 (1.40–1.78) | 1.63 (1.42–1.86) |
| V′O2 peak mL·kg·min−1 | 49.6 (43.2–52.3) | 47.5 (42.4–52.3) |
| Peak RQ | 1.03 (1.01–1.05) | 1.01 (0.99–1.02) |
| Maximum heart rate beats·min−1 | 197 (187–207) | 195 (186–202) |
| VT L·kg−1 | 25 (22–28) | 25 (22–28) |
| fR breaths·min−1 | 62 (53–70) | 60 (52–69) |
| Maximum V′E L·min−1·kg−1 | 1.55 (1.36–1.74) | 1.43 (1.30–1.67) |
| Breathing reserve % | 31.1 (26.4–35.6) | 35.5 (29.3–38.5) |
| Exercise symptoms | 21 (48%) | 23 (40%) |
| Docter diagnosed asthma ever | 23 (52%)* | 17 (29%) |
| Current asthma medication | 4 (9%) | 10 (17%)* |
Data are presented as mean±sd, median (interquartile range) or n (%), unless otherwise stated. Not all children completed every question in the symptom questionnaire. BPD: bronchopulmonary dysplasia; PMA: postmenstrual age; CPAP: continuous positive airway pressure; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; V′O2: oxygen uptake; RQ: respiratory quotient; VT: tidal volume; fR: respiratory frequency; V′E: minute ventilation. *: p<0.05.
TABLE 4.
Binary logistic regression for neonatal and spirometry variables, for presence of expiratory flow limitation
| Variable | Odds ratio (95% CI) | p-value | R2 |
| FEV1/FVC z-score | 0.184 (0.084–0.401)* | <0.001 | 0.477 |
| Gestational age (PMA) | 0.799 (0.640–0.997)* | 0.047 | |
| Supplemental oxygen days | 1.22 (0.674–2.200) | 0.514 |
FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; PMA: postmenstrual age. *: p<0.05.
Discussion
We investigated the impact of very preterm birth on the ventilatory response to exercise in school-aged children and determined the factors associated with presence of EFL in these children. We report that children with BPD have an altered breathing pattern during exercise, which is characterised by rapid, shallow breathing. Furthermore, we show that half of children born very preterm with BPD exhibit EFL during a maximal exercise test. We found that the presence of EFL was independently predicted by poorer lung function and a lower gestational age.
This study shows that preterm children with a neonatal classification of BPD have reduced minute ventilation due to a markedly decreased VT with an increased breathing frequency at peak exercise. These observations are consistent with a rapid and shallow breathing pattern and are in keeping with previous exercise studies in children born preterm [10, 12, 16, 17]. We hypothesised that this rapid and shallow breathing pattern would be associated with an increased prevalence of EFL. However, the absence of significant differences in VT, minute ventilation and breathing frequency between those with and without EFL during exercise suggests that EFL is not contributing to the altered breathing response to exercise.
Our study confirms that EFL is highly prevalent in children with BPD [12]. Our observations of the prevalence of EFL in preterm children with (53%) and without (26%) BPD are similar to the prevalence reported by MacLean et al. [12] (47% and 33% in children with and without BPD, respectively). Our study adds to the current literature in that we report this prevalence over a wider gestational age (up to 32 weeks gestational age compared with <29 weeks gestational age). Our data suggests that flow limitation during exercise is not limited to those children surviving extreme preterm birth. Selection of appropriate predicted values for lung function and exercise testing in a paediatric population is hampered by the lack of large normative datasets. We have previously confirmed that the Global Lung Function Initiative reference equations are valid for an Australian population [34]. While we cannot confirm that the predicted values for lung volumes are valid in our population, our inclusion of healthy term controls increases our ability to interpret our data and it is unlikely that the differences between groups reported here are associated with differences in group demographics.
The presence of EFL in adults with obstructive lung disease is often linked to an increase in operating lung volumes (EELV and EILV) and dynamic hyperinflation during exercise [18, 21]. However, the preterm children who developed EFL during this study (with reduced FEV1/FVC; table 4), did not show dynamic hyperinflation or changes in operating lung volumes. Preterm children with EFL were more likely to have had a doctor diagnosis of asthma in the past compared with those without; however, they were less likely to be currently using asthma medication. While the current use of asthma medication may blunt the ventilatory response to exercise, all children were assessed without the use of a short-acting bronchodilator medication prior to exercise. Given the lack of dynamic hyperinflation during exercise this suggests that current asthma may not be significantly contributing to the presence of EFL during peak exercise. We hypothesise the role of prematurity per se, rather than severity of neonatal lung disease or recent symptoms is the primary cause of EFL in children born very preterm. This is confirmed in the multivariate analysis demonstrating that the prevalence of EFL was significantly associated with lower gestational age and not markers of the severity of BPD, such as supplemental oxygen use (table 4).
In support of our hypothesis, Rideau Batista Novais et al. [17] identified that very low birth weight children had a rapid breathing pattern during exercise and reduced inspiratory muscle strength, suggesting that an increased inspiratory resistive load may lead to the early onset of inspiratory muscle fatigue. Inspiratory muscle load, inspiratory muscle fatigue and/or impaired contractile function of the respiratory muscles may result in the inability of preterm children to increase their operating lung volumes during maximal exercise rather than hyperinflation and hence to a higher prevalence of EFL. Furthermore, the damage associated with injurious tidal volumes during mechanical ventilation and altered peripheral lung development affects the mechanical properties of the lung [3]. Reduced pulmonary compliance increases elastic load on breathing. Increased load (work of breathing) that exceeds the ability of the respiratory muscles to generate sufficient force to increase the operating lung volumes when higher flows are required may result in EFL. In addition, the higher elastic work of increasing the lung volumes may not be tolerated in children born prematurely, preventing the maintenance of a higher operating lung volume. We reported a similar mechanism in obese children with an increased load on the chest wall and diaphragm resulting in a similar response to exercise to that seen in the preterm population, i.e. increased prevalence of EFL without any change in operating lung volumes [31].
Alternatively, the increased prevalence of EFL could be secondary to reduced lung and/or airway size. Children [30, 35] and women [36, 37] have increased EFL compared with adult males; both groups have a lower FEV1 and FEV1/FVC compared to adult males due to smaller lung volumes and airway size, a factor associated with increased prevalence and severity of EFL [36, 37]. However, as the preterm children with EFL did not exhibit differences in measured static lung volumes (FVC, TLC, FRC and residual volume; table 3 and table E2), it is unlikely that the EFL observed in this study is associated with inherent differences in lung size. Lung volumes in this study were assessed using multiple breath washout and it is known that this technique can underestimate actual FRC in the presence of significant airway obstruction leading to trapped gas. It is therefore feasible that our assessments of FRC (and hence TLC) may be underestimated, exploration of this mechanism using whole body plethysmography to measure static lung volumes may be of value. However, given that there were no changes in measures of dynamic lung volume (i.e. IC during exercise) we do not feel that this is a significant limitation to our study. The repeatability of EFL in the paediatric population has not been well described and is difficult to assess due to the need to repeat the exercise test. However, the measurement of EFL is dependent upon accurate measurement of IC and as we did not see any changes in the measurement of IC in this study we are confident this measurement is valid.
Children with BPD had an increased prevalence of EFL in the presence of a normal response to exercise and no differences in exercise symptoms; therefore, the clinical implications of this increased EFL are unclear. While we report no differences in exercise symptoms in children with EFL, we were unable to record rated perceived exertion scores or symptom scores at peak exercise as many children were unable to provide a clear answer. As such, we are unable to identify if there are differences in symptoms in children with EFL at peak exercise. We suggest that children born preterm may maintain normal physical activity by moderating their ventilatory response or work in shorter exercise bouts before EFL becomes clinically significant. Further investigation into the mechanisms driving EFL and the functional effects of EFL are necessary to fully identify its impact on exercise of different modes, intensities and durations, and the long-term impact of EFL as these children continue to grow.
Conclusion
We investigated the impact of EFL on aerobic capacity and the ventilatory response to a maximal exercise test in children born preterm with and without a neonatal diagnosis of BPD. We show that children born preterm have a higher prevalence of EFL than term-born controls, and that this was not associated with an altered ventilatory response to exercise. The prevalence of EFL in children born before 32 weeks gestation was associated with a lower gestational age and reduced lung function.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Online data supplement 00048-2018_Supplement (115.6KB, pdf)
Acknowledgements
Research participants and their families for giving up time for the study; the staff of the Department of Respiratory Medicine at Princess Margaret Hospital, Perth, Australia; Angela Jacques (School of Physiotherapy, Curtin University, Perth, Australia) and Max Bulsara (The University of Notre Dame, Fremantle, Australia) for statistical advice and assistance; and Maureen Verheggen (Princess Margaret Hospital for Children, Perth, Australia) for her assistance in recruiting the cohort.
Footnotes
This article has supplementary material available from openres.ersjournals.com
Author contributions: C.A O'Dea performed data measurement and interpretation, conducted the statistical analysis and drafted the initial versions of the manuscript. K. Logie and G.L. Banton performed data measurement and revision of the manuscript. S.J. Simpson performed data measurement, assisted with data analysis and interpretation, and revision of manuscript. A. Maiorana, A.C. Wilson, J.J. Pillow and G.L. Hall designed the study, obtained funding, had oversight of data collection and interpretation, and reviewed the manuscript.
Conflict of interest: K. Logie has nothing to disclose.
Conflict of interest: A. Maiorana has nothing to disclose.
Conflict of interest: G.L. Banton has nothing to disclose.
Conflict of interest: S.J. Simpson has nothing to disclose.
Conflict of interest: G.L. Hall has nothing to disclose.
Conflict of interest: C.A. O'Dea has nothing to disclose.
Conflict of interest: J.J. Pillow has nothing to disclose.
Conflict of interest: A.C. Wilson has nothing to disclose.
Support statement: Princess Margaret Hospital Foundation, Raine Medical Foundation and National Health and Medical Research Council (APP513730). G.L. Hall, S.J. Simpson and J.J. Pillow were supported by National Health and Medical Research Council Fellowships (APP1025550, APP1073301, APP1077691). Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Blencowe H, Cousens S, Chou D, et al. Born too soon: the global epidemiology of 15 million preterm births. Reprod Health 2013; 10: Suppl. 1, S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coalson JJ. Pathology of new bronchopulmonary dysplasia. Semin Neonatol 2003; 8: 73–81. [DOI] [PubMed] [Google Scholar]
- 3.Jobe AJ. The new BPD: an arrest of lung development. Pediatr Res 1999; 46: 641–643. [DOI] [PubMed] [Google Scholar]
- 4.Fawke J, Lum S, Kirkby J, et al. Lung function and respiratory symptoms at 11 years in children born extremely preterm: the EPICure study. Am J Respir Crit Care Med 2010; 182: 237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verheggen M, Wilson AC, Pillow JJ, et al. Respiratory function and symptoms in young preterm children in the contemporary era. Pediatr Pulmonol 2016; 51: 1347–1355. [DOI] [PubMed] [Google Scholar]
- 6.Simpson SJ, Logie KM, O'Dea CA, et al. Altered lung structure and function in mid-childhood survivors of very preterm birth. Thorax 2017; 72: 702–711. [DOI] [PubMed] [Google Scholar]
- 7.Korhonen P, Laitinen J, Hyödynmaa E, et al. Respiratory outcome in school-aged, very-low-birth-weight children in the surfactant era. Acta Paediatr 2004; 93: 316–321. [DOI] [PubMed] [Google Scholar]
- 8.Smith LJ, van Asperen PP, McKay KO, et al. Reduced exercise capacity in children born very preterm. Pediatrics 2008; 122: e287-93. [DOI] [PubMed] [Google Scholar]
- 9.Hennessy EM, Bracewell MA, Wood N, et al. Respiratory health in pre-school and school age children following extremely preterm birth. Arch Dis Child 2008; 93: 1037–1043. [DOI] [PubMed] [Google Scholar]
- 10.Welsh L, Kirkby J, Lum S, et al. The EPICure study: maximal exercise and physical activity in school children born extremely preterm. Thorax 2010; 65: 165–172. [DOI] [PubMed] [Google Scholar]
- 11.Burns YR, Danks M, O'Callaghan MJ, et al. Motor coordination difficulties and physical fitness of extremely-low-birthweight children. Dev Med Child Neurol 2009; 51: 136–142. [DOI] [PubMed] [Google Scholar]
- 12.MacLean JE, DeHaan K, Fuhr D, et al. Altered breathing mechanics and ventilatory response during exercise in children born extremely preterm. Thorax 2016; 71: 1012–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joshi S, Powell T, Watkins WJ, et al. Exercise-induced bronchoconstriction in school-aged children who had chronic lung disease in infancy. J Pediatr 2013; 162: 813–818. [DOI] [PubMed] [Google Scholar]
- 14.Clemm H, Roksund O, Thorsen E, et al. Aerobic capacity and exercise performance in young people born extremely preterm. Pediatrics 2012; 129: e97–e105. [DOI] [PubMed] [Google Scholar]
- 15.Kriemler S, Keller H, Saigal S, et al. Aerobic and lung performance in premature children with and without chronic lung disease of prematurity. Clin J Sport Med 2005; 15: 349–355. [DOI] [PubMed] [Google Scholar]
- 16.Kilbride HW, Gelatt MC, Sabath RJ. Pulmonary function and exercise capacity for ELBW survivors in preadolescence: effect of neonatal chronic lung disease. J Pediatr 2003; 143: 488–493. [DOI] [PubMed] [Google Scholar]
- 17.Rideau Batista Novais A, Matecki S, Jaussent A, et al. Hyperventilation during exercise in very low birth weight school-age children may implicate inspiratory muscle weakness. J Pediatr 2012; 160: 415–420. [DOI] [PubMed] [Google Scholar]
- 18.Regnis JA, Alison JA, Henke KG, et al. Changes in end-expiratory lung volume during exercise in cystic fibrosis relate to severity of lung disease. Am Rev Respir Dis 1991; 144: 507–512. [DOI] [PubMed] [Google Scholar]
- 19.Laveneziana P, Webb KA, Ora J, et al. Evolution of dyspnea during exercise in chronic obstructive pulmonary disease: impact of critical volume constraints. Am J Respir Crit Care Med 2011; 184: 1367–1373. [DOI] [PubMed] [Google Scholar]
- 20.Lougheed MD, Fisher T, O'Donnell DE. Dynamic hyperinflation during bronchoconstriction in asthma: implications for symptom perception. Chest 2006; 130: 1072–1081. [DOI] [PubMed] [Google Scholar]
- 21.O'Donnell DE, Laveneziana P. The clinical importance of dynamic lung hyperinflation in COPD. COPD 2006; 3: 219–232. [DOI] [PubMed] [Google Scholar]
- 22.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med 2001; 163: 1723–1729. [DOI] [PubMed] [Google Scholar]
- 23.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005; 26: 319–338. [DOI] [PubMed] [Google Scholar]
- 24.Wanger J, Clausen JL, Coates A, et al. Standardisation of the measurement of lung volumes. Eur Respir J 2005; 26: 511–522. [DOI] [PubMed] [Google Scholar]
- 25.Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J 2012; 40: 1324–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cook CD, Hamann JF. Relation of lung volumes to height in healthy persons between the ages of 5 and 38 years. J Pediatr 1961; 59: 710–714. [DOI] [PubMed] [Google Scholar]
- 27.Jones N. Clinical Exercise Testing. 8th edn Philadelphia, WB Saunders, 1988. [Google Scholar]
- 28.American Thoracic Society, American College of Chest Physicians. ATS/ACCP Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med 2003; 167: 211–277. [DOI] [PubMed] [Google Scholar]
- 29.Herdy AH, Ritt LE, Stein R, et al. Cardiopulmonary exercise test: background, applicability and interpretation. Arq Bras Cardiol 2016; 107: 467–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nourry C, Deruelle F, Fabre C, et al. Evidence of ventilatory constraints in healthy exercising prepubescent children. Pediatr Pulmonol 2006; 41: 133–140. [DOI] [PubMed] [Google Scholar]
- 31.Gibson N, Johnston K, Bear N, et al. Expiratory flow limitation and breathing strategies in overweight adolescents during submaximal exercise. Int J Obes (Lond) 2014; 38: 22–26. [DOI] [PubMed] [Google Scholar]
- 32.Johnson BD, Weisman IM, Zeballos RJ, et al. Emerging concepts in the evaluation of ventilatory limitation during exercise: the exercise tidal flow–volume loop. Chest 1999; 116: 488–503. [DOI] [PubMed] [Google Scholar]
- 33.Powell CV, McNamara P, Solis A, et al. A parent completed questionnaire to describe the patterns of wheezing and other respiratory symptoms in infants and preschool children. Arch Dis Child 2002; 87: 376–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hall GL, Thompson BR, Stanojevic S, et al. The Global Lung Initiative 2012 reference values reflect contemporary Australasian spirometry. Respirology 2012; 17: 1150–1151. [DOI] [PubMed] [Google Scholar]
- 35.Swain KE, Rosenkranz SK, Beckman B, et al. Expiratory flow limitation during exercise in prepubescent boys and girls: prevalence and implications. J Appl Physiol 2010; 108: 1267–1274. [DOI] [PubMed] [Google Scholar]
- 36.Dominelli PB, Guenette JA, Wilkie SS, et al. Determinants of expiratory flow limitation in healthy women during exercise. Med Sci Sports Exerc 2011; 43: 1666–1674. [DOI] [PubMed] [Google Scholar]
- 37.Smith JR, Rosenkranz SK, Harms CA. Dysanapsis ratio as a predictor for expiratory flow limitation. Respir Physiol Neurobiol 2014; 198: 25–31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Online data supplement 00048-2018_Supplement (115.6KB, pdf)