Abstract
Biomarker discovery and development for clinical research, diagnostics and therapy monitoring in clinical trials have advanced rapidly in key areas of medicine, notably oncology and cardiovascular diseases, allowing for rapid early detection and supporting the evolution of biomarker-guided precision medicine-based _targeted therapies.
In Alzheimer’s disease (AD), breakthroughs in biomarker identification and validation include the cerebrospinal fluid (CSF) and positron emission tomography (PET) markers of amyloid β (Aβ) and tau proteins, which are highly accurate in detecting the presence of pathophysiological and neuropathological changes of AD. However, their high cost, insufficient accessibility, or invasiveness may limit their use as viable first-line tools for detecting patterns of pathophysiology. Therefore, a multi-stage, tiered approach is needed, prioritizing development of an initial screen to exclude from these tests the high numbers of people with cognitive deficits who do not demonstrate evidence of underlying AD pathophysiology.
This perspective summarizes the efforts of a working group that aimed to survey the current landscape of blood-based AD biomarkers, and outlines operational steps for an effective academic-industry co-development and path forward from identification and assay development to validation for clinical use.
Introduction
Alzheimer’s disease
Alzheimer’s disease (AD) is a clinically and pathophysiologically heterogeneous complex neurodegenerative disease (ND). AD is the most common cause of age-related ND, impacting millions of individuals worldwide; currently, one out of nine people over the age of 65 are living with AD1 and the prevalence of AD is expected to grow exponentially over the next several decades1.
The pathogenesis of AD involves interacting pathophysiological cascades, including core events, i.e. accumulation of the 42-amino acid-long amyloid beta peptide (Aβ1–42) into amyloid plaques in the brain parenchyma and the formation of intraneuronal neurofibrillary tangles composed of hyperphosphorylated tau protein2. Emerging evidence stresses the existence of additional molecular pathophysiological pathways, such as axonal disintegration3, synaptic dysfunction and degeneration4, innate immune response and neuroinflammation5,6, vascular and cell membrane dysregulation7, and brain metabolic dysfunction8 – across the different stages of AD. Moreover, other proteinopathies and pathologies frequently co-exist in the ageing brain. These include TDP-43 or α-synuclein proteinopathies, non-AD tauopathies, vascular pathology, and hippocampal sclerosis9–12. For these reasons, establishing a definitive diagnosis and developing effective treatments of AD is complicated.
At present, the aetiology and pathogenesis of AD is the subject of ongoing research and debate. The amyloid cascade hypothesis proposes that the brain accumulation of aggregated forms of Aβ is the trigger and/or driver of the disease process13. However, recent studies raised questions about this hypothesis as the exclusive cause and/or intervening link between the pathophysiology of AD and its clinical phenotype. The notion that biochemical and cellular mechanisms generate complex cognitive alterations has renewed AD research, leading to replace the first descriptive studies with a molecular, mechanistic view. The exponential increase in knowledge on interacting pathogenic mechanisms in individuals suffering from AD holds promise for the development of future biomarker-guided _targeted therapies and prevention strategies14–17.
The potential impact of biomarkers on primary care and neurology for the detection and diagnosis of AD
Given the complex clinical phenomenology, clinical, neurological, and neuropsychological examinations are still an integral component of accurate late-stage detection of clinically symptomatic AD and other ND. However, waiting times for appointments with specialists in the U.S., U.K. and Ireland (and other countries) can be very long resulting in substantial and often critical delays for patients and providers18. Memory clinics or general neurology clinics in many countries receive a broad range of referrals covering many conditions and diseases, therefore the improved streamlining of referrals to specialty clinics can have a significant impact on health care utilization and costs18. As one example, recent U.S.-based legislation requires that elderly people aged 65 and older receive annual cognitive examinations as part of the Annual Wellness Visit (CMS.gov)19; however, older adults continue to be inadequately assessed for cognitive decline during primary care visits20. Given that the average duration of primary care visits for geriatric patients is 21 minutes21, this is perhaps not surprising. Additionally, cognitive examinations are regularly administered, scored, and interpreted incorrectly in primary care due to lack of training and expertise22,23 and there are significant differences between primary care and specialty care approaches to diagnosis, treatment, and social support24. Therefore, a process that aids primary care practitioners in deciding which patients should receive a referral to a memory clinic would be of substantial advantage to both specialists and general practitioners. Such a system would reduce the overall clinic and medical system burden by decreasing the numbers of unnecessary referrals and diagnostic procedures25,26. Biomarker-based diagnostics can greatly aid a multi-stage selection of patients into appropriate centres.
To date, the literature has focused on diagnostic biomarkers27,28 for specialty clinic settings with little attention to screening instruments required for broad-based implementation in primary care settings. Additionally, diagnostic paradigms continue to rely substantially on clinical symptoms, with only relatively recent research guidelines taking biomarkers of AD pathophysiology into account29,30. Up to the mid-2000s, diagnosis of AD had a “clinicopathological” basis, with no definitive positive diagnosis possible until post-mortem confirmation of the presence of Aβ plaques and neurofibrillary tangles, and clinical diagnosis being assigned after a patient had reached the late-stage syndromal dementia threshold, by exclusion of other aetiologies of dementia29. This concept of late-stage diagnosis of exclusion focusing on clinical phenotype showed profound limitations, given that clinical symptoms are by nature heterogeneous (including an increasing number of atypical clinical phenotypes) and vary regarding onset, progression, and sequence of events. They have considerable overlap with other central nervous system (CNS) proteinopathies causing dementia as well as with dementia due to cerebrovascular disease31. More recently, with the advent of reliable radiotracers with affinity for cerebral amyloid deposits32, and the availability of clinically well-validated assays for cerebrospinal fluid (CSF) detection of Aβ peptides and tau proteins3, there has been progress towards a “clinicobiological” diagnostic approach in which pathophysiological and topographic biomarkers are integrated into the diagnostic paradigm29 as adjuncts to core clinical criteria30. These biomarkers are clinically available in specialty clinic settings for some countries, providing the means for dementia specialty clinicians to confirm diagnosis with much higher certainty through the presence of characteristic features of AD pathophysiology, thereby allowing for a more conclusive aetiological diagnosis14,33,34. Nevertheless, use of these technologies is not yet the standard and they are restricted to specialty clinic settings. On the other hand, biomarkers are now being incorporated into the comprehensive clinical diagnostic process, which will result in improved overall accuracy in detection of pathophysiology. However, as was the case for more matured translational research areas of biomedicine, such as oncology and cardiovascular medicine, the field is in need of biomarker-based diagnostics that can accurately and reliably identify individuals at risk and patients as precise and early as possible in the disease process35–39, preferentially in primary care settings. The availability of these biomarker-based diagnostics (e.g. CSF Aβ1–42 and tau) is also expected to open the window for precision medicine and allow a shift away from the traditional “one-size-fits-all” approach with “magic bullet” drugs in neuroscience to the development of biomarker-guided _targeted therapies25,40–43.
Facilitation of the precision medicine paradigm in ND such as AD requires an array of converging advanced analysis methods. Through systems theory, it is possible to conceptualize original and innovative models to explicate all systems levels – assessed via systems biology and systems neurophysiology42 – and different data dimensions in space and time of the non-linear, dynamic, and chronically progressive nature of the clinically and biologically heterogeneous construct of polygenic AD35,44, historically developed after the clinical description of the first patients and the associated brain histopathological remarks45.
The agnostic, hypothesis-free systems theory approach seems appropriate to explain the complex and heterogeneous origin and time course of the pathophysiological failure underlying the different forms of AD35. For multifactorial diseases, comprehensive holistic systems-level approaches are required; this is the case of the systems biology model aiming at understanding the genotype-phenotype relationships and the mechanisms at the level of the genome/epigenome, transcriptome, microRNome, proteome/peptidome, metabolome/lipidome, microbiome, and environmental factors participating in complex cellular networks35,44,46. Longitudinal investigations using systems biology-based strategies help characterize the complex molecular pathophysiology of the subforms of AD, all evolving through the convergence of alterations of homeostasis and/or failures in many systems, networks, signalling pathways, or pathophysiological processes44.
One of the key objectives of precision medicine is to bring new models for prevention, early detection, differential diagnosis, and treatment of AD and other ND, according to individual biological differences reflected by multimodal biomarkers33,35,37. Therefore, following the advanced models of oncology and cardiovascular medicine, innovative biomarker studies are expected to detect specific diagnostic, prognostic, and predictive biomarker signatures to adapt the therapy to individual patients40. As reported by the Institute of Medicine (IOM) Committee Recommendations for Advancing Appropriate Use of Biomarker Tests (companion diagnostics) for Molecularly _targeted Therapies, the ultimate goal of precision medicine is to enhance both clinical outcomes and the quality of patient care47.
Precision medicine is being used in AD and in a growing number of ND as a result of: 1) the development of large-scale biological databases and 2) the evolution of advanced high-throughput “omics” sciences. Novel methods developed within the “omics” disciplines have been successfully applied in the more advanced cancer area and are expected to transfertilize to AD and other ND. For instance, validated microarray expression profiling and current RNAseq technologies allow identifying differential expression of whole genomes in any specific sample at any given time point. Numerous reports exploiting these transcriptomics methods to recognize biomarkers in specific cancer subtypes are present48. The participation of microRNAs (miRNAs) in crucial cellular processes including cell death and their role in negative control of the expression of various oncoproteins make them interesting candidate biomarkers for cancer. Cancer-specific markers are detected in the blood from the earlier stages of tumor development. Since their concentration increases as tumor advances over time, they are useful dynamic indicators of tumor growth49. In proteomics, innovative methods of immuno-PCR, based on conjugations of specific antibodies and nucleic acids and exploiting ultrasensitive PCR signal amplification, can result into a 100 to 10,000-fold increase in sensitivity compared with the analogous enzyme-amplified immunoassays, hence considerably increasing the sensitivity of protein biomarker detection50,51. Powerful computational and integrative network biology tools are needed to assimilate the large multimodal information generated by the “omics” exploratory analyses and to comprehensively understand the decompensations at systems level52. An advantage in the AD field is that many of the pathologies are well known. Hence hypothesis-free systems theory approaches may be advanced in synergy with the refinement of methods that directly reflect core pathologies, much like what has recently happened in the field of blood-based direct, Aβ-related biomarkers for amyloid pathology53–55 and blood biomarkers for axonal degeneration56,57.
Why are blood-based biomarkers needed for AD?
While advancements in positron emission tomography (PET) and CSF biomarker analyses have the potential to improve accuracy within the diagnostic process, these methods show limitations precluding their use as first-line diagnostic tools. These limitations would be countered by use of blood-based biomarkers28. If a sufficiently accurate and standardized blood test can be developed, it is highly likely to be more cost effective than PET scans, the substantially high cost of which limits accessibility, generalizability and availability58,59, particularly outside the U.S.A. Because of this high cost, PET testing in AD will likely only become reimbursed as an adjunct to other less expensive tests, as was previously the case with PET scans for cancer. In addition, blood testing is less invasive than CSF testing, which requires lumbar puncture60,61. Furthermore, blood testing is already a well-established part of clinical routines globally, requiring no further introduction and training for health care professionals (HCPs), and can be easily performed in a variety of relevant settings (including primary care, in community-based medicine centres or even in a patient’s home) and at repeated intervals62. Blood sample handling infrastructure is also well established, allowing for the shipping of samples during the initial development stages. Therefore, blood-based screening biomarkers for AD can meet the scalability needs required for primary care settings and even for the broad population-based screening approach that may evolve with the advancing innovative precision medicine framework. Finally, the use of blood-based biomarkers offers the potential for testing of a huge range of comprehensive exploratory and candidate pathophysiological biomarkers, reflecting the full spectrum of disease triggering and driving molecular mechanisms underlying polygenic AD, beyond the conventional amyloid- and tau-based tests.
Blood-based biomarkers are an ideal choice as the first-step of the multi-stage diagnostic process beginning in primary care settings, and provide the means to determine which individuals or patients should receive referral to assessment by specialists, including diagnostic CSF analysis, magnetic resonance imaging (MRI) or amyloid PET diagnostics26,63, which is analogous to the existing process for many other conditions. In addition to meeting the significant clinical need, the availability of these tools would provide a viable path towards regulatory64 and reimbursement approval, using the cancer paradigm as a model.
Physiological challenges of developing blood-based biomarkers for AD
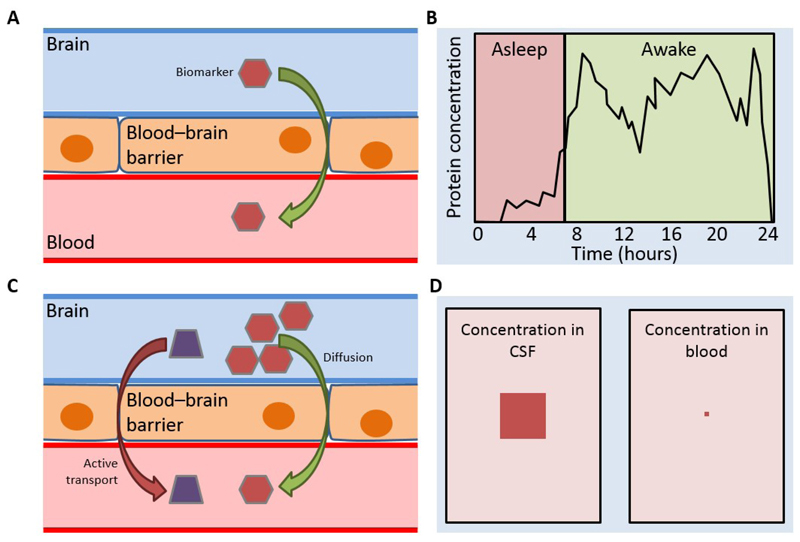
Identifying potential blood-based biomarkers for CNS diseases offers several challenges (Figure 1). First of all, the vast complexity of blood, which contains both cells and extracellular fluid, needs to be considered. Blood includes a range of different molecules (proteins, peptides, nucleic acids, lipids, metabolites) that can be detected in plasma, exosomes, and cellular compartments. The latter includes erythrocytes, leucocytes, and platelets, isolated into distinct cell subsets via flow cytometry or via buffy coat after density gradient centrifugation. Given the presence of different unique cellular compartments, each one is a potential source of biomarkers and may introduce variability to analyses62,65. The diversity of blood candidate biomarkers is relevant and includes: 1) protein concentrations/activity/isoforms and post-translational modifications; 2) metabolic products, such as amino acids, carbohydrates, lipids, organic acids; 3) nucleic acids. In the latter, single nucleotide polymorphisms (SNPs) – DNA sequence variations occurring when a single nucleotide in the genome differs between members of a species – can act as biological markers, helping localize genes associated with the disease66. When SNPs occur within a gene or in a regulatory region adjacent to a gene, they may affect the gene’s function, thus playing a key role in disease development. The growing application of high-throughput next-generation DNA sequencing technologies is playing a substantial role in screening whole genomes and, consequently, in identifying novel genetic variants affecting the risk of AD67,68. The 1000 Genomes Project69,70, supported by the U.S. National Human Genome Research Institute consortium, made significant progresses toward this aim.
Figure 1. Challenges in developing a blood-based biomarker of CNS disease.
There are several considerations when finding a biomarker for a CNS disease, particularly if the biomarker is to be blood-based.
Blood–brain barrier: biomarker must be able to cross the blood–brain barrier to allow detection (A). Diurnal differences in CSF and blood: diurnal differences in protein concentrations exist in both CSF and blood. Biomarker levels may not peak at the same time in CSF as in blood, and a biomarker assay will either require sampling at the peak concentration, or sufficient sensitivity to detect the biomarker throughout the day (B). Active/passive transport: biomarkers may originate in either CSF or blood. Those originating in CSF may enter the blood via active or passive transport, and understanding the exact nature of the derivation from CSF will be essential to develop an assay (C). Concentration differences: biomarker levels are not always similar in CSF and blood. For example, Aβ concentrations are 10-fold lower in plasma than in CSF (35 pg/ml vs. 350 pg/ml) (D).
Abbreviations: Aβ=amyloid beta; CNS=central nervous system; CSF=cerebrospinal fluid.
Secondly, the CNS is an effectively contained environment, and potential biomarkers may be present at very low concentrations in blood once they have crossed the blood–brain barrier (if they cross it at all as intact molecules)59,71. Indeed, there is minimal evidence of the peripheral effects of AD72, although there is some evidence that the blood–brain barrier may be increasingly compromised in normal aging and with increased AD progression73,74. Also, physiological mechanisms occurring peripherally may hamper the clinical utility of blood-based AD biomarkers, e.g. acute-phase or inflammatory proteins and small molecules, and metabolites present also in peripheral organs59. In particular, possible confounders include: significant biomarker dilution, considering the modest volume of the CSF and the extensive volume of the blood and extracellular fluid75; degradation in the liver or directly in the blood by proteases; matrix effects caused by adherence to plasma proteins or even blood cells; and excretion from the kidneys. These factors may substantially lower the concentrations of the biomarker, as well as decrease the time window for testing. A further major issue is the frequent existence of overlapping ND and co-morbidities in patients with AD, including cardiovascular, respiratory, hepatic, renal, and rheumatic disease, all of which may affect protein profiles in plasma59. On the other hand, since AD is a complex polygenic disease, amyloid and/or tau accumulation never occur in isolation of other relevant molecular/cellular pathophysiological mechanisms and brain systems failures in disease models. Elucidating this true complexity and heterogeneity, including through blood-based biomarker analyses, may substantially aid in a more comprehensive understanding of the disease for the generation of the precision medicine paradigm25,40,44.
Gathering consensus on blood-based biomarker development in AD
Given the strong rationale yet current challenges for the development of a blood-based biomarker for AD, an international, interdisciplinary expert working group was convened in March 2016 to discuss the ideal development process. The meeting was supported by Roche Diagnostics International. The working group was selected based on profiling of global experts and working groups that was done within the extensive biomarker literature search. Experts were selected based on their publications and involvement in research in the field of neurodegenerative biomarkers in CSF and in blood. The nature of support of Roche Diagnostics International was the assistance of organizing of scientific meetings, video, and teleconferences based on the scientific chair guidance and the support of the scientific writing agency. The meeting room as well as refreshments according to the guidance of the compliance guidelines were provided.
Process of the working group
Prior to the working group meeting, an in-depth comprehensive review of the literature was conducted (including “grey literature” – patents, press releases, and proprietary databases) to identify candidate biomarkers (see “Current landscape” below for further details). A structured and detailed pre-work survey of the meeting expert attendees provided additional material for analysis of the current blood-based biomarker landscape in AD and the most pressing challenges for the successful development of further markers. The aim of the survey was to identify and rank candidate blood-based biomarkers, which were selected from the landscape analysis, in order of priority for further development.
The working group itself provided a forum for definition of an ideal _target product profile for a blood-based biomarker in AD. A critical appraisal of the selected biomarkers was then performed to determine if they met this ideal profile, and to assess the general quality of research findings and their suitability for further development. Further considerations were the optimal validation process for a biomarker, the best route to determine the efficacy of a biomarker (predictive values versus accuracy) and the ideal cohorts in which biomarkers could be evaluated. In-depth discussions were also held on several candidate biomarkers, with the aim of defining key questions critical for evaluating any potential current or future candidate.
Current landscape
A landscape analysis was conducted to review all blood-based biomarkers in development for AD globally, and then assess these biomarkers on the basis of pre-defined selection criteria in order to prioritize those with the greatest likelihood of successful implementation. The data were sourced from publications (MEDLINE® database; 2011–2015), patents (worldwide patents, including PCT, USA and China; 2008–2015); press releases and conference abstracts from eight major neuroscience or AD conferences; and ChinaBio® proprietary databases.
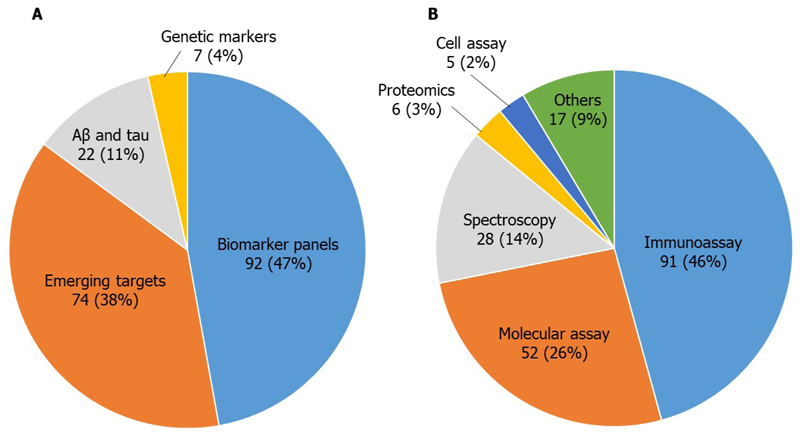
After screening more than 28,000 reports, 1,404 studies of blood-based biomarkers for AD were identified, 1,039 of which were from publications and conference abstracts. The 1,039 studies were categorized according to biomarker type; 18% of the studies were on the conventional AD biomarkers, Aβ and tau (or their associated peptides/proteins or variants), 19% were on genetic markers, 29% were on biomarker panels, and 34% were on markers related to emerging mechanisms such as inflammation, immune response, oxidative stress, DNA damage, mitochondrial dysfunction, and neuronal or microvascular injury. The identified biomarkers were then screened regarding intended use, asset type, technology platform, test type and analyte, and whether they included human/clinical data. A final screening was performed to focus only on high-quality candidates, according to novelty, cohort quality, presence and quality of validation study, diagnostic performance, and perceived quality of research. The final list contained 196 candidate biomarkers, with biomarker panels and emerging _targets being the most common categories (Figure 2A). Immunoassays and molecular assays were the most popular technology platforms utilized for high-quality AD blood-based biomarkers, accounting for more than 70% of studies (Figure 2B). A potential limitation of the search was the relatively narrow date range, which may have resulted in the exclusion of some potentially useful biomarkers that were published outside of this date range. The most promising biomarker candidates and the key studies, including those published since the original analysis, are summarized below.
Figure 2. Candidate blood-based biomarkers identified in the landscape analysis.
Technology (A) and platform (B) categories for the 196 high-quality studies identified in the landscape analysis.
Among the conventional AD biomarkers, the ratio of Aβ1–42/Aβ1–40 has shown potential as a screening/diagnostic marker in several studies. Most early studies on plasma Aβ1–42, Aβ1–40, and Aβ1–42/Aβ1–40 using enzyme-linked immunosorbent assay (ELISA) methods, found no or minor differences between AD and control groups76–78. More recently, a large cohort study using a novel ultrasensitive immunoassay technique (single molecule array (Simoa)), showed weak but significant correlations between plasma and CSF levels of Aβ1–42, Aβ1–40, and Aβ1–42/Aβ1–4055. In addition, plasma levels of Aβ1–42, Aβ1–40, and Aβ1–42/Aβ1–40 were lower in AD patients compared with cognitively healthy, mild cognitive impairment (MCI) and subjective cognitive decline (SCD) subjects55. An alternative technique for measuring Aβ peptides in plasma involves immunoprecipitation and mass spectrometry54,79. A pilot study found a trend for a reduction in both plasma Aβ1–42 and Aβ1–42/Aβ1–40 in AD compared with controls79. A separate study, using a modified method involving proteolytic digestion of Aβ peptides before mass spectrometry, found that plasma levels of Aβ1–42 and Aβ1–42/Aβ1–40 were reduced in amyloid PET-positive subjects54. Furthermore, plasma Aβ1–42/Aβ1–40 had a good diagnostic accuracy, as indicated by a receiver operating characteristic (ROC) area under the curve (AUC) value of 0.8865. A recent study further supports the use of immunoprecipitation–mass spectrometry methods to measure plasma Aβ peptides53. Nakamura and colleagues reported high performance of the ratios of plasma Aβ1–40/Aβ1–42 and amyloid precursor protein (APP)669–711/Aβ1–42, and their composite, for the prediction of amyloid PET burden53.
Results in the field have been mixed as Lewczuk and colleagues did not demonstrate plasma Aβ1–40/Aβ1–42 to be a useful diagnostic in a multi-center study80. Prior work also suggests that plasma amyloid markers may be impacted by cardiovascular and cerebrovascular factors81,82 and may impact the diagnostic and predictive of these markers83. Therefore, while promising, additional work is needed to determine if plasma Aβ has utility as a screening tool for brain amyloidosis and AD, including large clinical and prospective cohorts as well as direct comparisons of different pre-analytic and analytic methodologies since immunoprecipitation–mass spectrometry could be difficult to generalize for clinical use84.
Plasma levels of tau protein have also been successfully quantified using novel, highly sensitive immunoassay techniques, and different technologies have shown plasma tau to be increased in AD compared with controls85,86. Plasma tau levels showed a strong association with AD in a meta-analysis (average ratio of levels in AD versus controls: 1.95, 95% confidence interval 1.12–3.38, p=0.02)3. A study based on the large Alzheimer’s Disease Neuroimaging Initiative (ADNI) and Biomarkers For Identifying Neurodegenerative Disorders Early and Reliably (BioFINDER) cohorts confirmed an increase in plasma tau in AD dementia, but with a substantial overlap in levels with cogitively unimpaired elderly subjects87. Interestingly, longitudinal evaluations showed correlations between high baseline levels of plasma tau levels and future cognitive decline, increased atrophy rates (measured by MRI) and hypometabolism (measured by 18F-fluorodeoxyglucose-PET (18F-FDG-PET))87. Thus, current data suggest a minor increase in plasma tau in AD, although with too large overlap with controls to be diagnostically useful. In a recent study, elevated plasma tau levels were associated with cognitive decline, independent of elevated brain Aβ88, suggesting potential use as a non-disease specific screening tool.
Among the emerging _targets category is the axonal protein, neurofilament light (NF-L), which can be quantified using the ultrasensitive Simoa technique89. Serum NF-L levels are highly correlated with CSF levels, suggesting that blood measurements reflect brain pathophysiology90. A recent study on the ADNI cohort found a marked increase in plasma NF-L in AD cases with a ROC AUC value of 0.8757, which is comparable to the plasma Aβ1–42/Aβ1–40 ratio results reviewed above. Plasma NF-L was highest in MCI cases with positive amyloid PET scans, and predicted faster cognitive deterioration, higher rate of both future brain atrophy (measured by MRI) and hypometabolism (measured by 18F-FDG-PET)57. Interestingly, a study on familial AD (FAD) showed that blood NF-L was increased not only in symptomatic FAD cases, but also in pre-symptomatic mutation carriers with levels correlating with expected estimated year of symptom onset as well as cognitive and MRI measures of disease stage56. These findings suggest that plasma NF-L detects neurodegeneration in the preclinical phase of AD. However, high plasma (or CSF) NF-L is not specific for AD, but found in several neurodegenerative disorders such as frontotemporal dementia, progressive supranuclear palsy and corticobasal syndrome91–93. Thus, plasma NF-L might have an application as a screening test for neurodegeneration in the initial primary care evaluation of patients with cognitive disturbances.
Also in the emerging _targets category is β-site amyloid precursor protein cleaving enzyme 1 (BACE1), the β-secretase responsible for the first cleavage step required for the generation of Aβ peptides from APP94. Studies using ELISA-based methods have shown increased BACE1 activity in the plasma of AD patients compared with controls95,96. A recent study found that plasma BACE1 activity was increased in both MCI subjects and AD patients compared with healthy controls, and significantly higher in MCI subjects who progressed to AD (over 3 years’ follow-up) than in cognitively stable MCI subjects who did not progress96. These results suggest that plasma BACE1 activity has potential as a biomarker to predict progression from MCI to AD dementia, which could be valuable in the primary care and clinical trials settings; however, further studies are needed to validate these findings.
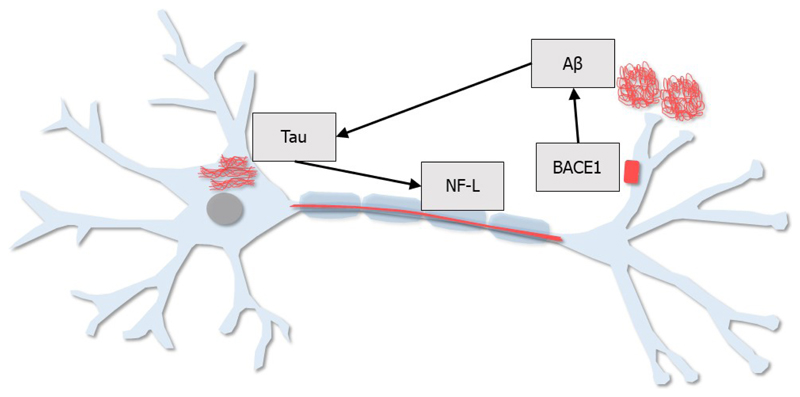
These four candidate blood-based biomarkers (Aβ1–42/Aβ1–40, tau, NF-L, and BACE1) and their roles in AD pathogenesis are depicted in Figure 3.
Figure 3. Schematic representation of promising blood-based biomarker candidates.
Four promising blood-based biomarker candidates are represented in this schematic:
Aβ: Aβ peptides, in particular Aβ1–42, are implicated in AD pathogenesis; however, it is the ratio of Aβ1–42/Aβ1–40 that appears to be the most promising Aβ-related biomarker in the blood.
BACE1: The first step in the generation of Aβ peptides is the cleavage of APP by the β-secretase, BACE1. Measurement of BACE1 activity in the blood may be useful for predicting progression from MCI to AD dementia.
Tau: Phosphorylated tau protein is a major component of intraneuronal neurofibrillary tangles, which are often present in AD. The abnormal phosphorylation of tau is thought to be driven by Aβ peptides. Tau levels in the blood may be useful as a predictor of future cognitive decline.
NF-L: NF-L is an axonal protein, released into the brain interstitial fluid following neuronal/axonal injury. NF-L levels in the blood are elevated in AD and other neurodegenerative diseases, therefore, blood-based NF-L could be useful as a biomarker of neurodegeneration.
Abbreviations: Aβ=amyloid beta; AD=Alzheimer’s disease; APP=amyloid precursor protein; BACE1=β-site amyloid precursor protein cleaving enzyme 1; MCI=mild cognitive impairment; NF-L=neurofilament light.
Blood-based biomarker panels are another area of interest for AD as a combination of markers might show better separation between groups than single biomarkers. In recent years, several protein panels have shown diagnostic or prognostic potential63,97–99, including a 21-protein panel for AD screening, which has demonstrated a positive predictive value (PPV) of 0.85 and a negative predictive value (NPV) of 0.94 in a preliminary validation63. Non-protein analytes such as amino acid100, miRNA101,102, and lipid panels103 have also shown promise but independent, large-scale validation studies are needed.
The road to the clinic
Historical difficulties with development
Assay reliability, and robust replication and validation of initial results are key issues for blood-based biomarker development26,71,104,105. Historically, an important limitation has also been the analytical sensitivity of available technologies; standard immunochemical methods have often not been sensitive enough to allow for the reliable quantification of CNS-derived molecules in the blood, a situation that has changed with the advent of ultrasensitive assays106. There is considerable variability between studies owing to inconsistencies in clinical cohorts (e.g. diagnostic evaluations, stage of disease), sample availability, difficulties in standardizing the samples themselves, and pre-analytical and analytical differences. Similar to CSF assays, consistent use of automated assays between studies may ameliorate analytical differences. However, the lack of standardization of pre-analytical protocols is also a major issue. For example, it is known that the majority of errors in proteomic analysis arise in the pre-analytical phase65,107. Pre-analytical variables can be divided into several main categories (Table 1)71,107. Initial steps towards standardization have already been made107, with the formation of a professional interest area focused on Biofluid Based Biomarkers (BBB-PIA) (in association with the Alzheimer’s Association’s International Society to Advance Alzheimer's Research and Treatment [ISTAART]). This group will drive towards consensus on harmonization of pre-analytical and analytical protocols, and will lead the development of a repository of clinical reference samples to enable assay harmonization and clinical performance assessment. However, further action is needed and communication of these issues to wider audiences is also warranted.
Table 1. Pre-analytical factors influencing results of blood-based biomarker measurements.
| Patient-related factors | Blood collection | Blood processing | Sample storage |
|---|---|---|---|
| Demographics (age, sex, weight, ethnicity) | Needle gauge | Time to storage | Storage temperature |
| Diet, including supplements | Needle composition | Centrifugation (presence and type of separator, temperature, number of rounds [single or double]) | Storage volume |
| Health status | Withdrawal site | Addition of protease inhibitor | Number of freeze-thaw cycles |
| Medication | Collection tube characteristics | Use of denaturation step or protein extraction | Duration |
| Drug/alcohol use | Anticoagulant use | Use of plasma or serum | |
| Medical conditions | Time of collection | ||
| Exercise | |||
| Posture/bed rest |
The experimental design of a biomarker evaluation study (including obtaining relevant cohorts with appropriate sample availability) will be important, as this has proved difficult in previous studies. Whilst many cohorts exist and potentially have samples suitable for use to develop a blood-based biomarker, these cohorts may not match the patient profile encountered in the _target setting. Finally, any candidate biomarker will need to offer excellent scalability to allow for large-scale use.
Ideal characteristics of a blood-based biomarker
The first steps in development of a blood-based biomarker for AD diagnosis should be to: a) finalize the specific context of use (COU) for regulatory purposes; and b) determine the _target product profile, which allows for _targeted development of the biomarker to match specific requirements for the intended COU. This prospective approach may enhance success compared with current approaches based on a discovery model, where biomarkers are first identified in case-control studies before being retrospectively adapted to an intended COU. In cases where a COU is prospectively defined, the original case-control study may not be fit-for-purpose to adequately test the COU. During the working group meeting, the consensus was that the COU for a blood-based biomarker in AD should be as a tool to assess patients reporting cognitive deficits in the primary care setting, allowing for identification of the subset of patients demonstrating biological signs consistent with AD who require further specialist diagnostic testing. Ideally, the biomarker could also be used to rule out other ND with symptoms similar and/or overlapping to those of AD. Validation against current clinical and biomarker-based diagnostic paradigms would also be required (ideally the IWG-2 diagnostic criteria29: evidence of cognitive impairment in conjunction with amyloid PET or the CSF “AD signature” [reduced Aβ and elevated tau CSF concentrations]). Alternatively, it may be possible to employ the recently proposed biomarker-guided A/T/N descriptive classification system108 in the validation stage.
The predictive accuracy requirements of a screening tool (PPV and NPV) are dependent on COU, in addition to the to the follow-up diagnostic resources, treatments and associated costs. A primary care-based screening tool would not be intended as a “diagnostic”, but would serve as a gatekeeper to the confirmatory diagnostic procedures utilized by specialty physicians. As is the case with all primary care screening tools, the primary concern is with high NPV. This is due to the base rates of the conditions in the general population (which are typically very low) and, therefore, the screening tool serves to preclude the vast majority of patients who are not in need of more invasive and expensive diagnostic procedures. For example, in mammography screening for breast cancer and in depression screening in primary care, PPVs are <30%63, but are counteracted by excellent NPVs (>90%). In primary care settings, it is commonplace to see tools with PPVs <40% (or even <20%) but NPVs >90% (or even >95%)63. The overall diagnostic accuracy of the primary care screening tool must be taken into account alongside the full diagnostic procedures in specialty clinics where available that include CSF and/or PET biomarkers (which have excellent PPV) for confirmatory diagnostic purposes. Once disease-modifying therapeutics become available (for prevention or treatment of symptomatic patients109), these primary care screening tools become required as there will be many more patients seeking services. Therefore, the primary care screen will be needed to cost-contain while simultaneously broadly opening up access to specialty diagnostics. The consensus in the working group was that a candidate biomarker for primary care screening should ideally have a PPV of >50%, with an aim for this to be as high as possible. However, given the ethical implications of false negatives, it would be more important to achieve a NPV of ≥90%, with 95–98% being an ideal _target63. On the other hand, a performance with a high PPV and an acceptable NPV may be desirable in a pharmaceutical setting that focuses on identifying as many amyloid-positive patients as possible for either inclusion in to clinical trials or a possible future treatment.
In terms of assay technology, the consensus indicated a strong preference for panel-based assays (i.e. a combination of biomarkers, either developed together or individually) over single biomarkers. Panels may offer wider applications beyond AD diagnosis, allowing more generalized testing for other ND, with each pathological condition having a unique protein signature. Several studies established panels of biomarkers to discriminate between cognitively healthy controls and AD participants and assessed large arrays of differently combined proteins to yield high specificity and sensitivity. Indeed, since there is a definite need for a holistic approach to standardize blood biomarkers for AD diagnosis, it is crucial to understand the link among various individual biomarkers and overcome the outdated approach of examining single candidate biomarkers at a time110. Given that various methods exist to detect biomarkers in blood28,110–112, it is crucial to standardize the technologies generating complex multidimensional data and the whole workflow. General consensus on both protocols and ultrasensitive analytical methods are needed across multicentre studies to set up a standardized panel of biomarkers for AD diagnosis110,111. In this regard, recent technical advances have provided ultrasensitive immunoassay methods that allow detecting and quantifying biomarkers at concentrations that are several-fold lower than those accessible by existing immunoassays. This may result in the development of a new array of blood biomarkers covering the entire spectrum of AD molecular pathophysiological mechanisms. Moreover, these technologies may serve as the foundation enabling the diagnosis of AD and other ND earlier than ever before113.
However, the number of biomarkers in the various types of panels so far developed increases the complexity of manufacture and commercial development, complicates validation and standardization processes, and leads to increased costs. This is a significant concern when considering scalability to meet global primary care needs. Panel-based assays also offer an additional challenge, as there are risks associated with analyzing large numbers of candidate molecules in small numbers of patients, which may result in data over-fitting and misleading results so that a panel seems to perform much better than individual biomarkers, but in fact does not114. This can be partly offset by use of a training set to initially reduce the dimensionality before validation in an independent cohort115. Therefore, while the working group agreed that a multi-marker panel may be needed, it was also agreed that the number of biomarkers should be restricted as much as possible within the NPV and PPV requirements as this would help in meeting the validation and regulatory hurdles.
Refinement and validation
Following identification of a prioritized candidate biomarker, the biomarker will require refinement and validation. Refinement will involve agreeing on a best-practice protocol, including blood collection procedures, pre-analytical sample handling and procedures for harmonization between different laboratories. Validation steps are outlined below.
Validation considerations and recommendations
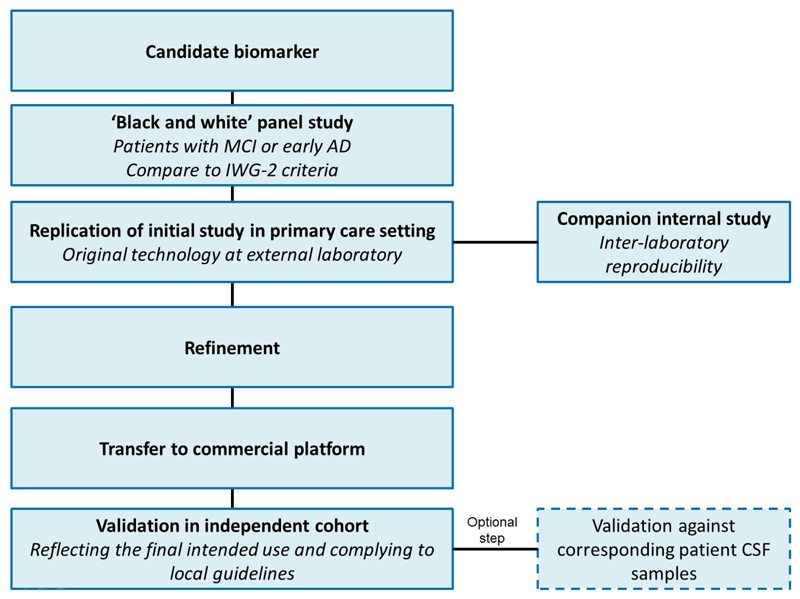
The consensus process for a pathway towards validation is shown in Figure 4. Prior to validation for widespread use, a biomarker should already have undergone initial clinical validation after the discovery analyses. Validation of _target biomarkers should begin with assessment in a “black-and-white” panel study, in which samples would be obtained from AD patients diagnosed according to the International Working Group-2 (IWG-2) criteria (amyloid PET or CSF AD signature), and also from cognitively normal (CN) controls. Samples would be divided approximately 50:50 CN:AD. This initial study would aim to establish concordance between the novel biomarker and gold standard methods, and would be performed using the original technology employed in the initial development of the biomarker test. Ideally, the study would be conducted by an external investigator blinded to the patient characteristics associated with each sample. Analysis of a black-and-white panel would not test the biomarker within the intended COU, but would instead attempt to independently validate the overall accuracy of the biomarker in a known group design. Notably, screening tests for AD may or may not detect MCI, and the accuracy of detection for MCI is likely to be lower, if even possible63. Therefore, multiple tests may be required if the _target population is to include both early AD and MCI.
Figure 4. Idealized validation process for blood-based biomarkers.
Abbreviations: AD=Alzheimer’s disease; CSF=cerebrospinal fluid; MCI=mild cognitive impairment.
Following the black-and-white panel, the next step would be to attempt to replicate the initial study results in a more representative sample set, i.e. one reflecting primary care. This second study would again employ the original technology from the developing laboratory while simultaneously beginning to transfer the technology to an existing diagnostic assay platform that is: a) globally available; b) capable of meeting the scalability needs for the numbers of assays needed when reaching primary care providers; and c) developed by an entity with prior experience of undergoing regulatory approval for diagnostics. Technology transfers are likely to be mandatory, as discovery platforms are unlikely to meet the stringent requirements for diagnostic assays as outlined by the Clinical Laboratory and Standards Institute (http://clsi.org/). Collection and assay of samples across both the original platform and the intended platform for use downstream in the regulatory pathway creates a built-in “bridge” cohort into the initial trial to test the technology within the intended COU. If strong concordance of results between internal and external laboratories is demonstrated following these studies, data acquired across the original training cohort and the new COU-specific test cohort (using the new technological platform) would be applicable for use in further refinement of the diagnostic algorithm, enabling the case for regulatory approval to be built. This would increase the reliability of the results. Finally, samples would be assayed on the intended platform for regulatory approval across multiple laboratories in order to establish inter-laboratory reproducibility.
Next, the full regulatory pathway, including all additional trials that might be required, would be established in conjunction with regulatory authorities116–118. An additional validation study within an independent primary care cohort would likely be required for regulatory approval. For US approval, this would need to comply with U.S. Food and Drug Administration (FDA) requirements for registrational studies (prospective data or robustly collected retrospective data). An optional further step would be validation using CSF samples collected from the same patients as included in the original study. Ideally, CSF samples would be collected from both blood-test-negative and blood-test-positive cases in the primary care setting to set the stage for studies examining the utility of the biomarker (or new biomarkers) in detecting amyloid positivity among cognitively normal elders. However, this latter use would require the entire process to begin anew, as this would represent an entirely different COU.
Need for a new cohort?
For the agreed intended use of the putative assay as a primary care screening tool, the ideal cohort to include in pivotal studies would align closely with the actual epidemiology of AD in primary care. The incidence of AD in this setting is ~10%1, a proportion which includes patients with either subjective or objective cognitive deficits. The ideal cohort should also provide access to PET and/or CSF data for all patients, and have known information for age, sex, APOE ε4 status and education to ensure that these are taken into account.
Current cohorts with both CSF and blood available are largely based around dementia specialty clinics and, as such, do not meet the needs of the _target product profile. Current cohorts are also limited by referral bias – they are enriched for patients with cognitive impairment and so do not echo the disease prevalence in primary care. An alternative to specifically examining primary care cohorts could be to use a yoked design, whereby the setting is a specialist centre with referrals from primary care where, in addition to referred patients, additional patients not meeting the referral criteria could be taken and included to make the cohort more representative. Additionally, it could be possible to work with primary care research networks.
In summary, it may be necessary to recruit new cohorts for the purposes of blood-based biomarker assay development. Alternatively, new large-scale cohorts, such as that created as part of the U.S. “All of Us Research Program”, evolved from the U.S. Precision Medicine Initiative (PMI)119 Cohort Program (available at https://www.nih.gov/research-training/allofus-research-program), or the UK Biobank120, could prove extremely valuable in the development of blood-based biomarkers, as these will represent real-world populations with a wide range of genetic and biomarker data available. Recently, in order to advance the development of the precision medicine paradigm in AD, the international Alzheimer Precision Medicine Initiative (APMI) and its planned pilot-cohort program (APMI-CP25,40–43) have been launched and thematically associated with the “All of Us Research Program”. The mono-centre pilot APMI cohorts, spanning from early asymptomatic preclinical populations to prodromal to late-stage dementia populations, allow for the standardized recruitment of both cognitively intact individuals at risk for AD and patients with a full spectrum of ND to provide an assortment of unique heterogeneous and multidimensional data. Particularly, the “INveStIGation of AlzHeimer’s PredicTors in Subjective Memory Complainers” (INSIGHT-preAD) study121 – a French mono-centric academic university-based cohort established at the Institute of Memory and Alzheimer’s disease (Institut de la Mémoire et de la Maladie d’Alzheimer, IM2A) at the Pitié-Salpêtrière University Hospital in Paris – is the key cohort of the APMI-CP25,40,41,43. The main goal of the INSIGHT-preAD study is to investigate the earliest preclinical stages of AD and its development, including influencing factors and markers of progression, in cognitively normal Caucasian individuals, recruited from the community in the wider Paris area, France, aged 70 to 85, with subjective complaint of memory dysfunction.
Does the landscape fit the _target product profile?
Following the landscape analysis, the 196 candidate biomarkers (Figures 2A and 2B) were further divided into tiers according to additional criteria (outlined in Callout Box 1). Of these 196 candidates, 19 were prioritized for further consideration by the working group (a table of all 19 assays is provided in the Supplementary Information); however, none were deemed to have met the agreed _target product profile.
Callout Box 1. Criteria for evaluation of identified biomarkers.
|
|
|
Along with an adequate, non-zero score on the other parameter
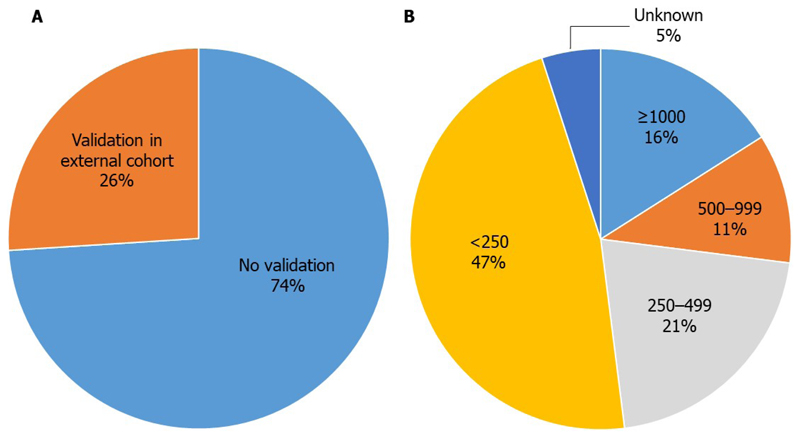
Most of the candidate biomarkers were limited by a lack of validation in independent cohorts (only five biomarker studies identified in the search have been followed up with validation studies) (Figure 5)97,98,122–124. This is regarded as a critical step in development and so is a conspicuous gap. Furthermore, where validation has been performed, it may not have been conducted by an independent group, and may not be based on a model published prior to the study105. This results in a risk of hypothesizing after results are known (HARKing), which can lead to selective reporting and inflated predictive accuracy125.
Figure 5. Validation status and patient numbers of the 19 prioritized biomarkers.
Few of the current blood-based biomarker assays in development have undergone validation in an external cohort. Of the 26% of assays that had some degree of external validation, only 2 (10·5%) were in large cohorts such as ADNI or AIBL (A). Studies are often underpowered, with small patient numbers (B).
Validation studies may require additional cohorts or, potentially, they could be validated within subgroups of the original cohort. However, appropriate cohorts for the ideal intended COU do not currently exist; validation between cohorts will therefore be a further hurdle in the development of assays. As previously discussed, the appropriate cohorts (stemming from patients in primary care with CSF and blood samples, as well as PET data) are not available and therefore the _target product profile could not be met by any study.
Additional issues present with some biomarkers are PPV and NPV values lower than those specified in the _target product profile, as well as small sample sizes (Figure 5). Studies using large and well-established cohorts such as ADNI and the Australian Imaging, Biomarker and Lifestyle Study of Ageing (AIBL) are more likely to meet the initial requirements. As discussed previously, biomarker panels are thought to offer the greatest chances of success, although panels featuring high numbers of markers will be difficult and expensive to develop and validate. In addition, most available technology platforms have a low ceiling on the number of biomarkers that can be contained within the assay, which is usually around five biomarkers. There are many emerging areas of interest (e.g. exosomes) that offer promise, although they are too early for consideration at this time. Additionally, blood-based assays for well-known biomarkers of AD, such as Aβ1–42/Aβ1–40, tau, NF-L, and BACE1, may have potential, and should be monitored closely as more information becomes available and novel technologies emerge to address the hurdles currently facing use of these biomarkers.
The future: blood-based biomarker diagnostics
Blood-based biomarkers have the potential to improve detection and diagnosis for AD by increasing convenience, acceptability and ease of testing, as well as reducing costs. There are a number of key areas in which they are likely to provide most benefit. The first and most important of these is testing for AD in the primary care setting, which will potentially allow identification of patients at the earliest stages of the disease. Ideally, this would be prior to the development of any noticeable cognitive deficits, but this will depend on future developments. The advent of testing in primary care would lead to substantial changes in the current treatment paradigm by allowing patients to be guided towards specialist diagnostics (e.g. CSF or PET scans) and access to care earlier in the course of the disease. This will be particularly important once disease-modifying therapies (DMTs) become available, but may provide benefit to patients and caregivers even at the present time126.
Once DMTs become available there will, undoubtedly, be a tremendous surge in numbers of patients in primary care settings seeking potential prescriptions. However, without a convenient primary care screen, confirmatory diagnostics will still be required (i.e. CSF, MRI or PET), which will act as a bottleneck in the diagnosis and treatment process, and will present a considerable burden in terms of costs. Availability of a multi-tiered diagnostic process commencing with a blood test in primary care would dramatically increase access to DMTs, while conveniently exclude from further diagnosis and treatment the vast majority of patients who would not need to undergo these expensive and invasive procedures. An additional potential use of blood-based AD biomarkers will be to provide a cost-effective and rapid test for AD pathology in order to determine the eligibility of patients for recruitment into clinical trials for DMTs127–129. These tests might also have applicability in the monitoring of disease progression and drug effects on AD pathophysiology during a trial130. As such, the availability of blood-based biomarker diagnostics may in fact accelerate the development of DMTs.
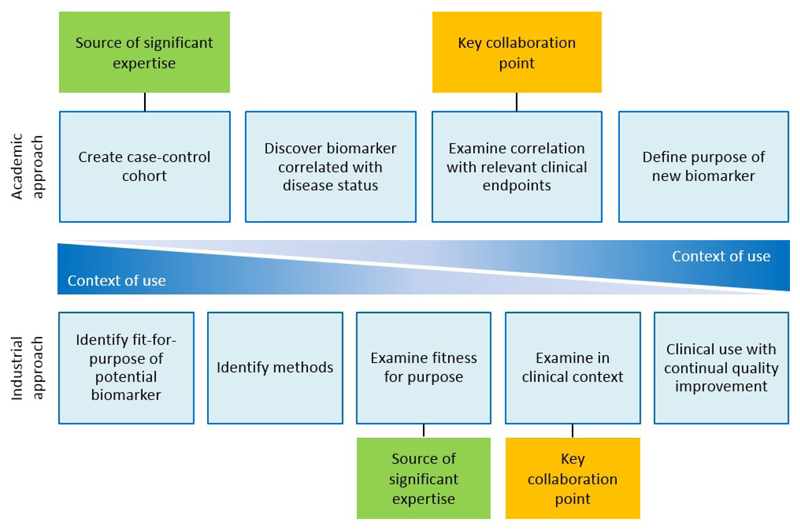
The development of blood-based biomarkers often stalls in the early stages due to a disconnection between academia, where biomarkers are identified, and industry, who have the resources to take biomarkers further and develop them commercially (Figure 6). This disconnect arises from the different needs of these different sectors and from mutual mistrust developed following a history of poor interactions. The current initiative by the working group and Roche Diagnostics International represents a step in an attempt to improve that process, both efficiently and economically.
Figure 6. Potential collaboration points between academia and industry.
Academic and industrial approaches to biomarker development are inherently different, but could be extremely useful if combined. Close collaboration between industry and academia would allow for sharing of expertise in product testing, access to cohorts and clinical data, as well as allow for sharing ideas and theories with regards to clinical endpoints and context. By merging the two approaches, a method where the context of use is the primary focus throughout the process can be established. This would allow for synergistic development of a new biomarker between academics and industrial partners, sharing a wealth of experience.
Conclusions
The last decade has unquestionably witnessed key progresses in the area of blood-based biomarkers research in AD and other ND. Human blood (plasma) holds the largest source of the proteome and technologies measuring even minor alterations of proteins and peptides are crucial tools to identify protein biomarkers in blood. For instance, mass spectrometry technologies can detect slight alterations of the protein concentrations and immunohistochemistry can recognize with high accuracy a definite protein in the living system. Compared with CSF markers, a validated blood-based AD biomarker would provide a fast, non-invasive, and cost-effective method of early detection and diagnosis for what is the most common age-related ND worldwide. In addition, venipuncture is a routine, safe procedure that does not pose any harm to the patient. As such – and differently from CSF sampling – the examination of blood biomarkers is accepted and more easily introduced in the clinical environment26. _targeting symptomatic patients in a primary care setting would help patients with early AD access care earlier. Development of biomarkers ideally reflecting all existing molecular pathophysiological mechanisms in the polygenic AD brain at distinct time points of progression will represent the foundation for personalized, tailored, biomarker-guided _targeted therapies and constitute a critical step towards the dissemination of precision medicine in AD25,40. Furthermore, the development and implementation of a multi-stage diagnostic approach beginning with a blood test in primary care will increase access to confirmatory diagnostic modalities (such as PET imaging and/or CSF sampling) and provide a clear path for regulatory approval. This tiered approach is expected to increase the access to DMTs, once these will ultimately be available.
Despite much research, many of the candidate blood-based biomarkers exhibit limitations currently preventing their use. Close cooperation and coordination is needed among academic institutions, industry partners, and regulatory bodies to accelerate the development of a blood-based biomarker assay suitable for clinical use. The assembly of novel cohorts may be required to allow the development of a new assay for the agreed ideal intended use of broad population-based screening for AD in patients in primary care.
Applying systems biology to inspect large multidimensional blood-based “omics” data will enable the stratification of patient populations into well-defined subsets of patients sharing a common pathophysiology that, in turn, can be further explored for _targeted interventions. The substantial use of systems biology methods to discover and validate diagnostic biomarkers of specific subsets of AD patients will considerably impact the progress of precision medicine in AD and facilitate the use of this paradigm for AD patients towards clinical reality26.
“Omics” sciences – providing a depiction of complex biological systems in a holistic and integrative manner – generate unavoidably large-scale and heterogeneous biomedical data. This led to open the era of “big data” in Biology and Medicine25,40,42, indicating the complexity and challenges presented by the combined analysis of data. These encompass an enormous amount of heterogeneous, disorganized, multidimensional data (from molecular/cellular data, to imaging, clinical, demographic, and environmental data) extensively produced by academic institutions, clinics, and, more recently, mobile devices131,132. The implementation of systems biology to attain novel insights into AD pathophysiology requires the assessment of big and deep multidimensional heterogeneous data. Performing the integrated analysis of big data depends on the accessibility to appropriate tools, both for data storage/management and for their modelling, according to disease pathophysiology. Therefore, effective and sophisticated methods will be essential to systematically screen for novel blood-based biomarkers associated with AD, as well as to gain insights into their spatiotemporal interactions with other biomarker categories. The integration of the results obtained with different experimental strategies is assumed to provide complementary information on the disease pathophysiology and provide insights into its clinical interpretation. Accordingly, the Big Data Research and Development Initiative (available at https://obamawhitehouse.archives.gov/blog/2012/03/29/big-data-big-deal), announced by the previous Obama Administration, is a crucial promoter of the implementation of precision medicine through the integration of big and deep biomedical data. The ability to deal with “big data science”, accompanied by the implementation of an integrative disease modelling133, is an essential aspect of the previously mentioned international APMI and other worldwide initiatives, including the previously mentioned Alzheimer’s Association ISTAART BBB-PIA71 and the recently established Cholinergic System Working Group (CSWG)134.
Supplementary Material
Callout Box 2. Checklist for developing a blood-based biomarker.
|
Glossary.
Technical definitions
α-Synuclein
α-Synuclein is a neuronal protein involved in regulating the synaptic vesicles pool size, trafficking, membrane dynamics, and neurotransmitters release. Intracellular aggregates of α-synuclein are typical of certain neurodegenerative diseases (α-synucleinopathies) including Parkinson's disease, dementia with Lewy bodies, and multiple system atrophy. α-Synuclein aggregates are also found in other neurodegenerative diseases, including AD.
ADNI
The Alzheimer’s Disease Neuroimaging Initiative (ADNI) is an ongoing, longitudinal, multicentre study, initiated in the U.S.A. in 2004, designed to develop and validate clinical, imaging, biochemical, and genetic, biomarkers for the early detection and tracking of AD.
AIBL
The Australian Imaging, Biomarker and Lifestyle Study of Ageing (AIBL) is an ongoing, longitudinal study, initiated in Australia in 2006, exploring biomarkers, cognitive characteristics, as well as health and lifestyle factors associated with AD.
Amyloid precursor protein (APP)
APP is a type I membrane protein abundantly expressed in the brain and implicated in neural growth and maturation. APP can be processed via the non-amyloidogenic pathway, where cleavage by α- and γ-secretases generate a secreted form of APP (sAPPα) and C-terminal fragments (CTF 83, p3 and AICD50). Alternatively, APP can be processed via the amyloidogenic pathway, where cleavage by β- and γ-secretases generate a secreted form of APP (sAPPβ), C-terminal fragments (CTF 99 and CTF 89) and Aβ peptides.
Amyloid beta (Aβ)
Aβ peptides are produced in variable length, including the 42-amino acid-long and the 40-amino acid-long amyloid beta peptides (Aβ1–40 and Aβ1–42), from the proteolytic cleavage of the amyloid precursor protein. Aβ1–42 exhibits a substantial propensity to form aggregates, including extracellular amyloid plaques, which are a pathological hallmark of AD.
A/T/N
The A/T/N scheme is a classification system that uses three binary biomarker categories reflecting AD pathophysiology. ‘A’ refers to biomarkers of amyloid beta pathology (CSF Aβ1–42 or amyloid PET), ‘T’ refers to biomarkers of tau pathology (CSF hyperphosphorylated tau or tau PET), and ‘N’ refers to biomarkers of neurodegeneration or neuronal injury (CSF total tau, 18F-fluorodeoxyglucose-PET (18F-FDG-PET), structural MRI).108
β-site amyloid precursor protein cleaving enzyme 1 (BACE1)
BACE1 is an endoprotease responsible for the first proteolytic cleaving step in the generation of Aβ peptides from the amyloid precursor protein.
BioFINDER
The Biomarkers For Identifying Neurodegenerative Disorders Early and Reliably (BioFINDER) study is an ongoing longitudinal study, initiated in Sweden in 2010, investigating the early diagnosis and underlying pathologies of AD and Parkinson’s disease based on neuroimaging, cerebrospinal fluid, blood-based, clinical and neuropsychological markers.
Cerebrospinal fluid (CSF)
CSF is the body fluid within the central canal of the spinal cord and the four ventricles of the brain.
Context of use (COU)
A COU is a statement that describes the manner and purpose of use for the biomarker in drug development. The supporting data and analyses submitted with the biomarker qualification determine the acceptability of the qualified COU.
IWG-2
The IWG-2 criteria are research diagnostic criteria for AD, developed by the International Working Group (IWG) for the diagnosis of AD.29
Mild cognitive impairment (MCI)
MCI causes a slight but noticeable and measurable decline in cognitive abilities, including memory and thinking skills. An individual with MCI is at an increased risk of developing AD or another form of dementia.
Negative predictive value (NPV)
The NPV is the probability a patient does not have the disease when the test result is negative.
Neurofilament light (NF-L)
NF-L chain protein is a component of neurofilaments, which are structural constituents of the neuronal cytoskeleton. It is highly expressed in large-calibre myelinated axons and is released into the brain interstitial fluid, following axonal injury.
Plasma
Plasma is the liquid constituent of blood obtained after removal of blood cells.
Positive predictive value (PPV)
The PPV is the probability a patient has the disease when the test result is positive.
Receiver operating characteristic (ROC)
The ROC curve is a plot of sensitivity versus 1-specificity for the different possible cut-off points of a diagnostic test. Accuracy of the diagnostic test is based on the area under the ROC curve; the closer the area under the ROC curve is to 1, the better the test.
Sensitivity
Diagnostic sensitivity is the probability a test result is positive when the disease is present.
Serum
Serum is the clear liquid constituent of blood characterized by the absence of the coagulation factors and blood cells
Specificity
Diagnostic specificity is the probability a test result is negative when the disease is absent.
Subjective cognitive decline (SCD)
SCD is a self-reported decline in cognition, undetected by standard neuropsychological tests.
Tau
Tau is a microtubule-associated protein that modulates the stability of axonal microtubules. In AD and other neurodegenerative diseases (tauopathies), aggregates of abnormally phosphorylated (hyperphosphorylated) tau proteins result in intraneuronal neurofibrillary tangles.
TDP-43
Transactive response DNA-binding protein 43 (TDP-43) binds both DNA and RNA and helps regulate mechanisms of RNA processing such as splicing, trafficking, and microRNA production. TDP-43 cytoplasmic inclusions are associated with definite neurodegenerative diseases, including frontotemporal dementia, amyotrophic lateral sclerosis, and AD.
Conceptual definitions
Big Data
Big Data is a repository of large amounts of data sets generated by data mining tools. Big Data includes information obtained through systems theory- and knowledge-based approaches, and clinical records.
Data science
Data science is an interdisciplinary field about processes and systems to extract knowledge from data in different forms – either structured or unstructured – which is a continuation of some of the data analysis fields including statistics, artificial intelligence, machine learning, data mining, and predictive analytics.
Homeostasis
Homeostasis is a spontaneous tendency towards a condition of a dynamic equilibrium based on a continuous counterbalance between regulatory-defense mechanisms and disrupting stress-induced signals. Homeostasis is common to any biological system. Homeostatic signalling is hierarchically organized from subcellular to cellular level, across organs, and, finally, systems. Homeostasis is essential for protecting all core biosynthetic processes necessary to optimal functioning and survival.
Integrative disease modelling
Integrative disease modelling is a multidisciplinary approach to standardize, manage, integrate, and interpret multiple sources of structured and unstructured quantitative and qualitative data across biological scales using computational models that assist decision making for translation of patient-specific molecular mechanisms into tailored clinical applications.
Network
A network is a set of recurring motifs; each motif is a pathway; each pathway, in turn, carries out specific dynamical functions and can be modulated, i.e. up-down regulated, either upstream or downstream or both. There is a large spectrum of biological cross-talks between pathways and networks inside a level of a given system and among systems.
“Omics”
“Omics” are high-throughput screening tools aimed at fully collecting, characterizing and quantifying pools of biological molecules (DNA sequences, transcripts, miRNAs, proteins/peptides, metabolites/lipids) that translate into the structure, function, and dynamics of an organism and/or whole organisms.
“One-size-fits-all” approach
The “one-size-fits-all” approach is the traditional approach used for the development of early detection, intervention, and prevention options, where biomarker candidates are being validated against the plethora of heterogeneous clinical operationalized syndromes, rather than against genetically (risk profile) and biologically (i.e., based on molecular mechanisms and cellular pathways) determined entities.
Precision medicine
Precision medicine is a translational science paradigm related to both health and disease. It is a biomarker-guided _targeted medicine on systems-levels taking into account methodological advancements and discoveries of the comprehensive pathophysiological profiles of complex polygenic, multi-factorial neurodegenerative diseases (proteinopathies of the brain). Precision medicine aims to optimize the effectiveness of disease prevention and therapy, by considering (customizing) an individual’s specific “biological make-up” (e.g. genetic, biochemical, phenotypic, lifestyle, and psychosocial characteristics) for _targeted interventions through P4M (Predictive, Preventive, Personalized, and Participatory) implementation.
Systems biology
Systems biology is an evolving hypothesis-free, exploratory, holistic (non-reductionistic), global, integrative, and interdisciplinary paradigm using advances in multimodal high-throughput technological platforms that enable the examination of networks of biological pathways where elevated amounts of structurally and functionally different molecules are simultaneously explored over time at a system level (i.e., at the level of molecules and subcellular compartments, cells, group of cells, tissues, organs, apparatuses, or even whole organisms). According to systems biology, organisms are made of systems which are entities consisting in hierarchically self-organized levels with increasing structural complexity resulting in different emerging properties.
Systems theory
Systems theory is a translational research theory of the precision medicine paradigm. It is an interdisciplinary conceptual framework allowing for the conceptualization of novel/original models to extract and explicate all systems levels and different spatiotemporal data types of complex polygenic diseases.
Acknowledgements
We acknowledge the following individuals who attended the working group meeting, the discussions from which formed the basis of this Personal View: Barbara Schaeuble (F. Hoffmann-La Roche Ltd, Basel, Switzerland), Bruce Jordan (F. Hoffmann-La Roche Ltd, Basel, Switzerland), Chiaki Yoda (F. Hoffmann-La Roche Ltd, Basel, Switzerland), Christian Czech (F. Hoffmann-La Roche Ltd, Basel, Switzerland), Estelle Vester-Blokland (F. Hoffmann-La Roche Ltd, Basel, Switzerland), Hai Zhang (F. Hoffmann-La Roche Ltd, Basel, Switzerland), Maryline Simon (Roche Diagnostics International, Rotkreuz, Switzerland) and Tobias Bittner (Genentech, Basel, Switzerland). Editorial support in the form of medical writing was provided by Jennifer Smith, PhD of MediTech Media Ltd (funded by Roche Diagnostics International).
Contributors
HH and KB provided the initial idea and outline of content for the manuscript. All authors contributed content, and critically reviewed and edited the manuscript.
Contributors to the Alzheimer Precision Medicine Initiative – Working Group (APMI–WG):
Lisi Flores AGUILAR (Montréal), Claudio BABILONI (Rome), Filippo BALDACCI (Pisa), Norbert BENDA (Bonn), Keith L. BLACK (Los Angeles), Arun L.W. BOKDE (Dublin), Ubaldo BONUCCELLI (Pisa), Karl BROICH (Bonn), René S. BUN (Paris), Francesco CACCIOLA (Siena), Juan CASTRILLO† (Derio), Enrica CAVEDO (Paris), Roberto CERAVOLO (Pisa), Patrizia A. CHIESA (Paris), Olivier COLLIOT (Paris), Cristina-Maria COMAN (Paris), Jean-Christophe CORVOL (Paris), Augusto Claudio CUELLO (Montréal), Jeffrey L. CUMMINGS (Las Vegas), Herman DEPYPERE (Gent), Bruno DUBOIS (Paris), Andrea DUGGENTO (Rome), Stanley DURRLEMAN (Paris), Valentina ESCOTT-PRICE (Cardiff), Howard FEDEROFF (Irvine), Maria Teresa FERRETTI (Zürich), Massimo FIANDACA (Irvine), Richard A. FRANK (Malvern), Francesco GARACI (Rome), Remy GENTHON (Paris), Nathalie GEORGE (Paris), Filippo S. GIORGI (Pisa), Manuela GRAZIANI (Roma), Marion HABERKAMP (Bonn), Marie-Odile HABERT (Paris), Harald HAMPEL (Paris), Karl HERHOLZ (Manchester), Eric KARRAN (Cambridge), Seung H. KIM (Seoul), Yosef KORONYO (Los Angeles), Maya KORONYO-HAMAOUI (Los Angeles), Foudil LAMARI (Paris), Todd LANGEVIN (Minneapolis-Saint Paul), Stéphane LEHÉRICY (Paris), Simone LISTA (Paris), Jean LORENCEAU (Paris), Mark MAPSTONE (Irvine), Christian NERI (Paris), Robert NISTICÒ (Rome), Francis NYASSE-MESSENE (Paris), Sid E. O’BRYANT (Fort Worth), George PERRY (San Antonio), Craig RITCHIE (Edinburgh), Katrine ROJKOVA (Paris), Simone ROSSI (Siena), Amira SAIDI (Rome), Emiliano SANTARNECCHI (Siena), Lon S. SCHNEIDER (Los Angeles), Olaf SPORNS (Bloomington), Nicola TOSCHI (Rome), Steven R. VERDOONER (Sacramento), Andrea VERGALLO (Paris), Nicolas VILLAIN (Paris), Lindsay A. WELIKOVITCH (Montréal), Janet WOODCOCK (Silver Spring), Erfan YOUNESI (Esch-sur-Alzette).
Declaration of Interest
HH reports personal fees from Roche Diagnostics International during the conduct of the study.
He received lecture fees from Biogen and Roche, research grants from Pfizer, Avid, and MSD Avenir (paid to the institution), travel funding from Functional Neuromodulation, Axovant, Eli Lilly and company, Takeda and Zinfandel, GE-Healthcare and Oryzon Genomics, consultancy fees from Jung Diagnostics, Cytox Ltd., Axovant, Anavex, Takeda and Zinfandel, GE Healthcare, Oryzon Genomics, and Functional Neuromodulation, and participated in scientific advisory boards of Functional Neuromodulation, Axovant, Eli Lilly and company, Cytox Ltd., GE Healthcare, Takeda and Zinfandel, Oryzon Genomics, and Roche Diagnostics.
HH is supported by the AXA Research Fund, the “Fondation partenariale Sorbonne Université” and the “Fondation pour la Recherche sur Alzheimer”, Paris, France. Ce travail a bénéficié d'une aide de l’Etat “Investissements d’avenir” ANR-10-IAIHU-06. The research leading to these results has received funding from the program “Investissements d’avenir” ANR-10-IAIHU-06 (Agence Nationale de la Recherche-10-IA Agence Institut Hospitalo-Universitaire-6).
HH is co-inventor in the following patents as a scientific expert and has received no royalties:
In Vitro Multiparameter Determination Method for The Diagnosis and Early Diagnosis of Neurodegenerative Disorders Patent Number: 8916388
In Vitro Procedure for Diagnosis and Early Diagnosis of Neurodegenerative Diseases Patent Number: 8298784
Neurodegenerative Markers for Psychiatric Conditions Publication Number: 20120196300
In Vitro Multiparameter Determination Method for The Diagnosis and Early Diagnosis of Neurodegenerative Disorders Publication Number: 20100062463
In Vitro Method for The Diagnosis and Early Diagnosis of Neurodegenerative Disorders Publication Number: 20100035286
In Vitro Procedure for Diagnosis and Early Diagnosis of Neurodegenerative Diseases Publication Number: 20090263822
In Vitro Method for The Diagnosis of Neurodegenerative Diseases Patent Number: 7547553
CSF Diagnostic in Vitro Method for Diagnosis of Dementias and Neuroinflammatory Diseases Publication Number: 20080206797
In Vitro Method for The Diagnosis of Neurodegenerative Diseases Publication Number: 20080199966
Neurodegenerative Markers for Psychiatric Conditions Publication Number: 20080131921
SO reports personal fees from Roche Diagnostics International and grants from the National Institute on Aging during the conduct of the study, as well as personal fees from Cx Precision Medicine outside the submitted work. In addition, SO has three patents licensed to Cx Precision Medicine. JLM reports personal fees from Roche Diagnostics International during the conduct of the study, and personal fees from IBL, Raman Health and Fujirebio outside the submitted work. HZ is a co-founder of Brain Biomarker Solutions in Gothenburg AB, a GU Ventures-based platform company at the University of Gothenburg, Sweden, and reports personal fees from Roche Diagnostics International during the conduct of the study. CLM reports personal fees from Roche Diagnostics International during the conduct of the study. SL received lecture honoraria from Roche. SJK reports personal fees from Roche Diagnostics International, grants from Medical Research Council (UK) and NIHR Biomedical Research Centre for Mental Health (BRC-MH) during the conduct of the study, and grants from Eli Lily outside the submitted work. RB is a current employee and stock holder of Roche Diagnostics International. KB reports personal fees from Roche Diagnostics International during the conduct of the study, and personal fees from Fujirebio Europe, IBL International, Roche Diagnostics, Eli Lilly and Alzheon outside the submitted work. In addition, KB has a European Patent application EP 16002379.2 pending and is co-founder of Brain Biomarker Solutions in Gothenburg AB, a GU Ventures-based platform company at the University of Gothenburg, Sweden.
References
- 1.Alzheimer's Association. 2016 Alzheimer's disease facts and figures. Alzheimers Dement. 2016;12:459–509. doi: 10.1016/j.jalz.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Scheltens P, et al. Alzheimer's disease. Lancet. 2016;388:505–517. doi: 10.1016/S0140-6736(15)01124-1. [DOI] [PubMed] [Google Scholar]
- 3.Olsson B, et al. CSF and blood biomarkers for the diagnosis of Alzheimer's disease: a systematic review and meta-analysis. Lancet Neurol. 2016;15:673–684. doi: 10.1016/S1474-4422(16)00070-3. [DOI] [PubMed] [Google Scholar]
- 4.Lista S, Hampel H. Synaptic degeneration and neurogranin in the pathophysiology of Alzheimer's disease. Expert Rev Neurother. 2017;17:47–57. doi: 10.1080/14737175.2016.1204234. [DOI] [PubMed] [Google Scholar]
- 5.Baldacci F, Lista S, Cavedo E, Bonuccelli U, Hampel H. Diagnostic function of the neuroinflammatory biomarker YKL-40 in Alzheimer's disease and other neurodegenerative diseases. Expert Rev Proteomics. 2017;14:285–299. doi: 10.1080/14789450.2017.1304217. [DOI] [PubMed] [Google Scholar]
- 6.Heneka MT, et al. Neuroinflammation in Alzheimer's disease. Lancet Neurol. 2015;14:388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iturria-Medina Y, et al. Early role of vascular dysregulation on late-onset Alzheimer's disease based on multifactorial data-driven analysis. Nat Commun. 2016;7 doi: 10.1038/ncomms11934. 11934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de la Monte SM, Tong M. Brain metabolic dysfunction at the core of Alzheimer's disease. Biochem Pharmacol. 2014;88:548–559. doi: 10.1016/j.bcp.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.James BD, et al. TDP-43 stage, mixed pathologies, and clinical Alzheimer's-type dementia. Brain. 2016;139:2983–2993. doi: 10.1093/brain/aww224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kovacs GG, et al. Non-Alzheimer neurodegenerative pathologies and their combinations are more frequent than commonly believed in the elderly brain: a community-based autopsy series. Acta Neuropathol. 2013;126:365–384. doi: 10.1007/s00401-013-1157-y. [DOI] [PubMed] [Google Scholar]
- 11.Rahimi J, Kovacs GG. Prevalence of mixed pathologies in the aging brain. Alzheimers Res Ther. 2014;6:82. doi: 10.1186/s13195-014-0082-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Attems J, Jellinger KA. The overlap between vascular disease and Alzheimer's disease--lessons from pathology. BMC Med. 2014;12:206. doi: 10.1186/s12916-014-0206-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hardy JA, Higgins GA. Alzheimer's disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 14.Blennow K, Hampel H, Weiner M, Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat Rev Neurol. 2010;6:131–144. doi: 10.1038/nrneurol.2010.4. [DOI] [PubMed] [Google Scholar]
- 15.Hampel H, Lista S. Alzheimer disease: from inherited to sporadic AD-crossing the biomarker bridge. Nat Rev Neurol. 2012;8:598–600. doi: 10.1038/nrneurol.2012.202. [DOI] [PubMed] [Google Scholar]
- 16.Kim D, Kim YS, Shin DW, Park CS, Kang JH. Harnessing cerebrospinal fluid biomarkers in clinical trials for treating Alzheimer's and Parkinson's diseases: potential and challenges. J Clin Neurol. 2016;12:381–392. doi: 10.3988/jcn.2016.12.4.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frisoni GB, et al. Strategic roadmap for an early diagnosis of Alzheimer's disease based on biomarkers. Lancet Neurol. 2017;16:661–676. doi: 10.1016/S1474-4422(17)30159-X. [DOI] [PubMed] [Google Scholar]
- 18.Patterson V, Humphreys J, Chua R. Email triage of new neurological outpatient referrals from general practice. J Neurol Neurosurg Psychiatry. 2004;75:617–620. doi: 10.1136/jnnp.2003.024489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American Medical Association. The ABCs of the Annual Wellness Visit (AMW) 2017 https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/AWV_chart_ICN905706.pdf.
- 20.The Gerontological Society of America. The Gerontological Society of America Workgroup on cognitive impairment detection and earlier diagnosis: report and recommendations. 2015 https://changeagents365.org/resources/ways-to-stay-engaged/the-gerontological-society-of-america/Cognitive%20Impairment%20Recommendations%20Report_GSA.pdf.
- 21.Chen LM, Farwell WR, Jha AK. Primary care visit duration and quality: does good care take longer? Arch Intern Med. 2009;169:1866–1872. doi: 10.1001/archinternmed.2009.341. [DOI] [PubMed] [Google Scholar]
- 22.Cannon P, Larner AJ. Errors in the scoring and reporting of cognitive screening instruments administered in primary care. Neurodegener Dis Manag. 2016;6:271–276. doi: 10.2217/nmt-2016-0004. [DOI] [PubMed] [Google Scholar]
- 23.Wojtowicz A, Larner AJ. General Practitioner Assessment of Cognition: use in primary care prior to memory clinic referral. Neurodegener Dis Manag. 2015;5:505–510. doi: 10.2217/nmt.15.43. [DOI] [PubMed] [Google Scholar]
- 24.Garcia-Ptacek S, et al. Differences in diagnostic process, treatment and social support for Alzheimer's dementia between primary and specialist care: results from the Swedish Dementia Registry. Age Ageing. 2017;46:314–319. doi: 10.1093/ageing/afw189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hampel H, et al. A precision medicine initiative for Alzheimer's disease: the road ahead to biomarker-guided integrative disease modeling. Climacteric. 2017;20:107–118. doi: 10.1080/13697137.2017.1287866. [DOI] [PubMed] [Google Scholar]
- 26.O'Bryant SE, et al. Blood-based biomarkers in Alzheimer disease: current state of the science and a novel collaborative paradigm for advancing from discovery to clinic. Alzheimers Dement. 2017;13:45–58. doi: 10.1016/j.jalz.2016.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lista S, et al. CSF Aβ1-42 combined with neuroimaging biomarkers in the early detection, diagnosis and prediction of Alzheimer's disease. Alzheimers Dement. 2014;10:381–392. doi: 10.1016/j.jalz.2013.04.506. [DOI] [PubMed] [Google Scholar]
- 28.Lista S, et al. Biomarkers in sporadic and familial Alzheimer's disease. J Alzheimers Dis. 2015;47:291–317. doi: 10.3233/JAD-143006. [DOI] [PubMed] [Google Scholar]
- 29.Dubois B, et al. Advancing research diagnostic criteria for Alzheimer's disease: the IWG-2 criteria. Lancet Neurol. 2014;13:614–629. doi: 10.1016/S1474-4422(14)70090-0. [DOI] [PubMed] [Google Scholar]
- 30.McKhann GM, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inekci D, Jonesco DS, Kennard S, Karsdal MA, Henriksen K. The potential of pathological protein fragmentation in blood-based biomarker development for dementia - with emphasis on Alzheimer's disease. Front Neurol. 2015;6:90. doi: 10.3389/fneur.2015.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vandenberghe R, Adamczuk K, Dupont P, Laere KV, Chetelat G. Amyloid PET in clinical practice: its place in the multidimensional space of Alzheimer's disease. Neuroimage Clin. 2013;2:497–511. doi: 10.1016/j.nicl.2013.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hampel H, et al. Perspective on future role of biological markers in clinical therapy trials of Alzheimer's disease: a long-range point of view beyond 2020. Biochem Pharmacol. 2014;88:426–449. doi: 10.1016/j.bcp.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 34.Hampel H, et al. Biomarkers for Alzheimer's disease: academic, industry and regulatory perspectives. Nat Rev Drug Discov. 2010;9:560–574. doi: 10.1038/nrd3115. [DOI] [PubMed] [Google Scholar]
- 35.Hampel H, Lista S, Khachaturian ZS. Development of biomarkers to chart all Alzheimer's disease stages: the royal road to cutting the therapeutic gordian knot. Alzheimers Dement. 2012;8:312–336. doi: 10.1016/j.jalz.2012.05.2116. [DOI] [PubMed] [Google Scholar]
- 36.Cavedo E, et al. The road ahead to cure Alzheimer's disease: development of biological markers and neuroimaging methods for prevention trials across all stages and _target populations. J Prev Alzheimers Dis. 2014;1:181–202. doi: 10.14283/jpad.2014.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hampel H, Lista S. Use of biomarkers and imaging to assess pathophysiology, mechanisms of action and _target engagement. J Nutr Health Aging. 2013;17:54–63. doi: 10.1007/s12603-013-0003-1. [DOI] [PubMed] [Google Scholar]
- 38.Lista S, et al. Evolving evidence for the value of neuroimaging methods and biological markers in subjects categorized with subjective cognitive decline. J Alzheimers Dis. 2015;48(Suppl 1):S171–191. doi: 10.3233/JAD-150202. [DOI] [PubMed] [Google Scholar]
- 39.Trojanowski JQ, Hampel H. Neurodegenerative disease biomarkers: guideposts for disease prevention through early diagnosis and intervention. Prog Neurobiol. 2011;95:491–495. doi: 10.1016/j.pneurobio.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hampel H, et al. Precision medicine - The golden gate for detection, treatment and prevention of Alzheimer's disease. J Prev Alzheimers Dis. 2016;3:243–259. doi: 10.14283/jpad.2016.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hampel H, et al. Precision pharmacology for Alzheimer's disease. Pharmacol Res. 2018;130:331–365. doi: 10.1016/j.phrs.2018.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hampel H, et al. Revolution of Alzheimer Precision Neurology. Passageway of systems biology and neurophysiology. J Alzheimers Dis. 2018 doi: 10.3233/JAD-179932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferretti M, et al. Sex-specific phenotypes of Alzheimer’s disease: the gateway to precision neurology. Nat Rev Neurol. doi: 10.1038/s41582-018-0032-9. (In press) [DOI] [PubMed] [Google Scholar]
- 44.Lista S, et al. Application of systems theory in longitudinal studies on the origin and progression of Alzheimer's disease. Methods Mol Biol. 2016;1303:49–67. doi: 10.1007/978-1-4939-2627-5_2. [DOI] [PubMed] [Google Scholar]
- 45.Alzheimer A. Über einen eigenartigen schweren erkrankungsprozeß der hirnrinde. Neurologisches Centralblatt. 1906;23:1129–1136. [Google Scholar]
- 46.Noorbakhsh F, Overall CM, Power C. Deciphering complex mechanisms in neurodegenerative diseases: the advent of systems biology. Trends Neurosci. 2009;32:88–100. doi: 10.1016/j.tins.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 47.Lyman GH, Moses HL. Biomarker tests for molecularly _targeted therapies--the key to unlocking precision medicine. N Engl J Med. 2016;375:4–6. doi: 10.1056/NEJMp1604033. [DOI] [PubMed] [Google Scholar]
- 48.Deyati A, Younesi E, Hofmann-Apitius M, Novac N. Challenges and opportunities for oncology biomarker discovery. Drug Discov Today. 2013;18:614–624. doi: 10.1016/j.drudis.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 49.Krutovskikh VA, Herceg Z. Oncogenic microRNAs (oncomiRs) as a new class of cancer biomarkers. Bioessays. 2010;32:894–904. doi: 10.1002/bies.201000040. [DOI] [PubMed] [Google Scholar]
- 50.Schröder H, Grösche M, Adler M, Spengler M, Niemeyer CM. Immuno-PCR with digital readout. Biochem Biophys Res Commun. 2017;488:311–315. doi: 10.1016/j.bbrc.2017.04.162. [DOI] [PubMed] [Google Scholar]
- 51.Niemeyer CM, Adler M, Wacker R. Immuno-PCR: high sensitivity detection of proteins by nucleic acid amplification. Trends Biotechnol. 2005;23:208–216. doi: 10.1016/j.tibtech.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 52.Castrillo JI, Oliver SG. Alzheimer's as a systems-level disease involving the interplay of multiple cellular networks. Methods Mol Biol. 2016;1303:3–48. doi: 10.1007/978-1-4939-2627-5_1. [DOI] [PubMed] [Google Scholar]
- 53.Nakamura A, et al. High performance plasma amyloid-beta biomarkers for Alzheimer's disease. Nature. 2018;554:249–254. doi: 10.1038/nature25456. [DOI] [PubMed] [Google Scholar]
- 54.Ovod V, et al. Amyloid β concentrations and stable isotope labeling kinetics of human plasma specific to central nervous system amyloidosis. Alzheimers Dement. 2017;13:841–849. doi: 10.1016/j.jalz.2017.06.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Janelidze S, et al. Plasma β-amyloid in Alzheimer's disease and vascular disease. Sci Rep. 2016;6 doi: 10.1038/srep26801. 26801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weston PSJ, et al. Serum neurofilament light in familial Alzheimer disease: a marker of early neurodegeneration. Neurology. 2017;89:2167–2175. doi: 10.1212/WNL.0000000000004667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mattsson N, Andreasson U, Zetterberg H, Blennow K, Alzheimer's Disease Neuroimaging Initiative Association of plasma neurofilament light with neurodegeneration in patients with Alzheimer disease. JAMA Neurol. 2017;74:557–566. doi: 10.1001/jamaneurol.2016.6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.O'Brien JT, Herholz K. Amyloid imaging for dementia in clinical practice. BMC Med. 2015;13:163. doi: 10.1186/s12916-015-0404-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Henriksen K, et al. The future of blood-based biomarkers for Alzheimer's disease. Alzheimers Dement. 2014;10:115–131. doi: 10.1016/j.jalz.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.de Almeida SM, et al. Incidence of post-dural puncture headache in research volunteers. Headache. 2011;51:1503–1510. doi: 10.1111/j.1526-4610.2011.01959.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schneider P, Hampel H, Buerger K. Biological marker candidates of Alzheimer's disease in blood, plasma, and serum. CNS Neurosci Ther. 2009;15:358–374. doi: 10.1111/j.1755-5949.2009.00104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thambisetty M, Lovestone S. Blood-based biomarkers of Alzheimer's disease: challenging but feasible. Biomark Med. 2010;4:65–79. doi: 10.2217/bmm.09.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.O'Bryant SE, et al. A blood screening test for Alzheimer's disease. Alzheimers Dement (Amst) 2016;3:83–90. doi: 10.1016/j.dadm.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van Gool AJ, et al. Bridging the translational innovation gap through good biomarker practice. Nat Rev Drug Discov. 2017;16:587–588. doi: 10.1038/nrd.2017.72. [DOI] [PubMed] [Google Scholar]
- 65.Lista S, Faltraco F, Prvulovic D, Hampel H. Blood and plasma-based proteomic biomarker research in Alzheimer's disease. Prog Neurobiol. 2013;101–102:1–17. doi: 10.1016/j.pneurobio.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 66.Naj AC, Schellenberg GD, Alzheimer's Disease Genetics Consortium (ADGC) Genomic variants, genes, and pathways of Alzheimer's disease: an overview. Am J Med Genet B Neuropsychiatr Genet. 2017;174:5–26. doi: 10.1002/ajmg.b.32499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bertram L, Hampel H. The role of genetics for biomarker development in neurodegeneration. Prog Neurobiol. 2011;95:501–504. doi: 10.1016/j.pneurobio.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 68.Pimenova AA, Raj T, Goate AM. Untangling genetic risk for Alzheimer's disease. Biol Psychiatry. 2018;83:300–310. doi: 10.1016/j.biopsych.2017.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Genomes Project, C. A map of human genome variation from population-scale sequencing. Nature. 2010;467:1061–1073. doi: 10.1038/nature09534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Genomes Project, C et al. A global reference for human genetic variation. Nature. 2015;526:68–74. doi: 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Snyder HM, et al. Developing novel blood-based biomarkers for Alzheimer's disease. Alzheimers Dement. 2014;10:109–114. doi: 10.1016/j.jalz.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Galasko D, Golde TE. Biomarkers for Alzheimer's disease in plasma, serum and blood - conceptual and practical problems. Alzheimers Res Ther. 2013;5:10. doi: 10.1186/alzrt164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zipser BD, et al. Microvascular injury and blood-brain barrier leakage in Alzheimer's disease. Neurobiol Aging. 2007;28:977–986. doi: 10.1016/j.neurobiolaging.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 74.Montagne A, et al. Blood-brain barrier breakdown in the aging human hippocampus. Neuron. 2015;85:296–302. doi: 10.1016/j.neuron.2014.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lewczuk P, et al. Electrophoretic separation of amyloid β peptides in plasma. Electrophoresis. 2004;25:3336–3343. doi: 10.1002/elps.200406068. [DOI] [PubMed] [Google Scholar]
- 76.Arvanitakis Z, Lucas JA, Younkin LH, Younkin SG, Graff-Radford NR. Serum creatinine levels correlate with plasma amyloid beta protein. Alzheimer Dis Assoc Disord. 2002;16:187–190. doi: 10.1097/00002093-200207000-00009. [DOI] [PubMed] [Google Scholar]
- 77.Fukumoto H, et al. Age but not diagnosis is the main predictor of plasma amyloid β-protein levels. Arch Neurol. 2003;60:958–964. doi: 10.1001/archneur.60.7.958. [DOI] [PubMed] [Google Scholar]
- 78.Fagan AM, et al. Cerebrospinal fluid tau/β-amyloid(42) ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol. 2007;64:343–349. doi: 10.1001/archneur.64.3.noc60123. [DOI] [PubMed] [Google Scholar]
- 79.Pannee J, et al. The amyloid-β degradation pattern in plasma--a possible tool for clinical trials in Alzheimer's disease. Neurosci Lett. 2014;573:7–12. doi: 10.1016/j.neulet.2014.04.041. [DOI] [PubMed] [Google Scholar]
- 80.Lewczuk P, et al. Amyloid beta peptides in plasma in early diagnosis of Alzheimer's disease: a multicenter study with multiplexing. Exp Neurol. 2010;223:366–370. doi: 10.1016/j.expneurol.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 81.Roeben B, et al. Association of plasma Abeta40 peptides, but not Abeta42, with coronary artery disease and diabetes mellitus. J Alzheimers Dis. 2016;52:161–169. doi: 10.3233/JAD-150575. [DOI] [PubMed] [Google Scholar]
- 82.Hilal S, et al. Plasma amyloid-beta levels, cerebral small vessel disease, and cognition: the Rotterdam Study. J Alzheimers Dis. 2017;60:977–987. doi: 10.3233/JAD-170458. [DOI] [PubMed] [Google Scholar]
- 83.Lopez OL, et al. Plasma amyloid levels and the risk of AD in normal subjects in the Cardiovascular Health Study. Neurology. 2008;70:1664–1671. doi: 10.1212/01.wnl.0000306696.82017.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lewczuk P, et al. Cerebrospinal fluid and blood biomarkers for neurodegenerative dementias: an update of the Consensus of the Task Force on Biological Markers in Psychiatry of the World Federation of Societies of Biological Psychiatry. World J Biol Psychiatry. 2018;19:244–328. doi: 10.1080/15622975.2017.1375556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tzen KY, et al. Plasma Aβ but not tau is related to brain PiB retention in early Alzheimer's disease. ACS Chem Neurosci. 2014;5:830–836. doi: 10.1021/cn500101j. [DOI] [PubMed] [Google Scholar]
- 86.Zetterberg H, et al. Plasma tau levels in Alzheimer's disease. Alzheimers Res Ther. 2013;5:9. doi: 10.1186/alzrt163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mattsson N, et al. Plasma tau in Alzheimer disease. Neurology. 2016;87:1827–1835. doi: 10.1212/WNL.0000000000003246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mielke MM, et al. Association of plasma total tau level with cognitive decline and risk of mild cognitive impairment or dementia in the Mayo Clinic Study on Aging. JAMA Neurol. 2017;74:1073–1080. doi: 10.1001/jamaneurol.2017.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gisslén M, et al. Plasma concentration of the neurofilament light protein (NFL) is a biomarker of CNS injury in HIV infection: a cross-sectional study. EBioMedicine. 2016;3:135–140. doi: 10.1016/j.ebiom.2015.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kuhle J, et al. Comparison of three analytical platforms for quantification of the neurofilament light chain in blood samples: ELISA, electrochemiluminescence immunoassay and Simoa. Clin Chem Lab Med. 2016;54:1655–1661. doi: 10.1515/cclm-2015-1195. [DOI] [PubMed] [Google Scholar]
- 91.Rojas JC, et al. Plasma neurofilament light chain predicts progression in progressive supranuclear palsy. Ann Clin Transl Neurol. 2016;3:216–225. doi: 10.1002/acn3.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rohrer JD, et al. Serum neurofilament light chain protein is a measure of disease intensity in frontotemporal dementia. Neurology. 2016;87:1329–1336. doi: 10.1212/WNL.0000000000003154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hansson O, et al. Blood-based NfL: a biomarker for differential diagnosis of parkinsonian disorder. Neurology. 2017;88:930–937. doi: 10.1212/WNL.0000000000003680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vassar R, et al. Beta-secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286:735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- 95.Wu G, et al. Characterization of plasma β-secretase (BACE1) activity and soluble amyloid precursor proteins as potential biomarkers for Alzheimer's disease. J Neurosci Res. 2012;90:2247–2258. doi: 10.1002/jnr.23122. [DOI] [PubMed] [Google Scholar]
- 96.Shen Y, et al. Increased plasma beta-secretase 1 may predict conversion to Alzheimer's disease dementia in individuals with mild cognitive impairment. Biol Psychiatry. 2018;83:447–455. doi: 10.1016/j.biopsych.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hye A, et al. Plasma proteins predict conversion to dementia from prodromal disease. Alzheimers Dement. 2014;10:799–807.e792. doi: 10.1016/j.jalz.2014.05.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.O'Bryant SE, et al. Validation of a serum screen for Alzheimer's disease across assay platforms, species, and tissues. J Alzheimers Dis. 2014;42:1325–1335. doi: 10.3233/JAD-141041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yu S, et al. Serum protein-based profiles as novel biomarkers for the diagnosis of Alzheimer's disease. Mol Neurobiol. 2017 doi: 10.1007/s12035-017-0609-0. [DOI] [PubMed] [Google Scholar]
- 100.Corso G, et al. Serum amino acid profiles in normal subjects and in patients with or at risk of Alzheimer dementia. Dement Geriatr Cogn Dis Extra. 2017;7:143–159. doi: 10.1159/000466688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Keller A, et al. Validating Alzheimer's disease micro RNAs using next-generation sequencing. Alzheimers Dement. 2016;12:565–576. doi: 10.1016/j.jalz.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 102.Guo R, et al. A 9-microRNA signature in serum serves as a noninvasive biomarker in early diagnosis of Alzheimer's disease. J Alzheimers Dis. 2017;60:1365–1377. doi: 10.3233/JAD-170343. [DOI] [PubMed] [Google Scholar]
- 103.Anand S, et al. Discovery and confirmation of diagnostic serum lipid biomarkers for Alzheimer's disease using direct infusion mass spectrometry. J Alzheimers Dis. 2017;59:277–290. doi: 10.3233/JAD-170035. [DOI] [PubMed] [Google Scholar]
- 104.Lista S, Faltraco F, Hampel H. Biological and methodical challenges of blood-based proteomics in the field of neurological research. Prog Neurobiol. 2013;101–102:18–34. doi: 10.1016/j.pneurobio.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 105.Kiddle SJ, Voyle N, Dobson R. A blood test for Alzheimer's disease: progress, challenges and recommendations. J Alzheimers Dis. doi: 10.3233/JAD-179904. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Andreasson U, Blennow K, Zetterberg H. Update on ultrasensitive technologies to facilitate research on blood biomarkers for central nervous system disorders. Alzheimers Dement (Amst) 2016;3:98–102. doi: 10.1016/j.dadm.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.O'Bryant SE, et al. Guidelines for the standardization of preanalytic variables for bloodbased biomarker studies in Alzheimer's disease research. Alzheimers Dement. 2015;11:549–560. doi: 10.1016/j.jalz.2014.08.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jack CR, Jr, et al. A/T/N: An unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology. 2016;87:539–547. doi: 10.1212/WNL.0000000000002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lista S, Dubois B, Hampel H. Paths to Alzheimer's disease prevention: from modifiable risk factors to biomarker enrichment strategies. J Nutr Health Aging. 2015;19:154–163. doi: 10.1007/s12603-014-0515-3. [DOI] [PubMed] [Google Scholar]
- 110.Gupta VB, Sundaram R, Martins RN. Multiplex biomarkers in blood. Alzheimers Res Ther. 2013;5:31. doi: 10.1186/alzrt185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lista S, Zetterberg H, O'Bryant SE, Blennow K, Hampel H. Evolving relevance of neuroproteomics in Alzheimer's disease. Methods Mol Biol. 2017;1598:101–115. doi: 10.1007/978-1-4939-6952-4_5. [DOI] [PubMed] [Google Scholar]
- 112.Brinkmalm A, et al. Explorative and _targeted neuroproteomics in Alzheimer's disease. Biochim Biophys Acta. 2015;1854:769–778. doi: 10.1016/j.bbapap.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 113.Blennow K, Zetterberg H. Understanding biomarkers of neurodegeneration: ultrasensitive detection techniques pave the way for mechanistic understanding. Nat Med. 2015;21:217–219. doi: 10.1038/nm.3810. [DOI] [PubMed] [Google Scholar]
- 114.Robin X, et al. Bioinformatics for protein biomarker panel classification: what is needed to bring biomarker panels into in vitro diagnostics? Expert Rev Proteomics. 2009;6:675–689. doi: 10.1586/epr.09.83. [DOI] [PubMed] [Google Scholar]
- 115.Hilario M, Kalousis A. Approaches to dimensionality reduction in proteomic biomarker studies. Brief Bioinform. 2008;9:102–118. doi: 10.1093/bib/bbn005. [DOI] [PubMed] [Google Scholar]
- 116.Hampel H, et al. Advances in the therapy of Alzheimer's disease: _targeting amyloid beta and tau and perspectives for the future. Expert Rev Neurother. 2015;15:83–105. doi: 10.1586/14737175.2015.995637. [DOI] [PubMed] [Google Scholar]
- 117.Broich K, Weiergräber M, Hampel H. Biomarkers in clinical trials for neurodegenerative diseases: regulatory perspectives and requirements. Prog Neurobiol. 2011;95:498–500. doi: 10.1016/j.pneurobio.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 118.Nistico G, Broich K, Hampel H. Need for new guidelines for Alzheimer's disease clinical trials. European Journal of Neurodegenerative Diseases. 2013;2:181–186. [Google Scholar]
- 119.Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015;372:793–795. doi: 10.1056/NEJMp1500523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.UK Biobank. About UK Biobank. 2018 http://www.ukbiobank.ac.uk/about-biobank-uk/
- 121.Dubois B, et al. Cognitive and neuroimaging parameters and brain amyloidosis in individuals at risk of Alzheimer's disease (INSIGHT-preAD): a longitudinal observational study. Lancet Neurology. doi: 10.1016/S1474-4422(18)30029-2. (In press) [DOI] [PubMed] [Google Scholar]
- 122.Doecke JD, et al. Blood-based protein biomarkers for diagnosis of Alzheimer disease. Arch Neurol. 2012;69:1318–1325. doi: 10.1001/archneurol.2012.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mapstone M, et al. Plasma phospholipids identify antecedent memory impairment in older adults. Nat Med. 2014;20:415–418. doi: 10.1038/nm.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Burnham SC, et al. A blood-based predictor for neocortical Aβ burden in Alzheimer's disease: results from the AIBL study. Mol Psychiatry. 2014;19:519–526. doi: 10.1038/mp.2013.40. [DOI] [PubMed] [Google Scholar]
- 125.Kerr NL. HARKing: hypothesizing after the results are known. Pers Soc Psychol Rev. 1998;2:196–217. doi: 10.1207/s15327957pspr0203_4. [DOI] [PubMed] [Google Scholar]
- 126.Robinson SM, Canavan M, O'Keeffe ST. Preferences of older people for early diagnosis and disclosure of Alzheimer's disease (AD) before and after considering potential risks and benefits. Arch Gerontol Geriatr. 2014;59:607–612. doi: 10.1016/j.archger.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 127.Ashton NJ, et al. Blood protein predictors of brain amyloid for enrichment in clinical trials? Alzheimers Dement (Amst) 2015;1:48–60. doi: 10.1016/j.dadm.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Aisen PS, Vellas B, Hampel H. Moving towards early clinical trials for amyloid-_targeted therapy in Alzheimer's disease. Nat Rev Drug Discov. 2013;12:324. doi: 10.1038/nrd3842-c1. [DOI] [PubMed] [Google Scholar]
- 129.Hampel H, et al. Biomarkers for Alzheimer's disease therapeutic trials. Prog Neurobiol. 2011;95:579–593. doi: 10.1016/j.pneurobio.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 130.Vellas B, et al. Designing drug trials for Alzheimer's disease: what we have learned from the release of the phase III antibody trials: a report from the EU/US/CTAD Task Force. Alzheimers Dement. 2013;9:438–444. doi: 10.1016/j.jalz.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 131.Hood L, Flores M. A personal view on systems medicine and the emergence of proactive P4 medicine: predictive, preventive, personalized and participatory. N Biotechnol. 2012;29:613–624. doi: 10.1016/j.nbt.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 132.Gligorijević V, Malod-Dognin N, Pržulj N. Integrative methods for analyzing big data in precision medicine. Proteomics. 2016;16:741–758. doi: 10.1002/pmic.201500396. [DOI] [PubMed] [Google Scholar]
- 133.Younesi E, Hofmann-Apitius M. From integrative disease modeling to predictive, preventive, personalized and participatory (P4) medicine. EPMA J. 2013;4:23. doi: 10.1186/1878-5085-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hampel H, et al. The cholinergic system in the pathophysiology and treatment of Alzheimer's disease. Brain. 2018 doi: 10.1093/brain/awy132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.