Abstract
Precision cardiology is a vision of a health-care approach that identifies the optimal course of care for each patient. Although precision cardiology is still in its nascent stage, new approaches and methodologies are being developed to achieve this goal and to overcome technical and implementation barriers. In 2018, several high-impact studies made progress in this direction.
Coronary artery disease (CAD) is a leading cause of death in the industrialized world1. Identifying individuals who are at high risk of CAD for the purpose of primary prevention remains a major health-care challenge. Given that nearly 50% of CAD cases are estimated to be heritable, stratifying patients for CAD risk could benefit tremendously from a comprehensive assessment of genetic susceptibility. However, the potential of genomic risk scores (GRS) to predict CAD outcomes has not been fully harnessed because previous studies had limited genomic scope and sample sizes, resulting in low generalizability. Overcoming these limitations, Inouye et al. constructed a powerful new CAD GRS with the use of a meta-analytic approach that combined large-scale, genome-wide and _targeted genetic association data2. The utility of the new GRS as a screening tool for primary prevention of CAD was evaluated on genome data from >480,000 individuals included in the UK Biobank (>22,000 with CAD; 460,000 without CAD). The new GRS had a larger association with CAD than any other score. The score stratified individuals into significantly different trajectories of CAD risk, in which those in the top 20% of GRS distribution had a hazard ratio of 4.17 compared with those in the bottom 20%. Furthermore, the GRS had a higher C-index for CAD than any of the conventional risk factors. This study is an important advance in the utility of genomic data to risk-stratify individuals who are susceptible to CAD and underscores the importance of addressing the heritable component of the CAD risk profile at a young age, as a complement to conventional risk-factor assessment in later life.
Obtaining and analysing information from wearable devices is an important aspect in realizing the promise of precision medicine. Atrial fibrillation (AF) is the most prevalent cardiac arrhythmia, and patients with AF have a higher mortality than healthy individuals owing to the ~5-fold increase in the risk of stroke associated with AF3. For individuals who have a stroke associated with AF, the arrhythmia is typically not diagnosed until stroke occurrence. Early identification of AF could result in initiation of prophylactic anticoagulation, reducing the risk of stroke. Two novel studies focused on the use of digital technology for early detection of AF. The mSToPS randomized clinical trial4 aimed to determine whether a home-based, self-applied, wearable electrocardiogram (ECG) patch could improve AF diagnosis compared with routine care. A total of 2,659 participants who had an increased risk of AF were randomly assigned to immediate, home-based monitoring with the ECG patch for 14 days or to monitoring delayed for 4 months. Immediate monitoring led to a significantly higher rate of AF diagnosis at 4 months (3.9% versus 0.9%). Individuals in the immediate-monitoring group were also more likely to be prescribed anticoagulation therapy earlier than unmonitored participants. The second study on early AF detection with the use of wearable devices, a multinational, remote-cohort study, leveraged the ubiquitous nature of smart watches and the new advancements in data science5. The investigators conducted a series of clinical studies to derive and validate a deep learning algorithm for automated AF detection using data from commercially available smart watches. An artificial neural network was trained to approximate RR intervals from pulse plethysmography in 9,750 participants. Validation was performed against 12-lead ECG in a separate cohort of 51 patients undergoing cardioversion for AF. In the validation cohort, the neural network could detect AF with high accuracy (C-statistic 0.97). These data support the proof of concept that a commercially available smart watch coupled with a deep neural network might ultimately be applied to perform AF detection at a large scale. The two studies reviewed above demonstrate that convenient, wearable devices and digital technologies that considerably increase user acceptance and data availability could facilitate early detection of AF and thereby help to prevent stroke.
In 2018, the field of computational modelling also made novel contributions to precision cardiology. In a proof-of-concept study of ablation of infarct-related ventricular tachycardia (VT), a personalized, virtual-heart methodology was used to determine the optimal ablation _targets and to guide patient treatment6. The study included a retrospective validation of the technology in 21 patients and prospective precision procedure guidance in five patients. This new technology seems poised to eliminate the need for invasive electrical mapping of VT circuits to determine the ablation _targets. The virtual-heart approach involved constructing 3D computer models of patients’ hearts with the use of noninvasive, contrast-enhanced MRI data and executing simulations to evaluate the patient-specific VTs, from which the optimal ablation _targets were then determined. Importantly, the virtual-heart approach _targeted termination not only of VTs that were clinically manifested or induced in the patient at the time of procedure, but also of all VTs that could arise in the patient’s infarct-remodelled heart, including those that might arise after the initial ablation procedure. This technology therefore aims to eliminate the need for repeated ablations and to offer long-term freedom from VT. The study is an important advance in asserting the potential of computational modelling to guide precision treatments for heart function disorders.
Computational modelling has also been used to guide engineering of cardiac tissues. The growth in the number of transcatheter valve replacement procedures and the disappointing performance of prosthetic valves had spurred interest in tissue-engineered heart valves generated with the patient’s own cells and therefore capable of self-repair, remodeling and regeneration7. The tissue-engineering technique involves implanting a valve scaffold and allowing the scaffold to be seeded by the patient’s cells. However, in animal models, these engineered valves have been shown to undergo uncontrolled tissue remodelling, particularly fibrosis and leaflet shortening due to excessive radial stress, which often translates into valve failure. In a study by Emmert et al., computational modelling was used to design valve morphologies that minimize leaflet radial compression (FIG. 1) and thereby ensure long-term functionality of the valve8. Engineered valves were implanted in a sheep translational model (n = 10) in the pulmonary valve position, and valve performance was analysed for 1 year on a monthly basis with the use of MRI and intracardiac echocardiography. In nine animals, the valves displayed quantitative measures of valve stenosis and regurgitation similar to that of native pulmonary valves. Four valves were examined after explant, revealing that the distribution of fibrosis corresponded well with that predicted from the computational model. This study demonstrated the importance of using computational modelling to optimize the design of patient-specific, tissue-engineered implants.
Fig. 1. Computational modelling to guide tissue engineering of personalized replacement valves.
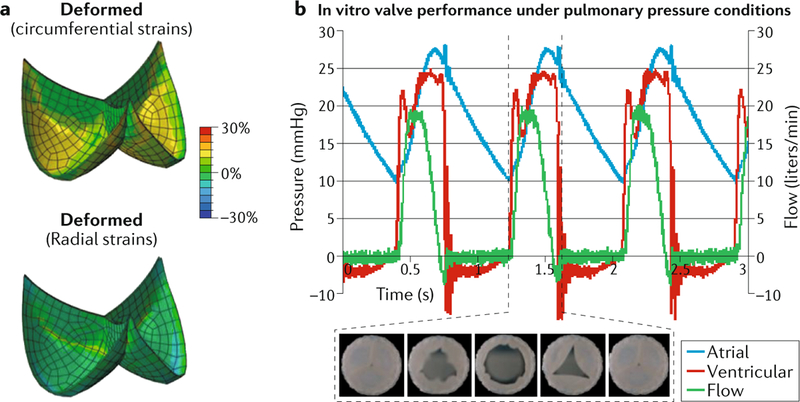
Computational modelling can be used to guide tissue engineering of valve morphologies that minimize radial compression of the leaflets. a | Simulated deformation of the designed leaflets demonstrating increased circumferential strain during deformation, without a significant radial strain. b | Representative flow and pressure curves during in vitro measurements, with representative images of the opening and closure of the engineered valves during the cardiac cycle. Adapted with permission from REF.8, AAAS.
The final study reviewed here presented a proof-of-concept of the design and utility of an individualized, 3D-printed heart device, in this case an occluder for the left atrial appendage (LAA)9. LAA occlusion is now commonly used to reduce the risk of thromboembolism in patients with AF10. However, current LAA-occlusion devices are designed with a fixed, rigid structure, despite the high variability in size and shape of human LAAs, frequently resulting in incomplete LAA occlusion. The investigators developed workflows for the design, fabrication and deployment of a patient-specific, soft LAA occluder. The geometry of the occluder was determined from non-invasive CT imaging so that the device matched the anatomy of the patient’s LAA. The workflow included 3D printing of a model with the patient-specific LAA geometry and using the model to create a flexible silicone occluder device, which was then coated with a polymer to allow endothelial cell attachment. Several in vitro studies of device deployment and LAA occlusion demonstrated the feasibility of the approach in a canine in vivo model. This study brings us one step closer to the fabrication of durable and haemocompatible personalized cardiovascular implants.
Key advances.
The utility of genomic data to risk-stratify individuals susceptible to coronary artery disease (CAD) has been demonstrated in a study of 480,000 adults, underscoring the importance of identifying the heritable component of CAD risk early in life2.
The mSToPS trial demonstrated that a home-based, self-applied, wearable electrocardiogram patch could improve diagnosis of atrial fibrillation (AF) and reduce the risk of stroke compared with routine care; similarly, early detection of AF could be achieved by a commercially available smart watch coupled with a deep neural network4,5.
The feasibility of using a personalized virtual-heart methodology to determine the optimal ablation _targets for infarct-related ventricular tachycardia and to directly guide patient treatment has been demonstrated6.
Computational modelling has been utilized to guide tissue-engineering of cardiac valves that uses patient cells, helping to overcome the limitations of earlier engineered valves8.
The concept of utilizing individualized, 3D-printed occluders for the left atrial appendage has been proven, bringing us a step closer to the fabrication of durable, personalized cardiovascular implants9.
Acknowledgements
N.T. acknowledges funding from the NIH (PD1 HL123271, R01 HL126802 and U01 HL141074) and a grant from Foundation Leducq. N.T. is grateful to Ashish Doshi (Johns Hopkins University, USA) for help in preparing this manuscript.
Footnotes
Competing interests
N.T. is a founder of CardioSolv, holds an equity ownership interest in the company and acts as its Chief Scientific Officer.
References
- 1.Fishman GI et al. Sudden cardiac death prediction and prevention: report from a National Heart, Lung, and Blood Institute and Heart Rhythm Society Workshop. Circulation 122, 2335–2348 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Inouye M et al. Genomic risk prediction of coronary artery disease in 480,000 adults: implications for primary prevention. J. Am. Coll. Cardiol 72, 1883–1893 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrade J, Khairy P, Dobrev D & Nattel S The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ. Res 114, 1453–1468 (2014). [DOI] [PubMed] [Google Scholar]
- 4.Steinhubl SR et al. Effect of a home-based wearable continuous ECG monitoring patch on detection of undiagnosed atrial fibrillation: the mSToPS randomized clinical trial. JAMA 320, 146–155 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tison GH et al. Passive detection of atrial fibrillation using a commercially available smartwatch. JAMA Cardiol 3, 409–416 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prakosa A et al. Personalized virtual-heart technology for guiding the ablation of infarct-related ventricular tachycardia. Nat. Biomed. Eng 2, 732 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yacoub MH & Takkenberg JJ Will heart valve tissue engineering change the world? Nat. Clin. Pract. Cardiovasc. Med 2, 60–61 (2005). [DOI] [PubMed] [Google Scholar]
- 8.Emmert MY et al. Computational modeling guides tissue-engineered heart valve design for long-term in vivo performance in a translational sheep model. Sci. Transl Med 10, eaan4587 (2018). [DOI] [PubMed] [Google Scholar]
- 9.Robinson SS et al. Patient-specific design of a soft occluder for the left atrial appendage. Nat. Biomed. Eng 2, 8 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Yu CM et al. Mechanical antithrombotic intervention by LAA occlusion in atrial fibrillation. Nat. Rev. Cardiol 10, 707–722 (2013). [DOI] [PubMed] [Google Scholar]


