Abstract
Substance P (SP) is a peptide that is present in unmyelinated primary afferents to the dorsal horn and is released in response to painful or noxious stimuli. Opiates active at the μ-opiate receptor (MOR) produce antinociception, in part, through modulation of responses to SP. MOR ligands may either inhibit the release of SP or reduce the excitatory responses of second-order neurons to SP. We examined potential functional sites for interactions between SP and MOR with dual electron microscopic immmunocytochemical localization of the SP receptor (NK1) and MOR in rat trigeminal dorsal horn. We also examined the relationship between SP-containing profiles and NK1-bearing profiles. We found that 56% of SP-immunoreactive terminals contact NK1 dendrites, whereas 34% of NK1-immunoreactive dendrites receive SP afferents. This result indicates that there is not a significant mismatch between sites of SP release and available NK1 receptors, although receptive neurons may contain receptors at sites distant from the peptide release site. With regard to opioid receptors, we found that many MOR-immunoreactive dendrites also contain NK1 (32%), whereas a smaller proportion of NK1-immunoreactive dendrites contain MOR (17%). Few NK1 dendrites (2%) were contacted by MOR-immunoreactive afferents. These results provide the first direct evidence that MORs are on the same neurons as NK1 receptors, suggesting that MOR ligands directly modulate SP-induced nociceptive responses primarily at postsynaptic sites, rather than through inhibition of SP release from primary afferents. This colocalization of NK1 and MORs has significant implications for the development of pain therapies _targeted at these nociceptive neurons.
Keywords: pain, electron microscopy, neuropeptide, analgesia, substantia gelatinosa, opioid receptors, tachykinin receptor, substance P, trigeminal nucleus caudalis, dental pain
Substance P (SP) is contained in many unmyelinated primary afferent axons that terminate in the dorsal horn of the spinal cord and caudal spinal trigeminal nuclei (Hökfelt et al., 1975; Barber et al., 1979). SP is released from primary afferent terminals by noxious or painful stimuli (Go and Yaksh, 1987; Duggan et al., 1988; Lerma et al., 1997) and is thought to be involved in pain transmission at the first central synapse. This hypothesis is supported by the observation that humans with diminished pain sensitivity appear to lack SP-containing fibers specifically in the dorsal horn area (Pearson et al., 1982). Furthermore, mice without the gene that encodes the SP precursor peptide show diminished responses to intensely painful stimuli (Cao et al., 1998). Anatomically, SP-containing terminals in the spinal dorsal horn preferentially _target neurons that encode noxious stimuli but not those that encode innocuous stimuli (De Koninck et al., 1992; Ma et al., 1997).
Ligands of the μ-opiate receptor (MOR), such as morphine, are highly effective in blocking or reducing nociceptive transmission at the spinal level (Yaksh and Rudy, 1976), and this antinociception is thought to be caused at least partially by diminished release of SP (Jessell and Iversen, 1977; Go and Yaksh, 1987; Mauborgne et al., 1987;Collin et al., 1992; Suarez-Roca and Maixner, 1992). These data, along with findings that dorsal rhizotomy can cause a significant reduction in MOR binding in the dorsal horn (LaMotte et al., 1976; Besse et al., 1992; Zhang et al., 1998), have led to suggestions that MOR ligands act at receptors that are localized mainly presynaptically on SP-containing axon terminals in the dorsal horn (Hökfelt et al., 1977; Jessell and Iversen, 1977; Besse et al., 1992).
Other evidence suggests that MOR agonists produce antinociception by decreasing the activity of neurons that receive afferent input from SP-containing axon terminals. First, studies using antibody-labeled microprobes to detect SP release within the spinal cord of intact cats have reported no effect of systemic morphine on SP release (Morton et al., 1990). Second, there is residual binding to MORs in the dorsal horn that remains stable long after rhizotomy (Besse et al., 1992). Third, MOR-immunoreactive somata and dendrites are present in the dorsal horn (Cheng et al., 1996; Zhang et al., 1998; Aicher et al., 2000). Fourth, identified nociceptive neurons in the dorsal horn are postsynaptic to SP-containing terminals and terminals that contain enkephalin, a peptide that is a potential endogenous ligand for MORs (Ma et al., 1997). We also showed recently that SP-containing terminals frequently contact dendrites containing MOR, but MOR is only rarely contained within SP terminals (Aicher et al., 2000). Together these results suggest that MORs may be located at sites postsynaptic to SP terminals in the dorsal horn. If this is true, MORs should be colocalized with the SP receptor, NK1.
We quantitatively examined the cellular distributions of MORs and NK1 in the medullary dorsal horn to determine whether there was morphological evidence supporting a presynaptic and/or postsynaptic model for the observed functional interactions between SP-receptive and MOR-containing cells. We used dual-labeling immunocytochemistry for NK1 and MOR with immunogold and immunoperoxidase detection of each antigen. Tissue sections were collected through laminae I and II (outer) of the rat spinal trigeminal caudalis, and randomly sampled profiles were examined with an electron microscope. The spinal trigeminal caudalis is functionally analogous to the spinal dorsal horn (Dubner and Bennett, 1983) and is enriched in SP-containing primary afferents and MOR binding sites (Jessell and Iversen, 1977; Priestley et al., 1982).
MATERIALS AND METHODS
Perfusion and tissue processing. The procedures used in these studies were approved by the Institutional Animal Care and Use Committee of Cornell University Medical College. All efforts were made to prevent animal suffering and to use the minimum number of animals needed to make sound scientific conclusions. Male Sprague Dawley rats (300–400 gm; n = 8) were deeply anesthetized with an overdose of sodium pentobarbital (150 mg/kg, i.p.) and perfused through the ascending aorta with 3.8% acrolein in 2.0% paraformaldehyde, followed by 2% paraformaldehyde [in 0.1 mphosphate buffer (PB), pH 7.4]. The medulla was removed and placed in 2% paraformaldehyde for 30 min, then sectioned (40 μm) on a vibrating microtome (Leica, Rockleigh, NJ) and collected into 0.1m PB.
Antisera. Well characterized commercial antibodies were used for these experiments. A rabbit anti-peptide antibody to NK1 (Novus Biologicals, Littleton, CO) was generated against a 15-residue portion (KTMTESSSFYSNMLA) of the C terminus of the rat NK1 receptor conjugated to bovine thyroglobulin. Staining is abolished by preabsorption with the antigenic peptide, and the staining is detected in tissues known to contain high levels of NK1. In our hands, the immunocytochemical distribution of NK1 was identical to that shown in previous studies of trigeminal dorsal horn (Brown et al., 1995). The NK1 antibody was used at a dilution of 1:1000 for peroxidase labeling and 1:100 for immunogold labeling. A rat monoclonal antiserum to SP was obtained from Sera Labs (Westbury, NY) (Milner et al., 1988). This antibody is secreted by a hybridoma formed by fusion of a mouse myeloma cell and a spleen cell from a Wistar rat immunized with substance P conjugated to BSA. The hybridoma secretes only specific heavy and light chains that recognize the C-terminal end of substance P. Dot blot immunocytochemistry showed that this antibody recognizes SP and also shows some binding to other members of the tachykinin family, including neurokinins A and B and physalaemin (Milner et al., 1988), but does not recognize neuromedin B or neuromedin C. This antibody was used at a dilution of 1:1000 for immunoperoxidase labeling. The guinea pig anti-MOR antiserum was obtained from Chemicon International (Temecula, CA). This anti-peptide antibody was produced using the a synthetic peptide (NHQLENLEAETAPLP) that corresponds to amino acids 384–398 of the C terminus of the cloned rat MOR1. The pattern of labeling obtained is identical to that seen with other MOR antibodies, and labeling is abolished by preabsorption with the antigenic peptide. The MOR antibody was used at a dilution of 1:5000 for immunogold silver labeling and 1:10,000 for immunoperoxidase localization.
The secondary antibodies used in these studies were obtained from commercial sources as follows: biotinylated donkey anti-rabbit IgG, donkey anti-rat IgG, and donkey anti-guinea pig IgG (Vector, Burlingame, CA); gold-conjugated goat anti-rabbit IgG and goat anti-guinea pig IgG (Amersham, Arlington Heights, IL). Secondary antibodies were used at a dilution of 1:400 for immunoperoxidase labeling and 1:50 for immunogold labeling.
The MOR localization that was obtained with this antibody is consistent with the localization of MOR as detected with receptor autoradiography (LaMotte et al., 1976; Besse et al., 1990; Besse et al., 1992) and with immunolabeling for other MOR antibodies (Arvidsson et al., 1995;Mansour et al., 1995). Therefore, the labeling described in this study is likely to represent MOR protein. The SP antibody has been characterized previously in this laboratory (Milner et al., 1988) and recognizes mainly SP, with more modest detection of neurokinins A and B and physalaemin, but does not recognize neuromedins A or C. Therefore, the SP labeling is mainly SP but probably includes some profiles that contain other tachykinins, which may also be involved in nociceptive transmission (Neugebauer et al., 1996). The NK1 receptor labeling is also consistent with that reported in other studies and appears to be selective for this receptor subtype (Liu et al., 1993; Routh and Helke, 1995; McLeod et al., 1998), although it may not recognize all of the isoforms of the receptor (Mantyh et al., 1996). Although the antibodies used in the present studies have been well characterized, it is always possible that a portion of the labeling obtained with each antibody may reflect detection of similar or identical peptides in a different protein. Thus, for all data and discussions, the term “labeling” should be understood to mean antigen-like immunoreactivity.
Dual labeling for NK1 and MOR. Details of the methods for combined immunoperoxidase and immunogold labeling have been published previously (Chan et al., 1990; Aicher et al., 1995, 1996, 1997). Tissue was processed using each detection method for the localization of NK1 and MOR antisera to control for differences in sensitivity of the two detection methods (Leranth and Pickel, 1989). Tissue sections were incubated in 1% sodium borohydride (30 min) to enhance immunoreactivity, then cryoprotected in a solution containing 12.5% sucrose and 1% glycerol for 15 min, then rapidly frozen in liquid nitrogen to increase membrane permeability before antibody incubations. Tissue sections were then incubated for 30 min in 0.5% bovine serum albumin (BSA) in 0.1 m Tris-saline (Tris, pH 7.6) to reduce nonspecific binding, followed by incubation in a mixture of two primary antibodies raised in different species (see above) at 4°C for 48 hr. This tissue was incubated in a secondary biotinylated IgG and placed in ABC solution (Elite kit, Vector) for 30 min at room temperature. The immunoperoxidase label was visualized by the DAB peroxidase method (Aicher et al., 1996). Subsequently, the tissue was incubated in the secondary gold-conjugated IgG for 2 hr at room temperature, and the second label was then visualized using immunogold detection and silver intensification (IntensEM kit, Amersham) of the secondary antisera (Chan et al., 1990).
Dual labeling for SP and NK1. Generally the procedures outlined above were used for these dual-labeling experiments as well, except that only one dilution of each antiserum was used. For this analysis, SP was always detected using the immunoperoxidase method, and NK1 was always detected using the immunogold method. Randomly sampled SP-labeled profiles were photographed from regions of the dorsal horn that contained both labels within at least 20 μm of each other.
Electron microscopy: tissue preparation. For electron microscopy, tissue was rinsed in PB, placed in 2.0% osmium tetroxide for 1 hr, dehydrated, and embedded in EMBed between two sheets of Aclar plastic. Ultrathin sections (70 nm) through laminae I and II (outer) of the dorsal horn of trigeminal nucleus caudalis (ventrolateral region) (5.3–5.6 mm caudal to the interaural line) (Paxinos and Watson, 1986) were collected from the surface of the tissue, placed on copper grids, and counterstained with uranyl acetate and Reynold's lead citrate.
Electron microscopy: sampling methods, labeling criteria, and data analysis. Ultrathin sections collected from 10 vibratome sections (from seven different animals) were examined by electron microscopy. All sections chosen for analysis contained both immunogold- and immunoperoxidase-labeled profiles and had excellent preservation of ultrastructural detail. Regions of the plastic/tissue interface containing both immunogold- and immunoperoxidase-labeled profiles within the same field (20 μm square) were viewed and photographed with a Philip's 201 electron microscope. The total area of the micrographs examined for this analysis was ∼17,000 μm2.
Immunogold–silver labeling for each receptor antigen was characterized as either plasmalemmal (on or within 20 nm of the plasma membrane) or cytoplasmic. The former would reflect potential functional sites accessible to extracellular ligands, whereas the latter may reflect internalized or newly synthesized receptor protein (Boudin et al., 1998). Plasmalemmal labeling was further classified as either synaptic (in contact with or within 80 nm of the postsynaptic density) or nonsynaptic (>80 nm away from the synaptic density). The background levels of immunogold–silver labeling (i.e., randomly scattered grains not associated with plasma membranes or cytoplasmic organelles) were minimal. Thus, for profiles <0.5 μm, the presence of one or more gold particles was considered positive labeling if at least one particle was located on the plasma membrane (Garzón et al., 1999). A stricter criterion of at least two particles was used for positive identification of larger profiles. In a recent study,Garzón et al. (1999) compared labeling criteria of one gold particle on a membranous structure versus at least two gold particles and showed that the distribution of the labeling in various types of profiles was similar using both criteria, but more profiles were detected using the less stringent criterion. In the present study using the criteria indicated above, similar distributions of profiles were seen using either the immunoperoxidase– or immunogold–silver methods for either NK1 or MOR, except for the profiles <0.4 μm in cross-sectional diameter (see Results). For most analyses, the data are pooled across both labeling methods (unless indicated otherwise).
Electron microscopy: morphological definitions. Labeled structures were classified as perikarya, dendrites, axons, axon terminals, or glia on the basis of morphological information available in the plane of section viewed (Peters et al., 1991). Axon terminals were defined by the presence of small clear vesicles, and synapses were defined by parallel membranes separated by a widened cleft and membrane specializations. Asymmetric synapses were defined by a prominent density on the postsynaptic side of the contact, whereas symmetric synapses showed equivalent densities on either side of the contact (Peters et al., 1991). Appositions between two profiles were defined by parallel membranes between them that lacked dense membrane specializations. Profiles were classified as dendrites that contained diffuse filaments in their cytoplasm and a few mitochondria and often received synaptic contacts. For quantitative analyses, dendrites were categorized on the basis of their minimum cross-sectional diameter. Astrocytic processes were identified by their amorphous shape, lack of vesicles or synaptic contacts, and occasional presence of glial microfilaments.
Preparation of figures. Electron micrographic prints were scanned using a Power Macintosh 8500/150 Computer (Apple Computers, Cupertino, CA) with an AGFA Arcus II scanner (Agfa-Gevaert, NV, Montsel, Belgium), Fotolook (Agfa-Gevaert, NV), and Adobe Photoshop (version 5.0; Adobe Systems, Mountain View, CA) software. The micrographs were adjusted only for saturation levels, sharpness, and contrast. Light microscopic images were captured using a Spot2 digital camera and software interfaced to a PC. Composite illustrations were composed and labeled using QuarkXPress (version 3.32; Quark, Denver, CO) and Adobe Illustrator (version 6.0; Adobe Systems) software.
RESULTS
SP, NK1, and MOR show overlapping distributions in trigeminal dorsal horn
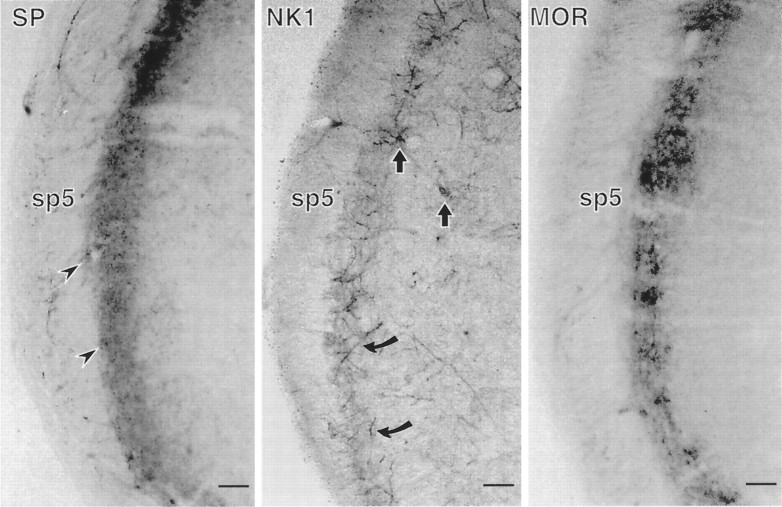
The light microscopic distribution of each of the antigens examined in this study is illustrated in Figure1. SP was located primarily in punctate profiles that we show correspond to axon terminals at the ultrastructural level. NK1 can be seen in cell bodies in lamina 1 and in deeper laminae but is primarily located in elongated profiles that correspond to dendrites at the ultrastructural level. MOR is concentrated in the outer laminae with a distribution very similar to that of SP. However, MOR labeling is rather diffuse, and isolated specific structures are difficult to discern with the light microscope.
Fig. 1.
Light micrographs showing immunoperoxidase labeling for SP, NK1, and MOR in the dorsal horn of spinal trigeminal caudalis. Labeling for SP and MOR was found almost exclusively in laminae I and II of the trigeminal dorsal horn, adjacent to the spinal trigeminal tract (sp5). SP labeling was seen primarily in punctate profiles resembling axon terminals (arrowheads). NK1 labeling was seen in a few cell bodies in both lamina I and deeper laminae (straight arrows) but was primarily found in long, slender processes resembling dendrites (curved arrows). MOR immunolabeling was diffuse, and it was difficult to identify specific structures at the light microscopic level, but most of the labeled structures were seen in the laminae that contained the highest density of SP and NK1 labeling. Scale bars, 0.2 mm.
MOR is located at both presynaptic and postsynaptic sites in trigeminal dorsal horn
Most of the MOR-labeled profiles in the trigeminal dorsal horn were dendrites or unmyelinated axons, with only a few axon terminals (Figs. 2, 3A, 7; Table1). MOR-immunoreactive perikarya were rarely encountered at the ultrastructural level, either reflecting the small number of somata as compared with dendrites within the neuropil or because the perikarya of these cells may be concentrated in slightly deeper laminae of the dorsal horn. MOR-immunoreactive dendrites were detected in this material using either immunogold or immunoperoxidase methods. When immunogold detection methods were used, MOR labeling was often detected in dendrites (Fig. 2A) and axon terminals (Fig. 2B) at nonsynaptic sites on the plasma membrane. In axon terminals, MOR immunoreactivity was sometimes associated with dense-core vesicles (Fig. 7).
Fig. 2.
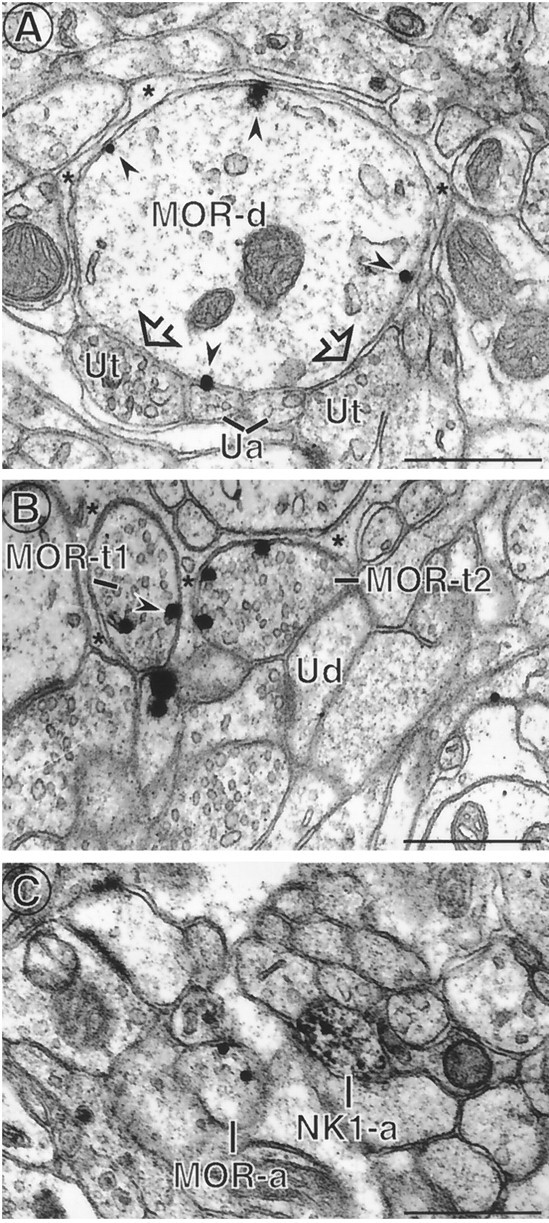
MOR immunoreactivity was detected in dendrites, axons, and axon terminals. A, A MOR-labeled dendrite (MOR-d) contains numerous immunogold particles (arrowheads) associated with the plasma membrane. A portion of the dendrite is apposed to unlabeled axon terminals (Ut) (open arrows) and unmyelinated axons (Ua), whereas the remaining surface is apposed to unlabeled astrocytic glial processes (asterisk).B, Two MOR immunogold-labeled axon terminals (MOR-t1 and MOR-t2) contain gold particles (arrowhead) associated with nonsynaptic portions of the plasma membrane. MOR-t2 is apposed to an unlabeled dendrite (Ud), but the other portions of the terminal are surrounded by unlabeled glial processes (asterisk).C, In a field containing many unmyelinated axons, an immungold-labeled unmyelinated axon (MOR-a) is near but not contacting an immunoperoxidase-labeled axon (NK1-a). Scale bars, 0.5 μm.
Fig. 3.
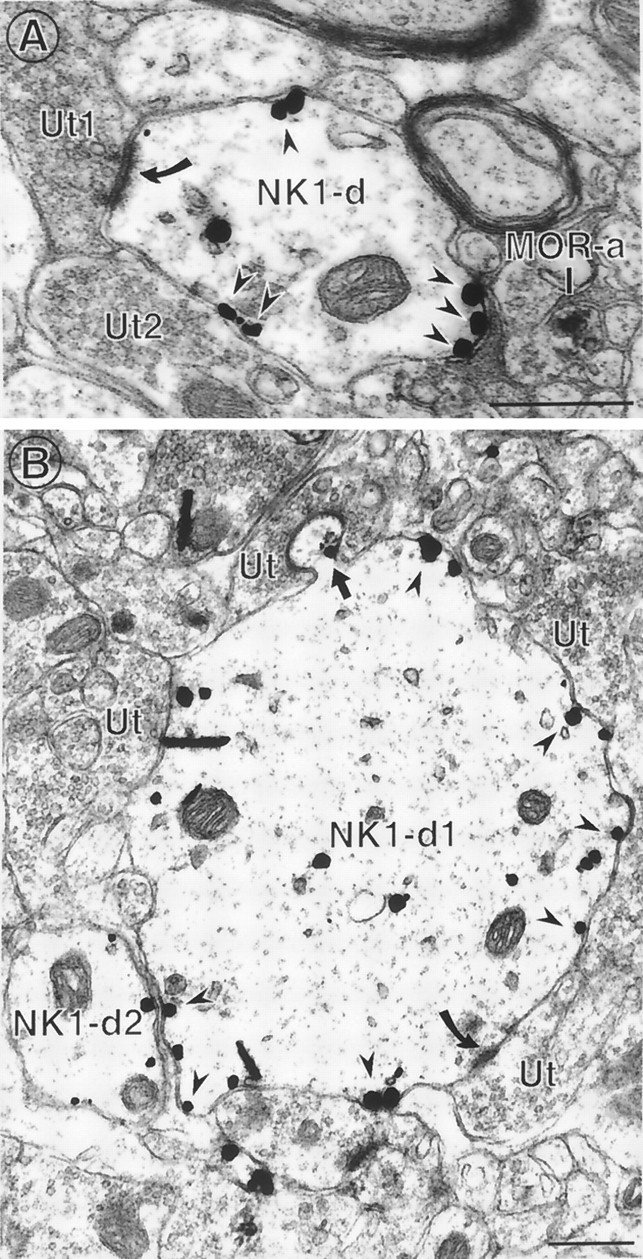
NK-1 immunoreactivity is primarily found in dendrites in trigeminal dorsal horn. A, An immunogold-labeled NK1 dendrite (NK1-d) contains several gold particles that are associated with nonsynaptic portions of the plasma membrane. This dendrite receives an asymmetric synapse (filled curved arrow) from an unlabeled terminal (Ut1) and is apposed to another unlabeled terminal (Ut2). A cluster of gold particles is located along the contact with Ut2, but no synaptic density is apparent between these membranes. A peroxidase-labeled MOR axon is separated from the NK1-labeled dendrite by an unlabeled axon interposed between these profiles. B, An immunogold-labeled NK1 dendrite (NK1-d) contains numerous gold particles associated with nonsynaptic portions of the plasma membrane (arrowheads). This dendrite is apposed by numerous unlabeled terminals (Ut), one of which forms an asymmetric synapse on the main part of the dendrite (filled curved arrow, bottom of figure) and one of which synapses with a small spinous extension of the dendrite (top of figure). An NK1 gold particle is located at the neck of this dendritic spine near the synapse (filled straight arrow). Another NK1-labeled dendrite (NK1-d2) contains gold particles that are exclusively associated with the plasma membrane and are all extrasynaptic in this plane of section. Scale bars, 0.5 μm.
Fig. 7.
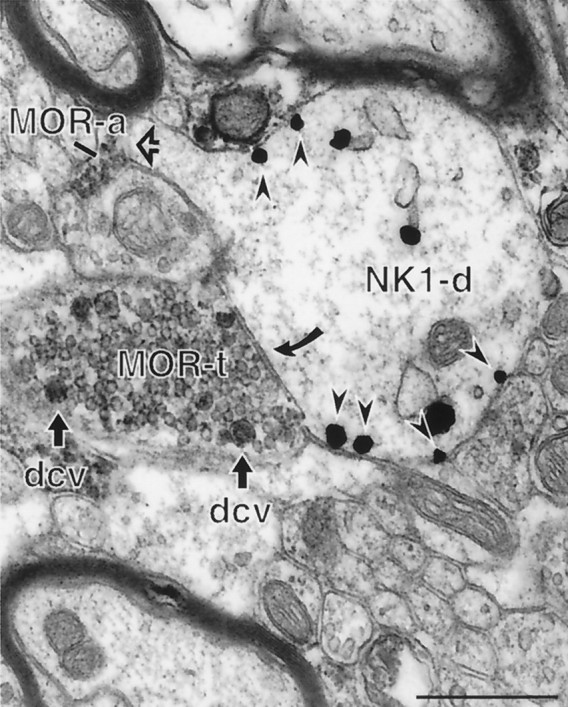
MOR-labeled axon terminals sometimes contact NK1-immunoreactive dendrites. An immunoperoxidase-labeled axon terminal (MOR-t) forms a symmetric synapse (filled curved arrow) with an immunogold-labeled dendrite (NK1-d). The MOR labeling is strongly associated with dense-core vesicles (dcv). The dendrite is also apposed to a MOR-labeled axon (MOR-a) at the head of a small spine (open arrow). Most of the immunogold particles (arrowheads) are associated with nonsynaptic portions of the plasma membrane. Scale bar, 0.5 μm.
Table 1.
Classification of neuronal profiles containing MOR immunoreactivity, NK1 immunoreactivity, or both MOR and NK1 in trigeminal dorsal horn
| Receptor | n | Somata | Dendrites | Axons | Axon terminals |
|---|---|---|---|---|---|
| MOR | 695 | 0 (0%)1-a | 311 (45%) | 348 (50%) | 36 (5%) |
| NK1 | 804 | 4 (<1%) | 713 (89%) | 81 (10%) | 6 (<1%) |
| MOR + NK1 | 149 | 1 (1%) | 145 (97%) | 3 (2%) | 0 (0%) |
Each cell in the table indicates numbers of profiles in that category for each antigen. Percentages indicate the breakdown across the table within that antigen group.
MOR somata were occasionally seen by light microscopy but were not observed in these randomly chosen fields.
NK1 is located almost exclusively in dendrites in trigeminal dorsal horn
The cellular distribution of NK1 was much more homogenous than that of MOR (Fig. 3, Table 1), with most of the labeling in dendrites of various sizes (Fig. 3), although profiles resembling unmyelinated axons were occasionally seen (Fig.2C). NK1 immunogold labeling was often associated with the plasma membrane of dendrites and usually located at nonsynaptic sites, even in dendrites that received asymmetric synaptic inputs (Fig.3A), but many NK1-immunoreactive dendrites (12%) did not receive any synaptic input in the plane of section viewed (Fig.3B, NK1-d2). NK1 immunogold particles were often associated with portions of the plasma membrane apposed by axons or axon terminals making symmetric contacts or lacking defined synaptic densities (Fig. 3A, Ut2), possibly suggesting localization at sites adjacent to synapses. Labeling adjacent to an asymmetric synapse was seen in some cases (Fig. 3B,top; see gold particle located at the neck of the spine emerging off the large dendrite).
MOR and NK1 often colocalize in dendrites
Given the virtually exclusive localization of NK1 in dendrites and the mixed distribution of MOR, we searched for two potential types of interactions: (1) the two receptors contained in the same dendrites or (2) MOR axons contacting NK1 dendrites. The most frequent interaction that we observed was colocalization of both receptors in large and small dendrites in the trigeminal dorsal horn (Fig.4, Table 1). Many of these dendrites received asymmetric synaptic contacts from axon terminals containing both small clear and dense-core vesicles and contained extrasynaptic labeling for NK1 (Fig. 4). Frequently, large portions of the dendritic surface were apposed to astrocytic glial processes (Fig.4B).
Fig. 4.
NK1 and MOR are often contained in the same dendrite. A, A dendrite containing both MOR and NK1 (MOR + NK1-d) receives an asymmetric synapse from an unlabeled terminal that contains both small clear vesicles (scv) and dense-core vesicles (dcv). The terminal and the dendrite are surrounded by unlabeled astrocytic processes (asterisk). Immunogold particles (arrowheads) representing NK1 are located at nonsynaptic plasma membrane sites. B, A dendrite containing both immunoperoxidase labeling for MOR and immunogold labeling for NK1 receives an asymmetric synapse (filled curved arrow) from an unlabeled terminal (Ut). NK1 immunogold particles (arrowheads) are located at nonsynaptic plasma membrane sites. The remainder of the dendritic surface is surrounded by unlabeled astrocytic processes (asterisk) containing glial filaments (f). Scale bars, 0.5 μm.
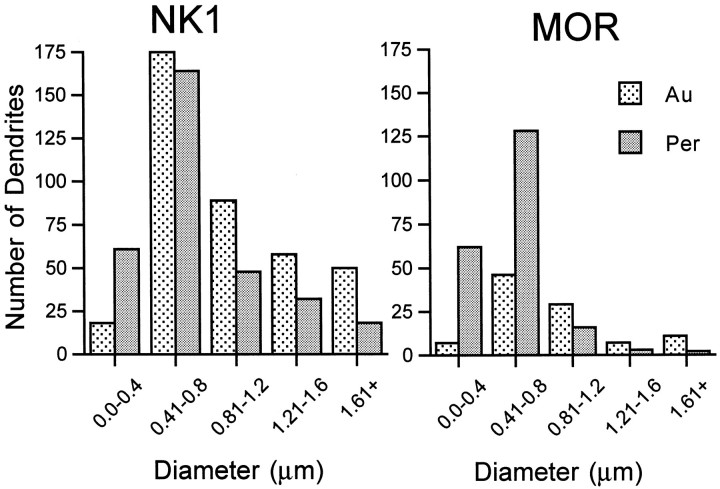
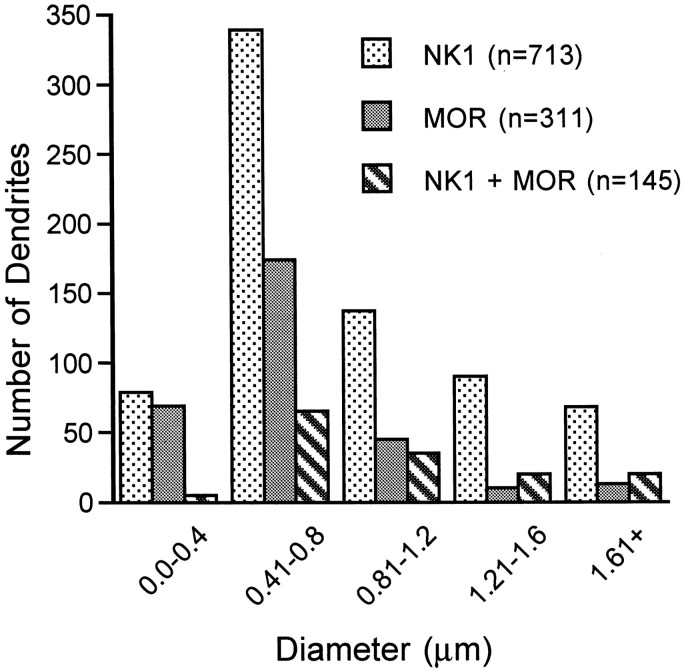
These dually labeled dendrites represented 17% of the total number of NK1-containing dendrites and 32% of the dendrites containing MOR. (The percentage of colocalization varied slightly with each detection method. When the immunogold method was used for MOR detection, 40% of the MOR dendrites also contained NK1, but when the immunoperoxidase method was used for detection of MOR, 27% of the MOR dendrites also contained NK1. These differences likely reflect the increased sensitivity of the immunoperoxidase method, which provides a more diffuse signal and would increase detection of a receptor such as MOR that has only limited distribution within labeled profiles.) To determine whether these receptors were more likely to be detected on smaller (presumably distal) or larger (presumably more proximal) dendrites (Vu and Krasne, 1992), profiles were categorized in 0.4 μm increments of minimal cross-sectional diameter. We found that most of the single-labeled dendrites containing either MOR or NK1 immunoreactivity were between 0.41 and 0.8 μm in diameter, but dendrites <0.4 μm were less likely to be detected using the immunogold detection method (Fig. 5). Therefore, it was not surprising that most of the dually labeled dendrites were between 0.4 and 0.8 μm in diameter and that dually labeled dendrites <0.4 μm were rarely detected (Fig.6). Also, it was noted that in dendrites >1.2 μm, dually labeled profiles were more numerous than profiles containing only MOR, but that profiles containing only NK1 were always more frequently detected. This finding is consistent with the idea that NK1 is present on a larger portion of the plasma membrane than is MOR and that MOR is more selectively _targeted to specific portions of the cell.
Fig. 5.
Most dendrites containing NK1 or MOR alone are medium-sized. Histograms show the number of dendrites containing only NK1 (left panel) or only MOR (right panel) that were identified using either immunogold (Au) or immunoperoxidase (Per) detection methods. The cross-sectional diameter of each dendrite was classified in a 0.4 μm bin (x-axis), and the number of dendrites in each size category for each detection method is represented along the y-axis. These values are for single-labeled profiles only. Profiles <0.4 μm were less frequently detected with the immunogold method compared with immunoperoxidase for both receptor types. The majority of labeled dendrites were 0.4–0.8 μm in diameter for both receptors using either detection method. Total numbers of profiles (n): n = 390 for NK1-Au, n = 323 for NK1-Per; n= 100 for MOR-Au, n = 211 for MOR-Per.
Fig. 6.
The distribution of NK1-, MOR-, and dual-labeled profiles varies with diameter. The data are pooled for immunogold and immunoperoxidase detection methods. Dually labeled profiles <0.4 μm were rarely detected, whereas the largest number of dually labeled profiles were 0.4–0.8 μm in diameter. The number of dually labeled profiles is greater than the number of MOR single-labeled profiles at diameters >1.2 μm, whereas single-labeled NK1 profiles were more frequently detected in all size categories.
MOR-containing axon terminals occasionally contact NK1-immunoreactive dendrites
Although very infrequent, we did find a few examples of MOR immunoreactive axons or axon terminals contacting an NK1-containing dendrites (Fig. 7). MOR immunoreactivity in these axon terminals was often associated with dense-core vesicles, and the NK1 receptor was located away from the MOR-containing afferents. Most of these MOR-labeled axon terminals formed either appositions or symmetric synapses with NK1-containing dendrites and may represent GABAergic or other inhibitory afferents. Of 858 NK1-containing dendrites (with or without MOR-labeling), most (86%) received unlabeled afferent input (Figs. 3A, 4), but only 18 dendrites (2%) were contacted by MOR axons or axon terminals (Fig.8). The remainder of these dendrites (12%) were not contacted by any afferents in the observed plane of section and were surrounded by astrocytic glial processes.
Fig. 8.
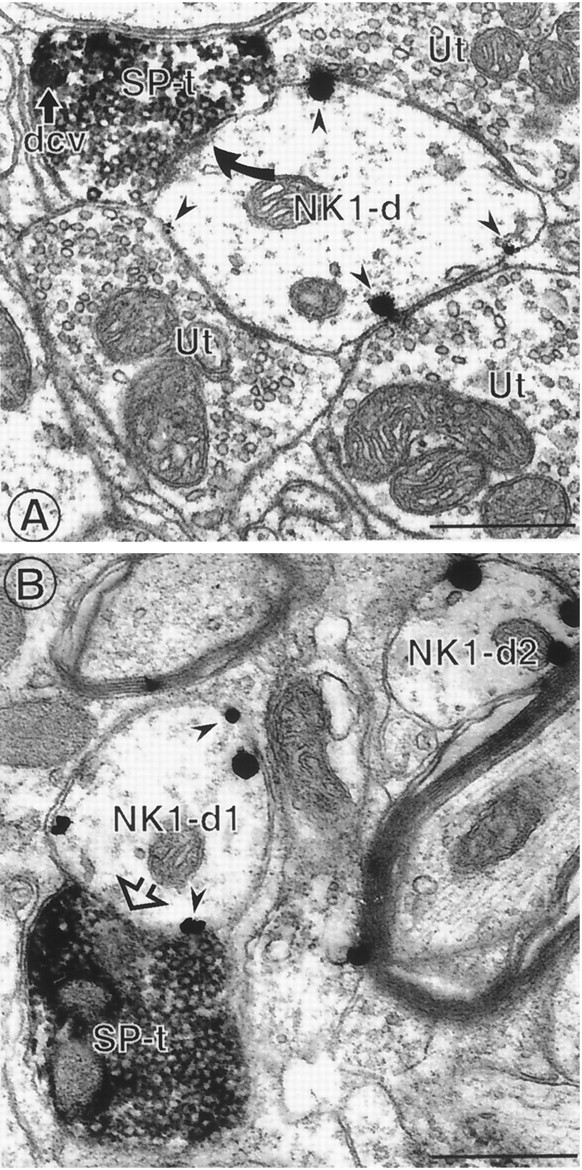
SP-containing terminals contact NK1-immunoreactive dendrites. A, An immunoperoxidase-labeled SP terminal (SP-t) forms an asymmetric synapse (filled curved arrow) with an immunogold-labeled NK1 dendrite (NK1-d). The peroxidase labeling is located throughout the axon terminal, which contains both small clear vesicles and at least one dense-core vesicle (dcv). The immunogold particles (arrowheads) are exclusively associated with nonsynaptic portions of the plasma membrane. This NK1-labeled dendrite is also apposed to three unlabeled axon terminals (Ut) containing small clear vesicles. B, An immunogold-labeled NK1 dendrite (NK1-d1) is contacted (open arrow) by an immunoperoxidase-labeled SP terminal (SP-t). Immunogold particles (arrowheads) in this dendrite and another dendrite in the field (NK1-d2) are associated with nonsynaptic portions of the plasma membrane. Scale bars, 0.5 μm.
SP-containing terminals contact NK1-immunoreactive dendrites
We showed previously that many MOR-immunoreactive dendrites in the trigeminal dorsal horn are contacted by SP-containing axon terminals (Aicher et al., 2000). It has been disputed whether NK1-containing dendrites are associated with SP-containing terminals (Liu et al., 1993; McLeod et al., 1998) or whether there is a mismatch between the peptide and its receptor (Herkenham, 1987). We examined regions of the trigeminal dorsal horn that contain both labels to determine their relative frequency of interaction. We found that 56% of the SP-containing terminals (n = 141) contacted a dendrite that contained NK1 (Fig. 8). In contrast, only 34% of the NK1 dendrites that receive presynaptic input were contacted by SP-containing axon terminals. This confirms that SP-containing terminals often contact dendrites that contain NK1 (Naim et al., 1997;McLeod et al., 1998) and MOR (Aicher et al., 2000) but also indicates that NK1 receptors are not selectively trafficked toward parts of the plasma membrane that receive synaptic input.
DISCUSSION
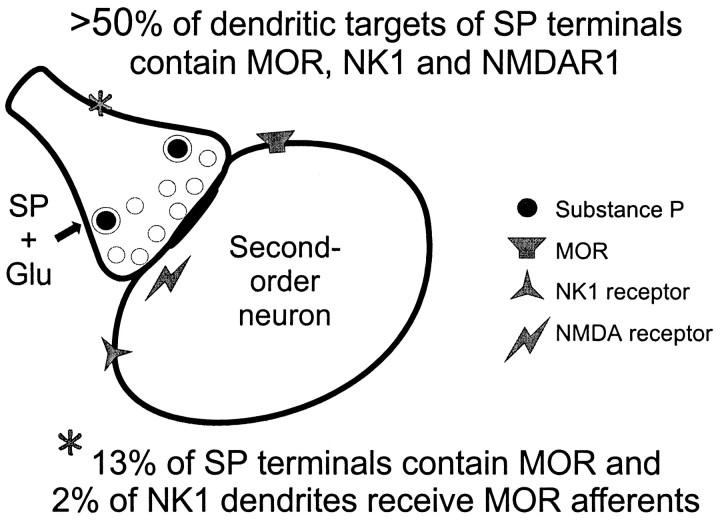
This study directly demonstrates that MOR-immunoreactive dendrites in the trigeminal dorsal horn of the rat often also contain the SP receptor, NK1. In addition, SP-containing terminals often contact dendrites that contain NK1, although many NK1-immunoreactive dendrites lack afferent input from SP. These results provide ultrastructural evidence that postsynaptic responses to SP, which are mediated primarily through NK1 receptors, may be modulated by MOR activation. Together with previous results, our findings suggest that MOR and NK1 receptors are often located in common regions of dendrites postsynaptic to SP afferent terminals, where they would be optimally positioned to modulate responses to SP and glutamate released from primary afferents in response to noxious stimuli (Fig.9).
Fig. 9.
Schematic summary of the localization of MOR, NK1, and NMDAR1 receptors relative to SP-containing axon terminals in the dorsal horn. This model is based on results from the present study and two previous studies on the localization of SP and each receptor in this area using similar ultrastructural methods and analyses.
MOR shows a mixed presynaptic and postsynaptic distribution in trigeminal dorsal horn
Consistent with other studies in the rat, we found MOR located in both axons and in dendrites in the trigeminal dorsal horn (Cheng et al., 1996; Zhang et al., 1998; Aicher et al., 2000). Most of the presynaptic MOR-immunoreactive profiles were unmyelinated axons. A similar distribution within the presynaptic compartment has also been observed in the striatum, where longitudinal sections of axons contiguous with axon terminals revealed a more prominent labeling for MOR in the axon (Wang and Pickel, 1998). In the dorsal horn, these MOR axons may represent axons of passage that ultimately terminate in other areas. Alternatively, the presence of many MOR-labeled axons and few MOR-containing terminals may represent an axonal receptor reserve that may be mobilized by appropriate stimuli.
The presence of MOR in many dendrites in the dorsal horn supports the idea that MOR ligands probably act primarily at postsynaptic sites in this region (Ma et al., 1997; Zhang et al., 1998; Trafton et al., 1999). In addition, we have found that many MOR-labeled dendrites (32%) also contain the SP receptor, NK1. Together with our previous study showing that 53% of SP-containing terminals in trigeminal dorsal horn contact MOR-labeled dendrites, these data support a primarily postsynaptic mechanism for the inhibition of SP-mediated nociceptive signals by MOR ligands.
NK1 is distributed almost exclusively to somata and dendrites in the dorsal horn
We found the SP receptor, NK1, to be located almost exclusively at neuronal postsynaptic sites in the trigeminal dorsal horn. This localization is similar to previous findings in trigeminal and spinal dorsal horn (Brown et al., 1995; Li et al., 1997; McLeod et al., 1998). NK1-containing dendrites were quite diverse in size, with the greatest proportion between 0.4 and 0.8 μm, similar to what we found for MOR. This finding suggests that although both NK1 and MORs are distributed throughout the dendritic tree, they are most common in medium-sized dendrites. In addition, we found that some NK1 dendrites received afferent contacts from SP-containing terminals but that many NK1 dendrites lacked contacts from axons or axon terminals, suggesting that this receptor is not selectively _targeted to dendritic regions innervated by SP or any other afferents.
SP-containing terminals in the dorsal horn _target dendrites containing MOR and NK1
We found SP primarily in unmyelinated axons and axon terminals in the trigeminal dorsal horn (Hökfelt et al., 1975; Pickel et al., 1977; Barber et al., 1979). It is likely that most of these SP-containing terminals in the dorsal horn arise from primary afferents (Knyihár-Csillik et al., 1990), although a number of lesion studies suggest that some of these terminals may arise from central pathways (Li et al., 1996), including local spinal interneurons (Barber et al., 1979). We found that most SP-containing terminals in the dorsal horn contacted NK1-containing dendrites; however, only 34% of NK1 dendrites received contacts from SP-containing terminals. This finding indicates that there is not a profound mismatch between the peptide and its receptor (Naim et al., 1997; McLeod et al., 1998), an issue that has been debated in a series of recent papers (Liu et al., 1993; Naim et al., 1997; McLeod et al., 1998). Our findings also show that the NK1 receptor is not _targeted to any particular portion of the receptive cell. NK1 receptors distant from SP release sites may be activated by diffusion of released peptide (Herkenham, 1987), which would allow for graded responsiveness of dendrites depending on the concentration of released SP (Allen et al., 1997) that reaches different receptor populations. SP and other peptides are contained in dense-core vesicles that do not appear to be released exclusively at synaptic sites (Pierce et al., 1999), in contrast to classical transmitters such as glutamate and GABA. Therefore, we should not expect peptide receptors to undergo specific clustering at synaptic sites, as is seen for these other transmitters (Craig et al., 1994).
Presynaptic MORs may modulate some input to NK1-containing cells
We found that only a very small fraction of MOR-containing axons or axon terminals contacted NK1-containing dendrites, and in our previous studies only a small percentage of SP-containing terminals contained MOR (Aicher et al., 2000). This finding appears to be at odds with observations that SP release is blocked by MOR ligands (Jessell and Iversen, 1977; Cano et al., 1999), which along with other data lead to the conclusion that MORs are located on SP-containing axons. These discrepancies may be explained by MOR effects on SP release from interneurons in the slice preparation (Ma et al., 1997) or by indirect actions of MOR ligands on SP release (e.g., presynaptic inhibition of SP release). Some of the MOR-containing axon terminals that contacted NK1-labeled dendrites formed symmetric synapses with these dendrites and may contain GABA or another inhibitory neurotransmitter.
Functional implications
Our data provide the first evidence that MOR ligands may directly affect the same dendrites that contain NK1, the SP receptor. This receptor colocalization provides strong evidence for direct interactions between MOR and SP receptor ligands on the same postsynaptic sites in nociceptive neurons. Functionally we would expect MOR ligands to alter the postsynaptic effects of SP agonists on second-order neurons. However, we would not necessarily expect MOR agonists to alter binding of SP to the NK1 receptor. A recent study shows that, in fact, MOR ligands do alter the degree of NK1 internalization evoked by either noxious stimuli or exogenously applied SP (Trafton et al., 1999).
Dendrites contacted by SP terminals, as well as those containing NK1, have been implicated in nociceptive processing in the dorsal horn (Liu et al., 1993; Bereiter et al., 1998; McLeod et al., 1998), making these processes potential sites for analgesic actions of opioids. In a previous study of SP-containing axon terminals in the dorsal horn, we found that NMDA-type glutamate receptors are frequently located in the postsynaptic _targets of SP terminals as well (Aicher et al., 1997). The combined results of our studies on this system suggest the following model, illustrated in Figure 9. Many SP terminals in the dorsal horn contact dendrites containing three distinct types of receptors: NMDA-type glutamate receptors, NK1-type tachykinin receptors, and MORs. Activation of either NMDA or NK1 receptors usually leads to excitation of the cell (Liu and Sandkühler, 1998), whereas MOR activation usually reduces excitability (Murase et al., 1998; Connor and Christie, 1999). Interestingly, the colocalization of NK1 and MOR in dendrites in the dorsal horn has important implications for recently proposed treatments for chronic pain. These treatments, which involve lesions of NK1-containing neurons (Mantyh et al., 1997), would also destroy a large proportion of the neurons in the dorsal horn containing MOR and could significantly reduce subsequent responsiveness to spinally administered opioid analgesics.
Footnotes
This work was supported by grants from the National Institute of Dental and Craniofacial Research (DE12640) and the National Heart, Lung and Blood Institute (HL56301). Special thanks to Sarita Sharma for assistance with the preparation of this manuscript and to Dr. Carrie T. Drake for helpful comments.
Correspondence should be addressed to Dr. Sue A. Aicher, Weill Medical College of Cornell University, Department of Neurology and Neuroscience, Division of Neurobiology, 411 E. 69th Street, New York, NY 10021. E-mail: saaicher@med.cornell.edu.
REFERENCES
- 1.Aicher SA, Reis DJ, Nicolae R, Milner TA. Monosynaptic projections from the medullary gigantocellular reticular formation to sympathetic preganglionic neurons in the thoracic spinal cord. J Comp Neurol. 1995;363:563–580. doi: 10.1002/cne.903630405. [DOI] [PubMed] [Google Scholar]
- 2.Aicher SA, Saravay RH, Cravo SL, Jeske I, Morrison SF, Reis DJ, Milner TA. Monosynaptic projections from the nucleus tractus solitarii to C1 adrenergic neurons in the rostral ventrolateral medulla: comparison with input from the caudal ventrolateral medulla. J Comp Neurol. 1996;373:62–75. doi: 10.1002/(SICI)1096-9861(19960909)373:1<62::AID-CNE6>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 3.Aicher SA, Sharma S, Cheng PY, Pickel VM. The N-methyl-d-aspartate (NMDA) receptor is postsynaptic to substance P-containing axon terminals in the rat superficial dorsal horn. Brain Res. 1997;772:71–81. doi: 10.1016/s0006-8993(97)00637-9. [DOI] [PubMed] [Google Scholar]
- 4.Aicher SA, Sharma S, Cheng PY, Liu-Chen LY, Pickel VM. Dual ultrastructural localization of μ-opiate receptors and substance P in the dorsal horn. Synapse. 2000;36:12–20. doi: 10.1002/(SICI)1098-2396(200004)36:1<12::AID-SYN2>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 5.Allen BJ, Rogers SD, Ghilardi JR, Menning PM, Kuskowski MA, Basbaum AI, Simone DA, Mantyh PW. Noxious cutaneous thermal stimuli induce a graded release of endogenous substance P in the spinal cord: imaging peptide action in vivo. J Neurosci. 1997;17:5921–5927. doi: 10.1523/JNEUROSCI.17-15-05921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arvidsson U, Riedl M, Chakrabarti S, Lee J-H, Nakano AH, Dado RJ, Loh HH, Law P-Y, Wessendorf MW, Elde R. Distribution and _targeting of a μ-opioid receptor (MOR1) in brain and spinal cord. J Neurosci. 1995;15:3328–3341. doi: 10.1523/JNEUROSCI.15-05-03328.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barber RP, Vaughn JE, Slemmon JR, Salvaterra PM, Roberts E, Leeman SE. The origin, distribution and synaptic relationships of substance P axons in rat spinal cord. J Comp Neurol. 1979;184:331–352. doi: 10.1002/cne.901840208. [DOI] [PubMed] [Google Scholar]
- 8.Bereiter DA, Bereiter DF, Tonnessen BH, Maclean DB. Selective blockade of substance P or neurokinin a receptors reduces the expression of c-fos in trigeminal subnucleus caudalis after corneal stimulation in the rat. Neuroscience. 1998;83:525–534. doi: 10.1016/s0306-4522(97)00433-8. [DOI] [PubMed] [Google Scholar]
- 9.Besse D, Lombard MC, Zajac JM, Roques BP, Besson JM. Pre- and postsynaptic distribution of μ, δ and k opioid receptors in the superficial layers of the cervical dorsal horn of the rat spinal cord. Brain Res. 1990;521:15–22. doi: 10.1016/0006-8993(90)91519-m. [DOI] [PubMed] [Google Scholar]
- 10.Besse D, Lombard MC, Besson JM. Time-related decreases in μ and δ opioid receptors in the superficial dorsal horn of the rat spinal cord following a large unilateral dorsal rhizotomy. Brain Res. 1992;578:115–121. doi: 10.1016/0006-8993(92)90237-4. [DOI] [PubMed] [Google Scholar]
- 11.Boudin H, Pélaprat D, Rostène W, Pickel VM, Beaudet A. Correlative ultrastructural distribution of neurotensin receptor proteins and binding sites in the rat substantia nigra. J Neurosci. 1998;18:8473–8484. doi: 10.1523/JNEUROSCI.18-20-08473.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown JL, Liu H, Maggio JE, Vigna SR, Mantyh PW, Basbaum AI. Morphological characterization of substance P receptor-immunoreactive neurons in the rat spinal cord and trigeminal nucleus caudalis. J Comp Neurol. 1995;356:327–344. doi: 10.1002/cne.903560302. [DOI] [PubMed] [Google Scholar]
- 13.Cano G, Arcaya JL, Gómez G, Maixner W, Suarez-Roca H. Multiphasic morphine modulation of substance P release from capsaicin-sensitive primary afferent fibers. Neurochem Res. 1999;24:1203–1207. doi: 10.1023/a:1020963120333. [DOI] [PubMed] [Google Scholar]
- 14.Cao YQ, Mantyh PW, Carlson EJ, Gillespie A, Epstein CJ, Basbaum AI. Primary afferent tachykinins are required to experience moderate to intense pain. Nature. 1998;392:390–394. doi: 10.1038/32897. [DOI] [PubMed] [Google Scholar]
- 15.Chan J, Aoki C, Pickel VM. Optimization of differential immunogold-silver and peroxidase labeling with maintenance of ultrastructure in brain sections before plastic embedding. J Neurosci Methods. 1990;33:113–127. doi: 10.1016/0165-0270(90)90015-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng PY, Moriwaki A, Wang JB, Uhl GR, Pickel VM. Ultrastructural localization of μ-opioid receptors in the superficial layers of the rat cervical spinal cord: extrasynaptic localization and proximity to Leu5-enkephalin. Brain Res. 1996;731:141–154. doi: 10.1016/0006-8993(96)00492-1. [DOI] [PubMed] [Google Scholar]
- 17.Collin E, Mauborgne A, Bourgoin S, Mantelet S, Ferhat L, Hamon M, Cesselin F. Kappa-/mu-receptor interactions in the opioid control of the in vivo release of substance P-like material from the rat spinal cord. Neuroscience. 1992;51:347–355. doi: 10.1016/0306-4522(92)90319-w. [DOI] [PubMed] [Google Scholar]
- 18.Connor M, Christie MJ. Opioid receptor signalling mechanisms. Clin Exp Pharmacol Physiol. 1999;26:493–499. doi: 10.1046/j.1440-1681.1999.03049.x. [DOI] [PubMed] [Google Scholar]
- 19.Craig AM, Blackstone CD, Huganir RL, Banker G. Selective clustering of glutamate and gamma-aminobutyric acid receptors opposite terminals releasing the corresponding neurotransmitters. Proc Natl Acad Sci USA. 1994;91:12373–12377. doi: 10.1073/pnas.91.26.12373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Koninck Y, Ribeiro-da-Silva A, Henry JL, Cuello AC. Spinal neurons exhibiting a specific nociceptive response receive abundant substance P-containing synaptic contacts. Proc Natl Acad Sci USA. 1992;89:5073–5077. doi: 10.1073/pnas.89.11.5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dubner R, Bennett GJ. Spinal and trigeminal mechanisms of nociception. Annu Rev Neurosci. 1983;6:381–418. doi: 10.1146/annurev.ne.06.030183.002121. [DOI] [PubMed] [Google Scholar]
- 22.Duggan AW, Hendry IA, Morton CR, Hutchison WD, Zhang ZQ. Cutaneous stimuli releasing immunoreactive substance P in the dorsal horn of the cat. Brain Res. 1988;451:261–273. doi: 10.1016/0006-8993(88)90771-8. [DOI] [PubMed] [Google Scholar]
- 23.Garzón M, Vaughan RA, Uhl GR, Kuhar MJ, Pickel VM. Cholinergic axon terminals in the ventral tegmental area _target a subpopulation of neurons expressing low levels of the dopamine transporter. J Comp Neurol. 1999;410:197–210. doi: 10.1002/(sici)1096-9861(19990726)410:2<197::aid-cne3>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 24.Go VL, Yaksh TL. Release of substance P from the cat spinal cord. J Physiol (Lond) 1987;391:141–167. doi: 10.1113/jphysiol.1987.sp016731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herkenham M. Mismatches between neurotransmitter and receptor localizations in brain: observations and implications. Neuroscience. 1987;23:1–38. doi: 10.1016/0306-4522(87)90268-5. [DOI] [PubMed] [Google Scholar]
- 26.Hökfelt T, Kellerth JO, Nilsson G, Pernow B. Substance P: localization in the central nervous system and in some primary sensory neurons. Science. 1975;190:889–890. doi: 10.1126/science.242075. [DOI] [PubMed] [Google Scholar]
- 27.Hökfelt T, Ljungdahl A, Terenius L, Elde R, Nilsson G. Immunohistochemical analysis of peptide pathways possibly related to pain and analgesia: enkephalin and substance P. Proc Natl Acad Sci USA. 1977;74:3081–3085. doi: 10.1073/pnas.74.7.3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jessell TM, Iversen LL. Opiate analgesics inhibit substance P release from rat trigeminal nucleus. Nature. 1977;268:549–551. doi: 10.1038/268549a0. [DOI] [PubMed] [Google Scholar]
- 29.Knyihár-Csillik E, Torok A, Csillik B. Primary afferent origin of substance P-containing axons in the superficial dorsal horn of the rat spinal cord: depletion, regeneration and replenishment of presumed nociceptive central terminals. J Comp Neurol. 1990;297:594–612. doi: 10.1002/cne.902970411. [DOI] [PubMed] [Google Scholar]
- 30.LaMotte C, Pert CB, Snyder SH. Opiate receptor binding in primate spinal cord: distribution and changes after dorsal root section. Brain Res. 1976;112:407–412. doi: 10.1016/0006-8993(76)90296-1. [DOI] [PubMed] [Google Scholar]
- 31.Leranth C, Pickel VM. Electron microscopic pre-embedding double immunostaining methods. In: Heimer L, Zaborszky L, editors. Neuroanatomical tract-tracing methods 2: recent progress. Plenum; New York: 1989. pp. 129–172. [Google Scholar]
- 32.Lerma J, Morales M, Vicente MA, Herreras O. Glutamate receptors of the kainate type and synaptic transmission. Trends Neurosci. 1997;20:9–12. doi: 10.1016/S0166-2236(96)20055-4. [DOI] [PubMed] [Google Scholar]
- 33.Li JL, Kaneko T, Shigemoto R, Mizuno N. Distribution of trigeminohypothalamic and spinohypothalamic tract neurons displaying substance P receptor-like immunoreactivity in the rat. J Comp Neurol. 1997;378:508–521. doi: 10.1002/(sici)1096-9861(19970224)378:4<508::aid-cne6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 34.Li YQ, Wang ZM, Zheng HX, Shi JW. Central origins of substance P-like immunoreactive fibers and terminals in the spinal trigeminal caudal subnucleus in the rat. Brain Res. 1996;719:219–224. doi: 10.1016/0006-8993(96)00071-6. [DOI] [PubMed] [Google Scholar]
- 35.Liu H, Brown JL, Jasmin L, Maggio JE, Vigna SR, Mantyh PW, Basbaum AI. Synaptic relationship between substance P and the substance P receptor: light and electron microscopic characterization of the mismatch between neuropeptides and their receptors. Proc Natl Acad Sci USA. 1993;91:1009–1013. doi: 10.1073/pnas.91.3.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu XG, Sandkühler J. Activation of spinal N-methyl-d-aspartate or neurokinin receptors induces long-term potentiation of spinal C-fibre-evoked potentials. Neuroscience. 1998;86:1209–1216. doi: 10.1016/s0306-4522(98)00107-9. [DOI] [PubMed] [Google Scholar]
- 37.Ma W, Ribeiro-da-Silva A, De Koninck Y, Radhakrishnan V, Cuello AC, Henry JL. Substance P and enkephalin immunoreactivities in axonal boutons presynaptic to physiologically identified dorsal horn neurons. An ultrastructural multiple-labelling study in the cat. Neuroscience. 1997;77:793–811. doi: 10.1016/s0306-4522(96)00510-6. [DOI] [PubMed] [Google Scholar]
- 38.Mansour A, Fox CA, Burke S, Akil H, Watson SJ. Immunohistochemical localization of the cloned μ opioid receptor in the rat CNS. J Chem Neuroanat. 1995;8:283–305. doi: 10.1016/0891-0618(95)00055-c. [DOI] [PubMed] [Google Scholar]
- 39.Mantyh PW, Rogers SD, Ghilardi JR, Maggio JE, Mantyh CR, Vigna SR. Differential expression of two isoforms of the neurokinin-1 (substance P) receptor in vivo. Brain Res. 1996;719:8–13. doi: 10.1016/0006-8993(96)00050-9. [DOI] [PubMed] [Google Scholar]
- 40.Mantyh PW, Rogers SD, Honore P, Allen BJ, Ghilardi JR, Li J, Daughters RS, Lappi DA, Wiley RG, Simone DA. Inhibition of hyperalgesia by ablation of lamina I spinal neurons expressing the substance P receptor. Science. 1997;278:275–278. doi: 10.1126/science.278.5336.275. [DOI] [PubMed] [Google Scholar]
- 41.Mauborgne A, Lutz O, Legrand J-L, Hamon M, Cesselin F. Opposite effects of δ and μ opioid receptor agonists on the in vitro release of substance P-like material from the rat spinal cord. J Neurochem. 1987;48:529–537. doi: 10.1111/j.1471-4159.1987.tb04125.x. [DOI] [PubMed] [Google Scholar]
- 42.McLeod AL, Krause JE, Cuello AC, Ribeiro-da-Silva A. Preferential synaptic relationships between substance P-immunoreactive boutons and neurokinin 1 receptor sites in the rat spinal cord. Proc Natl Acad Sci USA. 1998;95:15775–15780. doi: 10.1073/pnas.95.26.15775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Milner TA, Pickel VM, Abate C, Joh TH, Reis DJ. Ultrastructural characterization of substance P-containing neurons in the rostral ventrolateral medulla in relation to neurons containing catecholamine synthesizing enzymes. J Comp Neurol. 1988;270:427–445. doi: 10.1002/cne.902700311. [DOI] [PubMed] [Google Scholar]
- 44.Morton CR, Hutchison WD, Duggan AW, Hendry IA. Morphine and substance P release in the spinal cord. Exp Brain Res. 1990;82:89–96. doi: 10.1007/BF00230841. [DOI] [PubMed] [Google Scholar]
- 45.Murase K, Saka T, Terao S, Ikeda H, Asai T. Slow intrinsic optical signals in the rat spinal dorsal horn in slice. NeuroReport. 1998;9:3663–3667. doi: 10.1097/00001756-199811160-00018. [DOI] [PubMed] [Google Scholar]
- 46.Naim M, Spike RC, Watt C, Shehab SAS, Todd AJ. Cells in laminae III and IV of the rat spinal cord that possess the neurokinin-1 receptor and have dorsally directed dendrites receive a major synaptic input from tachykinin-containing primary afferents. J Neurosci. 1997;17:5536–5548. doi: 10.1523/JNEUROSCI.17-14-05536.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neugebauer V, Rumenapp P, Schaible HG. The role of spinal neurokinin-2 receptors in the processing of nociceptive information from the joint and in the generation and maintenance of inflammation-evoked hyperexcitability of dorsal horn neurons in the rat. Eur J Neurosci. 1996;8:249–260. doi: 10.1111/j.1460-9568.1996.tb01209.x. [DOI] [PubMed] [Google Scholar]
- 48.Paxinos G, Watson C. The rat brain in stereotaxic coordinates, Ed 2. Academic; London: 1986. [DOI] [PubMed] [Google Scholar]
- 49.Pearson J, Brandeis L, Cuello AC. Depletion of substance P-containing axons in substantia gelatinosa of patients with diminished pain sensitivity. Nature. 1982;295:61–63. doi: 10.1038/295061a0. [DOI] [PubMed] [Google Scholar]
- 50.Peters A, Palay SL, Webster HD. The fine structure of the nervous system: neurons and their supporting cells, Ed 3. Oxford; New York: 1991. [Google Scholar]
- 51.Pickel VM, Reis DJ, Leeman SE. Ultrastructural localization of substance P in neurons of rat spinal cord. Brain Res. 1977;122:534–540. doi: 10.1016/0006-8993(77)90463-2. [DOI] [PubMed] [Google Scholar]
- 52.Pierce JP, Kurucz OS, Milner TA. Morphometry of a peptidergic transmitter system: dynorphin B-like immunoreactivity in the rat hippocampal mossy fiber pathway before and after seizures. Hippocampus. 1999;9:255–276. doi: 10.1002/(SICI)1098-1063(1999)9:3<255::AID-HIPO6>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 53.Priestley JV, Somogyi P, Cuello AC. Immunocytochemical localization of substance P in the spinal trigeminal nucleus of the rat: a light and electron microscopic study. J Comp Neurol. 1982;211:31–49. doi: 10.1002/cne.902110105. [DOI] [PubMed] [Google Scholar]
- 54.Routh VH, Helke CJ. Tachykinin receptors in the spinal cord. Brain Res. 1995;104:93–108. doi: 10.1016/s0079-6123(08)61786-0. [DOI] [PubMed] [Google Scholar]
- 55.Suarez-Roca H, Maixner W. Morphine produces a multiphasic effect on the release of substance P from rat trigeminal nucleus slices by activating different opioid receptor subtypes. Brain Res. 1992;579:195–203. doi: 10.1016/0006-8993(92)90051-a. [DOI] [PubMed] [Google Scholar]
- 56.Trafton JA, Abbadie C, Marchand S, Mantyh PW, Basbaum AI. Spinal opioid analgesia: how critical is the regulation of substance P signaling? J Neurosci. 1999;19:9642–9653. doi: 10.1523/JNEUROSCI.19-21-09642.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vu ET, Krasne FB. Evidence for a computational distinction between proximal and distal neuronal inhibition. Science. 1992;255:1710–1712. doi: 10.1126/science.1553559. [DOI] [PubMed] [Google Scholar]
- 58.Wang H, Pickel VM. Dendritic spines containing μ-opioid receptors in rat striatal patches receive asymmetric synapses from prefrontal corticostriatal afferents. J Comp Neurol. 1998;396:223–237. [PubMed] [Google Scholar]
- 59.Yaksh TL, Rudy TA. Analgesia mediated by a direct spinal action of narcotics. Science. 1976;192:1357–1358. doi: 10.1126/science.1273597. [DOI] [PubMed] [Google Scholar]
- 60.Zhang X, Bao L, Shi TJ, Ju G, Elde R, Hökfelt T. Down-regulation of μ-opioid receptors in rat and monkey dorsal root ganglion neurons and spinal cord after peripheral axotomy. Neuroscience. 1998;82:223–240. doi: 10.1016/s0306-4522(97)00240-6. [DOI] [PubMed] [Google Scholar]