Highlights
-
•
Most of the COVID-19 cases are mild.
-
•
The estimated median duration of fever was 10 days.
-
•
Radiological improvement was observed on 14 days after onset of symptoms.
-
•
The median duration to negative reverse-transcriptase PCR tests of upper respiratory tract samples was 11 days.
Keywords: COVID-19, SARS-CoV-2, Clinical progression, Viral clearance, Clinical characteristics
Abstract
Background
Studies on the 2019 novel coronavirus disease (COVID-19) have generally been limited to the description of the epidemiology and initial clinical characteristics. We investigated the temporal progression in patients with COVID-19.
Methods
In this retrospective, single-center study, we included confirmed cases of COVID-19 from Jan 20 to Feb 6, 2020 in Shanghai. Final date of follow-up was February 25, 2020.
Results
Of the 249 patients enrolled, the median age was 51 years old, and 126 (50.6%) were male. The duration from onset of symptoms to hospitalization was 4(2–7) days in symptomatic patients. Fever was occurred in 235(94.3%) patients. A total of 215 (86.3%) patients had been discharged after 16(12–20) days hospitalization. The estimated median duration of fever in all the patients with fever was 10 days (95 confidential intervals [CIs]: 8–11 days) after onset of symptoms. Patients who were transferred to intensive care units (ICU) had significantly longer duration of fever as compared to those not in ICU (31 days v.s. 9 days after onset of symptoms, respectively, P <0.0001). Radiological aggravation of initial image was observed in 163 (65.7%) patients on day 7 after onset of symptoms. 154(94.5%) of these patients showed radiological improvement on day 14. The median duration to negative reverse-transcriptase PCR tests of upper respiratory tract samples was 11 days (95 CIs: 10–12 days). Viral clearance was more likely to be delayed in patients in ICU than those not in ICU (P <0.0001). In multivariate logistical analysis, age (Odds ratio [OR] = 1.06) and CD4 T cell count (OR = 0.55 per 100 cells/ul increase) were independently associated with ICU admission.
Conclusions
The majority of COVID-19 cases are mild. The clinical progression pattern suggests that early control of viral replication and application of host-directed therapy in later stage is essential to improve the prognosis of CVOID-19.
Introduction
In December 2019, a cluster of pneumonia cases of unknown origins occurred in Wuhan, Hubei Province, China.1 Thanks to the high-throughput sequencing technology, the pathogen that caused this series of pneumonia was soon identified.2 , 3 This novel beta coronavirus is now named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).4 The disease is also named 2019 novel coronavirus disease (COVID-2019).4
Similar to other coronavirus, human-to-human transmission of COVID-19 pneumonia has been confirmed in case reports.1 The disease has rapidly spread from Wuhan to other areas in China, as well as other countries including Japan, Korea, Italy, USA and Germany et al.5, 6, 7, 8 Therefore, the World Health Organization (WHO) has recently declared the COVID-2019 a public health emergency of international concern.9 As of Feb 29, 2020, a total of 85,403 cases of confirmed COVID-19 pneumonia have been reported worldwide.10
The clinical characteristics of the COVID-19 have been partially reported. Huang et al. first described 41 cases of COVID-19 in which most patients had a history of exposure to Huanan Seafood Wholesale Market.11 Subsequently, Chen et al. and Wang et al. reported findings from 99 cases and 138 cases of COVID-19 in Wuhan, respectively, suggesting that elderly with comorbidities were prone to develop acute respiratory depress syndrome (ARDS).12 , 13 Initial presentation of 13 confirmed patients outside Wuhan has also been revealed.14 Most recently, Guan et al. delineated the clinical characteristics of the largest case series of COVID-19 in China, which confirmed the epidemic spreads rapidly by human-to-human transmission and that the disease severity predict poor clinical outcomes.15 However, the clinical course of the COVID-19 is still not clear. Herein, we collected data from 249 laboratory confirmed cases, aiming to present the clinical progress of the COVID-19.
Methods
Study design and participants
For this retrospective, single-center study, we recruited patients from Jan 20 to Feb 6, 2020, at Shanghai Public Health Clinical Center (SPHCC), Shanghai, China. SPHCC is the pointed hospital that treat COVID-19 for adults in Shanghai. All patients with COVID-19 enrolled in this study were diagnosed according to Chinese national guideline for COVID-19 diagnosis and treatment, as well as the World Health Organization interim guidance.4 The study was approved by Ethics Committee of SPHCC and informed consent was obtained from patients involved before data were collected retrospectively. Informed consent was waived for patients unable to obtain an informed consent (i.e., those discharged or died).
Procedures
Epidemiological, demographic, underlying comorbidities, symptoms, signs, laboratory findings, X-ray, chest computed tomographic (CT) scans, treatment and outcome data were obtained from patients' medical records. The final date of follow-up was Feb 25, 2020. In clinical practice, body temperature was measured by means of digital thermometers. The parents were asked to take their body temperatures at least four times a day or even more frequently when the fever was high. Defervescence was defined as patients maintained normal body temperature without any antipyretics (e.g., nonsteroidal antiinflammatory drugs, corticosteroid).
PCR of SARS-CoV-2 for the upper respiratory specimens was not tested every day after admission. For patients with repeated PCR tests, the first date of the result was recorded if the patients had consecutive negative results, while the latest result and date were recorded for patients who had inconsistent results of the consecutive tests.
Laboratory confirmation and SARS-CoV-2 PCR
Laboratory confirmation of the SARS-CoV-2 was achieved by the Chinese Center for Disease Prevention and Control (CDC). Subsequent test of upper respiratory tract samples for SARS-CoV-2 after admission was performed by both SPHCC and CDC based on the recommendation by the National Institute for Viral Disease Control and Prevention (China) (http://ivdc.chinacdc.cn/kyjz/202001/t20200121_211337.html).
Statistical analysis
Depending on the distribution of the data, categorical variables were described as frequency rates and percentages, and continuous variables were described with mean, median, and interquartile range (IQR) values. Means for continuous variables were compared via independent group t tests when the data were normally distributed; otherwise, the Mann-Whitney test was used. Proportions for categorical variables were compared by the χ2 test. Wilcoxon ranksum tests were used for nonnormally distributed data. The median time to temperature normalization and viral clearance was examined by Kaplan–Meier estimates. The association between baseline clinical characteristics, laboratory results and intensive care unit (ICU) admission was calculated with logistical regression. All analyses were performed using STATA v12.0 (StataCorp, College Station, TX). The figures were constructed using GRAPHPAD PRISM 8.0 (GraphPad Software Inc., San Diego, CA).
Results
Clinical and laboratory characteristics of the study population on admission
As of Feb 6, 2020, a total of 249 patients were enrolled in this study. The median age was 51 years old (IQR, 36–64 years), and 126 (50.6%) were male. The duration from onset of symptoms to hospital admission was 4(2–7) days in patients with symptoms. Ninety patients (36.1%) had one or more coexisting chronic medical conditions. Cardiovascular and cerebrovascular diseases were the most common comorbidities (55[21.7%]), followed by endocrine system diseases (25[10.0%]). The most common symptoms at onset of illness were fever 217[87.1%], cough (91[36.5%]) and fatigue (39[15.7%]). Less common symptoms included dizziness and headache, shortness of breath, rhinorrhoea, sore throat, diarrhea and inappetence (Table 1 ). Seven patients (2.8%) who were close contacts of confirmed COVID-19 cases were asymptomatic while RT-PCR results were positive in their throat-swab samples.
Table 1.
Clinical presentation and pertinent laboratory findings of patient population with COVID-19 (N = 249).
| Clinical characteristics, symptoms or signs | |
|---|---|
| Age, years | |
| Median(interquartile range) | 51(36–64) |
| Sex | |
| Male (N,%) | 126 (50.6%) |
| Days from one set of symptoms (days) | 4(2–7) |
| Signs and symptoms at admission (N,%) | |
| Fever | 217(87.1%) |
| Cough | 91(36.5%) |
| Fatigue | 39(15.7%) |
| Dizziness and headache | 28(11.2%) |
| Shortness of breath | 19(7.6%) |
| Rhinorrhoea | 17(6.8%) |
| Sore throat | 16(6.4%) |
| Diarrhea | 8(3.2%) |
| Inappetence | 8(3.2%) |
| Asymptomatic | 7(2.8%) |
| Comorbidity (N,%) | 90(36.1%) |
| Cardiovascular and cerebrovascular diseases | 55(21.7%) |
| Endocrine system diseases | 25(10.0%) |
| Digestive system diseases | 9(3.6%) |
| Respiratory system diseases | 5(2.0%) |
| Chronic hepatitis B virus infection | 2(0.8%) |
| Malignant tumor | 1(0.4%) |
| Laboratory findings | |
| White blood cells (× 109 per L) | 4.71(3.80–5.86) |
| Lymphocytes (× 109 per L) | 1.12(0.79–1.49) |
| C-reactive protein (mg/L) | 12(4.4–29.4) |
| Erythrocyte sedimentation rate (mm/h) | 54(33–90) |
| Alanine aminotransferase (U/L) | 23(15–33) |
| Aspartate aminotransferase (U/L) | 25(20–33) |
| Albumin (g/L) | 40.8(37.9–43.0) |
| Lactate dehydrogenase (U/L) | 229(195–291) |
| Lactate (mmol/L) | 1.4(1.1–2.1) |
| Estimated glomerular filtration rate (mL/min/1.73m2) | 109.2(95.3–127.2) |
| CD4 T cells counts (cells/uL) | 431(299–637) |
| CD4/CD8 ratio | 1.68(1.24–1.33) |
On admission, leukopenia and lymphopenia were observed in 28.9% and 47.4% of the patients, respectively. Erythrocyte sedimentation rate (ESR) was increased in 85.5% of the patients, while C-reactive protein (CRP) was elevated in more than half of the patients. Elevated levels of alanine aminotransferase, aspartate aminotransferase were less common as well as decreased level of the estimated glomerular filtration rate. CD4 T cells count was decreased in 45.4% of the patients while CD4/CD8 ratio was normal in 92.8% of the patients.
Treatment and outcomes of the study population
All of these patients were admitted to negative pressure isolation wards. Patients were given supportive treatment after admission. Antiviral drugs (e.g., lopinavir/ritonavir, arbidol) were tried in a small proportion of patients. Corticosteroid was not used unless a panel discussion by experts considered necessary (e.g., ARDS). A total of 22(8.8%) patients were admitted in ICU 8.5 ± 4.0 days after onset of symptoms. A total of 8(3.2%) patients developed ARDS 4.8 ± 2.4 days after onset of symptoms. To the time of submission, a total of 215 (86.3%) patients were discharged after 16(12–20) days hospitalization, 13 patients were still in ICU and 19 patients in stable condition. A total of 2 patients died (0.8%).
Defervescence in the study population
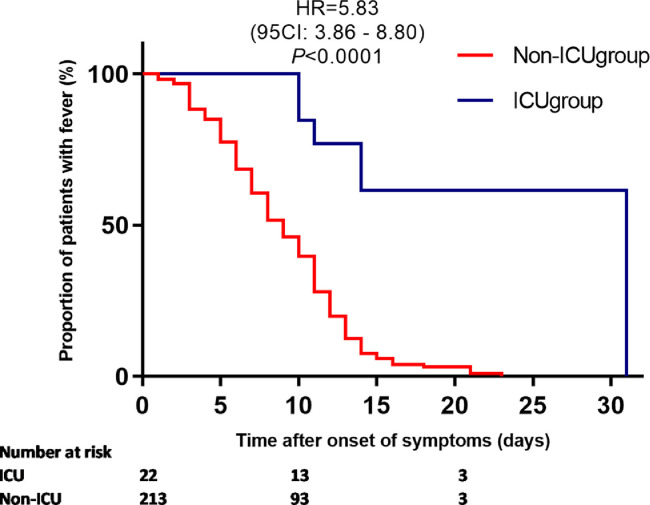
As fever was the most common symptoms among patients with COVID-19, we investigated the duration of fever in this population. Fever occurred in 18 of the 32 patients (56.2%) who were afebrile on admission, including one asymptomatic patient. Therefore, a total of 235(94.3%) patients had fever during their clinical course. In most of the patients (203[86.4%]), body temperature gradually dropped and returned normal with or without supportive therapy after admission. Corticosteroid was used in 32(12.9%) patients. The estimated median duration of fever in all the patients with fever was 10 days (95 confidential intervals [CIs: 8–11 days]) after onset of symptoms. Patients who were transferred to ICU had significantly longer duration of fever as compared to those who were stable (median duration of fever: 31 days v.s. 9 days after onset of symptoms, respectively, P <0.0001, Fig. 1 ). Corticosteroid was used in 13 patients in ICU. However, their body temperatures were not normalized until 20.7 ± 6.8 days after onset of symptoms. Most of the other symptoms including cough, fatigue and diarrhea also waived before defervescence.
Fig. 1.
Duration of fever in patients in ICU and not in ICU from onset of symptoms. ICU: intensive care units.
Radiological changes in disease progression
On admission, 203 patients manifested bilateral pneumonia in X-ray or chest computed tomography (Fig. 2 ) and 39 patients showed unilateral lesion. CT scan result was normal in another 6 patients. Of the 248 patients with repeated radiology examination, the image showed progression in 163 (65.7%) patients after a median of 3 days (that is the 7th day after onset of symptoms), including three patients whose images were normal on admission. The images presented shifting of radiological lesions, which was characterized by improvement of original lesions together with the appearance of new lesions. Some patients showed worsening of original lesions with or without development of new lesions. Images presented disease relief in 56(22.6%) patients and stable condition in 30(12.1%) patients, respectively. Among those patients with radiological worsening, 91 patients did another radiological examination after a median of 6 days (median 14 days after onset of symptoms). 154(94.5%) patients at this point showed improvement, while 1(0.6%) and 8(4.9%) patients showed stable condition and deterioration in images, respectively.
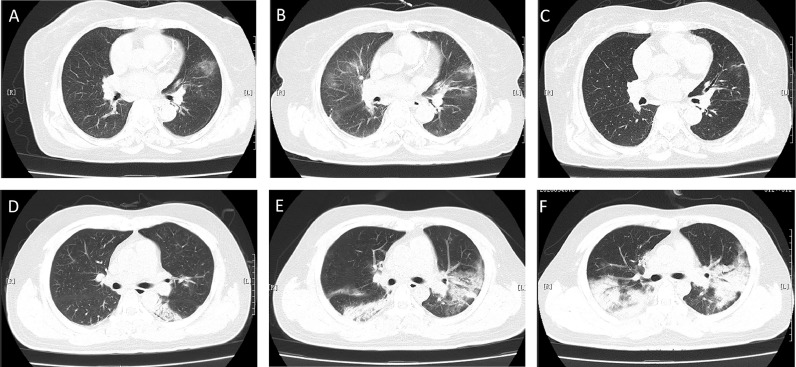
Fig. 2.
Computed tomographic (CT) scans of two patients.
Case 1: Chest CT scans on admission of a 75 years old female on day 3 after onset of symptoms (A). CT scan showed worsening on day 6 manifested by bilateral multiple ground-glass opacities (B); Chest CT scan on day 9 showed improved status (C).
Case 2: Chest CT scans on admission of a 30 years old on day 5 after onset of symptoms (D). Improvement of original lesions together with the appearance of new lesions on day 10 (E). Chest CT scan on day 13 showed worsening status (F).
Viral clearance in the upper respiratory route
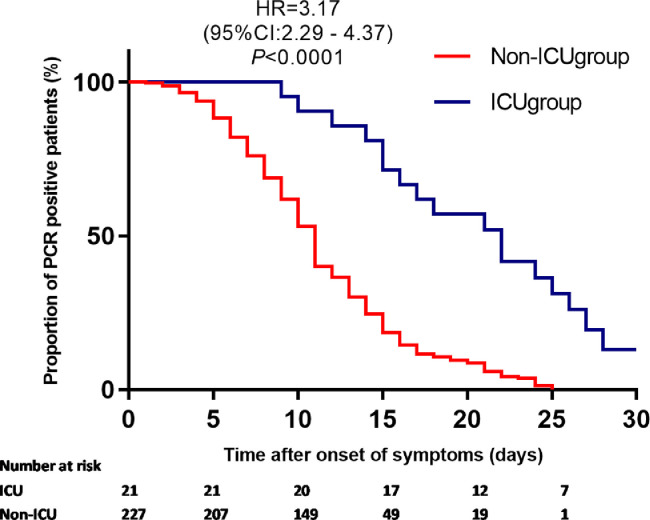
A total of 248 patients had PCR tested for SARS-COV-2 in upper respiratory tract specimens during this observation period. All of them had at least two repeated tests. The estimated median time from initiation of symptoms to PCR negative was 11 days (95 CIs: 10–12 days) in all the patients. In asymptomatic patients, PCR turned to be negative 2(1–3) days after admission. However, the estimated median time from initiation of symptoms to PCR conversation was significantly longer in patients in ICU than those not in ICU (Fig. 3 , P <0.0001). In a Cox proportional hazards regression model, CD4 T cell counts on admission (HR = 1.07 per 100cells/uL increase, P = 0.02), older age (HR = 0.99 per year, P = 0.02), and asymptomatic (HR = 14.3, P <0.0001) were all independently associated with early PCR conversion.
Fig. 3.
Time to a negative conversion of virus by PCR analysis of upper respiratory tract samples.
Factors associated with ICU admission
Trying to find factors that were associated with poor outcome, we then compared clinical and laboratory characters of patients admitted in ICU (N = 22) and non-ICU (N = 227). In univariate analysis, male gender, older age, high levels of white blood cell count, lymphocyte cell count, CRP, lactate dehydrogenase (LDH), low levels of albumin, estimated glomerular filtration rate and CD4 T cells count were all associated with the development of ARDS (Table 2 ). In multivariate logistical analysis, older age (OR=1.06), CD4 T cell counts (OR=0.55 per 100 cells/ul increase) were all independently associated with ICU admission (Table 2).
Table 2.
Factors associated with intensive care units admission.
| Univariate logistic regression |
Multivariate logistic regression |
|||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Age | 1.08 | 1.04–1.13 | <0.001 | 1.06 | 1.00–1.12 | 0.046 |
| Male | 7.10 | 2.04–24.07 | 0.002 | 3.38 | 0.77–14.9 | 0.11 |
| Comorbidity | 4.34 | 1.70–11.10 | 0.002 | 1.83 | 0.50–6.75 | 0.36 |
| Fever | 1.52 | 0.34–6.85 | 0.14 | |||
| White blood cells (× 109 per L) | 1.28 | 1.08–1.52 | 0.004 | 1.07 | 0.79–1.45 | 0.66 |
| Lymphocytes (× 109 per L) | 0.24 | 0.08–0.75 | 0.01 | 4.05 | 0.89–18.5 | 0.07 |
| C-reactive protein (mg/L) | 1.04 | 1.02–1.05 | <0.001 | 1.01 | 0.99–1.03 | 0.58 |
| Erythrocyte sedimentation rate (mm/h) | 1.00 | 0.99–1.02 | 0.58 | |||
| Albumin (g/L) | 0.75 | 0.66–0.85 | <0.001 | 0.95 | 0.78–1.16 | 0.60 |
| Alanine aminotransferase (U/L) | 1.01 | 1.0–1.03 | 0.15 | |||
| Aspartate aminotransferase (U/L) | 1.02 | 1.0–1.03 | 0.05 | 0.99 | 0.96–1.03 | 0.86 |
| Albumin (g/L) | 0.75 | 0.66–0.85 | <0.001 | 0.95 | 0.78–1.16 | 0.60 |
| Lactate dehydrogenase (U/L) | 1.01 | 1.0–1.02 | <0.001 | 1.01 | 1.0–1.02 | 0.08 |
| Lactate (mmol/L) | 1.23 | 0.88–1.70 | 0.22 | |||
| Estimated glomerular filtration rate (mL/min/1.73m2) | 0.98 | 0.96–0.99 | <0.001 | 0.99 | 0.89–1.02 | 0.89 |
| CD4 T cell counts (Per 100 cells/uL) | 0.45 | 0.31–0.64 | <0.001 | 0.55 | 0.33–0.92 | 0.02 |
| Radiological lesion | 4.46 | 0.62–31.9 | 0.14 | |||
CI: Confidential interval, OR: Odds ratio.
Discussion
Studies on COVID-19 have generally been limited to the description of the epidemiology characteristics, initial clinical, hematological and radiological findings.11, 12, 13, 14, 15, 16 To our knowledge, this is the first report to date that describes the temporal clinical progression of COVID-19.
In the current study, most of the cases were mild as evidenced by relatively low rates of ICU admission and high rate of cure rate. This is inconsistent with a recent report which showed more than 80% of COVID-19 cases were mild in China.17 However, nearly 10% were severe and critical, indicating that exploring the clinical progression and possible mechanisms is essential to the disease control.
The clinical progression of COVID-19 in our patients showed a biphasic pattern. The first phase was characterised by fever, cough, fatigue and other systemic symptoms. In consistent with previous studies, we found that fever was the most common symptom in patients with COVID-19. As high as 94.3% of the patients in this study, including those who were afebrile on admission had fever. Guan et al. recently showed that only 43.8% of the patients with COVID-19 on presentation but developed fever in 87.9% following hospitalization, indicating the afebrile patients may be at the early stage of the disease.15 During this first phase, consistent with fever, the disease progressed as evidenced by radiological worsening within 7 days after onset of symptoms. The disease progression during this time could be explained by uncontrolled viral replication as most majority of the patients showed positive results for SARS-CoV-2 PCR from their upper respiratory specimen.
As the disease progressed into week 2, symptoms began to relieve in most of the patients. 10 days after onset of symptoms, half of the patients were estimated to restore normal body temperature. The duration of fever was comparable to that in patients with SARS (11.4 days), but longer than those with MERS (8 days [range, 0–54 days]) and other coronavirus diseases.18, 19, 20 However, different from SARS, whose patients frequently had recurrence of fever in week 2, most of the patients with COVID-19 had normal temperature thereafter.21 In line with defervescence, half of the patients also became PCR negative with their upper respiratory tract samples. In addition, radiological improvement was observed in 94.5% of the patients whose radiological images showed worsening in the first week.
The median time to viral clearance in this study was 11 days after onset of symptoms, which was significantly shorter than that in MERS (17 days), indicating it is virulence maybe lower than MERS.19 Actually, most of the patients are able to clear the virus and their clinical course fit the biphasic model well. However, viral clearance was observed in only a small proportion of patients in ICU. Therefore, the clinical progression in these severe cases may not fit this biphasic model. Indeed, patients admitted to ICU were unable to defervesce without corticosteroid therapy or had significantly longer duration of fever compared with those in the non-ICU group. Persistent fever, lung damage and diseases progression could be partially explained by uncontrolled viral replication. To reduce the risk of disease progression and poor outcome, an effective antiviral to early reduce the viral load may be important. Several drugs are potential candidates to inhibit SARS-CoV-2 replication, including remdesivir which is under clinical trial.5
The host immune responses against the SARS-CoV-2 may also contribute substantially to COVID-19 pathogenesis. In the current study, both older age and lower CD4 T cells count, indicators of immunosuppression, were significantly associated with ICU admission. For our patients who stayed in ICU, elevated interleukin 6 levels had been observed (data not shown), which is in consistent with the previous reports.12 , 22 Therefore, the persistence of COVID-19 induced excessive but aberrant non-effective response which is associated with cytokine storm.11 , 23 A small proportion of patients developed ARDS early in the clinical course and five of the patients in ICU did not improve significantly after viral control. Consistently, inflammation persists after viral clearance in SARS.21 Taken together, these evidences indicate that disease progression may be a result of an over exuberant host response. Along this line, apart from effective antiviral agents which could decrease viral load at the early stage and in turn result in decreased immunopathological damage, the role of host-directed therapy could be an important tool to reduce the mortality.24
Theoretically, corticosteroid treatment could have a role in the treatment of COVID-19 as it suppress lung inflammation. Despite of fever persistence and radiological worsening in the first week, most of the patients had normal temperature and improvement in images with or without supportive therapy from the second week after onset of symptoms. Most importantly, corticosteroid had been shown to delay clearance of MERS-CoV from respiratory tract and SARS-CoV from blood, respectively.25 , 26 In the current study, delayed COVID-19 clearance was associated with ICU admission. Therefore, in agreement with the WHO guideline, we are against the usage of corticosteroid for the treatment of COVID-19.4 , 27
There are some limitations of our study. First, a small proportion the patients were still hospitalized at the time of manuscript submission. Therefore, clinical outcomes in these patients were not available and continued observations are still needed. Second, we did not test SARS-CoV-2 daily for everybody. Hence, the actual duration to viral clearance should be shorter than the estimated one. Meanwhile, we did not quantify the viral load of the SARS-CoV-2 and false negative of the PCR result for upper respiratory sample has also been reported.15 For that reason, studies on the dynamic changes of the viral load are still warranted.
In summary, the majority of COVID-19 cases are mild. The two phase pattern of the disease progression suggests that early control of viral replication and application of host-directed therapy at later stage is essential to improve the prognosis of CVOID-19.
Declaration of Competing Interest
The authors declare that they have no competing interests.
Acknowledgments
This study is funded by Fudan University (IDF162005); Shanghai Public Health Clinical Center (2020YJKY01); Shanghai major projects on infectious diseases (shslczdzk01102); Shanghai "Rising stars of Medical Talent" Youth Development Program, Specialist Program (No. 2019–72). We thank all patients involved in the study. We also thank Ling Gu and Danping Liu for their coordination.
References
- 1.Chan J.F., Yuan S., Kok K.H., To K.K., Chu H., Yang J. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020 doi: 10.1016/S0140-6736(20)30154-9. PubMed PMID: 31986261. Epub 2020/01/28. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020 doi: 10.1016/S0140-6736(20)30251-8. PubMed PMID: 32007145. Epub 2020/02/03. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu F., Zhao S., Yu B., Chen Y.M., Wang W., Song Z.G. A new coronavirus associated with human respiratory disease in China. Nature. 2020 doi: 10.1038/s41586-020-2008-3. PubMed PMID: 32015508. Epub 2020/02/06. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Organization World Health. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected. Geneva. 2020 Report No. [Google Scholar]
- 5.Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H. First case of 2019 novel coronavirus in the United States. New Engl J Med. 2020 doi: 10.1056/NEJMoa2001191. PubMed PMID: 32004427. Epub 2020/02/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim J.Y., Choe P.G., Oh Y., Oh K.J., Kim J., Park S.J. The first case of 2019 novel coronavirus pneumonia imported into Korea from Wuhan, China: Implication for infection prevention and control measures. J Korean Med Sci. 2020;35(5):e61. doi: 10.3346/jkms.2020.35.e61. PubMed PMID: 32030925. Epub 2020/02/08. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phan L.T., Nguyen T.V., Luong Q.C., Nguyen T.V., Nguyen H.T., Le H.Q. Importation and human-to-human transmission of a novel coronavirus in Vietnam. New Engl J Med. 2020 doi: 10.1056/NEJMc2001272. PubMed PMID: 31991079. Epub 2020/01/29. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rothe C., Schunk M., Sothmann P., Bretzel G., Froeschl G., Wallrauch C. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. New Engl J Med. 2020 doi: 10.1056/NEJMc2001468. PubMed PMID: 32003551. Epub 2020/02/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eurosurveillance Editorial T. Note from the editors: world health organization declares novel coronavirus (2019-nCoV) sixth public health emergency of international concern. Euro Surveill. 2020;25(5) doi: 10.2807/1560-7917.ES.2020.25.5.200131e. PubMed PMID: 32019636. Epub 2020/02/06. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. Coronavirus dieases 2019 (COVID-19) situation report - 40 2020[cited 2020 Mar 1st]. Available from:https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200229-sitrep-40-covid-19.pdf?sfvrsn=7203e653_2.
- 11.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 doi: 10.1016/S0140-6736(20)30183-5. PubMed PMID: 31986264. Epub 2020/01/28. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020 doi: 10.1016/S0140-6736(20)30211-7. PubMed PMID: 32007143. Epub 2020/02/03. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. PubMed PMID: 32031570. Epub 2020/02/08. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang Lin M, Wei L., Xie L., Zhu G., Dela Cruz C.S. Epidemiologic and clinical characteristics of novel coronavirus infections involving 13 patients outside Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1623. PubMed PMID: 32031568. Epub 2020/02/08. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical characteristics of coronavirus disease 2019 in China. New Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. PubMed PMID: 32109013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China. New Engl J Med. 2020 doi: 10.1056/NEJMoa2001017. PubMed PMID: 31978945. Epub 2020/01/25. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in china: summary of a report of 72 314 cases from the chinese center for disease control and prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 18.Zhong N.S., Zheng B.J., Li Y.M., Poon Xie ZH, Chan K.H. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, people's republic of China. Lancet. 2003;362(9393):1353–1358. doi: 10.1016/S0140-6736(03)14630-2. PubMed PMID: 14585636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi W.S., Kang C.I., Kim Y., Choi J.P., Joh J.S., Shin H.S. Clinical presentation and outcomes of middle east respiratory syndrome in the republic of Korea. Infect Chemother. 2016;48(2):118–126. doi: 10.3947/ic.2016.48.2.118. PubMed PMID: 27433382. Pubmed Central PMCID: 4945721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lau S.K., Woo P.C., Yip C.C., Tse H., Tsoi H.W., Cheng V.C. Coronavirus HKU1 and other coronavirus infections in Hong Kong. J Clin Microbiol. 2006;44(6):2063–2071. doi: 10.1128/JCM.02614-05. PubMed PMID: 16757599. Pubmed Central PMCID: 1489438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peiris J.S., Chu C.M., Cheng V.C., Chan K.S., Hung I.F., Poon L.L. Clinical progression and viral load in a community outbreak of coronavirus-associated sars pneumonia: a prospective study. Lancet. 2003 May 24;361(9371):1767–1772. doi: 10.1016/S0140-6736(03)13412-5. PubMed PMID: 12781535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wan S., Yi Q., Fan S., Lv J., Zhang X., Guo L., et al. Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP). doi: 10.1101/2020.02.10.20021832. [DOI]
- 23.Li G., Fan Y., Lai Y., Han T., Li Z., Zhou P. Coronavirus infections and immune responses. J Med Virol. 2020 doi: 10.1002/jmv.25685. PubMed PMID: 31981224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zumla A., Hui D.S., Azhar E.I., Memish Z.A., Maeurer M. Reducing mortality from 2019-nCoV: host-directed therapies should be an option. Lancet. 2020 doi: 10.1016/S0140-6736(20)30305-6. PubMed PMID: 32035018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee N., Allen Chan K.C., Hui D.S., Ng E.K., Wu A., Chiu R.W. Effects of early corticosteroid treatment on plasma SARS-associated coronavirus RNA concentrations in adult patients. J Clinical Virol Offic Publ Pan Am Soc Clin Virol. 2004;31(4):304–309. doi: 10.1016/j.jcv.2004.07.006. PubMed PMID: 15494274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arabi Y.M., Mandourah Y., Al-Hameed F., Sindi A.A., Almekhlafi G.A., Hussein M.A. Corticosteroid therapy for critically ill patients with middle east respiratory syndrome. Am J Respir Crit Care Med. 2018;197(6):757–767. doi: 10.1164/rccm.201706-1172OC. PubMed PMID: 29161116. [DOI] [PubMed] [Google Scholar]
- 27.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020 doi: 10.1016/S0140-6736(20)30317-2. PubMed PMID: 32043983. [DOI] [PMC free article] [PubMed] [Google Scholar]