Abstract
Objectives
To determine the viral epidemiology and clinical characteristics of patients with and without clinically apparent respiratory tract infection.
Methods
This prospective cohort study was conducted during the 2018 winter influenza season. Adult patients with fever/respiratory symptoms (fever/RS group) were age- and sex-matched with patients without fever/RS (non-fever/RS group) in a 1:1 ratio. Respiratory viruses were tested using NxTAG™ Respiratory Pathogen Panel IVD, a commercially-available multiplex PCR panel.
Results
A total of 214 acutely hospitalized patients were included in the final analysis, consisting of 107 with fever/RS (fever/RS group), and 107 age- and sex-matched patients without fever/RS (non-fever/RS group). Respiratory viruses were detected in 34.1% (73/214) of patients, and co-infection occurred in 7.9% (17/214) of patients. The incidence of respiratory virus was higher in the fever/RS group than in the non-fever/RS group (44.9% (48/107) versus 23.4% (25/107), p 0.001). Influenza B virus, enterovirus/rhinovirus and coronaviruses were detected more frequently in the fever/RS group, whereas parainfluenza virus 4B and adenovirus were detected more frequently in the non-fever/RS group. Among the non-fever/RS group, chest discomfort was more common among patients tested positive for respiratory viruses than those without respiratory virus detected (44% (11/25) versus 22% (18/82), p 0.04).
Conclusions
Respiratory viruses can be frequently detected among hospitalized patients without typical features of respiratory tract infection. These patients may be a source of nosocomial outbreaks.
Keywords: Adenovirus, Cardiac complications, Influenza, Parainfluenza virus, Respiratory tract infection
Introduction
Respiratory viruses are the leading cause of respiratory tract infection [1], [2]. Influenza virus and respiratory syncytial virus have been associated with high morbidity and mortality [3], [4], [5]. Rhinovirus has been found to be the most frequently detected respiratory virus among patients with community-acquired pneumonia, and has been associated with significant morbidity and mortality [1], [6], [7], [8]. Adenovirus and parainfluenza virus (PIV) were reported to have the highest hospitalization–fatality ratio [9].
Defining of the burden of respiratory viruses is one of the agendas in the WHO Battle against Respiratory Viruses (BRaVe) initiative [10]. Accurate data on the burden of respiratory virus infection is critical for patient management, infection control measures and public health policies. Current clinical guidelines suggest that respiratory virus should be tested in patients with respiratory symptoms, or those with myocarditis or encephalitis [2]. As a result, epidemiological data on respiratory virus infection mostly rely on studies analysing patients with clinical evidence of respiratory tract infection [1], [9], [11], [12], [13], [14], [15]. However, non-respiratory symptoms and extrapulmonary complications are common among patients with respiratory virus infection [15], [16], [17], [18]. Renal failure is particularly common among patients with Middle East respiratory syndrome coronavirus infection [19], [20]. Myocardial infarction and stroke can be triggered by respiratory virus infection [12], [14], [21]. Hence, epidemiological studies excluding patients without symptoms or signs of respiratory tract infection may underestimate the true burden of respiratory virus infection.
Here, our objective was to reveal the hidden burden of respiratory virus infection among patients requiring hospitalization without clinical suspicion of respiratory virus infection. We explored the differences in the incidence of different respiratory viruses between patients with or without clinical suspicion of respiratory tract infection. We analysed the clinical features associated with the detection of respiratory viruses among patients without fever or respiratory symptoms. To reduce patient discomfort, we collected saliva specimens for respiratory virus testing. We and others have demonstrated that there is a high concordance (>90%) between saliva and nasopharyngeal specimens in the detection of respiratory viruses [22], [23], [24].
Methods
Study setting and design
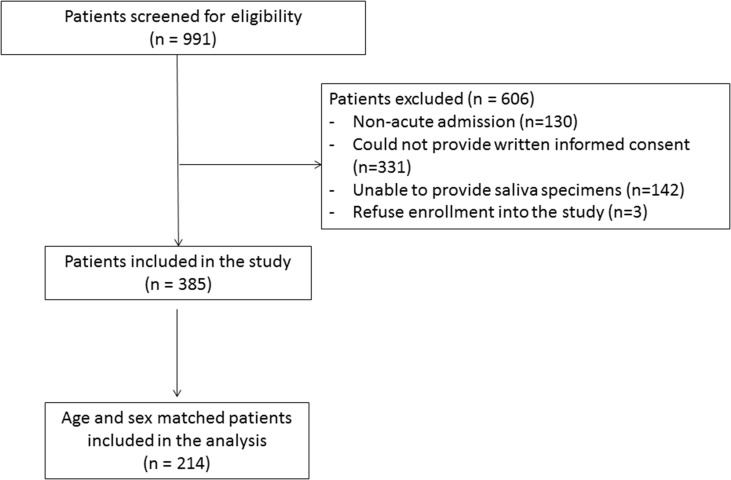
This was a prospective cohort study conducted in Queen Mary Hospital of Hong Kong, an acute-care university-affiliated teaching hospital with 1700 beds. All adult patients admitted to the acute medical wards from 9 January to 12 February, 2018 were screened for eligibility. Inclusion criteria were age ≥18 years, admission to any hospitals for <24 hours, competent and agreed to provide written informed consent. Patients were excluded if they could not provide adequate saliva. Written informed consent was obtained from all recruited patients. Saliva specimens were collected from all eligible patients.
Eligible patients with fever ≥38.0°C or any respiratory symptoms (RS) upon admission were classified as the fever/RS group, whereas those without fever or respiratory symptoms in the preceding 7 days before hospitalization were classified as non-fever/RS group. Respiratory symptoms included runny nose, sore throat, cough (including haemoptysis), sputum and shortness of breath. A saliva specimen was collected from all eligible patients. Each patient in the fever/RS group was matched with an age-matched (within 5 years) and sex-matched patient in the non-fever/RS group in a 1:1 ratio. All unmatched patients were excluded from further testing.
Saliva specimens were tested for respiratory viruses using NxTAG™ Respiratory Pathogen Panel IVD (Luminex, Austin, TX, USA) as described previously [22], [25]. Differentiation of rhinovirus and enterovirus was performed by sequence analysis of the VP4/VP2 gene region.
Data on fever or respiratory symptoms were collected by research nurses using a standardized questionnaire. Final diagnosis, outcome and laboratory investigation results were obtained from the Clinical Management System.
This study was approved by the Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster (UW 13-265). This study is reported using the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [26]. Please refer to Supplementary material (Appendix S1)for details on respiratory virus testing and sequencing, and specimen collection.
Statistical analysis
All statistical analysis was performed using SPSS 23.0. Mann–Whitney U test and Fisher's exact test were used for the comparison of continuous variables or categorical variables, respectively. A p value < 0.05 was considered statistically significant. The sample size chosen was based on feasibility.
Results
Patient characteristics
A total of 385 patients were included in the study, and 214 patients were included for respiratory virus testing in their saliva and final analysis, including 107 patients in the fever/RS group and 107 age- and sex-matched patients in the non-fever/RS group (Table 1 and Fig. 1 ). The median age was 67.5 years (range 18–93 years), and 42.1% (90/214) were female. There was no significant difference in the demographics and co-morbidities between the fever/RS group and the non-fever/RS group, except that chronic lung disease was more common among the fever/RS group than the non-fever/RS group (25.2% (27/107) versus 6.5% (7/107); p < 0.001). The number of patients in each week was not statistically different between the fever/RS group and the non-fever/RS group (p 0.489) (see Supplementary material, Table S1).
Table 1.
Demographics, clinical features and outcomes of all 214 patients
| Fever/RS group (n = 107) | Non-fever/RS group (n = 107) | p value | |
|---|---|---|---|
| Demographics | |||
| Median age in years (range) | 69 (18–92) | 66 (19–93) | 0.411 |
| Female | 45 (42.1) | 45 (42.1) | 1.000 |
| Underlying disease | |||
| Hypertension | 51 (47.7) | 57 (53.3) | 0.494 |
| Chronic heart disease | 33 (30.8) | 31 (29.0) | 0.881 |
| Chronic lung disease | 27 (25.2) | 7 (6.5) | <0.001 |
| Chronic liver disease | 14 (13.1) | 6 (5.6) | 0.098 |
| Chronic kidney disease | 12 (11.2) | 15 (14.0) | 0.681 |
| Neurological conditions | 13 (12.1) | 15 (14.0) | 0.840 |
| Peripheral vascular disease | 4 (3.7) | 2 (1.9) | 0.683 |
| Diabetes mellitus | 31 (29.0) | 31 (29.0) | 1.000 |
| Thyroid disease | 6 (5.6) | 5 (4.7) | 1.000 |
| Other endocrine or metabolic disorders | 7 (6.5) | 10 (9.3) | 0.614 |
| Gout or pseudogout | 3 (2.8) | 6 (5.6) | 0.498 |
| Autoimmune disease | 6 (5.6) | 5 (4.7) | 1.000 |
| Solid organ malignancy | 19 (17.8) | 13 (12.1) | 0.338 |
| Haematological malignancy | 6 (5.6) | 5 (4.7) | 1.000 |
| Transplant recipient | 0 (0) | 2 (1.9) | 0.498 |
| Respiratory symptoms or fever upon admission | |||
| Cough | 66 (61.7) | 0 (0) | <0.001 |
| Fever | 50 (46.7) | 0 (0) | <0.001 |
| Sputum | 42 (39.3) | 0 (0) | <0.001 |
| Shortness of breath | 35 (32.7) | 0 (0) | <0.001 |
| Runny nose | 33 (30.8) | 0 (0) | <0.001 |
| Sore throat | 19 (17.8) | 0 (0) | <0.001 |
| Non-respiratory presenting symptoms/reason for hospital admission | |||
| Chest discomfort | 24 (22.4) | 29 (27.1) | 0.527 |
| Palpitation or arrhythmia | 8 (7.5) | 19 (17.8) | 0.038 |
| Limb oedema | 11 (10.3) | 8 (7.5) | 0.632 |
| High blood pressure | 0 (0) | 4 (3.7) | 0.121 |
| Syncope | 4 (3.7) | 9 (8.4) | 0.252 |
| Seizure | 1 (0.9) | 4 (3.6) | 0.369 |
| Dizziness | 14 (13.1) | 22 (20.6) | 0.200 |
| Numbness or weakness | 3 (2.8) | 10 (9.3) | 0.082 |
| Headache | 2 (1.9) | 3 (2.8) | 1.000 |
| Confusion | 2 (1.9) | 1 (0.9) | 1.000 |
| Gastrointestinal symptomsa | 28 (26.2) | 20 (18.7) | 0.251 |
| Complications of endocrine disordersb or electrolyte disturbance | 4 (3.7) | 6 (5.6) | 0.748 |
| Trauma | 0 (0) | 4 (3.7) | 0.121 |
| Cardiac or neurological complications | |||
| Hypertension complications | 1 (0.9) | 6 (5.6) | 0.119 |
| Myocardial infarction or angina | 3 (2.8) | 3 (2.8) | 1.000 |
| Arrhythmia | 5 (4.7) | 11 (10.3) | 0.193 |
| Stroke/transient ischaemic attack | 1 (0.9) | 8 (7.5) | 0.035 |
| Outcome | |||
| Require oxygen supplement | 27 (25.2) | 4 (3.7)c | <0.001 |
| ICU or CCU admission | 0 (0) | 2 (1.8) | 0.498 |
| Death | 0 (0) | 1 (0.9) | 1.000 |
Abbreviations: CCU, coronary care unit; ICU, intensive care unit.
Data expressed as n (%) unless otherwise stated.
Gastrointestinal symptoms include nausea, bloating sensation, vomiting, diarrhoea, constipation, dysphagia and abdominal pain.
Complications of endocrine disorders include hypoglycaemia and thyroid storm.
Required oxygen supplementation after hospitalization.
Fig. 1.
Recruitment flow chart.
Among the fever/RS group, the most common respiratory symptom was cough (61.7%; 66/107) (Table 1). Sixteen patients (15.0%) only had fever but no respiratory symptoms. Among patients in the non-fever/RS group, the most common symptom was chest discomfort (27.1%; 29/107), followed by dizziness (20.6%; 22/107) and gastrointestinal symptoms (18.7%; 20/107) (Table 1). Three patients in the non-fever/RS group developed fever (n = 2) or respiratory symptoms (n = 1) during hospitalization after collection of saliva. There was no significant difference in the frequency of non-respiratory symptoms between the fever/RS group and the non-fever/RS group, except that palpitation was significantly more common in the non-fever/RS group (17.8% (19/107) versus 7.5% (8/107); p 0.038). Significantly more patients in the fever/RS group required oxygen supplementation when compared with those in the non-fever/RS group (25.2% (27/107) versus 3.7% (4/107); p < 0.001). Oseltamivir was only given empirically in the fever/RS group, not in the non-fever/RS group.
Respiratory virus detection by multiplex molecular assay
Respiratory viruses were detected in 34.1% (73/214) of patients (Table 2 ; and see Supplementary material, Table S2). The respiratory virus detection rate in the fever/RS group (44.9%; 48/107) was significantly higher than that in the non-fever/RS group (23.4%; 25/107) (p 0.001). For the 16 patients with fever only in the fever/RS group, seven (43.8%) had respiratory virus detected. Co-infection with two or more respiratory viruses was detected in 17 patients, including 10.3% (11/107) in the fever/RS group and 5.6% (6/107) in the non-fever/RS group, but the difference was not statistically significant (p 0.312). There was no significant difference in the proportion of patients with co-infection among different respiratory viruses (see Supplementary material, Fig. S1).
Table 2.
Comparison of respiratory virus detection between the fever/RS group and the non-fever RS group. Saliva specimens were tested for respiratory viruses by multiplex PCRa
| Virus | All patients (n = 214) | Fever/RS group (n = 107) | Non-fever/RS group (n = 107) | p value |
|---|---|---|---|---|
| Any virus detected | 73 (34.1) | 48 (44.9) | 25 (23.4) | 0.001 |
| Influenza virus | 19 (8.9) | 17 (15.9) | 2 (1.9) | <0.001 |
| Influenza Ab | 3 (1.4) | 2 (1.9)c | 1 (0.9)d | 1 |
| Influenza B | 17 (7.9) | 16 (15.0) | 1 (0.9) | <0.001 |
| Adenovirus | 17 (7.9) | 6 (5.6) | 11 (10.3) | 0.312 |
| Any PIV | 17 (7.9) | 6 (5.6) | 11 (10.3) | 0.312 |
| PIV-1 | 1 (0.5) | 1 (0.9) | 0 (0) | 1 |
| PIV-2 | 0 (0) | 0 (0) | 0 (0) | NA |
| PIV-3 | 2 (0.9) | 1 (0.9) | 1 (0.9) | 1 |
| PIV-4A | 1 (0.5) | 1 (0.9) | 0 (0) | 1 |
| PIV-4B | 13 (5.9) | 3 (2.8) | 10 (9.3) | 0.082 |
| Rhinovirus/enteroviruse | 16 (7.5) | 13 (12.1) | 3 (2.8) | 0.017 |
| Human metapneumovirus | 13 (6.1) | 9 (8.4) | 4 (3.7) | 0.252 |
| Any coronaviruses | 6 (2.8) | 6 (5.6) | 0 (0) | 0.029 |
| Coronavirus 229E | 1 (0.5) | 1 (0.9) | 0 (0) | 1 |
| Coronavirus NL63 | 3 (1.4) | 3 (2.8) | 0 (0) | 0.246 |
| Coronavirus OC43 | 2 (0.9) | 2 (1.9) | 0 (0) | 0.498 |
| Coronavirus HKU1 | 0 (0) | 0 (0) | 0 (0) | 1 |
| Any RSV | 2 (0.9) | 1 (0.9) | 1 (0.9)f | 1.000 |
| RSV-A | 2 (0.9) | 1 (0.9) | 1 (0.9)f | 1.000 |
| RSV-B | 0 (0) | 0 | 0 | NA |
| Bocavirus | 0 (0) | 0 (0) | 0 (0) | NA |
| More than one respiratory virus detected | 17 (7.9) | 11 (10.3) | 6 (5.6) | 0.312 |
Abbreviations: NA, not applicable; PIV, parainfluenza virus; RS, respiratory symptom; RSV, respiratory syncytial virus.
Data expressed as n (%).
Multiplex PCR was performed using NxTAG™ RPP IVD.
Either influenza A, H1 or H3 positive by NxTAG™ RPP IVD.
Both were influenza A virus subtype H3.
Influenza A virus subtype H1.
Rhinovirus and enterovirus cannot be differentiated with NxTAG™ RPP IVD. Differentiation of rhinovirus and enterovirus was achieved by sequence analysis of the VP4/VP2 region. Out of 16 specimens, VP4/VP2 sequencing was successful for nine specimens in the fever/RS group. Rhinovirus A, B and C were detected in three, one and five specimens, respectively.
This patient developed fever during hospitalization after the collection of the saliva specimen.
Overall, the most frequently detected respiratory viruses were adenovirus (7.9%; 17/214) and influenza B virus (7.9%; 17/214) (Table 2, and see Supplementary material, Fig. S1). For the fever/RS group, the most frequently detected respiratory viruses were influenza B virus (15.0%; 16/107) and enterovirus/rhinovirus (12.1%; 13/107) (Table 2). For the non-fever/RS group, the most frequently detected viruses were adenovirus (10.3%; 11/107) and PIV-4B (9.3%; 10/107). The detection rates of influenza B virus (15.0% (16/107) versus 0.9% (1/107), p < 0.001), enterovirus/rhinovirus (12.1% (13/107) versus 2.8% (3/107); p 0.017) and coronaviruses (5.6% (6/107) versus 0% (0/107); p 0.029) were significantly higher in the fever/RS group than in the non-fever/RS group (Fig. 2). Adenovirus (10.3% (11/107) versus 5.6% (6/107); p 0.312) and PIV-4B (9.3% (10/107) versus 2.8% (3/107); p 0.082) were more common in the non-fever/RS group than in the fever/RS group, but not reaching statistical significance.
Fig. 2.
Comparison of the detection of respiratory viruses between the fever/respiratory symptom (RS) group and the non-fever/RS group. *p < 0.05; ***p < 0.001.
We have further compared patients with and without virus detection among patients in the non-fever/RS group (Table 3 ). For co-morbidities, there were no significant differences between patients with respiratory virus detected than those without respiratory virus detected. None of the patients in the non-fever/RS group with respiratory virus detected had new chest radiograph changes suggestive of pneumonia. Chest discomfort was significantly more common among patients with respiratory virus detected than those without respiratory virus detected (44% (11/25) versus 22% (18/82); p 0.040). Adenovirus (20.7%) and PIV-4B (17.2%) were the most common respiratory viruses detected in patients with chest discomfort (see Supplementary material, Table S3).
Table 3.
Comparison between patients with or without respiratory virus detected in the non-fever/RS group
| Respiratory virus detected (n = 25) | No respiratory virus detected (n = 82) | p value | |
|---|---|---|---|
| Demographics | |||
| Median age in years (range) | 70 (39-93) | 64 (19-91) | 0.656 |
| Female | 15 (60.0) | 47 (57.3) | 1.000 |
| Underlying disease | |||
| Hypertension | 13 (52.0) | 44 (53.7) | 1.000 |
| Chronic heart disease | 10 (40.0) | 21 (25.6) | 0.209 |
| Chronic lung disease | 1 (4.0) | 6 (7.3) | 1.000 |
| Chronic liver disease | 2 (8.0) | 4 (4.9) | 0.623 |
| Chronic kidney disease | 3 (12.0) | 12 (14.6) | 1.000 |
| Neurological conditions | 1 (4.0) | 14 (17.1) | 0.184 |
| Peripheral vascular disease | 1 (4.0) | 1 (1.2) | 0.414 |
| Diabetes mellitus | 4 (16.0) | 27 (32.9) | 0.133 |
| Thyroid disease | 2 (8.0) | 3 (3.7) | 0.332 |
| Other endocrine disorders | 1 (4.0) | 9 (11.0) | 0.447 |
| Gout or pseudogout | 1 (4.0) | 5 (6.1) | 1.000 |
| Autoimmune disease | 2 (8.0) | 3 (3.7) | 0.332 |
| Solid organ malignancy | 2 (8.0) | 11 (13.4) | 0.728 |
| Haematological malignancy | 1 (4.0) | 4 (4.9) | 1.000 |
| Transplant recipient | 1 (4.0) | 1 (1.2) | 0.414 |
| Clinical features at presentation | |||
| Chest discomfort | 11 (44.0) | 18 (22.0) | 0.040 |
| Palpitation/arrhythmia | 5 (20.0) | 14 (17.1) | 0.768 |
| Limb oedema | 1 (4.0) | 7 (8.5) | 0.678 |
| High blood pressure | 2 (8.0) | 2 (2.4) | 0.232 |
| Syncope | 2 (8.0) | 7 (8.5) | 1.000 |
| Seizure | 0 (0) | 4 (4.9) | 0.571 |
| Dizziness | 6 (24.0) | 16 (19.5) | 0.587 |
| Numbness/weakness | 1 (4.0) | 9 (11.0) | 0.447 |
| Headache | 0 (0) | 3 (3.7) | 1.000 |
| Confusion | 0 (0) | 1 (1.2) | 1.000 |
| Gastrointestinal symptoms | 3 (12.0) | 17 (20.7) | 0.395 |
| Endocrine or electrolyte disturbance | 1 (4.0) | 5 (6.1) | 1.000 |
| Trauma | 0 (0) | 4 (4.9) | 0.571 |
| Initial laboratory findings, median (interquartile range) | |||
| Total white blood cell count, × 109 cells/L | 7.0 (5.8–8.5) | 7.1 (6.0–9.0) | 0.941 |
| Neutrophil count, × 109 cells/L | 4.7 (3.2–5.7) | 4.6 (3.9–6.7) | 0.397 |
| Lymphocyte count, × 109 cells/L | 1.64 (1.18–2.15) | 1.47 (1.04–1.96) | 0.383 |
| Platelet count, × 109 cells/L | 226 (187–251) | 206 (177–285) | 0.924 |
| Final diagnosis | |||
| Myocardial infarction or angina | 1 (4.0) | 2 (2.4) | 0.554 |
| Arrhythmia | 4 (16.0) | 7 (8.5) | 0.279 |
| Stroke/transient ischaemic attack | 1 (4.0) | 7 (8.5) | 0.678 |
| Poorly controlled hypertension | 2 (8.0) | 4 (4.9) | 0.623 |
| Outcome | |||
| Require oxygen supplement | 2 (8.0) | 2 (2.4) | 0.232 |
| ICU or CCU admission | 0 (0) | 2 (2.4) | 1.000 |
| Death | 0 (0) | 1 (1.2) | 1.000 |
Abbreviations: CCU, coronary care unit; ICU, intensive care unit.
Data expressed as n (%) unless otherwise stated.
Discussion
Principal findings
This study showed that respiratory viruses could be frequently detected among acutely hospitalized patients without fever or respiratory symptoms (23.4%), although less frequently than in patients with fever or respiratory symptoms (44.9%). For the fever/RS group, influenza B virus and enterovirus/rhinovirus were the most commonly identified viruses, which was similar to the surveillance data from the Public Health Laboratory Service in Hong Kong during the same period (see Supplementary material, Fig. S2). However, for the non-fever/RS group, adenovirus and PIV-4B were the most frequently detected viruses. Notably, adenovirus and PIV-4B were more common among patients in the non-fever/RS group than those in the fever/RS group. Among patients in the non-fever/RS group, chest discomfort was more frequently reported by patients with respiratory virus detected than those without respiratory virus detected.
Comparison with other studies
Previous studies have reported that respiratory symptoms predominated in patients with symptomatic infection, whereas non-respiratory symptoms were only present in a minority of patients [7], [15]. Many retrospective studies have demonstrated an association between respiratory virus infection and extrapulmonary complications [12], [14], [21]. However, because of the bias in testing patients with fever or respiratory symptoms, these reported results may not be accurate. Our results suggest that patients without respiratory symptoms should be enrolled in future studies on the role of respiratory viruses in extrapulmonary complications.
Adenovirus and PIV-4B were more frequently detected in the non-fever/RS group than in the fever/RS group. Adenovirus and PIV are generally perceived to be less important than influenza virus or respiratory syncytial virus because of the low incidence. However, a retrospective study showed that adenovirus and PIV had the highest hospitalization–fatality rate among different respiratory viruses [9]. It is possible that these respiratory viruses are transient, colonizing the patients' upper respiratory tract but not actually causing the symptoms or disease. However, although asymptomatic infections due to adenovirus and PIV are often found in children [11], [27], [28], adenovirus and PIV are rarely detected among healthy asymptomatic adults [11], [29].
This study was designed to minimize potential bias. First, we have recorded the respiratory symptom data from study participants using a standardized questionnaire. This is important because mild symptoms may not be actively reported by patients. Second, the fever/RS group and non-fever/RS group were matched for age and sex. The matching of age is particularly important because previous studies have shown that the proportion of patients with symptoms varies according to age [28]. Another unique feature in this study is that we have included patients with fever but without respiratory symptoms in the fever/RS group because many clinicians test respiratory viruses in febrile patients without respiratory symptoms.
In the current study, patients in the non-fever/RS group were admitted to hospital for acute non-respiratory symptoms. Hence, our patient cohort differs from studies involving asymptomatic adult individuals who were not clinically affected by the respiratory viruses [11], [27], [29]. Further studies are required to determine whether there is a causal relationship between the identified respiratory virus and the symptoms among patients in the fever/RS group.
This study was conducted during the influenza B epidemic. As expected, influenza B virus was the most commonly detected respiratory virus among the fever/RS group. Interestingly, influenza B virus was only found in one patient in the non-fever/RS group. The ratio in the detection rate of influenza B virus in the fever/RS group and non-fever/RS group was greatest among all viruses. Our results suggest that patients with influenza B virus infection are more likely to present with respiratory symptoms than those with other respiratory virus infections.
Limitations of this study
First, we only recruited patients from acute adult medical wards. Therefore, adult patients hospitalized with surgical conditions or paediatric patients would not be included. Second, we enrolled patients within 24 hours of hospitalization. The role of respiratory viruses for nosocomial complications should be further explored. Third, this study was conducted over a 1-month period during the influenza B winter season, and therefore the respiratory viruses detected in this study are not representative for the entire year. A long-term study should be conducted to capture the peak seasons of other respiratory viruses. Fourth, some patients in the non-fever/RS group may have prolonged viral shedding from a previous respiratory illness that occurred more than 7 days before admission. Finally, we studied acutely hospitalized patients, who would have more severe symptoms or complications when compared with patients seeking help in the community. Therefore, further studies should be performed at general practice in the community.
Conclusions and implications for clinical practice and research studies
This study showed that respiratory viruses, especially non-influenza viruses, can be detected in a substantial proportion of acutely hospitalized adult patients without clinically apparent respiratory tract infection. For future studies, the inclusion of hospitalized patients without clinically apparent respiratory tract infection will lead to a better understanding of the non-respiratory symptomatology and extrapulmonary complications of respiratory viruses.
The high rate of detection of respiratory viruses among the non-fever/RS group may be important for infection control and clinical management. Respiratory viruses are responsible for many nosocomial outbreaks. Patients without clinical evidence of respiratory illness may represent an ‘occult’ source of respiratory viruses for nosocomial outbreaks, and therefore these patients might have to be included in outbreak investigations. Furthermore, the detection of respiratory viruses in these patients may affect antiviral treatment decisions, especially when novel antivirals against non-influenza viruses become available [6], [30].
Transparency declaration
All authors declare no conflict of interest.
Funding
This work was supported by Consultancy Service for Enhancing Laboratory Surveillance of Emerging Infectious Diseases and Research Capability on Antimicrobial Resistance for the Department of Health of the HKSAR Government, donations from the Shaw Foundation Hong Kong, Richard Yu and Carol Yu, Michael Seak-Kan Tong, the Respiratory Viral Research Foundation Limited, the Hui Ming, Hui Hoy and Chow Sin Lan Charity Fund Limited.
Acknowledgements
We acknowledge Mr Chin-Ki Ng and Mr Joy-Yan Lam for their assistance in processing clinical specimens.
Editor: M. Paul
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2019.04.012.
Authors' contributions
KKWT, KHC, JH, VCCC, IFNH and KYY designed the study. KKWT, KHC, JH, PKPP, DTYH, ACHC, CWS and CCYY acquired the data. KKWT carried out the statistical analysis. All authors interpreted the data, revised the manuscript critically for important intellectual content and approved the final report.
Data sharing
The investigators will share data used in developing the results presented in this manuscript on request to the corresponding author. Anonymized record-level data will be made available on proposal for analysis by those who have received ethical clearance from their host institution.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Jain S., Self W.H., Wunderink R.G., Fakhran S., Balk R., Bramley A.M. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med. 2015;373:415–427. doi: 10.1056/NEJMoa1500245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Charlton C.L., Babady E., Ginocchio C.C., Hatchette T.F., Jerris R.C., Li Y. Practical guidance for clinical microbiology laboratories: viruses causing acute respiratory tract infections. Clin Microbiol Rev. 2019;32 doi: 10.1128/CMR.00042-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.To K.K., Chan J.F., Chen H., Li L., Yuen K.Y. The emergence of influenza A h7n9 in human beings 16 years after influenza A h5n1: a tale of two cities. Lancet Infect Dis. 2013;13:809–821. doi: 10.1016/S1473-3099(13)70167-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi T., McAllister D.A., O'Brien K.L., Simoes E.A.F., Madhi S.A., Gessner B.D. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet. 2017;390:946–958. doi: 10.1016/S0140-6736(17)30938-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iuliano A.D., Roguski K.M., Chang H.H., Muscatello D.J., Palekar R., Tempia S. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet. 2018;391:1285–1300. doi: 10.1016/S0140-6736(17)33293-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.To K.K., Yip C.C., Yuen K.Y. Rhinovirus—from bench to bedside. J Formos Med Assoc. 2017;116:496–504. doi: 10.1016/j.jfma.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 7.To K.K., Lu L., Fong C.H., Wu A.K., Mok K.Y., Yip C.C. Rhinovirus respiratory tract infection in hospitalized adult patients is associated with Th2 response irrespective of asthma. J Infect. 2018;76:465–474. doi: 10.1016/j.jinf.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Shorr A.F., Fisher K., Micek S.T., Kollef M.H. The burden of viruses in pneumonia associated with acute respiratory failure: an underappreciated issue. Chest. 2018;154:84–90. doi: 10.1016/j.chest.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Chan P.K., Tam W.W., Lee T.C., Hon K.L., Lee N., Chan M.C. Hospitalization incidence, mortality, and seasonality of common respiratory viruses over a period of 15 years in a developed subtropical city. Medicine (Balt) 2015;94 doi: 10.1097/MD.0000000000002024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. Research needs for the battle against respiratory viruses (brave). Available at: http://www.Who.Int/influenza/patient_care/clinical/brave_research_agenda_2013.Pdf?Ua=1. Accessed on 20 November 2017.
- 11.Self W.H., Williams D.J., Zhu Y., Ampofo K., Pavia A.T., Chappell J.D. Respiratory viral detection in children and adults: comparing asymptomatic controls and patients with community-acquired pneumonia. J Infect Dis. 2016;213:584–591. doi: 10.1093/infdis/jiv323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kwong J.C., Schwartz K.L., Campitelli M.A., Chung H., Crowcroft N.S., Karnauchow T. Acute myocardial infarction after laboratory-confirmed influenza infection. N Engl J Med. 2018;378:345–353. doi: 10.1056/NEJMoa1702090. [DOI] [PubMed] [Google Scholar]
- 13.Russell E., Yang A., Tardrew S., Ison M.G. Parainfluenza virus in hospitalized adults: a 7-year retrospective study. Clin Infect Dis. 2019;68:298–305. doi: 10.1093/cid/ciy451. [DOI] [PubMed] [Google Scholar]
- 14.Blackburn R., Zhao H., Pebody R., Hayward A., Warren-Gash C. Laboratory-confirmed respiratory infections as predictors of hospital admission for myocardial infarction and stroke: time-series analysis of English data for 2004–2015. Clin Infect Dis. 2018;67:8–17. doi: 10.1093/cid/cix1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.To K.K., Wong S.S., Li I.W., Hung I.F., Tse H., Woo P.C. Concurrent comparison of epidemiology, clinical presentation and outcome between adult patients suffering from the pandemic influenza A (h1n1) 2009 virus and the seasonal influenza a virus infection. Postgrad Med J. 2010;86:515–521. doi: 10.1136/pgmj.2009.096206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Minodier L., Masse S., Capai L., Blanchon T., Ceccaldi P.E., van der Werf S. Clinical and virological factors associated with gastrointestinal symptoms in patients with acute respiratory infection: a two-year prospective study in general practice medicine. BMC Infect Dis. 2017;17:729. doi: 10.1186/s12879-017-2823-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.To K.K., Hung I.F., Li I.W., Lee K.L., Koo C.K., Yan W.W. Delayed clearance of viral load and marked cytokine activation in severe cases of pandemic h1n1 2009 influenza virus infection. Clin Infect Dis. 2010;50:850–859. doi: 10.1086/650581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.To K.K., Lau S.K., Chan K.H., Mok K.Y., Luk H.K., Yip C.C. Pulmonary and extrapulmonary complications of human rhinovirus infection in critically ill patients. J Clin Virol. 2016;77:85–91. doi: 10.1016/j.jcv.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 19.Yeung M.L., Yao Y., Jia L., Chan J.F., Chan K.H., Cheung K.F. MERS coronavirus induces apoptosis in kidney and lung by upregulating smad7 and fgf2. Nat Microbiol. 2016;1:16004. doi: 10.1038/nmicrobiol.2016.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zumla A., Hui D.S., Perlman S. Middle east respiratory syndrome. Lancet. 2015;386:995–1007. doi: 10.1016/S0140-6736(15)60454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Warren-Gash C., Blackburn R., Whitaker H., McMenamin J., Hayward A.C. Laboratory-confirmed respiratory infections as triggers for acute myocardial infarction and stroke: a self-controlled case series analysis of national linked datasets from Scotland. Eur Respir J. 2018;51 doi: 10.1183/13993003.01794-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.To K.K., Lu L., Yip C.C., Poon R.W., Fung A.M., Cheng A. Additional molecular testing of saliva specimens improves the detection of respiratory viruses. Emerg Microbe. Infect. 2017;6:e49. doi: 10.1038/emi.2017.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.To K.K., Yip C.C., Lai C.Y., Wong C.K., Ho D.T., Pang P.K. Saliva as a diagnostic specimen for testing respiratory virus by a point-of-care molecular assay: a diagnostic validity study. Clin Microbiol Infect. 2019;25:372–378. doi: 10.1016/j.cmi.2018.06.009. [DOI] [PubMed] [Google Scholar]
- 24.Kim Y.G., Yun S.G., Kim M.Y., Park K., Cho C.H., Yoon S.Y. Comparison between saliva and nasopharyngeal swab specimens for detection of respiratory viruses by multiplex reverse transcription-PCR. J Clin Microbiol. 2017;55:226–233. doi: 10.1128/JCM.01704-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan K.H., To K.K.W., Li P.T.W., Wong T.L., Zhang R., Chik K.K.H. Evaluation of NxTAG respiratory pathogen panel and comparison with xTAG respiratory viral panel fast v2 and film array respiratory panel for detecting respiratory pathogens in nasopharyngeal aspirates and swine/avian-origin influenza A subtypes in culture isolates. Adv Virol. 2017;2017:1324276. doi: 10.1155/2017/1324276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.von Elm E., Altman D.G., Egger M., Pocock S.J., Gotzsche P.C., Vandenbroucke J.P. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007;4:e296. doi: 10.1371/journal.pmed.0040296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ip D.K., Lau L.L., Leung N.H., Fang V.J., Chan K.H., Chu D.K. Viral shedding and transmission potential of asymptomatic and paucisymptomatic influenza virus infections in the community. Clin Infect Dis. 2017;64:736–742. doi: 10.1093/cid/ciw841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Munywoki P.K., Koech D.C., Agoti C.N., Bett A., Cane P.A., Medley G.F. Frequent asymptomatic respiratory syncytial virus infections during an epidemic in a rural Kenyan household cohort. J Infect Dis. 2015;212:1711–1718. doi: 10.1093/infdis/jiv263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shaman J., Morita H., Birger R., Boyle M., Comito D., Lane B. Asymptomatic summertime shedding of respiratory viruses. J Infect Dis. 2018;217:1074–1077. doi: 10.1093/infdis/jix685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brendish N.J., Clark T.W. Antiviral treatment of severe non-influenza respiratory virus infection. Curr Opin Infect Dis. 2017;30:573–578. doi: 10.1097/QCO.0000000000000410. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.