Abstract
Here we report a case of a laboratory-confirmed 2019 novel coronavirus (2019-nCoV)-infected patient with COVID-19 (coronavirus disease 2019) who developed respiratory failure and shock accompanied by persistent diarrhoea despite conventional therapeutic interventions. The patient avoided mechanical ventilation and showed an immediate clinical and radiological improvement following treatment with intensive plasma exchange (PE) followed by intravenous immunoglobulin (IVIG). Successful therapeutic strategies in this case suggest that timely initiation of PE treatment followed by IVIG in critically ill patients with COVID-19 may prevent the disease from worsening and help to reduce the requirement for mechanical ventilation and intensive supportive care. Moreover, it may improve poor clinical outcomes of these patients.
Keywords: 2019 novel coronavirus, SARS-CoV-2, COVID-19, Plasma exchange, Immunoglobulin, Diarrhoea
1. Introduction
The prevalence of COVID-19 (coronavirus disease 2019), named by the World Health Organization (WHO) on 11 February 2020 and caused by infection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [previously known as 2019 novel coronavirus (2019-nCoV)], has become a growing concern worldwide and is causing increasing alarm [1]. As of 10 February 2020, more than 40 000 people have been infected. There are currently no specific treatments for COVID-19. Supportive treatment combined with existing antiviral agents are the mainstay treatments. More than 1000 people have lost their lives in the past 2 months. Mortality rates are as high as 15% in critically ill patients requiring intensive care unit (ICU) admission and oxygen therapy [1], suggesting an urgent need to try therapeutic interventions in addition to supportive treatment. Here we report a case of a laboratory-confirmed COVID-19 patient who failed to respond to conventional therapeutic interventions and who developed respiratory failure and shock accompanied by persistent diarrhoea. The patient avoided mechanical ventilation and showed an immediate clinical and radiological improvement following treatment with intensive plasma exchange (PE) followed by intravenous immunoglobulin (IVIG).
2. Case report
On 21 January 2020, a 50-year-old woman with laboratory-confirmed SARS-CoV-2 infection was transferred to our hospital for isolated treatment. She had experienced fever for 6 days prior to admission with an 8-day history of intermittent dry cough, myalgia, fatigue, headache and decreased appetite. She was previously healthy apart from a 1-month history of thyroid nodule. She had close contact 13 days previously with a permanent resident from Wuhan, China, where the new coronavirus pneumonia was spreading. She was admitted to another hospital approximately 8 h before and laboratory results revealed leukopenia and lymphocytopenia. Computed tomography (CT) revealed bilateral ground-glass opacity.
On admission [day of illness 9 (DOI 9)], her vital signs were within normal limits and no remarkable signs were found on physical examination. Laboratory results were remarkable for leukopenia and lymphocytopenia, with a white blood cell (WBC) count of 3.02 × 109/L, a lymphocyte count of 0.6 × 109/L and an absolute neutrophil count (ANC) of 2.2 × 109/L. Blood markers of kidney and liver function were within the normal range. Inflammatory indicators were almost normal, with a C-reactive protein (CRP) of 10.73 mg/L.
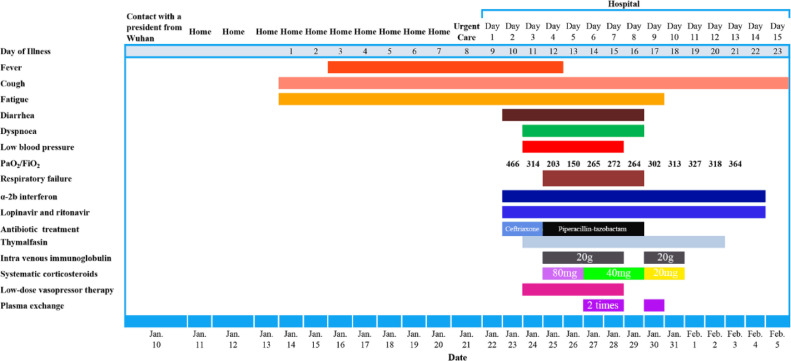
The patient's clinical course is shown in Fig 1 . Upon admission, she received low-flow oxygen therapy via a nasal catheter and supportive treatment. Antiviral therapy was initiated with inhaled interferon alfa-2b (IFN-α2b) followed by lopinavir/ritonavir (500 mg orally, twice daily) on the following day (DOI 10). In addition, empirical antibiotic treatment with ceftriaxone was initiated.
Fig. 1.
Clinical course of the patient according to day of illness (DOI) and day of hospitalisation, 10 January 2020 to 5 February 2020.
The next day after admission, the patient developed diarrhoea characterised by the passage of six to seven loose bowel movements per day, without abdominal pain, nausea or vomiting. No blood or mucus were observed in the faeces. Intravenous rehydration, oral probiotic supplement, antimotility agents and nutritional support were commenced.
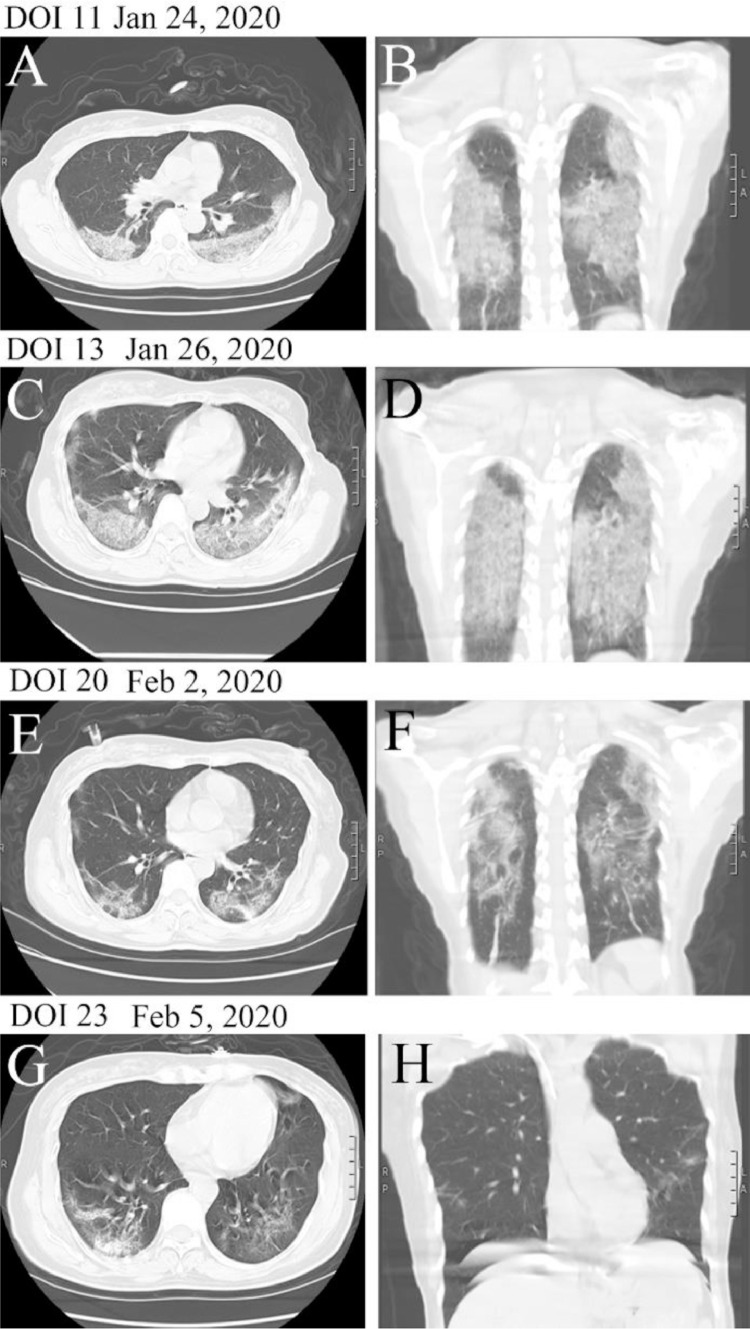
On 24 January 2020 (DOI 11), the patient was still suffering from diarrhoea and intermittent fever (maximum fever, 38.5 °C). No remarkable abnormity was found on stool examination and the admission blood culture was negative. Her condition began to deteriorate at midday when she reported intermittent cough accompanied by chest pain and her blood pressure fell to 96/54 mmHg. Based on her new symptoms, the patient immediately underwent abdominal and chest CT. Imaging revealed newly developed evidence of lung lesions (Fig. 2 ), whilst abdominal CT showed nothing noteworthy. Blood examinations revealed a progressive decrease in WBC and lymphocyte counts (WBC count 2.4 × 109/L, lymphocyte count 0.5 × 109/L and ANC 1.7 × 109/L). To increase the leukocyte count, the patient was administered a subcutaneous injection of 300 µg of human granulocyte-colony stimulating factor (G-CSF), which was discontinued 4 days later. The patient's blood pressure remained low despite adequate volume resuscitation (central venous pressure, 6–10 cmH2O) until low-dose vasopressor (dopamine) infusion.
Fig. 2.
Transverse and longitudinal chest computed tomography (CT) images of the patient from day of illness (DOI) 11 to 23.
On 25 January 2020 (DOI 12), the patient's blood pressure fell to 78/36 mmHg and her oxygenation status declined to a PaO2/FiO2 of 203 mmHg. Massive intravenous fluid infusion (~2.8 L in 24 h, including 200 mL of plasma) was immediately initiated. Together with intravenous administration of noradrenalin (4 µg/kg/min by continuous infusion maintaining blood pressure >100/60 mmHg). The patient was undoubtedly classified into the severe type of COVID-19 according to the Chinese ‘Novel coronavirus infection pneumonia diagnosis and treatment standards (5th edition)’. Given the exacerbation of the patient's symptoms, empirical IVIG (20 g) and thymalfasin were initiated. Corticosteroids (80 mg intravenous methylprednisolone) were also commenced, which was halved to 40 mg two days later. The patient's O2 requirement increased to 4–5 L/min while maintaining an oxygen saturation of 93%. Blood analysis demonstrated an obviously elevated WBC count and neutrophil percentage (Table 1 ), which might be related to the use of G-CSF and a possible nosocomial infection. Ceftriaxone was replaced with piperacillin/tazobactam (4.5 g intravenously every 8 h) based on the symptom deterioration and increased serum CRP (35.09 mg/L) of the patient.
Table 1.
Laboratory findings of the patient over time.
| Laboratory parameter | DOI 10 | DOI 13 | DOI 14 | DOI 15 | DOI 16 | DOI 17 | DOI 18 | DOI 22 |
|---|---|---|---|---|---|---|---|---|
| Creatine kinase (U/L) | 106.37 | 194 | 156.9 | 81.17 | 47.51 | 36.38 | 31.79 | 41.07 |
| CK-MB (IU/L) | 16.39 | 1.41 | 14.39 | 7.08 | 8.85 | 11.64 | 4.58 | 7.28 |
| Alanine aminotransferase (U/L) | 8.61 | 11.31 | 37.62 | 45.56 | 25.84 | 60.94 | 30.38 | 39.5 |
| Aspartate aminotransferase (U/L) | 42.6 | 42.47 | 129.23 | 160.21 | 57.38 | 171.55 | 74.82 | 31 |
| Lactate dehydrogenase (U/L) | 344.53 | 316.59 | 456.53 | 353.58 | 226.71 | 352.68 | 213 | 210 |
| Blood urea nitrogen (mmol/L) | 2 | 1.91 | 1.59 | 1.76 | 2.28 | 2.88 | 2.45 | 2.42 |
| Serum creatinine (µmol/L) | 82.53 | 84.36 | 75.22 | 81.92 | 70.96 | 59.39 | 75.66 | 70 |
| eGFR (mL/min/1.73 m2) | 78.29 | 66.4 | 71.24 | 50.99 | 49.09 | 43.39 | 64.94 | 78.59 |
| WBC count (× 109/L) | 2.6 | 16.8 | 14.7 | 12.7 | 11.6 | 9.2 | 8.4 | 7.73 |
| Platelet count (× 109/L) | 118 | 182 | 161 | 141 | 148 | 138 | 129 | 106 |
| Lymphocyte count (× 109/L) | 0.6 | 0.3 | 0.6 | 1.1 | 1 | 0.9 | 1.1 | 2.77 |
| Lymphocyte percentage (%) | 24.6 | 1.8 | 3.7 | 9 | 8.3 | 10.3 | 13.6 | 35.8 |
| ANC (× 109/L) | 1.7 | 15.9 | 13.9 | 11 | 10.2 | 7.6 | 6.7 | 4.39 |
| Neutrophil percentage (%) | 66.9 | 94.8 | 94.3 | 86.6 | 87.2 | 82.5 | 80.4 | 56.9 |
| C-reactive protein (mg/L) | 10.73 | 8.04 | 4.87 | 1.56 | <0.499 | 8.02 | 6.02 | 4.1 |
| Procalcitonin (ng/mL) | 0.08 | ND | 0.06 | 0.09 | ND | 0.08 | 0.03 | ND |
DOI, day of illness; CK-MB, creatine kinase myocardial band; eGFR, estimated glomerular filtration rate; WBC, white blood cell; ANC, absolute neutrophil count; ND, no data.
The patient was afebrile 1 day after starting methylprednisolone therapy, but her dyspnoea was aggravated with persistent diarrhoea. Despite administration of systematic corticosteroids and IVIG, the patient's PaO2/FiO2 decreased to 150 mmHg on DOI 13, suggesting that she had progressed to severe acute hypoxaemic respiratory failure. Laboratory data revealed progressive depletion of lymphocytes (lymphocyte count, 0.3 × 109/L). In addition, there were notable elevations of serum transaminase enzymes and creatinine (Table 1). Chest CT showed bilateral multiple patchy ground-glass opacities and high-density consolidation, which revealed rapidly progressive pulmonary lesions (Fig. 2). Nasal high-flow oxygen therapy was commenced. Furthermore, administration of three consecutive sessions of PE was initiated with 6000 mL of plasma (frozen plasma served as the sole replacement solution) followed by 20 g of IVIG from DOI 14 to DOI 17. The patient's symptoms were almost all alleviated following three consecutive sessions of PE treatment. No adverse events or complications were observed during PE treatment. The oxygenation index increased with an oxygen saturation of 96% while the patient was breathing ambient air oxygen and the blood pressure was restored and maintained on 29 January 2020 (DOI 16). Vasopressor therapy and antibiotic treatment were discontinued. Simultaneously, levels of serum transaminase enzymes and creatinine recovered. Although the clinical condition was improved, episodes of diarrhoea persisted (frequency of six to seven times per day; water lost, 700–1000 mL/day) on DOI 16. Final stool cultures showed no growth and the patient was prescribed intravenous anisodamine (10 mg daily).
After a fourth plasma exchange followed by IVIG on DOI 17, the patient made a prompt recovery with no further diarrhoea episodes and improved appetite. Her PaO2/FiO2 increased to 302 mmHg, coinciding with improving chest radiographic evidence. On DOI 18, the patient received the sixth IVIG treatment, with the dose of methylprednisolone halved to 20 mg and discontinued on DOI 19. Supplementary oxygen was discontinued on DOI 20, with the O2 saturation improved to 95% on room air. Swab samples collected on DOIs 16, 18 and 20 from the patient's throat and nares were negative for SARS-CoV-2. The patient was asymptomatic apart from a slight cough and was discharged from hospital on 5 February 2020 (DOI 23) with obvious improvement of chest radiographic evidence after 15 days of hospitalisation.
3. Discussion
To our knowledge, many patients who are classified into severe type COVID-19 may require intensive care therapy or even the use of mechanical ventilation for patients with respiratory failure. In the current case, the patient failed to respond to conventional interventions and progressed to persistent diarrhoea, acute respiratory failure and shock. Undoubtedly, she belongs to the severe type of COVID-19. Timely initiation of PE treatment followed by IVIG protected the patient from progressing to acute respiratory distress syndrome (ARDS) and multiple organ failure. Astoundingly, the patient made a prompt recovery following PE treatment without need for mechanical ventilation or intensive supportive care. The successful experience in this case suggests that timely treatment with PE followed by IVIG is worth trying, especially in those critically ill patients with COVID-19, since it may significantly improve the prognosis. This case report sheds new light on a therapeutic strategy for this potentially fatal disease.
Unlike previous reported cases, the patient described here developed severe diarrhoea, which was most likely attributable to the SARS-CoV-2 infection. Although diarrhoea might be a side effect of lopinavir/ritonavir, no further diarrhoeal episodes occurred after the fourth PE treatment (followed by IVIG), while lopinavir/ritonavir was continued. This suggests that severe diarrhoea is also a clinical manifestation of COVID-19, which may be further confirmed by positive test for SARS-CoV-2 in stool specimens [2].
Mechanisms accounting for the immediate improvement of the patient remain to be proven. It is most likely that timely treatment with PE followed by IVIG contributed to the prompt recovery of the patient with a favourable prognosis. It has been well documented that the ‘cytokine storm’ may be involved in disease progression of COVID-19 in critically ill patients. A previous study reported that COVID-19 patients requiring ICU admission have significantly higher plasma levels of cytokines and chemokines [1], suggesting that the immune system is activated and a cytokine storm is established. In the current case, PE followed by IVIG was initiated when signs of the cytokine storm appeared (i.e. decreased oxygenation index, progressive decline in lymphocyte count, increase in serum transaminase enzymes and creatinine levels, shock, high CRP levels). The patient presented an immediate clinical and laboratory improvement after initiating PE treatment followed by IVIG. Her lymphocyte count recovered gradually after intensive treatment of PE. Pathogenic substances, cytokines and inflammatory mediators might be cleared by PE and the subsequent supplementation of immunoglobulin exerted its immunoregulatory and antiviral effects. This perfect combination treatment produced a satisfactory effect in this case. Our speculation is supported by previous studies which demonstrated that PE could decrease serum cytokines as well as blood viral load in patients with certain viral infections [3,4]. The cytokine storm is also believed to be the basis of the pathophysiology of fatal influenza virus infection. Recovery of three critically ill patients with ARDS and severe shock due to 2009 pH1N1 influenza A virus infection after PE treatment [4] suggests that PE is still effective even in the later stages of cytokine storms. Application of IVIG was associated with decreased viral load and improved prognosis during the 2009 H1N1 influenza pandemic [5] and severe acute respiratory syndrome (SARS) epidemic, which is also caused by a coronavirus [6]. Unfortunately, we did not evaluate the viral load and cytokine levels before and after PE. Other mechanisms such as the broad-spectrum antibacterial and antiviral specificities of IVIG as well as their immunomodulatory effects [7] should also be considered in explaining the resolution of clinical signs in this case.
Several questions remained unanswered. First, the contribution of antiviral regimens cannot be completely ignored, as IFN-α2b and lopinavir/ritonavir were used throughout the course of the treatment. They previously helped to improve poor treatment outcomes associated with Middle East respiratory syndrome coronavirus (MERS-CoV) infections [8]. Second, the contribution of corticosteroids cannot be excluded since treatment with PE and IVIG overlapped with corticosteroid administration, which possesses anti-inflammatory effects.
4. Conclusion
Timely initiation of PE treatment followed by IVIG in critically ill COVID-19 patients may prevent the disease from worsening and help to reduce the requirements for mechanical ventilation and intensive supportive care. Moreover, it may improve the poor clinical outcomes of these patients. Randomised controlled trials are urgently required to confirm the effectiveness of PE combined with subsequent IVIG in critically ill COVID-19 patients.
Acknowledgments
Funding: This work was supported in part by grants from the NHC Key Laboratory of Pulmonary Immunological Diseases (Guizhou Provincial People's Hospital, Guiyang, Guizhou, China).
Competing interests: None declared.
Ethical approval: Not required. Written consent was signed by the patient.
Editor: Jean-Marc Rolain
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H., et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oh W.S., Heo S.T., Kim S.H., Choi W.J., Han M.G., Kim J.Y. Plasma exchange and ribavirin for rapidly progressive severe fever with thrombocytopenia syndrome. Int J Infect Dis. 2014;18:84–86. doi: 10.1016/j.ijid.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 4.Patel P., Nandwani V., Vanchiere J., Conrad S.A., Scott L.K. Use of therapeutic plasma exchange as a rescue therapy in 2009 pH1N1 influenza A—an associated respiratory failure and hemodynamic shock. Pediatr Crit Care Med. 2011;12:e87–e89. doi: 10.1097/PCC.0b013e3181e2a569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hung I.F.N., To K.K.W., Lee C.K., Lee K.L., Yan W.W., Chan K., et al. Hyperimmune IV immunoglobulin treatment: a multicenter double-blind randomized controlled trial for patients with severe 2009 influenza A(H1N1) infection. Chest. 2013;144:464–473. doi: 10.1378/chest.12-2907. [DOI] [PubMed] [Google Scholar]
- 6.Chong P.Y., Chui P., Ling A.E., Franks T.J., Tai D.Y., Leo Y.S., et al. Analysis of deaths during the severe acute respiratory syndrome (SARS) epidemic in Singapore: challenges in determining a SARS diagnosis. Arch Pathol Lab Med. 2004;128:195–204. doi: 10.1043/1543-2165(2004)128<195:AODDTS>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 7.Bayry J., Lacroix-Desmazes S., Kazatchkine M.D., Kaveri S.V. Intravenous immunoglobulin for infectious diseases: back to the pre-antibiotic and passive prophylaxis era? Trends Pharmacol Sci. 2004;25:306–310. doi: 10.1016/j.tips.2004.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zumla A., Azhar E.I., Arabi Y., Alotaibi B., Rao M., McCloskey B., et al. Host-directed therapies for improving poor treatment outcomes associated with the Middle East respiratory syndrome coronavirus infections. Int J Infect Dis. 2015;40:71–74. doi: 10.1016/j.ijid.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]