Abstract
Background: High-quality diabetes care is evidence-based, timely, and equitable. Sodium-glucose cotransporter-2 inhibitors (SGLT2i) are the most recently approved class of glucose-lowering medications with additional cardio- and renal-protective benefits and low risk of hypoglycemia. Cardiovascular and kidney disease are among the most common chronic diabetes complications, whereas hypoglycemia is the most prevalent adverse effect of glucose-lowering therapy. We examine the sociodemographic and clinical factors associated with early SGLT2i initiation and appropriateness of use based on contemporaneous scientific evidence.
Materials and Methods: Retrospective analysis of medical and pharmacy claims data from OptumLabs® Data Warehouse for commercially insured and Medicare Advantage adult beneficiaries with diabetes types 1 and 2, who filled any glucose-lowering medication between January 1, 2013 and December 31, 2016. Demographic (age, sex, race, income), clinical (comorbidities), and insurance-related factors affecting first prescription for a SGLT2i were examined using multivariable logistic regression.
Results: Among 1,054,727 adults with pharmacologically treated diabetes, 7.2% (n = 75,500) initiated a SGLT2i. Patients with prior myocardial infarction (MI) (odds ratio [OR]: 0.94, 95% confidence interval [CI]: 0.91–0.96), heart failure (HF) (OR: 0.93, 95% CI: 0.91–0.94), kidney disease (OR: 0.80, 95% CI: 0.78–0.81), and severe hypoglycemia (OR: 0.96, 95% CI: 0.94–0.98) were all less likely to start a SGLT2i; P < 0.001 for all. SGLT2i were also less likely to be started by patients ≥75 years (OR: 0.57, 95% CI: 0.55–0.59, vs. 18–44 years), Black patients (OR: 0.93, 95% CI: 0.91–0.95, vs. White), and those with Medicare Advantage insurance (OR: 0.63, 95% CI: 0.62–0.64, vs. commercial).
Conclusions: Younger, healthier, non-Black patients with commercial health insurance were most likely to start taking SGLT2i. Patients with MI, HF, kidney disease, and prior hypoglycemia were less likely to use SGLT2i, despite evidence supporting their preferential use in these patients. Efforts to address this treatment-risk paradox may help improve health outcomes among patients with type 2 diabetes.
Keywords: Diabetes mellitus, SGLT2 inhibitor, Evidence-based medicine, Pharmacoepidemiology, Health services research, Administrative claims data
Introduction
More than 30 million U.S. adults, or 12.2% of the adult population, have diabetes.1 High quality patient-centered diabetes care is predicated on managing glucose-lowering pharmacotherapy to reduce risk of immediate- and long-term diabetes complications, prevent adverse drug reactions, address pertinent comorbidities, and minimize burden of treatment while supporting a healthy lifestyle and addressing social determinants of health.2 Sodium-glucose cotransporter 2 inhibitors (SGLT2i) are the most recently approved class of glucose-lowering medications. Their use in select clinical contexts—specifically with concurrent hypertension, heart failure (HF), cardiovascular disease (CVD), or at-risk for hypoglycemia—is supported by scientific evidence3–6 and recommended by American Diabetes Association (ADA),7 American Association of Clinical Endocrinologists (AACE),8 European Association for the Study of Diabetes (EASD),9 and American Heart Association (AHA)10 clinical guidelines.
CVD is the most common comorbidity among people with diabetes and contributes the most to their morbidity and mortality.11 Nearly 74% of people with diabetes have hypertension,1 18% have coronary heart disease,12 and 9%–22% have HF.13 Similarly, severe hypoglycemia is a common, yet potentially preventable, adverse event in diabetes management, affecting as many as 17% of people with type 2 diabetes.14 SGLT2i may be a preferred agent to be considered in these contexts; yet, little is known about how they were incorporated into real-world diabetes management and, specifically, whether their early use was aligned with the clinical contexts for which they are most beneficial.
Three SGLT2i were approved by the U.S. Food and Drug Administration (FDA) before publication of cardiovascular outcome trials demonstrating improved CVD, HF, and kidney outcomes with their use3–6: canagliflozin (March 2013), dapagliflozin (January 2014), and empagliflozin (August 2014). They act by inhibiting SGLT2 in the proximal convoluted tubule, thereby preventing active glucose reabsorption and facilitating glycosuria in a glucose-dependent, insulin-independent fashion. Early on, it was apparent that SGLT2i are not associated with weight gain or hypoglycemia, but can lower blood pressure and weight, thereby making them particularly attractive treatment options for overweight or obese patients, patients with comorbid HF or hypertension, patients at risk for hypoglycemia, and the elderly.7 While approved as an add-on medication for the management of type 2 diabetes, SGLT2i have also been used off-label by patients with type 1 diabetes.15–17
The decision to initiate SGLT2i therapy is likely impacted by clinician familiarity,18–23 patient interest, insurance coverage and cost considerations, and adverse effect concerns. Most notable safety concerns that emerged early on included genitourinary tract infections, dehydration, acute kidney injury,24,25 as well as rare but serious events such as lower extremity amputations,5,26,27 bone density loss and fractures,28–30 and ketoacidosis.31 The goal of patient-centered diabetes pharmacotherapy is therefore to align the risk/benefit ratios of available treatment options to achieve maximal benefit with least probability of harm.
The degree to which this occurred for SGLT2i early in the course of their availability on the market following FDA approval, particularly in the context of emerging safety concerns, is unknown. Earlier studies revealed a risk/treatment paradox in the management of CVD (focused on statin and HF medications), whereby highest risk patients who are also most likely to benefit from the studied interventions, were least likely to receive them.32,33 To identify opportunities to better align glucose-lowering therapies with patient context and clinical necessity in a timely manner, we examined the patterns and corollaries of SGLT2i initiation by commercially insured and Medicare Advantage beneficiaries with diabetes (types 1 and 2) across the U.S. between 2013 and 2016.
Materials and Methods
Study design
We retrospectively analyzed medical and pharmacy claims from OptumLabs® Data Warehouse (OLDW), a deidentified dataset of privately insured and Medicare Advantage enrollees in a large, private, U.S. health plan (Appendix Methods).34,35 OLDW contains longitudinal health information on enrollees, representing 19% of commercially insured and 19% of Medicare Advantage beneficiaries, resulting in a diverse mixture of ages, races/ethnicities, and geographic regions across the U.S. The health plan provides comprehensive insurance coverage for physician, hospital, and prescription drug services. This study was exempt from review by the Mayo Clinic Institutional Review Board, as it involves research solely on preexisting and deidentified data.
Study population
The study population included adults (≥18 years) with diabetes mellitus type 1 or type 2 who filled less than one glucose-lowering medication (Appendix Table A1) between January 1, 2013 and December 31, 2016. Diabetes was ascertained using Healthcare Effectiveness Data and Information Set (HEDIS) claims-computable criteria36 applied to 12 months preceding the index medication fill date. All patients were required to have 12 months of continued medical and pharmacy enrollment before the index date. Patients with only gestational diabetes (International Classification of Diseases, 9th edition [ICD-9] codes 648.0, 648.8; ICD-10 O24.x) were not included.
Primary outcome
Initiation of SGLT2i (canagliflozin, dapagliflozin, and empagliflozin) therapy was defined as the first filled prescription for any SGLT2i with no fills 12 months prior. Use was characterized as first-line (no other glucose-lowering medication filled in preceding 12 months), add-on (one or more additional medication filled during the 120 days before and after SGLT2i initiation, and none discontinued), or replacement (one or more additional medication filled in the 120 days before SGLT2i initiation, and at least one discontinued after initiation).
Independent variables
Patient characteristics included age, sex, race/ethnicity, U.S. region of residency, and type of health plan (commercial vs. Medicare Advantage). Variables describing the clinical setting included prescriber specialty, number of evaluation and management (E&M) visits with clinicians (primary care, endocrinology, cardiology, other) during the preceding 12 months, diabetes type, and comorbidities. Comorbidities were ascertained using ICD codes from E&M visits in the 12 months preceding the index date. We measured the (1) overall comorbidity burden, measured by the Charlson Comorbidity Index37; (2) presence of diabetes complications, measured by an adaptation of the diabetes complications severity index and its individual components38; and (3) presence of myocardial infarction (MI), HF, cerebrovascular disease, chronic kidney disease (CKD), hypertension, and cancer (except for skin cancer). Patients with type 1 diabetes were included in the study to assess the degree to which SGLT2i were used off-label in this population. Diabetes type was ascertained on the basis of ICD codes and medications filled during 12 months preceding the index prescription fill date, consistent with previously published approaches.39,40 Specifically, type 1 diabetes was assumed for patients who had >0.5 ratio of type 1 diabetes to type 2 diabetes ICD codes, were treated with insulin, and had no fills for any glucose-lowering medications, except for metformin and SGLT2i. All other patients were considered to have type 2 diabetes. ICD codes indicative of type 1 diabetes included ICD-9 250.x1 and 250.x3, and ICD-10 codes E10.xxx and O24.0xx. ICD codes indicative of type 2 diabetes included ICD-9 250.x0 and 250.x2, and ICD-10 E11.xxx and O24.1xx.
Statistical analysis
SGLT2i-users were compared with patients who had no fills for a SGLT2i during the study period (nonusers). These patients were identified on the basis of the first pharmacy claim for any non-SGLT2i glucose-lowering drug, categorized as shown in Appendix Table A1. The two cohorts (SGLT2i users and nonusers) were mutually exclusive.
Baseline characteristics of SGLT2i and non-SGLT2i cohorts are reported as frequencies with percentages for categorical data and mean with standard deviations (SDs) for continuous variables. Differences in baseline characteristics of patients in the two cohorts were compared using χ2 tests for categorical variables and t-tests for continuous variables. Multivariable logistic regression was used to assess predictors of SGLT2i use with results presented as odds ratios (ORs) and 95% confidence intervals (CIs). In addition, multinomial logistic regression was used to assess predictors for SGLT2i initiation as add-on versus first-line therapy.
The monthly rates of new SGLT2i users per 1000 pharmacologically treated adults with diabetes over time were calculated by specific SGLT2i drug, as well as by insurance type, prescriber specialty, and diabetes type. All analyses were conducted using SAS 9.4 (SAS Institute, Inc., Cary, NC).
Results
The study cohort was composed of 1,054,727 adults with pharmacologically treated diabetes, of whom 7.2% (75,500) were treated with a SGLT2i (Table 1). The vast majority (70.0%) were taking canagliflozin. Index medications for the remaining 979,227 patients were metformin (37.5%), sulfonylurea (21.6%), insulin (22.7%), DPP-4 inhibitors (DPP-4i) (9.0%), GLP-1 receptor analogs (GLP-1RA) (2.3%), thiazolidinedione (3.5%), and others (0.6%). Diagnosis of type 1 diabetes was present in 1.2% of SGLT2i users and 3.7% of nonusers; P < 0.001. Most SGLT2i were prescribed by primary care clinicians (32% family medicine, 24% internal medicine), whereas 23.4% were prescribed by endocrinologists; P < 0.001.
Table 1.
Study Population
| SGLT2i (n = 75,500) | Non-SGLT2i (n = 979,227) | P | |
|---|---|---|---|
| Demographics | |||
| Age (years), mean (SD) | 56.5 (10.7) | 62.2 (13.3) | <0.001 |
| Age category (years), n (%) | <0.001 | ||
| 18–44 | 10,090 (13.4) | 101,111 (10.3) | |
| 45–64 | 49,302 (65.3) | 409,148 (41.8) | |
| 65–74 | 12,680 (16.8) | 291,780 (29.8) | |
| ≥75 | 3428 (4.5) | 177,188 (18.1) | |
| Sex, n (%) | <0.001 | ||
| Female | 33,620 (44.5) | 474,810 (48.5) | |
| Male | 41,880 (55.5) | 504,417 (51.5) | |
| Race/ethnicity, n (%) | <0.001 | ||
| White | 49,665 (65.8) | 615,336 (62.8) | |
| Black | 9265 (12.3) | 159,274 (16.3) | |
| Hispanic | 11,254 (14.9) | 122,937 (12.6) | |
| Asian | 2734 (3.6) | 41,051 (4.2) | |
| Other/unknown | 2582 (3.4) | 40,629 (4.1) | |
| U.S. census region, n (%) | <0.001 | ||
| Midwest | 18,239 (24.2) | 264,947 (27.1) | |
| Northeast | 7784 (10.3) | 145,145 (14.8) | |
| South | 40,988 (54.3) | 454,779 (46.4) | |
| West | 8449 (11.2) | 113,630 (11.6) | |
| Other/unknown | 40 (0.1) | 726 (0.1) | |
| Health insurance, n (%) | <0.001 | ||
| Commercial | 56,993 (75.5) | 458,627 (46.8) | |
| Medicare Advantage | 18,507 (24.5) | 520,600 (53.2) | |
| Diabetes type and complications | |||
| Diabetes type, n (%) | <0.001 | ||
| Type 1 diabetes | 1254 (1.7) | 36,052 (3.7) | |
| Type 2 diabetes | 74,246 (98.3) | 943,175 (96.3) | |
| Diabetes complications count, n (%) | <0.001 | ||
| 0 | 36,011 (47.7) | 441,826 (45.1) | |
| 1 | 21,338 (28.3) | 264,558 (27.0) | |
| 2 | 10,293 (13.6) | 142,133 (14.5) | |
| 3 | 4789 (6.3) | 75,308 (7.7) | |
| ≥4 | 3069 (4.1) | 55,402 (5.7) | |
| Diabetes complications, n (%) | |||
| Retinopathy | 10,801 (14.3) | 139,556 (14.3) | 0.68 |
| Nephropathy | 9591 (12.7) | 157,419 (16.1) | <0.001 |
| Neuropathy | 17,408 (23.1) | 190,195 (19.4) | <0.001 |
| Cerebrovascular disease | 3816 (5.1) | 78,965 (8.1) | <0.001 |
| Cardiovascular disease | 16,013 (21.2) | 277,269 (28.3) | <0.001 |
| Peripheral vascular disease | 6370 (8.4) | 108,852 (11.1) | <0.001 |
| Hyperglycemic events | 1311 (1.7) | 16,527 (1.7) | 0.32 |
| Hypoglycemic events | 4388 (5.8) | 53,166 (5.4) | <0.001 |
| Additional comorbidity burden | |||
| Charlson index, mean (SD) | 2.4 (1.8) | 2.6 (2.1) | <0.001 |
| Charlson index category, n (%) | <0.001 | ||
| 0–1 | 35,458 (47.0) | 431,401 (44.1) | |
| 2 | 9756 (12.9) | 137,802 (14.1) | |
| 3 | 15,003 (19.9) | 164,054 (16.8) | |
| ≥4 | 15,283 (20.2) | 245,970 (25.1) | |
| Key comorbidities, n (%) | |||
| Acute myocardial infarction | 1809 (2.4) | 34,070 (3.5) | <0.001 |
| Heart failure | 4125 (5.5) | 92,407 (9.4) | <0.001 |
| Cerebrovascular disease | 4452 (5.9) | 95,396 (9.7) | <0.001 |
| Chronic kidney disease | 5067 (6.7) | 114,389 (11.7) | <0.001 |
| Hypertension | 60,320 (79.9) | 776,973 (79.3) | <0.001 |
| Cancer | 3956 (5.2) | 75,407 (7.7) | <0.001 |
| Treatment regimen | |||
| Baseline insulin use | 32,761 (43.4) | 222,632 (22.7) | <0.001 |
| Number of drugs at baseline | <0.001 | ||
| 0 | 7215 (9.6) | 302,546 (30.9) | |
| 1 | 21,994 (29.1) | 400,817 (40.9) | |
| 2 | 27,141 (35.9) | 206,148 (21.1) | |
| ≥3 | 19,150 (25.4) | 69,716 (7.1) | |
| Number of drugs after index drug initiation | <0.001 | ||
| 1 | 6221 (8.2) | 609,283 (62.2) | |
| 2 | 23,526 (31.2) | 277,283 (28.3) | |
| 3 | 28,076 (37.2) | 80,497 (8.2) | |
| ≥4 | 17,677 (23.4) | 12,164 (1.2) | |
| Prescribing specialty, n (%) | <0.001 | ||
| Endocrinology | 17,659 (23.4) | 82,049 (8.4) | |
| Family medicine | 24,297 (32.2) | 373,344 (38.1) | |
| Internal medicine | 18,342 (24.3) | 325,997 (33.3) | |
| Cardiology | 353 (0.5) | 10,180 (1.0) | |
| Pediatrics | 119 (0.2) | 2684 (0.3) | |
| Other/unknown | 14,730 (19.5) | 184,973 (18.9) | |
Baseline patient characteristics at the time that either a SGLT2i or another glucose-lowering drug was first started.
SD, standard deviation; SGLT2i, sodium-glucose cotransporter-2 inhibitors.
SGLT2i users were younger than nonusers, with mean age 56.5 (SD, 10.7) versus 62.2 (SD, 13.3) years; P < 0.001 (Table 1) and were more likely to have commercial health insurance (75.5% vs. 46.8%; P < 0.001). Compared with other drugs, SGLT2i were prescribed less frequently to women (44.5% vs. 48.5%) and Black patients (12.3% vs. 16.3%); all P < 0.001.
Characteristics of patients receiving SGLT2i changed over time, as demonstrated in Appendix Table A2. The most notable change was the increase in the mean age of patients initiating SGLT2i therapy, with the proportion of patients ≥65 years increasing from 12.4% of those treated in 2013 to 27.5% in 2016; P < 0.001. The proportion of patients with Medicare Advantage health coverage similarly increased from 14.3% in 2013 to 31.8% in 2016; P < 0.001. Another substantial change was in the proportion of patients newly initiated on a SGLT2i who were treated with insulin, decreasing from 55.0% in 2013 to 31.6% in 2015; P < 0.001.
Trends in SGLT2i initiation over time
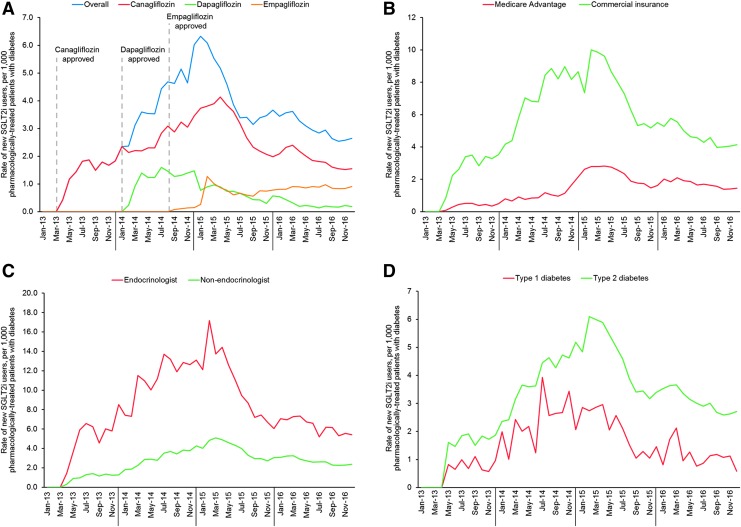
Rates of SGLT2i initiation increased in 2013 through 2015 but declined in 2016. Canagliflozin, the first SGLT2i to be approved by the FDA (March 2013), was the most frequently prescribed throughout the study period (Fig. 1A). Dapagliflozin began to be prescribed shortly after it was approved (January 2014), but its initiation rates began to decline after January 2015. Empagliflozin, the third SGLT2i to be approved, was rarely prescribed between its approval in August 2014 until December 2014 but started to be prescribed after January 2015 and has been slowly gaining in market share since.
FIG. 1.
Trends in SGLT2i initiation. Rates of SGLT2i initiation per 100,000 adults with pharmacologically treated diabetes are shown by drug (A), health insurance coverage (B), diabetes type (C), and prescriber specialty (D). SGLT2, sodium-glucose cotransporter-2.
Trends in SGLT2i initiation varied among patients with commercial and Medicare Advantage health coverage (Fig. 1B). Patients with commercial health insurance were started on SGLT2i earlier, and its use among them rose more rapidly, than among those with Medicare Advantage health plans. Endocrinologists began to prescribe SGLT2i earlier than other specialties and did so at a more rapid rate until early 2015, when the rate of SGLT2i initiation decreased more precipitously among endocrinologists than other specialties (Fig. 1C). Use among patients with type 1 diabetes paralleled that in type 2 diabetes, with initial increase between 2013 and 2015, and progressive decline in 2015 and 2016 (Fig. 1D).
Factors associated with SGLT2i initiation
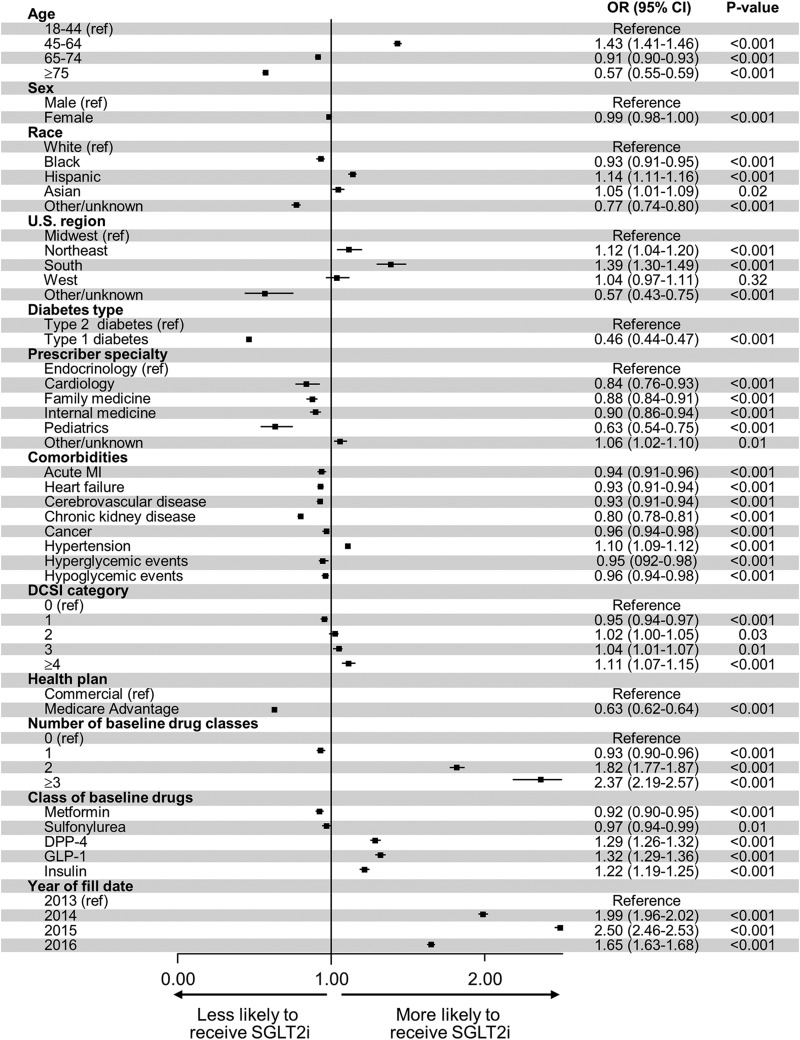
Endocrinologists were the most likely to start a SGLT2i, with odds of SGLT2i initiation 10% to 15% higher than for nonendocrine specialties; P < 0.001 for all specialties compared with endocrinology (Fig. 2). Middle-aged patients (age 45–64 years) were most likely to start a SGLT2i (OR: 1.43 [95% CI: 1.41–1.46] vs. 18–44 years), whereas patients ≥75 years were least likely (OR: 0.57, 95% CI: 0.55–0.59). Even after adjustment for age, comorbidities, and other factors, patients with Medicare Advantage health plans were less likely to start a SGLT2i than those with commercial insurance (OR: 0.63, 95% CI: 0.62–0.64). Type 1 diabetes decreased the odds of SGLT2i initiation only by half (OR: 0.46, 95% CI: 0.44–0.47), despite SGLT2i not being approved to use in type 1 diabetes. Compared with patients living in the Midwest, patients living in all other U.S. regions were more likely to start a SGLT2i, with the highest probability among patients living in the South (OR: 1.39, 95% CI: 1.30–1.49). Patient sex and race/ethnicity also affected the odds of SGLT2i initiation, with women less likely to be started on a SGLT2i than men, and Black patients less likely, but Hispanic and Asian patients more likely, to be started on a SGLT2i than White patients.
FIG. 2.
Predictors of SGLT2i initiation. Multivariable logistic regression analysis of the odds of SGLT2i initiation.
The presence of all comorbidities, with the sole exception of hypertension, decreased the odds of SGLT2i initiation. Patients with hypertension were more likely to be treated with a SGLT2i (OR: 1.10, 95% CI: 1.09–1.12). Patients with a history of severe hypoglycemia were less likely to be treated with a SGLT2i (OR: 0.96, 95% CI: 0.94–0.98), as were patients with a history of severe hyperglycemia (OR: 0.95, 95% CI: 0.92–0.98), after adjustment for other concurrent glucose-lowering therapies, including insulin.
We also examined whether the odds of starting a SGLT2i differed depending on whether it was the first-line or add-on medication (Appendix Table A3). The odds of first-line and second-line SGLT2i initiation were generally similar, except that patients 45–64 years of age were more likely to start a SGLT2i as an add-on agent (OR: 1.28, 95% CI: 0.12–1.31 compared with patients 18–44 years), but they were less likely to start it as a first-line agent (OR: 0.90, 95% CI: 0.85–0.96). Women were less likely to add a SGLT2i than men (OR: 0.95, 95% CI: 0.93–0.96), but were more likely to start it as a first-line agent (OR: 1.12, 95% CI: 1.06–1.17).
Clinical context of SGLT2i initiation
SGLT2i were started as first-line agents in 9.6% of patients (3.9% as monotherapy and 5.6% as combination therapy). They replaced other glucose-lowering medications in 4.3% of patients and were add-on agents in 86.1%. When the SGLT2i was started, 65.8% of patients were using metformin, 32.0% sulfonylurea, 24.3% DPP-4i, 16.7% GLP-1RA, and 43.4% insulin. With the initiation of SGLT2i therapy, 11.9% of metformin-treated patients discontinued metformin, 22.8% of sulfonylurea-treated patients discontinued sulfonylurea, 25.2% of DPP-4i-treated patients discontinued DPP-4i, 22.4% of GLP-1RA-treated patients discontinued GLP-RA, and 10.6% of insulin-treated patients discontinued their insulin.
Discussion
In this study of commercially insured and Medicare Advantage beneficiaries with type 2 or type 1 diabetes across the U.S., SGLT2i were initiated most often by lowest-risk patients (i.e., young, with fewer comorbidities), without the health conditions most likely to benefit from their preferential use (i.e., CVD, HF, CKD, hypoglycemia), and with apparent racial/ethnic, regional, and insurance-based disparities in use. Moreover, while SGLT2i are approved and guideline recommended as second-line agents for the management of type 2 diabetes, they were commonly prescribed as first-line agents and occasionally used by patients with type 1 diabetes. Temporal analyses of SGLT2i initiation also revealed potential impacts of FDA- and European Medical Association (EMA)-issued warnings,24,28,31,41,42 health plan formulary decisions, inclusion in clinical practice guidelines,43–45 and emerging cardiovascular outcome trial data3 on the rates of SGLT2i initiation.
Early in the course of SGLT2i introduction into clinical practice, they were first recommended for use by AACE/ACE as third-line agents in May 2013,46 as second-line agents by NICE in June 2014,47 and as second-line agents by ADA and AACE/ACE in January 2015.43,48 In January 2016, AACE/ACE recommended special consideration of SGLT2i in the context of HF and CVD49 in recognition of the recently published EMPA-REG trial.3 Yet, the presence of HF, MI, and prior hypoglycemia, all significantly decreased the odds of SGLT2i initiation. There are several potential explanations for this treatment/risk paradox, which was previously observed in the case of statin32 and ACE inhibitor/angiotensin receptor blocker33 therapies. Clinicians may not have been aware of emerging class-specific benefits of SGLT2i and as a result prescribed these new, costly agents to patients they believed warranted a more intensive approach to therapy: younger patients and those with less comorbidity. This awareness gap is expected to be greater among generalists as compared with endocrinologists, contributing to the much lower odds of SGLT2i initiation by internal medicine and family medicine clinicians. Still, even before publication of the cardiovascular outcome trials, SGLT2i were supported by the same level of evidence as the glucose-lowering medications considered in the comparator group, including GLP-1RA and DPP-4i. Similarly, patients with comorbidities may have been wary of starting a new medication, either due to existing polypharmacy or concerns about adverse effects.
Older patients and patients with Medicare Advantage (vs. commercial) health insurance coverage were less likely to start SGLT2i therapy, despite the higher propensity for hypoglycemia among older adults with type 2 diabetes. There are several potential explanations for this. Older patients are more likely to have multiple comorbidities, including but not limited to ones examined in our study, leading to cautious use of newly available drugs. Younger patients may be more likely to ask for new medications as a result of direct-to-consumer advertising.50 It may also be more difficult for people with Medicare Advantage health plans to afford brand name medications even though SGLT2i were on the included plans' formularies, as they are not eligible for the same discount programs as people with commercial insurance, and older patients are more likely to be taking and paying for multiple other medications already. Such financial barriers to diabetes management may disproportionately affect people with government-sponsored insurance, warranting examination of SGLT2i and other brand-name drug use by traditional Medicare and Medicaid enrollees, which could not be done in our data.
There were racial/ethnic differences in SGLT2i initiation, with Black patients significantly less likely to start a SGLT2i than people of other racial/ethnic backgrounds. This is consistent with other studies demonstrating decreased utilization of new drugs among Black patients,51,52 and may contribute to overall worse glycemic control and diabetes health outcomes in this population.53 Notably, Black patients requesting medications in response to direct-to-consumer advertising may be less likely to have them prescribed,50 reflecting potential clinician biases.
SGLT2i were frequently utilized as first-line agents and as monotherapy, despite clinical guidelines and the FDA explicitly recommending them as second-line drugs. Nearly 10% of patients were started on a SGLT2i as the first-line glucose-lowering agent and 8.2% were using it as monotherapy. Younger and healthier people were most likely to receive SGLT2i as first-line therapy; yet, these patients may also have tolerated metformin, the consistently recommended first-line drug,7 which was used by only 52.7% of the study population. It is therefore important to better understand the factors affecting choice of glucose-lowering therapy, including the consistently low rates of metformin use as the first-line agent as previously noted.54–56
We also found that 3.4% of enrollees with type 1 diabetes were started on a SGLT2i, comprising 1.7% of all SGLT2i initiators. SGLT2i are approved for the management of type 2 diabetes only, although off-label in type 1 diabetes has been described.15–17,57–62 SGLT2i initiation by patients with type 1 diabetes increased between 2013 and 2014 (when the fraction of SGLT2i users with type 1 diabetes reached 2.3%), but then decreased in 2015 down to 1.2% in 2016. This parallels the growing awareness that SGLT2i can increase the risk of ketoacidosis among insulin-treated patients, particularly with type 1 diabetes.63,64 SGLT2i initiation among insulin-treated patients in general declined over time, from 55% of patients starting an SGLT2i in 2013 to 32% in 2015. Warnings about ketoacidosis risk were issued by the FDA in May 201531 and the EMA in June 2015,42 corresponding to a pronounced decline in SGLT2i use overall and more noticeably among patients with type 1 diabetes. Still, continued use of SGLT2i by patients with type 1 diabetes merits scrutiny.
Temporal changes in the rates of SGLT2i initiation reflect formulary decisions as well as emerging evidence regarding drug safety. Canagliflozin was added to formularies of included commercial and Medicare Advantage health plans as Tier 2 drugs, generally with either metformin or another generic drug step therapy, in October–November 2014. This corresponded to a steep rise in canagliflozin initiation among Medicare Advantage beneficiaries, although patients with commercial insurance were being started on canagliflozin even before it came on formulary. Empagliflozin was added to formularies (also as Tier 2 with metformin or generic step) in January–February 2015, while dapagliflozin was simultaneously excluded from commercial plan formularies. This corresponded to a rapid drop in new dapagliflozin prescriptions paralleled by a rise in empagliflozin initiation. The apparent disillusionment with SGLT2i beginning in 2015 parallels emerging safety concerns, including ketoacidosis,31,42 fracture,28 amputation,41 and acute kidney injury.24 Empagliflozin is the only SGLT2i whose use has been gradually increasing since the second quarter of 2015, likely because it was the first in its class to demonstrate strong cardiovascular and renal benefits extending beyond its glucose-lowering capabilities.3
Study findings must be considered in the context of its limitations. We focused specifically on the early years of SGLT2i adoption, before publication of clinical trial data demonstrating their particular advantage in the context of HF and CKD,3–6 to specifically examine the integration of novel therapies into chronic disease management. Nonetheless, clinical benefits with regard to hypertension, diuresis, and hypoglycemia were already known and contemporaneous guidelines advised SGLT2i to be considered as add-on agents for type 2 diabetes in those contexts.43,46–48 Population-level use of SGLT2i may be different from that observed in our study, which relied on private and Medicare Advantage plans administered by one, although large, U.S. health plan, since treatment decisions are influenced by formulary design, which varies among health plans. Finally, we did not examine the impact of glycemic control and clinician characteristics on the likelihood of SGLT2i initiation, as these data are not available in OLDW.
Nonetheless, the discordance between guideline-recommended and evidence-based use of SGLT2i and their actual uptake in clinical practice, is striking. While SGLT2i have specific benefits among patients with CVD, HF, hypertension, CKD, and at risk for hypoglycemia, they were least likely to be used by them. There were also nonclinical disparities in SGLT2i use, with Black patients, older patients, and those with Medicare Advantage insurance significantly less likely to receive SGLT2i than White, younger, and privately insured patients. Patient and clinician education regarding individualized approaches to diabetes management, and health plan support of such evidence-based treatment strategies, may therefore help improve access to new diabetes therapeutics and improve health outcomes among patients living with this disease.
Supplementary Material
Acknowledgments
The authors sincerely thank the OptumLabs research team for providing them with information regarding formulary status of included SGLT2i medications throughout the study period. They also appreciate assistance of the Mayo Clinic Brand Strategy and Creative Studio for the creation of figure graphics.
Author Contributions
R.G.M. and N.D.S. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. R.G.M. designed the study, interpreted the data, and wrote the article. J.S.R., P.K.-M, V.M.M., and N.D.S. contributed to the discussion and reviewed/edited the article. H.D. analyzed the data and reviewed/edited the article, whereas L.S. supervised data analysis and also reviewed/edited the article. N.D.S. supervised study design and data interpretation, contributed to the discussion, and reviewed/edited the article.
Disclaimer
Study contents are the sole responsibility of the authors and do not necessarily represent the official views of NIH.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This effort was funded by the National Institute of Health National Institute of Diabetes and Digestive and Kidney Diseases grant K23DK114497 (R.G.M.), AHRQ's Comparative Health System Performance Initiative grant 1U19HS024075 (N.D.S.), and AHRQ's grant R01HS025164 (P.K.-M.). R.G.M. also receives support from the Mayo Clinic Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery. In the past 36 months, N.D.S. has received research support through Mayo Clinic from the FDA to establish Yale-Mayo Clinic Center for Excellence in Regulatory Science and Innovation (CERSI) program (U01FD005938); the Centers of Medicare and Medicaid Innovation under the Transforming Clinical Practice Initiative (TCPI); the Agency for Healthcare Research and Quality (R01HS025164; R01HS025402; R03HS025517); the National Heart, Lung, and Blood Institute of the National Institutes of Health (NIH) (R56HL130496; R01HL131535); the National Science Foundation; and the Patient-Centered Outcomes Research Institute (PCORI) to develop a Clinical Data Research Network (LHSNet). In the past 36 months, J.S.R. has received research support through Yale University from Johnson and Johnson to develop methods of clinical trial data sharing, from Medtronic, Inc. and the FDA to develop methods for postmarket surveillance of medical devices (U01FD004585), from the FDA to establish Yale-Mayo Clinic CERSI program (U01FD005938), from the Blue Cross Blue Shield Association to better understand medical technology evaluation, from the Centers of Medicare and Medicaid Services (CMS) to develop and maintain performance measures that are used for public reporting (HHSM-500-2013-13018I), from the Agency for Healthcare Research and Quality (R01HS022882), from the National Heart, Lung, and Blood Institute of the NIH (R01HS025164), and from the Laura and John Arnold Foundation to establish the Good Pharma Scorecard at Bioethics International and to establish the Collaboration for Research Integrity and Transparency (CRIT) at Yale. P.K.-M. serves as the Principal Investigator to several grants on prescription drug studies (NIA/P01AG005842; NIH/R56 HL130496 and Agency for Healthcare Research and Quality/R01 HS025164; American Cancer Society 131611-RSGI-17-154-01-CPHPS). In the past 36 months, she reports receiving consulting fees unrelated to this work from Tactile Medical and Precision Health Economics.
References
- 1. CDC. Centers for Disease Control and Prevention: National Diabetes Statistics Report, 2017. Centers for Disease Control and Prevention US Department of Health and Human Services, updated February 24, 2018. https://www.cdc.gov/diabetes/data/statistics/statistics-report.html (accessed July5, 2019) [Google Scholar]
- 2. ADA. American Diabetes Association: Section 4. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes-2019. Diabetes Care 2019;42:S34–S45 [DOI] [PubMed] [Google Scholar]
- 3. Zinman B, Wanner C, Lachin JM, et al. : Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–2128 [DOI] [PubMed] [Google Scholar]
- 4. Wanner C, Inzucchi SE, Lachin JM, et al. : Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 2016;375:323–334 [DOI] [PubMed] [Google Scholar]
- 5. Neal B, Perkovic V, Mahaffey KW, et al. : Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644–657 [DOI] [PubMed] [Google Scholar]
- 6. Wiviott SD, Raz I, Bonaca MP, et al. : Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019;380:347–357 [DOI] [PubMed] [Google Scholar]
- 7. ADA. American Diabetes Association: Section 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2019. Diabetes Care 2019;42:S90–S102 [DOI] [PubMed] [Google Scholar]
- 8. Garber AJ, Abrahamson MJ, Barzilay JI, et al. : Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2019 executive summary. Endocr Pract 2019;25:69–100 [DOI] [PubMed] [Google Scholar]
- 9. Davies MJ, D'Alessio DA, Fradkin J, et al. : Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018;41:2669–2701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dunlay SM, Givertz MM, Aguilar D, et al. : Type 2 diabetes mellitus and heart failure: a scientific statement from the American Heart Association and Heart Failure Society of America. J Card Fail 2019;140:e294–e324 [DOI] [PubMed] [Google Scholar]
- 11. Economic Costs of Diabetes in the U.S. in 2017: Diabetes Care 2018;41:917–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. CDC: Diabetes data and statistics. Diabetes atlas. Division of Diabetes Translation, Centers for Disease Control and Prevention U.S. Dept of Health and Human Services, updated March 14, 2018. https://gis.cdc.gov/grasp/diabetes/DiabetesAtlas.html (accessed April24, 2019) [Google Scholar]
- 13. Nichols GA, Gullion CM, Koro CE, et al. : The incidence of congestive heart failure in type 2 diabetes: an update. Diabetes Care 2004;27:1879–1884 [DOI] [PubMed] [Google Scholar]
- 14. Silbert R, Salcido-Montenegro A, Rodriguez-Gutierrez R, et al. : Hypoglycemia among patients with type 2 diabetes: epidemiology, risk factors, and prevention strategies. Curr Diab Rep 2018;18:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tamez HE, Tamez AL, Garza LA, et al. : Dapagliflozin as an adjunct therapy to insulin in the treatment of patients with type 1 diabetes mellitus. J Diabetes Metab Disord 2015;14:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pieber TR, Famulla S, Eilbracht J, et al. : Empagliflozin as adjunct to insulin in patients with type 1 diabetes: a 4-week, randomized, placebo-controlled trial (EASE-1). Diabetes Obes Metab 2015;17:928–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Henry RR, Rosenstock J, Edelman S, et al. : Exploring the potential of the SGLT2 inhibitor dapagliflozin in type 1 diabetes: a randomized, double-blind, placebo-controlled pilot study. Diabetes Care 2015;38:412–419 [DOI] [PubMed] [Google Scholar]
- 18. Tamblyn R, McLeod P, Hanley JA, et al. : Physician and practice characteristics associated with the early utilization of new prescription drugs [Erratum appears in Med Care 2003;41:1117]. Med Care 2003;41:895–908 [DOI] [PubMed] [Google Scholar]
- 19. Solomon DH, Schneeweiss S, Glynn RJ, et al. : Determinants of selective cyclooxygenase-2 inhibitor prescribing: are patient or physician characteristics more important? Am J Med 2003;115:715–720 [DOI] [PubMed] [Google Scholar]
- 20. Helin-Salmivaara A, Huupponen R, Virtanen A, Klaukka T: Adoption of celecoxib and rofecoxib: a nationwide database study. J Clin Pharm Ther 2005;30:145–152 [DOI] [PubMed] [Google Scholar]
- 21. Schneeweiss S, Glynn RJ, Avorn J, Solomon DH: A Medicare database review found that physician preferences increasingly outweighed patient characteristics as determinants of first-time prescriptions for COX-2 inhibitors. J Clin Epidemiol 2005;58:98–102 [DOI] [PubMed] [Google Scholar]
- 22. Garjon FJ, Azparren A, Vergara I, et al. : Adoption of new drugs by physicians: a survival analysis. BMC Health Serv Res 2012;12:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lo-Ciganic W-H, Gellad WF, Huskamp HA, et al. : Who were the early adopters of dabigatran? An application of group-based trajectory models. Med Care 2016;54:725–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. FDA. FDA Drug Safety Communication: FDA Strengthens Kidney Warnings for Diabetes Medicines Canagliflozin (Invokana, Invokamet) and Dapagliflozin (Farxiga, Xigduo XR). U.S. Food and Drug Administration (FDA), updated June 17, 2016. https://www.fda.gov/Drugs/DrugSafety/ucm505860.htm (accessed August21, 2018) [Google Scholar]
- 25. Perlman A, Heyman SN, Matok I, et al. : Acute renal failure with sodium-glucose-cotransporter-2 inhibitors: analysis of the FDA adverse event report system database. Nutr Metab Cardiovasc Dis 2017;27:1108–1113 [DOI] [PubMed] [Google Scholar]
- 26. Li D, Yang JY, Wang T, et al. : Risks of diabetic foot syndrome and amputation associated with sodium glucose co-transporter 2 inhibitors: a meta-analysis of randomized controlled trials. Diabetes Metab 2018;44:410–414 [DOI] [PubMed] [Google Scholar]
- 27. Udell JA, Yuan Z, Rush T, et al. : Cardiovascular outcomes and risks after initiation of a sodium glucose cotransporter 2 inhibitor: results from the EASEL Population-Based Cohort Study (evidence for cardiovascular outcomes with sodium glucose cotransporter 2 inhibitors in the real world). Circulation 2018;137:1450–1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. FDA. FDA Drug Safety Communication: FDA Revises Label of Diabetes Drug Canagliflozin (Invokana, Invokamet) to Include Updates on Bone Fracture Risk and New Information on Decreased Bone Mineral Density. U.S. Food and Drug Administration (FDA), updated January 15, 2016. https://www.fda.gov/Drugs/DrugSafety/ucm461449.htm (accessed August21, 2018) [Google Scholar]
- 29. Watts NB, Bilezikian JP, Usiskin K, et al. : Effects of canagliflozin on fracture risk in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab 2016;101:157–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bilezikian JP, Watts NB, Usiskin K, et al. : Evaluation of bone mineral density and bone biomarkers in patients with type 2 diabetes treated with canagliflozin. J Clin Endocrinol Metab 2016;101:44–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. FDA. U.S. Food and Drug Administration. Drug Safety Communication: FDA Warns that SGLT2 Inhibitors for Diabetes May Result in a Serious Condition of Too Much Acid in the Blood [Internet], 15 May 2015. www.fda.gov/downloads/Drugs/DrugSafety/UCM446954.pdf (accessed January17, 2018)
- 32. Ko DT, Mamdani M, Alter DA: Lipid-lowering therapy with statins in high-risk elderly patients: the treatment-risk paradox. JAMA 2004;291:1864–1870 [DOI] [PubMed] [Google Scholar]
- 33. Lee DS, Tu JV, Juurlink DN, et al. : Risk-treatment mismatch in the pharmacotherapy of heart failure. JAMA 2005;294:1240–1247 [DOI] [PubMed] [Google Scholar]
- 34. Wallace PJ, Shah ND, Dennen T, et al. : Optum Labs: building a novel node in the learning health care system. Health Aff (Millwood) 2014;33:1187–1194 [DOI] [PubMed] [Google Scholar]
- 35. Optum: Optum Research Data Assets. Optum, 2015. https://www.optum.com/content/dam/optum/resources/productSheets/5302_Data_Assets_Chart_Sheet_ISPOR.pdf (accessed July16, 2019) [Google Scholar]
- 36. NCQA: National Committee for Quality Assurance (NCQA) Healthcare Effectiveness Data and Information Set (HEDIS) Comprehensive Diabetes Care. Washington, D.C.: National Committee for Quality Assurance, 2015, 74–98 [Google Scholar]
- 37. Charlson ME, Pompei P, Ales KA, MacKenzie CR: A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–383 [DOI] [PubMed] [Google Scholar]
- 38. Chang HY, Weiner JP, Richards TM, et al. : Validating the adapted diabetes complications severity index in claims data. Am J Manag Care 2012;18:721–726 [PubMed] [Google Scholar]
- 39. Klompas M, Eggleston E, McVetta J, et al. : Automated detection and classification of type 1 versus type 2 diabetes using electronic health record data. Diabetes Care 2013;36:914–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schroeder EB, Donahoo WT, Goodrich GK, Raebel MA: Validation of an algorithm for identifying type 1 diabetes in adults based on electronic health record data. Pharmacoepidemiol Drug Saf 2018;27:1053–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. FDA. FDA Drug Safety Communication: Interim Clinical Trial Results Find Increased Risk of Leg and Foot Amputations, Mostly Affecting the Toes, with the Diabetes Medicine Canagliflozin (Invokana, Invokamet); FDA to Investigate. U.S. Food and Drug Administration (FDA), updated May 17, 2017. https://www.fda.gov/Drugs/DrugSafety/ucm500965.htm (accessed August21, 2018) [Google Scholar]
- 42. EMA. European Medicines Agency. Review of Diabetes Medicines Called SGLT2 Inhibitors Started: Risk of Diabetic Ketoacidosis to Be Examined [Internet], updated June 12, 2015. www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/SGLT2_inhibitors__20/Procedure_started/WC500187926.pdf (accessed January17, 2018)
- 43. ADA. American Diabetes Association: Standards of Medical Care in Diabetes-2015. Diabetes Care 2015;38:S1–S94 [DOI] [PubMed] [Google Scholar]
- 44. ADA. American Diabetes Association: Standards of Medical Care in Diabetes-2016. Diabetes Care 2016;39:S1–S11226696671 [Google Scholar]
- 45. ADA. American Diabetes Association: Standards of Medical Care in Diabetes-2014. Diabetes Care 2014;37:S14–S80 [DOI] [PubMed] [Google Scholar]
- 46. Garber AJ, Abrahamson MJ, Barzilay JI, et al. : American Association of Clinical Endocrinologists' comprehensive diabetes management algorithm 2013 consensus statement—executive summary. Endocr Pract 2013;19:536–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. NICE: Canagliflozin in Combination Therapy for Treating Type 2 Diabetes. National Institute for Health and Care Excellence (NICE) Technology Appraisal Guidance [TA315] Web Site, updated June 25, 2014. https://www.nice.org.uk/guidance/ta315 (accessed March27, 2019) [Google Scholar]
- 48. Handelsman Y, Bloomgarden ZT, Grunberger G, et al. : American association of clinical endocrinologists and American college of endocrinology—clinical practice guidelines for developing a diabetes mellitus comprehensive care plan-2015. Endocr Pract 2015;21:1–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Garber AJ, Abrahamson MJ, Barzilay JI, et al. : Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm-2016 executive summary. Endocr Pract 2016;22:84–113 [DOI] [PubMed] [Google Scholar]
- 50. Datti B, Carter MW: The effect of direct-to-consumer advertising on prescription drug use by older adults. Drugs Aging 2006;23:71–81 [DOI] [PubMed] [Google Scholar]
- 51. Wang J, Zuckerman IH, Miller NA, et al. : Utilizing new prescription drugs: disparities among non-Hispanic whites, non-Hispanic blacks, and Hispanic whites. Health Serv Res 2007;42:1499–1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jung J, Feldman R: Racial-ethnic disparities in uptake of new Hepatitis C drugs in medicare. J Racial Ethn Health Disparities 2017;4:1147–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Centers for Disease Control and Prevention: National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services, 2017 [Google Scholar]
- 54. Montvida O, Shaw J, Atherton JJ, et al. : Long-term trends in antidiabetes drug usage in the U.S.: real-world evidence in patients newly diagnosed with type 2 diabetes. Diabetes Care 2018;41:69–78 [DOI] [PubMed] [Google Scholar]
- 55. Berkowitz SA, Krumme AA, Avorn J, et al. : Initial choice of oral glucose-lowering medication for diabetes mellitus: a patient-centered comparative effectiveness study. JAMA Intern Med 2014;174:1955–1962 [DOI] [PubMed] [Google Scholar]
- 56. Lipska KJ, Yao X, Herrin J, et al. : Trends in drug utilization, glycemic control, and rates of severe hypoglycemia, 2006–2013. Diabetes Care 2016;40:468–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cherney DZ, Perkins BA, Soleymanlou N, et al. : Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 2014;129:587–597 [DOI] [PubMed] [Google Scholar]
- 58. Perkins BA, Cherney DZ, Partridge H, et al. : Sodium-glucose cotransporter 2 inhibition and glycemic control in type 1 diabetes: results of an 8-week open-label proof-of-concept trial. Diabetes Care 2014;37:1480–1483 [DOI] [PubMed] [Google Scholar]
- 59. Sands AT, Zambrowicz BP, Rosenstock J, et al. : Sotagliflozin, a dual SGLT1 and SGLT2 inhibitor, as adjunct therapy to insulin in type 1 diabetes. Diabetes Care 2015;38:1181–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Henry RR, Thakkar P, Tong C, et al. : Efficacy and safety of canagliflozin, a sodium-glucose cotransporter 2 inhibitor, as add-on to insulin in patients with type 1 diabetes. Diabetes Care 2015;38:2258–2265 [DOI] [PubMed] [Google Scholar]
- 61. Perkins BA, Cherney DZI, Soleymanlou N, et al. : Diurnal glycemic patterns during an 8-week open-label proof-of-concept trial of empagliflozin in type 1 diabetes. PLoS One 2015;10:e0141085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Argento NB, Nakamura K: Glycemic effects of SGLT-2 inhibitor canagliflozin in type 1 diabetes patients using the dexcom G4 platinum CGM. Endocr Pract 2016;22:315–322 [DOI] [PubMed] [Google Scholar]
- 63. Peters AL, Buschur EO, Buse JB, et al. : Euglycemic diabetic ketoacidosis: a potential complication of treatment with sodium–glucose cotransporter 2 inhibition. Diabetes Care 2015;38:1687–1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Erondu N, Desai M, Ways K, Meininger G: Diabetic ketoacidosis and related events in the canagliflozin type 2 diabetes clinical program. Diabetes Care 2015;38:1680–1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.