Abstract
In a prospective observational study (pre-AndroCoV Trial), the use of nitazoxanide, ivermectin and hydroxychloroquine demonstrated unexpected improvements in COVID-19 outcomes when compared to untreated patients. The apparent yet likely positive results raised ethical concerns on the employment of further full placebo controlled studies in early-stage COVID-19. The present analysis aimed to elucidate, through a comparative analysis with two control groups, whether full placebo-control randomized clinical trials (RCTs) on early-stage COVID-19 are still ethically acceptable. The Active group (AG) consisted of patients enrolled in the Pre-AndroCoV-Trial (n = 585). Control Group 1 (CG1) consisted of a retrospectively obtained group of untreated patients of the same population (n = 137), and Control Group 2 (CG2) resulted from a precise prediction of clinical outcomes based on a thorough and structured review of indexed articles and official statements. Patients were matched for sex, age, comorbidities and disease severity at baseline. Compared to CG1 and CG2, AG showed reduction of 31.5–36.5% in viral shedding (p < 0.0001), 70–85% in disease duration (p < 0.0001), and 100% in respiratory complications, hospitalization, mechanical ventilation, deaths and post-COVID manifestations (p < 0.0001 for all). For every 1000 confirmed cases for COVID-19, at least 70 hospitalizations, 50 mechanical ventilations and five deaths were prevented. Benefits from the combination of early COVID-19 detection and early pharmacological approaches were consistent and overwhelming when compared to untreated groups, which, together with the well-established safety profile of the drug combinations tested in the Pre-AndroCoV Trial, precluded our study from continuing employing full placebo in early COVID-19.
Keywords: Antiandrogen, clinical equipoise, COVID-19, dutasteride, hydroxychloroquine, ivermectin, nitazoxanide, proxalutamide, SARS-CoV-2, spironolactone
Highlights
-
•
Subjects with early COVID-19 treated with two-to-four drug combinations showed overwhelming improvements compared to untreated patients.
-
•
Improvements remained massive even when underestimating benefits of proposed treatments and underestimating risks of non-treated COVID-19.
-
•
Differences were sufficient to raise ethical concerns regarding the conduction of randomized clinical trials full-placebo in early COVID-19.
Abbreviations
- 5ARi
5alpha-reductase inhibitor
- ACEi
Angiotensin converter inhibitors
- ADHD
Attention deficiency and hyperactive disorders
- AGA
androgenetic alopecia
- ARB
Angiotensin-2 receptor blockers
- ASA
acetylsalicylic acid
- AZI
Azithromycin
- BCG
Bacillus Calmette-Guérin
- BMO
Body Mass Index
- BPH
Benign Prostate Hyperplasia
- CCB
Calcium channel blockers
- CNS
Central nervous system
- CHF
Chronic heart failure
- CKD
Chronic Kidney Disease
- COPD
Chronic Obstructive Pulmonary Disease
- COVID-19
infection caused by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)
- DPP4i
Di-peptidyl peptidase 4 inhibitors
- GI
Gastrointestinal
- GnRH
Gonadotropin-releasing hormone
- HCQ
Hydroxychloroquine
- IVE
Ivermectin
- NSAA
Non-steroidal antiandrogens
- NTZ
Nitazoxanide
- PCSK9i
Protein convertase subtilisin/kexin type 9 inhibitor
- rtPCR
Real time polymerase chain reaction
- SARS-CoV-2
Severe Acute Respiratory Syndrome Coronavirus 2
- SERM
Selective estrogen receptor modulator
- SNRI
Serotonin-noradrenaline reuptaker inhibitors
- SSRI
Selective serotonin reuptaker inhibitors
- SGLT2i
Sodium-glucose co-transporter 2 inhibitors
- T2DM
Type 2 diabetes mellitus
- URTI
Upper respiratory tract infection
- WHO
World Health Organization
Background
Coronavirus Disease 2019 (COVID-19) is a highly heterogeneous and multi-systemic infection caused by the novel Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) [1] that is particularly harmful to those aged above 60 y/o, with uncontrolled diabetes, hypertension, obesity, androgenetic alopecia (AGA), abuse of anabolic steroids in males and hyperandrogenism in females [[2], [3], [4], [5], [6], [7]].
Effective treatments to improve COVID-19 clinical outcomes, mortality and to prevent post-COVID manifestations are highly desired, while definitive solutions such as effective and safe vaccines are not universally available. Pharmacological interventions during the viral replication stage are likely the best timing to antagonize SARS-CoV-2 infectivity and prevent complications [1,3].
Hydroxychloroquine (HCQ), nitazoxanide (NIT) and ivermectin (IVE), in association with azithromycin (AZI), are popular drugs largely used as off label therapies for early COVID-19. Despite the demonstration of direct or indirect antiviral activity and positive preliminary observations when treatment is started before seven days of symptoms, all of these three drugs – HCQ [[8], [9], [10], [11], [12], [13]], IVE [[13], [14], [15], [16], [17], [18]] and NIT [[18], [19], [20], [21], [22]] lack definitive data regarding their efficacy for COVID-19.
Since the plausibility for their use in COVID-19 is based on their potential antiviral activity, these drugs failed to demonstrate benefits when were tested after the first stage, as expected [16,23], despite a few exceptions [24].
In addition to the above-mentioned drugs, antiandrogens could play a protective action against COVID-19 by the inhibition of transmembrane serine protease 2 (TMPRSS-2) expression, a critical protein that facilitates SARS-CoV-2 viral entry that finds in androgens its only known modulators [3,4,[25], [26], [27], [28], [29], [30], [31], [32]].
Indeed, chronic dutasteride use [[28], [29], [30]] and acute dutasteride [31] and proxalutamide [32] use demonstrated in randomized clinical trials (RCT) to protect against severe COVID-19 in a variety of male populations, which encourages the employment of antiandrogens in further RCTs for early COVID-19.
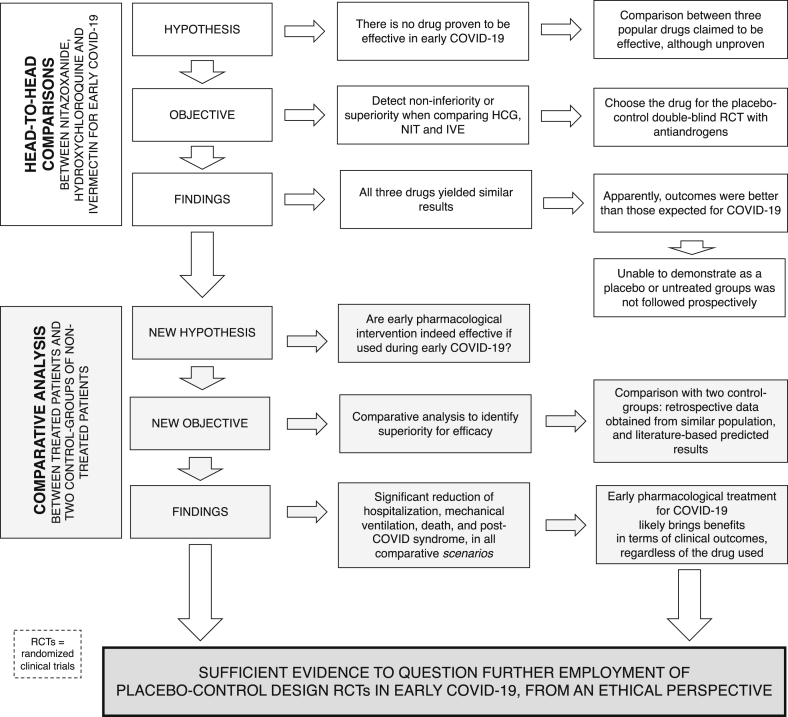
Our first objective was to compare antiandrogens, HCQ, NIT, IVE and placebo for early COVID-19, based on the assumption that none of these drugs presented actual benefits for any COVID-19 stage (null hypothesis). We first conducted a prospective open-label head-to-head comparison observational study with HCQ, NIT and IVE, with or without antiandrogens, to detect whether any of these drugs would demonstrate superior efficacy and then test the most effective options in double-blind placebo-controlled randomized clinical trials (RCTs), alone and combined with spironolactone (SPIRO), dutasteride (DUTA), or proxalutamide (PROXA) [[33], [34], [35]]. However, during the observational study, the interim analysis demonstrated that while HCQ, NIT and IVE showed similar effects, outcomes were seemingly more effective than those expected for COVID-19. The unexpected, seemingly positive results compelled us to question whether the employment of full placebo-control studies in early-stage COVID-19 would still remain ethically acceptable (Fig. 1).
Fig. 1.
Rationale for the ethical questioning on the employment of placebo-control design in RCTs for early COVID-19.
The objective of the present study was to elucidate whether a full placebo control RCT in early COVID-19 would still be ethically acceptable after the results obtained in the Pre-AndroCoV Trial. To answer this question, we performed independent comparisons with two distinct control-groups, matched for sex, age, comorbidities and disease characteristics, of untreated patients with COVID-19 from the same location as the subjects enrolled in the Pre-AndroCoV Trial and of a control group with the expected outcomes, based on data generated from a thorough and structured review of the literature. With these comparative analyses, we aimed to evaluate whether differences in COVID-19 outcomes actually exist between treated with different regimens and untreated subjects, how different COVID-19 outcomes were, the expected implications of these differences in terms of prevention of hospitalizations, mechanical ventilations, deaths, and post-COVID symptoms and if these discrepancies were sufficient to be considered as irrefutable, leading to mandatory changes in the design of further RCTs conducted on early COVID-19, in terms of no longer employing full placebo groups.
Materials and methods
Subject selection
The study was conducted in a single centre (Corpometria Institute, Brasilia, Brazil). Subjects of the Active Group (AG) (n = 585) were confirmed for COVID-19 through an rtPCR-SARS-CoV-2 (Abbott RealTime SARS-CoV-2 Assay, Abbott, USA; or Cobas SARS-CoV-2, Roche, Switzerland), aged 18 y/o and above, with less than seven days of symptoms and 72 hours of diagnosis, and absence of signs of COVID-19 complications. Patients of the AG consented in a formal written form, based on the consent form approved by the Ethics Committee of the National Board Ministry of Health, Brazil (CEP/CONEP: Parecer 4.173.074/CAAE: 34110420.2.0000.0008) that encompasses the use of the drugs of the present analysis.
Two control groups were employed, and both were adjusted for age, sex and presence of comorbidities. Control Group 1 (CG1) (n = 137) was a group of paired untreated patients randomly obtained retrospectively from the population of the same community that had confirmed diagnosis of COVID-19 during the same period of those included in the Pre-AndroCoV Trial, that either refused or did not receive specific treatments for COVID-19, including nitazoxanide, hydroxychloroquine or ivermectin.
A second control group (Control Group 2 - CG2) (paired for 585 patients) resulted from a precise estimative based on a thorough and structured review of articles indexed in PubMed and MEDLINE and statements by official government agencies and specific medical societies [2,[36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58]], in addition to the living systematic review of the British Medical Journal expected estimates for each outcome in untreated patients [11]. From data obtained, each parameter was estimated for range and median and the least negative data was employed, aiming to underestimate the risks of non-treatment to avoid overestimation of differences between treatment regimens and lack of treatment. When results had above 20% discrepancy between different sources, the least negative value was employed. When results had differences lower than 20% for the same parameter between studies, the value employed was one standard deviation (SD) below the median in favour of positive outcomes. When a specific parameter had differences above 100%, the range, instead of a specific value, was described (e.g., if the prevalence of a specific symptom was described as being between 10% and 80%, 10%–80% was described, instead of 45%). For these parameters, the least negative value was employed for comparison purposes with AG. The parameters of CG2 were estimated from the proportion between rates for those aged between 40 and 49 y/o with and without comorbidities, assuming a prevalence of comorbidities of 20% (compared to 30% of comorbidities in the AG). For CG2, we avoided statistical analyses from regions with higher case fatality ratios (CFR), like those observed in Northern Italy [69], as this could artificially increase the estimation of the number of preventable complications related to COVID-19.
Selected subjects were characterized for age, sex, the prevalence of obesity, hypertension, type 2 diabetes mellitus, overall comorbidities rate and adjusted accordingly. AG was additionally characterized for the presence of other 35 diseases and 20 drug classes. The medical records of the subjects were recorded individually, medically analyzed in an individual base, while data were compiled into a dataset and deidentified for statistical analysis purposes.
Procedures
A detailed description of the allocation process is described elsewhere [18]. Treatment was optional and all offered drugs that were within standard of care were provided since all early specific pharmacological approaches were based on the literature or as per the Brazilian Government. Drug options were offered in a quasi-randomized manner, i.e., options were randomly offered according to specific characteristics, including age, presence and number of comorbidities, number of days of symptoms.
Drugs offered included azithromycin 500mg daily for five days for all patients, in association with one of the following: hydroxychloroquine 400mg daily for five days, nitazoxanide 500mg twice a day for six days, or ivermectin 0.2mg/kg/day in a single daily dose for three days, In addition, repurposed drugs, including dutasteride 0.5mg/day for 15 days and spironolactone 100mg twice a day for 15 days, were optionally offered.
Vitamin D, vitamin C, zinc, apibaxan, rivaroxaban, enoxaparin and glucocorticoids were added according to clinical judgement, the risk for thrombosis and progression of the disease to the inflammatory stage.
Patients that decided to adhere to any treatment were included in the AG. All patients of AG and CG1 groups were followed longitudinally for 90 days for the occurrence of a new-onset or persistence of physical or mental manifestations.
Of the 585 subjects, all patients used azithromycin. A total of 357 patients used NIT, 159 used HCQ and 110 patients used IVE, alone with azithromycin or in combination with other drugs.
Of the 357 patients that used NIT, 69 used the same in combination with HCQ, 46 used in combination with IVE, 146 used in combination with SPIRO, and 27 males used in combination with DUTA.
Of the 110 patients that used IVE, 22 used in combination with HCQ, 82 used in combination with NIT, 66 used in combination with SPIRO and four males used in combination with DUTA.
Of the 159 patients that used HCQ, 21 used in combination with IVE, 113 used in combination with NIT, 86 used in combination with SPIRO and seven males used in combination with DUTA.
Parameters
Clinical outcomes were measured or directly obtained for AG and CG1 and estimated for CG2, including virologic duration (rtPCR-SARS-CoV-2, Abbott RealTime SARS-CoV-2 Assay, Abbott, USA; or Cobas SARS-CoV-2, Roche, Switzerland), time-to-clinical-remission, including and not including anosmia and ageusia, hospitalization rate, mechanical ventilation, deaths and prevalence of post-COVID syndrome, including mental, physical, or both types of manifestations.
CG1 and CG2 underwent pairwise comparative analysis to evaluate consistency between world data of untreated subjects and data generated from the local untreated population. Only parameters with minimally sufficient data and consisting findings between different studies were included. Amplitude effect – estimated number of preventable outcomes.
Since preliminary data demonstrated that hospitalizations, deaths and post-COVID syndrome were likely prevented in the AG, we calculated estimates for the number of patients that avoided progression to any of these complications with treatment regimens.
Statistical analysis
The sample size was determined based on the assumptions that its estimate for the chi-squared test would require 80% power to detect the difference in proportions at p = 0.05, at least 95% of subjects would complete the study, and hospitalization and death rates being between 3 and 20%, and 0.3 and 2.5%, respectively.
From these assumptions, we calculated a minimum of 45 and 125 patients for each group to detect safety and efficacy differences, respectively. Nonparametric ANOVA (Kruskal-Wallis) with adjusted Dunn’s test for pairwise analyses when overall p < 0.05, assuming that all parameters were distributed non normally. All statistical tests were performed using XLSTAT version 22.4.1 (Microsoft, USA).
Results
Baseline characteristics are presented in Table 1. The proportion between sex, age and comorbidities were similar between the treated population (AG) and both control group 1 (CG1) and control group 2 (CG2), except for obesity that was estimated to be more prevalent in the expected population (CG2) than in AG and CG1. Hypertension and type 2 diabetes were numerically more prevalent in the treated population, although not statistically significant.
Table 1.
Baseline characteristics
| Baseline characteristics | Treated population (AG) (n = 585) |
Control group 1 (CG1) – Untreated population (n = 137) | Control group 2 (CG2) – Expected population characteristics paired for 585 subjectsa | Overall p-value |
|---|---|---|---|---|
| Sex | ||||
| Male (M) | 315 (53.8%) | 77 (56.2%) | 322 (55%) | n/s |
| Female (F) | 270 (46.2%) | 60 (43.4%) | 263 (45%) | |
| Age (y/o) | ||||
| Median (95%CI) | 42 (0.9) | 44 (1.8) | 45 (2.0) | n/s |
| {Min – Max age} | [19-83] | [18–74] | [n/a] | |
| Hypertension | ||||
| Number (%) | 105 (17.9%) | 22 (16.0%) | 80 (13.7%) | n/s |
| Type 2 diabetes mellitus (T2DM) | ||||
| Number (%) | 59 (10.1%) | 11 (8.0%) | 30 (5.1%) | n/s |
| Obesity | ||||
| Number (%) | 104 (17.8%) | 23 (16.8%) | 177 (30.3%) (p < 0.05 vs treated patients and CG1) |
<0.0001 |
| Overall comorbidities (except obesity) | ||||
| Number (%) | 151 (25.8%) | 26 (21.2%) | 117 (20%) | n/s |
BMI = body mass index; 95% CI = 95% confidence interval; n/a = non-applicable; n/s = non-significant.
Based on the largest cohorts of COVID-19 patients.
The main clinical outcomes are summarized in Table 2. The percentage of asymptomatic subjects was 6.6% in the AG group, 13.3% in the CG1 group, and estimates vary between 15% and 80% of patients expected to be asymptomatic. The expected control population has estimates statistically significantly higher proportions of asymptomatic patients than the groups that were followed-up (overall p < 0.0001).
Table 2.
Clinical outcomes
| Clinical outcomes | Treated population (AG) (n = 585) | Control-group 1 (CG1) – Same population controls (n = 137) | Control-group 2 (CG2) – Expected outcomes paired for 585 subjects | Overall p-value | Estimated population protected or level of reduction with treatment (compared to untreated patients) |
|---|---|---|---|---|---|
|
Asymptomatic Number (%) |
9 (6.6%) | 78 (13.3%) (p < 0.05 vs treated patients) |
88 to 468 (15 to 80%) (p < 0.05 vs treated population and CG1) |
p < 0.0001 | n/a |
|
Duration of positive rtPCR (days) Median (95%CI) |
14 (0.5) | 21 (1.7) | 20 | p < 0.0001 | 31.5 to 36.5% reduction (in viral shedding duration) |
|
Remission not including anosmia (days) Median (95%CI) |
5 (0.4) | 18 (2.6) | 19 (3.5) | p < 0.0001 | 70 to 73% reduction (in time-to-remission) |
|
Remission including anosmia (days) Median (95%CI) |
8 (0.6) | 28 (3.3) | 30 to 60 | p < 0.0001 | 70 to 85% reduction reduction (in time-to-remission) |
|
Brescia COVID-19 Respiratory Severity Scale (0–4) in day 7 Median (95%CI) |
0 (0) | n/a | 1 (1) | p < 0.0001 | 100% reduction (in respiratory complication) |
|
Hospitalization Number (%) |
0 (0) | 27 (19.7%) | 41 (7%) | p < 0.0001 | 140 to 197 hospitalizations prevented for every 1000 patients treated |
|
Mechanical ventilation Number (%) |
0 (0) | 9 (6.6%) | 29 (4.9%) 11,6% (BMJ) |
p < 0.0001a | 50 to 66 mechanical ventilations prevented for every 1000 patients treated |
|
Death Number (%) |
0 (0) | 2 (1.4%) | 3 (0.5%) 13% of 5,1% |
p < 0.0001a | 5 to 14 deaths prevented for every 1000 patients treated |
|
Post-COVID Physical symptoms Number (%) |
6 (1.1%) | 42 (30.6%) | 322 (55%) 45 to 90% |
p < 0.0001 | 295 to 541 post-COVID physical manifestations prevented for every 1000 patients treated |
|
Post-COVID Mental symptoms Number (%) |
5 (0.8%) | 38 (27.7%) | 426 (72.8%) | p < 0.0001 | 269 to 719 post-COVID mental manifestations prevented for every 1000 patients treated |
|
Post-COVID Overall symptoms Number (%) |
11 (1.9%) | 58 (42.3%) | 523 (89.5%) | p < 0.0001 | 404 to 875 post-COVID syndrome prevented for every 1000 patients treated |
BMJ = living systematic review of drugs for COVID-19 in BMJ [11]; n/a = non-applicable.
Adjusted for the impacts on larger populations.
Duration of viral shedding (Median and 95% confidence interval – 95%CI) was 14 (0.5) days in the treated population, 21 (1.7) days in the untreated population that was followed up, and expected to demonstrate a duration of 20 days in external untreated patients. Duration of SARS-CoV-2 presence was significantly shorter in the treated population (p < 0.0001 for both comparisons), with a reduction in viral load between 31.5% and 36.5% compared to the untreated populations.
Time-to-remission of clinical symptoms excluding anosmia and ageusia was (Median – 95%CI) was 5 (0.6) days in treated patients, 18 (2.6) in untreated patients and 19 (3.5) days in the estimated population. COVID-19 clinical duration was significantly lower in treated patients (p < 0.0001 versus both untreated groups), with a 70% to 73% reduction in the duration of symptoms.
Time-to-remission of clinical symptoms, including anosmia and ageusia, was (Median – 95%CI) 8 (0.6) days in the AG group, 28 (3.3) days in the CG1 group, and expected to be between 30 and 60 days in the general untreated population. COVID-19 clinical duration, including anosmia, was 70% to 85% lower in treated compared to untreated groups (p < 0.0001).
There were no hospitalizations in the treated group, 19.7% of hospitalizations in untreated patients, and an estimate of 7% of hospitalizations in the paired untreated population. Hospitalizations were significantly lower in the untreated versus treated population (p < 0.0001 for both comparisons with CG1 and CG2). For every 100,000 people infected with COVID-19, between 14,000 and 19,700 hospitalizations may have been prevented.
While none of the treated patients required mechanical ventilation, 6.6% of the CG1 and 4.9% of the expected for the CG2 group required mechanical ventilation. For every 1000 patients infected with COVID-19, any of the early interventions could prevent between 50 and 66 patients from needing mechanical ventilation.
No treated patients and 1.4% of the CG1 group deceased. In the overall untreated population, a 0.5% mortality rate is expected. When adjusted for larger populations, the mortality rate would be significantly lower in the treated group, and approximately 5000 to 14,000 deaths are estimated to be prevented for every 1,000,000 cases.
Persisting physical manifestations after COVID-19 was present in 1.1% of treated patients, 30.6% of untreated patients that were followed up, and an estimate of 55% of the overall untreated population may present post-COVID physical manifestations (p < 0.0001 for treated versus both groups of untreated populations). For every 1000 infected subjects, between 295 and 541 patients are estimated to be prevented from post-COVID physical manifestations with early pharmacological approaches.
Persisting or new-onset mental symptoms after resolution of COVID-19 are present in 0.8%, 27.7% and expectedly 72.8% of AG, GC1 and GC2, respectively. Treated patients experienced significantly fewer post-COVID mental symptoms than untreated populations (p < 0.0001), with an expected reduction of 269 to 719 subjects experiencing post-COVID mental manifestations for every 1000 infected subjects.
The prevalence of post-COVID syndrome was 1.9% in treated patients, 42.3% in untreated patients, and estimated to be up to 89.5% of the population infected. The prevalence was significantly lower in untreated patients than treated groups (p < 0.0001). Early treatment is estimated to prevent post-COVID syndrome between 404 and 875 subjects for every 1000 infected subjects.
Discussion
Superiority of early pharmacological interventions for COVID-19: apparent or actual?
When we conducted the observational study comparing HCQ, NIT and IVE, we presumed that there were no actual effective options for early COVID-19, i.e., that none of the drugs would confer any protection. As per the design, we did not include patients that did not undergo any specific treatment since our primary objective was to perform a head-to-head comparison. However, once none of the patients were hospitalized, needed mechanical ventilation, or deceased, questions regarding the superiority of any treatment over none were raised, but could not be answered by the study, as we did not originally include untreated patients, precluding us from any conclusion regarding the overall efficacy. To respond to this question raised by the clear differences between treated and overall untreated populations, we performed the present comparative analysis based on two different control groups in order to detect reproducibility and consistency between both comparisons. To avoid the potential bias of overestimating our findings, we purposely underestimated risks, complications and negative data of untreated populations and underestimated the benefits of those treated for COVID-19. For the estimation of the CG2, we have considered slightly lower disease duration, hospitalization, mechanical ventilation, death and post-COVID syndrome prevalence than those described by the literature, and we avoided the use of studies that included hospitalized patients and that had median age above 55 y/o. Despite the underestimation of the benefits of our findings and underestimation or risks related to untreated subjects, the present comparative analysis revealed differences unlikely to be random for the most relevant clinical outcomes, including reductions of one-third in viral shedding, two-thirds in clinical duration and the ability to prevent 100% of hospitalizations, mechanical ventilation and deaths, which was consistent across comparisons with CG-1, CG-2 and both. Reduction of deaths and long-term consequences were meaningful when analyzed through a public health perspective. The numbers estimated from the present findings, with at least a moderate level of certainty, that from every 1,000,000 new COVID-19 cases, at least 70,000 hospitalizations, 5000 deaths and 250,000 long-term persistence of symptoms could be prevented with the use of any of the drug combinations presented in this analysis in the seven first days of COVID-19 symptoms. From the perspective of our findings, improvements were found to be dramatic and possibly conclusive when COVID-19 is diagnosed early and one of the three pharmacological options between HCG, IVE and NTZ combined with azithromycin is offered.
While differences were in subjects with a median age below 60 y/o, in subjects above 60 y/o, differences could be more pronounced. Since our study only had fewer than 15% of patients being asymptomatic, the expected CFR for our population was higher than for those obtained through the analysis of seroprevalence.
Our prevalence of comorbidities was higher compared to sex- and age-matched untreated populations, even with lower BMI (when compared to CG2, but no CG1), possibly because we have actively searched for comorbidities that could influence risks in COVID-19. This could have negatively influenced outcomes in the AG, although underdiagnosis of comorbidities in CG2 is possible.
Whether and tol what extent the change in COVID-19 detection towards a more sensitive diagnosis may have affected outcomes in a positive manner is unknown but possible. Correspondingly, a more aggressive approach to the patient suspected for COVID-19 may have been crucial for the better outcomes found in the AG when compared to CGs.
Post-COVID syndrome as an outcome
While mortality plays a key outcome in COVID-19, the notorious presence of persisting symptoms after COVID-19 remission has called attention to the chronic aspects, possibly mediated by the triggering of immunologic maladaptations. Persistent fatigue, brain fog, reduction of cognitive functions, impaired muscle recovery, decreased physical capacity, reduced fertility and sexual function and psychiatric manifestations not fully justified by post-traumatic stress disorder (PTSD), with substantial similarities with Chronic Fatigue Syndrome (CFS) and Burnout syndrome, are among the most commonly described symptoms and may affect up to 85% of patients [[59], [60], [61], [62], [63], [64], [65], [66], [67]]. Because of the potential long-term impairment of life quality, prevention of post-COVID symptoms should be considered as a major endpoint when approaching COVID-19.
In the present study, reductions in the prevalence of post-COVID symptoms in treated compared to untreated populations were greater than differences observed in any other parameter. This finding must be emphasized as an additional benefit that may overcome potential risks of the drug use per se, i.e., even in a hypothetical absence of other benefits, prevention of post-COVID syndrome may alone be sufficient to justify the use of early pharmacological approaches for COVID-19.
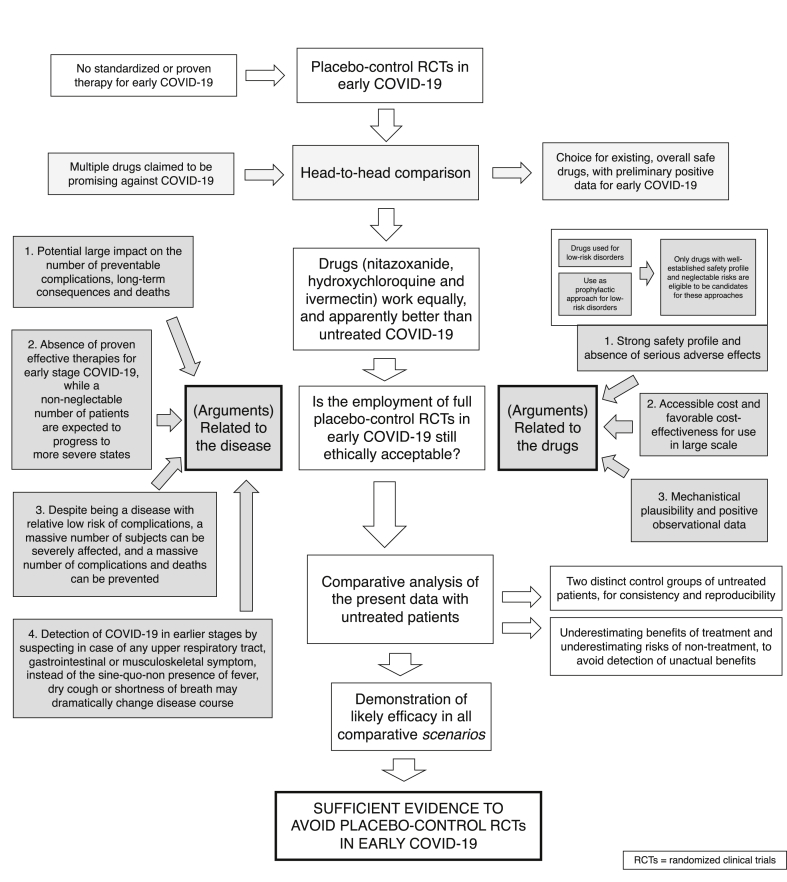
The decision of no longer use of placebo in early COVID-19
There is not a specific point from which it becomes ethically questionable to continue a clinical trial [68,69]. However, the fact that a placebo control is necessary to demonstrate efficacy is not sufficient to justify its employment in all circumstances.
While without double-blind placebo-controlled RCTs, it becomes harder to obtain evidence in terms of efficacy profile, from the perspectives of both percentage and an absolute number of patients prevented from complications, the use of safe options that demonstrated preliminary positive data and biological plausibility is highly recommended in the absence of established effective treatments.
The use of repurposed drugs presents several advantages in the context of a public health emergency: 1. The long-term safety profile is well-established, precluding from unexpected adverse effects and drug-related complications; 2. Whenever risks of adverse effects exist, these are known, which allows a directed monitoring and a more precise balancing between benefits and risks; 3. Cost-effectiveness tend to be beneficial due to lower costs of old, non-patented drugs; and 4. General physicians and healthcare providers are familiarized with already existing drugs, which allows their uses to be not only restricted to specialized centres. This last argument is particularly important in a pandemic with a massive number of new cases, which does not allow all cases to be managed within specialized centres. In the absence of proven therapies, the level of evidence required to recommend the use of drugs known to be safe and inexpensive should not be exceedingly high, at least until further definitive evidence is demonstrated for other drugs that are feasible to be administered in a large scale with accessible cost and sufficient production capacity.
In the case of HCQ, IVE and NIT use for early COVID-19, the prevention of 7%, 5% and 50% to 80% of subjects being hospitalized, intubated and suffering from chronic manifestations, respectively, their well-established safety profile for outpatient use and the social value of early treatment in a pandemic are arguments against the continuation of placebo-controlled studies for early COVID-19. In addition, the estimates for clinical outcomes in COVID-19 are largely based on population studies with positive rtPCR-SARS-CoV-2, not only tested due to symptoms or close contact with infected subjects. This is seen by the large percentage of asymptomatic patients expected for this group. This may underestimate the hospitalization and mortality rates in the estimates of the GC2.
Once hydroxychloroquine, nitazoxanide and ivermectin have been used for a wide range of diseases in the long-term for large populations with favourable cost-effectiveness even when used prophylactically, it is intuitive that their use for early COVID-19, when antiviral approaches tend to be more efficient, would be recommended, at least until evidence shows otherwise. Since the development of severe respiratory states can occur very rapidly in COVID-19, the timing to intervention is critical, and early pharmacological approaches showed to be likely efficient to prevent acute respiratory insufficiency.
Because COVID-19 is of public health importance, and the impact of the present findings is likely large, we were no longer ethically allowed to conduct studies with the employment of full placebo, and we found it mandatory to communicate our findings to the overall scientific community. Fig. 2 summarizes the rationale for the conclusions from the present analysis.
Fig. 2.
Ethics in the employment of full placebo-control RCTs in early COVID-19.
Limitations
This is a post-hoc comparative analysis that compared a group of treated patients with a variety of drug combinations with two control groups, one obtained retrospectively and one estimated for the population treated for COVID-19 with several biases, which is only able to offer evidence due to the overwhelming differences.
In particular, drugs that could disclose different results were all combined for comparison purposes. That included hydroxychloroquine, ivermectin and nitazoxanide. Although our previous observational study demonstrated similar outcomes, a formal randomized clinical trial for head-to-head comparisons have not been performed. In addition, all patients received a combination of one of these three drugs and azithromycin. Whether: 1. Azithromycin would work alone; and 2. Combination between hydroxychloroquine, ivermectin and nitazoxanide would yield better results are unknown. However, due to the complex pathophysiology, it is plausible that therapies with different _targets against SARS-CoV-2 may present synergistic activity and higher efficacy.
The last but important limitation is that this observational study was conducted during the occurrence of previous viral strains. The novel P.1 Variant of Concern (VOC) that surged in the country where the experiment was conducted, was shown to present higher infectivity, pathogenicity and poorer outcomes, with characteristics that could fulfil criteria to become the first Variant of High Consequence (VOHC) [70]. In this case, multidrug combined therapies tend to be more effective than testing or administering one or two drugs only.
Final discussion
Patients treated with azithromycin combined with nitazoxanide, hydroxychloroquine or ivermectin had significant reductions in virologic and clinical duration, hospitalization, mechanical ventilation, death and post-COVID symptoms, when compared to sex-, age- and comorbidity-matched untreated patients. The well-established safety profile of the drugs used in the present study, the likely benefits presented and the current absence of proven therapies for early COVID-19 bring ethical questions regarding the employment of placebo-control randomized clinical trials in early COVID-19. The medical decision-making on pharmacological interventions is particularly important for patients at high risk of developing severe COVID-19 and in regions where variants, mainly the P.1 variant, is highly prevalent when the natural disease course tends to be worse without pharmacological interventions.
Conclusion
Two-to four-drug treatment regimens for early COVID-19 that obligatorily including AZI, at least one between IVE, NIT and HCG, and optionally DUTA or SPIRO, were demonstrated to be very likely effective for hospitalization, deaths and prevention of post-COVID syndrome, and the use of full placebo for further RCTs on early COVID-19 should be a matter of ethical questioning.
Funding statement
The funding of present study was fully supported by Corpometria Institute (Brasilia, DF, Brazil) and Applied Biology Inc (Irvine, CA, USA).
Transparency declaration
The authors declare no conflict of interest with any of the pharmacological interventions proposed by the present study.
Authorship statement
Dr. Cadegiani was the principal investigator and contributed to the study conception and design, compiled and analyzed the data, and helped write the manuscript. Dr Goren contributed with the study design, analysis of the data, and review of the manuscript. Dr. Wambier performed the statistical analysis, analyzed the data, and helped write the manuscript. Dr. McCoy helped with the study design, helped to analyze the data, and reviewed the last version of the manuscript.
References
- 1.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;S0140–6736(20):30566–30573. doi: 10.1016/S0140-6736(20)30566-3. [published online ahead of print, 2020 Mar 11] [published correction appears in Lancet. 2020 Mar 12;:] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Booth A., Reed A.B., Ponzo S., Yassaee A., Aral M., Plans D., Labrique A., Mohan D. Population risk factors for severe disease and mortality in COVID-19: a global systematic review and meta-analysis. PLoS One. 2021 Mar 4;16(3) doi: 10.1371/journal.pone.0247461. PMID: 33661992; PMCID: PMC7932512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cadegiani F.A. Repurposing existing drugs for COVID-19: an endocrinology perspective. BMC Endocr Disord. 2020 Sep 29;20(1):149. doi: 10.1186/s12902-020-00626-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.a Gustavo Wambier C., Mehta N., Goren A., Cadegiani F. COVID-19, androgens, and androgenic alopecia. Dermatol Rev. 2021;2:146–153. doi: 10.1002/der2.50. [DOI] [Google Scholar]; b McCoy J., Wambier C.G., Herrera S., Vaño-Galván S., Gioia F., Comeche B., Ron R., Serrano-Villar S., Iwasiow R.M., Tayeb M.A., Cadegiani F.A., Mesinkovska N.A., Shapiro J., Sinclair R., Goren A. Androgen receptor genetic variant predicts COVID-19 disease severity: a prospective longitudinal study of hospitalized COVID-19 male patients. J Eur Acad Dermatol Venereol. 2021 Jan;35(1):e15–e17. doi: 10.1111/jdv.16956. Epub 2020 Oct 21. PMID: 32977355; PMCID: PMC7536899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cadegiani F., Lin E.M., Goren A., Wambier C.G. Potential risk for developing severe COVID-19 disease among anabolic steroid users. BMJ Case Rep. 2021 Feb 26;14(2) doi: 10.1136/bcr-2021-241572. PMID: 33637513; PMCID: PMC7919571. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Cadegiani F.A., Lim R.K., Goren A., McCoy J., Situm M., Kovacevic M., Vañó Galván S., Sinclair R., Tosti A., Wambier C.G. Clinical symptoms of hyperandrogenic women diagnosed with COVID-19. J Eur Acad Dermatol Venereol. 2021 Feb;35(2):e101–e104. doi: 10.1111/jdv.17004. Epub 2020 Nov 8. Erratum in: J Eur Acad Dermatol Venereol. 2021 Jul;35(7):1595. PMID: 33089570. [DOI] [PubMed] [Google Scholar]
- 7.Subramanian A., Anand A., Adderley N.J., Okoth K., Toulis K.A., Gokhale K., Sainsbury C., O'Reilly M.W., Arlt W., Nirantharakumar K. Increased COVID-19 infections in women with polycystic ovary syndrome: a population-based study. Eur J Endocrinol. 2021 May;184(5):637–645. doi: 10.1530/EJE-20-1163. PMID: 33635829; PMCID: PMC8052516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bansal P., Goyal A., Cusick A., Lahan S., Dhaliwal H.S., Bhyan P., Bhattad P.B., Aslam F., Ranka S., Dalia T., Chhabra L., Sanghavi D., Sonani B., Davis J M., 3rd Hydroxychloroquine: a comprehensive review and its controversial role in coronavirus disease 2019. Ann Med. 2020 Oct 23:1–35. doi: 10.1080/07853890.2020.1839959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lyngbakken M.N., Berdal J.E., Eskesen A., Kvale D., Olsen I.C., Rueegg C.S., Rangberg A., Jonassen C.M., Omland T., Røsjø H., Dalgard O. A pragmatic randomized controlled trial reports lack of efficacy of hydroxychloroquine on coronavirus disease 2019 viral kinetics. Nat Commun. 2020 Oct 20;11(1):5284. doi: 10.1038/s41467-020-19056-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Castelnuovo A., Costanzo S., Cassone A., Cauda R., De Gaetano G., Iacoviello L. Hydroxychloroquine and mortality in COVID-19 patients: a systematic review and a meta-analysis of observational studies and randomized controlled trials. Pathog Glob Health. 2021 Jun 15:1–11. doi: 10.1080/20477724.2021.1936818. Epub ahead of print. PMID: 34128772; PMCID: PMC8220439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartoszko J.J., Siemieniuk R.A.C., Kum E., Qasim A., Zeraatkar D., Ge L., Han M.A., Sadeghirad B., Agarwal A., Agoritsas T., Chu D.K., Couban R., Darzi A.J., Devji T., Ghadimi M., Honarmand K., Izcovich A., Khamis A., Lamontagne F., Loeb M., Marcucci M., McLeod S.L., Motaghi S., Murthy S., Mustafa R.A., Neary J.D., Pardo-Hernandez H., Rada G., Rochwerg B., Switzer C., Tendal B., Thabane L., Vandvik P.O., Vernooij R.W.M., Viteri-García A., Wang Y., Yao L., Ye Z., Guyatt G.H., Brignardello-Petersen R. Prophylaxis against covid-19: living systematic review and network meta-analysis. BMJ. 2021 Apr 26:373–n949. doi: 10.1136/bmj.n949. PMID: 33903131; PMCID: PMC8073806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.https://www.escardio.org/The-ESC/Press-Office/Press-releases/Study-shows-cardiac safety-of-hydroxychloroquine-in-COVID-19-patients
- 13.Rakedzon S., Neuberger A., Domb A.J., Petersiel N., Schwartz E. From hydroxychloroquine to ivermectin: what are the anti-viral properties of anti-parasitic drugs to combat SARS-CoV-2? J Travel Med. 2021 Feb 23;28(2):taab005. doi: 10.1093/jtm/taab005. PMID: 33480414; PMCID: PMC7928734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li N., Zhao L., Zhan X. Quantitative proteomics reveals a broad-spectrum antiviral property of ivermectin, benefiting for COVID-19 treatment. J Cell Physiol. 2020 Sep 22 doi: 10.1002/jcp.30055. doi: 10.1002/jcp.30055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jans D.A., Wagstaff K.M. Ivermectin as a broad-spectrum host-directed antiviral: the real deal? Cells. 2020 Sep 15;9(9):E2100. doi: 10.3390/cells9092100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roman Y.M., Burela P.A., Pasupuleti V., Piscoya A., Vidal J.E., Hernandez A.V. Ivermectin for the treatment of COVID-19: a systematic review and meta-analysis of randomized controlled trials. Clin Infect Dis. 2021 Jun 28:ciab591. doi: 10.1093/cid/ciab591. Epub ahead of print. PMID: 34181716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bryant A., Lawrie T.A., Dowswell T., Fordham E.J., Mitchell S., Hill S.R., Tham T.C. Ivermectin for prevention and treatment of COVID-19 infection: a systematic review, meta-analysis, and trial sequential analysis to inform clinical guidelines. Am J Ther. 2021 Jun 17 doi: 10.1097/MJT.0000000000001402. Epub ahead of print. PMID: 34145166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O Murchu E., Spillane S., Byrne P., O'Neill M., Harrington P., Ryan M. Interventions in an ambulatory setting to prevent progression to severe disease in patients with COVID-19: a systematic review. Ann Pharmacother. 2021 Jun 22 doi: 10.1177/10600280211028242. Epub ahead of print. PMID: 34157890. [DOI] [PubMed] [Google Scholar]
- 19.Hickson S.E., Margineantu D., Hockenbery D.M., Simon J.A., Geballe A.P. Inhibition of vaccinia virus replication by nitazoxanide. Virology. 2018 May;518:398–405. doi: 10.1016/j.virol.2018.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Son J., Huang S., Zeng Q., Bricker T.L., Case J.B., Zhou J., Zang R., Liu Z., Chang X., Harastani H.H., Chen L., Gomez Castro M.F., Zhao Y., Kohio H.P., Hou G., Fan B., Niu B., Guo R., Rothlauf P.W., Bailey A.L., Wang X., Shi P.Y., Whelan S.P.J., Diamond M.S., Boon A.C.M., Li B., Ding S. Nitazoxanide and JIB-04 have broad-spectrum antiviral activity and inhibit SARS-CoV-2 replication in cell culture and coronavirus pathogenesis in a pig model. bioRxiv. 2020 Sep 25 doi: 10.1128/mbio.03377-21. [Preprint] 2020.09.24.312165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rocco P.R.M., Silva P.L., Cruz F.F., Junior M.A.C.M., Tierno P.F.G.M.M., Moura M.A., De Oliveira L.F.G., Lima C.C., Dos Santos E.A., Junior W.F., Fernandes A.P.S.M., Franchini K.G., Magri E., de Moraes N.F., Gonçalves J.M.J., Carbonieri M.N., Dos Santos I.S., Paes N.F., Maciel P.V.M., Rocha R.P., de Carvalho A.F., Alves P.A., Modena J.L.P., Cordeiro A.T., Trivella D.B.B., Marques R.E., Luiz R.R., Pelosi P., E Silva JR Lapa, SARITA-2 investigators Early use of nitazoxanide in mild Covid-19 disease: randomised, placebo-controlled trial. Eur Respir J. 2021 Jan 14:2003725. doi: 10.1183/13993003.03725-2020. Epub ahead of print. PMID: 33361100; PMCID: PMC7758778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahmoud D.B., Shitu Z., Mostafa A. Drug repurposing of nitazoxanide: can it be an effective therapy for COVID-19? J Genet Eng Biotechnol. 2020 Jul 28;18(1):35. doi: 10.1186/s43141-020-00055-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Axfors C., Schmitt A.M., Janiaud P., Van't Hooft J., Abd-Elsalam S., Abdo E.F., Abella B.S., Akram J., Amaravadi R.K., Angus D.C., Arabi Y.M., Azhar S., Baden L.R., Baker A.W., Belkhir L., Benfield T., Berrevoets M.A.H., Chen C.P., Chen T.C., Cheng S.H., Cheng C.Y., Chung W.S., Cohen Y.Z., Cowan L.N., Dalgard O., de Almeida E Val F.F., de Lacerda M.V.G., de Melo G.C., Derde L., Dubee V., Elfakir A., Gordon A.C., Hernandez-Cardenas C.M., Hills T., Hoepelman A.I.M., Huang Y.W., Igau B., Jin R., Jurado-Camacho F., Khan K.S., Kremsner P.G., Kreuels B., Kuo C.Y., Le T., Lin Y.C., Lin W.P., Lin T.H., Lyngbakken M.N., McArthur C., McVerry B.J., Meza-Meneses P., Monteiro W.M., Morpeth S.C., Mourad A., Mulligan M.J., Murthy S., Naggie S., Narayanasamy S., Nichol A., Novack L.A., O'Brien S.M., Okeke N.L., Perez L., Perez-Padilla R., Perrin L., Remigio-Luna A., Rivera-Martinez N.E., Rockhold F.W., Rodriguez-Llamazares S., Rolfe R., Rosa R., Røsjø H., Sampaio V.S., Seto T.B., Shahzad M., Soliman S., Stout J.E., Thirion-Romero I., Troxel A.B., Tseng T.Y., Turner N.A., Ulrich R.J., Walsh S.R., Webb S.A., Weehuizen J.M., Velinova M., Wong H.L., Wrenn R., Zampieri F.G., Zhong W., Moher D., Goodman S.N., Ioannidis J.P.A., Hemkens L.G. Mortality outcomes with hydroxychloroquine and chloroquine in COVID-19 from an international collaborative meta-analysis of randomized trials. Nat Commun. 2021 Apr 15;12(1):2349. doi: 10.1038/s41467-021-22446-z. Erratum in: Nat Commun. 2021 May 14;12(1):3001. PMID: 33859192; PMCID: PMC8050319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blum V.F. Nitazoxanide superiority to placebo to treat moderate COVID-19 A Pilot prove of concept randomized double-blind clinical trial. EClinicalMedicine. 2021 doi: 10.1016/j.eclinm.2021.100981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cadegiani F.A. Can spironolactone be used to prevent COVID-19-induced acute respiratory distress syndrome in patients with hypertension? Am J Physiol Endocrinol Metab. 2020 May 1;318(5):E587–E588. doi: 10.1152/ajpendo.00136.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cadegiani F.A., Goren A., Wambier C.G. Spironolactone may provide protection from SARS-CoV-2: _targeting androgens, angiotensin converting enzyme 2 (ACE2), and renin-angiotensin-aldosterone system (RAAS) Med Hypotheses. 2020 Oct;143:110112. doi: 10.1016/j.mehy.2020.110112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cadegiani F.A., Wambier C.G., Goren A. Spironolactone: an anti-androgenic and anti-hypertensive drug that may provide protection against the novel coronavirus (SARS-CoV-2) induced acute respiratory distress syndrome (ARDS) in COVID-19. Front Med (Lausanne) 2020 Jul 28;7:453. doi: 10.3389/fmed.2020.00453. PMID: 32850920; PMCID: PMC7399048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCoy J., Cadegiani F.A., Wambier C.G., Herrera S., Vaño-Galván S., Mesinkovska N.A., Ramos P.M., Shapiro J., Sinclair R., Tosti A., Goren A. 5-alpha-reductase inhibitors are associated with reduced frequency of COVID-19 symptoms in males with androgenetic alopecia. J Eur Acad Dermatol Venereol. 2021 Apr;35(4):e243–e246. doi: 10.1111/jdv.17021. Epub 2020 Nov 22. PMID: 33135263. [DOI] [PubMed] [Google Scholar]
- 29.Goren A., Wambier C.G., Herrera S., McCoy J., Vaño-Galván S., Gioia F., Comeche B., Ron R., Serrano-Villar S., Ramos P.M., Cadegiani F.A., Kovacevic M., Tosti A., Shapiro J., Sinclair R. Anti-androgens may protect against severe COVID-19 outcomes: results from a prospective cohort study of 77 hospitalized men. J Eur Acad Dermatol Venereol. 2021 Jan;35(1):e13–e15. doi: 10.1111/jdv.16953. Epub 2020 Oct 21. PMID: 32977363; PMCID: PMC7536996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lazzeri Massimo, Duga Stefano, Azzolini Elena, Fasulo Vittorio, Buffi Nicolò, Saita Alberto. The humanitas COVID-19 task force, the humanitas gavazzeni COVID-19 task force, rodolfo hurle, alessandro nobili, maurizio cecconi, paolo casale, rosanna asselta impact of anti-androgen therapies on COVID-19 susceptibility: a case-control study in male population from two COVID-19 regional centers of lombardy (Italy) medRxiv. 2020 doi: 10.1101/2020.04.20.20068056. 04.20.20068056. [DOI] [Google Scholar]
- 31.Cadegiani F.A., McCoy J., Gustavo Wambier C., Goren A. Early antiandrogen therapy with dutasteride reduces viral shedding, inflammatory responses, and time-to-remission in males with COVID-19: a randomized, double-blind, placebo-controlled interventional trial (EAT-DUTA AndroCoV trial - biochemical) Cureus. 2021 Feb 1;13(2) doi: 10.7759/cureus.13047. PMID: 33643746; PMCID: PMC7885746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cadegiani F.A., McCoy J., Gustavo Wambier C., Vaño-Galván S., Shapiro J., Tosti A., Zimerman R.A., Goren A. Proxalutamide significantly accelerates viral clearance and reduces time to clinical remission in patients with mild to moderate COVID-19: results from a randomized, double-blinded, placebo-controlled trial. Cureus. 2021 Feb 22;13(2) doi: 10.7759/cureus.13492. PMID: 33633920; PMCID: PMC7899267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cadegiani F.A., Goren A., John McCoy, Wambier C.G. Hydroxychloroquine, nitazoxanide and ivermectin have similar effects in early COVID-19: a head-to-head515 comparison of the Pre-AndroCoV Trial. https://www.researchsquare.com/article/rs- 516 98106/v1
- 34.Cadegiani F.A., Goren A., McCoy John, Wambier C.G. Azithromycin with nitazoxanide, hydeoxychloroquine or ivermectin, with or without dutasteride, for early stage COVID-19: an open-label prospective observational study in males with mild-to-moderate COVID-19 (The Pre-AndroCoV Male Trial) https://www.researchsquare.com/article/rs-88952/v2
- 35.Cadegiani F.A., Goren A., Wambier C.G., McCoy John. An open-label prospective observational study of antiandrogen and non-antiandrogen early pharmacological approaches in females with mild-to-moderate COVID-19. The Pre-AndroCoV Female Trial. https://www.medrxiv.org/content/10.1101/2020.10.05.20206870v1.article-metrics
- 36.Petrilli C.M., Jones S.A., Yang J. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.https://www.uptodate.com/contents/coronavirus-disease-2019-covid-19-clinical features?topicRef=126981&source=see_link
- 38.Report of the WHO-China Joint Mission on Coronavirus DIsease 2019 (COVID-2019) February 16-24, 2020. http://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf [Google Scholar]
- 39.Verity R., Okell L.C., Dorigatti I. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis. 2020;20:669. doi: 10.1016/S1473-3099(20)30243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323:1775. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 42.Grasselli G., Pesenti A., Cecconi 541 M. Critical care utilization for the COVID-19 outbreak in lombardy, Italy: early experience and forecast during an emergency response. JAMA. 2020;323:1545. doi: 10.1001/jama.2020.4031. [DOI] [PubMed] [Google Scholar]
- 43.Meyerowitz-Katz G., Merone L. A systematic review and meta-analysis of published research data on COVID-19 infection-fatality rates. Int J Infect Dis. 2020 doi: 10.1016/j.ijid.2020.09.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Woolf S.H., Chapman D.A., Sabo R.T. Excess deaths from COVID-19 and other causes, march-april 2020. JAMA. 2020;324:510. doi: 10.1001/jama.2020.11787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stokes E.K., Zambrano L.D., Anderson K.N. Coronavirus disease 2019 case surveillance - United States, january 22-may 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:759. doi: 10.15585/mmwr.mm6924e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kragholm K., Andersen M.P., Gerds T.A. Association between male sex and outcomes of Coronavirus Disease 2019 (Covid-19) - a Danish nationwide, register553 based study. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Price-Haywood E.G., Burton J., Fort D., Seoane L. Hospitalization and mortality among black patients and white patients with covid-19. N Engl J Med. 2020;382:2534. doi: 10.1056/NEJMsa2011686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wortham J.M., Lee J.T., Althomsons S. Characteristics of persons who died with COVID-19 — United States, february 12–may 18, 2020. MMWR Morb Mortal Wkly Rep. 2020 doi: 10.15585/mmwr.mm6928e1. [DOI] [PubMed] [Google Scholar]
- 49.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goyal P., Choi J.J., Pinheiro L.C. Clinical characteristics of covid-19 in New York city. N Engl J Med. 2020;382:2372. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cohen P.A., Hall L.E., John J.N., Rapoport A.B. The 566 early natural history of SARS-CoV-2 infection: clinical observations from an urban, ambulatory COVID-19 clinic. Mayo Clin Proc. 2020;95:1124. doi: 10.1016/j.mayocp.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., Huang H., Zhang L., Zhou X., Du C., Zhang Y., Song J., Wang S., Chao Y., Yang Z., Xu J., Zhou X., Chen D., Xiong W., Xu L., Zhou F., Jiang J., Bai C., Zheng J., Song Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020 Jul 1;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.https://covidprotocols.org/protocols/clinical-course-and-epidemiology/#clinical-course
- 56.Young B.E., Ong S.W.X., Kalimuddin S., Low J.G., Tan S.Y., Loh J., Ng O.T., Marimuthu K., Ang L.W., Mak T.M., Lau S.K., Anderson D.E., Chan K.S., Tan T.Y., Ng T.Y., Cui L., Said Z., Kurupatham L., Chen M.I., Chan M., Vasoo S., Wang L.F., Tan B.H., Lin R.T.P., Lee V.J.M., Leo Y.S., Lye D.C. Singapore 2019 novel coronavirus outbreak research team. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA. 2020 Apr 21;323(15):1488–1494. doi: 10.1001/jama.2020.3204. Erratum in: JAMA. 2020 Apr 21;323(15):1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim G.U., Kim M.J., Ra S.H., Lee J., Bae S., Jung J., Kim S.H. Clinical characteristics of asymptomatic and symptomatic patients with mild COVID-19. Clin Microbiol Infect. 2020 Jul;26(7):948.e1–948.e3. doi: 10.1016/j.cmi.2020.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Almubark R.A., Memish Z.A., Tamim H., Alenazi T.H., Alabdulla M., Sanai F.M., BinDhim N.F., Alfaraj S., Alqahtani S.A. Natural history and clinical course of symptomatic and asymptomatic COVID-19 patients in the Kingdom of Saudi arabia. Saudi J Med Med Sci. 2021 May-Aug;9(2):118–124. doi: 10.4103/sjmms.sjmms_853_20. Epub 2021 Apr 29. PMID: 34084102; PMCID: PMC8152377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wijeratne T., Crewther S. 589 Post-COVID 19 Neurological Syndrome (PCNS); a novel syndrome with challenges for the global neurology community. J Neurol Sci. 2020 Oct 13;419:117179. doi: 10.1016/j.jns.2020.117179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sawadogo S.A., Dighero-Kemp B., Ouédraogo D.D., Hensley L., Sakandé J. How NETosis could drive "Post-COVID-19 syndrome" among survivors. Immunol Lett. 2020 Sep 29;228:35–37. doi: 10.1016/j.imlet.2020.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tolba M., Abo Omirah M., Hussein A., Saeed H. Assessment and characterization of post-COVID-19 manifestations. Int J Clin Pract. 2020 Sep 29 doi: 10.1111/ijcp.13746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Novak P. Post COVID-19 syndrome associated with orthostatic cerebral hypoperfusion syndrome, small fiber neuropathy and benefit of immunotherapy: a case report. eNeurologicalSci. 2020 Dec;21:100276. doi: 10.1016/j.ensci.2020.100276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Afrin L.B., Weinstock L.B., Molderings G.J. Covid-19 hyperinflammation and post-Covid-19 illness may be rooted in mast cell activation syndrome. Int J Infect Dis. 2020 Sep 10;100:327–332. doi: 10.1016/j.ijid.2020.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Donnelly S.C. Post COVID Syndrome (PCS) and healthcare workers: who cares for the carers? QJM. 2020 Sep 1;113(9):611. doi: 10.1093/qjmed/hcaa248. [DOI] [PubMed] [Google Scholar]
- 65.Iqbal F.M., Lam K., Sounderajah V., Clarke J.M., Ashrafian H., Darzi A. Characteristics and predictors of acute and chronic post-COVID syndrome: a systematic review and meta-analysis. EClinicalMedicine. 2021 May 24;36:100899. doi: 10.1016/j.eclinm.2021.100899. PMID: 34036253; PMCID: PMC8141371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fernández-de-Las-Peñas C., Palacios-Ceña D., Gómez-Mayordomo V., Florencio L.L., Cuadrado M.L., Plaza-Manzano G., Navarro-Santana M. Prevalence of post-COVID-19 symptoms in hospitalized and non-hospitalized COVID-19 survivors: a systematic review and meta-analysis. Eur J Intern Med. 2021 Jun 16;S0953–6205(21):208–209. doi: 10.1016/j.ejim.2021.06.009. Epub ahead of print. PMID: 34167876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Amdal C.D., Pe M., Falk R.S., Piccinin C., Bottomley A., Arraras J.I., Darlington A.S., Hofsø K., Holzner B., Jørgensen N.M.H., Kulis D., Rimehaug S.A., Singer S., Taylor K., Wheelwright S., Bjordal K. Health-related quality of life issues, including symptoms, in patients with active COVID-19 or post COVID-19; a systematic literature review. Qual Life Res. 2021 Jun 19:1–15. doi: 10.1007/s11136-021-02908-z. Epub ahead of print. PMID: 34146226; PMCID: PMC8214069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Millum J., Grady C. The ethics of placebo-controlled trials: methodological justifications. Contemp Clin Trial. 2013;36(2):510–514. doi: 10.1016/j.cct.2013.09.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.International ethical guidelines for biomedical research involving human subjects.Council for International Organizations of Medical Sciences. Bull Med Ethics. 2002 Oct;(182):17–23. [PubMed] [Google Scholar]
- 70.Imai M., Halfmann P.J., Yamayoshi S., Iwatsuki-Horimoto K., Chiba S., Watanabe T., Nakajima N., Ito M., Kuroda M., Kiso M., Maemura T., Takahashi K., Loeber S., Hatta M., Koga M., Nagai H., Yamamoto S., Saito M., Adachi E., Akasaka O., Nakamura M., Nakachi I., Ogura T., Baba R., Fujita K., Ochi J., Mitamura K., Kato H., Nakajima H., Yagi K., Hattori S.I., Maeda K., Suzuki T., Miyazato Y., Valdez R., Gherasim C., Furusawa Y., Okuda M., Ujie M., Lopes T.J.S., Yasuhara A., Ueki H., Sakai-Tagawa Y., Eisfeld A.J., Baczenas J.J., Baker D.A., O'Connor S.L., O'Connor D.H., Fukushi S., Fujimoto T., Kuroda Y., Gordon A., Maeda K., Ohmagari N., Sugaya N., Yotsuyanagi H., Mitsuya H., Suzuki T., Kawaoka Y. Characterization of a new SARS-CoV-2 variant that emerged in Brazil. Proc Natl Acad Sci U S A. 2021 Jul 6;118(27) doi: 10.1073/pnas.2106535118. e2106535118. PMID: 34140350. [DOI] [PMC free article] [PubMed] [Google Scholar]