Abstract
Background:
Dental pulp stem cells (DPSCs) are a unique source for future clinical application in dentistry such as periodontology or endodontics. However, DPSCs are prone to apoptosis under abnormal conditions. Taxifolin is a natural flavonoid and possesses many pharmacological activities including anti-hypoxic and anti-inflammatory. We aimed to elucidate the mechanisms of taxifolin protects DPSC under hypoxia and inflammatory conditions.
Methods:
DPSCs from human dental pulp tissue was purchased from Lonza (cat. no. PT-5025. Basel, Switzerland)) and identified by DPSC’s biomarkers. DPSC differentiation in vitro following the manufacturers’ instructions. ARS staining and Oil red staining verify the efficiency of differentiation in vitro after 2 weeks. The changes of various genes and proteins were identified by Q-PCR and western-blot, respectively. Cell viability was determined by the CCK-8 method, while apoptosis was determined by Annexin V/PI staining.
Results:
DPSC differentiation in vitro shows that hypoxia and TNF-α synergistically inhibit the survival and osteogenesis of DPSCs. A final concentration of 10 μM Taxifolin can significantly reduce the apoptosis of DPSCs under inflammation and hypoxia conditions. Taxifolin substantially increases carbonic anhydrase IX (CA9) expression but not HIF1a, and inhibitions of CA9 expression nullify the protective role of taxifolin under hypoxia and inflammatory condition.
Conclusion:
Taxifolin significantly increased the expression of CA9 when it inhibits DPSC apoptosis and taxifolin synergistically to protect DPSCs against apoptosis with CA9 under hypoxia and inflammatory conditions. Taxifolin can be used as a potential drug for clinical treatment of DPSC-related diseases.
Keywords: taxifolin, DPSCs, hypoxia and inflammation, CA9, apoptosis
Introduction
Stem cells have special ability to become more than one type of cell in the body and also to keep dividing and multiplying without limits 1 . According to their differentiation potential, stem cells can be divided into totipotent stem cells, pluripotent stem cells and unipotent stem cells 2,3 . Multipotent stem cells are of great interest in regenerative medicine due to their potential to repair damaged or diseased tissues, such as DPSCs have an ability to study multilineage differentiation in vitro and for therapeutic application 4 . What’s more, DPSCs are not only capable to be differentiated into cementoblasts, osteoblasts adipogenic, and chondrogenic lineages, but also affect the progression of translational regenerative medicine via modulating the immune response and pro-osteogenic microenvironment around them 5 –7 . A recent study has also demonstrated that DPSCs can serve as a potential treatment for periodontal regeneration 8 . In a word, DPSCs have been useful not only for dental diseases, but also for systemic other disease 9 .
But DPSCs are also highly vulnerable to a variety of damages, such as hypoxia and inflammatory, causing cell apoptosis. Hypoxia has been shown to promote apoptosis and to decrease osteogenic differentiation in a variety of cells, such as in MSCs and osteoblasts 10 –12 . Previously study implies hypoxia especially the mitochondria-dependent apoptosis pathways, which occur with a large release of cytochrome c, activated caspase-9, and caspase-3 13 . Study showed that TNF-α could induct mouse MSCs apoptosis by Fas apoptosis pathways 14 . Recent study further demonstrated the inflammatory microenvironment also to promote apoptosis and to reduce osteogenic differentiation in DPSCs 15 . Several efforts have been made to improve the property of MSCs against inflammation, including using scaffolds and cell communication 16 –18 , but there remain some limitations restrict the application of them.
Taxifolin is an active flavonoid, and it is the most prevalent class of naturally-occurring compound 10 –12 . Taxifolin has been shown to exert a wide range of biochemical and pharmacological effects, including anti-inflammatory, against oxidative stress-Induced apoptosis and it contributes to osteoclastogenesis in vitro and in vivo 13 –15 . Importantly, taxifolin exerts significant antioxidant effects that are critical in preventing the onset of apoptosis 19 .
However, potential protective effects of taxifolin to DPSC’s apoptosis and osteogenic differentiation have not been studied. Further, and the underlying molecular mechanisms have not been investigated in detail. Therefore, the present study was aimed to investigate protects of taxifolin in the apoptosis and osteogenic differentiation of DPSCs under hypoxia and inflammatory conditions, in order to reveal protects of the taxifolin signaling pathway in DPSCs anti-apoptosis and osteogenic differentiation. This may lay a foundation for the diagnosis and treatment of the future clinical application in dentistry and tissue regeneration.
Materials and Methods
Cell Culture
DPSCs from human dental pulp tissue was purchased from Lonza (cat. no. PT-5025. Basel, Switzerland)) and identified by DPSC’s biomarkers. Cryopreserved human DPSCs were recovered from liquid nitrogen using an AccuVital cell recovery kit (AccuRef Scientific, Goshen, NY, USA) and cultured as previously reported. Single-cell suspensions (0.01 to 1 × 105/well) of dental pulp were seeded into 6-well plates (Costar, USA) with alpha modification of Eagle’s medium (Gibco, BRL, USA) supplemented with 20% FBS, 1% Pen-Strept, 10 nM dexamethasone, 50 μg/ml L-ascorbic acid and 10 mM β-glycerophosphate,10 nM calcitriol (Sigma, USA) for 14 d to induce osteogenic differentiation and then incubated at 37°C in 5% CO2. For the induction of adipogenesis, alpha-minimum essential medium (MEM) supplemented with 10% FBS, 10% horse serum, 1% Pen-Strept, 100 nM dexamethasone, 0.45 mM isobutyl methyl xanthine, 3 μg/ml insulin (all purchased from Sigma, USA), and 1 μM rosiglitazone (BRL49653; Novo Nordisk, Denmark) was added to culture DPSCs for 14 d. For hypoxia treatment, DPSCs were cultured under 5% O2 in a hypoxia chamber (STEMCELL Technologies, Vancouver, USA) for 24 h at 37°C. For TNF-α treatment, cells were treated with 20 ng/ml TNF-α for 24 h before collection. The addition of Taxifolin is associated with hypoxia and TNF-α treatment.
Flow Cytometry
Cells were dissociated into single cells and then fixed with 1% (vol/vol) paraformaldehyde for 20 min at room temperature and stained with primary and secondary antibodies in PBS plus 0.1% (vol/vol) Triton X-100 and 0.5% (wt/vol) bovine serum albumin (Sangon, Shanghai, China). Flow cytometry sorting was performed to determine the expression levels of the cell surface markers CD44, CD146, and CD105. DPSCs were harvested from T75 flasks, washed and aliquoted equally into tubes. Cells were first incubated with a blocking solution containing 10% FBS at room temperature for 10 min followed by washing. Cells were then incubated with the specific antibodies conjugated with fluorochromes (Biolegends, San Diego, CA) at 4°C. After washing these cells, data were collected on a FACSCaliber flow cytometer (Beckton Dickinson, Sunnyvale, USA) and analyzed using FlowJo (Version 10.5.4, FlowJo, LLC).
DPSCs Characterization and Differentiation
Adipogenic and Osteogenic differentiation were induced as recommended by the manufacturer (R&D systems). DPSCs were confirmed using flow cytometry sorting and measuring gene expression of DPSCs markers, such as CD44, CD105, and CD146. And cells were grown in 10% FBS containing alpha minimum essential medium (α-MEM). The differentiation medium of adipocytes was changed every 3–4 days. The differentiation medium of osteoblasts was changed twice per week. DPSCs were differentiated into chondrocytes, osteoblasts and adipocytes in vitro following the protocol of differentiation. Osteoblasts and adipocytes should be observed at around 2 weeks after differentiation.
Alizarin Red S (ARS) Staining
DPSCs were seeded in 6-wells plates (1 × 105 cells/well) and cultured in osteogenic medium. After fixation with 4% PFA, the calcium deposits in the mineralization nodules formed were stained by ARS solution (Solarbio, China). To quantify ARS staining, the mineral stained was solubilized in 5% SDS in 0.5 ml 0.5 N HCl for 30 min, and the absorbance were further measured at 405 nm using a microplate reader.
Oil Red O Staining
DPSCs were seeded in 12-wells plates (1 × 105 cells/well) and cultured in adipose medium for 14 d to induce adipose differentiation. After fixation with 4% PFA, the samples were stained with Oil Red O. The staining procedure required ∼18 min for Oil Red O. To perform the Oil Red O staining, the slices were immersed in ready-to-use formalin for 1 minute, washed in running tap water, covered with the working solution for 12 min, washed in source water for 1 minute, covered with Mayer’s Hematoxylin for 1 minute, washed a second time in running tap water for 3 min, and mounted with watery media. A working solution composed of 8 mL of Oil Red O diluted with 5 mL of deionized water was prepared and left to rest for 10 min (Diapath, Bergamo, Italy). Lipids on adipose differentiation cells were highlighted in flesh red.
Cell Counting Kit-8 (CCK-8) Assay
Cells were seeded in 96-well flat-bottom microplates (Costar; Painted Post, NY, USA) with a density of 2 × 104 cells/well. After overnight incubation, the medium was replaced by DMEM containing 10% FBS. The cells were incubated for additional 24 hours, 48 hours, 72 hours, separately. At each time point, 100 µl CCK-8 reagent (EXINNO, Goshen, NY, USA) was added into each well. Cells were incubated at 37°C subsequently for 2 hours. Against a background control, the sample absorbance was tested at 450 nm via a microplate reader (Bio-Rad Laboratories, Inc.; Hercules, CA, USA).
Apoptosis Assay
DPSCs seeded in 6-well plates were cultured in vitro under conditions of hypoxia (5% O2, 24 h) and/or inflammation TNF-α (20 ng/ml, 24 h) after 24 hours. Then cells were collected after digestion with 0.25% typsin-EDTA solution (Cell biologics; Beijing, China), and subjected to apoptosis analysis using the Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) Apoptosis Kit (BD Biosciences; San Jose, CA, USA) following the manufacturer’s instructions. Briefly, after washing with PBS (phosphate-buffered saline) and the binding buffer for one time each, cells were stained with Annexin V-FITC/PI for 20 min at room temperature in a dark. After washing with the binding buffer once, the labeled cells were detected immediately by a flow cytometer (CytoFLEX; Beckman Coulter, Brea, CA, USA). Flow cytometry data were analyzed using expo32 software (Beckman Coulter).
Real-Time Quantitative PCR
Total RNA and miRNA were extracted with the TRIzol reagent (EXINNO) and the miRNeasy mini kit (Qiagen, Hilden, Germany), respectively, per the manufacturer’s protocols. Total RNA (1 µg each sample) was used to synthesize cDNA utilizing the PrimeScript® RT Master Mix Perfect Real Time Reagent Kit (Takara Bio Inc.). For miRNA reverse transcription, cDNA was synthesized using a universal tag by using a miScript II RT kit (Qiagen). Quantitative reverse transcription PCR (qRT-PCR) for miRNA and mRNA was performed using a standard protocol from the SYBR Green PCR kit (Toyobo, Osaka, Japan) on an AB7500 RT-PCR instrument (Applied Biosystems, Foster City, CA, USA). Relative quantification was determined by normalization to GAPDH. The primers for qRT-PCR analysis were as follows: ALP, forward 5′-CCATACAGGATGGCAGTGAAGG-3′, reverse 5′-TTGACCTCCTCGGAAGACACTC-3′; RUNX2, forward 5′-TGCTTTGGTCTTGAAATCACA-3′, reverse 5′-TCTTAGAACAAATTCTGCCCTTT-3′; OCN, forward 5′-TGCCTGGAGAGGAGCAGAACT-3′, reverse 5′-GGCGCTACCTGTATCAATGGC-3′; GAPDH, forward 5′-GGCATCCACTGTGGTCATGAG -3′, reverse 5′-TGCACCACCAACTGCTTAGC-3′; WNT3a, forward 5′-GTGAGGACATTGAATTTGGAGG-3′, reverse 5′-ACTTGAGGTGCATGTGACTG-3′; β-catenin, forward 5′-GCTACTGTTGGATTGATTCGAAATC-3′, reverse 5′-CCCTGCTCACGCAAAGGT-3′;The PCR reaction protocol consisted of two steps: step one, initial denaturation for 30 s at 95°C step two, denaturation for 5 s at 95°C, annealing and extension for 60 s at 60°C and fluorescence signal acquisition. The experiments were repeated for 3 times and each sample was run in triplicates. PCR product specificity was confirmed by melting curve analysis. Gene expression levels were calculated with the 2-ΔΔCT method.
Western Blot
Protein samples from DPSCs culture in vitro were obtained after cell lysis with the radioimmunoprecipitation assay lysis buffer (RIPA; EXINNO, Xi'an, China) and subsequent centrifugation at 13 000 g for 20 min at 4°C. Equal amount of protein samples (20 µg each) was separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), transferred to polyvinylidene difluoride (PVDF) membranes in an AccuRef fast transferring buffer (AccuRef Scientific), and incubated with indicated primary antibodies overnight. Antibody including anti-ALP (cat. no. ab83259), anti-RUNX2 (cat. no. ab236639), OCN (cat. no. ab133612), WNT3a (cat. no. ab219412), β-catenin (cat. no. ab32572), pro-casp3 (cat. no. ab32499), cleaved-casp3 (cat. no. ab32042), cleaved-casp-9 (cat. no. ab2324), anti-CA9 (cat. no. ab243660), anti-HIF-1a (cat. no. ab51608), GLUT1 (cat. no. ab115730), anti-pro-casp9 (cat. no ab138412), BAX (cat. no. ab32503) and the antibody for the loading control GAPDH (cat. No. ab8245) and β-actin (cat. no. ab8226) were all purchased from Abcam Company (Cambridge, UK). After binding with the secondary horseradish peroxidase (HRP)-conjugated anti-rabbit IgG (cat. no. ab7090, Abcam) or the secondary horseradish peroxidase (HRP)-conjugated anti-mouse IgG (cat. no. ab97040, Abcam), the membranes were processed with the enhanced chemiluminescence kit (EMD Millipore, Burlington, MA, USA). The expressions of _targeted proteins were quantified by densitometry using the ImageJ software (version 1.49; National Institutes of Health, Bethesda, MD, USA).
RNA Interference
CA9-specific small interfering RNAs (siRNA) (ON-_target plus Human CA9 siRNAs J-005244-05) were purchased from Dharmacon (GE Healthcare, Japan). 20 hours after DPSCs plated as monolayer in 2 × 104/cm2, DPSCs was incubated with siRNAs at 37°C for 24 hours. Cells were transfected with the CA9 siRNA oligos with Lipofectamine 3000 Transfection Reagent (Thermo Fisher Scientific, Waltham, MA, USA) following the manufacturer’s instructions. At 48 hours after transfection, cells were subjected to further analyses.
Statistics
All of the experiments in this study were repeated for at least 3 times, quantitative results are expressed as mean ± standard deviation (SD), and groups were compared using unpaired, two-tailed Student’s t-tests. Statistical analyses were performed using SPSS v. 19.0 statistical software (IBM Corporation; Armonk, NY, USA), and a P-value <0.05 was considered to be statistically significant.
Results
Differentiation of DPSCs into Osteoblasts and Adipocytes
DPSCs were expanded in a complete isolation/proliferation culture medium added with 1.25% HS. Following expansion, cell surface marker expression levels of CD44, CD105, and CD146 were evaluated for DPSCs in that the presence or absence (CD45, CD31, and CD34) of these markers are important determinants in defining “stemness” associated with DPSCs. Expansion conditions resulted in yielding DPSCs which expressed high levels of CD44 (>99.8%), CD105 (>99.9%), and CD146 (>99.7%), while expressed low levels of CD31 (<0.9%), CD34 (<1.1%), and CD45 (<1.2%) (Fig. 1A). Additionally, all these data confirmed the successful isolation of DPSCs. Isolated DPSCs from all individuals were differentiated into osteoblasts and adipocytes according toosteoblasts and adipocytes’ differentiation protocol as previously described. 0.1% ARS staining yielded a strong mineral staining at 2 wk after osteogenic differentiation (Fig. 1B). Oil red staining yielded bright-red droplets in differentiated adipocytes (Fig. 1C).
Figure 1.
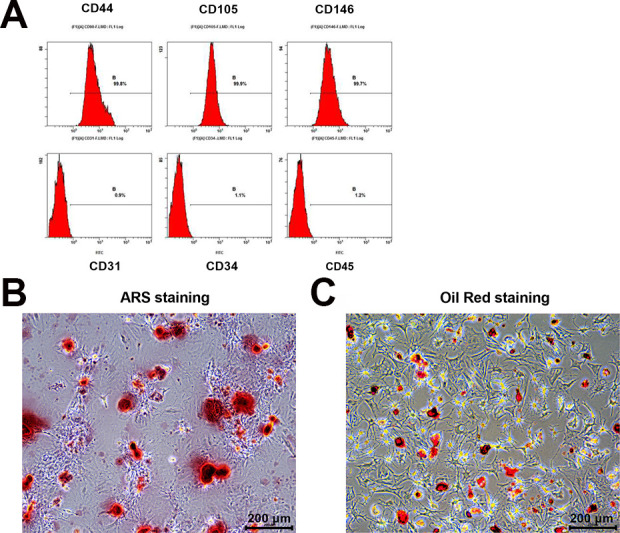
Isolation and characterization of DPSCs. (A) Representative flow cytometry data of undifferentiated DPSCs. Results indicating surface markers of DPSCs (n = 5). (B) ARS staining at 2 wk after osteogenic differentiation (n = 5). (C) Oil red staining at 2 wk after adipose differentiation (n = 5). Scale bars, 200 μm.
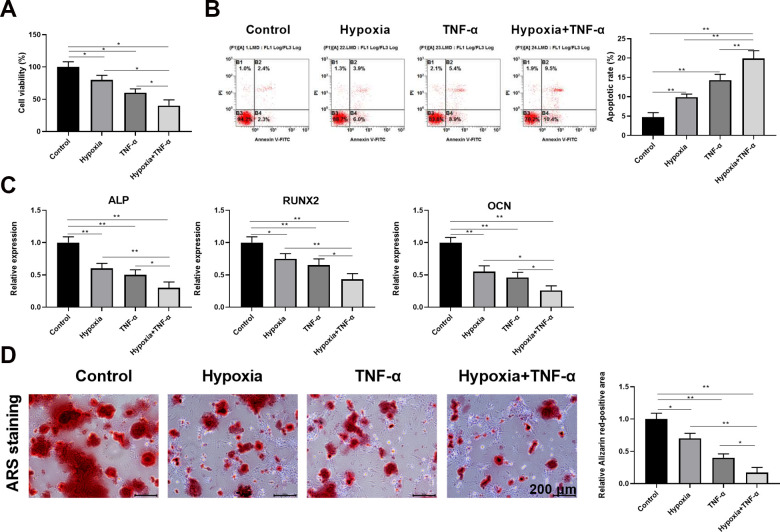
Hypoxia and TNF-α Synergistically Inhibit the Survival and Osteogenesis of DPSCs
It has been reported by other groups that DPSCs tend to apoptosis under abnormal culture conditions in vitro and in vivo. To determine what the specific phenotype of DPSC in abnormal culture conditions, we cultured and differentiated DPSCs under hypoxic and inflammatory conditions. CCK-8 analysis for the determination of cell viability in cell proliferation assays in day 30 post cultures and differentiation under normal conditions indicated 100% cell viability, while only 80% and 60% cell viability respectively under hypoxic and inflammatory conditions. Further, the cell viability is <50% under hypoxia and inflammation (Fig. 2A). The corresponding cell apoptosis results are match with the above cell viability respectively (Hypoxia+TNF-α >TNF-α >Hypoxia >Control) (Fig. 2B). Real time PCR data, expressed as differentiated DPSCs, showed an increased and comparable expression, 2 weeks of osteoblastic induction, for all the tested markers with significant differences deriving from the different proliferating conditions, as evidenced in Fig. 2C. In order to evaluate the effect of hypoxia and inflammation on osteogenic differentiation of DPSCs, proliferating DPSCs cultured in 1.25% HS were osteo-induced for 2 weeks. During the osteoblastic inductive period cells changed their osteoblasts’ morphology, 0.1% ARS staining showed that the efficiency of osteogenic differentiation under normal conditions is much greater than that of hypoxia and inflammation groups (Fig. 2D).
Figure 2.
Hypoxia and TNF-α synergistically inhibit the survival and osteogenesis of DPSCs. (A) CCK-8 analysis of cell viability under different conditions (n = 5). (B) Flow cytometric analysis of cell apoptosis in different conditions (n = 5). (C) Relative expression level of ALP, RUNX2, and OCN at 2 wk after osteogenic differentiation was determined by qRT-PCR (n = 5 for each group). (D) ARS staining showed that hypoxia and TNF-α synergistically inhibited osteoclast formation in vitro. Scale bars, 200 μm.
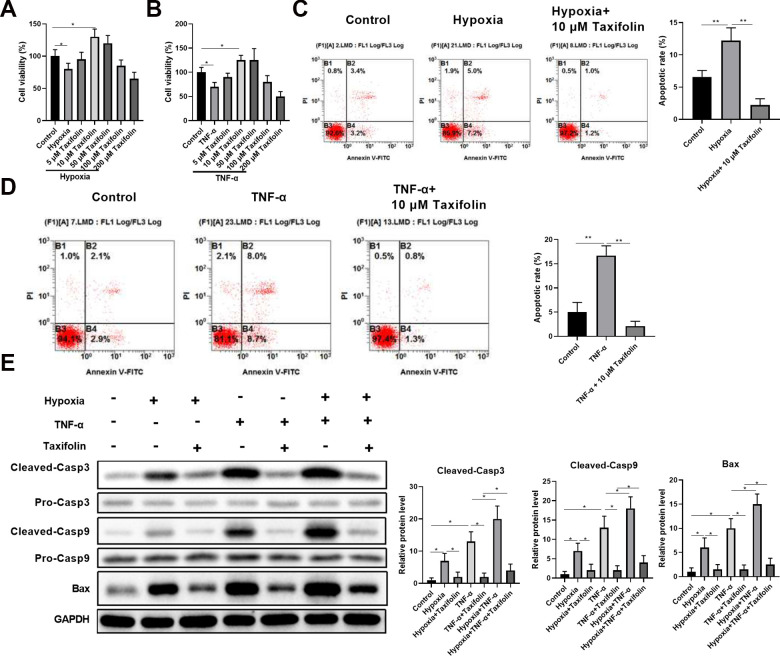
Taxifolin Protects DPSCs Against TNF-α and Hypoxia-Induced Apoptosis
To examine protective effects of taxifolin on apoptosis, DPSCs were incubated with different concentrations of taxifolin for 24 h and subsequently treated with CCK-8 for the determination of cell viability. Pretreatment with taxifolin showed a final concentration of 10 μM taxifolin can significantly improve the viability of DPSCs under inflammation- and hypoxia-induced conditions (Fig. 3A, B). Next, flow cytometric analysis showed that 10 μM taxifolin can significantly inhibit DPSCs apoptosis under hypoxic and inflammatory conditions (Fig. 3C, D). We then examined cleaved-Casp3, cleaved-Casp9and BAX protein levels by Western blotting to further analyze the potential influence of the taxifolin on inhibit DPSCs apoptosis activities. Hypoxia and TNF-α group exhibited increased ratio of these proteins, which were much higher than the taxifolin group (Fig. 3E).
Figure 3.
Taxifolin protects DPSCs against TNF-α and hypoxia-induced apoptosis. CCK-8 analysis of cell viability with the addition of different concentrations of taxifolin under hypoxia conditions (n = 5). (B) CCK-8 analysis of cell viability with the addition of different concentrations of taxifolin under TNF-α treatment (n = 5). (C, D) DPSCs (cultured with TNF-α and hypoxia) were treated with taxifolin 10 μM concentration. Flow cytometric analysis cell apoptosis in different proliferating conditions (n = 5). (E) Western blot analysis of apoptosis-related proteins. *P < 0.05; **P < 0.01 compared with indicated groups.
Taxifolin Promotes Osteogenic Differentiation of DPSCs Under Hypoxia and Inflammation Conditions
After we demonstrated that taxifolin inhibited the TNF-α and hypoxia decrease in DPSCs viability, we investigated whether taxifolin promotes ostegenic differentiation of DPSCs under hypoxia and inflammation conditions. We divided the DPSCs into seven groups: a control group, hypoxia group, TNF-α group, hypoxia and TNF-α group, hypoxia plus taxifolin (10 μM)-treated group, TNF-αplus taxifolin (10μM)-treated group, and hypoxia and TNF-α plus taxifolin (10 μM)-treated group. After incubation for 24 h, the seven groups were stained with ARS, and ARS staining was used to determine the osteogenic rate. control group, hypoxia plus taxifolin (10 μM)-treated group and TNF-αplus taxifolin (10 μM)-treated group were equivalent on ostegenic differentiation of DPSCs, which were much higher than the other groups (Fig. 4A, B). Moreover, the mRNA expression of osteogenesis differentiation markers such as ALP, OCN, Runx2, WNT3a, and β-catenin, were determined by quantitative real-time PCR, and the protein expression levels of ALP, OCN, Runx2, WNT3a, and β-catenin were determined by western blot. The results revealed that ALP, OCN, Runx2, WNT3a, and β-catenin mRNA levels were significantly elevated in DPSCs in the presence of taxifolin in a concentration dependent manner (Fig. 4C). Similarly, protein expression of ALP, OCN, Runx2, WNT3a, and β-catenin increased in the presence of taxifolin, especially at 10 μM taxifolin-treated group (Fig. 4D).
Figure 4.
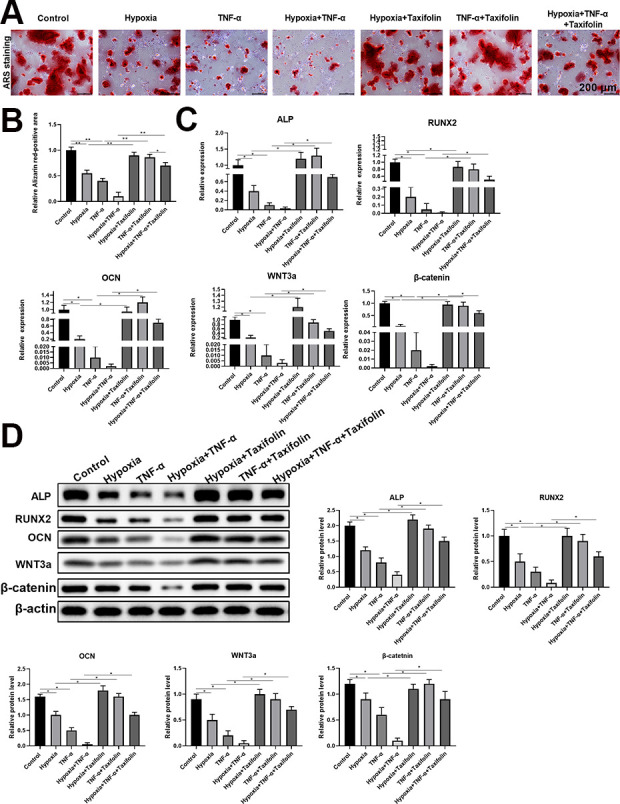
Taxifolin promotes osteogenic differentiation of DPSCs under hypoxia and inflammation conditions. (A) ARS staining at 2 wk after osteogenic differentiation (n = 5). Scale bars = 200 μm. Taxifolin significantly promoted osteogenic differentiation of DPSCs under hypoxia and inflammation conditions. (B) Quantitative data showed Alizarin red-positive areas quantified by ImageJ software. (C) The relative expression levels of ALP, OCN, RUNX2, WNT3a, and β-catenin were determined by real-time quantitative PCR. (D) The protein expression levels of ALP, OCN, RUNX2, WNT3a, and β-catenin were determined by western blot. Grayscale was quantified by ImageJ software. Data were shown as the mean ± SD (n = 5); *P < 0.05 and **P < 0.01 compared with indicated groups.
Molecular Mechanisms Involved in Taxifolin Protects DPSC
Carbonic anhydrase IX (CA9), as well as a membrane associated zinc metallo-enzyme, are reported to enhance hypoxic tumor cells to survive in the acidic microenvironment 20,21 . Therefore, we examined whether taxifolin could increase CA9 expression in a dose-dependent manner. We first determined protects of taxifolin in the DPSCs under difference conditions, the results of protects of taxifolin were significant (Fig. 5A). Western blotting results from our study showed that taxifolin significantly increased protein CA9 expression but not HIF1a, the corresponding mRNA suck as CA9, HIF-1a, and GLUT1, were determined by quantitative real-time PCR, match with Western blotting results (Fig. 5B).
Figure 5.
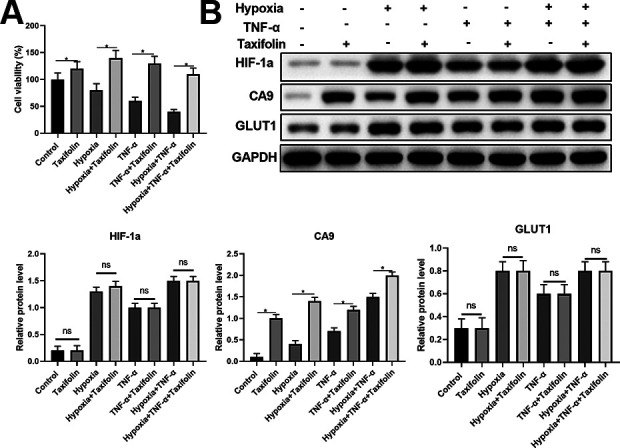
Taxifolin increases CA9 expression but not HIF1a. (A) CCK-8 assay showed the viability of DPSCs under different culture conditions with or without Taxifolin treatment. (B) The protein expression levels of HIF-1a, CA9, and GLUT1 were determined by western blot. Grayscale was quantified by ImageJ software. Data were shown as the mean ± SD (n = 5). *P < 0.05 compared with indicated groups.
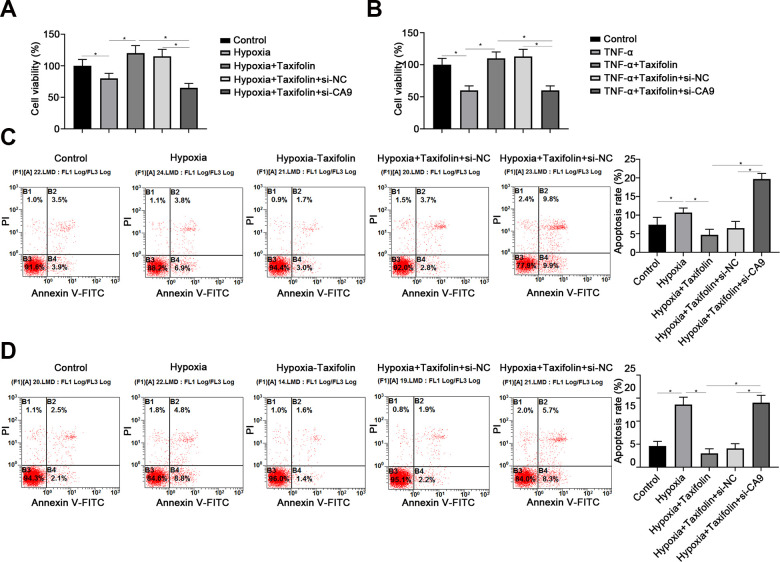
Further, to determine whether taxifolin and CA9 synergistically protect the survival and ostegenesis of DPSCs, in vitro RNAi inhibits CA9 protein expression were performed.
The results from our study demonstrated that inhibit CA9 protein expression cell viability cell survival decreased significantly, RNAi groups only 60%, while control groups have two-fold to RNAi groups (Fig. 6A, B). Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) Apoptosis Kit assay the apoptotic rate for difference groups. The results match with cell viability, which apoptosis rate of RNAi group is much higher than other groups (Fig. 6C, D).
Figure 6.
Inhibition of CA9 nullifies the protective role of Taxifolin under hypoxia and inflammation conditions. (A and B) CCK-8 assay showed the cell viability of DPSCs under hypoxia (A) or inflammation (B) conditions. Cells transfected with si-CA9 showed significant decrease of viability compared with cells transfected with scrambled siRNA (NC). (C, D) Flow cytometry analysis showed the apoptosis rate of DPSCs under hypoxia (C) or inflammation (D) conditions with or without downregulation of CA9. Data were shown as the mean ± SD (n = 5); *P < 0.05.
Discussion
Growing evidence indicates a protect action of taxifolin in cell viability and differentiation. However, the implications of taxifolin in DPSCs viability and differentiation remain unclear. Our study was the first to evaluate the protective effects of taxifolin in isolated DPSCs. We demonstrated that an important role of taxifolin in improving DPSCs cell viability, anti-hypoxic and anti-inflammatory stress and anti-apoptosis in a model of differentiation in vitro.
Stem cells area rare population of cells, which are found within the human body with the ability for proliferation, self-renewal, and differentiation into mature cells 22 . DPSCs express the special surface markers CD44, CD105, and CD146 but do not express CD34, CD31, CD45 23 . In the current study, according to the previous protocol, DPSCs has been identified by flow cytometry sorting, expanded, and cultured 24 . DPSCs cultured in vitro using this medium demonstrate multilineage differentiation capacity and protocol for adipose, myocytic, neuronal, and osteoblastic differentiation are included 25 . Our data demonstrate that osteoblastic and adipose differentiation potential is not negatively affected by our culture conditions, as evidenced by the ARS and Oil red staining data. But DPSCs is also very vulnerable to a variety of extreme culture conditions, such as hypoxia and inflammation. More and more evidence indicated that hypoxia may lead to cell apoptosis 26 . In addition, TNF-α has also been shown to enhance the apoptosis of osteoblast-like cells in vitro 27 . Therefore, we first evaluated the negative effects of hypoxia and inflammation by ARS and Oil red staining, and found that Hypoxia and TNF-α synergistically inhibit the survival and osteogenesis of DPSCs.
Taxifolin is a typical plant flavonoid, which are abundant in foods and herbs 28 . Taxifolin is also known to possess multiple pharmacological actions, such as anti-oxidant, anti-inflammatory, and anti-allergic actions 15,29 . From these reports, the compound, taxifolin, which has such as anti-apoptotic and anti-oxidant abilities, may provide the neuroprotective and Myocardial protective effect against the hypoxia-induced inflammatory response and apoptosis 30 –32 . However, the protections of taxifolin in DPSCs viability and differentiation have no reported. Results from the CCK8 assay revealed that a final concentration of 10 μM taxifolin treatment can significantly improve the viability of DPSCs, comparing with control group, hypoxia group and TNF-α group. Therefore, we chose a final concentration of 10 μM for research DPSCs protective effect of taxifolin.
We next investigated the mechanisms underlying taxifolin protects DPSCs. HIF-1a was important in cellular oxygen homeostasis and has been shown to mediate hypoxia-induced apoptosis 21,33 . So we want to know whether taxifolin exerts a protective effect through the HIF-1a pathway. But the results of western blotting and Q-PCR analysis indicated that the protein expression level of HIF-1a was not significant differences’ cells treated with taxifolin when compared to untreated cells.
HIF-1a and CA-9 are potential intrinsic markers of tumor hypoxia and predictor of an adverse disease prognosis 34 . As previously reported, CA9 is a transmembrane protein that regulates pH in tumor cells under hypoxia conditions and contributes to cell proliferation and viability 35 . Some study showed that HIF-1a activity leading to decreased expression of CA9 36 . Next, our aim investigated the CA9 expression to examine the mechanisms underlying the protective effects of taxifolin using western blot and Q-PCR. The indicated that the protein expression level of CA9 protein was markedly increased in DPSCs treated with taxifolin when compared to untreated DPSCs. Next results showed that nullify the protective role of Taxifolin under hypoxia and inflammation conditions when inhibition of CA9 expression uses siRNA method. The results from our study demonstrated that taxifolin synergistically to protect against DPSCs against apoptosis with CA9 under hypoxia and inflammatory conditions in vitro.
Some limitations of our study should be recognized. We analyzed only protects of taxifolin in vitro and didn’t analyze in vivo, such as model animals. Further research is necessary in order to reveal protects of taxifolin in dental pulp tissue in vivo. What’s more, we did not know how taxifolin synergistically to protect against DPSCs against apoptosis with CA9 under hypoxia and inflammatory conditions in cells. In the future, according to previously a conclusion, we designed related experiments to elaborate the details of the protection mechanism.
In conclusion, our study revealed a novel function of taxifolin to protect DPSCs against hypoxia and inflammatory stress, increase cell viability, and decrease cell apoptosis. The potential mechanism appears to involve up-regulation the CA9 expression and taxifolin synergistically to protect DPSCs against apoptosis with CA9 under hypoxia and inflammatory conditions. Our results provide important information on taxifolin as a possible drug for clinical treatment of DPSC-related diseases.
Supplemental Material
Supplemental Material, sj-tif-1-cll-10.1177_09636897211034452 for Taxifolin Protects Dental Pulp Stem Cells under Hypoxia and Inflammation Conditions by Xiaohui Fu, Yimiao Feng, Bingyi Shao and Yanzhen Zhang in Cell Transplantation
Footnotes
Availability of data and material: The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Authors’ Contributions: Conception and design: Xiaohui Fu, Yanzhen Zhang; Development of methodology: Yimiao Feng, Bingyi Shao; Acquisition of data: Xiaohui Fu, Yimiao Feng, Bingyi Shao; Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): Xiaohui Fu, Yimiao Feng, Bingyi Shao; Writing, review, and/or revision of the manuscript: Xiaohui Fu, Yanzhen Zhang; Study supervision: Yanzhen Zhang.
Authors Contribution: Xiaohui Fu, Yimiao Feng, the first two authors contributed equally.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was supported by the National Nature Science Foundation of China (grant number: 32000577).
ORCID iD: Yanzhen Zhang  https://orcid.org/0000-0003-4336-6283
https://orcid.org/0000-0003-4336-6283
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Ferro F, Spelat R, Baheney CS. Dental pulp stem cell (DPSC) isolation, characterization, and differentiation. Stem Cells and Tissue Repair: Springer; 2014;91–115. [DOI] [PubMed] [Google Scholar]
- 2. Shyh-Chang N, Ng H-H. The metabolic programming of stem cells. Genes Dev. 2017;31(4):336–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li L, Xie T. Stem cell niche: structure and function. Annu Rev Cell Dev Biol. 2005;21:605–631. [DOI] [PubMed] [Google Scholar]
- 4. Boroviak T, Loos R, Lombard P, Okahara J, Behr R, Sasaki E, Nichols J, Smith A, Bertone P. Lineage-specific profiling delineates the emergence and progression of naive pluripotency in mammalian embryogenesis. Dev Cell. 2015;35(3):366–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ballini A, Cantore S, Scacco S, Coletti D, Tatullo M. Mesenchymal stem cells as promoters, enhancers, and playmakers of the translational regenerative medicine 2018. Stem Cells Int. 2018;2018:6927401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Spagnuolo G, Codispoti B, Marrelli M, Rengo C, Rengo S, Tatullo M. Commitment of oral-derived stem cells in dental and maxillofacial applications. Dent J. 2018;6(4):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ballini A, Scacco S, Coletti D, Pluchino S, Tatullo M. Mesenchymal stem cells as promoters, enhancers, and playmakers of the translational regenerative medicine. Hindawi; 2017. [DOI] [PMC free article] [PubMed]
- 8. Lambrichts I, Driesen RB, Dillen Y, Gervois P, Ratajczak J, Vangansewinkel T, Wolfs E, Bronckaers A, Hilkens P. Dental pulp stem cells: their potential in reinnervation and angiogenesis by using scaffolds. J Endodont. 2017;43(9):S12–S16. [DOI] [PubMed] [Google Scholar]
- 9. Yamada Y, Nakamura-Yamada S, Kusano K, Baba S. Clinical potential and current progress of dental pulp stem cells for various systemic diseases in regenerative medicine: a concise review. Int J Mol Sci. 2019;20(5):1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim A, Nam YJ, Lee CS. Taxifolin reduces the cholesterol oxidation product-induced neuronal apoptosis by suppressing the Akt and NF-κB activation-mediated cell death. Brain Res Bullet. 2017;134:63–71. [DOI] [PubMed] [Google Scholar]
- 11. Tang Z, Yang C, Zuo B, Zhang Y, Wu G, Wang Y, Wang Z. Taxifolin protects rat against myocardial ischemia/reperfusion injury by modulating the mitochondrial apoptosis pathway. PeerJ. 2019;7:e6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang Y-J, Zhang H-Q, Han H-L, Zou Y-Y, Gao Q-L, Yang G-T. Taxifolin enhances osteogenic differentiation of human bone marrow mesenchymal stem cells partially via NF-κB pathway. Biochem Bioph Res Commu. 2017;490(1):36–43. [DOI] [PubMed] [Google Scholar]
- 13. Oi N, Chen H, Kim MO, Lubet RA, Bode AM, Dong Z. Taxifolin suppresses UV-induced skin carcinogenesis by _targeting EGFR and PI3 K. Cancer Prevent Res. 2012;5(9):1103–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Topal F, Nar M, Gocer H, Kalin P, Kocyigit UM, Gülçin İ, Alwasel SH. Antioxidant activity of taxifolin: an activity–structure relationship. J Enzyme Inhib Med Chem. 2016;31(4):674–683. [DOI] [PubMed] [Google Scholar]
- 15. Guo H, Zhang X, Cui Y, Zhou H, Xu D, Shan T, Zhang F, Guo Y, Chen Y, Wu D. Taxifolin protects against cardiac hypertrophy and fibrosis during biomechanical stress of pressure overload. Toxicol Appl Pharmacol. 2015;287(2):168–177. [DOI] [PubMed] [Google Scholar]
- 16. Bai H, Kyu-Cheol N, Wang Z, Cui Y, Liu H, Liu H, Feng Y, Zhao Y, Lin Q, Li Z. Regulation of inflammatory microenvironment using a self-healing hydrogel loaded with BM-MSCs for advanced wound healing in rat diabetic foot ulcers. J Tissue Eng. 2020;11:2041731420947242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thin Luu N, Mcgettrick HM, Buckley CD, Newsome PN, Ed Rainger G, Frampton J, Nash GB. Crosstalk between mesenchymal stem cells and endothelial cells leads to downregulation of cytokine-induced leukocyte recruitment. Stem cells. 2013;31(12):2690–2702. [DOI] [PubMed] [Google Scholar]
- 18. Marrelli M, Pujia A, Palmieri F, Gatto R, Falisi G, Gargari M, Caruso S, Apicella D, Rastelli C, Nardi GM. Innovative approach for the in vitro research on biomedical scaffolds designed and customized with CAD-CAM technology. Int J Immunopathol Pharmacol. 2016;29(4):778–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xu X, Wang Z, Liu N, Cheng Y, Jin W, Zhang P, Wang X, Yang H, Liu H, Zhang Y. Association between SOX9 and CA9 in glioma, and its effects on chemosensitivity to TMZ. Int J Oncol. 2018;53(1):189–202. [DOI] [PubMed] [Google Scholar]
- 20. Saka B, Ekinci O, Dursun A, Akyurek N. Clinicopathologic and prognostic significance of immunohistochemical expression of HIF-1α, CXCR4 and CA9 in colorectal carcinoma. Pathol Res Pract. 2017;213(7):783–792. [DOI] [PubMed] [Google Scholar]
- 21. Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010;40(2):294–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu TM, Lee EH, Lim B, Shyh-Chang N. Concise review: balancing stem cell self-renewal and differentiation with PLZF. Stem Cells. 2016;34(2):277–287. [DOI] [PubMed] [Google Scholar]
- 23. Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci. 2000;97(25):13625–13630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hilkens P, Gervois P, Fanton Y, Vanormelingen J, Martens W, Struys T, Politis C, Lambrichts I, Bronckaers A. Effect of isolation methodology on stem cell properties and multilineage differentiation potential of human dental pulp stem cells. Cell Tissue Res. 2013;353(1):65–78. [DOI] [PubMed] [Google Scholar]
- 25. Ferro F, Spelat R, Beltrami AP, Cesselli D, Curcio F. Isolation and characterization of human dental pulp derived stem cells by using media containing low human serum percentage as clinical grade substitutes for bovine serum. PLoS One. 2012;7(11):e48945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Iwai T, Obara K, Ito C, Furukawa H, Oka J-I. Hydroxyobtustyrene protects neuronal cells from chemical hypoxia-induced cell death. J Nat Med. 2018;72(4):915–921. [DOI] [PubMed] [Google Scholar]
- 27. Longcope C, Angerer B, Schröder D, Struppler A. Effect of 1alpha,25(OH)2-vitamin D3 on TNF alpha-mediated apoptosis of human primary osteoblast-like cells in vitro. Horm Metab Res. 1999;31(12):653–656. [DOI] [PubMed] [Google Scholar]
- 28. Pozharitskaya ON, Karlina MV, Shikov AN, Kosman VM, Makarova MN, Makarov VG. Determination and pharmacokinetic study of taxifolin in rabbit plasma by high-performance liquid chromatography. Phytomedicine. 2009;16(2-3):244–251. [DOI] [PubMed] [Google Scholar]
- 29. Weidmann AE. Dihydroquercetin: More than just an impurity? Eur J Pharmacol. 2012;684(1-3):19–26. [DOI] [PubMed] [Google Scholar]
- 30. Yuan M, Zhang L, You F, Zhou J, Ma Y, Yang F, Tao L. MiR-145-5p regulates hypoxia-induced inflammatory response and apoptosis in cardiomyocytes by _targeting CD40. Mol Cell Bioch. 2017;431(1):123–131. [DOI] [PubMed] [Google Scholar]
- 31. Zhang C, Zhan J, Zhao M, Dai H, Deng Y, Zhou W, Zhao L. Protective mechanism of Taxifolin for chlorpyrifos neurotoxicity in BV2 cells. Neurotoxicology. 2019;74:74–80. [DOI] [PubMed] [Google Scholar]
- 32. Chen X, Gu N, Xue C, Li BR. Plant flavonoid taxifolin inhibits the growth, migration and invasion of human osteosarcoma cells. Mol Med Rep. 2018;17(2):3239–3245. [DOI] [PubMed] [Google Scholar]
- 33. Wang X, Li J, Wu D, Bu X, Qiao Y. Hypoxia promotes apoptosis of neuronal cells through hypoxia-inducible factor-1α-microRNA-204-B-cell lymphoma-2 pathway. Exp Biol Med. 2016;241(2):177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Logsdon DP, Grimard M, Luo M, Shahda S, Jiang Y, Tong Y, Yu Z, Zyromski N, Schipani E, Carta F. Regulation of HIF1α under hypoxia by APE1/Ref-1 impacts CA9 expression: dual _targeting in patient-derived 3D pancreatic cancer models. Mol Cancer Ther. 2016;15(11):2722–2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kaluz S, Kaluzová M, Liao S-Y, Lerman M, Stanbridge EJ. Transcriptional control of the tumor-and hypoxia-marker carbonic anhydrase 9: A one transcription factor (HIF-1) show? Biochimica Biophys Acta (BBA)-Rev Cancer. 2009;1795(2):162–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fishel ML, Jiang Y, Rajeshkumar N, Scandura G, Sinn AL, He Y, Shen C, Jones DR, Pollok KE, Ivan M. Impact of APE1/Ref-1 redox inhibition on pancreatic tumor growth. Mol Cancer Therapeut. 2011;10(9):1698–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-tif-1-cll-10.1177_09636897211034452 for Taxifolin Protects Dental Pulp Stem Cells under Hypoxia and Inflammation Conditions by Xiaohui Fu, Yimiao Feng, Bingyi Shao and Yanzhen Zhang in Cell Transplantation